Abstract
Metabolic addiction, an organism that is metabolically addicted with a compound to maintain its growth fitness, is an underexplored area in metabolic engineering. Microbes with heavily engineered pathways or genetic circuits tend to experience metabolic burden leading to degenerated or abortive production phenotype during long-term cultivation or scale-up. A promising solution to combat metabolic instability is to tie up the end-product with an intermediary metabolite that is essential to the growth of the producing host. Here we present a simple strategy to improve both metabolic stability and pathway yield by coupling chemical addiction with negative autoregulatory genetic circuits. Naringenin and lipids compete for the same precursor malonyl-CoA with inversed pathway yield in oleaginous yeast. Negative autoregulation of the lipogenic pathways, enabled by CRISPRi and fatty acid-inducible promoters, repartitions malonyl-CoA to favor flavonoid synthesis and increased naringenin production by 74.8%. With flavonoid-sensing transcriptional activator FdeR and yeast hybrid promoters to control leucine synthesis and cell grwoth fitness, this amino acid feedforward metabolic circuit confers a flavonoid addiction phenotype that selectively enrich the naringenin-producing pupulation in the leucine auxotrophic yeast. The engineered yeast persisted 90.9% of naringenin titer up to 324 generations. Cells without flavonoid addiction regained growth fitness but lost 94.5% of the naringenin titer after cell passage beyond 300 generations. Metabolic addiction and negative autoregulation may be generalized as basic tools to eliminate metabolic heterogeneity, improve strain stability and pathway yield in long-term and large-scale bioproduction.
Keywords: Synthetic biology, Metabolic addiction, Negative autoregulation, Strain stability, Cell fitness, Metabolic heterogeneity
1. Introduction
Metabolic heterogeneity has been found to play an essential role in determining cellular performance and pathway efficiency (Xiao et al., 2016; Ceroni et al., 2018; Rugbjerg et al., 2018). Traditional bioprocess engineering strategies, including modulating agitation, temperature, pH, dissolved oxygen (DO), dilution rate, and feeding of limiting nutrients, are largely employed nowadays to maintain metabolic homeostasis and improve the titer, yield, and productivity (TYP) of the engineered cell (Qiao et al., 2017; Xu et al., 2017). Fermentation could be viewed as an evolutionary process with productive cells constantly dividing into multiple lineages that are deviating from the parental strains (Xu, 2018). In particular, heavily engineered strains with artificial pathways or genetic circuits tend to lose the production phenotype in long-term fermentations due to metabolic burden, enzyme/cofactor imbalance, toxic chemical accumulation, and genetic instability (Wu et al., 2016). Metabolic burden drives subpopulations of cell to escape the selection pressure, regain growth fitness, propagate and dominate the producing cell line (Lv et al., 2019). Moreover, this metabolic instability is hard to comply with the high-standard of GMP guidelines in pharmaceutical and biotechnology industry (Madzak, 2015).
The decrease and even loss of production has been recently ascribed to nongenetic cell-cell variations or genetic population heterogeneity (Xiao et al., 2016; Rugbjerg et al., 2018; Rugbjerg et al., 2018). To solve this challenge, we may borrow the concept of “intelligent control” from mechanical or electrical engineering, to encode decision-making feedback functions at the population level and improve the community-level cellular performance. In such a way, the engineered cell could sense the metabolic stress and autonomously adjust cell metabolism to reinforce growth fitness. Indeed, dynamic feedback control has been successfully applied to various organisms to autonomously partition carbon flux and optimize cell metabolism for chemical production in recent years. The development of the metabolator (Fung et al., 2005), malonyl-CoA inverter gate (Liu et al., 2015), malonyl-CoA oscillatory switches (Xu et al., 2014; Xu et al., 2014; Johnson et al., 2017; Xu, 2019), burden-driven feedback control (Ceroni et al., 2015; Ceroni et al., 2018), quorum-sensing based genetic circuits (Gupta et al., 2017; Doong et al., 2018; Dinh and Prather, 2019), antisense RNA-based control (Yang et al., 2018), and dCas9-CRIRPRi-based circuits (Berckman and Chen, 2019; Wu et al., 2020) have been implemented to relieve metabolic stress and boost cell's productivity in recent years. To combat cellular heterogeneity, one promising area that is largely underexplored is to consider the mutualistic social interactions and enhance microbial cooperation. We can apply social “punishment-and-reward” rules to eliminate the non-productive cell and incentivize the productive cell (Xiao et al., 2016; Lv et al., 2019). For example, one can link the end-product with cell fitness to selectively enrich the growth of the producing cell-lines (Rugbjerg et al., 2018), or use a xenobiotic chemical (i.e., a rare nitrogen/phosphate source or an antibiotics) to confer a competitive growth advantage to the producing cell (Shaw et al., 2016) and eliminate the growth of the cheater cells during cultivations (Xiao et al., 2016). In such a way, we may mitigate the metabolic heterogeneity and stabilize the overproduction phenotype.
The lipogenic acetyl-CoA and malonyl-CoA flux have been repurposed for production of β-carotenoids (Gao et al., 2017; Larroude et al., 2018), polyketides (Markham et al., 2018; Liu et al., 2019; Lv et al., 2019; Palmer et al., 2020), and terpenoids (Jin et al., 2019; Zhang et al., 2019) in Yarrowia lipolytica. However, substantial portion of acetyl-CoA and malonyl-CoA is channeled into lipid synthesis in the engineered strains. These nutraceuticals and lipids compete for the same precursors (acetyl-CoA or malonyl-CoA) with inversed pathway yield in oleaginous yeast, which is a common problem when Y. lipolytica is used as chassis to produce acetyl-CoA-derived chemicals (Beopoulos et al., 2011). A large portfolio of engineering work has been dedicated to mitigate lipogenesis or redirect lipid synthesis for heterologous chemical production (Ledesma-Amaro and Nicaud, 2016; Markham et al., 2018), but the heavily engineered strains, even the chromosomally-integrated cell lines, are often difficult to maintain high performance during long-term cultivations (Roth et al., 2009; Xu et al., 2017; Wei et al., 2019). To solve this challenge, we took advantage of the transcriptional activity of metabolite-responsive promoters (Skjoedt et al., 2016; D'Ambrosio and Jensen, 2017; Wan et al., 2019), aiming to develop an end-product addiction circuit to rewire cell metabolism in Y. lipolytica. Coupled with CRISPRi-assisted lipogenic negative autoregulation, the end-product (flavonoid) addiction circuit drives gene expression (leucine synthesis) that is advantageous to productive cell or deleterious to cheater cell. In such a way, we reinforced the fitness of the productive cell and enhanced the overall metabolic performance. Specifically, we have engineered a bilayer dynamic control circuit to autonomously partition metabolic flux and improve strain stability in Y. lipolytica. By coupling metabolic addiction with lipogenic negative autoregulation, we improved cell fitness and pathway yield, and the flavonoid-producing phenotype was sustained up to 320 generations. These results highlight the importance of applying dynamic population control and microbial cooperation to improve the community-level metabolic performance.
2. Materials and methods
2.1. Plasmids and strains
Plasmids pYLXP′-Cre, pYLXP′-Nluc, and pYLXP′-dCas9 were frozen stocks in our lab. The dCas9 contains an SV40 nuclear localization sequence at C-terminal. Escherichia coli NEB 5α was used for plasmid construction and maintenance. Y. lipolytica Po1f/Δku70 is a derivative of Y. lipolytica Po1f (ATCC MYA-2613, MATA ura 3-302 leu2-270 xpr2-322 axp2-deltaNU49 XPR2:SUC2) by removing KU70 gene. Y. lipolytica Po1f/Δku70 and NarPro/ASC were used as the chassis.
2.2. Molecular biology and genetic cloning
The plasmid pYLXP′-URA3-loxP was constructed in the previous work (Lv et al., 2019). The site-directed integration plasmids pΔleu2loxP and pΔxpr2loxP were constructed as follows. The leu2 upstream and downstream homologous arms leu2-up and leu2-down were amplified from Y. lipolytica Po1f genome DNA using primer pairs leu2-up F/leu2-up R and leu2-down F/leu2-down R, respectively. The 600-bp leu2-up fragment was assembled with AvrII digested pYLXP′-URA3-loxP using Gibson Assembly, resulting in pΔleu2loxP-up. The 600-bp leu2-down fragment was assembled with NotI digested pΔleu2loxP-up using Gibson Assembly, resulting in pΔleu2loxP. The xpr2 upstream and downstream homologous arms xpr2-up and xpr2-down were amplified from Y. lipolytica Po1f genome DNA using primer pairs xpr2-up F/xpr2-up R and xpr2-down F/xpr2-down R, respectively. The 414-bp xpr2-up fragment was assembled with AvrII digested pYLXP′-URA3-loxP using Gibson Assembly, resulting in pΔxpr2loxP-up. The 552-bp xpr2-down fragment was assembled with NotI digested pΔxpr2loxP-up using Gibson Assembly, resulting in pΔxpr2loxP. pΔleu2loxP and pΔxpr2loxP are YaliBrick plasmids, which can utilize the isocaudarners for fast assembly (Wong et al., 2017).
Plasmids containing fatty acid inducible promoters were constructed as follows. The 1591-bp POX2(1591) promoter was amplified from the genome DNA of Y. lipolytica Po1f using primer pair pPOX2 (1591) F/pPOX2 (1591) R. The purified PCR product was assembled with AvrII/XbaI digested pYLXP′ using Gibson Assembly to yield plasmid pPOX2 (1591). The 601-bp A1R1 and 438-bp A3-core promoter were amplified from Y. lipolytica Po1f genome DNA using primer pairs pPOX2 (1591) F/A1R1 R and A3 F2/pPOX2 (1591) R, respectively. The purified A1R1 and A3-core promoter fragments were assembled with AvrII/XbaI digested pYLXP′ using Gibson Assembly to yield plasmid pA1R1A3. The 601-bp A1R1-1 and 438-bp A3-core promoter were amplified from Y. lipolytica Po1f genome DNA using primer pairs pPOX2 (1591) F/A1R1 R1 and A3 F/pPOX2 (1591) R, respectively. The 601-bp A1R1-2 fragment was amplified from plasmid pA1R1A3 using primer pair A1R1 F2/A1R1 R2. The purified A1R1-1, A1R1-2, and A3-core promoter fragments were assembled with AvrII/XbaI digested pYLXP’ to yield plasmid p (A1R1)x2A3. The resulting plasmids pPOX2 (1591), pA1R1A3, and p (A1R1)x2A3 will drive the transcription of gRNAs under the control of PPOX2(1591), PA1R1A3, and P(A1R1)x2A3 promoters, respectively.
In order to analyze these inducible promoters, the luciferase encoding gene Nluc was used as reporter. Nluc was amplified from plasmid pYLXP′-Nluc using primer pair Nluc F/Nluc R. The purified Nluc fragment was assembled with SnaBI digested pPOX2 (1591), pA1R1A3, and p (A1R1)x2A3 to yield pPOX2 (1591)-Nluc, pA1R1A3-Nluc, and p (A1R1)x2A3-Nluc, respectively. The AvrII/SalI digested donor plasmids pPOX2 (1591)-Nluc, pA1R1A3-Nluc, and p (A1R1)x2A3-Nluc were ligated to NheI/SalI digested destination plasmid pΔleu2loxP to yield pΔleu2loxP-POX2 (1591)-Nluc, pΔleu2loxP-A1R1A3-Nluc, and pΔleu2loxP -(A1R1)x2A3-Nluc, respectively. Plasmids used in this paper were listed in Supplementary Table 2. These plasmids were used to integrate the Nluc expressing cassette to the leu2 site.
Fatty acid synthesis genes FAS1, FAS2, and FabD were used as targeting genes to auto-regulate fatty acid synthesis. To optimize the transcription efficiency, 3 gRNAs targeting different locations were designed for each gene. The gRNA sequences and targeting locations were listed in Supplementary Table 1. The 645-bp gRNA_FAS1-1 was synthesized by Genewiz (Frederick, MD). Overlapping sequences were designed at 5′ and 3′ terminals for Gibson Assembly. The 5′ hammer-head ribozyme and 3′ HDV ribozyme sites flanking the gRNA were designed to generate mature gRNAs (Wong et al., 2017). gRNA_FAS1-1 was assembled with XbaI/SpeI digested p (A1R1)x2A3 using Gibson Assembly to yield plasmid p (A1R1)x2A3-FAS1-1. The gRNA upstream xxx-up and downstream xxx-down fragments were amplified using primer pairs gRNA F/gRNA-xxx R and gRNA-xxx F/gRNA R, respectively. Fragments xxx-up and xxx-down were assembled with AvrII/SalI digested p (A1R1)x2A3 using Gibson Assembly to yield plasmid p (A1R1)x2A3-xxx. “xxx” here referred to gRNA. For instance, the upstream fragment FAS1-2-up and downstream fragment FAS1-2-down were amplified from plasmid p (A1R1)x2A3-FAS1-1 using primer pairs gRNA F/gRNA-FAS1-2 R and gRNA-FAS1-2 F/gRNA R, respectively (primers used in this study were listed in Supplementary Table 3). FAS1-2-up and FAS1-2-down were assembled with AvrII/SalI digested p (A1R1)x2A3 to yield p (A1R1)x2A3-FAS1-2. For all plasmids, the 5′ hammer-head ribozyme and 3′ HDV ribozyme sites flanking the gRNA were designed to generate mature gRNA (Wong et al., 2017). The AvrII/SalI digested donor plasmid pYLXP′-dCas9 was ligated to the NheI/SalI digested destination plasmid p (A1R1)x2A3-xxx to yield p (A1R1)x2A3-xxx-dCas9. The AvrII/SalI digested donor plasmid p (A1R1)x2A3-xxx-dCas9 was subsequently ligated to NheI/SalI digested destination plasmid pΔleu2loxP to yield pΔleu2loxP-xxx-dCas9. “xxx” here referred to gRNA(s). These plasmids were used to integrate at the leu2 site. Plasmids used in this paper were listed in Supplementary Table 2.
The product addiction plasmids were constructed as follows. The hybrid promoters are composed of FdeR binding sequence FdeO and core promoters (Skjoedt et al., 2016). FdeO was placed at the upstream of the TATA box of TEF(111), LEU2(78), and GAPDH(88), yielding hybrid promoters POTEF(111), POLEU2(78), and POGAPDH(88), respectively. A 25-bp proximal motif was added between FdeO and core TEF promoter, yielding hybrid promoter POTEF(136) (Hussain et al., 2016). These hybrid promoters were synthesized by Genewiz (Frederick, MD), and inserted to pYLXP′2 at AvrII/XbaI site to replace the original TEF promoter, resulting in plasmids pOTEF (111), pOTEF (136), pOLEU2 (78), and pOGAPDH (88). Luciferase encoding gene Nluc was amplified from pYLXP′-Nluc, and inserted into pOTEF (111), pOTEF (136), pOLEU2 (78), and pOGAPDH (88) at SnaBI site using Gibson assembly, resulting in pOTEF (111)-Nluc, pOTEF (136)-Nluc, pOLEU2 (78)-Nluc, and pOGAPDH (88)-Nluc, respectively. LEU2 was amplified from pYLXP′ using primer pair LEU2 F/LEU2 R, and inserted into pOTEF (111), pOTEF (136), pOLEU2 (78), and pOGAPDH (88) at SnaBI site using Gibson Assembly, resulting in pOTEF (111)-LEU2, pOTEF (136)-LEU2, pOLEU2 (78)-LEU2, and pOGAPDH (88)-LEU2. FdeR containing an SV40 nuclear localization sequence at C-terminal was synthesized by Genewiz (Frederick, MD), and inserted into pYLXP′, pYaliJ1, pYaliL1, pOTEF (111), pOTEF (136), pOLEU2 (78), and pOGAPDH (88) at SnaBI site using Gibson assembly, resulting in pYLXP′-FdeR, pYaliJ1-FdeR, pYaliL1-FdeR, pOTEF (111)-FdeR, pOTEF (136)-FdeR, pOLEU2 (78)-FdeR, and pOGAPDH (88)-FdeR, respectively (Wong et al., 2017). The recombination of these plasmids was achieved by ligating AvrII/SalI digested donor plasmid to NheI/SalI digested destination plasmid. Plasmids used in this paper were listed in Supplementary Table 2.
2.3. Site-directed integration
The pΔleu2loxP and pΔxpr2loxP derived plasmids were digested with AvrII/NotI. The linearized genetic circuits were transformed into Y. lipolytica Po1f, NarPro/ACS, or NarPro/ACS_Rep. The uracil drop-out CSM-Ura plate was used to screen transformants. The primer pairs leu2_Inte F/leu2_Inte R and xpr2_Inte F/leu2_Inte R were used to analyze the integration at leu2 and xpr2 sites using colony PCR, respectively. The forward primers leu2_Inte F and xpr2_Inte F prime the upstream sequence of the genome, while reverse primer leu2_Inte R primes URA3 gene in the circuit. The positive colony containing integration at leu2 site will yield a 1368-bp specific band, while the positive colony containing integration at xpr2 site will yield a 1556-bp specific band in the colony PCR analysis. The URA3 marker was rescued by transient expressing Cre recombinase using pYLXP′-Cre, which was subsequently removed by culturing in YPD medium at 30 °C for 48 h (Lv et al., 2019).
2.4. Fatty acid and naringenin inducible promoter analysis
The luciferase encoding gene Nluc was used as reporter. The transformant cells were harvested by centrifugation after grown in YPD medium for 24 h. After washing twice using 20 mM PBS, the cells were resuspended in fresh YPD medium. Oleic acid or naringenin was added to concentrations as indicated. The luminescence was recorded using a Cytation 3 microtiter plate reader. The RLU was calculated by integrating the read-out data by time.
2.5. Transcriptional assay of fatty acid biosynthesis genes
Reverse transcription - quantitative polymerase chain reaction (qRT-PCR) was used to analysis the transcriptional levels of fatty acid biosynthesis gene. Total RNA was extracted using ToTALLY RNA™ Kit (Ambion, Inc.) at 48 h. DNA was removed using TURBO DNA-free™ Kit (Invitrogen, Vilnius, Lithuania). Reverse transcription was carried out using an iScript™ cDNA Synthesis kit (Bio-Rad, Hercules, CA). Real time PCR was carried out using an iTaq™ Universal SYBR® Green Supermix (Bio-Rad, Hercules, CA) using the Bio-Rad iCycler iQ Real-Time PCR Detection System (Bio-Rad, Hercules, CA). The fluorescence results were analyzed using Real-time PCR miner. Actin encoding gene ACT1 (GRYC ID: YALI0D08272g) was used as reference gene (Tai and Stephanopoulos, 2013). Relative changes in gene transcriptional level were calculated using ΔΔCq method (Livak and Schmittgen, 2001). Primers used for qRT-PCR were listed in Supplementary Table 3.
2.6. Month-long fermentation test of the naringenin-producing cell line
The month-long fermentation testing was implemented by serial passaging in shaking flasks (Xiao et al., 2016; Rugbjerg et al., 2018). Inoculum seedling culture aging from 0 h to 720 h were individually harvested and stocked in −80 °C freezer before the inoculum was used as seed culture. Then the individually harvested seeds were revived on solid agar minimal media (CSM-Leu) before inoculation into 3 ml liquid media. To avoid cell density variations, the liquid seed with an initial OD 0.1 (normalized by the individual culture) was inoculated into 25 ml fresh CSM-Leu medium in 250 ml shaking flask. Samples were taken and stored in −80 °C for flavonoid analysis. To analyze the naringenin productivity, 1 ml of the −80 °C frozen culture harvested at 120 h was used to analyze naringenin titer with HPLC (Lv et al., 2019).
3. Results and discussion
3.1. Construction of a fatty acid-driven inverter gate
We previously engineered the flavonoid biosynthetic pathway in Y. lipolytica by overexpressing the acetyl-CoA carboxylase and supplementing media with the expensive FAS inhibitor (1 mg/L cerulenin) (Lv et al., 2019). However, the engineered cell still contained more than 35% (w/w) of oil (primarily neutral lipids, triacyl glycerides) (Beopoulos et al., 2011), indicating a substantial portion of malonyl-CoA was used for lipid synthesis. Consistent with this study, mitigating lipogenesis competition has been reported as effective strategies to improve malonyl-CoA-derived end product synthesis in oleaginous yeast (Wu et al., 2014; Yang et al., 2015). Since lipogenesis is essential to membrane synthesis and cellular function, it is important to balance the flux between lipogenesis and heterologous product synthesis.
As dynamic control has emerged as effective strategy to regulate metabolic flux (Xu, 2018), we sought to build a fatty acid-driven inverter device to autonomously tune down lipids synthesis when there is sufficient fatty acids in the cell. To autonomously tune down fatty acid synthesis, we engineered a fatty acid inducible promoter in combination with the CRISPRi elements to repress lipogenic pathways (Fig. 1). Previous report has validated the essential molecular components of a fatty acid inducible promoter POX2, which is composed of upstream activating sequences (A1, A2, and A3), regulatory sequences (R1 and R2), and the core promoter sequence (Fig. 2a) (Hussain et al., 2017). Both the intact POX2 promoter and its derivatives have been reported to be inducible by fatty acids (Hussain et al., 2016; Hussain et al., 2017). We tested a number of genetic configurations of the POX2 promoter to improve the dynamic output range and operational range (Fig. 2). When induced by 2% (v/v) oleic acids, the hybrid promoter (A1R1)x2A3 exhibited the highest gene expression fold change with an engineered luciferase (encoded by Nluc) as the reporter (Fig. 2b). Moreover, the hybrid promoter (A1R1)x2A3 was characterized to respond to oleic acids ranging from 0.1% (v/v) to 5.0% (v/v) (Fig. 2c). Considering the dynamic output and the operational range of the hybrid promoter, the hybrid promoter P(A1R1)x2A3 was chosen to drive the expression of guide RNAs (gRNAs) that were specifically designed to target the lipogenic pathways. The 5′ hammer-head ribozyme (5′ HHR) and 3’ HDV ribozyme sites flanking the gRNAs were used to generate mature gRNA (Wong et al., 2017). Due to plasmid instability in Y. lipolytica, we integrated the inverter device at the leu2 loci by using site-specific integration plasmid pΔleu2loxP (Supplementary Fig. 1a, 1b and 1c) (Le Dall, Nicaud et al., 1994). The integration efficiency was determined to be 67.7% (21/31) in KU70 deficient strain (Supplementary Fig. 1b). By transiently expressing the Cre recombinase, we were able to efficiently cure the URA3 marker with 100% (23/23) efficiency (Supplementary Fig. 1c) (Lv et al., 2019). This site-specific gene integration enable us to develop a stable inverter device to autonomously control lipid synthesis, and integration of the inverter gate at leu2 loci will further block the native leucine biosynthetic pathway, rendering us the opportunity to link leucine auxotroph (growth fitness) with flavonoid synthesis (which will be described in section 2.4).
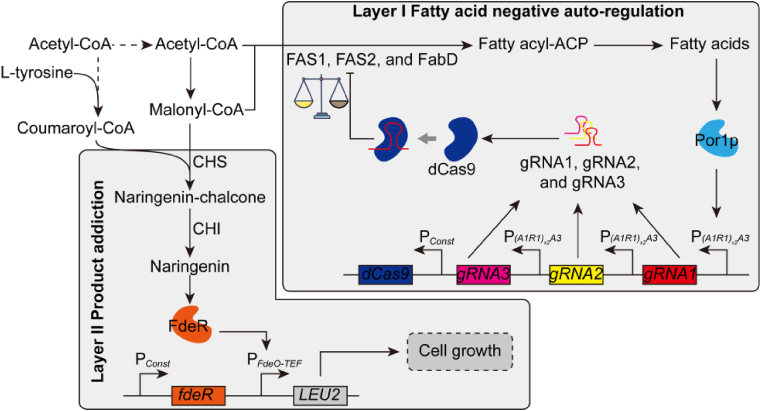
Fig. 1.
Demonstration of integrating fatty acid negative auto-regulation with product-addiction. Layer I: CRISPRi based fatty acid negative auto-regulation. The fatty acid inducible promoter P(A1R1)x2A3 was used to control the transcription of gRNAs, which were used to guide dCas9 to FAS1, FAS2, and FabD. Layer II: End-product addiction. PFdeO-TEF is a hybrid promoter, which is composed of FdeR binding site FdeO and the TEF core promoter. PConst refers to the constitutive promoter. In the presence of naringenin, FdeR bands FdeO site and activates PFdeO-TEF. The expression of LEU2 gene confers the leucine-auxotrophic host cell growth in the leucine drop-out medium.
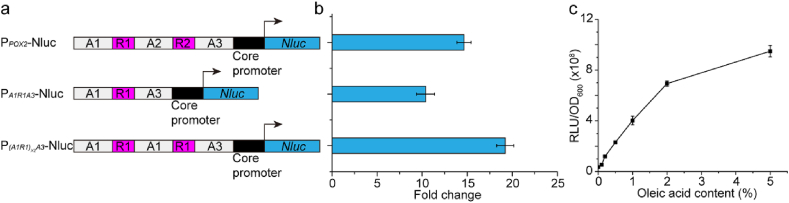
Fig. 2.
Analysis of fatty acid inducible promoters. a Structures of the fatty acid inducible promoters. b Dynamic output range of fatty acid inducible promoters. The fold change was calculated by dividing the RLU/OD600 with 2% (v/v) oleic acid by the RLU/OD600 without oleic acid. c Operational range of hybrid promoter (A1R1)x2A3. The oleic acid content was the volume content.
3.2. CRISPR interference to inhibit fatty acid synthesis
Yeast uses polypeptide fatty acid synthetases Fas1p and Fas2p (encoded by FAS1 and FAS2) to assemble acetyl-CoAs and malonyl-CoAs into long-chain saturated fatty acids (Schweizer et al., 1986). The Y. lipolytica homologue of malonyl-CoA-ACP [acyl-carrier protein] transacylase (encoded by FabD, GRYC ID: YALI0E18590g) was also reported to play a major role in fatty acid synthesis (Schneider et al., 1997). For these reasons, FAS1, FAS2, and FabD were selected as endogenous CRISPRi targets to repress fatty acid synthesis. In order to achieve gradient repression levels, we tested 3 gRNAs targeting different locations for each gene (Supplementary Table 1). The hybrid promoter P(A1R1)x2A3 was used to drive the transcription of gRNAs. The mature gRNAs, which were used to guide dCas9 to the target locations, were produced by endogenous processing of the flanking 5′ hammer-head ribozyme and 3’ HDV ribozyme sites after transcription (Fig. 3a–c).
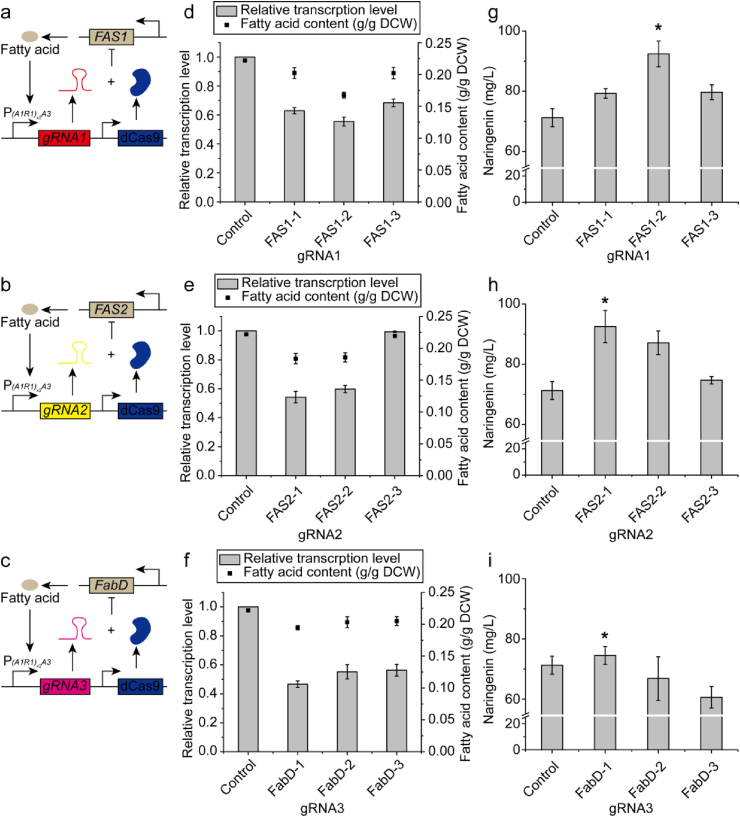
Fig. 3.
Effects of different gRNAs on relative transcription level, fatty acid content, and naringenin production. a - c Mechanism of gRNA1, gRNA2, and gRNA3 guided fatty acid synthesis auto-regulation. d - f Effects of different gRNA1, gRNA2, and gRNA3 on relative transcription level and fatty acid content. The relative transcription level of FAS1, FAS2, and FabD was normalized by the transcription level of FAS1, FAS2, and FabD without auto-regulation (the control). g - i Effects of repressing FAS1, FAS2, and FabD using different gRNA1, gRNA2, and gRNA3 on naringenin titer. Strain NarPro/ASC without auto-regulation circuit was used as control. gRNA1 refers to gRNA FAS1-1, FAS1-2, or FAS1-3. gRNA2 refers to gRNA FAS2-1, FAS2-2, or FAS2-3. gRNA3 refers to FabD-1, FabD-2, or FabD-3.
CRISPRi experiments showed that different gRNAs resulted in differential transcriptional repression for each gene (Fig. 3d–f). The gRNAs FAS1-2, FAS2-1, and FabD-1 showed the highest transcriptional repression efficiency, resulting in relative transcription levels of 0.55, 0.54, and 0.47, respectively, as quantified by qRT-PCR. Consistent with these transcriptional data, the gRNAs FAS1-2, FAS2-1, and FabD-1 guided dCas9 decreased fatty acid content by 24.5%, 17.2%, and 12.3% respectively (Fig. 3d–f). gRNAs targeting FabD was less efficient in decreasing fatty acid accumulation, possibly due to the fact that FabD activity was compensated by the pentafunctional Fas1p (Schweizer et al., 1986; Kottig et al., 1991). Both gRNAs FAS1-2 and FAS2-1 were found to increase naringenin titer by 29.9%, while the gRNA FabD-1 did not have obvious effect on naringenin titer (Fig. 3g–i). These results indicated that naringenin production was improved by dCas9 interfering with fatty acid synthesis when the gRNAs were controlled by the fatty acid inducible promoter.
3.3. Fatty acid synthesis negative autoregulation by multiplexed gRNAs-CRISPRi tuning
To further divert lipogenic flux toward the flavonoid pathway, we sought to use gRNAs in combination to target FAS1, FAS2, and FabD and test whether the multiplexed gRNAs could improve naringenin production (Fig. 4a). The results showed that combinatorially repressing FAS1-2 and FAS2-1 decreased fatty acid dramatically comparing with repressing single gene, while the duplex gRNAs targeting FAS1-FabD or FAS2-FabD did not result in significant fatty acid reduction comparing with solo repression of FAS1 or FAS2 (Fig. 4b). Duplex gRNAs targeting FAS1-FAS2 increased naringenin titer by 52.8%, and triplex gRNAs targeting FAS1-FAS2-FabD increased naringenin titer by 56.5% (Fig. 4b). We further confirmed that naringenin titer was negatively correlated with fatty acid content (R2 = 0.98) (Fig. 4c). These results indicated that naringenin production was improved with the multiplexed gRNAs targeting the fatty acid pathway. Specifically, the strain NarPro/ASC_Rep produced 111.4 mg/L naringenin (Fig. 4b), when triplex gRNAs (FAS1-2-FAS2-1-FabD-1) were used to repress the fatty acid pathway.
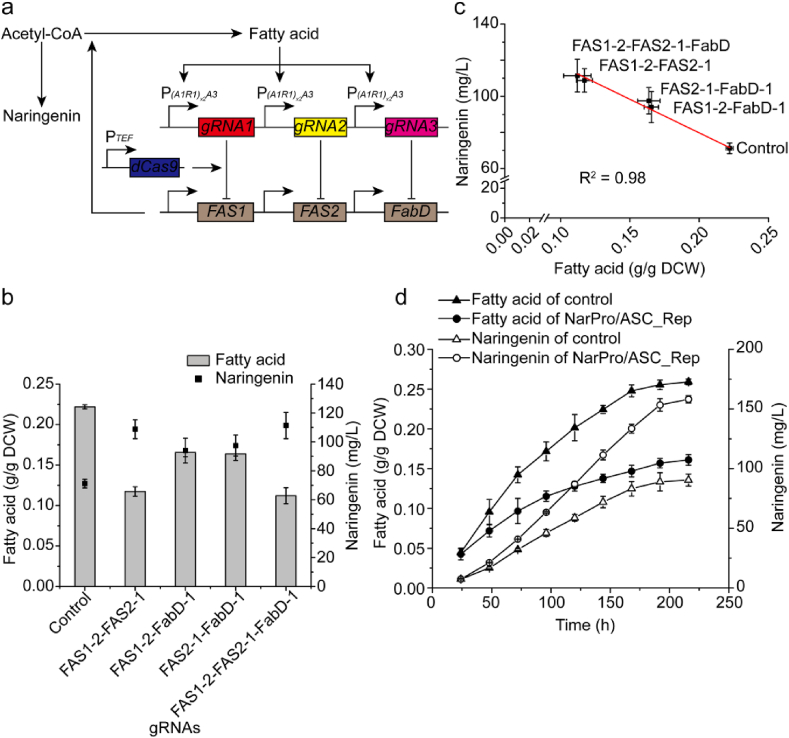
Fig. 4.
Combinatory repression analysis and time course of fatty acid and naringenin production. a Mechanism of auto-regulating fatty acid synthesis by combinatory repressing FAS1, FAS2, and/or FabD. b Effects of combinatory repressing FAS1, FAS2, and/or FabD on fatty acid accumulation and naringenin titer. c Analysis of the tradeoff between fatty acid and naringenin synthesis. The red line refers to the linear fit to the mean values. c Time course of fatty acid accumulation and naringenin production. Strain NarPro/ASC, which is identical to NarPro/ASC_Rep but without the negative auto-regulation circuit, was used as control. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We further analyzed the time course of fatty acid and naringenin production using 250-ml shaking flask in fed-batch fermentation. Interestingly, fatty acid accumulation was reduced further in NarPro/ASC_Rep than the control strain. At the end of the fermentation, fatty acid in NarPro/ASC_Rep decreased by 37.9% (Fig. 4d). Naringenin production rate was increased substantially in the NarPro/ASC_Rep strain. At the end of the fermentation, NarPro/ASC_Rep produced 158.0 mg/L naringenin, which was 74.8% higher than that of the control (Fig. 4d). The inverse correlation between naringenin and fatty acid confirmed that the triplex FAS1-2-FAS2-1-FabD-1 gRNAs effectively guided dCas9 to divert the lipogenic carbons toward the naringenin pathway. In summary, the fatty acid-driven negative auto-regulation inverter gate provided a promising solution to mitigate precursor flux competition from lipogenesis in Y. lipolytica.
3.4. Construction of naringenin inducible promoters
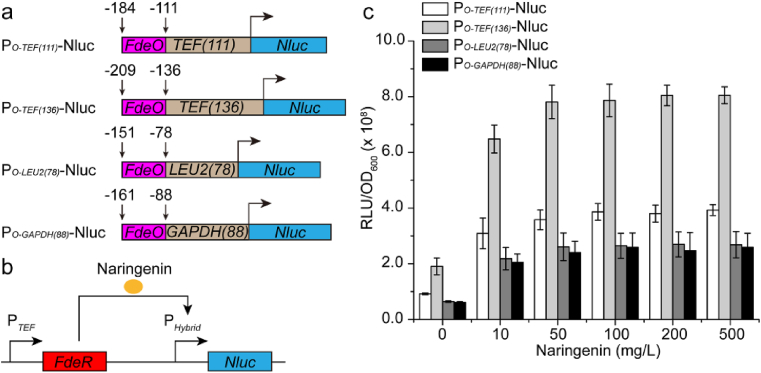
Despite the improvement of naringenin production by mitigating fatty acid synthesis, we constantly observed that the engineered yeast gradually lost the production phenotype after several generations of cultivation. To solve this problem, we attempted to engineer an end-product (naringenin) addiction circuit, which will link cell growth fitness with naringenin production. This competitive growth advantage will encourage the proliferation of the high naringenin-producing populations, while suppress or eliminate the growth of the low naringenin-producing populations during long-term cultivation. To link cell fitness with the end-product, we sought to place an essential gene under the control of naringenin inducible circuit. We firstly ruled out the antibiotic resistant genes, because the antimicrobial property of both antibiotics and naringenin may make the addiction circuit difficult to validate. Because the LEU2 gene is a ready to use (the chassis is leucine-auxotrophic) and reliable selective marker, we firstly tried this non-conditionally essential gene. Cultivated with the leucine drop-out synthetic medium, genetic variants with high naringenin titer will produce more leucine and outcompete the growth of the low naringenin-producing strain. As a result, the high naringenin-producing strains will gradually dominate the cell populations and low naringenin-producing cells will be suppressed (Layer II in Fig. 1). To build the naringenin-inducible genetic circuits, we firstly sought to use the well-characterized transcriptional activator FdeR and its cognate DNA binding site FdeO (Skjoedt et al., 2016) to control the expression of a reporter gene. A panel of synthetic promoters PO-TEF(111), PO-TEF(136), PO-LEU2(78), and PO-GAPDH(88) were constructed by fusing FdeO with the core promoters to drive the expression of the Nluc luciferase (Fig. 5a). The nuclear localization signal SV40 was fused at the C-terminal of FdeR to facilitate nuclear transportation. With naringenin as the effector molecule, FdeR will bind to FdeO site and activate the transcription of the reporter gene (Fig. 5b) (Siedler et al., 2014). Experimental results demonstrated that all our constructed promoters were inducible by naringenin with an operational range from 0 mg/L to 50 mg/L (Fig. 5c). The induction reached saturation when naringenin concentration was beyond 100 mg/L. These naringenin-inducible promoters will be used to control the expression of essential gene LEU2 that dictates cell fitness.
Fig. 5.
Naringenin inducible promoter analysis. a Structures of the hybrid promoters. The hybrid promoters OTEF(111), OTEF(136), OLEU2(78), and OGAPDH(88) were composed of FdeR binding site FdeO and the core promoters of TEF(111), TEF(136), LEU2(78), and GAPDH(88) respectively. b Mechanism of the naringenin inducible transcription. PHybrid refers to the hybrid promoters of PO-TEF(111), PO-TEF(136), PO-LEU2(78), and PO-GAPDH(88). Nluc was used as the reporter gene. c The result of induction of the hybrid promoters. The luminescence was recorded using Cytation 3 microtiter plate reader. The RLU was calculated by integrating the read-out data by time.
3.5. Linking flavonoid production with cell growth fitness
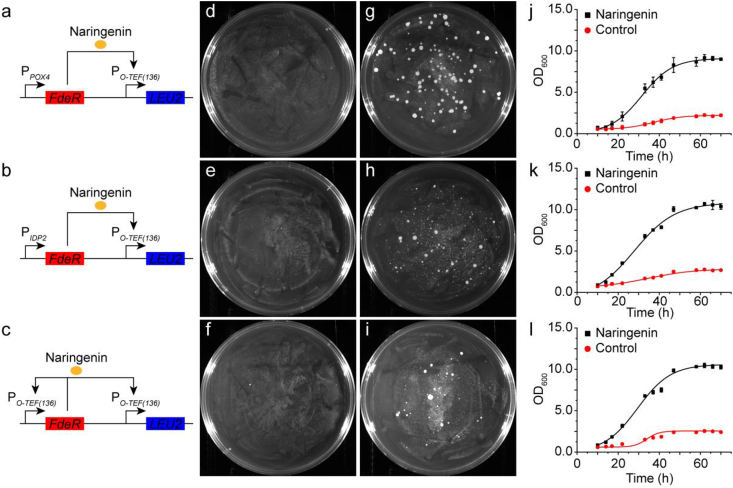
One important strategy in metabolic engineering is to engineer competitive growth advantage that links cell growth with product formation. To enable naringenin inducible growth, we placed the leucine biosynthesis gene LEU2 under the control of these naringenin-inducible promoters (Supplementary Figs. 2a–d). Upon transformation into the leu2-deficient Y. lipolytica Po1f chassis, we obtained comparable number of colonies on the plates with naringenin (CSM-Leu + Naringenin) or without naringenin (CSM-Leu) (Supplementary Fig. 2e-l), indicating that cell growth is independent of naringenin. We speculate this might be arising from the basal activity of FdeR, which was excessively expressed from the strong and constitutive TEF promoter. To overcome this limitation, we next sought to express FdeR with weak promoters POX4 and IDP2 (Wong et al., 2017). Indeed, we observed naringenin-inducible growth: FdeR expression from the weak promoters (PPOX4 and PIDP2) effectively activated the leucine circuits (PO-TEF(136)-LEU2) and supported a distinguishable growth phenotype in the presence of naringenin (Fig. 6d, e, g, and h). However, when leucine circuits PO-TEF(111)-LEU2, PO-LEU2(78)-LEU2, and PO-GAPDH(88)-LEU2 were tested, none of them could grow on CSM-Leu or CSM-Leu + naringenin plates, indicating an intricate interaction between FdeR and these synthetic promoters. Interestingly, FdeR expression from the naringenin-inducible promoter (PO-TEF(136)) also conferred naringenin-inducible growth (Fig. 6f and i), this possibly created a positive feedforward loop that may amplify the sensor input-output relationship. We next confirmed the naringenin-inducible growth in liquid media. Indeed, Y. lipolytica strains carrying the naringenin inducible circuits grew faster in CSM-Leu + naringenin medium than those in CSM-Leu medium (Fig. 6j and l), indicating the naringenin inducible circuits conferred a competitive growth fitness for the engineered cell, albeit flavonoids have been reported as antimicrobial agents in plant signaling. These results highlighted the importance of adjusting promoter strength to achieve the desirable end-product addiction phenotype. For example, excess expression of FdeR instead led to naringenin-independent expression of LEU2, causing indistinguishable cell fitness. While too less FdeR is not sufficient to activate the LEU2 circuits and support cell growth. As for the LEU2 expression, it is important to select the naringenin-inducible promoter with broad operational range as well as minimal leaky expression.
Fig. 6.
Analysis of naringenin addiction circuits in Y. lipolytica Po1f. a-c Mechanism of addiction circuits of PO-TEF(136)-LEU2-PPOX4-FdeR, PO-TEF(136)-LEU2-PIDP2-FdeR, and PO-TEF(136)-LEU2-PO-TEF(136)-FdeR. d-f Growth of Y. lipolytica Po1f transformants with PO-TEF(136)-LEU2-PPOX4-FdeR, PO-TEF(136)-LEU2-PIDP2-FdeR, and PO-TEF(136)-LEU2-PO-TEF(136)-FdeR circuits on CSM-Leu plates. Pictures were taken 3 days after transformation. g-i Growth of Y. lipolytica Po1f transformants with PO-TEF(136)-LEU2-PPOX4-FdeR, PO-TEF(136)-LEU2-PIDP2-FdeR, and PO-TEF(136)-LEU2-PO-TEF(136)-FdeR circuits on CSM-Leu + Naringenin plates. Pictures were taken 3 days after transformation. j-l Growth curves of Y. lipolytica Po1f equipped with PO-TEF(136)-LEU2-PPOX4-FdeR, PO-TEF(136)-LEU2-PIDP2-FdeR, and PO-TEF(136)-LEU2-PO-TEF(136)-FdeR circuits in liquid CSM-Leu medium with or without naringenin. The control set was in CSM-Leu liquid medium without naringenin. The experimental set was in CSM-Leu liquid medium with 5 mg/L naringenin.
To link cell fitness with naringenin production, we next transformed the naringenin-inducible genetic circuit into the naringenin producing strain NarPro/ASC_Rep with fatty acid negative autoregulation. The fatty acid negative autoregulatory circuits were integrated at the leu2 genomic loci. To validate naringenin-inducible growth, we observed that strains carrying the naringenin-addicting circuits grew normally on leucine drop-out plates (Supplementary Figs. 3e–h). However, the strains grew poorly or did not grow at all when the transcriptional activator FdeR was removed from the system (Supplementary Fig. 3i-l), indicating the tight control of the LEU2-expressing fitness circuits by FdeR. In the liquid media, strains carrying the addiction circuits enabled the cell reach 3–4 fold higher cell density (OD600) with 5 mg/L naringenin than the same strain without naringenin (Fig. 6 j, k, l).
3.6. Stabilizing naringenin-production phenotype by integrating metabolic addiction with negative autoregulatory circuits
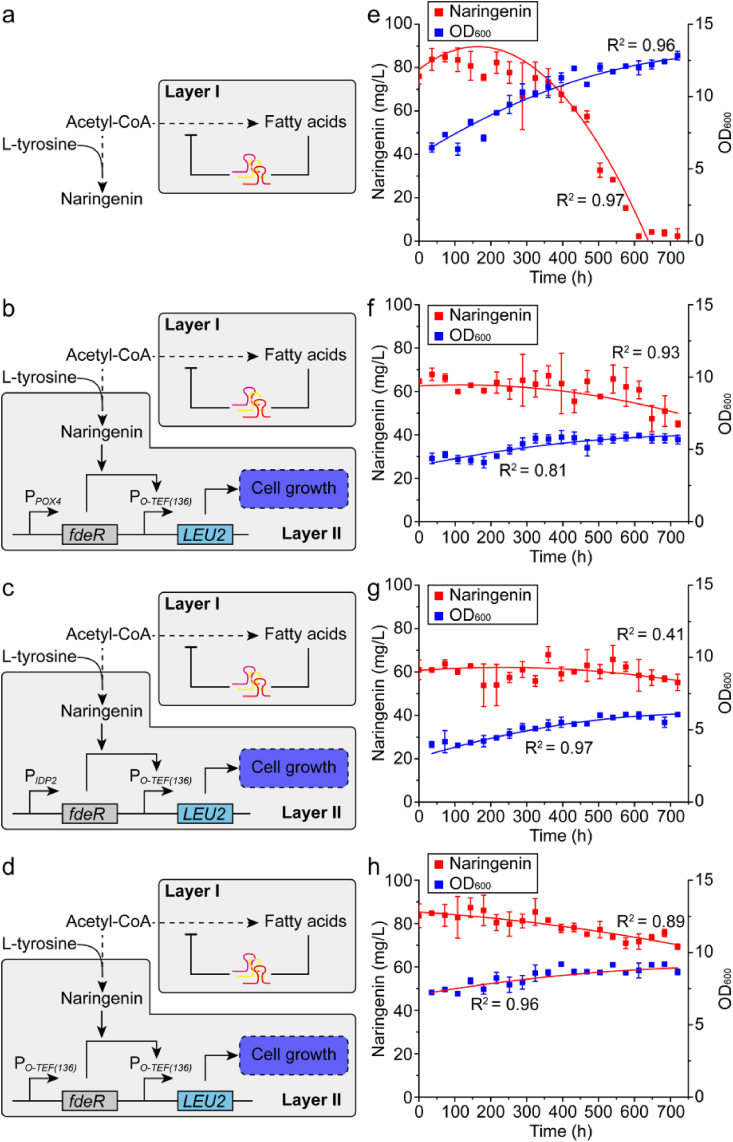
We next coupled the metabolic addiction with negative autoregulation, and validated the long-term robustness of the engineered strains with a month-long fermentation experiment. Three naringenin addiction circuits (PO-TEF(136)-LEU2-PPOX4-FdeR, PO-TEF(136)-LEU2-PIDP2-FdeR, and PO-TEF(136)-LEU2-PO-TEF(136)-FdeR) were introduced to the naringenin producing strain with fatty acid negative autoregulation (NarPro/ASC_Rep chassis), which drives leucine synthesis in a naringenin-dependent manner. By incorporating the naringenin-addiction circuits, we locked the naringenin production phenotype with cell growth fitness. The NarPro/ASC_Rep chassis with blank pYLXP’ plasmid was used as control. These engineered strains were undergone serial passage in paralleled shaking flasks experiments (Rugbjerg et al., 2018). Cells harvested from 0 to 720 h (1 month, about 360 generations) were individually stocked and used as seed culture to inoculate the leucine dropout media (CSM-Leu). We confirmed that the strains carrying the addiction circuits sustained the naringenin production phenotype compared with the control strains (Fig. 7a, b, 7c and 7d), albeit the control strain (without naringenin-addiction circuit) regained growth fitness and reached relatively higher cell density (Fig. 7a). For example, the control stain and all the addiction strains produced similar amount of naringenin (80–90 mg/L) for the inoculum aging from 0 to 180 generations (2 h per generation, which is about 360 h or 15 days). After 200 generations, naringenin production declined rapidly in the control strain, compared with the strains with addiction circuits (Fig. 7b, c and 7d). For the inoculum at 648 h (which is about 324 generations), all the three strains carrying the addiction circuits maintained 90.9% of naringenin production compared with the inoculum at 0 h. On the contrary, the control strain (without the naringenin addiction circuits) almost lost all the naringenin, only produced less than 5.5% of naringenin compared with the fresh inoculum (0 h). By linking leucine synthesis with flavonoid production, our coupling strategies effectively force the cell to maintain a selective growth advantage to enrich the flavonoid-producing populations, but suppress the mutant cells that produce less naringenin. These results demonstrated that the end-product addiction circuits are invaluable tools to maintain community-level strain stability, which is a critical index to stabilize recombinant metabolite production and prevent genetic degeneration during bioprocess scale-up and long-term fermentation.
Fig. 7.
Stability analysis of naringenin producing strains equipped with or without naringenin-addiction circuits. The metabolic addiction were coupled with fatty acid negative autoregulation by equipping naringenin addiction circuits (PO-TEF(136)-LEU2-PPOX4-FdeR, PO-TEF(136)-LEU2-PIDP2-FdeR, and PO-TEF(136)-LEU2-PO-TEF(136)-FdeR) to the NarPro/ACS_Rep chassis, which is a naringenin producing strain with fatty acid negative autoregulatory circuit. a. Stability analysis of the control strain (NarPro/ACS_Rep chassis with blank pYLXP′ plasmid). b. Stability analysis of NarPro/ACS_Rep equipped with PO-TEF(136)-LEU2-PPOX4-FdeR. c. Stability analysis of NarPro/ACS_Rep equipped with PO-TEF(136)-LEU2-PIDP2-FdeR. d. Stability analysis of NarPro/ACS_Rep equipped with PO-TEF(136)-LEU2-PO-TEF(136)-FdeR. Long-term fermentation was carried out in CMS-leu synthetic drop out medium. The strains were passaged at exponential phase (every 36 h). Before every passaging, OD600 was measured and samples were taken for frozen stocks. To analyze the naringenin production, the frozen stocks of each sample were re-inoculated into CSM-leu synthetic drop out medium (4% (v/v)). Naringenin titer was measured at 120 h. The lines were the polynomial fits to the means.
Nonetheless, the overall naringenin production is decreased after we combine the fatty acid negative autoregulatory circuit and the flavonoid addiction circuit (Fig. 7a and b). The decrease in naringin production is possibly due to the metabolic overloading or burden effect (Wu et al., 2016) due to the expression of multiple transcriptional regulators (CRISPRi, FdeR and Por1p, and three gRNAs). From an evolutionary perspective, there is a tradeoff between metabolic stability and pathway yield. Metabolites (i.e. leucine or naringenin in this study) cross-feeding could largely elicit metabolic heterogeneity (Evans et al., 2020), for example, leucine secreted from the high naringenin-producing strain may be assimilated by the low naringenin-producing strain, therefore causing a faulted cross-talk between the sub-populations of the engineered cell. To restrict leucine cross-feeding, we may need to further delete the gene encoding leucine permease (Bap 2 homologs) to sequestrate leucine and reinforce the naringenin production phenotype. Single cell imaging and genetic analysis will be critical to help us understand the source of this metabolic heterogeneity and propose novel genetic or process engineering solutions to inhibit such metabolic heterogeneity (Hartline et al., 2020).
4. Conclusions
Metabolic heterogeneity has become a major issue that compromises the cellular performance and pathway yield. To overcome this limitation, it is important to rewire cellular logics to maintain metabolic homeostasis and improve the community-level cell performance. Social reward-punishment rules to incentivize the production cell and punish the nonproduction cell may be applied to combat this fitness loss. Such rules could be biologically implemented by conferring a selective growth advantage to the production cell, in such a way, the population of the production cell will be enriched and therefor the production phenotype might be sustained. Most of the reported strategies have focused on nongenetic cell-to-cell variations to confer competitive fitness. Genetic underpinnings that are associated with metabolic heterogeneity remain a challenging area. Single-cell analysis and microfluidic-integrated optogenetic tools might be a promising area to solve these conundrums. In this work, we have engineered lipogenic negative autoregulation with metabolite addiction to redistribute carbon flux and improve strain stability in Y. lipolytca. The flavonoid-producing phenotype was sustained for up to 320 generations. These results highlight the importance of applying dynamic population control and microbial cooperation to improve the community-level metabolic performance, which might be useful to combat metabolic heterogeneity and stabilize overproduction phenotype for long-term cultivation in industrial biotechnology settings.
Author contributions
PX and YL conceived the topic and designed the study. YL performed genetic engineering and fermentation experiments with output from YG. YL and PX wrote the manuscript. YL is a joint scientist supervised by JWZ, JLX and PX.
Declaration of competing interest
A provisional patent has been filed based on the results of this study.
Acknowledgement
This work was supported by the Cellular & Biochem Engineering Program of the National Science Foundation under grant no.1805139 and the Bill and Melinda Gates Foundation (OPP1188443). YL would like to thank the China Scholarship Council, China Postdoctoral Science Foundation (Grant No. 2019M662535), and Science and Technology Project of Henan Province (Grant No. 202102310019) for funding support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymben.2020.05.005.
Contributor Information
Jingliang Xu, Email: xujl@zzu.edu.cn.
Jingwen Zhou, Email: zhoujw1982@jiangnan.edu.cn.
Peng Xu, Email: pengxu@umbc.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- Beopoulos A., Nicaud J.M., Gaillardin C. An overview of lipid metabolism in yeasts and its impact on biotechnological processes. Appl. Microbiol. Biotechnol. 2011;90(4):1193–1206. doi: 10.1007/s00253-011-3212-8. [DOI] [PubMed] [Google Scholar]
- Berckman E.A., Chen W. Exploiting dCas9 fusion proteins for dynamic assembly of synthetic metabolons. Chem. Commun. 2019;55(57):8219–8222. doi: 10.1039/c9cc04002a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceroni F., Algar R., Stan G.B., Ellis T. Quantifying cellular capacity identifies gene expression designs with reduced burden. Nat. Methods. 2015;12(5):415–418. doi: 10.1038/nmeth.3339. [DOI] [PubMed] [Google Scholar]
- Ceroni F., Boo A., Furini S., Gorochowski T.E., Borkowski O., Ladak Y.N., Awan A.R., Gilbert C., Stan G.B., Ellis T. Burden-driven feedback control of gene expression. Nat. Methods. 2018;15:387–393. doi: 10.1038/nmeth.4635. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio V., Jensen M.K. Lighting up yeast cell factories by transcription factor-based biosensors. FEMS Yeast Res. 2017;17(7) doi: 10.1093/femsyr/fox076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinh C.V., Prather K.L.J. Development of an autonomous and bifunctional quorum-sensing circuit for metabolic flux control in engineered Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2019;116(51):25562–25568. doi: 10.1073/pnas.1911144116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doong S.J., Gupta A., Prather K.L.J. Layered dynamic regulation for improving metabolic pathway productivity in Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 2018;115(12):2964–2969. doi: 10.1073/pnas.1716920115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C.R., Kempes C.P., Price-Whelan A., Dietrich L.E.P. Metabolic heterogeneity and cross-feeding in bacterial multicellular systems. Trends Microbiol. 2020 doi: 10.1016/j.tim.2020.03.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung E., Wong W.W., Suen J.K., Bulter T., Lee S.G., Liao J.C. A synthetic gene-metabolic oscillator. Nature. 2005;435(7038):118–122. doi: 10.1038/nature03508. [DOI] [PubMed] [Google Scholar]
- Gao S.L., Tong Y.Y., Zhu L., Ge M., Zhang Y.A., Chen D.J., Jiang Y., Yang S. Iterative integration of multiple-copy pathway genes in Yarrowia lipolytica for heterologous β-carotene production. Metab. Eng. 2017;41:192–201. doi: 10.1016/j.ymben.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Gupta A., Reizman I.M.B., Reisch C.R., Prather K.L.J. Dynamic regulation of metabolic flux in engineered bacteria using a pathway-independent quorum-sensing circuit. Nat. Biotechnol. 2017;35:273. doi: 10.1038/nbt.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartline C.J., Mannan A.A., Liu D., Zhang F., Oyarzún D.A. Metabolite sequestration enables rapid recovery from fatty acid depletion in <span class="named-content genus-species" id="named-content-1">Escherichia coli</span>. mBio. 2020;11(2) doi: 10.1128/mBio.03112-19. e03112-03119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M.S., Gambill L., Smith S., Blenner M.A. Engineering promoter architecture in oleaginous yeast Yarrowia lipolytica. ACS Synth. Biol. 2016;5(3):213–223. doi: 10.1021/acssynbio.5b00100. [DOI] [PubMed] [Google Scholar]
- Hussain M.S., Wheeldon I., Blenner M.A. A strong hybrid fatty acid inducible transcriptional sensor built from Yarrowia lipolytica upstream activating and regulatory sequences. Biotechnol. J. 2017;12(10) doi: 10.1002/biot.201700248. [DOI] [PubMed] [Google Scholar]
- Jin C.-C., Zhang J.-L., Song H., Cao Y.-X. Boosting the biosynthesis of betulinic acid and related triterpenoids in Yarrowia lipolytica via multimodular metabolic engineering. Microb. Cell Factories. 2019;18(1):77. doi: 10.1186/s12934-019-1127-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A.O., Gonzalez-Villanueva M., Wong L., Steinbuchel A., Tee K.L., Xu P., Wong T.S. Design and application of genetically-encoded malonyl-CoA biosensors for metabolic engineering of microbial cell factories. Metab. Eng. 2017;44:253–264. doi: 10.1016/j.ymben.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Kottig H., Rottner G., Beck K.F., Schweizer M., Schweizer E. The pentafunctional FAS1 genes of Saccharomyces cerevisiae and Yarrowia lipolytica are co-linear and considerably longer than previously estimated. Mol. Gen. Genet. 1991;226(1–2):310–314. doi: 10.1007/BF00273618. [DOI] [PubMed] [Google Scholar]
- Larroude M., Celinska E., Back A., Thomas S., Nicaud J.M., Ledesma-Amaro R. A synthetic biology approach to transform Yarrowia lipolytica into a competitive biotechnological producer of β-carotene. Biotechnol. Bioeng. 2018;115(2):464–472. doi: 10.1002/bit.26473. [DOI] [PubMed] [Google Scholar]
- Le Dall M.T., Nicaud J.M., Gaillardin C. Multiple-copy integration in the yeast Yarrowia lipolytica. Curr. Genet. 1994;26(1):38–44. doi: 10.1007/BF00326302. [DOI] [PubMed] [Google Scholar]
- Ledesma-Amaro R., Nicaud J.M. Yarrowia lipolytica as a biotechnological chassis to produce usual and unusual fatty acids. Prog. Lipid Res. 2016;61:40–50. doi: 10.1016/j.plipres.2015.12.001. [DOI] [PubMed] [Google Scholar]
- Liu D., Xiao Y., Evans B.S., Zhang F.Z. Negative feedback regulation of fatty acid production based on a malonyl-CoA sensor-actuator. ACS Synth. Biol. 2015;4(2):132–140. doi: 10.1021/sb400158w. [DOI] [PubMed] [Google Scholar]
- Liu H., Marsafari M., Wang F., Deng L., Xu P. Engineering acetyl-CoA metabolic shortcut for eco-friendly production of polyketides triacetic acid lactone in Yarrowia lipolytica. Metab. Eng. 2019;56:60–68. doi: 10.1016/j.ymben.2019.08.017. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lv Y., Edwards H., Zhou J., Xu P. Combining 26s rDNA and the Cre-loxP system for iterative gene integration and efficient marker curation in Yarrowia lipolytica. ACS Synth. Biol. 2019;8(3):568–576. doi: 10.1021/acssynbio.8b00535. [DOI] [PubMed] [Google Scholar]
- Lv Y., Marsafari M., Koffas M., Zhou J.W., Xu P. Optimizing oleaginous yeast cell factories for flavonoids and hydroxylated flavonoids biosynthesis. ACS Synth. Biol. 2019;8(11):2514–2523. doi: 10.1021/acssynbio.9b00193. [DOI] [PubMed] [Google Scholar]
- Lv Y., Qian S., Du G., Chen J., Zhou J., Xu P. Coupling feedback genetic circuits with growth phenotype for dynamic population control and intelligent bioproduction. Metab. Eng. 2019;54:109–116. doi: 10.1016/j.ymben.2019.03.009. [DOI] [PubMed] [Google Scholar]
- Madzak C. Yarrowia lipolytica: recent achievements in heterologous protein expression and pathway engineering. Appl. Microbiol. Biotechnol. 2015;99(11):4559–4577. doi: 10.1007/s00253-015-6624-z. [DOI] [PubMed] [Google Scholar]
- Markham K.A., Palmer C.M., Chwatko M., Wagner J.M., Murray C., Vazquez S., Swaminathan A., Chakravarty I., Lynd N.A., Alper H.S. Rewiring Yarrowia lipolytica toward triacetic acid lactone for materials generation. Proc. Natl. Acad. Sci. U.S.A. 2018;115(9):2096–2101. doi: 10.1073/pnas.1721203115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer C.M., Miller K.K., Nguyen A., Alper H.S. Engineering 4-coumaroyl-CoA derived polyketide production in Yarrowia lipolytica through a β-oxidation mediated strategy. Metab. Eng. 2020;57:174–181. doi: 10.1016/j.ymben.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Qiao K.J., Wasylenko T.M., Zhou K., Xu P., Stephanopoulos G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 2017;35(2):173–177. doi: 10.1038/nbt.3763. [DOI] [PubMed] [Google Scholar]
- Roth R., Moodley V., van Zyl P. Heterologous expression and optimized production of an Aspergillus aculeatus endo-1,4-beta-mannanase in Yarrowia lipolytica. Mol. Biotechnol. 2009;43(2):112–120. doi: 10.1007/s12033-009-9187-3. [DOI] [PubMed] [Google Scholar]
- Rugbjerg P., Myling-Petersen N., Porse A., Sarup-Lytzen K., Sommer M.O.A. Diverse genetic error modes constrain large-scale bio-based production. Nat. Commun. 2018;9:787. doi: 10.1038/s41467-018-03232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugbjerg P., Sarup-Lytzen K., Nagy M., Sommer M.O.A. Synthetic addiction extends the productive life time of engineered Escherichia coli populations. Proc. Natl. Acad. Sci. U.S.A. 2018;115(10):2347–2352. doi: 10.1073/pnas.1718622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider R., Brors B., Burger F., Camrath S., Weiss H. Two genes of the putative mitochondrial fatty acid synthase in the genome of Saccharomyces cerevisiae. Curr. Genet. 1997;32(6):384–388. doi: 10.1007/s002940050292. [DOI] [PubMed] [Google Scholar]
- Schweizer M., Roberts L.M., Holtke H.J., Takabayashi K., Hollerer E., Hoffmann B., Muller G., Kottig H., Schweizer E. The pentafunctional FAS1 gene of yeast: its nucleotide sequence and order of the catalytic domains. Mol. Gen. Genet. 1986;203(3):479–486. doi: 10.1007/BF00422073. [DOI] [PubMed] [Google Scholar]
- Shaw A.J., Lam F.H., Hamilton M., Consiglio A., MacEwen K., Brevnova E.E., Greenhagen E., LaTouf W.G., South C.R., van Dijken H., Stephanopoulos G. Metabolic engineering of microbial competitive advantage for industrial fermentation processes. Science. 2016;353(6299):583–586. doi: 10.1126/science.aaf6159. [DOI] [PubMed] [Google Scholar]
- Siedler S., Stahlhut S.G., Malla S., Maury J., Neves A.R. Novel biosensors based on flavonoid-responsive transcriptional regulators introduced into Escherichia coli. Metab. Eng. 2014;21:2–8. doi: 10.1016/j.ymben.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Skjoedt M.L., Snoek T., Kildegaard K.R., Arsovska D., Eichenberger M., Goedecke T.J., Rajkumar A.S., Zhang J., Kristensen M., Lehka B.J., Siedler S., Borodina I., Jensen M.K., Keasling J.D. Engineering prokaryotic transcriptional activators as metabolite biosensors in yeast. Nat. Chem. Biol. 2016;12(11):951–958. doi: 10.1038/nchembio.2177. [DOI] [PubMed] [Google Scholar]
- Tai M., Stephanopoulos G. Engineering the push and pull of lipid biosynthesis in oleaginous yeast Yarrowia lipolytica for biofuel production. Metab. Eng. 2013;15:1–9. doi: 10.1016/j.ymben.2012.08.007. [DOI] [PubMed] [Google Scholar]
- Wan X., Marsafari M., Xu P. Engineering metabolite-responsive transcriptional factors to sense small molecules in eukaryotes: current state and perspectives. Microb. Cell Factories. 2019;18(1):61. doi: 10.1186/s12934-019-1111-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H., Wang W., Alper H.S., Xu Q., Knoshaug E.P., Van Wychen S., Lin C.-Y., Luo Y., Decker S.R., Himmel M.E., Zhang M. Ameliorating the metabolic burden of the co-expression of secreted fungal cellulases in a high lipid-accumulating Yarrowia lipolytica strain by medium C/N ratio and a chemical chaperone. Front. Microbiol. 2019;9(3276) doi: 10.3389/fmicb.2018.03276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong L., Engel J., Jin E., Holdridge B., Xu P. YaliBricks, a versatile genetic toolkit for streamlined and rapid pathway engineering in Yarrowia lipolytica. Metabolic Engineering Communications. 2017;5:68–77. doi: 10.1016/j.meteno.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G., Yan Q., Jones J.A., Tang Y.J., Fong S.S., Koffas M.A.G. Metabolic burden: cornerstones in synthetic biology and metabolic engineering applications. Trends Biotechnol. 2016;34(8):652–664. doi: 10.1016/j.tibtech.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Wu J., Yu O., Du G., Zhou J., Chen J. Fine-tuning of the fatty acid pathway by synthetic antisense RNA for enhanced (2S)-naringenin production from L-tyrosine in Escherichia coli. Appl. Environ. Microbiol. 2014;80(23):7283–7292. doi: 10.1128/AEM.02411-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Chen T., Liu Y., Tian R., Lv X., Li J., Du G., Chen J., Ledesma-Amaro R., Liu L. Design of a programmable biosensor-CRISPRi genetic circuits for dynamic and autonomous dual-control of metabolic flux in Bacillus subtilis. Nucleic Acids Res. 2020;48(2):996–1009. doi: 10.1093/nar/gkz1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Bowen C.H., Liu D., Zhang F.Z. Exploiting nongenetic cell-to-cell variation for enhanced biosynthesis. Nat. Chem. Biol. 2016;12(5):339–344. doi: 10.1038/nchembio.2046. [DOI] [PubMed] [Google Scholar]
- Xu P. Production of chemicals using dynamic control of metabolic fluxes. Curr. Opin. Biotechnol. 2018;53:12–19. doi: 10.1016/j.copbio.2017.10.009. [DOI] [PubMed] [Google Scholar]
- Xu P. Branch point control at malonyl-CoA node: a computational framework to optimize the controller architecture toward ideal metabolic switches. bioRxiv. 2019:847947. [Google Scholar]
- Xu P., Li L.Y., Zhang F.M., Stephanopoulos G., Koffas M. Improving fatty acids production by engineering dynamic pathway regulation and metabolic control. Proc. Natl. Acad. Sci. U.S.A. 2014;111(31):11299–11304. doi: 10.1073/pnas.1406401111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P., Qiao K.J., Stephanopoulos G. Engineering oxidative stress defense pathways to build a robust lipid production platform in Yarrowia lipolytica. Biotechnol. Bioeng. 2017;114(7):1521–1530. doi: 10.1002/bit.26285. [DOI] [PubMed] [Google Scholar]
- Xu P., Wang W., Li L., Bhan N., Zhang F., Koffas M.A. Design and kinetic analysis of a hybrid promoter-regulator system for malonyl-CoA sensing in Escherichia coli. ACS Chem. Biol. 2014;9(2):451–458. doi: 10.1021/cb400623m. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lin Y., Li L., Linhardt R.J., Yan Y. Regulating malonyl-CoA metabolism via synthetic antisense RNAs for enhanced biosynthesis of natural products. Metab. Eng. 2015;29:217–226. doi: 10.1016/j.ymben.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lin Y., Wang J., Wu Y., Zhang R., Cheng M., Shen X., Wang J., Chen Z., Li C., Yuan Q., Yan Y. Sensor-regulator and RNAi based bifunctional dynamic control network for engineered microbial synthesis. Nat. Commun. 2018;9(1):3043. doi: 10.1038/s41467-018-05466-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Zhang Y., Wang Y., Yao M., Zhang J., Liu H., Zhou X., Xiao W., Yuan Y. Pregnenolone overproduction in Yarrowia lipolytica by integrative components pairing of the cytochrome P450scc system. ACS Synth. Biol. 2019;8(12):2666–2678. doi: 10.1021/acssynbio.9b00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.