Abstract
The disease produced by the new coronavirus known as SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2), named COVID-19 (Coronavirus Disease-2019) has recently been classified as a pandemic by the World Health Organization (WHO). However, scarce clinical data is available and generally limited to the Chinese population due to the first cases were identified in Wuhan (Hubei, China).
This article describes the rationale and design of the HOPE COVID-19 (Health Outcome Predictive Evaluation for COVID 19) registry (ClinicalTrials.gov Identifier: NCT04334291). With an ambispective cohort design, eligible patients are those discharged, deceased or alive, from any hospital center with a confirmed diagnosis or a COVID-19 high suspicion. With a current recruitment of more than 7000 cases, in 46 hospitals in 8 countries, since it is not possible to estimate the sample size based on literature reports, the investigators will try to get the maximum numbers of patients possible. The study primary objective is all cause mortality and aims to characterize the clinical profile of patients infected in order to develop a prognostic clinical score allowing, rapid logistic decision making. As secondary objectives, the analysis of other clinical events, the risk-adjusted influence of treatments and previous comorbidities of patients infected with the disease will be performed.
The results of HOPE COVID-19 will contribute to a better understanding of this condition. We aim to describe the management of this condition as well as the outcomes in relation to the therapy chosen, in order to gain insight into improving patient care in the coming months.
Clinical Trial registration
ClinicalTrials.gov. Unique identifier: NCT04334291.
Keywords: COVID-19, Mortality, Score, Registry, Prognosis
1. Introduction
The disease caused by the new respiratory virus (coronavirus) designated as SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus 2) has recently been deemed as a pandemic by the World Health Organization (WHO) [1,2].
With an increasing number of confirmed cases in most countries worldwide, it is responsible for a significant morbidity and mortality and has motivated the implementation of measures at national and international levels with a great impact on the way of life of people throughout the whole planet.
In addition, this condition currently threatens many countries with the breakdown of health systems, producing serious logistical problems due to extensive affectation of the population, which can worsen the outcomes of those primarily affected by Coronavirus Disease-2019 (COVID-19) and secondarily the other patients with different pathologies who may suffer difficulties to get healthcare.
Limited clinical information is available and generally limited to the Chinese population, since the first cases were identified in Wuhan (Hubei, China) [3].
2. Purpose
The main objective of the present study is to characterize the clinical profile of patients infected with COVID-19 in order to develop a simple prognostic clinical score allowing a rapid logistic decision making: discharge with follow-up, referral to provisional/field hospitals or admission to regular hospital centers.
As secondary objectives, the analysis of events and the risk-adjusted influence of treatments and previous comorbidities of patients infected with the disease will be performed.
3. Design and statistical analysis
Ambispective international registry, real-life cohort “all comers” type, with voluntary participation. Without conflicts of interest, it is an investigator initiated study with advanced methodological support from the IMAS foundation (Institute for the Improvement of Health Care, Madrid, Spain).
4. Participants protocol and inclusion criteria
The study has been approved by the coordinating center Ethic's Committee (20/241-E) and the institutional board of each participating center. It has received a National drugs agency (AEMPS) classification EPA-0D.
Herein, the protocol proposes to select all the patients attended in any health center, with in hospital beds, who have been discharged or have died at the time of the evaluation.
All will be considered eligible with a positive COVID 19 test, after a RT-PCR, or if their attending physicians consider them highly likely to have presented the infection upon clinical and epidemiological evaluation in a pandemic environment. Consecutive recruitment is strongly warranted.
Given the anonymous characteristics of the registry and the health alarm situation generated by the virus, in principle, written informed consent was waived.
There are no exclusion criteria, except for the patient's or relatives’ (in intubated patients) explicit refusal to participate.
5. Data base
An anonymized database is presented in electronic format, to be filled in at each participating center (www.HopeProjectMD.com). In theory, all information could be obtained from electronic records (medical history). If deemed necessary, the investigator may call patients in order to establish their vital status (strongly warranted), as well as the results of the RT-PCR test (or others diagnostic tests available: antibodies), if they were pending during their stay.
6. Sample size
We consider it is not possible to estimate a precise sample size based on literature reports. Thus, HOPE will aim to get the maximum numbers of patients possible.
7. Outcomes
-
-
Primary: All-cause mortality. The major contributors of increased mortality will be assessed.
-
-Secondary: In stay events. Definitions available at the website.
-
∙Mechanical ventilation (invasive/non invasive).
-
∙In hospital stay.
-
∙Intensive Care admission.
-
∙Heart failure.
-
∙Renal failure.
-
∙Respiratory Insufficiency.
-
∙Upper respiratory tract involvement.
-
∙Pneumonia.
-
∙Sepsis.
-
∙Systemic inflammatory response Syndrome.
-
∙Clinically relevant bleeding.
-
∙Hemoptysis.
-
∙Embolic event
-
∙Other complications.
-
∙Causes of death.
-
∙
7.1. Statistics
Qualitative variables will be reported as their frequencies and quantitative data as the mean and standard deviation or median and interquartile range, as appropriate. Univariate analysis will be performed for qualitative variables by a mixed-model of country and reported as odds ratios (OR) with 95% confidence interval (CI). Mixed-logistic regression models will be adjusted by backward-stepwise regression based on the maximum likelihood estimators. The likelihood-ratio and its significance will be calculated for each variable according to criteria for entry (P < 0.05) and removal (P > 0.10). Thus, we will select risk factors showing a P < 0.10 in the univariate analysis or that were clinically relevant. Possible collinearity and interactions will be evaluated with the introduction of multiplicative terms.
Discriminative capacity for the models will be assessed by the area under the ROC curve (AUC) and its 95%CI. The model calibration assessing will be performed by comparing predicted versus observed probabilities after their calculation from the adjusted model coefficients. We will use the Hosmer–Lemeshow test to estimate model's goodness-of-fit. The model with the greatest discriminative power, good calibration, viable capacity, and meeting the principle of parsimony, explaining the maximum variability outcome variable with the smallest number of parameters included, is to be selected. Finally, internal validity estimation with bootstrap of 1000 samples and optimistic value will be produced.
We aim to provide a mortality risk-score from the point estimate for each variable using the OR of the final model. AUCs of the scores will be assessed with the nonparametric ROC Mann–Whitney U test. Propensity-score matching (PSM) will serve to determine how near subjects were to each other by using estimated treatment probabilities. Variables included in the PSM generally will be those identified by the multivariate analysis. In all cases, the distribution of the variable would be checked against theoretical models and the assumption of homogeneity of variance tested. In all hypothesis, tests the null hypothesis will be rejected with a type I error or α error <0.05.
7.2. HOPE-COVID-19 organization and timeline
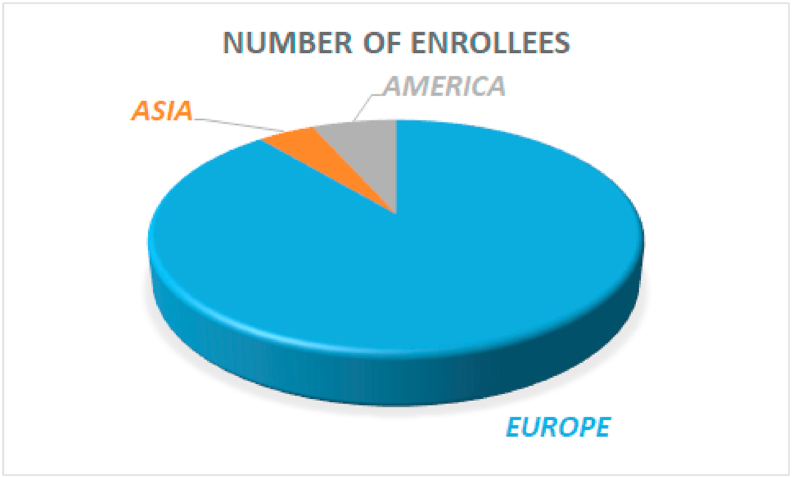
The present registry is conducted by a multinational independent research group and led by a steering committee comprised of internal medicine specialists, cardiologists and statisticians. Appendix table 1 depicts a list of centers with their principal investigators and Appendix table 2 list the multidisciplinary scientific committee. The first patient was admitted in February 2020. As of May 21ST 2020, 7170 patients from 47 sites, in 33 cities, and 8 countries (Canada, China, Chile, Cuba, Ecuador, Germany, Italy and Spain) have been recruited, Fig. 1. Final recruitment period ends on May 31st.
Fig. 1.
Enrollment stratified by area.
8. Discussion
We present, to the best of our knowledge, the rationale and design of the largest registry assessing COVID-19 patients after discharge. The international multicentric character of this investigation would provide additional and informative advantages, since most of current available data is derived from Chinese patients [3]. The first reports of COVID 19 presented a low mortality rate [[3], [4], [5]] and the disease was compared with the seasonal flu, even in older people [6]. However, actually, the death rate in Europe, United States and several other countries seems to be higher with and explosive pandemic dynamic. Thus, COVID-19 is the biggest public health problem with global dimensions we have seen in decades.
Several factors could be involved there. Among others, the race, the epidemiological measures, the way of counting, the saturation of very populated hospitals, with intensive care units collapsed and depleted availability of respirators could be of paramount importance. Therefore, it is very important to have first-hand patient level data in order to develop and produce proper logistic measures recommendations, both in countries at a current high point of the pandemic curve and in those that are starting with their cases.
In the nosocomial field, another relevant aspect is the collaboration among the medical specialties, leaded by, in normal conditions, the primary professionals in charge of theses patients: internal medicine, infectious disease specialists, pneumologist, general practitioners … These days the saturated hospitals have witnessed how the COVID-19 wards were on charge of all other specialties of physicians. COVID-19 patients could have seen these days, as primary attendings, by cardiologists, ophthalmologists, dermatologists, otorhinolaryngologists, surgeons, and so on … who had to change their professional routine meaningfully providing a so called in some places “medicine war” in a catastrophic environment. This fact mainly derived by the circumstance that hospitals, in some countries, during the hardest times in the pandemic curve, have become giants wards of COVID-19 and stopping most of other diseases admissions and delaying no fundamental/urgent procedures (surgeries, diagnostics tests, imaging …) [7]. Probably, the home confinement, the professionals attempt to attend patients by telephone to prevent them from visiting flooded hospitals or offices and more importantly, the fear of patients and relatives to go to the dreaded and “contagious” emergency rooms explain an intense decrease in admission of no COVID-19 conditions, with even the delay in presentation of other urgent diseases, like acute myocardial infarction [7], with dramatic results.
9. Limitations
We recognize some limitations in the registry, inherent to this type of observational design. The focus will be put in the most severe patients, those needing admission, so the extrapolation to the general population would be complex but otherwise allowing the identification of most complications. With the current participating centers distribution, we feel the analysis would be closer to reality for Western Europe Caucasians and perhaps for Latins/Hispanic people, but not so close/certain for Asians or Blacks. Nevertheless, its data probably reflect the real-life practice in certain countries at a concrete point of the pandemic curve.
10. Conclusion
The results of HOPE COVID-19 will contribute to a better understanding of this condition, primarily focusing in mortality. It will define a precise epidemiological profile of COVID-19 patients with higher severity and thus, admitted to a hospital. We aim to describe the management of this condition as well as the outcomes in relation to the therapy chosen, in order to gain insight into improving patient care in the coming months.
HOPE COVID-19 will be open to further collaborations and subanalysis with other international research groups.
Funding
Non conditioned grant (Fundación Interhospitalaria para la Investigación cardiovascular, FIC. Madrid, Spain). This nonprofit institution had no role in the study design; collection, analysis, interpretation of data; in the writing of the report; nor in the decision to submit the paper for publication.
Declaration of competing interest
1) We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
2) None coauthor has any disclosure.
Acknowledgements
Cardiovascular Excellence SL, for their essential support in the database and HOPE webpage. All HOPE researchers.
Appendix data.
Table 1.
Principal investigators, country, city and centers with at least 1 enrollee at the moment.
| NAME | SURNAME | HOSPITAL | CITY | COUNTRY |
|---|---|---|---|---|
| Rodrigo | Bagur | London Health Sciences Center | London | Canada |
| Daniela | Coca | Hospital de Quilpue | Quilpue | Chile |
| Jia | Huang | The Second Affiliated Hospital of Southern University of Science and Technology | Shenzhen | China |
| Emilio | Alfonso | Institute of Cardiology and Cardiovascular Surgery | Havana | Cuba |
| Alex Fernando | Castro Mejía | Hospital General del norte de Guayaquil IESS Los Ceibos | Guayaquil | Ecuador |
| Jorge | Jativa Mendez | H de Especialidades de las Fuerzas Armadas | Quito | Ecuador |
| Jorge Luis | Valdez | Hospital Pablo Arturo Suárez | Quito | Ecuador |
| Adelina | González | Hospital Universitario Infanta Sofia. San Sebastián de los Reyes.Madrid | Madrid | Spain |
| Luis | Buzón | Hospital Universitario de Burgos | Burgos | Spain |
| Christoph | Liebetrau | Kerckhoff Heart and Thorax Center | Bad Nauheim | Germany |
| Ibrahim | El-Battrawy | University Medical Center Mannheim | Mannheim | Germany |
| Bernardo | Cortese | San Carlo Clinic | Milan | Italy |
| Federico | Guerra | Ospedali Riuniti “Umberto I - Lancisi - Salesi" | Ancona | Italy |
| Daniela | Trabattoni | Centro Cardiologico Monzino, IRCCS | Milan | Italy |
| Fabrizio | D'Ascenzo | San Giovanni Battista | Turin | Italy |
| Danilo | Buonsenso | Policlinico Universitario Agostino Gemelli IRCSS | Rome | Italy |
| Massimo | Mancone | Policlinico Umberto I | Rome | Italy |
| Fabrizio | Ugo | Sant'Andrea Hospital | Vercelli | Italy |
| Enrico | Cerrato | San Luigi Gonzaga University Hospital, Orbassano and Rivoli Infermi Hospital, Rivoli (Turin), Italy | Turin | Italy |
| Martino | Pepe | Azienda ospedaliero-universitaria consorziale policlinico di Bari | Bari | Italy |
| Andrea | Rognoni | A.O.U. Maggiore dellaq CaritÃ, novara | Novara | Italy |
| Maurizio | Bertaina | Martini Hospital, via Tofane, 71 — TORINO | Turin | Italy |
| Mario | Iannaccone | San Giovanni Bosco, ASL Citta di Torino, Turin, Italy | Turin | Italy |
| Francesco | Santoro | ASL BAT | Andria | Italy |
| Enrico | Pinotti | Policlinico San Pietro | Ponte San Pietro | Italy |
| Álvaro | López Masjuán | Hospital Universitario Juan Ramó³n Jimenez | Huelva | Spain |
| Aitor | Uribarri | Hospital Clínico Universitario de Valladolid | Valladolid | Spain |
| Oscar | Fabregat-Andres | Hospital IMED Valencia | Valencia | Spain |
| Victor Manuel | Becerra-Muñoz | Hospital Clínico Universitario Virgen de la Victoria | Málaga | Spain |
| José | Moreu | complejo hospitalario de toledo | Toledo | Spain |
| Gisela | Feltes | Nuestra Señora de América | Madrid | Spain |
| Javier | Lopez-Pais | Hospital Clínico de Santiago de Compostela | Santiago de Compostela | Spain |
| María C. | Viana-LLamas | Hospital Universitario Guadalajara | Guadalajara | Spain |
| Jaime | Signes-Costa | Clinico de Valencia | Valencia | Spain |
| Vicente | Estrada | Clínico San Carlos | Madrid | Spain |
| Inmaculada | Fernández Rozas | Hospital Universitario Severo Ochoa | Leganés | Spain |
| Alfredo | Bardaji | Joan XXIII | Tarragona | Spain |
| Francisco | Marín | Hospital Clínico Universitario Virgen de la Arrixaca | Murcia | Spain |
| Thamar | Capel Astrua | Hospital Virgen del Mar | Madrid | Spain |
| Rodolfo | Romero | Hospital Universitario Getafe | Getafe (Madrid) | Spain |
| Sergio | Raposeiras Roubin | H Álvaro Cunqueiro | Vigo | Spain |
| Charbel | Maroun Eid | HOSPITAL UNIVERSITARIO LA PAZ. Instituto de Investigación Hospital Universitario La Paz (IdiPAZ). | Madrid | Spain |
| Marcos | García Aguado | Hospital Puerta de Hierro de Majadahonda | Majadahonda. Madrid | Spain |
| Miguel | Corbi-Pascual | Hospital General de Albacete | Albacete | Spain |
| Carolina | Espejo Paeres | Hospital Universitario PrÃncipe de Asturias, Madrid, Spain. | Madrid | Spain |
| Juan Fortunato | Garcia Prieto | Hospital de Manises | Valencia | Spain |
Table 2.
Scientific Committee:
| Ivan J Nuñez Gil | Hospital Clinico San Carlos, Madrid | SPAIN | Cardiology |
|---|---|---|---|
| Carlos Macaya | Hospital Clinico San Carlos, Madrid | SPAIN | Cardiology |
| Asunción Guerri | Hospital Nuestra Señora de América, Madrid | SPAIN | Internal Medicine |
| Charles Lefranc | Hospital Nuestra Señora de América, Madrid | SPAIN | Intensive Care Medicine |
| David Orgaz | Atención Primaria, Madrid | SPAIN | GP |
| Enma Gil-Higes | Atención Primaria, Madrid | SPAIN | GP |
| Juan Conesa | Hospital Clinico San Carlos, Madrid | SPAIN | Intensive Care Medicine |
| Antonio Fernández Ortiz | Hospital Clinico San Carlos, Madrid | SPAIN | Cardiology |
| Harish Ramakhrisna | Mayo Clinic, Minneapolis | United States | Anesthesiology and Perioperative Medicine |
| Julio Jimenez | SUMMA 112, Madrid | SPAIN | Out of hospital Care |
| Vanessa Garcia de Biedma | Hospital Universitario de Fuenlabrada | SPAIN | Internal Medicine |
| Ruth Sendino | Atención Primaria, Vitoria | SPAIN | GP |
| Gisela Feltes | Hospital Nuestra Señora de América, Madrid | SPAIN | Cardiology |
| Sergio Raposeiras Roubin | Hospital Alvaro Cunqueiro, Vigo | SPAIN | Cardiology |
| Giusseppe Biondi Zoccai | Sapienza University of Rome, Latina, Italy | ITALY | Cardiology |
| Angel Molino | Hospital Clinico San Carlos, Madrid | SPAIN | Internal Medicine |
| Cristina Fernandez | Hospital Clinico San Carlos, Madrid | SPAIN | Preventive Medicine and Bioestadistics |
| Vicente Estrada | Hospital Clinico San Carlos, Madrid | SPAIN | Internal Medicine |
| Fabrizio D'Ascenzo | Città della Salute e della Scienza, Turin | ITALY | Cardiology |
| Enrico Cerrato | San Luigi Gonzaga University Hospital, Rivoli, Turin. | ITALY | Cardiology |
| F Javier Martin-Sanchez | Hospital Clinico San Carlos, Madrid | SPAIN | Geriatrics. Emergency Medicine. |
References
- 1.WHO. WHO statement regarding cluster of pneumonia cases in Wuhan, China [Available from: https://www.who.int/china/news/detail/09-01-2020-who-statement-regarding-cluster-of-pneumonia-cases-in-wuhan-china.
- 2.WHO. http://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/3/who-announces-covid-19-outbreak-a-pandemic..
- 3.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese center for disease control and prevention. J. Am. Med. Assoc. 2020;323(13):1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- 5.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. Feb 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen T., Dai Z., Mo P. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): a single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020 Sep 16;75(9):1788–1795. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Leor O., Cid-Alvarez Belen, Ojeda S., Martin Moreiras J., Rumoroso J.R., Lopez-Palop R. 2020 Apr 4. Impacto de la pandemia de COVID-19 sobre la actividad asistencial en cardiología intervencionista en España. REC: interventional cardiology. (English version will be available soon) [DOI] [Google Scholar]