Presentation of Case
Dr. Matthew B. Roberts: A 66-year-old man who had undergone orthotopic liver transplantation because of end-stage liver disease was admitted to this hospital because of fever and cough during the pandemic of coronavirus disease 2019 (Covid-19), the disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Two days before this admission, fever, chills, and diffuse myalgias developed. The patient also noted a dry cough and nausea and that he had reduced his fluid intake. On the day of admission, the patient learned that his sister had received a diagnosis of Covid-19, and he was concerned that his symptoms might also be caused by infection with SARS-CoV-2, prompting him to present to a neighborhood health center.
At the health center, the patient reported mild dyspnea. He appeared comfortable, with unlabored breathing. The temperature was 37.8°C, the pulse 125 beats per minute, the blood pressure 127/78 mm Hg, the respiratory rate 18 breaths per minute, and the oxygen saturation 97% while he was breathing ambient air. Auscultation of the lungs revealed normal breath sounds. Further evaluation in the emergency department of this hospital was recommended.
On arrival at the emergency department, the patient reported ongoing cough and nausea but no chest pain, abdominal pain, vomiting, or diarrhea. He had a history of hypertension, diabetes, hyperlipidemia, obesity, peripheral neuropathy, chronic kidney disease, and end-stage liver disease due to alcohol use disorder and hepatitis B virus infection. Twenty-two months earlier, renal replacement therapy had been initiated for worsening renal function due to the hepatorenal syndrome, and shortly after, the patient had undergone orthotopic liver transplantation. Combined liver and kidney transplantation had been planned, but the donor kidney was unsuitable for transplantation because of extensive dissection of the renal artery. After the liver transplantation, the patient’s renal function improved, and renal replacement therapy was discontinued. Histopathological examination of tissue from the explanted native liver revealed moderately differentiated hepatocellular carcinoma without evidence of intravascular invasion.
Current medications included tacrolimus, mycophenolate, amlodipine, doxazosin, gabapentin, omeprazole, ursodiol, and magnesium oxide. There were no known allergies. The patient was widowed and lived with his sister and several friends in an apartment in an urban area of New England. He was born in Central America and had immigrated to the United States three decades earlier. He had smoked tobacco in the past but had quit 14 years earlier. He had stopped drinking alcohol 3 years earlier; during periods of heavy drinking, he had used cocaine and marijuana occasionally.
On examination, the patient appeared comfortable. The temperature was 38.0°C, the pulse 99 beats per minute, the blood pressure 134/73 mm Hg, the respiratory rate 20 breaths per minute, and the oxygen saturation 94% while he was breathing ambient air. The body-mass index (the weight in kilograms divided by the square of the height in meters) was 32.1. The respiratory effort and breath sounds were normal, as was the remainder of the physical examination. Nucleic acid testing of a nasopharyngeal swab for influenza viruses and respiratory syncytial virus was negative. A nasopharyngeal swab for nucleic acid testing for SARS-CoV-2 RNA was obtained. Urinalysis revealed a pH of 5.5, a specific gravity of 1.008, 1+ blood, and 1+ protein. A urine test for legionella antigen was negative. Gram’s staining of a sputum specimen revealed rare polymorphonuclear leukocytes and few mixed gram-positive and gram-negative organisms with no specific type predominating; sputum and blood samples were obtained for culture. Other laboratory test results are shown in Table 1. An electrocardiogram showed sinus tachycardia and nonspecific ST-segment and T-wave abnormalities that were unchanged from a study that had been performed 6 months earlier; the corrected QT interval was 424 msec.
Table 1. Laboratory Data.*.
| Variable | Reference Range, Adults† |
Before Transplantation |
On Admission |
Peak Value during Hospitalization | |
|---|---|---|---|---|---|
| Value | Hospital Day | ||||
| White-cell count (per μl) | 4500–11,000 | 5390 | 8910 | 13 | |
| Differential count (per μl) | |||||
| Neutrophils | 1800–7700 | 4540 | 8150 | ||
| Lymphocytes | 1000–4800 | 470 | 150 | ||
| Monocytes | 200–1200 | 380 | 300 | ||
| Prothrombin time (sec) | 11.5–14.5 | 13.1 | 14.9 | 16 | |
| Prothrombin-time international normalized ratio | 0.9–1.1 | 1.0 | 1.2 | 16 | |
| d-dimer (ng/ml) | <500 | 1074 | 2977 | 16 | |
| Fibrinogen (mg/dl) | 150–400 | 580 | 1134 | 15 | |
| Ferritin (μg/liter) | 20–300 | 1361 | 5798 | 11 | |
| Erythrocyte sedimentation rate (mm/hr) | 0–13 | 23 | 124 | 15 | |
| C-reactive protein (mg/liter) | <8.0 | 126.5 | 240.7 | 12 | |
| Procalcitonin (ng/ml) | 0.00–0.08 | 0.24 | 0.49 | 12 | |
| Creatinine (mg/dl) | 0.6–1.5 | 2.0 | 2.8 | 6 | |
| Aspartate aminotransferase (U/liter) | 10–55 | 65 | 65 | 1 | |
| Alanine aminotransferase (U/liter) | 10–40 | 60 | 75 | 8 | |
| Lactate dehydrogenase (U/liter) | 110–210 | 238 | 365 | 8 | |
| Creatine kinase (U/liter) | 60–400 | 247 | 339 | 2 | |
| High-sensitivity troponin T (ng/liter) | 0–14 | 37 | 45 | 8 | |
| Triglycerides (mg/dl) | 40–150 | 206 | |||
| Interleukin-6 (pg/ml) | <1.8 | 24.7 | 4 | ||
| Mycobacterium tuberculosis interferon-γ release assay | Negative | Negative | Negative | ||
| Hepatitis B virus core antibody | Negative | Positive | Positive | ||
| Hepatitis B virus surface antigen | Negative | Positive | Positive | ||
| Cytomegalovirus IgG | Negative | Positive | |||
| Cytomegalovirus DNA | Negative | Negative | |||
| Epstein–Barr virus viral capsid antigen IgG | Negative | Positive | |||
| Influenza A virus DNA | Negative | Negative | |||
| Influenza B virus DNA | Negative | Negative | |||
| Respiratory syncytial virus DNA | Negative | Negative | |||
| Legionella pneumophila serogroup 1 urinary antigen | Negative | Negative | |||
To convert the values for creatinine to micromoles per liter, multiply by 88.4. To convert the values for triglycerides to millimoles per liter, multiply by 0.01129.
Reference values are affected by many variables, including the patient population and the laboratory methods used. The ranges used at Massachusetts General Hospital are for adults who are not pregnant and do not have medical conditions that could affect the results. They may therefore not be appropriate for all patients.
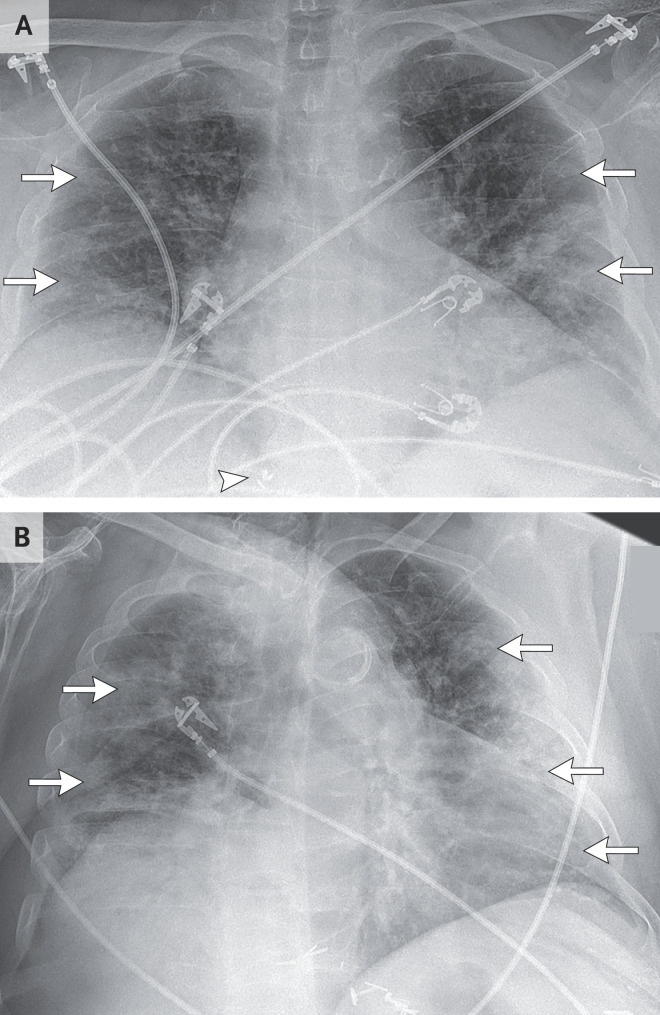
Dr. Eric W. Zhang: Chest radiography (Figure 1A) revealed low lung volumes with patchy, confluent airspace opacities in the mid-to-lower lungs that were more prominent on the left side than on the right side, with peripheral predominance. There were no radiographically significant pleural effusions.
Figure 1. Chest Radiographs.
A portable anteroposterior chest radiograph that was obtained on admission (Panel A) shows low lung volumes with bilateral airspace opacities in the mid-to-lower lung zones that are more prominent on the left side than on the right side, with peripheral predominance (arrows). No radiographically significant pleural effusions are seen. Surgical clips related to the previous liver transplantation are visible in the right upper abdomen (arrowhead). A portable anteroposterior chest radiograph that was obtained on hospital day 5 (Panel B) shows persistent low lung volumes with increased bilateral consolidative airspace opacities, which are now diffuse (arrows). No radiographically significant pleural effusions are seen.
Dr. Roberts: Intravenous normal saline, intravenous ceftriaxone, and oral azithromycin were administered, and the patient was admitted to the hospital. On admission, the oxygen saturation was 88% while he was breathing ambient air, and auscultation of the lungs revealed coarse crackles. Supplemental oxygen was administered through a nasal cannula at a rate of 2 liters per minute. Amlodipine and doxazosin were discontinued. The next morning, the polymerase-chain-reaction test for SARS-CoV-2 RNA showed a positive result. Atorvastatin, oral prednisone, and hydroxychloroquine were initiated, and the dose of mycophenolate was decreased.
During the next 2 days, the temperature increased to 39.2°C. Chills, myalgias, nausea, and cough continued; dyspnea increased; and headache, diarrhea, and occasional vomiting developed. Blood cultures obtained at the time of admission remained negative, and the sputum culture grew normal respiratory flora. Acetaminophen and ondansetron were administered, and the rate of supplemental oxygen was increased to 6 liters per minute. The patient was placed in the prone position in bed.
On the fourth hospital day, the serum level of interleukin-6 was 24.6 pg per milliliter (reference value, <1.8); the levels of inflammatory markers and other laboratory test results are shown in Table 1. On the morning of the fifth hospital day, the oxygen saturation fell below 90% while the patient was receiving supplemental oxygen through a nasal cannula at a rate of 6 liters per minute. The patient was unable to speak in full sentences because of dyspnea, and auscultation of the lungs revealed decreased air entry.
Dr. Zhang: Chest radiography (Figure 1B) revealed low lung volumes with increased diffuse multifocal airspace opacities. There were no radiographically significant pleural effusions.
Dr. Roberts: Supplemental oxygen was administered through a nonrebreather face mask at a rate of 15 liters per minute. The patient was transferred to the medical intensive care unit (ICU), the trachea was intubated, and mechanical ventilation was initiated. On admission to the ICU, the patient was enrolled in a placebo-controlled trial of the interleukin-6 receptor antagonist sarilumab (ClinicalTrials.gov number, NCT04315298).
Management decisions were made.
SARS-CoV-2 Infection after Solid-Organ Transplantation
Dr. Jay A. Fishman: This 66-year-old man who had undergone orthotopic liver transplantation had several risk factors for severe Covid-19, including diabetes, obesity, chronic renal insufficiency, and immunosuppression.1-4
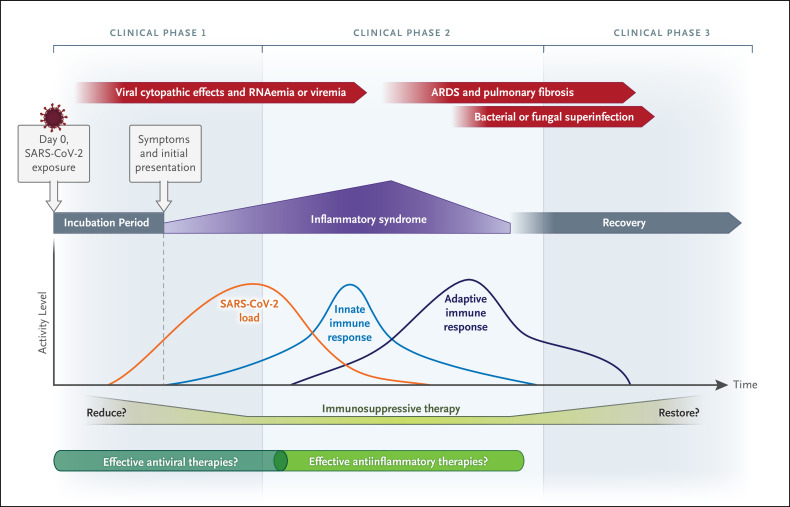
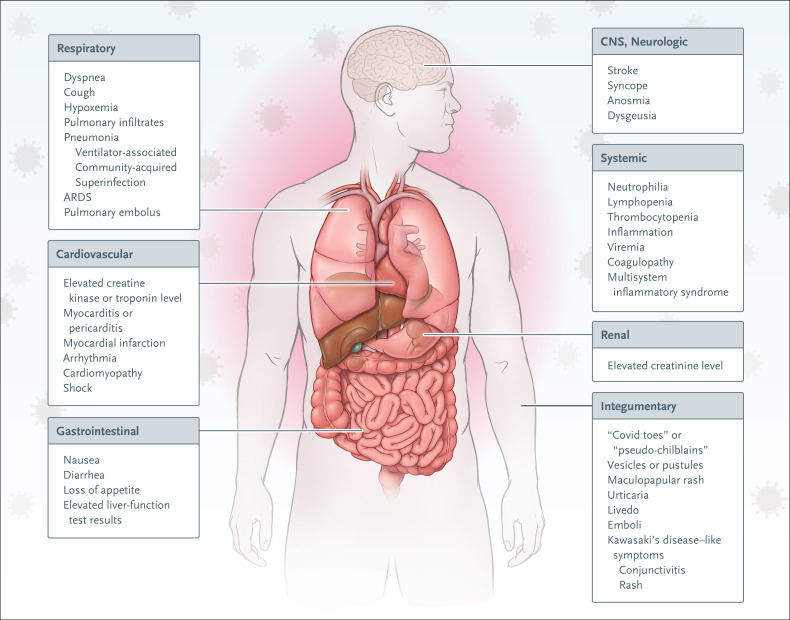
Initial presentations of Covid-19 vary in both normal and immunocompromised hosts.5,6 The heterogeneous progression of SARS-CoV-2 infection in the context of solid-organ transplantation emphasizes the role of the immune response as a driver of clinical progression (Figure 2). Clinical symptoms at presentation include combinations of fever, dry cough, shortness of breath, myalgias, nausea, vomiting, diarrhea, anosmia, headache, and sputum production (Figure 3).1,3,4,8,9 Some patients have had syncope or stroke.2,8 Initial chest radiographs may appear normal, although bilateral peripheral patchy consolidative or ground-glass opacities, which develop in most patients, are best seen on computed tomographic (CT) imaging of the chest.2
Figure 2. SARS-CoV-2 Infection in Organ Transplant Recipients.
After infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), viral replication ensues in the respiratory epithelium, followed by viremia and systemic spread to organs by means of the angiotensin-converting–enzyme 2 receptor. Symptoms develop 3 to 5 days after exposure, with initiation of the innate immune response (first clinical phase). Elaboration of inflammatory cytokines and chemokines precedes the development of adaptive immunity (antibodies and T-lymphocyte activation). Lung injury results from systemic inflammation, viral cytopathic effects, and immune responses to virally infected cells, including type II pneumocytes (second clinical phase). Fibrosis and acute respiratory distress syndrome (ARDS) may result; superinfection of injured lung tissue may occur, notably among patients receiving mechanical ventilation. Recovery among patients who receive mechanical ventilation is often protracted (third clinical phase). Research regarding the appropriate adjustment of exogenous immunosuppression for transplant patients, the timely start of antiviral therapies, and the deployment of antiinflammatory and immunomodulatory therapies in Covid-19 is in progress.
Figure 3. Signs and Symptoms of SARS-CoV-2 Infection.
The varied presentation of SARS-CoV-2 infection reflects diversity in host immune responses, notably in immunosuppressed transplant recipients. Studies of viral strains and receptors are in progress. Acral lesions, which are purpuric macules, papules, vesicles, or pustules, may be described as “Covid toes” or “pseudo-chilblains,” among other terms.7 ARDS denotes acute respiratory distress syndrome, and CNS central nervous system.
Pulmonary coinfection at the time of presentation is uncommon; however, other community-acquired respiratory viruses, cytomegalovirus, and pneumocystis, pseudomonas, and aspergillus species have been identified in patients with Covid-19.10,11 Many solid-organ transplant recipients with mild Covid-19–related symptoms recover at home and are followed remotely, without the need for hospitalization. However, the clinical manifestations of Covid-19 in transplant recipients may occur as a biphasic process before recovery (Figure 2).12
In the first phase of illness in a solid-organ transplant recipient, symptoms are typically mild, and the patient’s condition remains relatively stable. However, in some transplant recipients, such as this patient, abrupt clinical deterioration occurs, and of the patients who are hospitalized, more than half receive intensive care.13
The second phase of illness appears to be driven by the development of innate and adaptive immune responses to viral infection, accompanied by a concurrent exuberant inflammatory response to infection that amplifies the viral cytopathic lung injury.14,15 It is not known whether the period of initial stability (the first phase) is related to exogenous immunosuppression and whether empirical reduction in immunosuppression contributes to the pathogenesis of the inflammatory processes of the second phase, as well as to graft rejection. Mortality is high among symptomatic solid-organ transplant recipients (10 to 28%) and intubated solid-organ transplant recipients (52 to 75%).9,16,17 This may reflect immunosuppression and the preponderance of coexisting risk factors — including older age, obesity, diabetes, cardiovascular disease, and renal dysfunction — for poor outcomes among transplant recipients.18 The disproportionate burden of severe Covid-19 among Black and Hispanic populations persists among transplant recipients.
In immunocompromised hosts, laboratory data — notably biomarkers associated with inflammation and Covid-19 — have less intrinsic value in predicting a patient’s clinical course than do trends.1-4,8,9 This patient, like most solid-organ transplant recipients, presented with pulmonary infiltrates, an elevated lactate dehydrogenase level, lymphopenia (lymphocyte count, <1000 per microliter), and high levels of inflammatory markers (Table 1). In transplant recipients, an elevation in the C-reactive protein level predicts the need for intensive care. Procalcitonin levels generally remain low (<0.5 to 0.8 ng per milliliter) for the first 7 to 10 days of SARS-CoV-2 infection and may subsequently rise, even in the absence of superinfection.19
This patient had progressive elevations in procalcitonin and ferritin levels and in the erythrocyte sedimentation rate later in his hospitalization that were concurrent with the subsequent development of ventilator-associated pneumonia with Pseudomonas aeruginosa. He had moderate renal dysfunction, which is common among solid-organ transplant recipients, particularly those who have undergone kidney transplantation or who have preexisting renal dysfunction. Renal dysfunction may reflect the effects of calcineurin inhibitors, hypotension, or graft rejection after a reduction in immunosuppression; the roles of viral cytopathic effects, endotheliitis, and immune responses to viral antigens in the kidney remain to be clarified.20,21 In one report, acute renal failure occurred in 27% of otherwise healthy persons with Covid-19 in China.22 Graft rejection and toxic effects from calcineurin inhibitors may be difficult to distinguish from viral-mediated effects, complement deposition, or thrombotic microangiopathy; microvascular thrombosis has been seen in some patients with d-dimer levels above 2500 ng per milliliter and low platelet counts.23,24 Small-vessel thrombosis may affect multiple organs, including the small bowel, lungs, heart, and kidneys.
Trials of immunomodulatory therapies are in progress. This patient was enrolled in a randomized, placebo-controlled trial of the interleukin-6 receptor antagonist sarilumab. In general, the use of antiinflammatory therapies after the development of severe pneumonia may not ameliorate progression; once a patient undergoes intubation or receives extracorporeal membrane oxygenation, recovery is slow (clinical phase 3, Figure 2).
Previous Coronavirus Outbreaks
Dr. Deepali Kumar: Transplant recipients are uniquely susceptible to the complications of both community and novel coronaviruses. In this population, community coronaviruses often cause both upper and lower respiratory tract illness. In a study involving 26 patients who had previously undergone stem-cell transplantation and had lower respiratory disease resulting from community coronaviruses, the 90-day mortality from respiratory causes was 35%.25 A follow-up analysis of this cohort showed prolonged viral shedding.26
SARS-CoV
In 2003, a novel coronavirus, SARS-CoV, affected various parts of the world, including China, Hong Kong, and Canada. Unlike in the current Covid-19 pandemic, testing for SARS-CoV was not widely available, and the virus was not identified until several hundred persons had become infected. Clinical presentations were mostly severe with acute pneumonia, and most affected persons were hospitalized. The rate of asymptomatic infection was low, with one meta-analysis showing an overall seropositivity rate of only 0.1% among asymptomatic at-risk persons.27 The incubation period was similar to that of Covid-19, with a median of 4.6 days,28 but viral loads and infectivity tended to peak later in the course of disease, when patients were hospitalized; this feature may have been a contributing factor to the marked susceptibility of health care workers to infection.29 From a virologic perspective, SARS-CoV and SARS-CoV-2 share 79% genomic homology30; both use the angiotensin-converting–enzyme 2 (ACE2) receptor for host-cell entry, and both have been isolated from stool and urine. Although no randomized trials were conducted to evaluate treatments for SARS-CoV infection, observational cohort studies were unable to show clinically significant benefits of ribavirin, lopinavir in combination with ritonavir, pulse glucocorticoids, interferon, or convalescent plasma. SARS-CoV ultimately infected 8098 persons and was associated with a mortality of 9.6%. The outbreak was controlled with the use of strict infection-control measures, case identification, and contact tracing.
SARS developed in 3 patients who had undergone organ transplantation through a transplantation program in Toronto. Two cases were fatal. One of the patients who died was a liver transplant recipient whose infection may have been associated with a “super spreader” event in which more than 70 persons were exposed and 10 persons subsequently had illness compatible with SARS.31 The second patient who died was a lung transplant recipient who had infected a health care worker during a bronchoscopy. The SARS-CoV tissue viral loads were measured at autopsy and were 4 log copies per milliliter greater than, or 10,000 times, the viral loads seen in a cohort of immunocompetent persons who had died from SARS.32 The third patient was a kidney transplant recipient who had been hospitalized and had recovered.
Middle East Respiratory Syndrome Coronavirus
Middle East respiratory syndrome coronavirus (MERS-CoV), which was described in 2012, has affected approximately 2500 persons, with a mortality of 34.4%. MERS-CoV shares 50% genomic homology with SARS-CoV-230 and uses the dipeptidyl peptidase 4 receptor to gain entry into the host cell, a feature that probably leads to differences in infectivity and clinical presentation. Two solid-organ (kidney) transplant recipients with MERS have been described.33 The first presented with pneumonia and acute kidney injury and was found to have MERS-CoV in both nasopharyngeal and blood specimens. The patient received pegylated interferon alfa-2a with ribavirin but eventually died. The second patient had a milder presentation but also had acute kidney injury; immunosuppression was reduced, and he recovered without specific antiviral therapy.
The outbreak of SARS-CoV-2 shares many features with the previous coronavirus outbreaks in transplant recipients. As was the case with SARS and MERS, a substantial proportion of transplant recipients with Covid-19 have lower respiratory disease and are hospitalized, with frequent need of intensive care. Extrapulmonary manifestations such as renal, neurologic, or gastrointestinal disease were not well described with SARS or MERS; however, coronaviruses can cause multisystem disease. Autopsy findings in the case of a lung transplant recipient with SARS showed SARS-CoV in almost every tissue examined, with the greatest level of virus in the lungs and small intestine.32 One important aspect of the Covid-19 pandemic is the finding that more transplant recipients are asymptomatic or mildly symptomatic than in the other coronavirus outbreaks, and the treatment of those patients has been successfully managed on an outpatient basis.
Virologic and Immunologic Features of SARS-CoV-2
Dr. Hans H. Hirsch: On the basis of the virologic features of SARS-CoV-2, a novel species of the Coronaviridae family, ample opportunities are predicted for antiviral drug targets.34 SARS-CoV-2 has the highest genetic homology with bat coronaviruses, suggesting a zoonotic origin.4,30,35 The most striking feature of SARS-CoV-2 is its efficient human-to-human transmission, which is similar to that of annually circulating human coronaviruses that affect transplant recipients36; it has a reproductive number of 2 to 4, indicating a high level of infectivity, which is responsible for the current pandemic. The reservoir and intermediate hosts of SARS-CoV-2 are still elusive. A review of the transmission dynamics suggests that the spread of the virus occurred months before the initial clusters of Covid-19–related pneumonia were detected, after synchronized transmissions during preparations for the Lunar New Year.37
The great speed of pandemic spread is consistent with a short viral incubation period of 3 to 5 days, after which high SARS-CoV-2 loads (several million copies) become detectable in respiratory secretions38,39; this often occurs before symptoms are noted,40,41 although chest CT may already reveal bilateral peripheral patchy ground-glass opacities.42 These findings suggest that there is little preexisting or cross-reactive virus-specific adaptive immunity in the human population during the Covid-19 pandemic,39 in contrast with the 2009 pandemic influenza A (H1N1) outbreak, in which older populations appeared to be protected from severe outcomes.43 The absence of protective neutralizing immunity to coronaviruses is notable.
The mutation rate during viral replication44 is substantially lower with SARS-CoV than with other RNA viruses (e.g., human immunodeficiency virus or influenza virus), owing to the 3′ to 5′ exonuclease proofreading function, which slows antigenic evolution in the absence of homologous genome recombination. SARS-CoV mutations allow construction of clades and the monitoring of changes over time, as well as attribution to geographic regions, but overall mutation rates are low.45 The 12-nucleotide insertion encoding the novel furin target is in the viral spike protein and probably required complex genetic events, even among coinfected bats. The acquisition of the novel furin target sequence is reminiscent of genetic changes seen in the shift from low pathogenic to highly pathogenic avian influenza.44 Thus, SARS-CoV-2 may have acquired key virulence properties for human infection before the first spillover into the human population.
Together, viral factors and the lack of preexisting SARS-CoV-2–specific immune memory are likely to account for the high viral loads and rapid progression to the lower respiratory tract. Activation of innate immune responses, including resident and invading macrophages, results in increasing levels of cytokines such as interleukin-6 and inducing acute-phase responses in the liver and rising C-reactive protein levels, as described in this patient. Early radiologic changes evolve with progressive inflammation and edema.46 In approximately 5 to 15% of clinically severe cases of Covid-19, the SARS-CoV-2 genome is detected in blood (RNAemia),8 correlating with higher interleukin-6 levels47 and systemic spread with endotheliitis, coagulation, and complement activation. Stimulation of virus-specific T-cell responses and neutralizing antibodies in immunocompetent hosts within 2 to 3 weeks after infection can eliminate virus-producing cells and prevent new infection, thus reducing inflammation and promoting recovery (Figure 2).48 The low infection rates among young children and the benign clinical course of SARS-CoV-2 infection among younger adults may be related to ACE2 receptor levels,49 whereas the roles of competing respiratory viral infections in children39 and heterologous immunity, complement activation, and distorted immune repertoires of immunosenescence among the elderly warrant further study.
Given these pathophysiological considerations, therapeutic strategies include interrupting viral damage, reducing innate inflammation, and promoting virus-specific immunity (Figure 2). Experience with antiviral treatment for uncomplicated influenza among immunocompetent adults suggests that the clinical benefit is greatest when potent antiviral agents are administered less than 48 hours after symptoms start.50 Convalescent plasma and antiinflammatory treatments, which dampen macrophages but spare lymphocyte function, may be considered early in the course of clinical progression, although data are limited and further study is needed. Interleukin-6 inhibition may be preferred over glucocorticoid treatment to spare the functional lymphocyte count, since adaptive immune responses are sufficiently triggered by innate inflammation. In transplant recipients, reduced immunosuppression may exacerbate inflammatory responses.
Dr. Kathy M. Tran (Medicine): Dr. Maggiore, would you tell us how you would manage this patient’s immunosuppressive regimen?
Management of Immunosuppressive Therapy
Dr. Umberto Maggiore: A major challenge in the treatment of SARS-CoV-2 infection in patients who have undergone solid-organ transplantation is the management of maintenance immunosuppression — a topic that remains controversial. Various approaches have been used, ranging from no change or a minimal reduction in immunosuppressive treatment to stopping all immunosuppressive drugs except for glucocorticoids, which are usually continued at an increased dose.17,21,51-53
Most current immunosuppressive drugs target T cells, the main component of the immune system that is responsible for viral clearance (Table 2). The potential benefit of reducing immunosuppression in a patient such as this one is associated with augmenting the antiviral response. It has been shown that a suboptimal T-cell response contributes to the pathological changes observed in SARS.61 Among convalescent patients with Covid-19, neutralizing antibody titers correlate with the numbers of virus-specific T cells.48 Finally, SARS-CoV-2–reactive CD4+ T cells are found in 40 to 60% of unexposed persons, suggesting some cross-reactive T-cell recognition between circulating “common cold” coronaviruses and SARS-CoV-2.62 However, the pathophysiological processes of SARS-CoV-2 infection involve the initiation of an uncontrolled inflammatory response, which contributes to the development of acute respiratory distress syndrome, thrombotic complications, and eventually pulmonary fibrosis (Figure 2). Therefore, the use of immunosuppressive agents may provide benefit by reducing inflammation and associated injury. Such drugs may also exert direct antiviral effects; in vitro evidence suggests that calcineurin inhibitors may have activity against SARS-CoV-2.63 In addition, calcineurin inhibitors are the only immunosuppressive drugs that could be safely used as monotherapy to prevent graft rejection.64,65
Table 2. Use of Immunosuppressive Therapy during SARS-CoV-2 Infection.*.
| Immunosuppressive Therapy | Potential Benefits | Possible Side Effects | Current Evidence |
|---|---|---|---|
| Calcineurin inhibitors (e.g., tacrolimus or cyclosporine) |
|
|
|
| Mycophenolate sodium or mycophenolate mofetil |
|
|
|
| Azathioprine |
|
|
|
| mTOR inhibitors (e.g., sirolimus or everolimus) |
|
|
|
| Belatacept |
|
|
|
| Glucocorticoids |
|
ARDS denotes acute respiratory distress syndrome, MERS-CoV Middle East respiratory syndrome coronavirus, mTOR mammalian target of rapamycin, and SARS-CoV-2 severe acute respiratory syndrome coronavirus 2.
In recipients of a non-lifesaving organ who are at risk for the rapid development of severe respiratory impairment despite ongoing maintenance immunosuppressive treatment, the dose of calcineurin inhibitors may be reduced until clinical improvement occurs, at which time the use of other antiinflammatory agents could be considered for controlling lung injury.56 Temporary discontinuation of calcineurin inhibitors should be considered if the patient is receiving additional antiinflammatory and immunosuppressive drugs, such as intravenous glucocorticoids, interleukin-6 receptor inhibitors (e.g., tocilizumab or sarilumab) with or without concomitant glucocorticoids, or signal transduction inhibitors such as JAK-STAT (Janus-associated kinase–signal transducer and activator of transcription) inhibitors.56 In contrast, continuation of calcineurin inhibitors, even at a reduced dose, is usually recommended in recipients of lifesaving organs (especially organs with the highest immunogenicity, such as the heart and lungs), irrespective of the degree of severity of the viral disease and of any additional immunosuppressive treatment, because of the potential life-threatening consequences of acute rejection.
The most controversial issue regarding immunosuppressive treatment in patients with SARS-CoV-2 infection is the appropriate management of glucocorticoids. Glucocorticoids should be continued if they are part of the maintenance immunosuppression regimen and may be introduced in transplant recipients who are receiving glucocorticoid-free maintenance regimens.56 In the absence of viable alternatives, the early use of higher-dose glucocorticoids can be considered in transplant recipients receiving respiratory support.52,53,59,66 Treatment with mycophenolate, azathioprine, or mTOR (mammalian target of rapamycin) inhibitors (e.g., sirolimus or everolimus) may be stopped, or the dose reduced, initially because of their unfavorable side-effect profiles (Table 2). The effect of belatacept on SARS-CoV-2 infection is unknown. Atypical presentations of viral infections have been observed in solid-organ transplant recipients who were receiving belatacept.67 In patients in whom moderate-to-severe SARS-CoV-2 infection develops, the administration of belatacept may be delayed until clinical improvement is observed; alternatively, transient switches to low-dose calcineurin inhibitors may be considered.
Although the use of antiinflammatory drugs (e.g., high-dose glucocorticoids or interleukin-6 receptor antagonists) in solid-organ transplant recipients may have the additional benefit of protecting against rejection among patients who are receiving tapering courses of the immunosuppressive agents, especially when calcineurin inhibitors are discontinued because of severe disease, their efficacy in the context of solid-organ transplantation warrants testing in clinical trials. Moreover, few of the largest case series published to date have reported on the incidence of rejection, as may have occurred in this patient, given the short follow-up period and the practical difficulties of assessing the cause of acute kidney injury in kidney transplant recipients. Therefore, the effects of various strategies on the risks of acute and chronic rejection are currently unknown.
Follow-up
Dr. Roberts: After prolonged intensive care management that included mechanical ventilatory support and that was complicated by P. aeruginosa ventilator-associated pneumonia, the patient’s trachea was extubated successfully on hospital day 24. On hospital day 26, the patient had elevated results of liver-function tests, which aroused concern about allograft rejection. The liver-function test abnormalities normalized after the dose of tacrolimus was increased and with the continued administration of prednisone and mycophenolate. The patient’s condition gradually improved, and he was discharged to an inpatient rehabilitation facility on hospital day 34.
Final Diagnosis
Severe acute respiratory syndrome coronavirus 2 infection.
Acknowledgments
We thank the members of the American Society of Transplantation, the Transplantation Society, and the European Society for Organ Transplantation for their participation in the case presentation.
Disclosure Forms
Footnotes
This case was presented at the combined Medical–Surgical Grand Rounds.
Dr. Fishman reports receiving consulting fees from Bain Capital, Roche, and eGenesis, advisory board fees and stock options from Jura Bio and Be Well Medical Center, consulting fees and fees for serving on a data and safety monitoring board from CTI BioPharma, and advisory board fees from Sfunga Therapeutics; and Dr. Maggiore, receiving lecture fees from Sandoz. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061-1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xu X-W, Wu X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606-m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China — key questions for impact assessment. N Engl J Med 2020;382:692-694. [DOI] [PubMed] [Google Scholar]
- 6.Fishman JA, Grossi PA. Novel Coronavirus-19 (COVID-19) in the immunocompromised transplant recipient: #Flatteningthecurve. Am J Transplant 2020;20:1765-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hernandez C, Bruckner A. Focus on “COVID toes”. JAMA Dermatol 2020. June 25 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 8.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernández-Ruiz M, Andrés A, Loinaz C, et al. COVID-19 in solid organ transplant recipients: a single-center case series from Spain. Am J Transplant 2020;20:1849-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin D, Liu L, Zhang M, et al. Co-infections of SARS-CoV-2 with multiple common respiratory pathogens in infected patients. Sci China Life Sci 2020;63:606-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim D, Quinn J, Pinsky B, Shah NH, Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA 2020;323:2805-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fishman JA. The immunocompromised transplant recipient and SARS-CoV-2 infection. J Am Soc Nephrol 2020;31:1147-1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alberici F, Delbarba E, Manenti C, et al. Management of patients on dialysis and with kidney transplant during SARS-COV-2 (COVID-19) pandemic In Brescia, Italy. Kidney Int Rep 2020;5:580-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He L, Ding Y, Zhang Q, et al. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J Pathol 2006;210:288-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira MR, Mohan S, Cohen DJ, et al. COVID-19 in solid organ transplant recipients: initial report from the US epicenter. Am J Transplant 2020;20:1800-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akalin E, Azzi Y, Bartash R, et al. Covid-19 and kidney transplantation. N Engl J Med 2020;382:2475-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The OpenSAFELY Collaborative. OpenSAFELY: factors associated with COVID-19-related hospital death in the linked electronic health records of 17 million adult NHS patients. May 8, 2020. (https://www.medrxiv.org/content/10.1101/2020.05.06.20092999v1). preprint.
- 19.Roberts MB, Izzy S, Tahir Z, Al Jarrah A, Fishman JA, El Khoury J. COVID-19 in solid organ transplant recipients: dynamics of disease progression and inflammatory markers in ICU and non-ICU admitted patients. Transpl Infect Dis 2020. July 12 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puelles VG, Lütgehetmann M, Lindenmeyer MT, et al. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med 2020;383:590-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Columbia University Kidney Transplant Program. Early description of coronavirus 2019 disease in kidney transplant recipients in New York. J Am Soc Nephrol 2020;31:1150-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diao B, Wang C, Wang R, et al. Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. April 10, 2020. (https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4). preprint. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020;395:1417-1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: a report of five cases. Transl Res 2020;220:1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ogimi C, Waghmare AA, Kuypers JM, et al. Clinical significance of human coronavirus in bronchoalveolar lavage samples from hematopoietic cell transplant recipients and patients with hematologic malignancies. Clin Infect Dis 2017;64:1532-1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ogimi C, Greninger AL, Waghmare AA, et al. Prolonged shedding of human coronavirus in hematopoietic cell transplant recipients: risk factors and viral genome evolution. J Infect Dis 2017;216:203-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung GM, Lim WW, Ho L-M, et al. Seroprevalence of IgG antibodies to SARS-coronavirus in asymptomatic or subclinical population groups. Epidemiol Infect 2006;134:211-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung GM, Hedley AJ, Ho L-M, et al. The epidemiology of severe acute respiratory syndrome in the 2003 Hong Kong epidemic: an analysis of all 1755 patients. Ann Intern Med 2004;141:662-673. [DOI] [PubMed] [Google Scholar]
- 29.Cheng PKC, Wong DA, Tong LKL, et al. Viral shedding patterns of coronavirus in patients with probable severe acute respiratory syndrome. Lancet 2004;363:1699-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kumar D, Tellier R, Draker R, Levy G, Humar A. Severe acute respiratory syndrome (SARS) in a liver transplant recipient and guidelines for donor SARS screening. Am J Transplant 2003;3:977-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kumar D, Humar A. Emerging viral infections in transplant recipients. Curr Opin Infect Dis 2005;18:337-341. [DOI] [PubMed] [Google Scholar]
- 33.AlGhamdi M, Mushtaq F, Awn N, Shalhoub S. MERS CoV infection in two renal transplant recipients: case report. Am J Transplant 2015;15:1101-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parks JM, Smith JC. How to discover antiviral drugs quickly. N Engl J Med 2020;382:2261-2264. [DOI] [PubMed] [Google Scholar]
- 35.Chan JF-W, Yuan S, Kok K-H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet 2020;395:514-523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ison MG, Hirsch HH. Community-acquired respiratory viruses in transplant patients: diversity, impact, unmet clinical needs. Clin Microbiol Rev 2019;32(4):e00042-19-e00042-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med 2020;382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zou L, Ruan F, Huang M, et al. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med 2020;382:1177-1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leuzinger K, Roloff T, Gosert R, et al. Epidemiology of SARS-CoV-2 emergence amidst community-acquired respiratory viruses. J Infect Dis 2020. July 29 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pan X, Chen D, Xia Y, et al. Asymptomatic cases in a family cluster with SARS-CoV-2 infection. Lancet Infect Dis 2020;20:410-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med 2020;382:2081-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, Hong H, Yoon SH. Diagnostic performance of CT and reverse transcriptase-polymerase chain reaction for coronavirus disease 2019: a meta-analysis. Radiology 2020. April 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hancock K, Veguilla V, Lu X, et al. Cross-reactive antibody responses to the 2009 pandemic H1N1 influenza virus. N Engl J Med 2009;361:1945-1952. [DOI] [PubMed] [Google Scholar]
- 44.Claas EC, Osterhaus AD, van Beek R, et al. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet 1998;351:472-477. [DOI] [PubMed] [Google Scholar]
- 45.Nextstrain. Genomic epidemiology of novel coronavirus — global subsampling (https://nextstrain.org/ncov/global?l=clock).
- 46.Pan Y, Guan H, Zhou S, et al. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol 2020;30:3306-3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chen X, Zhao B, Qu Y, et al. Detectable serum SARS-CoV-2 viral load (RNAaemia) is closely correlated with drastically elevated interleukin 6 (IL-6) level in critically ill COVID-19 patients. Clin Infect Dis 2020. April 17 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ni L, Ye F, Cheng M-L, et al. Detection of SARS-CoV-2-specific humoral and cellular immunity in COVID-19 convalescent individuals. Immunity 2020;52(6):971-977.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA 2020;323:2427-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hayden FG, Sugaya N, Hirotsu N, et al. Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018;379:913-923. [DOI] [PubMed] [Google Scholar]
- 51.Husain SA, Dube G, Morris H, et al. Early outcomes of outpatient management of kidney transplant recipients with coronavirus disease 2019. Clin J Am Soc Nephrol 2020;15:1174-1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alberici F, Delbarba E, Manenti C, et al. A single center observational study of the clinical characteristics and short-term outcome of 20 kidney transplant patients admitted for SARS-CoV2 pneumonia. Kidney Int 2020;97:1083-1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu L, Gong N, Liu B, et al. Coronavirus disease 2019 pneumonia in immunosuppressed renal transplant recipients: a summary of 10 confirmed cases in Wuhan, China. Eur Urol 2020;77:748-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nair V, Jandovitz N, Hirsch JS, et al. COVID-19 in kidney transplant recipients. Am J Transplant 2020;20:1819-1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cheng K-W, Cheng S-C, Chen W-Y, et al. Thiopurine analogs and mycophenolic acid synergistically inhibit the papain-like protease of Middle East respiratory syndrome coronavirus. Antiviral Res 2015;115:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Maggiore U, Abramowicz D, Crespo M, et al. How should I manage immunosuppression in a kidney transplant patient with COVID-19? An ERA-EDTA DESCARTES expert opinion. Nephrol Dial Transplant 2020;35:899-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lieberthal W, Fuhro R, Andry CC, et al. Rapamycin impairs recovery from acute renal failure: role of cell-cycle arrest and apoptosis of tubular cells. Am J Physiol Renal Physiol 2001;281:F693-F706. [DOI] [PubMed] [Google Scholar]
- 58.Xu H, Perez SD, Cheeseman J, Mehta AK, Kirk AD. The allo- and viral-specific immunosuppressive effect of belatacept, but not tacrolimus, attenuates with progressive T cell maturation. Am J Transplant 2014;14:319-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid-19 – preliminary report. N Engl J Med 2020;xxx:xxx-xxx. 32678530 [Google Scholar]
- 60.Fadel R, Morrison AR, Vahia A, et al. Early short course corticosteroids in hospitalized patients with COVID-19. Clin Infect Dis 2020. May 19 (Epub ahead of print). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao J, Zhao J, Perlman S. T cell responses are required for protection from clinical disease and for virus clearance in severe acute respiratory syndrome coronavirus-infected mice. J Virol 2010;84:9318-9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Grifoni A, Weiskopf D, Ramirez SI, et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 2020;181(7):1489-1501.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pfefferle S, Schöpf J, Kögl M, et al. The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors. PLoS Pathog 2011;7(10):e1002331-e1002331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vitko S, Klinger M, Salmela K, et al. Two corticosteroid-free regimens — tacrolimus monotherapy after basiliximab administration and tacrolimus/mycophenolate mofetil — in comparison with a standard triple regimen in renal transplantation: results of the Atlas study. Transplantation 2005;80:1734-1741. [DOI] [PubMed] [Google Scholar]
- 65.Ponticelli C, Tarantino A, Segoloni GP, et al. A randomized study comparing three cyclosporine-based regimens in cadaveric renal transplantation. Italian Multicentre Study Group for Renal Transplantation (SIMTRe). J Am Soc Nephrol 1997;8:638-646. [DOI] [PubMed] [Google Scholar]
- 66.Low-cost dexamethasone reduces death by up to one third in hospitalized patients with severe respiratory complications of COVID-19. JIC. June 16, 2020 (https://www.invasivecardiology.com/news/low-cost-dexamethasone-reduces-death-one-third-hospitalized-patients-severe-respiratory-complications-covid-19).
- 67.Vincenti F, Blancho G, Durrbach A, et al. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 2010;21:1587-1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.