Abstract
Background
Surveillance with annual mammography and breast MRI is recommended for female survivors of childhood cancer treated with chest radiation, yet benefits, harms and costs are uncertain.
Objective
To compare the benefits, harms and cost-effectiveness of breast cancer screening strategies in childhood cancer survivors.
Design
Collaborative simulation modeling using two Cancer Intervention and Surveillance Modeling Network breast cancer models.
Data Sources
Childhood Cancer Survivor Study, published data.
Target Population
Women aged 20+ with a history of chest radiation.
Time Horizon
Lifetime.
Perspective
Payer.
Interventions
Annual MRI +/− mammography, starting at ages 25, 30 or 35.
Outcome Measures
Breast cancer deaths averted, false-positive screens, benign biopsies, incremental cost-effectiveness ratios (ICERs).
Results of Base-Case Analysis
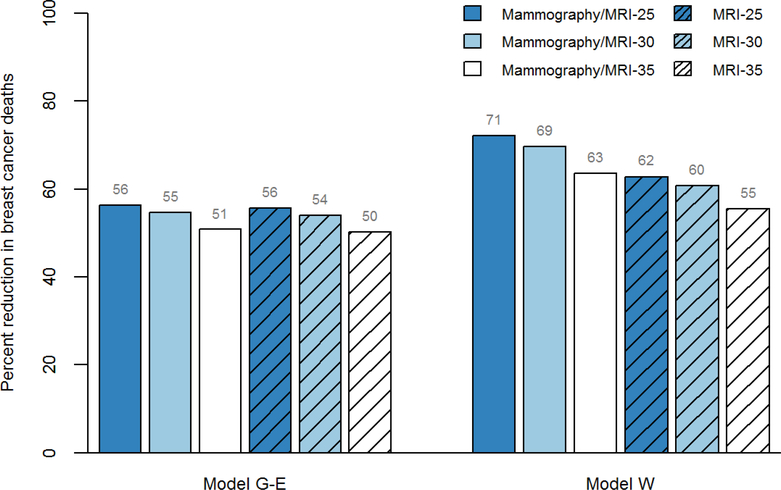
Lifetime breast cancer mortality risk without screening was 10%−11% across models. Compared to no screening, starting at age 25, annual mammography with MRI averted the most deaths (56%−71%), and annual MRI (without mammography) averted 56%−62%. Both had the most screening tests, false-positives, and benign biopsies. For an ICER threshold of <$100,000 per quality-adjusted life year, starting at age 30 was preferred.
Results of Sensitivity Analysis
Assuming lower screening performance, the benefit of adding mammography to MRI increased in both models, although the conclusions about preferred start age remained unchanged.
Limitations
Elevated breast cancer risk was based on survivors diagnosed with childhood cancer between 1970 and 1986.
Conclusions
Early initiation (at ages 25–30) of annual breast cancer screening with MRI with or without mammography could reduce breast cancer mortality for survivors of childhood cancer by half or more.
INTRODUCTION
Improvements in treatment for childhood cancer over the past five decades have resulted in remarkable survival increases, with more than 80% of children diagnosed today expected to survive five years or longer (1, 2). Despite this success, survivors face very high risks for treatment-related mortality (3) and late-effects, including secondary breast cancer (4, 5). This includes ~55,000 women in the US who have been treated with ≥20 Gray (Gy) chest radiation (6). Similar to BRCA1 mutation carriers, an estimated 30% of these survivors will develop breast cancer by age 50 (7). At the same time, overall competing mortality is higher among survivors than women without childhood cancer, reflecting the burden of comorbidities (8, 9), which may reduce health benefits from breast cancer screening and treatment.
Because of this elevated breast cancer risk, earlier initiation and more intensive screening is recommended for these women (10–13). For example, the Children’s Oncology Group (COG) recommends annual mammography screening with adjunct breast magnetic resonance imaging (MRI) starting at age 25 (or 8 years after chest radiation) in survivors of childhood, adolescent or young adult cancer who received ≥20 Gy chest radiation (14). However, fewer than 50% of at-risk survivors undergo recommended screening (15, 16) and clinicians who care for adult survivors are often unfamiliar with surveillance guidelines (17, 18). Recently, the International Late Effects Of Childhood Cancer Guideline Harmonization Group noted the substantial uncertainty about the balance between the benefits and harms of mammography and MRI in this high-risk population (19).
Decision modeling can facilitate evidence synthesis and provide data to inform guidelines in circumstances when randomized clinical trials are infeasible (20, 21). For example, modeling work by the Cancer Intervention and Surveillance Modeling Network (CISNET) informed the US Preventive Services Task Force (USPSTF) recommendations for breast cancer screening for average-risk women by assessing the benefits and harms for clinically relevant strategies (22, 23). Building upon this work, we used data from the Childhood Cancer Survivor Study (CCSS) (24) and two CISNET breast cancer simulation models to estimate the clinical benefits, harms, and cost-effectiveness of breast cancer screening among childhood cancer survivors previously treated with chest radiation.
METHODS
Overview
To examine breast cancer screening outcomes in survivors of childhood cancer, we used data from the CCSS (25) and adapted two CISNET breast cancer models (26): Model G-E (Georgetown University Medical Center and Albert Einstein College of Medicine) and Model W (University of Wisconsin-Madison) (27, 28) (Appendix Figure 1). The models share common model inputs such as screening test performance and competing mortality risks but vary in their approaches to modeling unobservable breast cancer natural history, including tumor onset and progression (29). Examining results across models thereby provides a range of estimates on breast cancer screening outcomes and helps determine the robustness of the conclusions to structural uncertainty resulting from different approaches to modeling disease natural history. Representative of the larger US population of survivors (25), the CCSS is a multi-institutional cohort study with longitudinal follow-up of North American five-year survivors of common childhood and adolescent cancers diagnosed prior to age 21 between 1970 and 1999 (24). The study was determined not to be human subjects research (Boston Children’s Hospital) or exempt from human subjects review (Georgetown University Medical Center; University of Wisconsin-Madison) by each Institutional Review Board.
Screening strategies
For a cohort of female survivors of childhood cancer with a history of chest radiation, the models evaluated the following strategies: 1) no screening; 2) digital mammography with MRI screening starting at age 25 (current COG recommendations), 30 or 35 and continuing to age 74, and 3) MRI only starting at age 25, 30 or 35 to age 74. Digital mammography alone was not considered as none of the current guidelines recommend mammography alone as a surveillance strategy in this high-risk population. To estimate efficacy, we assumed 100% adherence to screening and that women will receive the most effective therapy available at the time of breast cancer diagnosis, based on calendar year, age, stage, and estrogen receptor/ human epidermal growth factor 2 status.
Computer Simulation Models
Both Models G-E and W are discrete-event system microsimulation models of US women. Model G-E is a state-transition model that simulates breast cancer natural history without explicitly modeling tumor growth (27). Each breast cancer is assigned a time period in which the cancer can be detected prior to clinical symptoms; screening benefit is a function of detection at younger ages and earlier stage (i.e., stage-shift). Treatment benefits are based on a hazard reduction (i.e., due to lower stage of disease from detection at younger age). Model W simulates breast cancer natural history using a continuous tumor growth model (28). Screening benefit occurs through detection at younger age and smaller tumor size. Treatment benefit is modeled as lifelong “cure” for a proportion of those diagnosed (i.e., no possibility of dying from breast cancer) and no cure for the remainder. In both models, a subset of cancers are nonprogressive (Model G-E) or have limited malignant potential (Model W) (27, 28). Appendix Table 1 provides more detailed model overviews, also available at https://cisnet.cancer.gov/ and previously described (27, 28, 30). Coding for both models underwent extreme value testing to ensure that results changed in the expected directions. Both models reproduce US temporal trends in incidence and mortality for average-risk women (31) and also have predictive validity by replicating the UK Age Trial results (22, 32).
Model parameters
Breast Cancer Incidence
CCSS participants who were female at time of childhood cancer diagnosis between 1970 and 1986 (median age, 13 years) and treated with ≥20 Gy of chest radiation (74%) are at a 21.9-fold (95% CI, 19.1 to 25.2) higher risk of developing breast cancer compared to average-risk women based on Surveillance, Epidemiology, and End Results Program (SEER) data (7). These women were diagnosed with Hodgkin lymphoma (55%), Wilms (12%), non-Hodgkin lymphoma (8%) and other tumors. To reflect this elevated risk, we applied age-specific standardized incidence ratios from CCSS participants (relative to age- and calendar year-specific SEER rates) to breast cancer incidence rates in Models G-E and W (Table 1).
Table 1.
Model Input Parameters
| Parameter | Description | Data Source |
|---|---|---|
| Natural history of breast cancer | ||
| Incidence in the absence of screening | Age-period-cohort model calibrated to observed SEER program rates. Adjusted to reflect the elevated risk among childhood cancer survivors with a history of chest radiation using age-specific standardized incidence ratios (SIR): 40.2 (95%CI, 25.3, 63.7) for 20–29 years old, 30.6 (95%CI, 25.1, 37.2) for 30–39 year old, 16.6 (95% CI, 13.2–20.7) for 40–49 years old, and 15.3 (95% CI, 8.2, 28.4) for ≥50 years old. | (7, 33) |
| Stage distribution* | Stage distribution among women with clinically-, interval- and screen-detected cancer by age group (<50, 50–64, ≥65). Based on data for digital mammography. | BCSC |
| ER/HER2 joint distribution | Probability of ER/HER2 status conditional on age and stage/tumor size at diagnosis | BCSC |
| Sojourn time | Sojourn time, defined as the period of time preclinical disease is detectable by screening but asymptomatic, by joint ER/HER2 status and age. Assumed tumor characteristics in survivors similar to those in average-risk women. | (34) |
| Mean stage dwell time/tumor progress rates | Dwell time, defined as the time spent within a stage, varies by age and ER/HER2 status, and by model. Assumed tumor characteristics in survivors similar to those in average-risk women. | (27–29) |
| Breast cancer screening | ||
| Sensitivity/detection rates | Sensitivity by age group for digital mammography with MRI (<50 years: 0.932 [95%CI, 0.793, 0.980]; ≥50 years: 0.941 [95% CI, 0.777, 0.987] and MRI (without mammography) (<50 years: 0.857 [95%CI, 0.694, 0.941]; ≥50 years: 0.844 [95% CI, 0.618–0.948]. | (35) |
| Specificity | Specificity by age group for mammography with MRI (<50 years: 0.787 [95%CI, 0.706, 0.850]; ≥50 years: 0.853 [95% CI, 0.785, 0.902] and MRI (<50 years: 0.835 [95%CI, 0.776, 0.881]; ≥50 years: 0.885 [95% CI, 0.835, 0.922]. Assuming 75% of false positive screens were due to MRI, the proportion of women undergoing additional imaging without biopsy (6.7% vs. 6.1%) or having a benign biopsy (14.4% vs. 9.4%) was higher for mammography with MRI versus MRI only. | (22, 23, 35, 36) BCSC |
| Breast cancer treatment | ||
| Treatment use | Assume receipt of and adherence to the most effective available treatment specific to age, stage and ER/HER2 status. Optimal therapy assumed similar efficacy for surgery (lumpectomy or mastectomy) with or without radiation. | (37) |
| Treatment effects | Meta-analysis of clinical trial results. Modeled as a reduction in breast cancer-specific mortality risk or increase in proportion cured in the absence of adjuvant treatment. | (38) |
| Survival | ||
| Breast cancer survival | Long-term breast cancer survival before adjuvant treatment by joint ER/HER2 status, age group, and stage or tumor size. | (34) |
| Non-breast cancer mortality | Age- and cohort-specific all-cause mortality rates by year, modified to include survivor-specific late mortality risks based on female CCSS participants diagnosed between 1970 and 1999 treated with chemotherapy and radiation. | (39, 40) |
| Costs, US 2018 dollars | ||
| Screening mammography | $141 | CMS |
| Screening MRI | $550 | CMS |
| Work-up after false-positive screen result | Imaging costs: $157 (all ages). Biopsy costs by age for mammography: $1040 for ages 20–49, $1508 for ages 50–64, $1516 for ages 65–74, and $1606 for ages 75+. Assumed biopsy costs for MRI were 30% higher than for mammography. | (41) |
| Work-up after true-positive screen result | By age: $2556 for ages 20–49, $2400 for ages 50–64, $2412 for ages 65–74, and $2034 for ages 75+. | (41) |
| Breast cancer treatment | By stage during initial treatment: $14,440 for DCIS, $23,573 for local stage, $40,215 for regional stage, and $54,446 for distant stage. During the last year of life among women with cancer that was not cured/progressed, depending on stage at diagnosis: $55,428 for DCIS, $57,912 for local stage, $62,741 for regional stage, and $79,411 for distant stage. | (42–44) |
| Utilities | ||
| Survivors | Age- and sex-specific quality-of-life utilities among survivors of childhood cancer. | (45) |
| Screening mammography | Utility weight of 0.994 for 1 week. | (46) |
| Screening MRI | Assumed disutility for MRI, including potential side effects from gadolinium, was 2-fold higher than mammography, for utility weight of 0.988. | (46) |
| Diagnostics after positive screen | Utility weight of 0.895 for 5 weeks for mammography. Assuming disutility is proportional to biopsy rate, utility weight of 0.79 for 5 weeks for MRI and 0.815 for mammography with MRI for 5 weeks. | (46) |
| Cancer treatment | By stage: Utility weight of 0.9 or 2 years for DCIS and local stage, 0.75 for 2 years for regional stage, and 0.6 until death for distant stage. | (47) |
Abbreviations: BCSC, Breast Cancer Surveillance Consortium; CCSS, Childhood Cancer Survivor Study; CMS, Centers for Medicaid and Medicare Services; DCIS, ductal carcinoma in situ; ER, estrogen receptor; HER2, human epidermal growth factor 2; MRI, magnetic resonance imaging.
Stage based on Surveillance, Epidemiology, and End Results (SEER) historical staging for Model W and American Joint Committee on Cancer (version 6) for Model G-E.
Breast Cancer Characteristics
We assumed that the following natural history parameters were similar for breast cancers in childhood cancer survivors as for average-risk women (48): 1) probability and time of disease progression from preclinical to clinical disease, 2) stage distribution among clinically-detected and screen-detected cancers, 3) estrogen receptor/ human epidermal growth factor 2 distribution, and 3) breast cancer-specific mortality rates.
Screening Test Performance
Due to the limited available data on mammography and MRI screening performance specific to childhood cancer survivors, we based estimates of sensitivity and specificity on a meta-analysis which pooled individual-level data from six screening studies on BRCA 1/2 mutation carriers (35), estimated to have cancer risk similar to survivors previously exposed to chest radiation (7). We assumed that past radiation did not affect test performance.
To estimate additional imaging and biopsy rates for combined modality screening, we assumed that 75% of false positive screens were due to MRI (36). Thus, the proportion of women undergoing additional imaging without biopsy (6.7% vs. 6.1%) or having a benign biopsy (14.4% vs. 9.4%) was higher for mammography with MRI versus MRI alone, respectively.
Mortality
Childhood cancer survivors face general population risks of dying but also may develop multiple late complications. We therefore added the excess mortality risks associated with late recurrence of childhood cancer and development of comorbidities (40) to competing causes of mortality (39).
Costs
The costs of screening and diagnostic evaluation of a positive screen were based on US 2018 Medicare reimbursement rates and published estimates (41) (Table 1). For false-positive screen results, we assumed MRI findings led to 30% higher biopsy costs than mammography ones because of higher costs for MRI guidance. For true-positives, we assumed work-up costs were similar for all screening modalities. Cancer treatment costs varied by cancer stage and treatment phase and reflect updated SEER-Medicare costs from a prior analysis (42–44).
Quality of Life
To reflect quality of life among survivors living with late-effects, we used age- and sex-specific utility (quality of life preference) weights for childhood cancer survivors (45). We also incorporated utility deductions for undergoing screening, having false-positive screen results, and undergoing breast cancer treatment (by stage) (46, 47) (Table 1). Due to lack of published estimates for multi-modality screening, we inflated previously published disutility weights for screening mammography (46) by 2-fold for MRI only and 3-fold for mammography with MRI.
Analyses
Both Models GE and W simulated a cohort of 20-year-old female survivors of childhood cancer with a history of chest radiation undergoing breast cancer screening. Model outcomes included lifetime clinical benefits (reduction in breast cancer deaths, gains in life years and quality-adjusted life years), potential harms (number of false-positive screen results, benign biopsies, overdiagnosed cases), and costs. Overdiagnosed cases were defined as those that would not be clinically detected in the absence of screening.
To illustrate the tradeoffs, we calculated the following harm-benefit ratios: screening tests per death averted, false-positive screens per death averted, benign biopsies per death averted and overdiagnosed cases per death averted. For context, we compared these estimates to published estimates following USPSTF recommendations for biennial mammography for average-risk women aged 50–74 (22, 23). We evaluated the relative performance of strategies, calculating incremental cost-effectiveness ratios (ICERs), defined as the additional cost of a strategy divided by the additional clinical benefit, compared with the next least expensive strategy, and expressed as cost per quality-adjusted life year (QALY) gained. Analyses were conducted from a payer perspective following established recommendations (49, 50).
To reflect uncertainty in key model parameters on results, we conducted sensitivity analyses on the elevated risk of breast cancer associated with chest radiation among survivors, screening performance, and MRI-related costs and disutility weights. Plausible ranges were based on 95% confidence intervals for data used in the base case and expert opinion.
Role of Funding Source
The American Cancer Society and the National Cancer Institute funded this research. The funding sources had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
RESULTS
Clinical benefit and harms of current COG recommendations
Without screening, the lifetime risk of dying from breast cancer among childhood cancer survivors previously treated with chest radiation was 10%−11% across models (Table 2). For all strategies, the estimated benefits were greater in Model W than Model G-E. Compared to no screening, annual mammography and breast MRI starting at age 25 averted the most (56%−71%) breast cancer deaths and increased life years gained by 884–1990 years per 1000 women. Over their lifetimes, 1000 women would have 4188–4878 false-positive screens 1340–1561 benign biopsies. Appendix Table 2 provides estimates of overdiagnosed cases.
Table 2.
Lifetime Benefits and Harms of Screening Strategies Varying by Modality and Start Age
| Strategy* | Breast cancer deaths per 1000 women, n | Reduction in breast cancer deaths, %† | Life years gained per 1000 women, LYs†‡ | QALYs gained per 1000 women, QALYs†║ | Screening tests per 1000 women, n† | False-positive screens per 1000 women, n† | Benign biopsies per 1000 women, n† | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model G-E | Model W | Model G-E | Model W | Model G-E | Model W | Model G-E | Model W | Model G-E | Model W | Model G-E | Model W | Model G-E | Model W | |
| No screening | 101.5 | 112.1 | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- |
| MRI-35 | 50.6 | 50.7 | 50.2 | 54.8 | 1109 | 1774 | 475 | 851 | 16,928 | 13,901 | 2289 | 1909 | 870 | 726 |
| Mammo/MRI-35 | 49.9 | 41.8 | 50.8 | 62.7 | 1122 | 2095 | 479 | 1079 | 33,798 | 26,750 | 2969 | 2418 | 950 | 774 |
| MRI-30 | 46.7 | 44.9 | 54.0 | 60.0 | 1242 | 2034 | 564 | 1030 | 21,351 | 17,840 | 3002 | 2550 | 1141 | 969 |
| Mammo/MRI-30 | 46.0 | 35.0 | 54.7 | 68.8 | 1255 | 2408 | 566 | 1294 | 42,644 | 34,566 | 3892 | 3244 | 1245 | 1038 |
| MRI-25 | 45.0 | 42.6 | 55.7 | 62.0 | 1304 | 2148 | 599 | 1101 | 26,053 | 22,310 | 3764 | 3283 | 1430 | 1248 |
| Mammo/MRI-25 | 44.4 | 32.3 | 56.3 | 71.2 | 1317 | 2544 | 599 | 1380 | 52,048 | 43,466 | 4879 | 4188 | 1561 | 1340 |
G-E, Georgetown-Einstein; LYs, life years; mammo, mammography; MRI, magnetic resonance imaging; QALYs, quality-adjusted life years; W, Wisconsin.
Strategies rank ordered by increasing reduction in breast cancer deaths in Model G-E
Compared to no screening.
Life years per 1000 women for no screening was 4347 in Model W and 4523 in Model G-E.
Quality-adjusted life years per 1000 women for no screening was 3195 in Model W and 3318 in Model G-E.
Alternative screening strategies: Harm-benefit tradeoffs
In both models, all screening strategies, regardless of start age and screening modality reduced breast cancer deaths by 50% or more (Figure 1). Similarly, adding mammography to MRI or starting screening earlier at age 25 averted more breast cancer deaths, but with greater absolute reductions in Model W than Model G-E, especially when examining start age (Table 2).
Figure 1. Reduction in Breast Cancer Mortality for Screening Strategies Varying by Modality and Start Age among Childhood Cancer Survivors.
Shown are estimates for the reduction in breast cancer deaths for each screening strategy varying by modality (mammography with MRI, MRI only) and start age (25, 30 and 35) compared to no screening. G-E, Georgetown-Einstein; MRI, magnetic resonance imaging; W, Wisconsin.
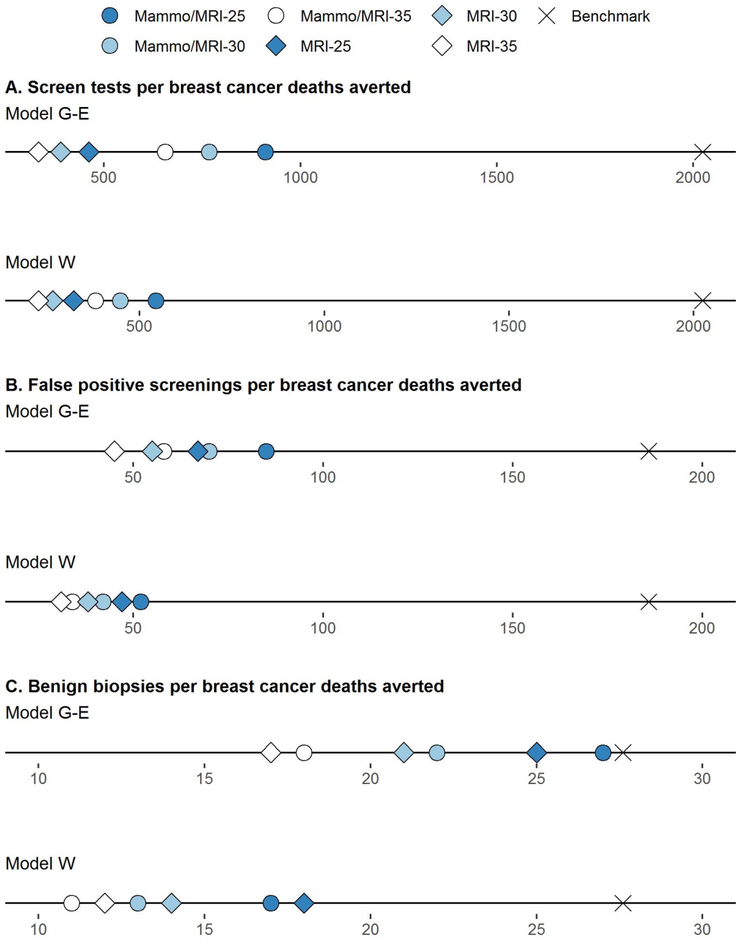
For all strategies, the number of false-positive screens per death averted ranged from 31 to 85 per 1000 women and for benign biopsies per death averted, 11 to 27 per 1000 women across models (Appendix Table 3). These harm-benefit ratios were considerably lower (i.e., more favorable) than benchmarks for average-risk women undergoing USPSTF recommendations for biennial screening (Figure 2). Estimates of overdiagnosed cases per death averted were also lower than average-risk benchmarks (Appendix Table 3).
Figure 2. Harm-Benefit Ratios for Screening Strategies Varying by Modality and Start Age among Childhood Cancer.
Shown are estimates for number of screening tests per breast cancer death averted (Panel A), false positive screens per breast cancer death averted (Panel B) and benign biopsies per breast cancer death averted (Panel C) for each screening strategy. For context, benchmark published estimates for harm-benefit ratio are shown for average-risk women in the general population undergoing USPSTF screening recommendations (biennial mammography between ages 50 and 74) (22, 23) Estimates for all screening strategies in both Model W and G-E were more favorable than benchmark ratios for average-risk women. G-E, Georgetown-Einstein; mammo, mammography; MRI, magnetic resonance imaging; W, Wisconsin.
Cost-effectiveness
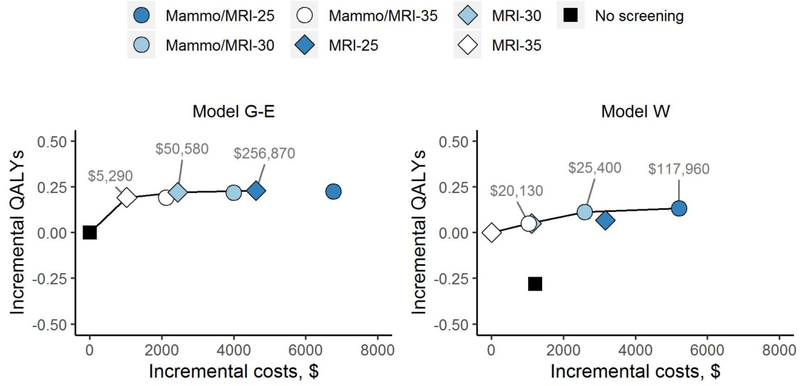
When examining COG recommendations, the ICER for annual mammography with MRI starting at age 25 versus no screening was cost-effective at the common threshold of <$100,000/QALY gained ($28,890/QALY in Model G-E and $9,160/QALY in Model W). When considering all screening and start age strategies falling below this threshold, the preferred screening modality was mammography with MRI starting at age 30 (Table 3). ICERs for screening starting at age 25 increased above the threshold relative to starting at age 30, reflecting decreasing marginal gains in QALYs relative to higher incremental costs (Figure 3). For example, in Model W, mammography with MRI screening starting at age 30 (vs. 35) increased QALYs by an additional 215. Starting at age 25 (vs. 30), the gain was only 86 QALYs (Table 2), with even smaller relative increases when discounted to calculate ICERs (Table 3).
Table 3.
Cost-Effectiveness of Breast Cancer Screening for Strategies Varying by Modality and Start Age: Cost per QALY
| Strategy* | Model G-E | Model W | ||||
|---|---|---|---|---|---|---|
| Discounted costs per 1000 women, $† | Discounted QALYs per 1000 women, QALYs† | ICER, $ per QALY† | Discounted costs per 1000 women, $† | Discounted QALYs per 1000 women, QALYs† | ICER, $ per QALY† | |
| No screening | $13,495,990 | 17378.0 | Baseline comparator | $11,923,450 | 17089.9 | ‡ |
| MRI-35 | $14,516,990 | 17571.4 | $5,290 | $10,716,760 | 17369.9 | Baseline comparator |
| Mammo/MRI-35 | $15,615,310 | 17571.1 | $11,000║ | $11,745,380 | 17421 | $20,130 |
| MRI-30 | $15,928,260 | 17599.3 | $50,580 | $11,822,100 | 17420.6 | $21,800§ |
| Mammo/MRI-30 | $17,494,210 | 17597.7 | $113,200¶ | $13,302,130 | 17482.3 | $25,400 |
| MRI-25 | $18,111,660 | 17607.8 | $256,870 | $13,871,490 | 17438.7 | $120,120** |
| Mammo/MRI-25 | $20,256,890 | 17604.6 | $816,720†† | $15,920,920 | 17504.5 | $117,960 |
G-E, Georgetown-Einstein; ICER, incremental cost-effectiveness ratio; mammo, mammographyMRI, magnetic resonance imaging; QALYs, quality-adjusted life years; W, Wisconsin.
Strategies rank ordered by increasing reduction in breast cancer deaths in Model G-E
Costs and benefits discounted 3% annually.
Strategy was dominated by Mammo/MRI-35, MRI-35 and Mammo/MRI-30 (i.e. more costly and less effective).
Strategy was dominated by Mammo/MRI-35, however because the difference in discounted costs and QALYs between the two strategies are close, the ICER where the reference case is No screening is reported.
Strategy was dominated by Mammo/MRI-35, however because the difference in discounted costs and QALYs between the two strategies are close, the ICER where the reference case is MRI-35 is reported.
Strategy was dominated by MRI-30, however because the differences in discounted costs and QALYS between the two strategies are close, the ICER where the reference case is MRI-35 is reported.
Strategy was dominated by Mammo/MRI-30, however because the difference in discounted costs and QALYs between the two strategies are close, the ICER where the reference case is Mammo/MRI-35 is reported.
Strategy was dominated by MRI-25, however because the differences in discounted costs and QALYs between the two strategies are close, the ICER where the reference case is MRI-30 is reported.
Figure 3. Cost-Effectiveness Efficiency Frontier for Screening Strategies Varying by Modality and Start Age.
Shown are incremental discounted costs per gain in discounted QALYS for each screening strategy compared to the baseline strategy in Model W (Panel A) and Model G-E (Panel B). Because of the greater estimated reduction in breast cancer deaths in Model W vs. Model GE, no screening was dominated (and eliminated) by MRI-35 and the baseline comparator was MRI-35 in Model W and no screening in Model G-E. Strategies on the efficiency frontier (solid line) have incremental cost-effectiveness ratios (ICERs), expressed as cost per QALY gained, as shown and offer both higher effectiveness and lower cost than those strategies below it. Both costs and benefits were discounted 3% annually. G-E, Georgetown-Einstein; mammo, mammography; MRI, magnetic resonance imaging; QALY, quality-adjusted life year; W, Wisconsin.
Sensitivity analyses
In both models, ICERs for annual screening starting at age 25 became more favorable if the lifetime risk of breast cancer associated with prior chest radiation increased to 64%−67% (vs. 56%−59% in the base case), and less favorable with a lower 43–44% lifetime risk (Appendix Tables 4; Appendix Figure 2). Results were largely unchanged if the elevated risk was 50% lower starting at age 60. If changes in radiotherapy (e.g., decreased volume, dose, field) were assumed to reduce the elevated breast cancer risk by 50% at all ages, ICERs for screening starting at age 25 (compared to no screening) remained below the $100,000/QALY threshold ($86,500/QALY in Model G-E and $38,330/QALY in Model W). When all strategies were considered, the ICER for mammography with MRI starting at age 30 remained below the threshold in Model W but not Model G-E (Appendix Figure 3).
Model W results were more sensitive to changes in test sensitivity and specificity than Model G-E, although overall conclusions about screening strategies and start ages remain unchanged in both models (Appendix Table 4). For example, the 95% lower bound test characteristics for MRI only and MRI with Mammography (35) increased the benefit of adding mammography to MRI in both models, but the ICERs for screening starting at age 30 remained below the $100,000/QALY threshold (Appendix Figure 4).
Additionally, if the disutilities associated with MRI-related screening and false-positive results were similar to those for mammography and MRI costs were halved, ICERs for screening starting at age 25 (compared to 30) improved in both models, but remained above the $100,000/QALY threshold in Model G-E. If breast cancer treatment was assumed less effective in survivors (a proxy for possible higher breast cancer-specific mortality among survivors (8)), ICERs for all screening strategies fell as the relative benefits of early detection became greater (Appendix Figure 5).
DISCUSSION
In this collaborative modeling study, we found that in the absence of screening, approximately 1 in 10 female childhood cancer survivors with a history of chest radiation will die from breast cancer compared with 1 in 40 among average-risk women in the general population (23). Screening with mammography and MRI starting at age 25, as currently recommended by the COG, is projected to avert half of expected breast cancer deaths among these high-risk survivors. With this annual schedule, a survivor will have an average of 4 to 5 false-positive screens and 1 to 2 benign biopsies over the course of her lifetime. However, due to the large survival benefits, the harm-benefit tradeoffs for survivors appear to be appropriate, resulting in more favorable harm-benefit ratios than published benchmarks for average-risk women (following USPSTF recommendations). To our knowledge, our study is the first to estimate these harm-benefit ratios for breast cancer screening among survivors of childhood cancer. Further, our findings suggest that starting screening at 30 is cost-effective given commonly cited cost-effectiveness thresholds (51, 52).
Recognizing that screening may be associated with potential anxiety from screening tests and benign biopsies, we evaluated alternative screening strategies which focused on initiating screening at later ages and/or with breast MRI only. We found that all strategies averted more than an estimated 50% of breast cancer deaths among survivors with favorable harm-benefit ratios. However, when both costs and quality of life were considered, ICERs for initiating screening at age 25 (compared to age 30) were considerably higher, reflecting the relatively small incremental benefit (e.g., 2–3 per 1000 women) in breast cancer deaths averted from initiating screening 5 years earlier. We also found that the additional mortality benefit of adding mammography to MRI varied by model, reflecting differences in model assumptions about the impact of detecting smaller or earlier-stage tumors. This is consistent with prior work by the models, with Model G-E generally finding small additional benefits from improvements in screening sensitivity versus more appreciable benefits in Model W. Although not directly comparable given differences in strategies evaluated, our findings are consistent with previous studies which found early initiation of screening among survivors to reduce breast cancer mortality (53) and be cost-effective (54).
Our findings underscore the importance of making sure that young women previously treated with chest radiation are informed about their elevated breast cancer risk and the benefits of routine screening. Both primary care providers and oncologists who care for survivors should discuss breast cancer screening with survivors. Screening guidelines should emphasize the importance of MRI screening (with or without mammography) among survivors. Our findings also highlight the importance of ensuring survivors have access to health insurance coverage for MRI screening. Of note, highlighting the challenges in augmenting rates in these women, the EMPOWER study found only marginal increases in MRI screening rates within 12 months among survivors who received a tailored telephone-delivered motivational interview(16).
Importantly, we did not account for the risk of radiation-induced breast cancer from mammography screening as the additional radiation exposure from mammograms between ages 25 and 39 is small (<0.3%) relative to the total radiation dose in women previously treated with 20 Gy of chest radiation (6). While the risks of radiation in survivors are likely smaller than for BRCA mutation carriers (who may be more sensitive to radiation-induced DNA damage due to the role of these genes in DNA repair), the use of mammography under the age of 30 remains controversial, and is currently not recommended by the American Cancer Society (11) nor the National Comprehensive Cancer Network (12). Our findings suggest that even without accounting for these additional potential risks, the benefit of adding mammography to MRI screening at these young ages is uncertain, and MRI alone may be a reasonable screening strategy at younger ages.
Additional limitations to our study include using data from survivors diagnosed between 1970 and 1986 to inform the elevated risk of subsequent breast cancer in adulthood now. Our findings suggest that even if the risk declines by half with changes in radiation dose/delivery, initiating screening earlier at younger ages in survivors remains favorable. Second, we based screening test performance on a meta-analysis on BRCA1/2 mutation carriers, who are known to be at higher risk for more aggressive breast cancer subtypes. However, results were stable in sensitivity analyses across a range of test performance (55, 56). We also did not consider digital breast tomosynthesis; its improved specificity (57) may lead to more favorable ICERs. Third, we assumed tumor characteristics and cancer treatment among survivors were similar to average-risk women (6, 48). Treatment options (e.g., use of radiotherapy or cardiotoxic chemotherapy agents) may be more limited for some survivors due to prior treatment exposures, although we found that with lower breast cancer treatment effectiveness, ICERs were even more favorable. Fourth, we assumed that in situ and invasive cancers were equally detectable with mammography or MRI; prior studies have shown that invasive cancers are more likely to be detected by MRI, and ductal carcinoma in situ by mammography. Fifth, we recognize that some childhood cancer survivors are unable to receive MRI and therefore undergo screening mammography only. We did not evaluate this strategy as MRI is recommended by all professional societies for this high-risk cohort and test performance data for mammography screening (without MRI) in high-risk women are limited. Screening costs for young survivors may also be higher than Medicare rates, especially if they are underinsured (58). Additionally, we found considerable uncertainty in estimated overdiagnosed cases between models. However, we note that the biology of overdiagnosis may differ among survivors given prior chest radiation and that estimated cases of overdiagnosis varied little across strategies in both models. Rates of overdiagnosis therefore provide less useful information on the tradeoffs in potential benefits and harms associated with different screening strategies for this unique group of women. Sixth, because of the large number of model parameters and computation time needed, we did not conduct probabilistic sensitivity analyses to evaluate the uncertainty surrounding all input parameters. However, we used two alternative natural history models of breast cancer to understand structural uncertainty and found qualitatively similar results. Lastly, we did not evaluate recently identified genetic markers of susceptibility for secondary breast cancer among survivors (59, 60). Survivors without a history of chest radiation have also been shown to be at elevated risk for breast cancer (56, 61, 62). Future planned analyses include using modeling to understand how this information can refine and inform screening guidelines for at-risk survivors.
In conclusion, female childhood cancer survivors previously treated with chest radiation are at high risk from dying from breast cancer and this early mortality can be averted with initiation of annual breast cancer screening. Our models suggest that annual screening with MRI (with or without mammography) starting at ages 25–30 can avert half or more of the expected deaths, with an acceptable rate of false-positive screens. Our findings highlight the importance of MRI in reducing deaths from breast cancer among young women previously exposed to chest radiation. Identifying effective policies and interventions to reduce barriers to screening should be priorities for policymakers to ensure comprehensive and coordinated care for these high-risk survivors.
Supplementary Material
Reproducible Research Statement.
Study protocol
Available from Dr. Yeh (email, jennifer.yeh@childrens.harvard.edu).
Computer code
Detailed information about the models is available online at https://cisnet.cancer.gov/breast/profiles.html and available in references (27) and (28).
Analytic dataset
Output data from models available from Dr. Yeh (email, jennifer.yeh@childrens.harvard.edu).
Acknowledgments
FUNDING SOURCE
American Cancer Society (Research Scholar Grant, RSG-16-018-01 – CPHPS, J.M. Yeh, Principal Investigator) and the National Cancer Institute at the National Institutes of Health (U01CA199218, J. Mandelblatt, Clyde Schechter, and Amy Trentham-Dietz, Principal Investigators; U24CA55727, G.T. Armstrong, Principal Investigator; R01CA134722, K. Oeffinger and J. Ford, Principal Investigators). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese-Syrian Associated Charities (ALSAC).
Footnotes
This is the prepublication, author-produced version of a manuscript accepted for publication in Annals of Internal Medicine. This version does not include post-acceptance editing and formatting. The American College of Physicians, the publisher of Annals of Internal Medicine, is not responsible for the content or presentation of the author-produced accepted version of the manuscript or any version that a third party derives from it. Readers who wish to access the definitive published version of this manuscript and any ancillary material related to this manuscript (e.g., correspondence, corrections, editorials, linked articles) should go to Annals.org or to the print issue in which the article appears. Those who cite this manuscript should cite the published version, as it is the official version of record.
REFERENCES
- 1.Institute of Medicine and National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, D.C.: The National Academies Press; 2005. [Google Scholar]
- 2.Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA Cancer J Clin. 2014;64(2):83–103. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong GT, Chen Y, Yasui Y, Leisenring W, Gibson TM, Mertens AC, et al. Reduction in Late Mortality among 5-Year Survivors of Childhood Cancer. N Engl J Med. 2016;374(9):833–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. [DOI] [PubMed] [Google Scholar]
- 5.Turcotte LM, Liu Q, Yasui Y, Arnold MA, Hammond S, Howell RM, et al. Temporal Trends in Treatment and Subsequent Neoplasm Risk Among 5-Year Survivors of Childhood Cancer, 1970–2015. JAMA. 2017;317(8):814–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henderson TO, Amsterdam A, Bhatia S, Hudson MM, Meadows AT, Neglia JP, et al. Systematic review: surveillance for breast cancer in women treated with chest radiation for childhood, adolescent, or young adult cancer. Ann Intern Med. 2010;152(7):444–55; W144–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moskowitz CS, Chou JF, Wolden SL, Bernstein JL, Malhotra J, Novetsky Friedman D, et al. Breast cancer after chest radiation therapy for childhood cancer. J Clin Oncol. 2014;32(21):2217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moskowitz CS, Chou JF, Neglia JP, Partridge AH, Howell RM, Diller LR, et al. Mortality After Breast Cancer Among Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37(24):2120–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Milano MT, Li H, Gail MH, Constine LS, Travis LB. Long-term survival among patients with Hodgkin’s lymphoma who developed breast cancer: a population-based study. J Clin Oncol. 2010;28(34):5088–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Children’s Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent, and Young Adult Cancers. Version 5.0 - October 2018. Children’s Oncology Group. [Google Scholar]
- 11.Saslow D, Boetes C, Burke W, Harms S, Leach MO, Lehman CD, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007;57(2):75–89. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Breast Cancer Screening and Diagnosis Version 1; 2019. [DOI] [PubMed] [Google Scholar]
- 13.Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast Cancer Screening in Women at Higher-Than-Average Risk: Recommendations From the ACR. J Am Coll Radiol. 2018;15(3 Pt A):408–14. [DOI] [PubMed] [Google Scholar]
- 14.Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, et al. Development of risk-based guidelines for pediatric cancer survivors: the Children’s Oncology Group Long-Term Follow-Up Guidelines from the Children’s Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–90. [DOI] [PubMed] [Google Scholar]
- 15.Yan AP, Chen Y, Henderson TO, Oeffinger KC, Hudson MM, Gibson TM, et al. Adherence to Surveillance for Second Malignant Neoplasms and Cardiac Dysfunction in Childhood Cancer Survivors: A Childhood Cancer Survivor Study. J Clin Oncol. 2020:JCO1901825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oeffinger KC, Ford JS, Moskowitz CS, Chou JF, Henderson TO, Hudson MM, et al. Promoting Breast Cancer Surveillance: The EMPOWER Study, a Randomized Clinical Trial in the Childhood Cancer Survivor Study. J Clin Oncol. 2019;37(24):2131–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Suh E, Daugherty CK, Wroblewski K, Lee H, Kigin ML, Rasinski KA, et al. General internists’ preferences and knowledge about the care of adult survivors of childhood cancer: a cross-sectional survey. Ann Intern Med. 2014;160(1):11–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nathan PC, Daugherty CK, Wroblewski KE, Kigin ML, Stewart TV, Hlubocky FJ, et al. Family physician preferences and knowledge gaps regarding the care of adolescent and young adult survivors of childhood cancer. J Cancer Surviv. 2013;7(3):275–82. [DOI] [PubMed] [Google Scholar]
- 19.Mulder RL, Kremer LC, Hudson MM, Bhatia S, Landier W, Levitt G, et al. Recommendations for breast cancer surveillance for female survivors of childhood, adolescent, and young adult cancer given chest radiation: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2013;14(13):e621–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Habbema JD, Wilt TJ, Etzioni R, Nelson HD, Schechter CB, Lawrence WF, et al. Models in the development of clinical practice guidelines. Ann Intern Med. 2014;161(11):812–8. [DOI] [PubMed] [Google Scholar]
- 21.Owens DK, Whitlock EP, Henderson J, Pignone MP, Krist AH, Bibbins-Domingo K, et al. Use of Decision Models in the Development of Evidence-Based Clinical Preventive Services Recommendations: Methods of the U.S. Preventive Services Task Force. Ann Intern Med. 2016;165(7):501–8. [DOI] [PubMed] [Google Scholar]
- 22.Mandelblatt JS, Cronin K, de Koning H, Miglioretti DL, Schechter CS, Stout N. Modeling Technical Report. Collaborative modeling of U.S. breast cancer screening strategies. AHRQ Publication No. 14–05201-EF-4. April 2015. 2015. [Google Scholar]
- 23.Mandelblatt JS, Stout NK, Schechter CB, van den Broek JJ, Miglioretti DL, Krapcho M, et al. Collaborative Modeling of the Benefits and Harms Associated With Different U.S. Breast Cancer Screening Strategies. Ann Intern Med. 2016;164(4):215–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. J Clin Oncol. 2009;27(14):2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips SM, Padgett LS, Leisenring WM, Stratton KK, Bishop K, Krull KR, et al. Survivors of childhood cancer in the United States: prevalence and burden of morbidity. Cancer Epidemiol Biomarkers Prev. 2015;24(4):653–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alagoz O, Berry DA, de Koning HJ, Feuer EJ, Lee SJ, Plevritis SK, et al. Introduction to the Cancer Intervention and Surveillance Modeling Network (CISNET) Breast Cancer Models. Med Decis Making. 2018;38(1_suppl):3S–8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schechter CB, Near AM, Jayasekera J, Chandler Y, Mandelblatt JS. Structure, Function, and Applications of the Georgetown-Einstein (GE) Breast Cancer Simulation Model. Med Decis Making. 2018;38(1_suppl):66S–77S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alagoz O, Ergun MA, Cevik M, Sprague BL, Fryback DG, Gangnon RE, et al. The University of Wisconsin Breast Cancer Epidemiology Simulation Model: An Update. Med Decis Making. 2018;38(1_suppl):99S–111S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Broek JJ, van Ravesteyn NT, Mandelblatt JS, Cevik M, Schechter CB, Lee SJ, et al. Comparing CISNET Breast Cancer Models Using the Maximum Clinical Incidence Reduction Methodology. Med Decis Making. 2018;38(1_suppl):112S–25S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mandelblatt JS, Near AM, Miglioretti DL, Munoz D, Sprague BL, Trentham-Dietz A, et al. Common Model Inputs Used in CISNET Collaborative Breast Cancer Modeling. Med Decis Making. 2018;38(1_suppl):9S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, et al. Association of Screening and Treatment With Breast Cancer Mortality by Molecular Subtype in US Women, 2000–2012. JAMA. 2018;319(2):154–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van den Broek JJ, van Ravesteyn NT, Mandelblatt JS, Huang H, Ergun MA, Burnside ES, et al. Comparing CISNET Breast Cancer Incidence and Mortality Predictions to Observed Clinical Trial Results of Mammography Screening from Ages 40 to 49. Med Decis Making. 2018;38(1_suppl):140S–50S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gangnon RE, Sprague BL, Stout NK, Alagoz O, Weedon-Fekjaer H, Holford TR, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev. 2015;24(6):905–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz D, Near AM, van Ravesteyn NT, Lee SJ, Schechter CB, Alagoz O, et al. Effects of screening and systemic adjuvant therapy on ER-specific US breast cancer mortality. J Natl Cancer Inst. 2014;106(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Phi XA, Houssami N, Obdeijn IM, Warner E, Sardanelli F, Leach MO, et al. Magnetic resonance imaging improves breast screening sensitivity in BRCA mutation carriers age >/= 50 years: evidence from an individual patient data meta-analysis. J Clin Oncol. 2015;33(4):349–56. [DOI] [PubMed] [Google Scholar]
- 36.Lee JM, Ichikawa L, Valencia E, Miglioretti DL, Wernli K, Buist DSM, et al. Performance Benchmarks for Screening Breast MR Imaging in Community Practice. Radiology. 2017;285(1):44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. 2015.
- 38.Early Breast Cancer Trialists’ Collaborative G, Peto R, Davies C, Godwin J, Gray R, Pan HC, et al. Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100,000 women in 123 randomised trials. Lancet. 2012;379(9814):432–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gangnon RE, Stout NK, Alagoz O, Hampton JM, Sprague BL, Trentham-Dietz A. Contribution of Breast Cancer to Overall Mortality for US Women. Med Decis Making. 2018;38(1_suppl):24S–31S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeh JM, Ward ZJ, Chaudhry A, Liu Q, Yasui Y, Armstrong GT, et al. Life Expectancy of Adult Survivors of Childhood Cancer Over 3 Decades. JAMA Oncol. 2020. doi: 10.1001/jamaoncol.2019.5582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stout NK, Lee SJ, Schechter CB, Kerlikowske K, Alagoz O, Berry D, et al. Benefits, Harms, and Costs for Breast Cancer Screening After US Implementation of Digital Mammography. J Natl Cancer Inst. 2014;106(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lowry KP, Trentham-Dietz A, Schechter CB, Alagoz O, Barlow WE, Burnside ES, et al. Long-term Outcomes and Cost-effectiveness of Breast Cancer Screening with Digital Breast Tomosynthesis in the United States. J Natl Cancer Inst. 2019;pii: djz184. doi: 10.1093/jnci/djz184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mariotto AB, Warren JL, Zeruto C, Coughlan D, Barrett MJ, Zhao L, et al. Cancer-Attributable Medical Costs for Colorectal Cancer Patients by Phases of Care: What Is the Effect of a Prior Cancer History? JNCI Monographs. 2020;2020(55):22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mariotto A, Enewold L, Zhao J, Zeruto C, Yabroff KR. Medical Care Costs Associated with Cancer Survivorship in the United States. Cancer Epidemiol Biomarkers Prev. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yeh JM, Hanmer J, Ward ZJ, Leisenring WM, Armstrong GT, Hudson MM, et al. Chronic Conditions and Utility-Based Health-Related Quality of Life in Adult Childhood Cancer Survivors. J Natl Cancer Inst. 2016;108(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Haes JC, de Koning HJ, van Oortmarssen GJ, van Agt HM, de Bruyn AE, van Der Maas PJ. The impact of a breast cancer screening programme on quality-adjusted life-years. Int J Cancer. 1991;49(4):538–44. [DOI] [PubMed] [Google Scholar]
- 47.Stout NK, Rosenberg MA, Trentham-Dietz A, Smith MA, Robinson SM, Fryback DG. Retrospective cost-effectiveness analysis of screening mammography. J Natl Cancer Inst. 2006;98(11):774–82. [DOI] [PubMed] [Google Scholar]
- 48.Elkin EB, Klem ML, Gonzales AM, Ishill NM, Hodgson D, Ng AK, et al. Characteristics and outcomes of breast cancer in women with and without a history of radiation for Hodgkin’s lymphoma: a multi-institutional, matched cohort study. J Clin Oncol. 2011;29(18):2466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG. Cost-Effectiveness in Health and Medicine. Second Edition. New York: Oxford University Press; 2017. [Google Scholar]
- 50.Caro JJ, Briggs AH, Siebert U, Kuntz KM. Modeling good research practices--overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force−−1. Value Health. 2012;15(6):796–803. [DOI] [PubMed] [Google Scholar]
- 51.Eichler HG, Kong SX, Gerth WC, Mavros P, Jonsson B. Use of cost-effectiveness analysis in health-care resource allocation decision-making: how are cost-effectiveness thresholds expected to emerge? Value Health. 2004;7(5):518–28. [DOI] [PubMed] [Google Scholar]
- 52.Neumann PJ, Sandberg EA, Bell CM, Stone PW, Chapman RH. Are pharmaceuticals cost-effective? A review of the evidence. Health Aff (Millwood). 2000;19(2):92–109. [DOI] [PubMed] [Google Scholar]
- 53.Hodgson DC, Cotton C, Crystal P, Nathan PC. Impact of Early Breast Cancer Screening on Mortality Among Young Survivors of Childhood Hodgkin’s Lymphoma. J Natl Cancer Inst. 2016;108(7). [DOI] [PubMed] [Google Scholar]
- 54.Tessier L, Furzer J, Hodgson D, Cotton C, Nathan PC, Gupta S, et al. Cost-Utility of Early Breast Cancer Surveillance in Survivors of Thoracic Radiation-Treated Adolescent Hodgkin’s Lymphoma. J Natl Cancer Inst. 2019:pii: djz037. doi: 10.1093/jnci/djz037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chiarelli AM, Blackmore KM, Muradali D, Done SJ, Majpruz V, Weerasinghe A, et al. Performance measures of magnetic resonance imaging plus mammography in the High Risk Ontario Breast Screening Program. J Natl Cancer Inst. 2019:pii: djz079. doi: 10.1093/jnci/djz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ehrhardt MJ, Howell CR, Hale K, Baassiri MJ, Rodriguez C, Wilson CL, et al. Subsequent Breast Cancer in Female Childhood Cancer Survivors in the St Jude Lifetime Cohort Study (SJLIFE). J Clin Oncol. 2019;37(19):1647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conant EF, Barlow WE, Herschorn SD, Weaver DL, Beaber EF, Tosteson ANA, et al. Association of Digital Breast Tomosynthesis vs Digital Mammography With Cancer Detection and Recall Rates by Age and Breast Density. JAMA Oncol. 2019;5(5):635–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Park ER, Kirchhoff AC, Nipp RD, Donelan K, Leisenring WM, Armstrong GT, et al. Assessing Health Insurance Coverage Characteristics and Impact on Health Care Cost, Worry, and Access: A Report From the Childhood Cancer Survivor Study. JAMA Intern Med. 2017;177(12):1855–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morton LM, Sampson JN, Armstrong GT, Chen T, Hudson MM, Karlins E, et al. Genome-Wide Association Study to Identify Susceptibility Loci That Modify Radiation-Related Risk for Breast Cancer After Childhood Cancer. J Natl Cancer Inst. 2017;109(11):djx058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Best T, Li D, Skol AD, Kirchhoff T, Jackson SA, Yasui Y, et al. Variants at 6q21 implicate PRDM1 in the etiology of therapy-induced second malignancies after Hodgkin’s lymphoma. Nat Med. 2011;17(8):941–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Henderson TO, Moskowitz CS, Chou JF, Bradbury AR, Neglia JP, Dang CT, et al. Breast Cancer Risk in Childhood Cancer Survivors Without a History of Chest Radiotherapy: A Report From the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34(9):910–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Teepen JC, van Leeuwen FE, Tissing WJ, van Dulmen-den Broeder E, van den Heuvel-Eibrink MM, van der Pal HJ, et al. Long-Term Risk of Subsequent Malignant Neoplasms After Treatment of Childhood Cancer in the DCOG LATER Study Cohort: Role of Chemotherapy. J Clin Oncol. 2017;35(20):2288–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.