Abstract
Background
Clinical trial monitoring is an essential activity for quality assurance (QA) to ensure the protection of human rights and the reliability and transparency of the data collection process. The purpose of this article is to enhance the understanding of monitoring process and major findings in clinical trials of complementary and alternative medicine (CAM).
Methods
Based on International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use (ICH-GCP), we summarized main concept of monitoring process. Personal experiences on monitoring for CAM studies were also narratively described.
Results
In this brief article, the basic concept of QA and quality control (QC), various monitoring activities during the study process, and major findings regarding clinical trials of CAM are suggested in an effort to improve understanding of monitoring in clinical research on CAM.
Conclusion
When performing clinical trials for CAM-related interventions, the monitoring recommended in GCP is needed to be recognized as a mandatory element in the course of CAM research.
Keywords: Monitoring, Complementary and alternative medicine, CAM, Quality assurance, Quality control, Clinical study
Introduction
"Safeguarding the health of the people" is the primary mission of the medical profession.1 In line with this proposition, clinical research should be designed and implemented ethically and scientifically. Clinical trials in humans are inevitable in new drug or medical device development to ensure the efficacy and safety of the intervention. However, protection of human rights is paramount.
Clinical research relies on the dedication of participants who willingly take expected or unexpected risks. The principal aim of a clinical trial should be the contribution that can be made to human health and well-being by expansion of medical knowledge. Therefore, the results of clinical trials should be transparent and reliable.
Monitoring in the context of a clinical trial entails many types of systematic activity to ensure that the study is conducted and data are acquired according to the planned protocol in compliance with Good Clinical Practice (GCP) and relevant legislation.2 The reliability of the data collected cannot be ensured by the investigators' efforts alone and is only possible through systematic planned supervision of research procedures. From this point of view, monitoring has come to be recognized as an important procedure in clinical research.3
The research purpose and strategy in clinical studies involving complementary and alternative medicine (CAM) are different from those in trials of pharmacological interventions conducted for the purpose of gaining regulatory approval to market a new drug entity. The majority of CAM interventions have long been used without supporting evidence from clinical trials, and most researches have been focused mainly on establishing the safety of CAM and its effectiveness relative to conventional treatments in an effort to establish clinical evidence for CAM interventions.4 Protection of human rights, overseeing the research steps in the study plan, and confirming the accuracy of the data collected are also essential in clinical trials of CAM. The purpose of this brief review is to enhance the understanding of researchers about monitoring process in general and major considerations of CAM trials.
Basic concepts of quality assurance and quality control in clinical trials
A clinical trial should be conducted based on a predefined study protocol, and the data generated need to be documented accurately. Furthermore, the trial data must be analyzed and reported according to the study plan. Of particular importance is that the ethical conduct of the study should be in compliance with relevant regulations. Quality assurance (QA) is defined as any type of planned systematic activity intended to ensure transparency in the conduct of clinical trials, reliability of study data, and protection of human rights.
While QA is a comprehensive principle, quality control (QC) refers to specific operational techniques and activities that ensure the quality of research.2 The quality of clinical trials reflects multidimensional factors that range from the design of the study to reporting of the results, whereas QC and QA directly reflect how rigorously the study was conducted according to the research protocol and accurate collection of research data.5 Ensuring the quality of clinical trials is important, given that when pharmaceutical and medical device companies develop a new chemical entity or device, they cannot conduct the necessary clinical trials themselves. Instead, they must take a sponsorship role and rely on hospital-based investigators who are responsible for clinical practice and day-to-day conduct of a clinical trial. Therefore, it is necessary to have a detailed study plan and a strategy for determining whether or not the study is being conducted correctly. QA and QC are needed to ensure that a clinical trial is performed in accordance with the established protocol, standard operating procedures (SOPs), GCP, and relevant regulations set out by the sponsor.2 Using a strict monitoring procedure, the sponsor can evaluate the overall status of research under way at a participating institution and address any problems identified in monitoring reports so that the study is well conducted. Thus, monitoring is essential to guarantee internal validity of clinical trials.2
Procedures and methods used for trial monitoring
According to the International Conference on Harmonization of technical requirements for registration of pharmaceuticals for human use (ICH-GCP), on-site monitoring is recommended before, during, and after the trial procedure, whereas central monitoring is advised only in exceptional circumstances, which often leads to misunderstandings regarding whether or not on-site monitoring is mandatory and has priority for all cases.6 However, there is evidence suggesting that intensive on-site monitoring is not always effective in identifying errors and has a significant cost burden.3 In response, the current ICH-GCP version (R2) has added the potential benefit and role of central monitoring to its addendum section.7 The 2020 COVID-19 pandemic has rendered regular on-site monitoring impossible at present, and reliance on central monitoring is increasing. However, there are still some logistical inadequacies that make it difficult to rely on central monitoring alone. Central monitoring must include the ability to identify adverse reactions in research participants, but it is impossible to obtain the relevant data without visiting the study site. Therefore, a better QC strategy is needed to resolve the significant challenges of data monitoring in the current climate.
Sponsors need to have SOPs for clinical trials that include detailed procedures and designated personnel to perform the necessary monitoring activities. GCP states that sponsors need to decide the nature and extent of monitoring based on the specific features of the study, such as objectives, design, complexity, and size.2 Sponsors may directly employ full-time monitors or appoint a contract research organization to perform monitoring activities on their behalf. The monitors should be trained and have appropriate knowledge on the investigational product, study protocol, how to complete informed consent forms, the sponsor’s SOP, and regulatory requirements, including GCP and the relevant legislation.
Monitors should be appropriately qualified in accordance with GCP2 and usually have an academic degree in a health-related discipline such as nursing. The main roles of a monitor are defined by GCP, and are classified according to the principal purpose of monitoring as follows: first, they must ensure protection of human rights during the trial by checking that all study participants have provided written informed consent; second, they must ensure that the data collected are accurate and complete by checking the source documents; and third, they must confirm whether or not the study is conducted according to the protocol, SOP, GCP, and other regulatory requirements by verifying the qualifications of investigators, checking the status of investigational products and performance of research personnel, and confirming the management status of trial documents. Another important role is to provide a channel of communication between sponsors and investigators. Monitors need to check whether the investigators are receiving appropriate information about the trial and all necessary supplies from the sponsors. They also need to be able to report the recruitment status of the trial at any time point and the results of their monitoring activities to the sponsor.2
Monitoring activity
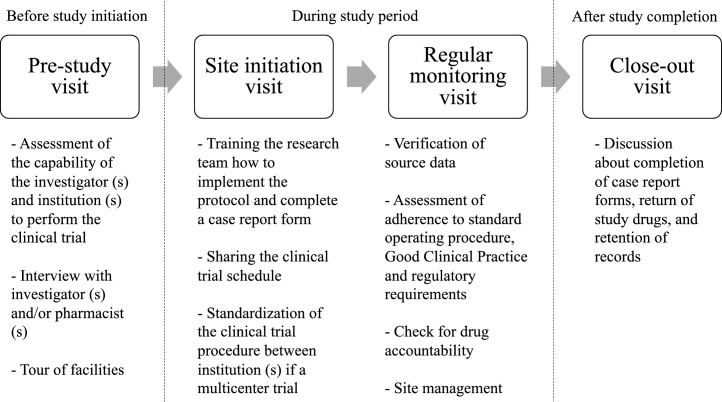
On-site monitoring is classified according to the status of a clinical trial (Fig. 1). The pre-study site visit recommended by GCP is not mandatory for monitoring. Sponsors identify the most appropriate research center for conducting a clinical trial and send a trained representative who is either employed directly by the sponsor or a clinical research associate affiliated to a contract research organization that performs trial-related duties and provides expertise as part of their contract with the sponsor.2
Fig. 1.
On-site monitoring in the different stages of a clinical trial.
During the pre-study site visit, the research capability of the site is assessed considering the adequacy of facilities, recruiting ability, and the expertise of the investigator (s). After the study protocol is approved by the institutional review board and local regulatory agency (if necessary) and the investigational products are ready for delivery, a site initiation visit is made to train the research team and prepare the necessary documents and equipment needed at the institution.
The site initiation visit is the last opportunity to check that all study-related issues have been addressed and that preparation is complete before recruitment starts. Essential documents for evaluation of trial conduct and the quality of the study data according to ICH-GCP2 need to be updated and kept secure in the research institution and sponsor site in a timely manner. The clinical research associate usually prepares packages including informed consent forms, investigator site files, and worksheets for distribution at the site initiation visit.
Monitoring visits, which are the most important activity in terms of the QA and QC of a clinical trial, start after the first participant is recruited and end when the final subject has been evaluated. Source document verification (SDV), which is not clearly referred to in ICH-GCP, is a systematic process for identifying the completeness, accuracy, and validity of data collected for each study participant and requires comparison between the source data and the case report form (CRF).8 Source data include all types of information in the original or certified copies of original records obtained during clinical trials. They are contained in the source documents, which are medical charts, laboratory reports, or work sheets, including instruments or tools for patient-reported outcomes.2
A CRF is specially prepared for each participant in a clinical trial and records all information that will be used for the analysis.2 An SDV is necessary to confirm that the data collected in the CRF are accurate and reliable and that none have been incorrectly entered or omitted because of transcription errors. It is not always possible to review every single item of study data during the SDV, and there is no clear evidence that an SDV significantly improves the integrity of the data. Therefore, partial on-site SDVs combined with centralized, risk-based monitoring are considered acceptable.9 When using this approach, monitors usually categorize data into critical (e.g., related to informed consent, evaluation for selection of participants, and adverse events) or noncritical. Critical data are monitored in all study participants, whereas noncritical data are checked in only a small sample (for example, 25%) of CRFs at a monitoring visit.8
The entire monitoring process needs to be documented, and monitoring reports must be submitted to the sponsors.2 A close-out visit is conducted generally after the last follow-up visit for the final study subject. All queries that arise during the SDV need to be resolved, and all documents related to the clinical trials should be checked at this visit. Any documents that are missing in the investigator site file or trial master file should be tracked and included at this visit. Finally, the clinical research associate must return any unused study equipment and investigational drugs or medical devices to the sponsor.
Common findings in clinical trials in complementary and alternative medicine
When CAM research is monitored by an experienced clinical research associate (CRA), there is little difference in the major findings in clinical trials between biomedicine and CAM. Problems that arise during monitoring often stem from misunderstanding of the clinical trial protocol and procedures on the part of the researchers themselves. Even for CRAs and coordinators, the most difficult part of the job is to understand and answer questions about study protocols and procedures.10 In this sense, findings of protocol deviation or trial misconducts are common in CAM trials. Based on the empirical evidence, minor findings such as deviations of window visit are the most frequent findings which can be observed in most of the CAM studies. Although important items, such as correct application of inclusion and exclusion criteria are thoroughly checked, important examination and laboratory data requested as part of the study protocol are missing sometimes. Omissions of laboratory tests and violations of random allocation are very rare but are actually observed findings. Although some of the missing data are the result of non-attendance of a study participant at a planned visit, some are related to non-compliance with the study protocol on the part of the researchers, such that no tests or evaluations are performed when the study participant attends a visit.
There is often a difference in documentation between the general informed consent form and the consent form required for collection of biological samples. Questionnaires and patient diaries, which are completed by the study participants themselves, could contain errors that need to be corrected using appropriate criteria. Researchers require education and training in how to adjust for such errors so that they can assess outcomes in a consistent way. Another common problem is lack of efficient management of clinical trial documents; for example, the investigator site file is not updated in many cases. Furthermore, essential CRF documents are sometimes missing because the information was not entered at the time of the subject’s visit. All these deviations can be prevented by education and training of researchers and monitoring to improve the quality and credibility of the study data through a QA plan (Table 1).
Table 1.
Common findings of protocol deviation or trial misconducts in complementary and alternative medicine trials.
| Type | Frequency |
|---|---|
| Deviation of window visit | Very frequent |
| Errors observed in the clinical trial document | Frequent |
| Missing in the laboratory tests | Rare |
| Omission of outcome assessments | Rare |
| Misconduct in the screening process | Very rare |
| Misconduct during the informed consent process | Very rare |
Conclusion
Monitoring is a major component of QA that can ensure the transparency and credibility of data acquired in clinical trials. Given that clinical studies in the field of CAM are on the rise and the increasing need to improve the quality of clinical studies along with quantitative growth, sponsors and researchers conducting clinical studies have to ensure a study monitoring plan. When performing clinical trials for development of CAM-related interventions, the QA monitoring recommended in GCP should be a mandatory element in the course of CAM research.
Author contributions
Conceptualization: S-YJ, JWK and T-HK Methodology: S-YJ, JWK and T-HK. Formal Analysis: S-YJ, JWK and T-HK. Investigation: S-YJ, JWK and T-HK. Resources: S-YJ, JWK and T-HK. Writing – Original Draft: S-YJ, JWK and T-HK. Writing – Review & Editing: S-YJ, JWK and T-HK. Supervision: T-HK. Project Administration: T-HK.
Conflicts of interest
There is no conflict of interest in all the authors.
Funding
This research did not receive funding from any source.
Ethical statement
There is no data which might be related to the ethical issue.
Data availability
There is no available data as this work used available literature.
References
- 1.Association W.M. Human experimentation. Code of ethics of the World Medical Association (Declaration of Helsinki) Br Med J. 1964;2:177. doi: 10.1136/bmj.2.5402.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Food U, Administation D. E6 (R2) good clinical practice: Integrated addendum to ICH E6 (R1)2018.
- 3.Baigent C., Harrell F.E., Buyse M., Emberson J.R., Altman D.G. Ensuring trial validity by data quality assurance and diversification of monitoring methods. Clin Trials. 2008;5:49–55. doi: 10.1177/1740774507087554. [DOI] [PubMed] [Google Scholar]
- 4.Fønnebø V., Grimsgaard S., Walach H. Researching complementary and alternative treatments–the gatekeepers are not at home. BMC Med Res Methodol. 2007;7:7. doi: 10.1186/1471-2288-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jüni P., Altman D.G., Egger M. Assessing the quality of controlled clinical trials. BMJ. 2001;323:42–46. doi: 10.1136/bmj.323.7303.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith C.T., Stocken D.D., Dunn J. The value of source data verification in a cancer clinical trial. PLoS One. 2012;7 doi: 10.1371/journal.pone.0051623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guideline I.H. Integrated addendum to ICH E6 (R1): guideline for good clinical practice E6 (R2) Curr Step. 2015;2:1–60. [Google Scholar]
- 8.Schuyl M.L., Engel T. A review of the source document verification process in clinical trials. Drug Inf J. 1999;33:789–797. [Google Scholar]
- 9.Olsen R., Bihlet A.R., Kalakou F., Andersen J.R. The impact of clinical trial monitoring approaches on data integrity and cost—a review of current literature. Eur J Clin Pharmacol. 2016;72:399–412. doi: 10.1007/s00228-015-2004-y. [DOI] [PubMed] [Google Scholar]
- 10.Kim S-m. Pharmaceutical Medicine and Industry, Yensei University; Seoul: 2011. The Study of Workload and Task Difficulty for Clinical Research Associate (CRA) and Clinical Research Coordinator (CRC) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
There is no available data as this work used available literature.