Abstract
Purpose
Impaired function of gonadotropin-releasing hormone (GnRH) neurons can cause a phenotypic spectrum ranging from delayed puberty to isolated hypogonadotropic hypogonadism (IHH). We sought to identify a new genetic etiology for these conditions.
Methods
Exome sequencing was performed in an extended family with autosomal dominant, markedly delayed puberty. The effects of the variant were studied in a GnRH neuronal cell line. Variants in the same gene were sought in a large cohort of individuals with IHH.
Results
We identified a rare missense variant (F900V) in DLG2 (which encodes PSD-93) that co-segregated with the delayed puberty. The variant decreased GnRH expression in vitro. PSD-93 is an anchoring protein of NMDA receptors, a type of glutamate receptor that has been implicated in the control of puberty in laboratory animals. The F900V variant impaired the interaction between PSD-93 and a known binding partner, Fyn, which phosphorylates NMDA receptors. Variants in DLG2 that also decreased GnRH expression were identified in 3 unrelated families with IHH.
Conclusion
The findings indicate that variants in DLG2/PSD-93 cause autosomal dominant delayed puberty and may also contribute to IHH. The findings also suggest that the pathogenesis involves impaired NMDA receptor signaling and consequently decreased GnRH secretion.
Keywords: DLG2, PSD-93, NMDA receptors, puberty, hypogonadotropic hypogonadism
Introduction
Puberty results from reactivation of gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus.1 In patients with isolated hypogonadotrophic hypogonadism (IHH), puberty either fails to occur completely, is incomplete, or occurs during adulthood, rather than during adolescence.2 The disorder can be inherited in an X-linked, autosomal dominant, autosomal recessive, or oligogenic fashion.3 IHH is caused by variants in genes that regulate the embryonic migration of GnRH neurons into the hypothalamus and/or the function of GnRH neurons. Variants in more than 40 genes have been implicated in the etiology.3,4
Isolated, self-limited delayed puberty is a milder condition in which puberty occurs at an abnormally late age in adolescence.2 It is often inherited in an autosomal dominant fashion, although other modes of inheritance have also been described.4,5 IHH and self-limited delayed puberty can occur within the same family, suggesting that the same genetic defects can underlie both conditions.2,5 Consistent with this concept, individuals with self-limited delayed puberty have an increased frequency of potentially pathogenic variants in IHH genes.2,5 Recent studies have implicated variants in several genes involved in GnRH migration and function in the pathogenesis of delayed puberty, including EAP16, HS6ST1,7 and IGSF10.8 However, in the great majority of families with delayed puberty, the genetic etiology remains unknown.
Here we studied an extended family with markedly delayed puberty and found a rare missense variant (F900V) in DLG2 that co-segregated with the phenotype. We found evidence that this variant impairs GnRH expression in vitro. We also identified rare missense variants in DLG2 that impaired GnRH expression in vitro in 3 nuclear families with IHH. Interestingly, PSD-93, the protein encoded by DLG2, serves as an anchoring protein for NMDA receptors, and NMDA signaling has been strongly implicated in the regulation of puberty in laboratory animals.1 We found that that the variant identified in the family with delayed puberty interfered with binding of PSD-93 to Fyn, a non-receptor type protein kinase which phosphorylates NMDA receptors to promote signaling. Taken together, the evidence strongly suggests that DLG2/PSD-93 participates in regulating the onset of human puberty and reproduction and, consequently, that variants in this gene cause pubertal disorders.
Subjects, Materials, and Methods
Subjects
An extended family with extremely delayed puberty
We evaluated an extended family in which multiple members showed a marked delay in the onset of puberty. The proband was evaluated at the NIH Clinical Center and the extended family members were evaluated either at the Clinical Center or through phone interviews.
Subjects with isolated hypogonadotropic hypogonadism (IHH)
Subjects were enrolled in a study at the Massachusetts General Hospital to investigate the genetic causes of hypogonadotropic hypogonadism, which included exome sequencing of probands and parents for the genetic causes of IHH (ClinicalTrials.gov Identifier: NCT00494169).
This study was approved by IRBs at participating institutions. All adult subjects and parents of minors provided written informed consent and children provided written assent.
This study was approved by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institute of Neurological Disorders and Stroke, and Massachusetts General Hospital.
Exome sequencing
Exome sequencings were performed at three different sequencing centers: NIH Intramural Sequencing Center (NISC), Broad Institute, or NICHD Molecular Genomics Core (MGC) depending on where the subjects were recruited. For the 3 subjects with IHH, the BAM files that contained the sequence alignment data were carefully examined on Integrative Genomics Viewer (IGV) to seek any significant variant in 40 genes associated with IHH (gene list is available in Supplemental method 1).9–10
Sanger sequencing
The identified variants were confirmed by Sanger sequencing. The primer sets used for PCR amplification and sequencing are listed in Supplemental method 2.
Expression vectors and mutagenesis
Dlg2 cDNA was cloned from 7-day old mouse brain mRNA and subcloned into expression vector, pcDNA3.1(−) (Invitrogen, USA). The identified variants were introduced using a site-direct mutagenesis kit (Invitrogen, USA), and sequences of the entire insert were confirmed with Sanger sequencing. The position in the murine ortholog that corresponds to the human F900V variant is chr7: 92438043 T>G (ENSMUST00000107196.9, GRCm38/mm10), and the evolutionary conservation is shown in Supplemental figure 1.
Pull-down experiment, mass spectrometry, co-immunoprecipitation, and western blot
For pull-down experiments, lysates of HEK293T cells expressing GFP alone or PSD-93-GFP (protein encoded by wild-type Dlg2 and tagged with GFP) or F900V-PSD-93- GFP (protein encoded by F900V Dlg2 and tagged with GFP) were mixed sequentially with rat hypothalamus lysates and GFP antibody that were then bound to protein A-Sepharose beads. The eluted proteins were analyzed using mass spectrometry at the National Institute of Neurological Disorders and Stroke (NINDS) mass spectrometry core facility. An identified protein, Fyn, was co-expressed with wild-type Dlg2 or F900V Dlg2 in HEK293T cells, and the interaction was analyzed by co-immunoprecipitation and western blot. The detailed methods are described in Supplemental method 3.
Assessment of GluN2B phosphorylation
HEK293T cells were transfected with GluN1, GluN2B, Fyn, and WT PSD-93 or the F900V variant. After 4 hours, APV (50 μM) and MgCl2 (20 mM) were added to the medium to block cell death caused by excitotoxicity. After 24 hours following transfection, cells were lysed with 1% SDS lysis buffer and analyzed by western blot with antibodies to phosphorylated-GluN2B (rabbit anti-pY1472-GluN2B, Millipore, cat. AB5403) and GluN2B (mouse anti-GluN2B, NeuroMab).
Culture and transfection of GnRH-expressing cell lines (GT1–7 and HEK293)
GT1–7 was a generous gift from Dr. Pamela Mellon.11 GT1–7 cells (passage 5–15) were cultured in DMEM with Glutamax (Gibco, USA) medium containing 10% fetal bovine serum (FBS) and 1% pen-strep. The medium was changed every 3 days. 0.3 million cells/well were plated in 12-well plates and after 24 hours the cells were transfected with empty vector (EV), mutant (Mut) Dlg2/PSD-93 or wild-type (WT) Dlg2/PSD-93 expression vector using lipofectamine 3000 (Invitrogen, USA) in Opti-MEM (Invitrogen, USA) for 4 hours. Then, the medium was changed to regular culture medium. The cells were collected after 48 hours for RNA extraction or GnRH immunoassay. The above experiments were also performed in HEK293 cells, which express GNRH1 (http://amp.pharm.mssm.edu/Harmonizome/gen), except that the cells were collected at 24 hours post-transfection for analysis.
GnRH competitive ELISA assay
GT1–7 cells were transfected with empty vector, wild-type Dlg2/PSD-93 vector, or mutant Dlg2/PSD-93 expression vector as described above, and after 24 hours the culture medium was removed and cells were lysed by 2 freeze-thaw cycles. GnRH immunoassay was performed as previously described12 using a commercially available kit (Phoenix Pharmaceuticals, Inc, USA) following the manufacturer’s instruction. The reported detection limit is 10 pg/mL and reported intra-assay variation and inter-assay coefficients of variation were 10% and 15%, respectively.
mRNA expression of Dlg2 in rat preoptic area (POA)
RNA from rat preoptic area was extracted using an RNeasy kit (Qiagen) following the manufacturer’s protocol and was reverse transcribed into cDNA. The expression of Gnrh1/GNRH1, membrane-associated guanylate kinases (MAGUKs: Dlg1, Dlg2, Dlg3, Dlg4) and NMDARs (GluN1/NR1, GluN2A/NR2A, GluN2B/NR2B) was measured by real-time RT-PCR using Taqman probes (Invitrogen, USA). Expression values were normalized to 18s rRNA. The detailed probes used and methods are described in Supplemental method 4 and 5.
Protein expression of PSD-93 in mouse hypothalamus
Hippocampus and hypothalamus were dissected from mice at different ages, then analyzed by western blot. The detailed probes used and methods are described in Supplemental method 4 and 5.
Statistical Analysis
Western blot bands after co-immunoprecipitation were quantified using Image J13 and compared using Student’s t-test. The raw data for empty vector, wild-type Dlg2/PSD-93 and mutant Dlg2/PSD-93 from each experiment were log-transformed to better fit the normal distribution and compared using one-way ANOVA followed by pair-wise comparisons using the Holm-Sidak correction for multiple comparisons. P-values less than 0.05 were considered statistically significant.
Results
Clinical presentation in an extended family with markedly delayed puberty
The proband (Figure 1A, III.7) was a young man who presented at 16 years of age with delayed puberty. He had Tanner stage 2 pubic hair, testicular volumes of 4 mL bilaterally, testosterone of 40 ng/dL, and significantly delayed bone age of 13 years 2 months. The patient’s mother (II.5) and maternal grandmother (I.2) had menarche at age 18 years, and the maternal aunt (II.2) reported a history of breast development beginning at age 14 years, menarche at age 16.5 year and 3 inches of height gain after 18 years of age. They had no history of infertility. The proband’s older brother (III.6) had late pubertal onset at age 16 years with prolonged linear growth until age 21 years. Unaffected females in the family had menarche at approximately 12 – 14 years of age, and unaffected males also had pubertal onset (determined by detailed questionnaire) at a mid-normal age. I.2, II.2 and II.5 subjects had no problems with fertility. Subjects’ hormonal profiles and imaging studies are summarized in Supplemental Table 1.
Figure 1. A family with markedly delayed puberty.

A. Pedigree. Arrow indicates proband. Closed symbol, delayed puberty; open symbol, normal timing of puberty; F900V, a variant in DLG2/PSD-93; WT, wild-type DLG2/PSD-93.
Identification of a variant in DLG2/PSD-93
Exome sequencing of affected family members (I.2, II.2, II.5, III.6, and III.7) and unaffected family members (II.3, III.1, III2, III.3, and IIII.5) revealed a single rare, nonsynonymous variant that co-segregated precisely with the delayed puberty phenotype. This missense variant (c.2698T>G:p.F900V, NM_001142699.1) occurred in DLG2 which encodes PSD-93. The variant was confirmed by Sanger sequencing (Supplemental figure 2). It was found in only 1 individual in gnomAD and was predicted to be deleterious to protein function by all 4 applied in silico analyses (CADD, SIFT, MutationTaster, PolyPen2).14–17 An extensive search for any rare variants in 40 genes known to cause hypogonadotropic hypogonadism or delayed puberty revealed only a single, heterozygous, rare, missense variant (c.568C>G:p.P190A, predicted to be deleterious by multiple silico analyses) in TACR3 (an autosomal recessive cause of IHH) in the proband (III.7) and some affected family members (I.2, II.5, III.6). However, the TACR3 variant was present in unaffected members (II.3 and III.5) and absent in affected subject II.2; thus, it failed to co-segregate with the delayed puberty phenotype.
The F900V variant decreases Gnrh1/GNRH1 expression in vitro
We studied the effect of the F900V variant on Gnrh1 expression in the mouse hypothalamic GnRH cell line GT1–7 (between 13–15 passages in culture). In these cells, endogenous Dlg2 expression was minimal compared to mouse brain tissue (by real-time RT PCR, Supplemental Figure 3). Expression of wild-type PSD-93 stimulated both Gnrh1 mRNA and peptide expression compared to empty vector (P<0.001 for both mRNA and peptide, Figure 2). F900V PSD-93 stimulated expression less than did wild-type (P=0.003 for mRNA, P=0.008 for peptide, Figure 2). Similar mRNA results were obtained in GT1–7 cells studied after 5–7 passages and in HEK293 cells (Supplemental Figure 4). These findings suggest that DLG2/PSD-93 participates in the regulation of Gnrh1 expression in GnRH neurons and that the identified variant, F900V, causes a partial loss of function in DLG2/PSD-93.
Figure 2. Effect of F900V DLG2/PSD-93 variant on Gnrh1 expression in GT1–7 cells.

In GT1–7 cells (between 13–15 passages in culture), wild-type (WT) PSD-93 expression stimulated Gnrh1 expression compared to empty vector (EV) for both mRNA (normalized to 18s rRNA, Panel A, P<0.001, n=10) and GnRH peptide expression (normalized to total protein, Panel B, P<0.001, n=14). The F900V PSD-93 mutant impaired this stimulation both in mRNA levels (P=0.003, Panel A) and intracellular peptide levels (P=0.008, Panel B).
The F900V variant decreases binding of PSD-93 to Fyn and decreases GluN2B phosphorylation
DLG2 encodes the postsynaptic density protein, PSD-93. The identified variant is located in the guanylate kinase domain, which is not enzymatically active but instead serves as a binding site for other interacting proteins, such as MAP1A.18 Pull-down experiments using the wild-type and F900V mutant PSD-93 followed by mass spectrometry were used to identify differentially bound proteins and suggested that the F900V PSD-93 variant had decreased binding to Fyn. This effect on Fyn binding was confirmed by co-immunoprecipitation (P = 0.005, Figure 3A&B). Fyn is a non-receptor tyrosine protein kinase, which phosphorylates GluN2B (NMDA receptor type 2B) to stabilize surface expression and to activate NMDA receptor signaling.19–20 This tyrosine phosphorylation of GluN2B is enhanced by interaction with PSD-93.19–20 We therefore co-expressed Fyn, GluN1, GluN2B, and PSD-93 in HEK293 cells and found that the F900V variant decreased the phosphorylation of GluN2B (Figure 3C&D). Thus, our findings support the following pathogenic mechanism – the F900V variant decreases PSD-93 binding to Fyn, which decreases Fyn phosphorylation of NMDA receptors, decreases NMDA receptor activity, and decreases GnRH expression, causing delayed puberty.
Figure 3.

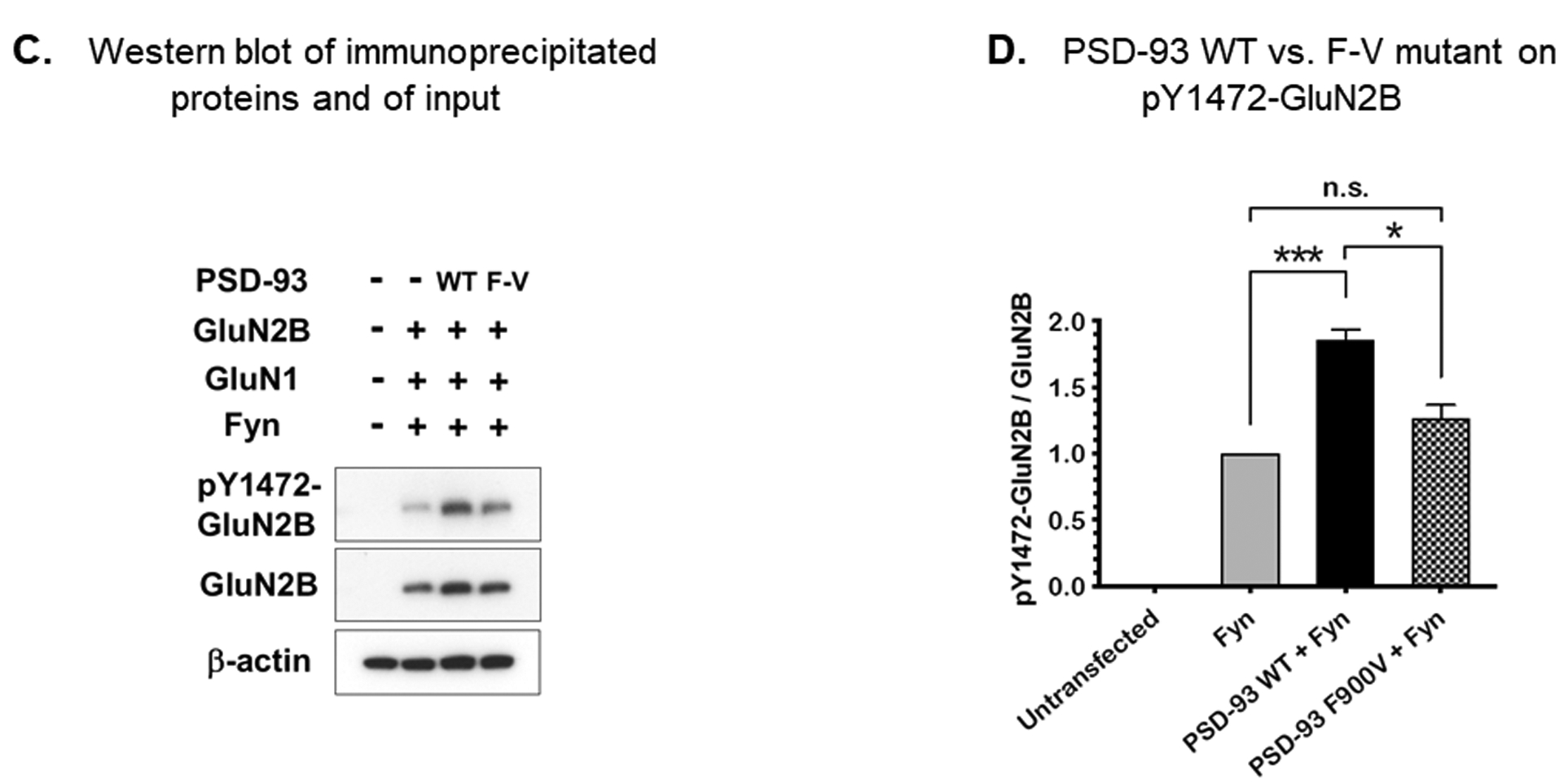
A and B) Co-immunoprecipitation of PSD-93 and myc-Fyn in HEK293T cells. Fyn tagged with myc and either wild-type or F900V mutant PSD-93 were co-expressed in HEK293T cells and immunoprecipitated with antibody against PSD-93. The input protein (prior to immunoprecipitation) and immunoprecipitated protein were analyzed by western blot (3A) using antibody against the myc tag. The immunoprecipitated myc-Fyn bands were quantified and normalized to input (3B). The findings indicate that the variant in PSD-93 decreased binding to Fyn (n=3, P=0.005). WT, wild-type; F-V, F900V variant; IP, immunoprecipitation. C and D) Phosphorylation of GluN2B. Fyn, GluN1, GluN2B, and either wild-type or F900V PSD-93 were co-expressed in HEK293T cells and the phosphorylation of GluN2B at Y1472 was assessed by western blot. The F900V variant decreased phosphorylation (n=3). ***; P = 0.0004, *; P = 0.01.
Identification of variants in DLG2/PSD-93 in a large cohort of subjects with isolated hypogonadotropic hypogonadism (IHH)
We next hypothesized that DLG2/PSD-93 variants might contribute not only to pubertal delay but also to more severe pubertal disorders, such as IHH. We therefore analyzed the exome sequence data from 1,367 IHH patients who had been studied at the Massachusetts General Hospital and identified 7 unrelated subjects who each carried a rare sequence variant in DLG2/PSD-93. Among these, there were 3 intronic variants and 4 missense variants. Vectors designed to express each of the 4 identified missense variants were transfected in GT1–7 cells. As noted in previous experiments, wild-type Dlg2/PSD-93 stimulated Gnrh1 mRNA expression (Figure 4). Three of the 4 identified variants diminished this stimulation, indicating that the variants caused a loss of function (Figure 4). Co-immunoprecipitation experiments showed that the 3 variants found in IHH patients that diminished Gnrh1 expression also impaired the interaction of PSD-93 with Fyn (Supplemental figure 5). Of the 3 families with variants that affected protein function in vitro, Family A had normosmic IHH and Family B and C had Kallmann Syndrome (IHH with olfactory dysfunction) (Table 1, Supplemental Table 1). In Family A, two brothers had IHH. The detailed medical history of family A is not available, and the father was deceased. A missense variant (p.E140K) in DLG2, which diminished Gnrh1 mRNA expression (Figure 4), was identified in both brothers. Neither had any significant sequence variant in 40 genes previously associated with IHH. In family B, two male siblings had bilateral undescended testes, failure to undergo spontaneous puberty, and anosmia with olfactory nerve hypoplasia documented in the older brother. Their father had a history of small phallus but reported a normal timing of puberty. A missense variant in DLG2 (p.I901V), which diminished Gnrh1 mRNA expression (Figure 4), was found in both brothers and the father. A rare sequence variant in a known gene for IHH, FGFR1, was found in the father, and both brothers (Table 1). In family C, the proband failed to undergo spontaneous puberty. She failed a smell test, but her head MRI was reported as normal. Her father had a history of delayed puberty which required testosterone treatment. He had no problem conceiving. The proband and her father had a missense variant in DLG2 (p.Q166H) which diminished Gnrh1 mRNA expression (Figure 4) and also rare sequence variants in two genes associated with IHH, TAC3 and PCSK1 (Table 1).
Figure 4. Three unrelated families with variants in DLG2/PSD-93.

Family A. Two siblings with unaffected parents presented with normosmic hypogonadotropic hypogonadism. Each sibling was heterozygous for an E140K variant in DLG2/PSD-93. Neither DNA nor a pubertal history was available for the father of the affected individuals. Family B. Two siblings with unaffected parents presented with Kallmann syndrome. Both siblings and the unaffected father were heterozygous for a I901V variant in DLG2/PSD-93. Family C. A patient presented with Kallmann syndrome. She and her father with delayed puberty were heterozygous for a Q166H variant in DLG2. Below each pedigree is shown the results of in vitro studies of the specific Dlg2/PSD-93 variant found in that family. Empty vector (EV), wild-type (WT) Dlg2/PSD-93 and mutant Dlg2/PSD-93 were expressed in GT1–7 cells and Gnrh1 mRNA expression was measured. The three Dlg2/PSD-93 variants suppressed the stimulation of Gnrh1 compared to wild-type Dlg2/PSD-93 (n=8). Arrow indicates probands. Left upper black quadrant, IHH; right lower black quadrant, anosmia; right upper black quadrant, delayed puberty.
Table 1.
Characteristics of subjects with variants in DLG2
| ID | Index proband III-7 | Family A | Family B | Family C |
|---|---|---|---|---|
| Phenotype | Delayed puberty | nIHH¶ | KS† | KS† |
| First pubertal sign | Testicular enlargement at 16 years | None | None | Pubic hair at age 16 years |
| Cessation of growth | Still growing at 20 y | NA | NA | 18–19 y |
| Sex of affected individual(s) | Male | Male/female | Male/male | Female |
| Variant in DLG2/PSD-93 | c.2698T>G p.F900V | c.418G>A p.E140K | c.2701A>G p.I901V | c.498G>C p.Q166H |
| Affected siblings carrying the same variant | Yes | Yes | Yes | No sibling |
| Inherited from | Mother with delayed puberty | Presumably father Unknown timing of puberty | Father with history of small phallus | Father with delayed puberty |
| Domain in PSD-93 | Guanylate kinase | Pre-PDZ | Guanylate kinase | Pre-PDZ |
| Allele frequency in gnomAD | 1 | 11 | Not found | 1 |
| Other variants in genes associated with IHH* | TACR3(P190A) | None | FGFR1(T340A) | TAC3 (R80C) PCSK1(W35R) |
| Found in unaffected family members | . | Found in father with small phallus | Found in affected father with delayed puberty | |
| Other clinical findings | None | NAǂ | On hormone replacement | Hashimoto thyroiditis on hormone replacement |
nIHH, Normosmic isolated hypogonadotropic hypogonadism
KS, Kallmann syndrome
NA, not available.
IHH, isolated hypogonadotropic hypogonadism
Temporal Expression of Dlg2/PSD-93 in the preoptic area
We observed mRNA expression of Dlg2, Dlg4, and NMDA receptors in the rat preoptic area and protein expression in mouse hypothalamus (Supplemental figure 6, 7, and 8) which varied with age. However, the temporal pattern of expression did not match the temporal pattern of reproductive maturation suggesting that, although Dlg2/PSD-93 may be important for normal puberty, rising expression may not be a trigger for pubertal onset.
Discussion
We report an extended family with extremely delayed puberty whose affected family members had a rare variant, F900V, in DLG2/PSD-93. Multiple lines of evidences indicate that the variant in DLG2/PSD-93 is a cause of delayed puberty: 1) The F900V variant co-segregated with delayed puberty in the extended family; 2) Experimental estimates of exchangeability suggest that F to V substitutions, which are conservative, have less tendency to impair protein function than do radical amino acid substitutions but still frequently do have an effect.21 Three in silico prediction tools predicted that this specific variant alters protein function based on evolutionary conservation and protein structure considerations. 3) In GnRH-expressing cell lines, wild-type PSD-93 upregulated Gnrh1 expression and the F900V mutant impaired this stimulatory effect; 4) PSD-93 is an anchoring protein for NMDA receptors and has an important role in NMDA receptor signaling. NMDA receptors have been strongly implicated in regulating the timing of puberty in rodents and primates1; 5) The identified F900V variant impaired the ability of PSD-93 to bind Fyn and to stimulate NMDA receptor phosphorylation by Fyn; 6) Recently, genome-wide association (GWA) studies have implicated the DLG2 gene locus in the normal timing of puberty in males22 and females (Supplemental figure 9).23
Other variants in DLG2/PSD-93, which also impaired Gnrh1 expression in vitro, were found in 3 families with IHH, suggesting that variants in this gene may also contribute to IHH, with and without anosmia. This shared genetic etiology between delayed puberty and IHH has also been observed for other genes that regulate GnRH neurons.2 However, in the families that we studied with IHH, there appeared to be incomplete penetrance, suggesting that other genetic factors likely contribute to these more severe phenotypes. Indeed, in these families, we also identified variants in other genes that are associated with IHH (FGFR1, TAC3, and PCSK1). Although it is not known whether these other gene variants affect protein function, it is possible that they may have also contributed to the phenotype. This possibility is consistent with previous evidence that IHH may have an oligogenic cause.24–25 In contrast, in our extended family with self-limited delayed puberty, which is a milder phenotype, the inheritance appeared to be monogenic. The proband carried a rare deleterious variant in TACR3, a gene in which biallelic variants cause IHH,26 However, the TACR3 variant was present in unaffected members (II.3 and III.5) and absent in affected subject II.2. Thus, it failed to co-segregate with the delayed puberty phenotype, indicating that it is neither necessary nor sufficient to explain the phenotype. However, the data do not exclude the possibility that the TACR3 variant could have contributed to the phenotype in those subjects carrying both variants.
PSD-93, the protein encoded by DLG2, serves as an anchoring protein for NMDA receptors, a specific class of ionotropic glutamate receptors, and there is strong evidence that NMDA receptors promote the reactivation of gonadotropin-releasing hormone (GnRH) neurons in the hypothalamus which leads to puberty.27–31 NMDA receptors are expressed by GnRH neurons in the medial aspects of the rostral preoptic area, which are activated at the time of the pubertal GnRH surge.27 Furthermore, in several species, glutamate stimulates GnRH release from the adult hypothalamus via activation of NMDA and kainate receptors.27 Similarly, NMDA receptor stimulation by agonist results in precocious puberty in infantile rats28 and in pre-pubertal monkeys29 and the administration of an NMDA receptor blocker delays the onset of puberty in rats, confirming the importance of NMDA receptor signaling in the timing of puberty.30–31 NMDA receptors in the postsynaptic membrane are anchored to postsynaptic density proteins, including PSD-93. In mice, targeted ablation of Dlg2 alters cell-surface NMDA receptor expression and reduces NMDA receptor-mediated postsynaptic signaling,32 confirming the role of PSD-93 in NMDA signaling. These mice appear to be fertile, but the effects on timing of puberty and reproductive function have not, to our knowledge, been otherwise studied.32 The phenotypic consequence of Dlg2 variants in mice and humans may be mitigated by redundancy in the function of MAGUK family members (DLG1, 2, 3, and 4) for NMDA receptor signaling.33 Interestingly, the suppression of Dlg4/PSD-95 expression in the preoptic region of female rats disrupted estrous cyclicity, suggesting a role of PSD-95 in GnRH regulation.34
Therefore, in combination with previous evidence, the current study suggests that DLG2 loss-of-function variants may delay or prevent pubertal development by decreasing excitatory neuronal NMDA receptor signaling. We did not directly test the effects of the variant on NMDA signaling. However, we found that the F900V variant in Dlg2/PSD-93 decreased the interaction of PSD-93 with Fyn. Fyn is a member of the Src family of tyrosine kinases which phosphorylates GluN2B to activate NMDA receptor signaling.20 This phosphorylation of GluN2B is enhanced by interaction with PSD-93.20 Thus, taken together, the evidence suggests that the F900V variant in DLG2/PSD-93 may reduce GluN2B phosphorylation by Fyn, causing decreased NMDA receptor signaling.
Genetic defects found in IHH can affect GnRH production not only by functionally modulating levels of GnRH expression and/or secretion by GnRH neurons but also by affecting the embryonic migration of GnRH neurons along the olfactory tract to the hypothalamus, and some genetic defects cause combined IHH and anosmia, termed Kallmann syndrome. The finding of Kallmann syndrome in two of the families found to have functional variants in DLG2 suggests that neuronal migration may be also disrupted, resulting in fewer GnRH neurons in the hypothalamus. There is evidence that normal GnRH neuronal migration is dependent on AMPA receptors,35 a different class of glutamate receptors, and evidence that DLG2/PSD-93 also anchors AMPA receptors,36 suggesting a possible mechanism by which variants in DLG2/PSD-93 might affect neuronal migration. However, further study is required to confirm the possible role of DLG2/PSD-93 on GnRH migration.
Normosmic IHH and Kallmann syndrome have also been associated with variants in NSMF (NMDA receptor synaptonuclear signaling and neuronal migration factor), also known as NELF (Nasal embryonic LHRH factor).37–38 NSMF is expressed in the olfactory nerves and migratory GnRH cells during embryonic development and is important for neuronal migration.37 The protein encoded by NSMF is phosphorylated upon synaptic NMDA receptor activation and is subsequently translocated to the nucleus to coordinate synapse-to-nucleus signaling.39 Therefore, variants in both DLG2 and NSMF may cause IHH and Kallmann syndrome through a shared pathway involving NMDA receptor signaling. The importance of NMDA receptor signaling on the timing of puberty in humans is also supported by a report that an infant with high glycine concentration in cerebrospinal fluid due to nonketotic hyperglycinemia developed precocious puberty, with evidence that glycine in high concentration activates NMDA receptors.40
One limitation of our study is that the in vitro cell lines, GT1–7 and HEK293 used to measure the impact of variants in DLG2 do not express all components of NMDA receptors, although GT1–7 cells have been used to study the effect of NMDA on GnRH release.41 Therefore, further in vivo studies will be required to elaborate the mechanisms by which DLG-2 affects GnRH expression.
In conclusion, we found evidence that variants in DLG2/PSD-93, an anchoring protein of NMDA receptors, can cause pubertal disorders. Combined with previous studies demonstrating that NMDA receptor signaling is required for normal puberty in mammals, our findings suggest that the observed variants in DLG2/PSD-93 may delay puberty by interfering with the interaction of PSD-93 and Fyn, decreasing NMDA receptor signaling, and suppressing GnRH secretion.
Supplementary Material
Acknowledgments
We appreciate Prof. Ken Ong for providing the locuszoom plot for DLG2 SNP. The research is supported by NIH intramural research grant.
Footnotes
Conflicts of interest
Disclosure: The authors declare no conflict of interest.
References
- 1.Plant TM. 60 YEARS OF NEUROENDOCRINOLOGY: The hypothalamo-pituitary-gonadal axis. J Endocrinol 2015. 226(2):T41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J, Choa RE, Guo MH, Plummer L, Buck C, Palmert MR, Hirschhorn JN, Seminara SB, Chan YM. A shared genetic basis for self-limited delayed puberty and idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2015. 100(4):E646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Young J, Xu C, Papadakis GE, Acierno JS, Maione L, Hietamäki J, Raivio T, Pitteloud N. Clinical Management of Congenital Hypogonadotropic Hypogonadism. Endocr Rev. 2019. 40(2):669–710. [DOI] [PubMed] [Google Scholar]
- 4.Howard SR. Genes underlying delayed puberty. Mol Cell Endocrinol. 2018. 476:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Howard SR. The Genetic Basis of Delayed Puberty Front Endocrinol (Lausanne: ). 2019. 10:423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancini A, Howard SR, Cabrera CP, Barnes MR, David A, Wehkalampi K, Heger S, Lomniczi A, Guasti 1, Ojeda SR, Dunkel L EAP1 regulation of GnRH promoter activity is important for human pubertal timing. Hum Mol Genet. 2019. 28(8):1357–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Howard SR, Oleari R, Poliandri A, Chantzara V, Fantin A, Ruiz-Babot G, Metherell LA, Cabrera CP, Barnes MR, Wehkalampi K, Guasti L, Ruhrberg C, Cariboni A, Dunkel L. HS6ST1 Insufficiency Causes Self-Limited Delayed Puberty in Contrast With Other GnRH Deficiency Genes. J Clin Endocrinol Metab. 2018. 103(9):3420–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard SR, Guasti L, Ruiz-Babot G, Mancini A, David A, Storr HL, Metherell LA, Sternberg MJ, Cabrera CP, Warren HR, Barnes MR, Quinton R, de Roux N, Young J, Guiochon-Mantel A, Wehkalampi K, André V, Gothilf Y, Cariboni A, Dunkel L. IGSF10 mutations dysregulate gonadotropin-releasing hormone neuronal migration resulting in delayed puberty. EMBO Mol Med. 2016. 8(6):626–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zwaveling-Soonawala N, Alders M, Jongejan A, Kovacic L, Duijkers FA, Maas SM, Fliers E, van Trotsenburg ASP, Hennekam RC. Clues for Polygenic Inheritance of Pituitary Stalk Interruption Syndrome From Exome Sequencing in 20 Patients. J Clin Endocrinol Metab. 2018. February 1;103(2):415–428. [DOI] [PubMed] [Google Scholar]
- 10.Amato LGL, Montenegro LR, Lerario AM, Jorge AAL, Guerra Junior G, Schnoll C, Renck AC, Trarbach EB, Costa EMF, Mendonca BB, Latronico AC, Silveira LFG. New genetic findings in a large cohort of congenital hypogonadotropic hypogonadism. Eur J Endocrinol. 2019. August 1;181(2):103–119. [DOI] [PubMed] [Google Scholar]
- 11.Mellon PL, Windle JJ, Goldsmith PC, Padula CA, Roberts JL, Weiner RI. Immortalization of hypothalamic GnRH neurons by genetically targeted tumorigenesis. Neuron 1990;5(1):1–10. [DOI] [PubMed] [Google Scholar]
- 12.Zhang G1, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 2013;497(7448):211–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012;9(7):671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kircher M, Witten DM, Jain P, O’Roak BJ, Cooper GM, Shendure J. A general framework for estimating the relative pathogenicity of human genetic variants. Nat Genet. 2014. March;46(3):310–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaser R, Adusumalli S, Leng SN, Sikic M, Ng PC. SIFT missense predictions for genomes. Nat Protoc. 2016. January;11(1):1–9. [DOI] [PubMed] [Google Scholar]
- 16.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014. April;11(4):361–2. [DOI] [PubMed] [Google Scholar]
- 17.Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, Bork P, Kondrashov AS, Sunyaev SR. A method and server for predicting damaging missense mutations. Nat Methods. 2010. April;7(4):248–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenman JE, Topinka JR, Cooper EC, McGee AW, Rosen J, Milroy T, Ralston HJ, Bredt DS. Localization of postsynaptic density-93 to dendritic microtubules and interaction with microtubule-associated protein 1A. J Neurosci. 1998. November 1;18(21):8805–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sato Y, Tao YX, Su Q, Johns RA. Post-synaptic density-93 mediates tyrosine-phosphorylation of the N-methyl-D-aspartate receptors. Neuroscience 2008;153(3):700–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang M, Li Q, Chen L, Li J, Zhang X, Chen X, Zhang Q, Shao Y, Xu Y. PSD-93 deletion inhibits Fyn-mediated phosphorylation of NR2B and protects against focal cerebral ischemia. Neurobiol Dis 2014;68:104–11. [DOI] [PubMed] [Google Scholar]
- 21.Yampolsky LY, Stoltzfus A. The exchangeability of amino acids in proteins. Genetics. 2005. 170(4): 1459–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Day FR, Helgason H, Chasman DI, Rose LM, Loh PR, Scott RA, Helgason A, Kong A, Masson G, Magnusson OT, Gudbjartsson D, Thorsteinsdottir U, Buring JE, Ridker PM, Sulem P, Stefansson K, Ong KK, Perry JRB. Physical and neurobehavioral determinants of reproductive onset and success. Nat Genet 2016;48(6):617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Day FR, Thompson DJ, Helgason H, Chasman DI, Finucane H, Sulem P, Ruth KS, Whalen S, Sarkar AK, Albrecht E, Altmaier E, Amini M, Barbieri CM, Boutin T, Campbell A, Demerath E, Giri A, He C, Hottenga JJ, Karlsson R, Kolcic I, Loh PR, Lunetta KL, Mangino M, Marco B, McMahon G, Medland SE, Nolte IM, Noordam R, Nutile T, Paternoster L, Perjakova N, Porcu E, Rose LM, Schraut KE, Segrè AV, Smith AV, Stolk L, Teumer A, Andrulis IL, Bandinelli S, Beckmann MW, Benitez J, Bergmann S, Bochud M, Boerwinkle E, Bojesen SE, Bolla MK, Brand JS, Brauch H, Brenner H, Broer L, Brüning T, Buring JE, Campbell H, Catamo E, Chanock S, Chenevix-Trench G, Corre T, Couch FJ, Cousminer DL, Cox A, Crisponi L, Czene K, Davey Smith G, de Geus EJCN, de Mutsert R, De Vivo, Dennis J, Devilee P, Dos-Santos-Silva, Dunning AM, Eriksson JG, Fasching PA, Fernández-Rhodes L, Ferrucci L, Flesch-Janys D, Franke L, Gabrielson M, Gandin I, Giles GG, Grallert H, Gudbjartsson DF, Guénel P, Hall P, Hallberg E, Hamann U, Harris TB, Hartman CA, Heiss G, Hooning MJ, Hopper JL, Hu F, Hunter DJ, Ikram MA, Im HK, Järvelin MR, Joshi PK, Karasik D, Kellis M, Kutalik Z, LaChance G, Lambrechts D, Langenberg C, Launer LJ, Laven JSE, Lenarduzzi S, Li J, Lind PA, Lindstrom S, Liu Y, Luan J, Mägi R, Mannermaa A, Mbarek H, McCarthy MI, Meisinger C, Meitinger T, Menni C, Metspalu A, Michailidou K, Milani L, Milne RL, Montgomery GW, Mulligan AM, Nalls MA, Navarro P, Nevanlinna H, Nyholt DR, Oldehinkel AJ, O’Mara TA, Padmanabhan S, Palotie A, Pedersen N, Peters A, Peto J, Pharoah PDP, Pouta A, Radice P, Rahman I, Ring SM, Robino A, Rosendaal FR, Rudan I, Rueedi R, Ruggiero D, Sala CF, Schmidt MK, Scott RA, Shah M, Sorice R, Southey MC, Sovio U, Stampfer M, Steri M, Strauch K, Tanaka T, Tikkanen E, Timpson NJ, Traglia M, Truong T, Tyrer JP, Uitterlinden AG, Edwards DRV, Vitart V, Völker U, Vollenweider P, Wang Q, Widen E, van Dijk KW, Willemsen G, Winqvist R, Wolffenbuttel BHR, Zhao JH, Zoledziewska M, Zygmunt M, Alizadeh BZ, Boomsma DI, Ciullo M, Cucca F, Esko T, Franceschini N, Gieger C, Gudnason V, Hayward C, Kraft P, Lawlor DA, Magnusson PKE, Martin NG, Mook-Kanamori DO, Nohr EA, Polasek O, Porteous D, Price AL, Ridker PM, Snieder H, Spector TD, Stöckl D, Toniolo D, Ulivi S, Visser JA, Völzke H, Wareham NJ, Wilson JF; LifeLines Cohort Study; InterAct Consortium; kConFab/AOCS Investigators; Endometrial Cancer Association Consortium; Ovarian Cancer Association Consortium; PRACTICAL consortium, Spurdle AB, Thorsteindottir U, Pollard KS, Easton DF, Tung JY, Chang-Claude J, Hinds D, Murray A, Murabito JM, Stefansson K, Ong KK, Perry JRB. Genomic analyses identify hundreds of variants associated with age at menarche and support a role for puberty timing in cancer risk. Nat Genet 2017;49(6):834–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitteloud N, Quinton R, Pearce S, Pearce S, Raivio T, Acierno J, Dwyer A, Plummer L, Hughes V, Seminara S, Cheng YZ, Li WP, Maccoll G, Eliseenkova AV, Olsen SK, Ibrahimi OA, Hayes FJ, Boepple P, Hall JE, Bouloux P, Mohammadi M, Crowley W. Digenic mutations account for variable phenotypes in idiopathic hypogonadotropic hypogonadism. J Clin Invest 2007;117(2):457–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF Jr, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A. 2010. August 24;107(34):15140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Topaloglu AK, Reimann F, Guclu M, Yalin AS, Kotan LD, Porter KM, Serin A, Mungan NO, Cook JR, Imamoglu S, Akalin NS, Yuksel B, O’Rahilly S, Semple RK. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for Neurokinin B in the central control of reproduction. Nat Genet 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parent AS, Matagne V, Bourguignon JP. Control of puberty by excitatory amino acid neurotransmitters and its clinical implications. Endocrine 2005;28(3):281–6. [DOI] [PubMed] [Google Scholar]
- 28.Smyth C, Wilkinson M. A critical period for glutamate receptor-mediated induction of precocious puberty in female rats. J Neuroendocrinol 1994;6(3):275–84. [DOI] [PubMed] [Google Scholar]
- 29.Plant TM, Gay VL, Marshall GR, Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci U S A 1989;86(7):2506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iremonger KJ, Constantin S, Liu X, Herbison AE. Glutamate regulation of GnRH neuron excitability. Brain Res 2010;1364:35–43. [DOI] [PubMed] [Google Scholar]
- 31.Gay VL, Plant TM. Sustained intermittent release of gonadotropin-releasing hormone in the prepubertal male rhesus monkey induced by N-methyl-DL-aspartic acid. Neuroendocrinology 1988;48(2):147–52. [DOI] [PubMed] [Google Scholar]
- 32.Tao YX, Rumbaugh G, Wang GD, Petralia RS, Zhao C, Kauer FW, Tao F, Zhuo M, Wenthold RJ, Raja SN, Huganir RL, Bredt DS, Johns RA. Impaired NMDA receptor-mediated postsynaptic function and blunted NMDA receptor-dependent persistent pain in mice lacking postsynaptic density-93 protein. J Neurosci 2003;23(17):6703–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Levy JM, Hou A, Winters C, Azzam R, Sousa AA, Leapman RD, Nicoll RA, Reese TS. PSD-95 family MAGUKs are essential for anchoring AMPA and NMDA receptor complexes at the postsynaptic density. Proc Natl Acad Sci U S A 2015;112(50):E6983–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.d’Anglemont de Tassigny X, Campagne C, Dehouck B, Leroy D, Holstein GR, Beauvillain JC, Buée-Scherrer V, Prevot V. Coupling of neuronal nitric oxide synthase to NMDA receptors via postsynaptic density-95 depends on estrogen and contributes to the central control of adult female reproduction. J Neurosci 2007;27(23):6103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simonian SX, Herbison AE. Differing, spatially restricted roles of ionotropic glutamate receptors in regulating the migration of gnrh neurons during embryogenesis. J Neurosci 2001;21(3):934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elias GM, Elias LA, Apostolides PF, Kriegstein AR, Nicoll RA. Differential trafficking of AMPA and NMDA receptors by SAP102 and PSD-95 underlies synapse development. Proc Natl Acad Sci U S A 2008;105(52):20953–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miura K, Acierno JS Jr, Seminara SB. Characterization of the human nasal embryonic LHRH factor gene, NELF, and a mutation screening among 65 patients with idiopathic hypogonadotropic hypogonadism (IHH). J Hum Genet 2004;49(5):265–8. [DOI] [PubMed] [Google Scholar]
- 38.Sykiotis GP, Plummer L, Hughes VA, Au M, Durrani S, Nayak-Young S, Dwyer AA, Quinton R, Hall JE, Gusella JF, Seminara SB, Crowley WF Jr, Pitteloud N. Oligogenic basis of isolated gonadotropin-releasing hormone deficiency. Proc Natl Acad Sci U S A3 2010;107(34):15140–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karpova A, Mikhaylova M, Bera S, Bär J, Reddy PP, Behnisch T, Rankovic V, Spilker C, Bethge P, Sahin J, Kaushik R, Zuschratter W, Kähne T, Naumann M, Gundelfinger ED, Kreutz MR. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell 2013;152(5):1119–33. [DOI] [PubMed] [Google Scholar]
- 40.Bourguignon JP, Jaeken J, Gerard A, de Zegher F. Amino acid neurotransmission and initiation of puberty: evidence from nonketotic hyperglycinemia in a female infant and gonadotropin-releasing hormone secretion by rat hypothalamic explants. J Clin Endocrinol Metab 1997;82(6):1899–903. [DOI] [PubMed] [Google Scholar]
- 41.El-Etr M, Akwa Y, Baulieu EE, Schumacher M. The neuroactive steroid pregnenolone sulfate stimulates the release of gonadotropin-releasing hormone from GT1–7 hypothalamic neurons, through N-methyl-D-aspartate receptors. Endocrinology 2006;147(6):2737–43. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


