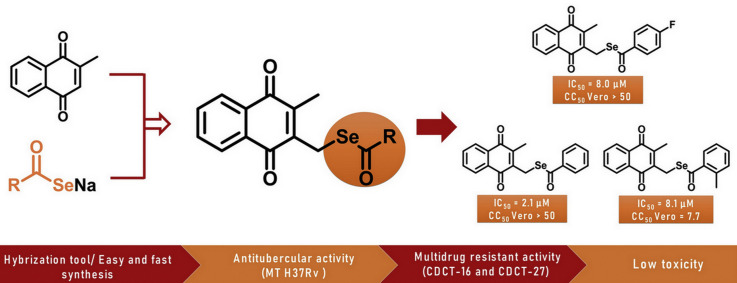
Abstract
Tuberculosis (TB) is one of the most fatal diseases and is responsible for the infection of millions of people around the world. Most recently, scientific frontiers have been engaged to develop new drugs that can overcome drug-resistant TB. Following this direction, using a designed scaffold based on the combination of two separate pharmacophoric groups, a series of menadione-derived selenoesters was developed with good yields. All products were evaluated for their in vitro activity against Mycobacterium tuberculosis H37Rv and attractive results were observed, especially for the compounds 8a, 8c and 8f (MICs 2.1, 8.0 and 8.1 μM, respectively). In addition, 8a, 8c and 8f demonstrated potent in vitro activity against multidrug-resistant clinical isolates (CDCT-16 and CDCT-27) with promising MIC values ranging from 0.8 to 3.1 μM. Importantly, compounds 8a and 8c were found to be non-toxic against the Vero cell line. The SI value of 8a (>23.8) was found to be comparable to that of isoniazid (>22.7), which suggests the possibility of carrying out advanced studies on this derivative. Therefore, these menadione-derived selenoesters obtained as hybrid compounds represent promising new anti-tubercular agents to overcome TB multidrug resistance.
Keywords: Naphthoquinones, Selenoesters, Tuberculosis, Multi-drug-resistant tuberculosis, Mycobacterium tuberculosis, Antimycobacterial activity, Cytotoxicity, Drug development, MIC
Abbreviations: NMR, nuclear magnetic resonance; HMRS, high resolution mass spectrometry; HR-ESIMS, High-resolution electrospray ionisation mass spectrometry; TB, tuberculosis; MDR-TB, multidrug-resistant TB; GPx, glutathione peroxidase; MIC, minimum inhibitory concentration; SI, selectivity index; CC50, cytotoxic concentration
Graphical abstract
Highlights
-
•
New menadione-derived selenoesters were synthesized.
-
•
The compounds demonstrated excellent activity against M. tuberculosis H37Rv.
-
•
8a, 8c and 8f showed potent activity against multidrug resistant clinical isolates.
-
•
Compounds 8a and 8c were found to be non-toxic.
-
•
These organoselenium compounds represent promising new anti-tubercular agents.
1. Introduction
Tuberculosis (TB) is a major public health concern worldwide representing the principal cause of death from a single infectious agent, Mycobacterium tuberculosis. Each year, approximately 10 million people become ill with TB, with the prevailing incidence in Southeast Asia and Africa. The risk of developing TB is high, considering an estimated 1.7 billion people globally infected with these mycobacteria [1,2]. In 2018, 7 million of new cases and 11.1 million of the estimated incident cases were reported. Therefore, it is important to highlight the gap between underreporting and underdiagnoses cases between the new and the estimated incident cases. This observed gap is due to the global range of people with TB without access to health care [2].
TB represents a difficult challenge with multiple aggravating factors. The drug resistance in TB, the poor tolerability and efficacy of therapeutic regimen together with the difficulty in accessing therapy and incomplete treatment increase the complexity of disease resolution [3]. The spread of infection by M. tuberculosis strains resistant to first and second-line anti-TB drugs through the population is another factor that makes it difficult to fight the disease. World Health Organization (WHO) published, in 2018 there were approximately half a million new cases of resistance to rifampicin (RR-TB) worldwide, of which 78% had multidrug-resistant TB (MDR-TB) [2]. The emergence and spread of multidrug-resistant (MDR; defined as strains resistant to at least rifampicin and isoniazid) and extensively drug-resistant (XDR; as MDR plus additional resistance to at least one fluoroquinolone and 1 s-line injectable drug) M. tuberculosis strains are associated with the ineffectiveness of the available treatments for TB and are a major public health concern. MDR and XDR strains are often associated with low cure and high mortality rates [1].
The treatment of drug-susceptible TB is complicated due to the use of drug cocktails composed of first-line drugs (rifampicin, isoniazid, pyrazinamide and ethambutol) and more toxic, less efficacious and more expensive second- or third-line agents (aminoglycosides, fluoroquinolones, clofazimine, amoxicillin plus clavulanate) [4,5]. At the same time, cases of extensively drug-resistant TB and drug-resistant tuberculosis (TDR-TB) have been reported [[5], [6], [7]]. Consequently, the development of new drugs for the treatment of TB appears to be extremely important and natural products may be a valid resource for drug discovery.
In this context, naphthoquinones are a characteristic group of quinones that exist as secondary metabolites largely present in plants, lichens and microorganisms. Some of them can be extracted and used in traditional medical practices and folk medicine [8]. Naphthoquinones present a chemical structure of naphthalene with two ketone groups at position C-1 and C-2 (1,2-naphthoquinones) or C-1 and C-4 (1,4-naphthoquinones) [9]. The considerable interest in these compounds is based on their pharmacological activities, especially as antimicrobial [[8], [9], [10], [11]] and antitumor agents [[12], [13], [14], [15]]. Most recently, 1,4-naphthoquinones, such as menadione (vitamin K3), have aroused a lot of interest due to relevant anticancer activity [16]. With specific attention on the anti-tubercular activity, studies have reported on the potential of using naphthoquinones as active agents against drug-resistant M. tuberculosis strains, although their mechanism of action has yet to be unveiled [[17], [18], [19]].
Since the 1970s, the identification of different selenoproteins and their involvement in mammalian biochemistry have motivated intense studies in the synthesis and biological properties of organoselenium compounds [20,21]. Notably, many studies have shown the potential applications of selenium compounds for the treatment of infectious diseases. The antimicrobial efficacy of selenium compounds has been tested against a great variety of microorganisms, demonstrating their activity as antibacterial [22], antifungal [23], antiviral [24] and antiparasitic agents [25]. Ebselen (1) is the most well-known and well-studied seleno-derivative, especially for its antioxidant effects, GPx-like activity [26] and antimicrobial activity [27]. Antitubercular activities of ebselen (1) and its derivatives have consistently been demonstrated and considering the capacity of ebselen (1) to act as an allosteric inhibitor of the M. tuberculosis antigen 85 complex [28,29], confirming the potential use of selenium compounds in MDR-TB. Recently, ebselen (1) was described among 10.000 molecules as the best non-reversible inhibitor of the SARS-CoV-2 encoded protease (Mpro), showing again the biological relevance of organoselenium compounds [30].
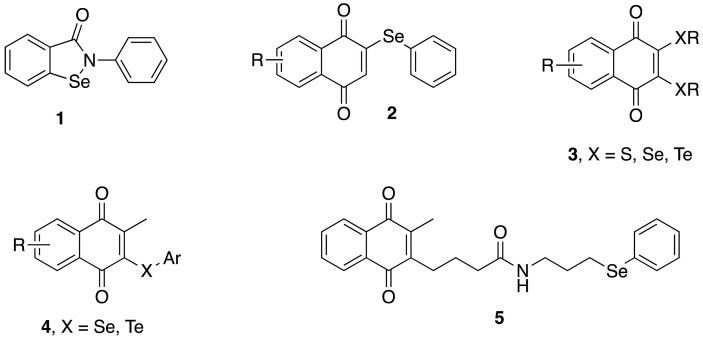
Considering the important chemical interest and potential biological applications of 1,4-naphthoquinones and chalcogen derivatives, different research groups have concentrated their efforts to develop new compounds possessing these two structural moieties [31]. A method for the synthesis of selenonaphthoquinones (2) by phenylselenide ions has been reported in the literature by Sakakibara [32], and others chalcogenium-naphthoquinones were successively explored by Jacob to obtain new redox agents (3-5) as anti-cancer drugs [[33], [34], [35], [36]], as shown in Fig. 1 .
Fig. 1.
Chemical structures of already known chalcogenium-naphthoquinones (1-5).
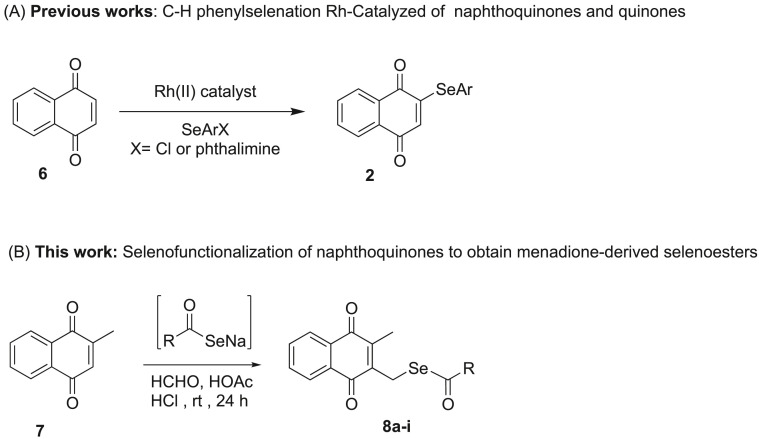
In the last three years, synthetic methodologies to coupling selenium groups involving direct selenilation of the quinone ring have been described through Rh-catalyzed C-H phenylselenation, as shown in Fig. 2 . For example, using N-(phenylseleno)-phthalimide or phenyl hypochloroselenoites as an electrophile, the C-2 selenation of naphthoquinone can be performed, as well as the Rh-catalyzed selenation of benzoquinone (Fig. 2A) [[37], [38], [39]].
Fig. 2.
Overview of the different synthetic methodologies to selenofunctionalization of quinones and naphthoquinones, through Rh-catalyzed C-H bond activation (Panel A) and to obtain menadione-derived selenoesters (Panel B).
We herein report on the synthesis of a new series of naphthoquinone derivatives 8a-i by selenofuncionalization of menadione (7) and their evaluation against the drug-susceptible M. tuberculosis H37Rv strain and multidrug-resistant clinical isolates (CDCT-16 and CDCT-27) (Fig. 2B).
2. Experimental section
2.1. Chemistry
The reagents were purchased from Sigma-Aldrich Brazil and were used without further purification. Column chromatography was performed with silica gel 60 (Merck 70–230 mesh). Analytical thin layer chromatography was performed with silica gel plates (Merck, TLC silica gel 60 F254), and the plates were visualized using UV light. The indicated yields refer to homogeneous materials purified by chromatography and confirmed by spectroscopic techniques. Melting points were obtained on a Thermo scientific 9100 apparatus and were uncorrected. Infrared spectra were collected using KBr pellets on a PerkinElmer model 1420 FT-IR spectrophotometer, and the spectra were calibrated relative to the 1601.8 cm−1 absorbance of polystyrene. 1H and 13C Nuclear magnetic resonance (NMR) were recorded at room temperature using a Varian VXR Unity 300 or 500 MHz, in the DMSO‑d 6. Chemical shifts (δ) are given in ppm and coupling constants (J) in Hertz. The chemical shift data were reported in units of δ (ppm) downfield from solvent, and the solvent was used as an internal standard; coupling constants (J) are reported in hertz and refer to apparent peak multiplicities. High-resolution mass spectra (HRMS) were recorded on a MICROMASS Q-TOF mass spectrometer (Waters) and the deviation of the measured mass from the theoretical mass (Δ) was inserted in ppm.
X-ray diffraction data for cubebin was carried out with radiation MoKa (λ = 0.71073 Å) in Bruker D8 Venture diffractometer at room temperature The data collection and cell refinement were carried out using APEX3 (ref: Bruker APEX3, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA The structure was solved using Intrinsic Phasing and refinement package using Least Squares Methods with the SHELX programs [40]. Non-hydrogen atoms were refined with anisotropic displacement parameters, and the hydrogen atoms were positioned geometrically using the riding model. Molecular graphics draw using SHELXL [41].
The protocol for preparing 2-(chloromethyl)-3-methylnaphthalene-1,4-dione (9) was performed as described by Ferreira and cols [42].
2.2. General procedure for the synthesis of selenonaftoquinones 8a-i
To a two-necked round-bottom flask, under an argon atmosphere, containing Se0 (1.5 mmol) in 5 mL of ethanol was added portion wise to sodium borohydride (2.0 mmol). Then, the appropriate acyl chloride (1.5 mmol) was added and the reaction mixture was kept under stirring for 30 min. After this, 2-(chloromethyl)-3-methylnaphthalene-1,4-dione (1.0 mmol) was added and the mixture was left under stirring for 24 h and extracted with ethyl acetate (3 x 15 mL), dried over anhydrous sodium sulfate and concentrated under vacuum. The crude product was purified by flash chromatography using gradient ethyl acetate:hexane as the eluent.
2.2.1. Se-((3-methyl-1,4-dioxo-1,4-dihidronafaleno-2-yl)methyl)-benzoselenoate (8a)
Yellow solid, m.p. 105–107 °C, yield 75%. 1H NMR (300.00 MHz, DMSO‑d 6) δ ppm: 8.03-7.99 (m, 2H), 7.89-7.82 (m, 4H), 7.73-7.70 (m, 1H), 7.58-7.55 (m, 2H), 4.28 (s, 2H), 2.23 (s, 3H). 13C NMR (75.00 MHz, DMSO‑d 6) δ ppm: 193.32, 183.92, 183.27, 143.53, 143.11, 137.62, 134.30, 133.96, 133.81, 131.55, 131.20, 129.22, 126.77, 125.91, 125.77, 19.81, 12.59. HR-ESIMS [M+H]+ m/z calcd. for C19H15O3Se: 371.0181. Found: 371.0192. Δ 3.0 ppm.
2.2.2. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-4-methylbenzoselenoate (8b)
Yellow solid, m.p. 109–111 °C, yield 58%. 1H NMR (500.00 MHz, DMSO‑d 6) δ ppm: 8.10-8.05 (m, 2H), 7.77 (d, J 1.5 Hz, 2H), 7.72-7.66 (m, 2H), 7.23 (d, J 1.5 Hz, 2H), 4.30 (s, 2H), 2.38 (s, 3H), 2.32 (s, 3H). 13C NMR (75.00 MHz, CDCl3) δ ppm: 193.43, 185.04, 184.42, 145.15, 144.47, 144.25, 136.07, 133.84, 133.70, 132.40, 132.10, 129.75, 127.58, 126.66, 126.57, 21.92, 20.14, 13.22. HR-ESIMS [M+Na]+ m/z calcd. for C20H16NaO3SeNa+: 407.0157. Found: 407.0173. Δ 3.7 ppm.
2.2.3. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-2-methylbenzoselenoate (8c)
Yellow solid, m.p. 125–127 °C, yield 32%. 1H NMR (300.00 MHz, DMSO‑d 6) δppm: 8.15-8.09 (m, 2H), 7.79-7.71 (m, 3H), 7.46-7.40 (td, J 3.0 and 9.0 Hz, 1H), 7.30-7.27 (td, J 3.0 and 9.0 Hz, 2H), 4.32 (s, 2H), 2.53 (s, 3H), 2.37 (s, 3H). 13C NMR (75.00 MHz, CDCl3) δppm: 195.77, 184.88, 184.23, 144.36, 144.00, 138.40, 136.18, 133.68, 133.55, 132.22, 132.20, 131.92, 131.83, 128.78, 126.49, 126.41, 126.05, 20.98, 20.72, 13.04. HR-ESIMS [M+Na]+ m/z calcd. for C20H16NaO3SeNa+: 407.0157. Found: 407.0150. Δ 2.1 ppm.
2.2.4. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-4-ethylbenzoselenoate (8d)
Yellow solid. m.p. 95–97 °C, yield 25%. 1H NMR (300.00 MHz, CDCl3) δ ppm: 8.10-8.05 (m, 2H), 7.79 (d, J 1.5 Hz, 2H), 7.71-7.66 (m, 2H), 7.25 (d, J 1.5 Hz, 2H), 4.30 (s, 2H), 2.67 (q, J 7.6 Hz, 2H), 2.32 (s, 3H), 1.23 (t, J 7.6 Hz, 3H). 13C NMR (75.00 MHz, CDCl3) δppm: 193.51, 185.10, 184.49, 151.35, 144.53, 144.28, 133.88, 132.43, 132.13, 131.94, 128.83, 128.62, 127.71, 126.70, 126.61, 29.23, 20.16, 15.29, 13.25. HR-ESIMS [M+Na]+ m/z calcd. for C21H18NaO4SeNa+: 421.0313. Found: 421.0326. Δ 2.7 ppm.
2.2.5. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-4-metoxybenzoselenoate (8e)
Yellow solid, m.p. 118–120 °C. Yield 24%. 1H NMR (300.00 MHz, CDCl3) δ ppm: 8.03-8.00 (m, 2H), 7.78 (d, J 8.9 Hz, 2H), 7.64-7.62 (m, 2H), 6.84 (d, J 8.9 Hz, 2H), 4.23 (s, 2H), 3.78 (s, 3H), 2.26 (s, 3H). 13C NMR (75.00 MHz, CDCl3) δ ppm: 191.77, 184.90, 184.28, 164.22, 144.39, 144.02, 133.65, 133.51, 132.23, 131.93, 131.22, 129.62, 126.47, 126.38, 114.08, 55.58, 19.90, 13.03. HR-ESIMS [M+Na]+ m/z calcd. for C20H16NaO4SeNa+: 423.0110. Found: 423.0106. Δ 0.6 ppm.
2.2.6. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-4-fluorobenzoselenoate (8f)
Orange solid, m.p. 124–126 °C, yield 51%. 1H NMR (500.00 MHz, DMSO) δ ppm: 8.00-7.92 (m, 4H), 7.84-7.80 (m, 2H), 7.39-7.30 (m, 2H), 4.28 (s, 2H), 2.22 (s, 3H). 13C NMR (75.00 MHz, DMSO‑d 6) δppm: 192.42, 184.53, 183.86, 166.08 (d, J 253.1 Hz), 144.27, 143.68, 135.07, 134.52, 134.38, 132.24, 131.90, 131.41 (dd, J 9.7 Hz), 126.50, 126.36, 116.41 (dd, J 22.2 Hz), 20.66, 13.11. HR-ESIMS [M+Na]+ m/z calcd. for C19H13FNaO3SeNa+: 410.9906. Found: 410.9923. Δ 4.0 ppm.
2.2.7. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-4-chlorobenzoselenoate (8g)
Yellow solid, m.p. 158–160 °C, Yield 26%. 1H NMR (500.00 MHz, DMSO‑d 6) δ ppm: 8.04-8.01 (m, 2H), 7.75 (d, J 1.6 Hz, 2H), 7.65-7.62 (m, 2H), 7.36 (d, J 1.6 Hz, 2H), 4.26 (s, 2H), 2.27 (s, 3H). 13C NMR (75.00 MHz, CDCl3) δ 192.99, 184.99, 184.42, 144.48, 144.09, 140.57, 136.98, 133.95, 133.80, 132.42, 132.07, 129.45, 128.78, 126.74, 126.62, 20.62, 13.29. Δ = 1.1 ppm. HR-ESIMS [M+Na]+ m/z calcd. for C19H13ClNaO3SeNa+: 426.9611. Found: 426.9609. Δ 0.2 ppm.
2.2.8. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-pentaneselenoate (8h)
Yellow oil, yield 24%. 1H NMR (500.00 MHz, CDCl3) δ ppm: 8.98-7.94 (m, 2H), 7.61-7.58 (m, 2H), 4.02 (s, 2H), 2.55 (t, J 10.0 Hz, 2H), 2.16 (s, 3H), 1.57 (q, J 10.0 Hz, 2H), 1.28 (sextet, J 10.0 Hz, 2H), 0.82 (t, J 10.0 Hz, 3H). 13C NMR (125.00 MHz, CDCl3) δ ppm: 200.92, 184.70, 183.99, 144.22, 143.78, 133.60, 133.47, 132.12, 131.81, 126.39, 126.30, 47.25, 27.45, 21.98, 19.79, 13.68, 12.83. HR-ESIMS [M+Na]+ m/z calcd. for C17H18NaO3SeNa+: 373.0313. Found: 373.0329. Δ 4.0 ppm.
2.2.9. Se-((3-methyl-1,4-dioxo-1,4-dihydronaphthalen-2-yl)methyl)-2-methylpentanoselenoate (8i)
Yellow oil, yield 37%. 1H NMR (500.00 MHz, CDCl3) δppm: 7.99-7.96 (m, 2H), 7.61-7.59 (m, 2H), 4.02 (s, 2H), 2.63 (sextet, J 6.9 Hz, 1H), 2.16 (s, 3H), 1.64-1.60 (m, 1H), 1.32-1.25 (m, 3H), 1.09 (d, J 6.9 Hz, 3H), 0.81 (t, J 7.3 Hz, 3H). 13C NMR (126.00 MHz, CDCl3) δppm: 205.64, 184.79, 184.06, 144.41, 143.78, 133.60, 133.48, 132.16, 131.86, 126.41, 126.33, 51.74, 35.98, 20.23, 19.45, 17.18, 13.97, 12.81. HR-ESIMS [M+Na]+ m/z calcd. for C18H20NaO3SeNa+: 387.0470. Found: 387.0483. Δ 3.1 ppm.
2.3. Biological evaluation
Vero cell line (ATCC N° CCL-81) were purchased from the Rio de Janeiro Cell Bank, Brazil. Dulbecco’s Modified Eagle’s Medium (DMEM), Hank’s Balanced Salt Solution, fetal bovine serum (FBS), antibiotic solution (10,000 U/mL penicillin, 10 mg/mL streptomycin), antimycotic solution (25–30 μg/mL amphotericin B), trypsin-ethylenediaminetetraacetic acid (EDTA) solution (2.5 mg/mL trypsin, 0.2 mg/mL EDTA) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were all supplied by Sigma-Aldrich (São Paulo, Brazil). Dimethyl sulfoxide (DMSO) and other reagents were analytical grade.
2.3.1. Mycobacterium tuberculosis inhibition assay
The minimum inhibitory concentration (MIC) for each tested compound was determined by the resazurin reduction microplate assay (REMA), as thoroughly described before [43,44]. Isoniazid (INH, control drug) and compound solutions were solubilized at 1 mg/mL in neat DMSO, followed by dilution in Middlebrook 7H9 broth, supplemented with 10% ADC (albumin, dextrose, and catalase) and 5% DMSO, to a concentration of 50 μg/mL. Ten-point, two-fold serial dilutions were prepared directly in 96-well plates, giving a concentration range of 25.0 to 0.05 μg/mL. It is important to mention that all dilutions formed real solutions. A growth control (without compounds) and a sterility control (without bacterial inoculum) were included in each plate. Compounds were tested against the drug-sensitive M. tuberculosis H37Rv strain (ATCC 27294) and two drug-resistant clinical isolates (named CDCT-16 and CDCT-27). CDCT-16 is a multidrug-resistant clinical isolate resistant to isoniazid, ethionamide, rifampicin, streptomycin and ethambutol, while CDCT-27 is resistant to isoniazid and ethambutol only. The MIC was considered as the lowest drug concentration that prevented a color change from blue (no growth) to pink (growth). Three independent experiments were carried out, and MIC values reported here were observed in at least two experiments, or were the highest value observed among the three assays.
2.3.2. Cell line culture conditions
Vero cells were maintained in DMEM supplemented with 4.5 mg/mL glucose, 0.1 mg/mL penicillin, 0.14 mg/ml streptomycin and 10% inactivated FBS. Cultured cells were maintained at 37 °C in an atmosphere containing 95% air and 5% CO2. Cells were sub-cultivated every 48 h using trypsin-EDTA solution.
2.3.3. Toxicity to the Vero cell line using the MTT assay
Metabolically active cells were assessed using the MTT reduction colorimetric assay, as previously reported by Mosmann (1983) [45] and Alley (1988) [46]. Cells were seeded in 96-well plates (Corning) at density of 30,000 cells/well, distributed in a total volume of 200 μL/well. Plates were kept in the incubator at 37 °C and 5% CO2 for 24 h. After incubation, cells were put in contact with the samples (0–50 μM) for 24 h. 10% FBS DMEM containing 1% DMSO was used as the control treatment. Isoniazid (INH) was used as the comparative treatment. The samples were kept in contact with the cells for 3 h and then aspirated and treated with sterile MTT reagent solution (0.5 mg/mL in HBSS) by adding 125 μL per well. The plates containing the cells were incubated with MTT for 3 h at 37 °C and 5% CO2. At the end of the incubation time, MTT was aspirated and the cells were washed with phosphate buffered saline (PBS) solution (pH 7.4). The phosphate buffer was then aspirated and 100 μL/well of DMSO was added to disrupt cell membranes and solubilize formazan crystals. The absorbance readings were performed on an iMARK™ Microplate Absorbance Reader (Bio-Rad Laboratories Srl, Segrate, Italy), with reference to 570 nm–690 nm after vigorous shaking for 30 s.
Cytotoxicity was expressed as the percentage of cells surviving in relation to untreated cells. Drug concentration required to inhibit growth by 50% (CC50) and selectivity index (SI) were calculated with GraphPad Prism 5 (version 5.00; GraphPad Software, Inc., San Diego, CA, USA).
3. Results and discussion
3.1. Chemistry
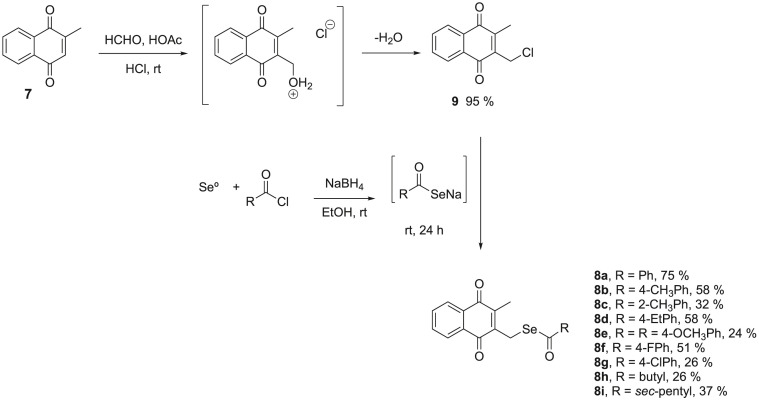
Our strategy was based on obtaining a series of novel menadione-derived selenoesters using a designed scaffold formed by two fragments through the combination of two separate pharmacophoric groups with analogous activity into one compound. The combination of 1,4-naphthoquinone and a selenium group in the same molecule was an interesting strategy to obtain hybrid compounds with synergistic effects as potent anti-tubercular agents, especially with the aim of overcoming TB multidrug resistance (Fig. 3 ). This selenofunctionalization of menadione is described for the first time in the literature and the methodology that we have developed is economical and fast compared with the other procedures used in the selenilation of naphthoquinones.
Fig. 3.
Synthesis of menadione-derived selenoesters 8a-i.
The synthetic approach used to obtain selenium-containing menadione derivatives 8a-i initially provided some synthetic steps described in the literature [42] and was based on a two-step pathway entailing the use of commercial menadione (7). The synthetic route started with the first reaction of the insertion of the -CH2Cl group in menadione, in excellent yield (95%), through the reaction with formaldehyde, glacial acetic acid and dry hydrogen chloride, obtaining 2-(chloromethyl)-3-methylnaphthalene-1,4-dione (7) (Fig. 3).
Then, we promoted the selenofuncionalization of 9 through the reaction with the respective selenocarboxylate that was generated in situ from benzoyl chloride and Se0 (Fig. 3). Using different acyl chlorides and benzoylchlorides, a series of menadione-derived selenoesters in moderate to good yields ranged between 24 and 75% was obtained, as reported in Fig. 3.
For example, compound 8b, with p-methylbenzoyl chloride, was obtained in 58% yield, while the best yield was represented by compound 8a with 75% (Fig. 3). Exchanging the aromatic for aliphatic selenocarboxylate led to a decrease in the yield, as shown in Fig. 3. Additionally, we found that a change in the electron-donating group in the aromatic ring to an electron withdrawer group did not produce significant variation in the product yield. The synthetic approach chosen shows that overall returns ranged from 23 to 71%.
The structure of all products synthetized was confirmed by 1H and 13C nuclear magnetic resonance (NMR) spectroscopy and HRMS spectrometry experiments. The 1H NMR experiment shows that the typical signals of hydrogens of the methyl group at C-3 of quinoidic rings are registered in the range of ca. 2.21–2.43 ppm and 2.17–2.19 ppm as singlets, integrating for three hydrogens, in case of benzoselenoates and pentaneselenoates, respectively. Another typical signal is represented by the singlet of two hydrogens of methyl group that connected the 1,4-dioxo-1,4-dihidronaphthalen core with the selenoate moiety. These protons are influenced by the presence of the selenium atom and they appear between 4.24-4.30 ppm and 4.04–4.05 ppm for benzoselenoates and pentaneselenoates, respectively. The presence of these signals confirmed the actual effectiveness of our synthetic method to obtain the desired selenonaphthoquinones [[47], [48], [49]].
The aromatic protons of 1,4-dihidronaphthalen nucleus respect the characteristic range of signals reported in literature.50 The 13C NMR spectra shows the typical signal of the carbonyl selenoester moiety that appears between 191.74 and 195.75 ppm for benzoselenoates and between 200.89 and 205.86 ppm for pentaneselenoates [50].
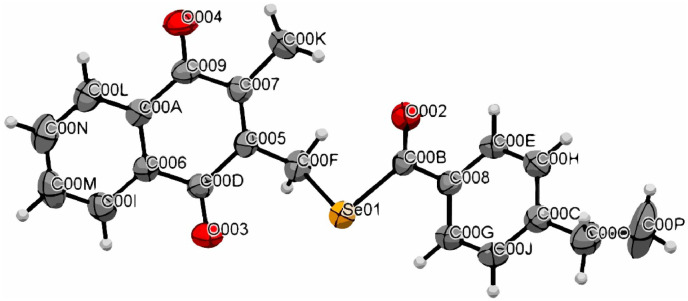
Structure of 8d was confirmed by X-ray diffraction and Ortep-3 diagram of the product is shown in Fig. 4 . Bond lengths and angles are available in supporting information, being that all the interatomic distances and angles are within the expected values for similar chemical bondings [51].
Fig. 4.
ORTEP-3 representation of compound 8d (CCDC 2006682).
Table 1 lists main crystallographic parameters. For compound 8d a total of 54362 reflections to a maximum 2θ of 25.4° were measured. The crystal structure for compound was solved by direct methods and refined anisotropically with full matrix least square on F2 using SHELXL-97 program [52]. The bond angles between atoms are 121.4(2)º for Se01–C00B–O002 and 115.4(2)º for Se01–C00B–C008. All H atoms were located by geometric considerations placed (C–H = 0.93–0.97 Å).
Table 1.
Crystallographic parameters for 8d.
| Empirical formula | C21H18O3Se |
|---|---|
| Formula weight | 397.3 |
| Temperature (K) | 296 |
| Crystal system | Monoclinic |
| Space group | P 21/c |
| a (Å) | 12.2127 |
| b (Å) | 18.5981 |
| c (Å) | 8.3327 |
| α (º) | 90 |
| β (º) | 109.622 (1) |
| γ (º) | 90 |
| Volume (Å3) ] |
1782.72 (11) |
| Z | 4 |
| Calculated density (Mg/m−3) ] |
1.480 |
| μ (mm−1) | 2.12 |
| F (000) | 808.0 |
| Crystal size (mm) ] |
0.16 × 0.14 × 0.07 |
| Radiation (Å) | Mo Kα (λ = 0.71073) |
| hkl range | −14≤ h ≤ 14, −22 ≤ k ≤ 22, −10 ≤ l ≤ 10 k = −2222 l = −1010 |
| θ (º) | 2.6–25.4 |
| Reflections collected | 54362 |
| Independent reflections | 3281 |
| R(int) | 0.038 |
| Final R indices | R1 = 0.033 |
| [I > 2sigma(I)] | wR2 = 0.079 |
| S | 1.07 |
| Largest difference peak and hole (e/Å3) ] |
0.52/-0.48 |
Crystallographic data for compound 8d have been deposited with the Cambridge Crystallographic Data Center as Supplementary Publication No. CCDC 2006682. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CH21EZ, UK (fax: þ44 1223 336 033 or e-mail: deposit@ccdc.cam.ac.uk).
3.2. Biological evaluation
3.2.1. Mycobacterium tuberculosis inhibition assay
Considering the emergence and spread of MDR and XDR-TB, new drug candidates have been proposed for treating these phenomena, as such as bedaquiline, delamanid and pretomanid, but possible cardiotoxicity and resistance to these new treatments were also rapidly reported [53]. Herein, we evaluated the effect of new selenium-containing menadione derivatives on the growth of drug-sensitive and -resistant strains of M. tuberculosis. As shown in Table 2 , the compounds exhibited inhibitory activity for the H37Rv M. tuberculosis strain from 2.1 to 71.4 μM. Compound 8a exhibited comparable activity to that of the related compound isoniazid; in fact, the MIC obtained for isoniazid under these experimental conditions was 2.2 μM, while that one determined for 8a analog was 2.1 μM. Compounds 8c and 8f also demonstrated significant inhibitory activity, with MICs of 8.1 and 8.0 μM, respectively. These more active compounds show the phenyl group as a substituent of the carbonyl attached to selenium. The results show that the phenyl group at this position has an important role in the biological activity since the exchange of substituents significantly alter the results. Interestingly, only exchanging the fluor atom at the C-4 position of the phenyl group with a chlorine atom generated a molecule less active (compound 8f versus 8g).
Table 2.
CLogP values, in vitro activity against three M. tuberculosis strains (H37Rv, CDCT-16 and CDCT-27), CC50 values for Vero cell line, and calculated selectivity index (SI) values for the selenium-containing naphthoquinones.
| Compounds | miLogP | MIC (μM) |
CC50 Vero (μM) | SI |
||||
|---|---|---|---|---|---|---|---|---|
| H37Rv | CDCT-16 | CDCT- 27 | H37Rv | CDCT-16 | CDCT- 27 | |||
| 8a | 4.39 | 2.1 | 0.8 | 0.8 | >50 | >23.8 | >62.5 | >62.5 |
| 8b | 4.89 | >10 | >10 | >10 | – | – | – | – |
| 8c | 4.89 | 8.1 | 3.1 | 3.1 | 7.7 | 0.9 | 2.5 | 2.5 |
| 8d | 5.42 | >10 | >10 | >10 | – | – | – | – |
| 8e | 4.31 | >10 | >10 | >10 | – | – | – | – |
| 8f | 4.53 | 8.0 | 0.8 | 3.1 | >50 | >6.2 | >62.5 | >16.1 |
| 8g | 5.10 | >10 | >10 | >10 | – | – | – | – |
| 8h | 6.48 | >10 | >10 | >10 | – | – | – | – |
| 8i | 6.88 | >10 | >10 | >10 | – | – | – | – |
| Isoniazid | −0.67 | 2.2 | 100 | 25 | >50 | >22.7 | – | – |
aClogP calculated by ChemBioDraw Ultra version 13.0.0.3015.
Compounds with MIC <10 μM for the drug-sensitive strain (8a, 8c and 8f) were evaluated against two multidrug-resistant clinical isolates (CDCT-16 and CDCT-27). CDCT-16 is an MDR-TB strain that holds mutations in the rpoB (D516V) and katG (S315T) genes, as well as in the inhA promoter sequence [C(-15)T]. CDCT-27 carries the same mutation in the katG gene, which confers cross-resistance to isoniazid [54]. As shown in Table 2, none of these clinical isolates exhibited resistance to compounds 8a, 8c and 8f, with MIC values ranging from 0.8 to 3.1 μM. These data suggest a possible capacity of menadione derivatives to evade the most common mechanisms of resistance and the potential interest to study these compounds in pre-clinical tests to develop new antimycobacterial agents. Finally, the absence of a resistant phenotype by the two clinical isolates suggests that these compounds do not share a mechanism of action similar to the first-line drugs isoniazid and rifampicin. However, how these compounds act to disrupt the mycobacterial growth and to inhibit their molecular target(s), as well as the contribution of each pharmacophoric group to the biological activity, has yet to be elucidated.
According to the importance of estimating the distribution and diffusion of drugs to across the cell membrane and tissue and their solubilization in an aqueous medium, the CLogP was calculated. In particular, it has been well established that the lipophilicity of anti-TB drug candidates is positively correlated with their ability to permeate through M. tuberculosis cell wall [55]. As reported in Table 2, the compounds 8a-i showed CLogP values in the range of 4.31–6.88. Notably, steric and/or conformational properties seem to be directly related to the antimycobacterial activity exhibited by the compounds rather than the physicochemical parameters such as ClogP. Changing the methyl group from 4- to 2-position of the benzene ring greatly changed the activity of naphthoquinones 8b and 8c while their CLogP values are identical. It is noteworthy that the most effective compounds (8a, 8c, and 8f) present ClogP values consistent with the classic rule of five [56]. In addition, the number of hydrogen bonds donors (<5) and hydrogen bonds acceptors (<10) as well as the molecular mass (<500 D) were also respected, suggesting that these molecules may have adequate bioavailability when orally administered.
3.2.2. Toxicity of menadione derivatives to the Vero cell line
Menadione derivatives and isoniazid were tested by the MTT reduction assay in the Vero cell line at 5, 10, 25 and 50 μM. Among evaluated derivatives, 8c showed the lowest CC50 value (7.7 μM) and consequently a low SI (selective index) value (Vero/H37Rv 0.9). Derivatives 8a and 8f showed CC50 above the highest dose (>50 μM), which pointed to SI values (Vero/H37Rv) above 23.8 and 6.2, respectively, as reported in Table 2. Isoniazid showed an SI (>22.7) very similar to 8a, which represents a starting point for advanced studies of this derivative, given the correlation between toxicity in Vero cells, isolated renal tissue and nephrotoxicity in rats [57].
4. Conclusions
In summary, we report in this paper a new series of selenium-containing naphthoquinones and the evaluation of their biological activity against M. tuberculosis H37Rv and MDR clinical isolates (CDCT-16 and CDCT-27) that were isolated from patients undergoing clinical treatments. The compounds were designed as hybrids by combining two pharmacophoric groups (naphthoquinone and selenoester) known to have similar biological activities. The hybrid compounds were synthesized in adequate yields and showed good profiles of anti-tubercular activity against multidrug-resistant strains of M. tuberculosis. A convenient synthesis of compounds, as well as in biological activity and toxicity, suggest the continuation of this study for in vivo tests, to overcome the phenomenon of resistance to multiple drugs of TB, and in the synthesis of new hybrid selenoesters with menadione that may have better profiles of anti-tubercular activity with low toxicity.
Considering the promising results obtained in terms of synthesis, yields, biological activity and toxicity, this work suggests the potential application of this series of selenoesters derived from menadione in subsequent biological and structure-activity studies (SAR). Although the mechanism of action of the synthesized and tested compounds is still not known, these represent interesting starting points for the development of new antimycobacterial agents, in particular for overcoming the phenomenon of resistance to multiple TB drugs. The next steps to understand the mechanism of action and to determine the molecular target(s) of the selenium-containing naphthoquinones will include the selection of spontaneous mutants, the whole-genome sequencing of the obtained mutants, and the use of genetic approaches to validate the gene(s) involved in the mechanism of action and resistance. Besides, considering that ebselen is known to bind and inhibit the antigen 85 (Ag85) complex activity, which is involved in the biosynthesis of trehalose dimycolate and mycolylarabinogalactan, both essential components of the mycomembrane [28], attempts will be made to evaluate the capacity of our compounds to inhibit in vitro the activity of enzymes of this complex and the synthesis of different mycolates by growing cultures of M. tuberculosis.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank CNPq (305454/2017-0), CAPES (scholarships), and FAPERJ (E-26/202.911/2019, E-26/203.191/2017) for financial support. Prof. Pedro N. Batalha (UFF - Brazil) is acknowledged for supporting elucidation of the X-ray structure of 8d. This work was supported by Banco Nacional de Desenvolvimento Econômico e Social (BNDES) [grant number February 14, 0914.1] and the National Institute of Science and Technology on Tuberculosis (CNPq-FAPERGS-CAPES) [grant numbers: 421703-2017-2 and 17-1265-8]. C. V. Bizarro, L. A. Basso, and P. Machado are Research Career Awardees of the National Research Council of Brazil (CNPq). We also acknowledge Dr. Silvana Spindola de Miranda from Federal University of Minas Gerais (Belo Horizonte, Brazil) for providing the CDCT-16 (630/08) and CDCT-27 (0128/09) Mycobacterium tuberculosis clinical isolates.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejmech.2020.112859.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization Global tuberculosis report 2018 2019. https://apps.who.int/iris/bitstream/handle/10665/274453/9789241565646-eng.pdf?sequence=1&isAllowed=y (accessed 3 June 2020)
- 2.World Health Organization Global tuberculosis report 2019 2019. https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf?ua=1 (accessed 3 June 2020)
- 3.Koch A., Cox H., Mizrahi V. Drug-resistant tuberculosis: challenges and opportunities for diagnosis and treatment. Curr. Opin. Pharmacol. 2018;42 doi: 10.1016/j.coph.2018.05.013. 7–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zumla A., Nahid P., Cole S.T. Advances in the development of new tuberculosis drugs and treatment regimens. Nat. Rev. Drug Discov. 2013;12:388–404. doi: 10.1038/nrd4001. [DOI] [PubMed] [Google Scholar]
- 5.Hoagland D., Liu J., Lee R.B., Lee R.E. New agents for the treatment of drug-resistant Mycobacterium tuberculosis. Adv. Drug Deliv. Rev. 2016;102:55–72. doi: 10.1016/j.addr.2016.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falzon D., Mirzayev F., Wares F., Garcia Baena I., Zignol M., Linh N., Weyer K., Jaramillo E., Floyd K., Raviglione M. Multidrug-resistant tuberculosis around the world: what progress has been made? Eur. Respir. J. 2015;45:150–160. doi: 10.1183/09031936.00101814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parida S.K., Axelsson-Robertson R., Rao M.V., Singh N., Master I., Lutckii A., Keshavjee S., Andersson J., Zumla A., Maeurer M. Totally drug-resistant tuberculosis and adjunct therapies. J. Intern. Med. 2015;277:388–405. doi: 10.1111/joim.12264. [DOI] [PubMed] [Google Scholar]
- 8.Futuro D., Ferreira P.G., Nicoletti C.D., Borba-Santos L.P., da Silva F.C., Rozental S., Ferreira V.F. The antifungal activity of naphthoquinones: an integrative review, An. Acad. Bras. Cienc. 2018;90:1187–1214. doi: 10.1590/0001-3765201820170815. [DOI] [PubMed] [Google Scholar]
- 9.da Silva F.C., Ferreira V.F. Natural naphthoquinones with great importance in medicinal chemistry. Curr. Org. Synth. 2016;13:334–371. doi: 10.2174/1570179412666150817220343. [DOI] [Google Scholar]
- 10.Riffel A., Medina L.F., Stefani V., Santos R.C., Bizani D., Brandelli A. In vitro antimicrobial activity of a new series of 1,4-naphthoquinones. Braz. J. Med. Biol. Res. 2002;35:811–818. doi: 10.1590/s0100-879x2002000700008. [DOI] [PubMed] [Google Scholar]
- 11.Janeczko M., Demchuk O.M., Strzelecka D., Kubiński K., Masłyk M. New family of antimicrobial agents derived from 1,4-naphthoquinone. Eur. J. Med. Chem. 2016;124:1019–1025. doi: 10.1016/j.ejmech.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 12.Coelho-Cerqueira E., Netz P.A. V.P. do Canto, A.C. Pinto, C. Follmer, beyond topoisomerase inhibition: antitumor 1,4-naphthoquinones as potential inhibitors of human monoamine oxidase. Chem. Biol. Drug Des. 2014;83:401–410. doi: 10.1111/cbdd.12255. [DOI] [PubMed] [Google Scholar]
- 13.Menacho-Márquez M., Rodríguez-Hernández C.J., Villaronga M.A., Pérez-Valle J., Gadea J., Belandia B., Murguía J.R. eIF2 kinases mediate β-lapachone toxicity in yeast and human cancer cells. Cell Cycle. 2015;14:630–640. doi: 10.4161/15384101.2014.994904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiu H.Y., Wang P.F., Lin H.Y., Tang C.Y., Zhu H.L., Yang Y.H. Naphthoquinones: a continuing source for discovery of therapeutic antineoplastic agents, Chem. Biol. Drug Des. 2018;91:681–690. doi: 10.1111/cbdd.13141. [DOI] [PubMed] [Google Scholar]
- 15.Costa D.C.S., de Almeida G.S., Rabelo V.W., Cabral L.M., Sathler P.C., Alvarez Abreu P., Ferreira V.F., Pereira da Silva C.R., da Silva F.C. Synthesis and evaluation of the cytotoxic activity of furanaphthoquinones tethered to 1H-1,2,3-triazoles in Caco-2, Calu-3, MDA-MB231 cells. Eur. J. Med. Chem. 2018;156:524–533. doi: 10.1016/j.ejmech.2018.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Prasad C.V., Nayak V.L., Ramakrishna S., Mallavadhani U.V. Novel menadione hybrids: synthesis, anticancer activity, and cell-based studies. Chem. Biol. Drug Des. 2018;91:220–233. doi: 10.1111/cbdd.13073. [DOI] [PubMed] [Google Scholar]
- 17.Uc-Cachón A.H., Borges-Argáez R., Said-Fernández S., Vargas-Villarreal J., González-Salazar F., Méndez-González M., Cáceres-Farfán M., Molina-Salinas G.M. Naphthoquinones isolated from Diospyros anisandra exhibit potent activity against pan-resistant first-line drugs Mycobacterium tuberculosis strains. Pulm. Pharmacol. Therapeut. 2014;27:114–120. doi: 10.1016/j.pupt.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 18.Sharma M.C. A structure-activity relationship study of naphthoquinone derivatives as antitubercular agents using molecular modeling techniques, Interdiscip. Sci. 2015;7:346–356. doi: 10.1007/s12539-015-0011-4. [DOI] [PubMed] [Google Scholar]
- 19.Halicki P.C.B., Ferreira L.A., De Moura K.C.G., Carneiro P.F., Del Rio K.P., Carvalho T.D.S.C., Pinto M.D.C.F.R., da Silva P.E.A., Ramos D.F. Naphthoquinone derivatives as scaffold to develop new drugs for tuberculosis treatment, Front. Microbiol. 2018;9:673. doi: 10.3389/fmicb.2018.00673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberto E.E., Nascimento V., Braga A.L. Catalytic application of selenium and tellurium compounds as glutathione peroxidase enzyme mimetics. J. Braz. Chem. Soc. 2010;21:2032–2041. doi: 10.1590/S0103-50532010001100004. [DOI] [Google Scholar]
- 21.Nogueira C.W., Rocha J.B.T. Toxicology and pharmacology of selenium: emphasis on synthetic organoselenium compounds, Arch. Toxicology. 2011;85:1313. doi: 10.1007/s00204-011-0720-3. [DOI] [PubMed] [Google Scholar]
- 22.Macegoniuk K., Grela E., Palus J., Rudzinska-Szostak E., Grabowiecka A., Biernat M., Berlicki L. 1,2-Benzisoselenazol-3(2H)-one derivatives as a new class of bacterial urease inhibitors. J. Med. Chem. 2016;59:8125–8133. doi: 10.1021/acs.jmedchem.6b00986. [DOI] [PubMed] [Google Scholar]
- 23.Loreto E.S., Mario D.A.N., Santurio J.M., Alves S.H., Nogueira C.W., Zeni G. In vitro antifungal evaluation and structure-activity relationship of diphenyl diselenide and synthetic analogues. Mycoses. 2011;54:572–576. doi: 10.1111/j.1439-0507.2010.01994.x. [DOI] [PubMed] [Google Scholar]
- 24.Iraci N., Tabarrini O., Santi C., Sancineto L. NCp7: targeting a multitask protein for next-generation anti-HIV drug development part 2. Noncovalent inhibitors and nucleic acid binders, Drug Discov. Today Off. 2018;23:687–695. doi: 10.1016/j.drudis.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 25.Font M., Baquedano Y., Plano D., Moreno E., Espuelas S., Sanmartín C., Palop J.A. Molecular descriptors calculation as a tool in the analysis of the antileishmanial activity achieved by two series of diselenide derivatives. An insight into its potential action mechanism. J. Mol. Graph. Model. 2015;60:63–78. doi: 10.1016/j.jmgm.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Bhabak K.P., Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc. Chem. Res. 2010;43:1408–1419. doi: 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
- 27.Wójtowicz H., Kloc K., Maliszewska I., Młochowski J., Pietka M., Piasecki E. Azaanalogues of ebselen as antimicrobial and antiviral agents: synthesis and properties. Farmaco. 2004;59:863–868. doi: 10.1016/j.farmac.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Favrot L., Grzegorzewicz E., Lajiness D.H., Marvin R.K., Boucau J., Isailovic D., Jackson M., Ronning D.R. Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen. Nat. Commun. 2013;4:2748. doi: 10.1038/ncomms3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gustafsson T.N., Osman H., Werngren J., Hoffner S., Engman L., Holmgren A. Ebselen and analogs as inhibitors of Bacillus anthracis thioredoxin reductase and bactericidal antibacterials targeting Bacillus species, Staphylococcus aureus and Mycobacterium tuberculosis. Biochim. Biophys. Acta. 2016;1860:1265–1271. doi: 10.1016/j.bbagen.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 30.Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu H., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020 doi: 10.1038/s41586-020-2223-y. InPress. [DOI] [PubMed] [Google Scholar]
- 31.da Cruz E.H.G., Silvers M.A., Jardim G.A.M., Resende J.M., Cavalcanti B.C., Bomfim I.S., Pessoa C., de Simone C.A., Botteselle G.V., Braga A.L., Nair D.K., Namboothiri I.N.N., Boothman D.A., da Silva Júnior E.N. Synthesis and antitumor activity of selenium-containing quinone-based triazoles possessing two redox centres, and their mechanistic insights. Eur. J. Med. Chem. 2016;122:1–6. doi: 10.1016/j.ejmech.2016.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakakibara M., Watanabe Y., Toru T., Ueno Y.J. New selenenylation Method. Synthesis of selenonaphthoquinones and selenoquinolinequinones mediated by phenyl selenide ion. Chem. Soc. Perkin Trans. 1991;1:1231–1234. doi: 10.1039/P19910001231. [DOI] [Google Scholar]
- 33.Fry F.H., Holme A.L., Giles N.M., Giles G.I., Collins C., Holt K., Pariagh S., Gelbrich T., Hursthouse M.B., Gutowskic N.J., Jacob C. Multifunctional redox catalysts as selective enhancers of oxidative. Org. Biomol. Chem. 2005;3:2579–2587. doi: 10.1039/b502197a. [DOI] [PubMed] [Google Scholar]
- 34.Mecklenburg S., Shaaban S., Ba L.A., Burkholz T., Schneider T., Diesel B., Kiemer A.K., Roseler A., Becker K., Reichrath J., Stark A., Tilgen W., Abbas M., Wessjohann L.A., Sasse F., Jacob C. Exploring synthetic avenues for the effective synthesis of selenium- and tellurium-containing multifunctional redox agents. Org. Biomol. Chem. 2009;7:4753–4762. doi: 10.1039/b907831b. [DOI] [PubMed] [Google Scholar]
- 35.Doering M., Ba L.A., Lilienthal N., Nicco C., Scherer C., Abbas M., Zada A.A.P., Coriat R., Burkholz T., Wessjohann L., Diederich M., Batteux F., Herling M., Jacob C. Synthesis and selective anticancer activity of organochalcogen based redox catalysts. J. Med. Chem. 2010;53:6954–6963. doi: 10.1021/jm100576z. [DOI] [PubMed] [Google Scholar]
- 36.Jamier V., Ba L.A., Jacob C. Selenium- and tellurium-containing multifunctional redox agents as biochemical redox modulators with selective cytotoxicity. Chem. Eur J. 2010;16:10920–10928. doi: 10.1002/chem.201000884. [DOI] [PubMed] [Google Scholar]
- 37.Jardim G.A.M., Bower J.F., da Silva Júnior E.N. Rh-catalyzed reactions of 1,4-benzoquinones with electrophiles: C-H iodination, bromination, and phenylselenation. Org. Lett. 2016;18:4454–4457. doi: 10.1021/acs.orglett.6b01586. [DOI] [PubMed] [Google Scholar]
- 38.Jardim G.A.M., Silva T.L., Goulart M.O.F., de Simone C.A., Barbosa J.M.C., Salomão K., de Castro S.L., Bower J.F., da Silva Júnior E.N. Rhodium-catalyzed C-H bond activation for the synthesis of quinonoid compounds: significant Anti-Trypanosoma cruzi activities and electrochemical studies of functionalized quinines. Eur. J. Med. Chem. 2017;136:406–419. doi: 10.1016/j.ejmech.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Jardim G.A.M., da Cruz E.H.G., Valença W.O., Lima D.J.B., Cavalcanti B.C., Pessoa C., Rafique J., Braga A.L., Jacob C., da Silva Júnior E.N. Synthesis of selenium-quinone hybrid compounds with potential antitumor activity via Rh-catalyzed C-H bond activation and click reactions. Molecules. 2018;23:83. doi: 10.3390/molecules23010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheldrick G.M. SHELXT-Integrated space-group and crystal-structure determination, Acta Cryst. A C71. 2015:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolomanov O.V., Bourhis L.J., Gildea R.J., Howard J.A.K., Puschmann H. Olex 2: a complete structure solution, refinement and analysis program. J. Appl.Cryst. 2009;42:339–341. doi: 10.1107/S0021889808042726. [DOI] [Google Scholar]
- 42.Ferreira V.F., Pinto A.V., Pinto M.F.R., Santos S.C. The Diels-Alder reaction with O-2, 3-dimethylene-1, 4-naphthoquinone: a useful intermediate for the synthesis of the B ring of anthracyclinones, J. Braz. Chem. Soc. 1996;7:169–172. doi: 10.5935/0103-5053.19960026. [DOI] [Google Scholar]
- 43.Couto Giacobbo B., Pissinate K., Rodrigues-Junior V., Drumond Villela A., Silveira Grams E., Lopes Abbadi B., Teixeira Subtil F., Sperotto N., Valim Trindade R., Back D.F., Campos M.M., Basso L.A., Machado P., Santos D.S. New insights into the SAR and drug combination synergy of 2-(quinolin-4-yloxy)acetamides against Mycobacterium tuberculosis. Eur. J. Med. Chem. 2017;216:491–501. doi: 10.1016/j.ejmech.2016.11.048. [DOI] [PubMed] [Google Scholar]
- 44.Borsoi A.F., Paz J.D., Abbadi B.L., Macchi F.S., Sperotto N., Pissinate K., Rambo R.S., Ramos A.S., Machado D., Viveiros M., Bizarro C.V., Basso L.A., Machado P. Design, synthesis, and evaluation of new 2-(quinoline-4-yloxy)acetamide-based antituberculosis agents, Eur. J. Med. Chem. 192. 2020;112179 doi: 10.1016/j.ejmech.2020.112179. [DOI] [PubMed] [Google Scholar]
- 45.Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and citotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- 46.Alley M.C., Scudiero D.A., Monks A., Hursey M.L., Czerwinski M.J., Fine D.L., Abbott B.J., Mayo J.G., Shoemaker R.H., Boyd M.R. Feasibility of drug screening with panels of human tumor cell lines using a microculture tetrazolium assay. Canc. Res. 1988;48:589–601. [PubMed] [Google Scholar]
- 47.Barbosa F.A.R., Canto R.F.S., Saba S., Rafique J., L.Braga A. Synthesis and evaluation of dihydropyrimidinone-derived selenoesters as multi-targeted directed compounds against Alzheimer’s disease. Bioorg. Med. Chem. 2016;24:5762–5770. doi: 10.1016/j.bmc.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 48.Wang L., Qiao J., Wei J., Liang Z., Xu X., Li N. Air-stable binuclear Titanium(IV) salophen perfluorobutanesulfonate with zinc power catalytic system and its application to C–S and C–Se bond formation. Tetrahedron. 2020;7 doi: 10.1016/j.tet.2019.130750. [DOI] [Google Scholar]
- 49.Thomson R.H., Worthington R.D. Quinones. Part 9. Side-chain alkylthiolation of methyl-1,4-naphthoquinones. J. Chem. Soc. Perkin Trans. 1980;1:282–288. doi: 10.1039/P19800000282. [DOI] [Google Scholar]
- 50.Ning X., Qi H., Li R., Li Y., Jin Y., McNutt M.A., Liu J., Yin Y. Discovery of novel naphthoquinone derivatives as inhibitors of the tumor cell specific M2 isoform of pyruvate kinase. Eur. J. Med. Chem. 2017;138:343–352. doi: 10.1016/j.ejmech.2017.06.064. [DOI] [PubMed] [Google Scholar]
- 51.Allen F.H., Kennard O., Watson D.G., Brammer L., Orpen A.G., Taylor R., Chem J. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. Soc. Perkin Trans. 1987;2:S1–S19. https://doi-org.ez24.periodicos.capes.gov.br/10.1039/P298700000S1 [Google Scholar]
- 52.Sheldrick G.M. Univ. of Göttingen; Göttingen, Germany: 1997. SHELXL-97. Program for Crystal Structures Analysis. [Google Scholar]
- 53.Tadolini M., Lingtsang R.D., Tiberi S., Enwerem M., D’Ambrosio I., Sadutshang T.D., Centis R., Migliori G.B. First case of extensively drug-resistant tuberculosis treated with both delamanid and bedaquiline. Eur. Respir. J. 2016;48:935–938. doi: 10.1183/13993003.00637-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abbadi B.L., Villela A.D., Rodrigues-Junior V.S., Subtil F.T., Dalberto P.F., Pinheiro A.P.S., Santos D.S., Machado P., Basso L.A., Bizarro C.V. Revisiting activation of and mechanism of resistance to compound IQG-607 in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018;62 doi: 10.1128/aac.02222-17. 02222–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ekins S., Freundlich J.S., Choi I., Sarker M., Talcott C. Computational databases, pathway and cheminformatics tools for tuberculosis drug discovery. Trends Microbiol. 2011;19:65–74. doi: 10.1016/j.tim.2010.10.005. https://doi:10.1016/j.tim.2010.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 57.Rao M., Kumar M.M., Rao M.A. In vitro and in vivo effects of phenolic antioxidants against cisplatin-induced nephrotoxicity. J. Biochem. 1999;125:383–390. doi: 10.1093/oxfordjournals.jbchem.a022298. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.