Abstract
Alphitobius diaperinus Panzer, 1797 is a major pest in poultry production and easily observed in poultry litter. We have determined mitochondrial genome of A. diaperinus collected in Chungcheongbuk-do, Republic of Korea. The circular mitogenome of A. diaperinus is 15,511 bp long which is longer than that of Z. atratus but shorter than that of T. obscurus. It includes 13 protein-coding genes, two ribosomal RNA genes, and 22 transfer RNAs. The base composition was AT-biased (72.4%). Phylogenetic tree displays that tribe Alphitobiini is clustered with tribes Helopini and Diaperini with enough supportive values of three phylogenetic trees.
Keywords: Mitochondrial genome, Alphitobius diaperinus, Coleoptera, Tenebrionidae, South Korea
The darkling beetle, Alphitobius diaperinus Panzer, 1797 (Coleoptera: Tenebrionidae), is a significant pest in poultry worldwide. Though these beetles are small, their importance as a poultry pest is enormous. It is also considered a primary structural pest in the poultry industry, causing extensive damage to broiler housing, which has led to increased heating and repair costs for poultry producers (Axtell and Arends 1990).
To understand its genetic background, we completed mitogenome of A. diaperinus, as the first mitochondrial genome in tribe Alphitobiini, collected in Daeso-myeon, Eumseong-gun, Chungcheongbuk-do, Republic of Korea (36°96′77″N, 127°51′61″E; the specimen and its DNA were deposited at the Sunchon National University, Korea; Accession number: 190925HK004). DNA was extracted using DNeasy Blood &Tissue Kit (QIAGEN, Hilden, Germany). Raw sequences from Illumina HiSeqX (Macrogen, Korea) were filtered by Trimmomatic 0.33 (Bolger et al. 2014) and de novo assembled using Velvet 1.2.10 (Zerbino and Birney 2008), SOAPGapCloser 1.12 (Zhao et al. 2011), BWA 0.7.17 (Li 2013), and SAMtools 1.9 (Li et al. 2009). Geneious R11 11.1.5 (Biomatters Ltd, Auckland, New Zealand) was used to annotate based on several mitogenomes including Zophobas atratus (NC_041101; Bai et al. 2019), Tenebrio obscurus (NC_037196; Bai et al. 2018), and Tenebrio molitor (NC_024633; Li-Na and Cheng-Ye 2014).
Alphitobius diaperinus mitogenome (GenBank accession is MT165524) is 15,511 bp long, which is longer than that of Z. atratus (15,494 bp) but shorter than those of T. obscurus (15,771 bp) and T. molitor (15,785 bp). It contains 13 protein-coding genes (PCGs), 37 tRNAs, and two rRNAs. The base composition was AT-biased (72.4%) and gene order was identical to 23 available Tenebrionidae mitogenomes.
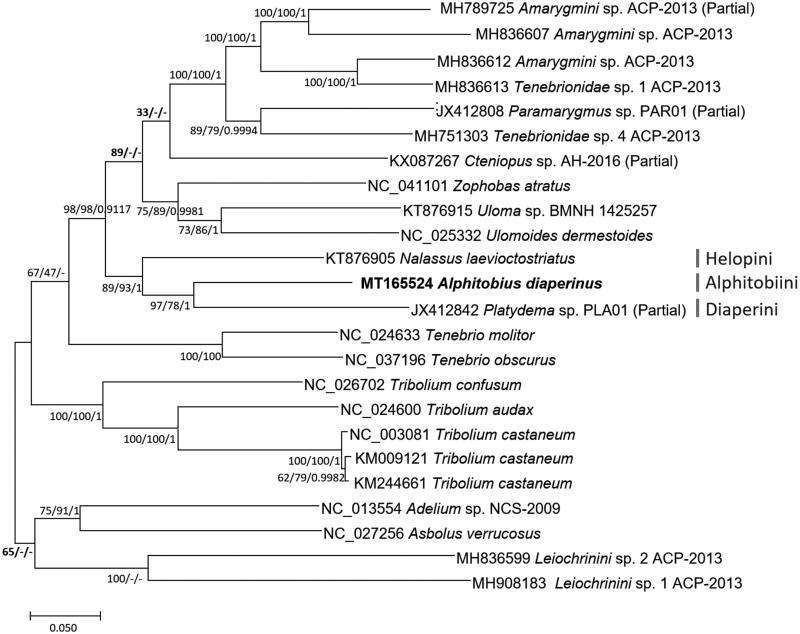
We inferred the phylogenetic relationship of 24 Tenebrionidae mitogenomes, including A. diaperinus mitogenome. Complete mitochondrial genomes were aligned by MAFFT 7.450 (Katoh and Standley 2013) after rearranging sequences including utilizing reverse complement sequences and moving unaligned sequences to the end of mitogenomes. Multiple sequence alignment was used for constructing bootstrapped neighbor-joining, maximum-likelihood, and Bayesian inference phylogenetic trees with MEGA X (Kumar et al. 2018) and Mr. Bayes (Huelsenbeck and Ronquist 2001), respectively. Phylogenetic tree displays that tribe Alphitobiini is clustered with tribes Helopini and Diaperini with enough supportive values of three phylogenetic trees (Figure 1). Some of higher clades not supported by three phylogenetic trees (see bold supportive values in Figure 1) indicate that the phylogenetic relationship of species in Tenebrioninae should be investigated more with additional mitochondrial genomes which will be available in near future. In addition, three mitochondrial genomes of Tribolium castaneum display high supportive values of three phylogenetic trees, which is similar to those of Laodelphax striatellus (Park, Jung, et al. 2019; Seo et al. 2019) and Stegobium paniceum (Park et al., under review); while it is different from those of Nilaparvata lugens (Choi et al. 2019; Park, Kwon, et al. 2019; Choi et al., under review).
Figure 1.
Bayesian inference (1,000,000 generations), maximum-likelihood (1000 bootstrap repeats), and neighbor-joining (10,000 bootstrap repeats) phylogenetic trees of 24 Tenebrionidae mitochondrial genomes: Alphitobius diaperinus (MT165524), Amarygmini sp. (MH789725; Partial mitochondrial genome; MH836607, and MH836612), Tenebrionidae sp. (MH836613 and MH751303), Paramarygmus sp. (JX412808; Partial mitochondrial genome), Cteniopus sp. (KX087267; Partial mitochondrial genome), Zophobasatratus (NC_041101), Uloma sp. (KT876915), Ulomoides dermestoides (NC_025332), Nalassus laevioctostriatus (KT876905), Platydema sp. (JX412842), Tenebrio molitor (NC_024633), Tenebrio obscurus (NC_037196), Tribolium confusum (NC_026702), Tribolium audax (NC_024600), Tribolium castaneum (NC_003081, KM009121, and KM244661), Adelium sp. (NC_013554), Asbolus verrucosus (NC_027256), and Leiochrinini sp. (MH836599 and MH908183). Phylogenetic tree was drawn based on maximum-likelihood tree. The numbers above branches indicate bootstrap support values of maximum-likelihood and neighbor-joining phylogenetic trees and posterior probability value of Bayesian inference tree, respectively. Tribe names were displayed as light gray color. Bolded supportive values indicate nodes that all three phylogenetic trees show different topologies.
Funding Statement
This study was supported by the R&D project ‘Study on infestation status and control measures of darkling beetle in broiler house [project number: Z-1543084-2018-19-01]’.
Disclosure statement
The authors declare that they have no competing interests.
Data availability statement
The data that support the findings of this study are openly available in NCBI (National Center for Biotechnology Information) at https://www.ncbi.nlm.nih.gov/nuccore/MT165524.
References
- Axtell RC, Arends JJ. 1990. Ecology and management of arthropod pests of poultry. Annu Rev Entomol. 35:101–125. [DOI] [PubMed] [Google Scholar]
- Bai Y, Li C, Yang M, Liang S. 2018. Complete mitochondrial genome of the dark mealworm Tenebrio obscurus Fabricius (Insecta: Coleoptera: Tenebrionidae). Mitochondrial DNA B. 3(1):171–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Wang H, Li G, Luo J, Liang S, Li C. 2019. Complete mitochondrial genome of the super mealworm Zophobas atratus (Fab.) (Insecta: Coleoptera: Tenebrionidae). Mitochondrial DNA B. 4(1):1300–1301. [Google Scholar]
- Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 30(15):2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi NJ, Lee B-C, Park J, Park J. 2019. The complete mitochondrial genome of Nilaparvata lugens (Stål, 1854) captured in China (Hemiptera: Delphacidae): investigation of intraspecies variations between countries. Mitochondrial DNA B. 4(1):1677–1678. [Google Scholar]
- Choi N-J, Lee B-C, Park J, and Park J. (Under review). The complete mitochondrial genome of Nilaparvata lugens (Stål, 1854) captured in Guangxi province, China (Hemiptera: Delphacidae): identification of the origin of N. lugens migrated to Korea, Mitochondrial DNA Part B, 5:2, 1960–1961. [Google Scholar]
- Huelsenbeck JP, Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 17(8):754–755. [DOI] [PubMed] [Google Scholar]
- Katoh K, Standley DM. 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol Biol Evol. 30(4):772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Li M, Knyaz C, Tamura K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 35(6):1547–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li-Na L, Cheng-Ye W. 2014. Complete mitochondrial genome of yellow meal worm (Tenebrio molitor). Zool Res. 35(6):537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. 2013. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. arXiv preprint arXiv:13033997. [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics. 25(16):2078–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Jung JK, Ho Koh Y, Park J, Seo BY. 2019. The complete mitochondrial genome of Laodelphax striatellus (Fallén, 1826) (Hemiptera: Delphacidae) collected in a mid-Western part of Korean peninsula. Mitochondrial DNA B. 4(2):2229–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Kwon W, Park J, Kim H-J, Lee B-C, Kim Y, Choi NJ. 2019. The complete mitochondrial genome of Nilaparvata lugens (stål, 1854) captured in Korea (Hemiptera: Delphacidae). Mitochondrial DNA B. 4(1):1674–1676. [Google Scholar]
- Park J, Lee J, and Park J, The complete and intraspecific characteristics of mitochondrial genome of Stegobium paniceum (Linnaeus, 1758) (Coleoptera: Anobiidae) assembled from NGS raw reads of truffle, Tuber melanosporum, PLoS One, under revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BY, Jung JK, Ho Koh Y, Park J. 2019. The complete mitochondrial genome of Laodelphax striatellus (Fallén, 1826) (Hemiptera: Delphacidae) collected in a southern part of Korean peninsula. Mitochondrial DNA B. 4(2):2242–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. 2008. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 18(5):821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao QY, Wang Y, Kong YM. 2011. Optimizing de novo transcriptome assembly from short-read RNA-Seq data: a comparative study. BMC Bioinformatics;12(Suppl 14):S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available in NCBI (National Center for Biotechnology Information) at https://www.ncbi.nlm.nih.gov/nuccore/MT165524.