Abstract
Background
Pulmonary arterial hypertension (PAH) is a progressive chronic disease with poor outcomes. One reason for poor prognosis is the lack of understanding regarding individual variability in response to treatment. Idiopathic PAH (IPAH) patients with bone morphogenetic protein receptor type 2 (BMPR2) mutations have distinct phenotypes that are crucial for individualized therapy but evidence regarding their prevalence and clinical features in the Korean population is lacking. Therefore, the present study aimed to screen Korean IPAH patients for BMPR2 mutations and analyze their clinical phenotypes.
Methods
We enrolled 73 unrelated IPAH patients for BMPR2 mutation screening between March 2010 to November 2015 from 11 hospitals in Korea. Thirty-three lineal family members from 6 families of BMPR2 mutation carriers were also screened.
Results
Among 73 patients, 16 (22%) had BMPR2 mutations. Mutation carriers were younger (27 vs. 47 years; p = 0.02) and had a higher mean pulmonary arterial pressure (mPAP) than non-carriers (64 vs. 51 mmHg; p<0.05). Of the 16 individuals with mutations, 5 deletion, 2 splice-site, 6 nonsense, and 3 missense mutations were found, among which, 9 were newly identified mutation types. Patients less than 30 years old had more BMPR2 mutations (44 vs. 14%; p = 0.04) and a higher mPAP (64 vs. 50 mmHg; p = 0.04) compared with those equaled to or over 30 years old. There were no differences in hemodynamic profiles or the proportion of BMPR2 mutation carriers between groups according to sex.
Conclusion
The prevalence of BMPR2 mutations in Korean IPAH patients was 22%. Mutation carriers were younger and had a poorer hemodynamic profile compared with the non-carriers.
Clinical trial registration
Clinicaltrials.gov NCT01054105
Introduction
Pulmonary arterial hypertension (PAH) is a rare and fatal disease characterized by pulmonary vascular cell proliferation and a sustained increase in mean pulmonary artery pressure (mPAP), leading to right heart failure and eventually death [1, 2]. Among the World Health Organization (WHO) types of pulmonary hypertension (PH), idiopathic PAH (IPAH) is in group 1 and includes sporadic and familial cases with or without known germline mutations [3, 4]. An altered transforming growth factor-beta (TGF-β) related receptor signaling via the bone morphogenetic protein receptor type 2 (BMPR2) is the most common germline mutation [3]. Bone morphogenetic proteins are part of a TGF-β superfamily cytokine group regulating growth and differentiation of bone and cartilage that affect various cell types [5]. BMPR2 is a type II receptor with a long carboxyl-terminal sequence following the intracellular kinase domain [6]. Mutations in the BMPR2 gene result in loss of function and reduced downstream signaling [7]. Such mutations are prevalent in cases of IPAH, where 55% to 75% of individuals with familial history and up to 40% of individuals with idiopathic cases were found to be BMPR2 mutation carriers [4, 8, 9].
Targeted therapy with the use of endothelin receptor antagonists, phosphodiesterase-5 inhibitors, and prostanoids have markedly improved the likelihood of survival [10]. Despite such advances, poor prognosis is a reality for most individuals, in part due to the lack of understandings regarding individual phenotypic variability [11, 12]. One of the most well-described and most prevalent examples of individual variability in IPAH is the BMPR2 mutation [13–15]. Heterozygous BMPR2 mutation carriers usually present 10 years earlier than non-carriers [14] and are associated with more severe hemodynamic deterioration at diagnosis [14–16], respond poorly to vasodilators [16, 17], and are at higher risk of lung transplantation, although evidence regarding survival is debatable [7, 18, 19]. Understanding the phenotypic variabilities of BMPR2 mutations may be crucial for individualized treatment strategies to improve outcomes.
Although there have been several case reports regarding BMPR2 mutations in Korean IPAH patients [20], there is no established nationwide Korean multi-center cohort to study BMPR2 mutations to date. Therefore, this study aimed to screen Korean IPAH patients for BMPR2 mutations and investigate the mutation prevalence, clinical characteristics, and hemodynamic profile of carriers.
Materials and methods
Subjects
The Effect of BMPR2 Gene Mutations on Hemodynamic Response by Iloprost Inhalation in Pulmonary Arterial Hypertension (PILGRIM) cohort is a prospective, investigator-initiated, multi-institutional clinical trial (NCT01054105). The study protocol was reviewed and approved by the institutional review board of each participating center (GIRBA-2278; Gachon University Gil Medical Center, Seoul National University Hospital, Severance Cardiovascular Hospital at Yonsei University College of Medicine, Samsung Medical Center, Seoul National University Bundang Hospital, Wonkwang University Hospital, Seoul St. Mary’s Hospital of The Catholic University, Sejong General Hospital, Soonchunhyang University Hospital, Chonnam National University Hospital, and Hanyang University Medical Center).
The PILGRIM study was designed with two phases. The first phase was to evaluate the prevalence, hemodynamic features, and short-term outcomes of Korean individuals with IPAH. The second phase of the study will focus on the hemodynamic response to iloprost inhalation. Herein, we present the first phase of the PILGRIM study.
All patients gave informed consents for participation. Clinical data were acquired from electronic medical records. Telephone interviews were conducted for patients lost during follow-up. Consecutive patients diagnosed with IPAH at 11 participating hospitals were enrolled and followed between March 1, 2010, and November 30, 2015. The inclusion criteria were: (1) patients aged between 20 to 80 years; (2) those with WHO group I PH (mPAP >25 mmHg and pulmonary artery wedge pressure [PAWP] <15 mmHg) confirmed by right heart catheterization (RHC) or those who satisfied the echocardiographic criteria (peak pulmonary arterial pressure >40mmHg and mPAP >30mmHg); (3) previously diagnosed PAH patients refractory to conventional treatment excluding iloprost inhalation solution (Ventavis™), and; (4) able to undergo a low-intensity exercise test (bicycle or walking). Exclusion criteria were patients with PH belonging to WHO groups II-V and patients concurrently using other pulmonary artery vasodilators such as an inhaled nitric oxide (NO) or endothelin antagonists except for phosphodiesterase-5 inhibitors. Detailed inclusion and exclusion criteria are shown in S1 Table. Other test modalities such as pulmonary function testing, chest computed tomography, perfusion scan, human immunodeficiency virus blood tests, and connective tissue disease markers were used to rule out other specific causes among those without WHO group I PH.
The definition of IPAH in the current study
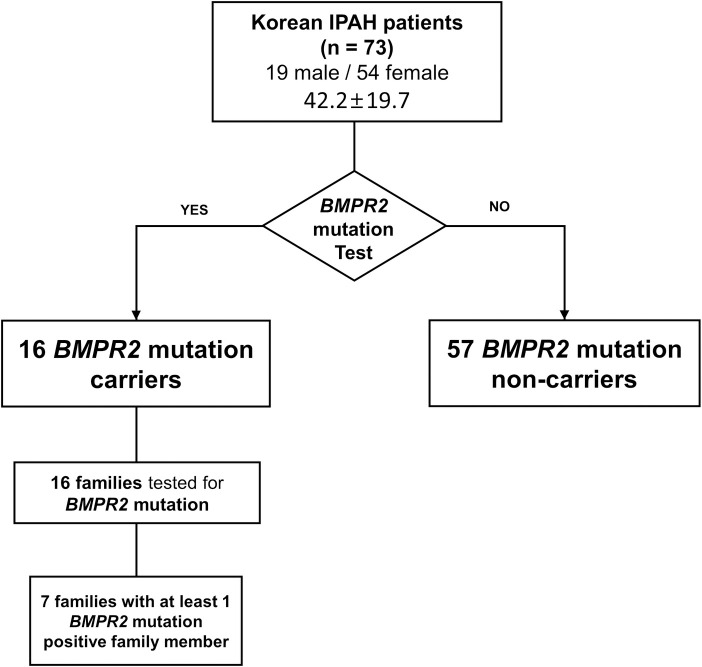
In this study, IPAH was defined as individuals with WHO group I PH without apparent cause regardless of family history, which included all cases of sporadic or familial PAH. The sporadic and familial cases were not separately analyzed because they have indistinguishable histopathological and clinical phenotypes [21]. Additionally, BMPR2-associated PAH is an autosomal dominant disease with variable penetrance, indicating that all BMPR2 mutation carriers have the heritable disease regardless of the clinical presentation of family members [13]. Thus, a total of 33 lineal family members of all BMPR2 mutation-positive patients (33 lineal family members among 16 families of 16 mutation carriers) were examined. A diagram for enrollment is shown in Fig 1.
Fig 1. Diagram for detailed enrollment.
The study population included 73 individuals with IPAH who were evaluated for BMPR2 mutations. Sixteen individuals were positive for BMPR2 mutation, among which 7 had at least one BMPR2 mutation-positive family member. Fifty-seven individuals were BMPR2 mutation carriers, whereas 17 were non-carriers. Abbreviations: PAH, pulmonary arterial hypertension; BMPR2, bone morphogenetic protein receptor type 2; IPAH, idiopathic pulmonary arterial hypertension.
Hemodynamic measurements
All diagnoses were confirmed by RHC. RHC was performed to measure hemodynamic values at initial diagnosis for all patients. The mPAP, PAWP, cardiac output, and pulmonary vascular resistance (PVR) were recorded.
Molecular methods
Blood samples of patients were collected in vacuum blood collection tubes containing ethylenediaminetetraacetic acid. Genomic DNA was extracted from the blood or buffy coat samples using an Exgene Blood SV mini-kit (GeneAll, Korea) according to the manufacturer’s protocol. All exons and the flanking intronic sequences of the BMPR2 gene were amplified by polymerase chain reaction using PrimeSTAR HS DNA Polymerase (TAKARA, Japan) and the appropriate primer sets. The primers used in this study are shown in S2 Table. Polymerase chain reaction products were purified using an Expin Combo GP kit (GeneAll, Korea). The purified samples were subsequently subjected to direct sequencing using a BigDye Terminator v3.1 cycle sequencing kit (Life Technologies, USA) and ABI 3730xl DNA Analyzer (Applied Biosystems, USA). The GenBank accession numbers for the reference sequences used in this study were NM_001204.6 for cDNA and NC_000002.12 for the genomic DNA of BMPR2.
Literature search
We obtained cohort or meta-analysis data regarding individuals with IPAH with or without BMPR2 mutations through a systematic search of the Cochrane Library, PubMed/MEDLINE, and Web of Science databases. The search terms were: 1) “BMPR2” OR “bone morphogenetic protein receptor type 2”; AND 2) “idiopathic PAH,” OR “heritable PAH” OR “sporadic PAH,” OR “familial PAH.” As a result, 11 studies contained relevant data with more than 50 patients enrolled.
Statistical analysis
Data are presented as mean±standard deviation for normally distributed data, whereas continuous non-normally distributed data are given as a median and interquartile range (IQR). Discrete data are given as numbers and/or percentages. P-values less than 0.05 were considered statistically significant. The clinical and hemodynamic parameters between BMPR2 mutation carriers and non-carriers were compared The Pearson χ2 test and Mann-Whitney U-test were used to compare normally and non-normally distributed data, respectively. The median follow-up was analyzed among those who did not die during follow-up. Mortality rates between groups were not analyzed due to a lack of statistical power. Statistical analyses were performed using SPSS v. 23.0 for Windows (IBM Corp., Armonk, NY, USA).
Results
Patient characteristics
We enrolled a total of 73 Korean patients diagnosed with IPAH from 11 health centers in Korea. Table 1 summarizes the demographic variables, functional/hemodynamic status, and drug regimen in BMPR2 mutation carriers and non-carriers. The mean age at initial diagnosis regardless of etiology was 42.7±19.8 years and 27% were men. The mean age at diagnosis for carriers was lower than non-carriers by approximately 20 years (27.2±11.1 vs. 46.6±19.6 years; p = 0.022). The mutation carriers showed a higher mPAP than the non-carriers (63.7±17.3 vs. 50.5±15.2 mmHg; p < 0.05). Additionally, the PAWP in the carriers was significantly lower than in the non-carriers (6.8±2.2 vs. 10.8±3.5 mmHg; p = 0.020). Other hemodynamic profiles including PVR and cardiac output showed no significant differences between the two groups. The proportion of patients on PAH targeted therapy, including prostaglandin I2 (PGI2), phosphodiesterase-5 inhibitors, endothelin receptor antagonists, and tyrosine kinase inhibitors were similar between the two groups (Table 1). The median follow-up duration of the cohort was 1.5 years (IQR, 1.0 to 2.2) (Table 1). The median follow-up of the carrier and non-carrier groups was 1.0 and 1.3 years, respectively (p = 0.806). Zero carriers but 4 non-carriers died during follow-up (Table 1).
Table 1. Demographic and clinical characteristics of BMPR2 carriers and non-carriers.
| BMPR2 mutation | ||||
|---|---|---|---|---|
| Total (n = 73) | Carrier (n = 16) | Non-carrier (n = 57) |
P-value | |
| Demography | ||||
| Men, n (%) | 19 (26) | 4 (25) | 15 (26) | 0.857 |
| Age (y), mean±SD | 42.2±19.7 | 27.2±11.1 | 46.6±19.6 | 0.022* |
| Functional and hemodynamic status | ||||
| NYHA functional class ≥II, n (%) | 52 (71) | 10 (63) | 42 (74) | 0.270 |
| mPAP, mmHg | 53.3±16.5 | 63.7±17.3 | 50.5±15.2 | <0.001* |
| PAWP mmHg | 15.8±12.3 | 6.8±2.2 | 10.8±3.5 | 0.020* |
| PVR, mmHg/L/min | 10.2±8.9 | 11.5±9.6 | 9.9 ±8.8 | 0.722 |
| Cardiac output, L/min | 4.2±2.2 | 3.7±1.2 | 4.3±2.4 | 0.092 |
| PAH targeted therapy | ||||
| Prostaglandin I2, n (%) | 20 (27) | 2 (13) | 18 (32) | 0.391 |
| PDE5i, n (%) | 14 (19) | 3 (19) | 11 (19) | 0.854 |
| ERA, n (%) | 38 (52) | 10 (63) | 28 (49) | 0.649 |
| TKI, n (%) | 2 (3) | 0 (0) | 2 (4) | 0.587 |
| Follow-up data | ||||
| Median follow-up years (IQR)† | 1.5 (1.0–2.2) | 1.0 (1.0–2.1) | 1.3 (0.9–2.3) | N/A |
| Death, n (%) | 4 (5) | 0 (0) | 4 (7) | N/A |
Abbreviations: BMPR2, bone morphogenetic protein receptor type 2; SD, standard deviation; NYHA, New York Heart Association; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance; PDE5i, phosphodiesterase-5 inhibitors; ERA, Endothelin receptor antagonists; TKI, tyrosine kinase inhibitors. IQR, interquartile range
*P < 0.05
#Mann-Whitney test
† Median follow-up information was gathered among survivors only
A total of 33 lineal family members of the 16 IPAH BMPR2 mutation-positive patients agreed to undergo screening examinations (Fig 1). One to five members in addition to the proband of each family were evaluated. Seven families had at least 1-member tested positive for the BMPR2 mutation (S3 Table). Penetrance of the BMPR2 mutation was not evaluated due to the lack of information regarding the clinical symptoms of each family member.
BMPR2 mutations
We found BMPR2 mutations in 21.9% (16/73) of Korean individuals with IPAH (Table 2). Among the 16 mutations, 6 (37.5%) were nonsense, 5 (31.3%) were deletion, 2 (12.5%) were splice-site, and 3 (18.8%) were missense types. Six (37.5%) mutation types were previously reported [7, 22–24] and 2 nonsense mutations were identified in 2 different individuals. Of the 9 novel mutation types identified in the current study, 5 were deletion, 2 were splice site, one was nonsense, and one was missense (Table 2).
Table 2. Description of BMPR2 mutation-positive genes.
| Patient | Classification | Location | Mutation type | Nucleotide change | Protein change | Reference Number |
|---|---|---|---|---|---|---|
| 1 | idiopathic | exon 6 | nonsense | c.631C>T | p.Arg211X | [10] |
| 2 | idiopathic | exon 8 | nonsense | c.994C>T | p.Arg332X | [10] |
| 3 | idiopathic | exon 12 | nonsense | c.2695C>T | p.Arg899X | [4] |
| 4 | idiopathic | 5’UTR-exon1 | deletion | c.1-1_8del | p.? | novel |
| 5 | idiopathic | exon 9 | missense | c.1258T>C | p.Cys420Arg | [26] |
| 6 | heritable | exon 4 | deletion | c.451del | p.Ile151fs | novel |
| 7 | idiopathic | exon 8 | deletion | c.1042-1047del | p.Val348_Ile349del | novel |
| 8 | heritable | intron 5 | splice site | c621+1G>T | p.? | novel |
| 9 | heritable | exon 8 | deletion | c.1028del | p.Asn343fs | novel |
| 10 | heritable | intron6 | splice site | c.853-2A>C | p.? | novel |
| 11 | heritable | exon12 | nonsense | c.2695C>T | p.Arg899X | [4] |
| 12 | heritable | exon11 | deletion | c.1448delGT | p.Cys483fs | novel |
| 13 | idiopathic | exon 6 | nonsense | c.846T>G | p.Tyr282X | novel |
| 14 | idiopathic | exon 9 | missense | c.1226T>C | p.Leu409Pro | novel |
| 15 | heritable | exon11 | missense | c.1471C>T | p.Arg491Trp | [28] |
| 16 | idiopathic | exon 8 | nonsense | c.994C>T | p.Arg332X | [10] |
Abbreviation: UTR, untranslated region
Subgroup analysis
Further stratified analysis was performed according to age and sex (Table 3). In patients under the age of 30, the proportion of BMPR2 mutation carriers were significantly higher than the non-carriers (43.8% vs. 15.7%; p<0.001). The mean age difference between the younger and older groups was approximately 30 years. All deaths occurred in the older group (Table 3). There were no significant differences in age, hemodynamic profiles, or death between the sexes (Table 3).
Table 3. Demographic characteristics, BMPR2 mutation status, hemodynamic profile, and clinical outcomes of subgroups.
| Age at diagnosis | |||
| < 30 (n = 16) | ≥ 30 (n = 57) | P-value | |
| Age (y), mean±SD | 20±7 | 50±17 | <0.001 |
| BMPR2 mutation carriers, n (%) | 7 (43.8) | 8 (14.0) | 0.035 |
| mPAP, mmHg | 64±15 | 50±16 | 0.003 |
| PAWP mmHg | 20±12 | 15±13 | 0.314 |
| PVR, mmHg/L/min | 12±8 | 10±9 | 0.433 |
| Cardiac output, L/min | 3.9±1.1 | 4.3±2.4 | 0.652 |
| Sex | |||
| Male (n = 19) | Female (n = 54) | P-value | |
| Age (y), mean±SD | 49±21 | 41±19 | 0.13 |
| BMPR2 mutation carriers, n (%) | 4 (21.0) | 11 (20.0) | 9.86 |
| mPAP, mmHg | 50±14 | 54±17 | 0.363 |
| PAWP mmHg | 15±10 | 16±13 | 0.882 |
| PVR, mmHg/L/min | 7.8±4.8 | 11.1±9.8 | 0.258 |
| Cardiac output, L/min | 5.1±2.4 | 3.9±2.1 | 0.093 |
Abbreviations: SD, standard deviation; BMPR2, bone morphogenetic protein receptor type 2; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVR, pulmonary vascular resistance
Discussion
According to the present study, the prevalence of BMPR2 mutations in Korean individuals with IPAH is 21.9%. In addition, Korean BMPR2 mutation carriers are younger and have poorer hemodynamic profiles at diagnosis than non-carriers. Survival analysis was not performed in the current study due to the lack of statistical power.
The discovery of the association between PAH and BMPR2 mutations has led to a better understanding of the pathobiology of PAH. BMPR2 mutations increase the susceptibility to apoptosis in the endothelial cells and promote the proliferation of pulmonary arterial vascular smooth muscle cells [25]. These changes in the vasculature are thought to accelerate the development of PAH [26]. Previous studies report that BMPR2 mutation carriers present at initial diagnosis at an earlier age and have more severe hemodynamic profile than non-carriers [14–16, 27]. The age of PAH diagnosis for the BMPR2 gene mutation carriers has been reported to be 10 years earlier than in non-carriers, with the mean age ranging between 28 and 38.5 years [7, 13, 14, 16, 17, 19, 22–24, 28]. However, the present study data show that the BMPR2 mutation carriers were diagnosed at the age of 27.9±11.4 years, which is approximately 10 years younger than the age of diagnosis in all international cohorts except for the Chinese [7, 13, 15–17, 19, 22, 24, 28, 29]. Thus, Korean BMPR2 mutation carriers were 20 years younger than their non-carrier counterparts.
Despite the age gap between the two groups, BMPR2 mutation carriers in the present study showed the highest gap of mPAP (13.2 mmHg) between carriers and non-carriers compared with other cohorts, including meta-analysis data, which only showed a 2 to 7 mmHg gap (Table 4) [7, 13, 15–17, 19, 22, 24, 28, 29]. Although the present study showed similar trends of higher mPAP at diagnosis in BMPR2 mutation carriers compared to non-carriers, the uniquely high mPAP warrants further investigation. These results also indicate the importance of early screening for BMPR2 for the initiation of aggressive treatment in patients to ensure better outcomes [10]. This has been previously suggested for the Japanese population which possesses similar genetic background [30].
Table 4. Comparison of clinical and hemodynamic features of BMPR2 mutation carriers and non-carriers.
| Country | PAH type | BMPR2 mutation | Male/Female | Age, y | mPAP, mmHg | PVRI, | CI, L/min/m2 | Familial cases, % | Reference |
|---|---|---|---|---|---|---|---|---|---|
| mmHg/L/min/m2 | |||||||||
| Korea | IPAH | Present (N = 16) | 1/3.0 | 27.2±11.1 | 63.7±17.3 | 10.1±2.4 | 2.1±0.4 | - | - |
| Absent (N = 57) | 1/2.8 | 46.6±19.6 | 50.5±15.2 | 8.3±1.1 | 2.3±0.4 | ||||
| Japan | IPAH and HPAH | Present (N = 26) | 1/4.8 | 35±13 | 57.7±14.5 | 19.1±7.3* | 1.9±0.5 | - | Isobe [28] |
| Absent (N = 36) | 1/1.8 | 34±11 | 55.7±15.1 | 16.9±9.0* | 2.2±1.0 | ||||
| Japan | IPAH and HPAH | Present (N = 18) | 1/2.2 | 37.4±12.7 | 60.8±15.4 | 21.5±9.4 | - | 18 | Kabata [19] |
| Absent (N = 31) | 1/1.7 | 25.9±11.3 | 58.8±12.0 | 18.6±8.6 | - | ||||
| China | IPAH and HPAH | Present (N = 50) | 1/1.3 | 28±2 | 67±6 | 17.1±2* | 2.0±0.2 | - | Liu [22] |
| Absent (N = 255) | 1/2.9 | 32±4 | 60±6 | 14.6±2* | 2.3±0.2 | ||||
| China | IPAH and HPAH | Present (N = 37) | 1/2.2 | 27.2±9.9 | 60.2±15.3 | 17.3±8.0 | 2.6±0.9 | 6 | Yang [24] |
| Absent (N = 154) | 1/1.7 | 31.6±10.5 | 54.9±14.7 | 13.0±5.2 | 3.0±0.9 | ||||
| US | IPAH and HPAH | Present (N = 41) | 1/2.2 | 36.1±1.4 | 58.6±1.7 | 18.1±2.0* | 1.9±0.1 | 74 | Austin [13] |
| Absent (N = 106) | 1/3.1 | 42±2.3 | 58.3±1.7 | 14.0±0.8* | 1.8±0.2 | ||||
| US | IPAH and HPAH | Present (N = 27) | 1/1.0 | 37.1±12.0 | 60.7±10.5 | 14.6±5.1 | 2.1±0.6 | 18 | Elliott [17] |
| Absent (N = 40) | 1/6.0 | 38.2±10.9 | 56.9±11.3 | 12.3±6.1 | 2.3±0.7 | ||||
| US | IPAH and HPAH | Present (N = 23) | - | - | 61±13 | 26±14 | 2.0±1.1 | 22 | Rosenzweig [16] |
| Absent (N = 124) | - | - | 59±20 | 21±14 | 2.4±1.5 | ||||
| France | IPAH and HPAH | Present (N = 68) | 1/2.0 | 36.5±14.5 | 64±13 | 17.4±6.1 | 2.1±0.7 | 16 | Sztrymf [15] |
| Absent (N = 155) | 1/2.6 | 46.0±16.1 | 56±13 | 12.7±6.6 | 2.5±0.7 | ||||
| Germany | IPAH and HPAH | Present (N = 49) | 1/1.9 | 38.5±11.8 | 62.6±9.9 | 28.8 ±9.6 | 1.7±0.3 | 10 | Pfarr [14] |
| Absent (N = 179) | 1/3.0 | 45.8±11.3 | 53.4±12.2 | 18.8±8.4 | 2.1±0.5 | ||||
| Meta-analysis | IPAH, HPAH, and anorexigen | Present (N = 448) | 1/3.2 | 35±15 | 60.5±13.8 | 16.6±8.3* | 2.1±0.7 | 15 | Evans [7] |
| Absent (N = 1102) | 1/3.6 | 42±18 | 56.4±15.3 | 12.9±8.3* | 2.5±0.9 |
Abbreviations: BMPR2, bone morphogenetic protein receptor type 2; mPAP, mean pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; PVRI, pulmonary vascular resistance index; CI, confidence interval; IPAH, idiopathic pulmonary arterial hypertension; HPAH, heritable pulmonary arterial hypertension
*Pulmonary vascular resistance is presented due to the lack of data regarding pulmonary vascular resistance index (units: mmHg/L/min)
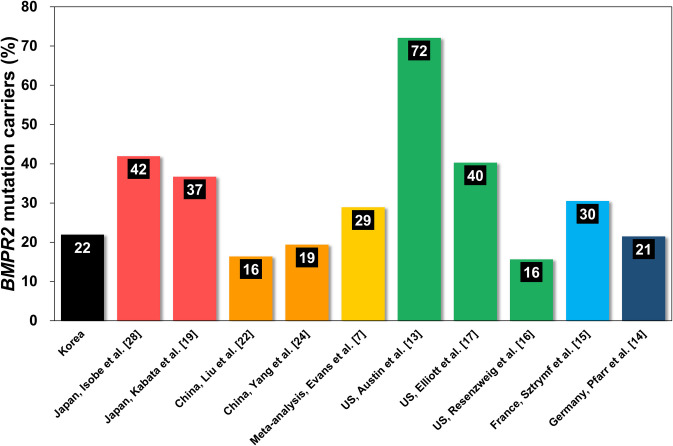
The prevalence of BMPR2 mutations and the differences between characteristics of BMPR2 carriers and non-carriers in the present study were similar to most cohorts in the literature, representing the United States (US), France, Germany, China, and Japan [7, 14–17, 19, 22, 24, 28], except for a single cohort from the US which mainly recruited familial cases (125 out of 169, 74%), as shown in Table 4 and Fig 2 [13]. All other cohorts enrolled familial cases to a similar degree (6 to 22%). The overall prevalence of BMPR2 mutations in Korean IPAH patients was 22%, which is slightly higher than observed in Chinese and lower than in Japanese cohorts (Fig 2) [19, 22, 24, 28]. Data from Asian populations are limited, and the prevalence of BMPR2 mutation differs between Japanese (36–41%) and Chinese (16–29%) studies (Fig 2) [19, 25, 27, 29]. This may be partly explained by the Chinese cohort having a relatively low percentage of familial cases (6%) compared with the Japanese (18%) [19, 24], considering that familial cases of PAH have a much higher prevalence of mutation carriers (70%) than sporadic cases (20%) [31]. It may also be suggested that the higher prevalence of BMPR2 mutation carriers in Japanese cohorts may be due to the underestimation of familial cases because Asian patients are generally reluctant to share information about inherited diseases because they are considered a source of shame.
Fig 2. The worldwide prevalence between BMPR2 mutation carriers and non-carriers in patients with idiopathic or heritable pulmonary arterial hypertension.
The overall prevalence of BMPR2 mutation carriers in IPAH patients in Korea was 22%, which is higher than in China and lower than in Japan. BMPR2 = bone morphogenetic protein receptor type 2.
The percentage of patients carrying BMPR2 mutations was significantly higher in patients younger than 30, compared with patients aged 30 or higher. This was consistent with the meta-analysis data, suggesting that BMPR2 mutation may play a crucial role in the early development of PAH and severe hemodynamic symptoms [7]. Our study also shows that PAH patients were predominantly women (male:female, 1:2.8), although there was no difference in the proportion of BMPR2 mutation carriers, hemodynamic profiles, or death according to sex [32].
The influence of BMPR2 mutations on clinical outcomes has been a topic of controversy. Earlier studies reported that BMPR2 mutation status does not affect overall survival or risk for lung transplantation [33]. A Japanese study found that the overall survival of BMPR2 mutation carriers may be better than non-carriers due to ethnicity or the introduction of PGI2 infusion therapy [28]. On the other hand, a study with a Chinese cohort demonstrated that BMPR2 mutation carriers had a significantly poorer survival, although the use of PGI2 or other medication was not specified [22]. According to a recent meta-analysis, mutation carriers had poorer outcomes especially in younger patients; however, information regarding comorbidities or PAH-targeted medication was not investigated [7]. Although we did not include a survival analysis in the present study due to the lack of statistical power, the BMPR2 mutation carrier group had a higher percentage of deaths compared with the non-carrier group. It is most likely that this is by chance considering the small sample size and short follow-up. However, the trend is consistent with Japanese studies [10] that share a common east Asian heritage [34]. Also, the BMPR2 mutation carriers in the current study were approximately 8 years younger than those in the meta-analysis [7].
The main limitation of this study was the small sample size and lack of information regarding family history. All 11 centers experienced difficulties enrolling patients, as the majority of the patients and family members were reluctant to share their genetic information and symptoms, which can be used against them in Korean society. In Asian societies, sharing information regarding heritable diseases is taboo because it is generally perceived as a shame. Therefore, we were not able to gather hemodynamic profiles or data on symptoms from all family members, hampering the effort to include individuals with heritable PAH. The Genetic Information Nondiscrimination Act (GINA) is a law that protects individuals from being discriminated against by insurance companies or employers after participating in research or genetic testing [35]. As Korean society has no protective measures against genetic discrimination, such as the GINA in the US, fear of being discriminated against dissuaded many patients from participating in this study. Legislative initiatives for genetic nondiscrimination are necessary for the continuation of genetic research in Korea. Another limitation of the study was that the phenotypic expression of each genetic mutation was not investigated. Future studies involving multi-omics data and deep phenotyping in Korean individuals with PAH are warranted [11].
Conclusions
In the PILGRIM study, the BMPR2 mutation carriers were 20 years younger and had higher mPAP than the non-carriers. Despite these discrepancies in baseline characteristics upon initial diagnosis, there was no statistical difference in all-cause mortality among the two groups.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by grants by Bayer & Co (https://www.bayer.co.kr/, Grant number: GCU-2010-2278) and partially supported by the Research of Korea Centers for Disease Control and Prevention (http://www.cdc.go.kr/, Grant number: 2018-ER6304-00 and 2018-ER6304-01) and the Korean Society of Cardiology (https://www.circulation.or.kr:4443/eng/, Grant number: KSC 200403-17). Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
References
- 1.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Survival in patients with idiopathic, familial, and anorexigen-associated pulmonary arterial hypertension in the modern management era. Circulation. 2010;122(2): 156–163. 10.1161/CIRCULATIONAHA.109.911818 [DOI] [PubMed] [Google Scholar]
- 2.Chung WJ, Park YB, Jeon CH, Jung JW, Ko KP, Choi SJ, et al. Baseline characteristics of the Korean Registry of Pulmonary Arterial Hypertension. J Korean Med Sci. 2015;30(10): 1429–1438. 10.3346/jkms.2015.30.10.1429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrell NW, Aldred MA, Chung WK, Elliott CG, Nichols WC, Soubrier F, et al. Genetics and genomics of pulmonary arterial hypertension. Eur Respir J. 2019;53(1): 1801899 10.1183/13993003.01899-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1): 1801913 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyazono K, Maeda S, Imamura T. BMP receptor signaling: transcriptional targets, regulation of signals, and signaling cross-talk. Cytokine Growth F R. 2005;16(3): 251–263. 10.1016/j.cytogfr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- 6.Morrell NW. Pulmonary hypertension due to BMPR2 mutation: a new paradigm for tissue remodeling? Proc Am Thorac Soc. 2006;3(8): 680–686. 10.1513/pats.200605-118SF [DOI] [PubMed] [Google Scholar]
- 7.Evans JD, Girerd B, Montani D, Wang XJ, Galie N, Austin ED, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4(2): 129–137. 10.1016/S2213-2600(15)00544-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morisaki H, Nakanishi N, Kyotani S, Takashima A, Tomoike H, Morisaki T. BMPR2 mutations found in Japanese patients with familial and sporadic primary pulmonary hypertension. Hum Mutat. 2004;23(6): 632 10.1002/humu.9251 [DOI] [PubMed] [Google Scholar]
- 9.Thomson JR, Machado RD, Pauciulo MW, Morgan NV, Humbert M, Elliott GC, et al. Sporadic primary pulmonary hypertension is associated with germline mutations of the gene encoding BMPR-II, a receptor member of the TGF-beta family. J Med Genet. 2000;37(10): 741–745. 10.1136/jmg.37.10.741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang AY, Chung WJ. Current status of pulmonary arterial hypertension in Korea. Korean J Intern Med. 2019;34(4): 696–707. 10.3904/kjim.2019.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jang AY, Kim S, Park SJ, Choi H, Oh PC, Oh S, et al. A Nationwide multicenter registry and biobank program for deep phenotyping of idiopathic and hereditary pulmonary arterial hypertension in Korea: the PAH platform for deep phenotyping in Korean subjects (PHOENIKS) cohort. Clin Hypertens. 2019;25: 21 10.1186/s40885-019-0126-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Savale L, Guignabert C, Weatherald J, Humbert M. Precision medicine and personalising therapy in pulmonary hypertension: seeing the light from the dawn of a new era. Eur Respir Rev. 2018;27(148). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin ED, Phillips JA, Cogan JD, Hamid R, Yu C, Stanton KC, et al. Truncating and missense BMPR2 mutations differentially affect the severity of heritable pulmonary arterial hypertension. Respir Res. 2009;10: 87 10.1186/1465-9921-10-87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfarr N, Szamalek-Hoegel J, Fischer C, Hinderhofer K, Nagel C, Ehlken N, et al. Hemodynamic and clinical onset in patients with hereditary pulmonary arterial hypertension and BMPR2 mutations. Respir Res. 2011;12: 99 10.1186/1465-9921-12-99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sztrymf B, Coulet F, Girerd B, Yaici A, Jais X, Sitbon O, et al. Clinical outcomes of pulmonary arterial hypertension in carriers of BMPR2 mutation. Am J Respir Crit Care Med. 2008;177(12): 1377–1383. 10.1164/rccm.200712-1807OC [DOI] [PubMed] [Google Scholar]
- 16.Rosenzweig EB, Morse JH, Knowles JA, Chada KK, Khan AM, Roberts KE, et al. Clinical implications of determining BMPR2 mutation status in a large cohort of children and adults with pulmonary arterial hypertension. J Heart Lung Transplant. 2008;27(6): 668–674. 10.1016/j.healun.2008.02.009 [DOI] [PubMed] [Google Scholar]
- 17.Elliott CG, Glissmeyer EW, Havlena GT, Carlquist J, McKinney JT, Rich S, et al. Relationship of BMPR2 mutations to vasoreactivity in pulmonary arterial hypertension. Circulation. 2006;113(21): 2509–2515. 10.1161/CIRCULATIONAHA.105.601930 [DOI] [PubMed] [Google Scholar]
- 18.Humbert M, Sitbon O, Chaouat A, Bertocchi M, Habib G, Gressin V, et al. Pulmonary arterial hypertension in France: results from a national registry. Am J Respir Crit Care Med. 2006;173(9): 1023–1030. 10.1164/rccm.200510-1668OC [DOI] [PubMed] [Google Scholar]
- 19.Kabata H, Satoh T, Kataoka M, Tamura Y, Ono T, Yamamoto M, et al. Bone morphogenetic protein receptor type 2 mutations, clinical phenotypes and outcomes of Japanese patients with sporadic or familial pulmonary hypertension. Respirology. 2013;18(7): 1076–1082. 10.1111/resp.12117 [DOI] [PubMed] [Google Scholar]
- 20.Ahn KJ, Jang AY, Park SJ, Chung WJ. 15 years journey of idiopathic pulmonary arterial hypertension with BMPR2 mutation. Clin Hypertens. 2019;25: 22 10.1186/s40885-019-0127-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaine SP, Rubin LJ. Primary pulmonary hypertension. The Lancet. 1998;352(9129): 719–725. 10.1016/s0140-6736(98)02111-4 [DOI] [PubMed] [Google Scholar]
- 22.Liu D, Wu WH, Mao YM, Yuan P, Zhang R, Ju FL, et al. BMPR2 mutations influence phenotype more obviously in male patients with pulmonary arterial hypertension. Circ Cardiovasc Genet. 2012;5(5): 511–518. 10.1161/CIRCGENETICS.111.962209 [DOI] [PubMed] [Google Scholar]
- 23.Sztrymf B, Francoual J, Sitbon O, Labrune P, Jambou M, Pous C, et al. Clinical, haemodynamic and genetic features of familial pulmonary arterial hypertension. Rev Mal Respir. 2004;21(5 Pt 1): 909–915. 10.1016/s0761-8425(04)71472-2 . [DOI] [PubMed] [Google Scholar]
- 24.Yang H, Zeng Q, Ma Y, Liu B, Chen Q, Li W, et al. Genetic analyses in a cohort of 191 pulmonary arterial hypertension patients. Respir Res. 2018;19(1). 10.1186/s12931-018-0789-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Roman-Campos D, Austin ED, Eyries M, Sampson KS, Soubrier F, et al. A novel channelopathy in pulmonary arterial hypertension. N Engl J Med. 2013;369(4):351–61. 10.1056/NEJMoa1211097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res. 2014;115(1): 165–175. 10.1161/CIRCRESAHA.113.301141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Humbert M, Sitbon O, Yaici A, Montani D, O'Callaghan DS, Jais X, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J. 2010;36(3): 549–555. 10.1183/09031936.00057010 [DOI] [PubMed] [Google Scholar]
- 28.Isobe S, Kataoka M, Aimi Y, Gamou S, Satoh T, Fukuda K. Improved survival of patients with pulmonary arterial hypertension with BMPR2 mutations in the last decade. Am J Respir Crit Care Med. 2016;193(11): 1310–1314. 10.1164/rccm.201601-0158LE [DOI] [PubMed] [Google Scholar]
- 29.Pfarr N, Fischer C, Ehlken N, Becker-Grunig T, Lopez-Gonzalez V, Gorenflo M, et al. Hemodynamic and genetic analysis in children with idiopathic, heritable, and congenital heart disease associated pulmonary arterial hypertension. Respir Res. 2013;14:3 10.1186/1465-9921-14-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tokunaga N, Ogawa A, Ito H, Matsubara H. Rapid and high-dose titration of epoprostenol improves pulmonary hemodynamics and clinical outcomes in patients with idiopathic and heritable pulmonary arterial hypertension. J Cardiol. 2016;68(6): 542–547. 10.1016/j.jjcc.2015.11.012 [DOI] [PubMed] [Google Scholar]
- 31.Machado RD, Eickelberg O, Elliott CG, Geraci MW, Hanaoka M, Loyd JE, et al. Genetics and genomics of pulmonary arterial hypertension. J Am Coll Cardiol. 2009;54(1 Suppl): S32–42. 10.1016/j.jacc.2009.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ge X, Zhu T, Zhang X, Liu Y, Wang Y, Zhang W. Gender differences in pulmonary arterial hypertension patients with BMPR2 mutation: a meta-analysis. Respir Res. 2020;21(1): 44 10.1186/s12931-020-1309-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Girerd B, Montani D, Eyries M, Yaici A, Sztrymf B, Coulet F, et al. Absence of influence of gender and BMPR2 mutation type on clinical phenotypes of pulmonary arterial hypertension. Respir Res. 2010;11(1): 73 10.1186/1465-9921-11-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cai Z, Camp NJ, Cannon-Albright L, Thomas A. Identification of regions of positive selection using Shared Genomic Segment analysis. Eur J Hum Genet. 2011;19(6):667–71. 10.1038/ejhg.2010.257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Equal Employment Opportunity C. Genetic Information Nondiscrimination Act. Final rule. Fed Regist. 2016;81(95): 31143–59. . [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.