Abstract
Chronic wounds represent a growing clinical problem for which limited treatment strategies exist. Defects in immune cell-mediated healing play an important role in chronic wound development, presenting an attractive clinical target in the treatment of chronic wounds. However, efforts to improve healing through the application of growth factors and cytokines have been limited by the rapid degradation and diffusion of these molecules in the wound environment. In this study we sought to overcome the challenge of rapid diffusion through the development of a hydrogel delivery system in which protein cargo can be released into the wound environment at a constant and tunable rate. This system was used to deliver the intercellular adhesion molecule-1 (ICAM-1) in order to target endogenous cells upstream of growth factor and cytokine production and circumvent the issue of their rapid degradation. We demonstrated that our delivery system was able to release cargo at different and highly controllable rates and thereby improved cargo retention in the wound environment. Additionally, treatment with ICAM-1 in the delivery system improved healing in both ICAM-1-deficient mice and an aged mouse model of delayed healing, highlighting a potential clinical benefit for this protein in the treatment of chronic wounds.
INTRODUCTION
Chronic wounds are estimated to affect 6.5 million patients in the US each year,1 a number that is expected to rise with increasing rates of diabetes and an aging population.2 These wounds are typically complex with a high associated cost of treatment.1, 3–4 A wide variety of treatment approaches have been developed including vacuum assisted closure, skin substitutes, and bioactive dressings.5–6 However, a large proportion of patients still fail to respond to treatment7 and amputations are a relatively common outcome.3 Additional approaches are urgently needed to more effectively treat this rising clinical group.
Normal wound healing proceeds through an ordered series of events that begins with an initial inflammatory phase, followed by new tissue formation and eventual remodeling.8–9 Immune cells perform critical roles at each of these stages, clearing infection, removing cellular debris, and producing a variety of cytokines and growth factors in coordinated waves that are important for the healing process.10–13 The immune-mediated wound response spans the innate and adaptive branches of the immune system with neutrophils,14–15 macrophages,16–17 and T cells18–20 all critical for efficient healing. The key role of the immune system in wound healing makes it an attractive target for wound treatment strategies. However, previous trials administering immune cell-derived cytokines and growth factors into chronic wounds have shown variable results.12, 21–22 Currently, platelet derived growth factor (PDGF)23 is the only growth factor approved for use in wound treatment in the US, and no cytokine-based therapies have been approved.12
Less focus has been devoted to targets upstream of the immune cells that are present in the wound environment. Activating these immune cells in vivo has the potential to initiate the sustained production of a variety of cytokines and growth factors that together could be more effective than the application of individual proteins. Indeed, a number of cell-based wound dressings including amniotic and placental membranes, human skin allografts, and human skin substitutes have shown promising results in clinical trials.6, 24 These successes highlight the benefits of cell-mediated production of growth factors and cytokines directly in the chronic wound environment. However, cell-based therapies come with a high cost24 and the risk of an alloreactive response25 that could be avoided by directly targeting immune cells in situ with immune-stimulatory compounds.
A significant challenge in administering soluble molecules into wounds is the rapid diffusion that occurs from the site of application in a moist environment. In order to overcome this barrier, we have developed a system to deliver protein cargo from a degradable hydrogel. Hydrogels have gained increasing attention in the treatment of chronic wounds due to their low toxicity and immunogenicity, high water content, and potential to release drugs in a variety of ways.26–29 Here we employ gels based on polyethylene glycol (PEG) and dextran using thiol adducts of oxanorbornadiene dicarboxylates (ONDs), which degrade at different rates based on their chemical structures rather than by reaction with an external reagent such as acid or base.30 This setup allows for control of the release of cargo proteins contained in the gel material over a wide range of rates. This study represents the first time these materials have been used for protein delivery in vivo.
We wished to explore the focused delivery of intercellular adhesion molecule-1 (ICAM-1) to the wound environment. ICAM-1 is a cell adhesion molecule expressed on endothelial and epithelial cells that is critical for efficient neutrophil and macrophage mediated wound healing.16, 31 Mice devoid of ICAM-1 are known to have delayed healing32–34 and so were chosen as an initial model for testing in vivo delivery of wound-modulatory proteins in the hydrogel delivery system. Additionally, our delivery system was tested in an aged mouse model since defective ICAM-1 upregulation has been reported in the wounds of elderly individuals.35
RESULTS AND DISCUSSION
Hydrogel synthesis and characterization
The fundamental chemistry of the OND linkage is shown in Figure 1a, featuring a fast conjugate addition of thiol (or thiolate) to the reactive double bond of the OND electrophile, followed by first-order retro-Diels-Alder fragmentation.36–37 We have previously described fast-setting hydrogels from multi-armed PEG-thiols and PEG-OND reagents, which undergo programmed decomposition at rates determined by the nature of the oxanorbornadiene linkage.30 For the purposes of this application, we required hydrogels with smaller average pore dimensions so as to retain unmodified ICAM-1 in the gel matrix, to be released upon degradation of the gel network. We therefore turned to dextran, a well-accepted biopolymer for a wide variety of biocompatible hydrogels.
Figure 1.
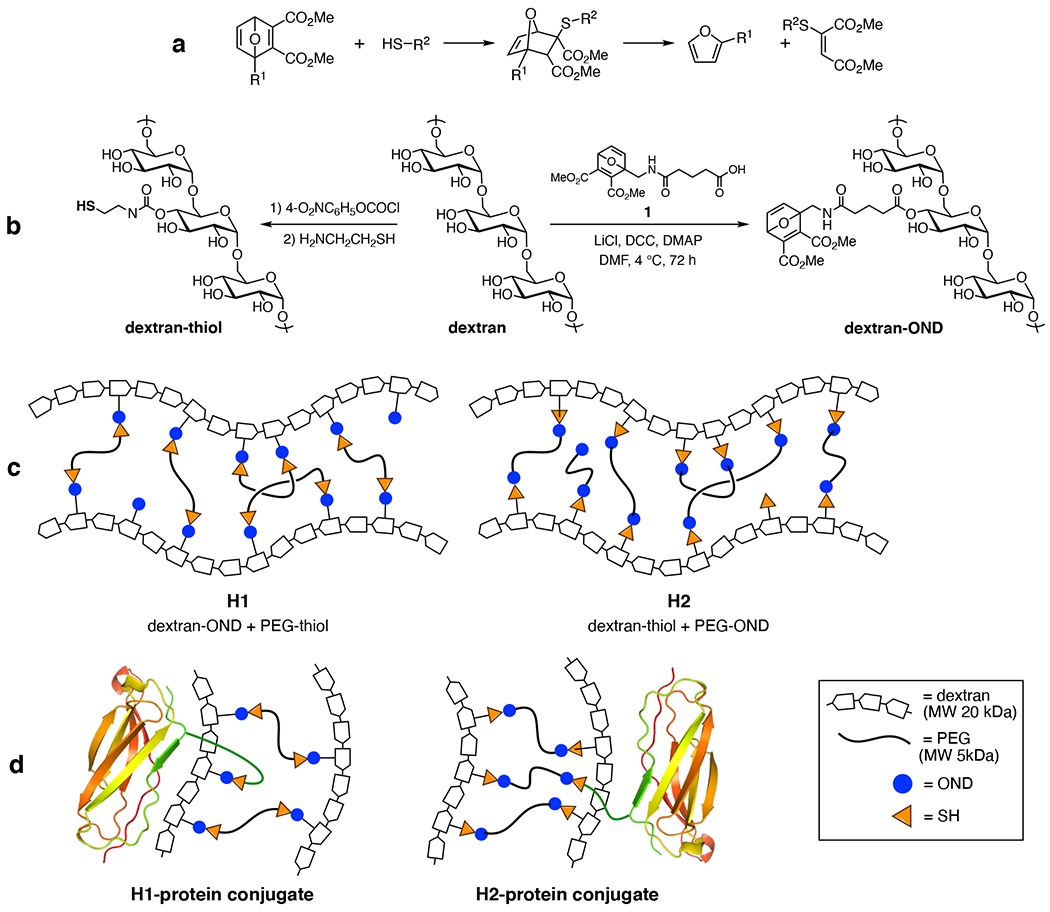
Oxanorbornadiene-based hydrogel components and construction. (a) Conjugate addition and retro-Diels-Alder cleavage of the OND moiety. (b) Functionalization of 20-kDa dextran with both nucleophilic (thiol) and electrophilic (OND) moieties. Note that these functional groups are likely to be attached to a mixture of hydroxyl positions on the glycan rings; only 4-OH modification is shown for clarity. (c) Hydrogel compositions described here. (d) Covalent attachment of protein-thiol to hydrogel networks via OND linkages.
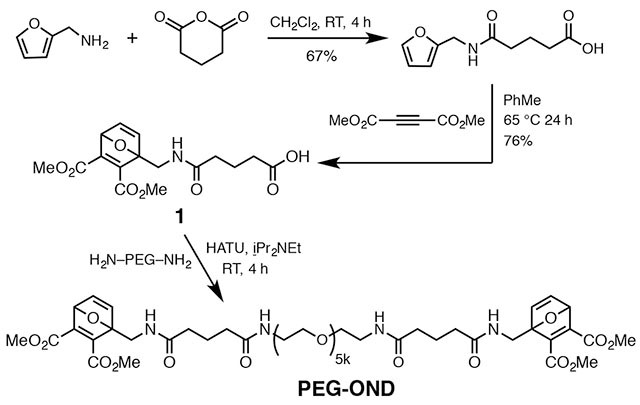
Commercial dextran (average molecular weight = 20 kDa) was treated to append thiol groups to approximately 20% of its hydroxyls, according to a published procedure (Figure 1b).38 Similarly, DCC/DMAP-based Steglich reaction of the same starting dextran with OND derivative 1 (Figure 1b) converted approximately 25% of the hydroxyl groups to ester linkages bearing an OND electrophile. A similar procedure was used to cap diaminopoly(ethylene oxide) (PEG diamine, MW 5000 kDa) with 1 to provide the reagent designated PEG-OND.
An extensive exploration of gelation rates and properties vs. composition was performed and will be described elsewhere. In the present case, we chose two combinations involving dextran and PEG (Figure 1c) which gave a suitable range of properties, swapping which polymer bore the thiol and OND groups. For this proof-of-concept study, we used a readily-available OND derived from furfurylamine and dimethylacetylene dicarboxylate, with a substitution pattern previously reported to give rise to a retro-Diels-Alder half-life of 9 hours at 37°C.39 The synthetic methods illustrated in Figure 1b show that dextran can be conveniently functionalized with either the nucleophilic or electrophilic components. We did not expect the two combinations to behave exactly the same, due to the somewhat different density of functionalization on the two dextran components which would be expected to provide for differences in the extent of crosslinking, a factor to which the overall performance of hyperbranched gels is highly sensitive. In addition, thiol nucleophile or OND electrophile reactivity can be affected to a significant degree by the scaffold to which these reactive groups are attached.39
As shown in Figure 1c, 10 wt% gels were made in dilute potassium phosphate buffer as a generally biocompatible medium at rates dependent on pH (Figure 2). Gelation times could be tuned by variation of pH (faster at higher pH because of the resulting increase in concentration of the deprotonated thiol nucleophile) or buffer concentration (faster at higher concentration, as noted previously for thiol-maleimide hydrogels).40 Thus, hydrogels prepared in pH 7-8 buffers all took less than 20 seconds to gel, whereas most hydrogels prepared at pH 5.5-7 required as long as 10 minutes to form self-supporting gels. A gelation time of approximately 2 minutes was judged to be ideal so as to allow time for a viscous mixture to be deposited by pipette before setting of the gel. While we focused here on 10 wt% gels, gelation could be achieved at polymer content as low as 2.5 wt%. When prepared in buffer containing dye-labeled BSA, approximately 80% of the added protein was found to be entrained in the interior of the gel, leaving approximately 20% that could be washed away immediately.
Figure 2.
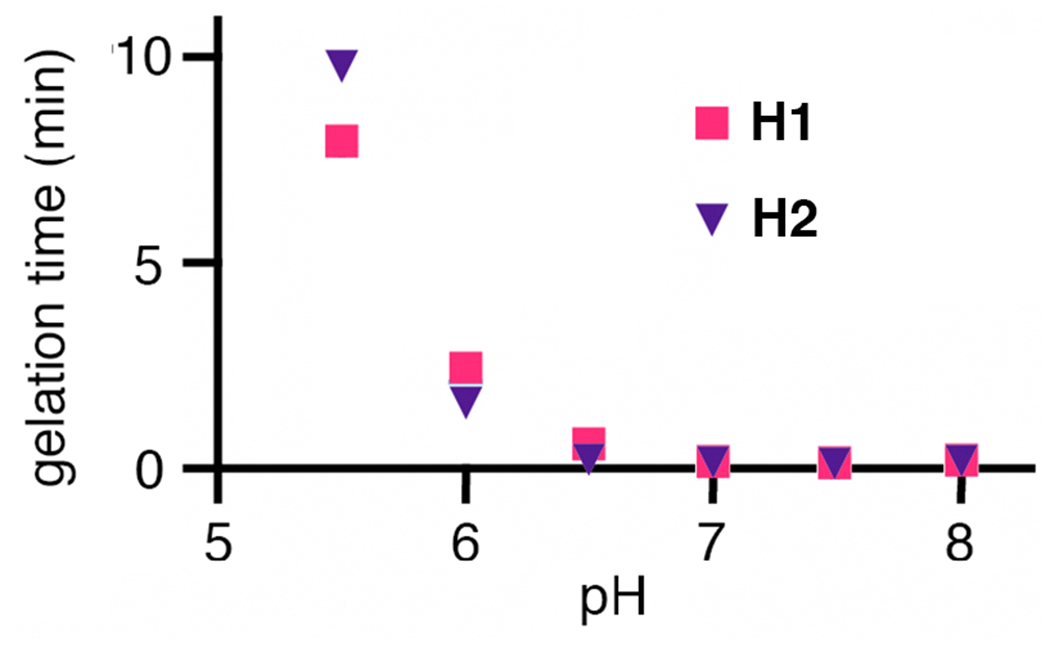
Dependence of pH on gelation time for hydrogel compositions noted in Figure 1b. Hydrogels were prepared by first dissolving the OND and thiol components separately in 0.001 M potassium phosphate buffer and then combining the two solutions in a 1:1 ratio of OND to thiol. Gelation was determined to be complete by the inversion test when the gel material was observed to be no longer flowing; the times reported were reproducible within ±10% for at least three replicate experiments.
Representative time-dependent rheological characterization of the H1 gel material is shown in Figure 3. Gelation occurs at the point at which G’ (storage modulus) becomes greater than G” (loss modulus), and the material rapidly reaches a stable solid-like form, as indicated by the relatively flat G’ and G” curves over time (Figure 3a). The gel retains significant deformability (Figure 3b) at this stage; continued storage and loss moduli measurements showed gel failure (transition to a flowing viscous liquid) at approximately 2.3 days, indicated by the decrease in G’ to values comparable or less than G” (Figure 3a). Note that release of entrained cargo is expected to occur before gel failure as the polymer network fragments and pore sizes increase. The swelling ratio of a hydrogel describes the weight increase of the hydrogel due to water absorption, and is usually inversely dependent on the number of crosslinks within the hydrogel network: the more crosslinks, the less the swelling. If swelling ratios are very large (typical values for hydrogels are in the tens to hundreds41–43) then release of entrained and immobilized cargo is less dependent on degradation of the hydrogel network and is instead more dependent on diffusion, which is known to give burst release profiles.44 Consequently, lower swelling ratios are desirable.45 The gel fraction of a hydrogel describes the degree to which the starting material was incorporated within the hydrogel network. The swelling ratios and gel fractions were found to be: H1 = swelling ratio 2.6, gel fraction 0.5; H2 = swelling ratio 1.7, gel fraction 0.4.
Figure 3.

Rheological characterization of hydrogel H1; time = 0 is the time of mixing of the components. (a) Time dependence of G’ and G” for hydrogel H1 assembled at pH 6; oscillatory rheology measurement at constant strain of 25% and an angular frequency of 1 rad/s. Approximate gelation and gel failure are indicated by the arrows. (b) Oscillatory strain sweep of hydrogel H1 at an angular frequency of 1 rad/s.
To demonstrate their degradability, gels H1 and H2 were prepared in the presence of Qβ virus-like particles, which are 28 nm in diameter and thus are relatively easy to trap compared to smaller proteins. In this case, the particles were engineered to contain approximately 10 copies of red fluorescent protein to make monitoring of release from the gel easy.46 As shown in Figure 4, the H2 hydrogel (OND-PEG and dextran-SH) underwent relatively rapid burst release, freeing all of the nanoparticle cargo within 12 hours. In contrast, hydrogel H1 exhibited a much more gradual release profile, requiring approximately 30 hours overall.
Figure 4.
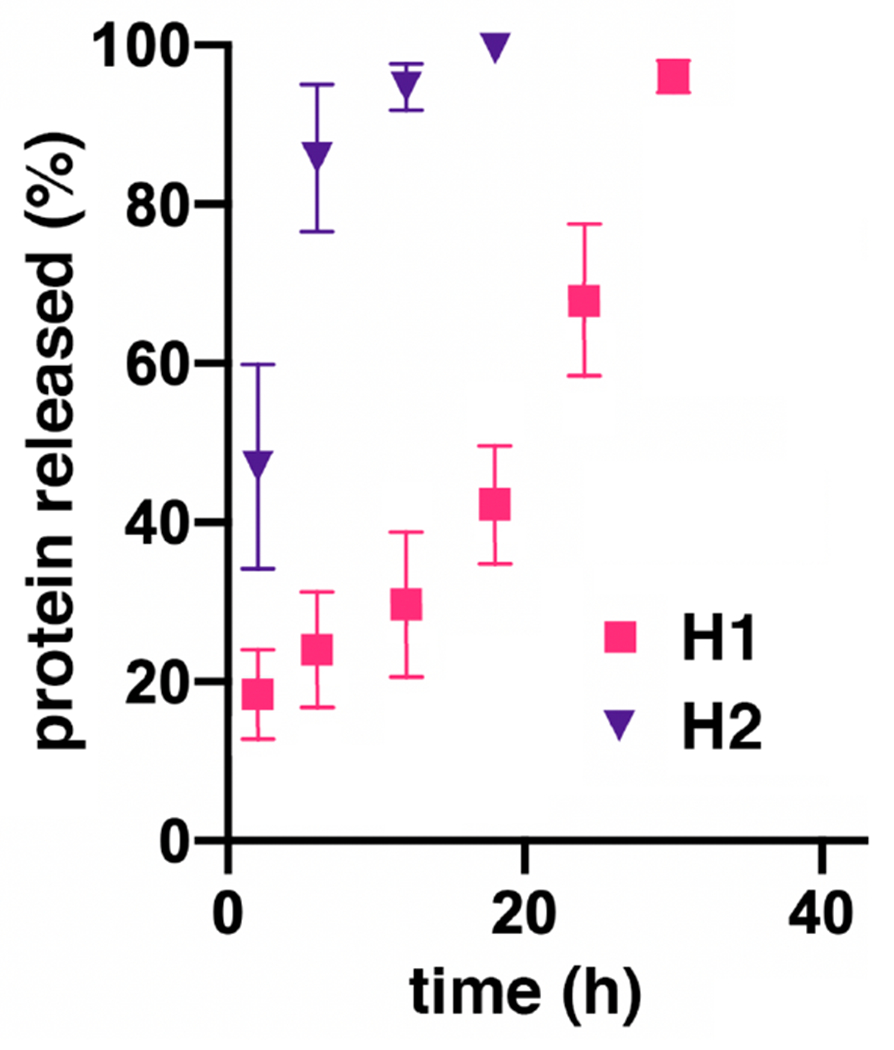
Release of entrained Qβ viruslike particles from methyl ester OND hydrogels. Experimental error is represented as the standard deviation from at least three replicate experiments.
Toxicity testing of hydrogel material
To test for toxicity in the wound environment, wounds were created in the backs of C57BL/6J mice using a 3mm biopsy punch. Hydrogel H2 containing no cargo protein was administered into these wounds, and tissue was collected from a 1mm margin around the wound edge following complete gel degradation at 24 hours. Epidermal cells were isolated from this tissue and stained with viability dye and annexin V to identify dead and dying cells. No difference in cell death was observed in epidermal cells surrounding wounds treated with hydrogel material compared to those treated with PBS (Figure 5a). Additionally, wound tissue embedded in paraffin and stained with hematoxylin and eosin showed no changes in morphology or leukocyte infiltration (Figure 5b). Taken together these results suggest that the hydrogel material itself does not affect cell survival or inflammation in the wound environment. This is consistent with previous reports for synthetic hydrogels, which are typically well tolerated.5, 29 Indeed, hydrogels based on natural products such as dextran, collagen, chitosan, and alginate have received special attention for wound care due to their natural promotion of wound healing.26–27, 29 However, in order for our material to be used as an effective protein delivery platform, proteins must be released from the material in such a way that they are retained in the wound environment long enough to have a biological effect.
Figure 5.
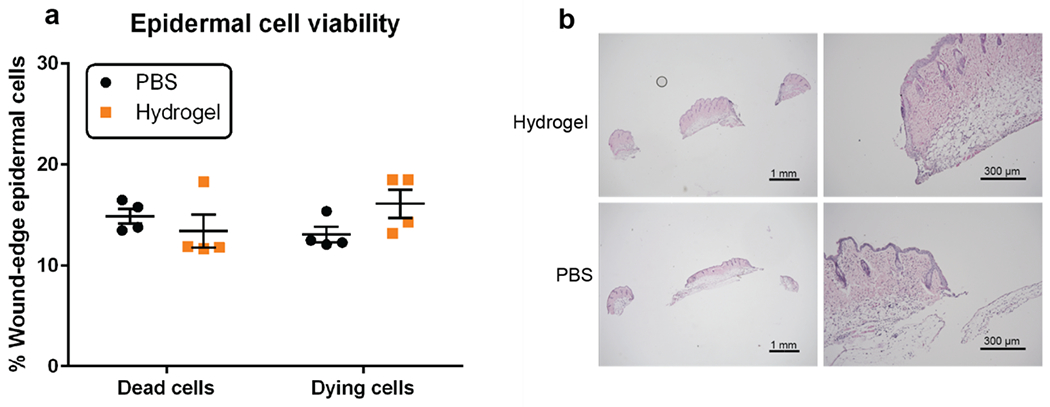
Hydrogel effects on cells in the wound environment. (a) Percent of dead and dying epidermal cells at the wound edge in hydrogel H2-treated and PBS buffer-treated mice. (b) Hematoxylin and eosin staining of tissue 24 hours after hydrogel and buffer treatment; no significant differences were noted.
Analysis of protein retention in the wound environment following hydrogel delivery
The retention of protein cargo in the wound environment was initially tested with bovine serum albumin (BSA, molecular weight 66 kDa) covalently labeled with Texas Red dye, regarded as a suitable model for the similarly-sized functional cargo. We tested two methods of retention in the hydrogel: covalent attachment of a protein thiol to OND residues displayed on either the dextran (H1) or PEG (H2) components (illustrated in Figure 1d), and physical entrainment in the gel network. When BSA, which has one free thiol residue,39 was covalently anchored into the H1 hydrogel material, it was retained much better in the wound environment at 5 and 25 hours after application compared to application of the protein in buffer solution (Figure 6a). In this method, the protein cargo was connected to one of the hydrogel component polymers by a single degradable OND linkage. Release of this cargo therefore required both the fragmentation of that linkage (at a pre-programmed first-order rate) and the diffusion of the released material through the matrix,30 the porosity of which increases over time as the crosslinking OND-based linkages also undergo spontaneous retro-Diels-Alder cleavage.
Figure 6.
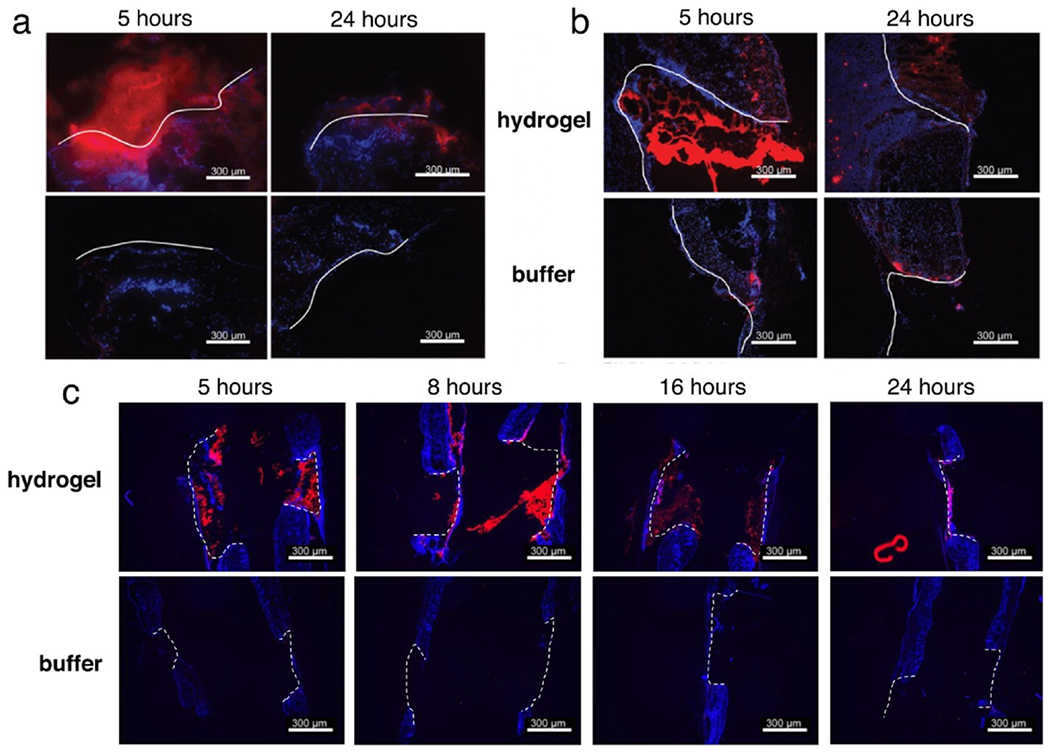
Protein retention in the wound environment following hydrogel delivery. (a) Covalent connection of Texas Red-labeled BSA to hydrogel H1. (b) Noncovalent entrainment of dye-labeled BSA within hydrogel H1. (c) Noncovalent entrainment of dye-labeled BSA within hydrogel H2. Scale bars = 300 μm, white lines = outlines of the wound bed, blue = DAPI, red = BSA-TxRed.
Very similar results were observed for the physically entrained material in H1 (Figure 6b), whereas BSA trapped hydrogel H2 was released faster, as expected (Figure 6c). Covalent anchoring would be required for the delivery of smaller cargo; indeed, the need for improved release mechanisms for such cases has been identified as an ongoing challenge.28, 47 Examples include the growth factors GM-CSF,48–49 TGFβ,50 and VEGF51 for which delayed release mechanisms have been shown to improve treatment effectiveness. However, the entrainment method is conceptually simpler, requiring only diffusion for the escape of the cargo. Since this method gave acceptable retention of the BSA model cargo in the wound environment, in vivo tests of the effectiveness of the similarly-sized ICAM-1-Fc cargo in wound healing assays were performed with this system.
Effects of hydrogel delivery of protein cargo on in vivo wound healing
While the immune system plays a central role in the wound healing response12, 52, the complexity of overlapping cellular mechanisms has hindered progress in targeting immune cells in the treatment of chronic wounds. A number of recent studies have worked to address this complexity through the concurrent application of multiple immune-stimulatory treatments.53–55 Here we took a simpler approach by targeting a molecular signal upstream of endogenous immune cells through the application of ICAM-1. ICAM-1 is expressed on keratinocytes, endothelial cells, and fibroblasts in the wound environment56–57 and interacts with LFA-1 expressed on neutrophils, macrophages, and T cells to recruit them to the site of injury.32–34 Targeting the recruitment of these cells has the potential to increase the native production of growth factors and cytokines in the correct sequences and combinations without the need to identify and apply each factor individually. For these experiments we used commercially available soluble ICAM-1 fused to an Fc-tag for purification purposes (total molecular weight 77 kDa). This molecule was selected for in vivo wound delivery because its upregulation in the first 24 hours following wounding is known to be important for an efficient immune-mediated healing response,32–34 and the faster-releasing hydrogel H2 was selected as the carrier to ensure maximum delivery during this early time period. As anticipated, analysis of in vivo ICAM-1-Fc retention demonstrated that H2-based delivery resulted in significantly greater amounts of protein in the wound environment after 5 hours compared to addition of a buffer solution of the protein (Figure 7a).
Figure 7.
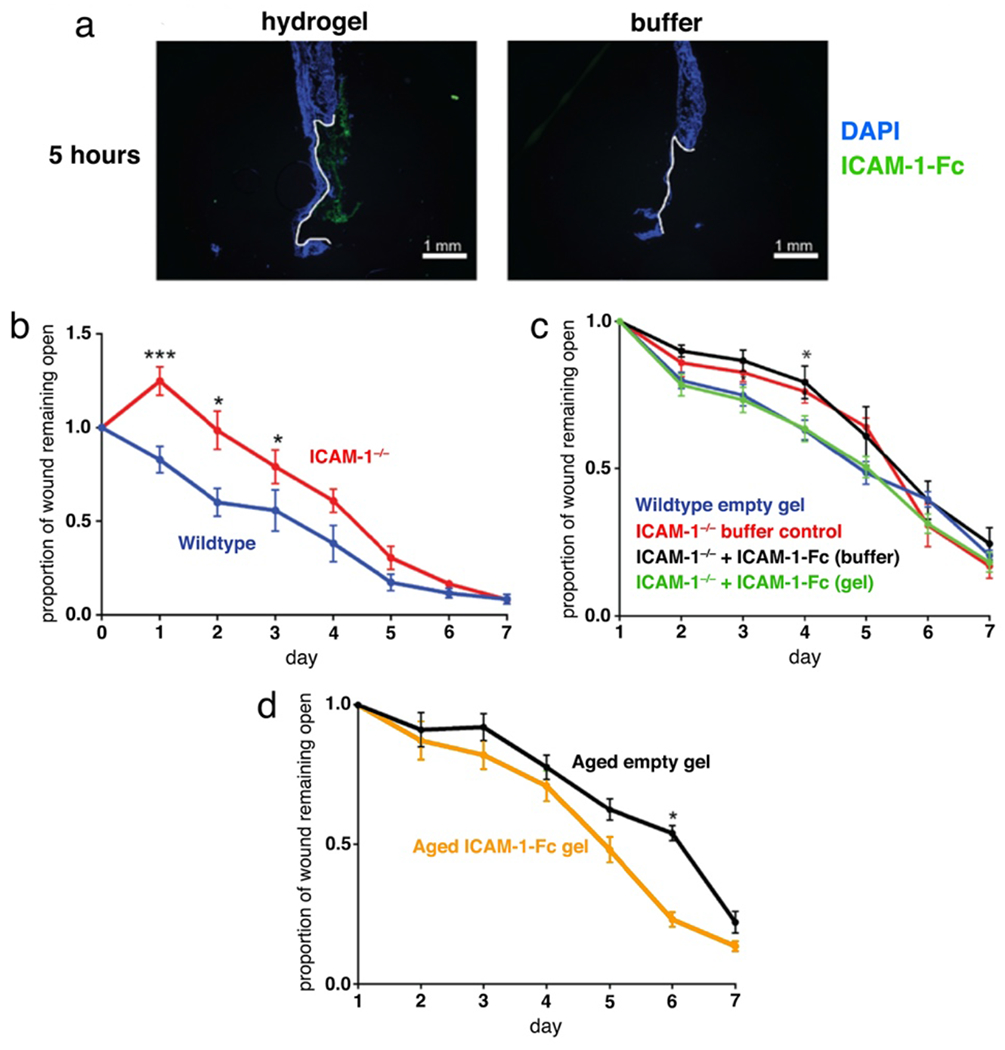
Effects of delivery of ICAM-1 on wound healing. (a) Delivery of AlexaFluor 488-labeled ICAM-1-Fc (5 μg) by noncovalent entrainment within hydrogel H2. (b) Wound healing in ICAM-1−/− mice. (c) Effect of hydrogel delivery on the impact of ICAM-1-Fc treatment (d) Treatment of aged mice with ICAM-1-Fc in the hydrogel delivery system.
We next tested how hydrogel-based delivery impacts the effectiveness of treatment with an immune-modulatory protein using ICAM-1−/− mice as a model of delayed wound healing. In previous studies ICAM-1−/− mice have been shown to exhibit delays in wound healing linked to defective recruitment of neutrophils, macrophages, and T cells.32–34 In agreement with previous reports, we observed delayed healing in ICAM-1−/− mice (Figure 7b). Gratifyingly, we found that treatment with ICAM-1-Fc (10 μg/wound) entrained in the H2 hydrogel, but not in solution, reversed this delay in healing (Figure 7c) indicating that extended presence of the missing endogenous protein in the wound environment is important for ICAM-1-mediated wound healing functions. Additionally, these results provide support for the hypothesis that exogenous ICAM-1 can be sufficient to induce a normal healing response in mice, effectively replacing the natural cell-surface bound form of the protein.
Wound healing is known to be delayed with age in humans58–59 and mice,60 and impaired wound healing in the elderly is a growing clinical concern.61 From a mechanistic perspective, there is evidence for defects in neutrophil and macrophage functions in aged wounds,14, 60–62 as well as a decrease in ICAM-1 upregulation following wounding and infection in aged epithelium.35, 63 We thus sought to determine if the application of ICAM-1 could improve the rate of wound repair in an aged mouse model of delayed healing. Aged mice that were treated with ICAM-1-Fc in the H2 hydrogel showed improved healing at day 6 compared with those treated with the same gel lacking the protein cargo (Figure 7d) indicating a benefit for increased ICAM-1 availability in this model as well. This result is significant as the aged mouse model more closely mimics the complex environment of a chronic wound, and improved healing with ICAM-1 treatment suggests this protein as a potential clinical target for the treatment of non-healing wounds. While the exact mechanisms remain to be determined, it is possible that addition of exogenous ICAM-1 results in improved recruitment and/or retention of neutrophils and macrophages in an aged wounds, helping to overcome the functional defects reported in these subsets. Additional work is warranted to elucidate the cellular mechanisms behind this shift and to determine if ICAM-1 could be beneficial in the treatment of chronic wounds outside of the aging context.
CONCLUSION
Oxanorbornadiene (OND)-based materials may be programmed to degrade and release entrained or attached cargo independent of the pH of the medium or the presence of other chemical signals. This makes such materials ideal for applications in vivo in which control over the rate of drug release is needed. Here we demonstrate the use of such hydrogel materials to improve the effectiveness of an immune-stimulatory protein in the wound environment. This system overcomes the typical limitation of rapid diffusion out of topical wounds and may be easily adapted to the delivery of a wide range of proteins and other agents. Overall, we introduce here a unique cargo delivery system that is easy to apply, well tolerated, and improves protein retention in the wound environment. This system is well suited to the targeting of immune cells and was successfully used to modulate ICAM-1 mediated wound healing in vivo. The properties of the hydrogels described here are highly tunable and can easily be adapted for future use with a wide variety of cargo proteins and delivery timeframes.
METHODS
Synthesis
Glutaroyl furfurylamine.
A solution of glutaric anhydride (4 g, 35 mmol, 1.7 equiv) in dry CH2Cl2 (25 mL) was slowly added to a solution of furfurylamine (2 g, 20.6 mmol) in dry CH2Cl2 (20 mL). The resulting solution was stirred for 4 hours at room temperature. The product was filtered, washed with cold CH2Cl2, and dried under vacuum (2.9 g, 67% yield). 1H NMR (400 MHz, CD3OD) δ 7.43 (dd, J = 1.9, 0.9 Hz, 1H), 6.35 (dd, J = 3.3, 1.9 Hz, 1H), 6.25 (dd, J = 3.3, 0.9 Hz, 1H), 4.36 (s, 2H), 2.34 (t, J = 7.4 Hz, 2H), 2.28 (t, J = 7.5 Hz, 2H), 1.95 – 1.85 (m, 2H). 13C NMR (101 MHz, CD3OD) δ 175.36, 173.75, 151.70, 141.86, 109.92, 106.61, 35.70, 34.54, 32.62, 20.81. HRMS (ESI-TOF) C10H13NO4 [M + H+] calcd 212.0923, found 212.0967.
Compound 1, 5-(((2,3-bis(methoxycarbonyl)-7-oxabicyclo[2.2.1]hepta-2,5-dien-1-yl)methyl)-amino)-5-oxopentanoic acid.
A solution of glutaroyl furfurylamine (1.5 g, 7.1 mmol, 1 equiv) and dimethyl acetylenedicarboxylate (1.51 g, 10.65 mmol, 1.5 equiv) in methanol (1 mL) was stirred at 65°C for 20 hours. The resulting solution was cooled and poured into diethyl ether (50 mL) to yield a pale yellow precipitate, which was filtered and dried under vacuum to give 1 (1.9 g, 76% yield). 1H NMR (400 MHz, CDCl3) δ 7.23 (dd, J = 5.3, 1.9 Hz, 1H), 7.02 (d, J = 5.3 Hz, 1H), 6.23 (s, 1H), 5.66 (d, J = 1.9 Hz, 1H), 4.18 (dd, J = 14.8, 6.3 Hz, 1H), 4.03 (dd, J = 14.8, 5.3 Hz, 1H), 3.82 (d, J = 14.6 Hz, 6H), 2.41 (t, J = 7.1 Hz, 2H), 2.30 (t, J = 7.4 Hz, 2H), 1.97 (q, J = 7.2 Hz, 2H). 13C NMR (101 MHz, CDCl3) δ 177.27, 172.72, 163.90, 162.59, 153.43, 152.91, 145.31, 142.91, 96.90, 83.68, 52.64, 52.44, 37.76, 35.04, 32.91, 20.56. HRMS (ESI-TOF) C16H19NO8 [M + H+] calcd 354.1189, found 354.1122.
PEG-OND.
Compound 1 (18 mg, 0.05 mmol, 2.5 equiv) was dissolved in 1.5 mL of DMF. HATU (52 mg, 0.14 mmol, 3 equiv) and N,N-diisopropylethylamine (29.2 mg, 0.23 mmol, 5 equiv.) were added to the OND solution followed by addition of PEG diamine (HCl salt) (225 mg, 0.045 mmol, 1 equiv). The reaction was stirred at room temperature for 4 hours followed by precipitation into diethyl ether. The resulting off-white solid was collected by vacuum filtration, rehydrated in 1 mL of water, and dialyzed (MW cutoff 3500 Da) against water for 2 days. The retentate was filtered, frozen, and lyophilized to yield PEG-OND as a white fluffy powder. 1H NMR (400 MHz, D2O) δ 7.39 (dd, J = 5.3, 2.1 Hz, 2H), 7.18 (d, J = 5.3 Hz, 2H), 5.84 (d, J = 2.0 Hz, 2H), 4.34 (d, J = 15.2 Hz, 4H), 3.86 (d, J = 13.0 Hz, 12H), 3.73 (s, 605H), 3.65 (t, J = 5.4 Hz, 6H), 3.56 (d, J = 4.5 Hz, 2H), 3.42 (t, J = 5.4 Hz, 4H), 2.29 (t, J = 7.5 Hz, 8H), 1.93 – 1.85 (m, 4H). 13C NMR (126 MHz, D2O) δ 175.55, 175.46, 165.12, 163.67, 153.58, 153.13, 145.13, 142.09, 96.89, 83.44, 69.42, 69.36, 69.27, 69.17, 68.58, 52.85, 52.69, 38.67, 37.07, 34.55, 34.34, 21.50.
Dextran-OND.
Dextran (20 kD, 334 mg, 2.3 mmol glucose units, 1 equiv) and lithium chloride (600 mg, 14 mmol, 6 equiv) were added to 30 mL DMF and stirred at 90°C for 30 minutes. The resulting solution was cooled to 0°C and N,N’-dicyclohexylcarbodiimide (518 mg, 2.5 mmol, 1.1 equiv), OND 1 (0.4 equiv), and 4-(dimethylamino)pyridinium 4-toluenesulfonate (77.5 mg, 0.27 mmol, 0.14 equiv) were added to the cooled solution under stirring. The resulting solution was stirred at 4°C for 3 days, after which the solution was filtered through a 0.2 μm syringe filter and subsequently precipitated in 150 mL acetone with stirring. The white precipitate was collected by vacuum filtration, rehydrated in 9 mL water, and dialyzed (MW cutoff 3500 Da) against water for 2 days at 4°C. The solution was filtered, frozen, and lyophilized to yield a white fluffy powder. The analysis below showed the degree of substitution to be 18–25%. 1H NMR (400 MHz, D2O) δ 7.42 – 7.32 (m, 1H), 7.18 (d, J = 5.2 Hz, 1H), 5.87 – 5.74 (m, 1H), 5.18 (s, 1H), 5.01 (d, J = 4.5 Hz, 3H), 4.32 (s, 2H), 4.08 – 3.92 (m, 7H), 3.86 (d, J = 14.5 Hz, 6H), 3.68 – 3.49 (m, 7H), 2.52 (s, 2H), 2.34 (s, 2H), 1.93 (s, 2H). 13C NMR (126 MHz, D2O) δ 174.29, 165.05, 163.65, 153.61, 153.06, 145.15, 142.15, 97.46, 96.84, 83.45, 73.15, 71.16, 69.94, 69.26, 65.26, 52.89, 52.73, 37.13, 34.23, 32.85, 32.58, 20.48.
Gel formation.
Hydrogels were prepared by first dissolving the OND material and thiol material separately in 0.001 M potassium phosphate buffer and then combining the two solutions in a 1:1 ratio of OND to thiol. Gelation was determined to be complete by the inversion test when the gel material was observed to be no longer flowing. Once the OND and thiol solutions were mixed, the hydrogels were formed in as little as 2 seconds. Gelation times could be tuned as a function of pH or buffer concentration. It was found that increasing the buffer concentration sped up gelation significantly. Hydrogels prepared in 7 to 8 pH buffers all took less than 20 seconds to gel, whereas most hydrogels prepared in buffers at pH 5.5-7 were still able to form gels but took as long as 18 minutes to form self-supporting gels. Gels reported were prepared as 10 wt% gels in 0.001 M potassium phosphate buffer, however, gelation could be achieved at ratios as low as 2.5 wt%.
Determination of swelling ratio and gel fraction
Swelling ratios were determined by suspending hydrogels in water and comparing the hydrogel mass after 24 hours to the original hydrogel mass. Specifically, 50 μL of the gel of interest was prepared in 0.001 M potassium phosphate buffer as described above. The gels were allowed to cure for 5 minutes at room temperature, weighed (M0), and then suspended in 1 mL of deionized water. The gels were stored at 4 °C to prevent significant degradation of the hydrogel network. Hydrogel swelling was monitored periodically by decanting, gently wiping any excess water off of the gel, and weighing. After 24 hours, when swelling had reached equilibrium, the masses of the gels were recorded as Meq. The equilibrium mass swelling ratio is defined as the ratio Meq/M0.
Gel fractions were determined by comparing the lyophilized mass of a gel (Mres) to the mass of the OND and thiol materials that were used to prepare it (Min). Once the equilibrium mass swelling ratio was determined, the gels were frozen, lyophilized and weighed to determine Mres. Salts present in the gel were removed during exchange with the deionized water solvent for the swelling measurements.
Oscillatory rheology
The viscoelastic properties of different Dextran-OND and PEG-OND gels were assessed using oscillatory rheology. 100 μL 10 wt% gels were prepared in 0.001 M potassium phosphate buffer in a 1:1 ratio. The solution was quickly mixed and then dispensed onto the center of the rheometer plate. The cone was brought into contact with the gel and any excess gel was trimmed. The storage (G’) and loss (G”) moduli were then measured overtime at a constant strain of 25%, which is well within the linear regime, and an angular frequency of 1 rad/s to monitor the gel formation and quantify the gelation and degradation times.
Release of VLPs
Qβ virus-like particles containing an average of 10 copies of red fluorescent protein per particle were prepared as a 5.5 mg/mL solution in 0.001 M potassium phosphate buffer. To make 100 μL of 10 wt% gel, separate vials of OND and thiol material were each dissolved in 50 μL of the VLP solution, which were then chilled on ice for 1 minute. The thiol solution was added to the OND solution, the mixture was pipetted up and down and then transferred to molds. The gels were allowed to cure for 5 minutes and then were removed from their molds, transferred to vials, and suspended in 800 μL of deionized water. The gels were incubated at 37°C and release of VLPs into the supernatant was monitored by fluorescence with excitation at 585 nm and emission at 605 nm.
Mice
Unless otherwise noted, C57BL/6J mice were 8-12 week old male and female mice obtained from the Scripps Research Rodent Breeding Colony. Aged C57BL/6J mice were 6-12 months old. ICAM-1−/− (B6.129S4-Icam1tm1Jcgr/J) mice were obtained from JAX® Mice. All animal experiments were conducted in accordance with the Scripps Research Institute Institutional Care and Use Committee.
Wounding and hydrogel application
Mice were anesthetized and hair was plucked from a 2cm2 area on the back of each mouse. A 3mm biopsy punch was used to make 2 full-thickness wounds in the plucked area. Hydrogel components were mixed immediately prior to use as described above and 5μl pipetted into each wound bed. Following hydrogel polymerization, 3M™ Tegaderm™ was used to cover the wounds and prevent drying. 24 hours post-hydrogel application, at which point hydrogel degradation was complete, the Tegaderm™ was removed. For wound closure experiments, wounds were imaged on day 1 (24 hours post-wounding) through day 7. 4-5 mice per group were included for each experiment. ImageJ software64 was used to quantify wound area at each time point and the wound proportion remaining open was calculated. Samples were analyzed with Prism GraphPad software using multiple t-tests to compare treatment groups at each time point and determine the statistical significance of results. Significance was denoted as follows: *= P<0.05, **=P<0.01, ***=P<001.
Protein retention analysis
High-purity BSA (3-5 mg/mL) was reacted with Texas Red N-hydroxysuccinimide ester (2 molar equivalents relative to total protein) in phosphate buffer (pH=7) for 18 hours at room temperature, followed by gravity separation from small-molecule reagents using a PD-10 desalting column (GE Healthcare) according to the manufacturer’s protocol. For covalent hydrogel attachment, Texas Red-labeled BSA (2 mg/mL) was further treated with excess dithiothreitol (10 mM) at 37°C for 1 hour, purified by gravity separation on a PD-10 desalting column, and concentrated using a 3 kDa centrifugal filter (4,000x g, 10 min) prior to hydrogel incorporation. ICAM-1-Fc was acquired from R&D Systems and fluorescently labeled using a ThermoFisher Alexa Fluor® 488 microscale labeling kit according to manufacturer’s instructions.
The hydrogel material (5 μL) containing attached or entrained BSA (at 5-10 mg/mL) or entrained ICAM-1-Fc (at 1 mg/mL) was administered to wounds at the time of wounding. Tissue was collected at the indicated time points by excision and embedding in Tissue-Tek® O.C.T. compound on dry ice. 8 μm sections were fixed for 15 minutes in ice cold acetone followed by a DAPI stain at 1:1000 in PBS for 10 minutes. Sections were mounted with Dako Fluorescence Mounting Medium and imaged with a Keyence BZX700 all-in-one fluorescence microscope. The longer duration of cargo release with hydrogel H1 is shown by the additional images in Figure S2 (Supporting Information).
Epidermal cell isolation and viability stain
Empty hydrogel material was administered into mouse wounds as described above using 8-12 week-old male and female mice. Skin was collected from a 1mm diameter surrounding each wound. These full thickness skin pieces were floated for 2 hours at 37°C on trypsin/GNK solution (0.3% trypsin, 150mM NaCl, 5mM KCl, 5.5 mM glucose), after which epidermis was separated from dermis as previously described65. Epidermal sheets were then incubated for 20 minutes at 37°C in trypsin/GNK solution containing 0.01% DNase with occasional shaking to release epidermal cells from the tissue. Following incubation, samples were vortexed and strained through Sera-Separa® columns (Evergreen Scientific) to remove undigested epidermal tissue. Cells were stained with eBioscience™ Fixable Viability Dye eFluor™ 780 to identify dead cells followed by staining with eBioscience™ Annexin V Apoptosis Detection Kit according to manufacturer’s instructions. Data were acquired on a BD™ LSR II flow cytometer, and analysis was conducted using FlowJo software.
Tissue preparation and hematoxylin and eosin staining
Following hydrogel treatment, full-thickness tissue sections surrounding wounds were excised and trimmed to leave approximately 2mm margins along wound edges. Tissue was then left overnight in zinc buffered formalin to fix. Fixed samples were then dehydrated by subsequent incubation in 70%, 95%, and 100% ethanol (2-24 hours per step), cleared with a 2-hour incubation xylene, and embedded in paraffin. 5μm sections were cut and stained with hematoxylin (2 min) and eosin (3 min). Images were taken with a Keyence BZX700 all-in-one fluorescence microscope.
Supplementary Material
ACKNOWLEDGEMENTS
This research was supported by NIH grants AI119971 (MGF and WLH) and AI064811 (WLH). Jessica Lloyd was supported by the NIH BioMat T32 program, award number EB006343. Margarete Johnson was supported by a TL1 training award (TR001113-05) and by the ARCS foundation. We thank Dr. Soumen Das for production of the RFP-encapsulating virus-like particles.
Footnotes
This paper is dedicated to Prof. Wendy Havran, who passed away during its writing, in boundless gratitude for her insights, inspiration, and friendship.
REFERENCES
- 1.Singer AJ; Clark RA, Cutaneous wound healing. N Engl J Med 1999, 341, 738–46. [DOI] [PubMed] [Google Scholar]
- 2.Makrantonaki E; Wlaschek M; Scharffetter-Kochanek K, Pathogenesis of wound healing disorders in the elderly. J. Dtsch. Dermatol. Ges 2017, 15, 255–275. [DOI] [PubMed] [Google Scholar]
- 3.Sen CK; Gordillo GM; Roy S; Kirsner R; Lambert L; Hunt TK; Gottrup F; Gurtner GC; Longaker MT, Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen 2009, 17, 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Driver VR; Fabbi M; Lavery LA; Gibbons G, The costs of diabetic foot: the economic case for the limb salvage team. J Am Podiatr Med Assoc 2010, 100, 335–41. [DOI] [PubMed] [Google Scholar]
- 5.Dhivya S; Padma VV; Santhini E, Wound dressings - a review. Biomedicine (Taipei) 2015, 5, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powers JG; Higham C; Broussard K; Phillips TJ, Wound healing and treating wounds: Chronic wound care and management. J Am Acad Dermatol 2016, 74, 607–25; quiz 625-6. [DOI] [PubMed] [Google Scholar]
- 7.Fife CE; Carter MJ, Wound Care Outcomes and Associated Cost Among Patients Treated in US Outpatient Wound Centers: Data From the US Wound Registry. Wounds 2012, 24, 10–7. [PubMed] [Google Scholar]
- 8.Gurtner GC; Werner S; Barrandon Y; Longaker MT, Wound repair and regeneration. Nature 2008, 453, 314–21. [DOI] [PubMed] [Google Scholar]
- 9.Eming SA; Martin P; Tomic-Canic M, Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med 2014, 6, 265sr6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martin P, Wound healing--aiming for perfect skin regeneration. Science 1997, 276, 75–81. [DOI] [PubMed] [Google Scholar]
- 11.Ellis S; Lin EJ; Tartar D, Immunology of Wound Healing. Curr Dermatol Rep 2018, 7, 350–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larouche J; Sheoran S; Maruyama K; Martino MM, Immune Regulation of Skin Wound Healing: Mechanisms and Novel Therapeutic Targets. Adv Wound Care (New Rochelle) 2018, 7, 209–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Strbo N; Yin N; Stojadinovic O, Innate and Adaptive Immune Responses in Wound Epithelialization. Adv Wound Care (New Rochelle) 2014, 3, 492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishio N; Okawa Y; Sakurai H; Isobe K, Neutrophil depletion delays wound repair in aged mice. Age (Dordr) 2008, 30, 11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang J, Neutrophils in tissue injury and repair. Cell Tissue Res 2018, 371, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mahdavian Delavary B; van der Veer WM; van Egmond M; Niessen FB; Beelen RH, Macrophages in skin injury and repair. Immunobiology 2011, 216, 753–62. [DOI] [PubMed] [Google Scholar]
- 17.Leibovich SJ; Ross R, The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975, 78, 71–100. [PMC free article] [PubMed] [Google Scholar]
- 18.Toulon A; Breton L; Taylor KR; Tenenhaus M; Bhavsar D; Lanigan C; Rudolph R; Jameson J; Havran WL, A role for human skin-resident T cells in wound healing. J Exp Med 2009, 206, 743–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jameson J; Ugarte K; Chen N; Yachi P; Fuchs E; Boismenu R; Havran WL, A role for skin gammadelta T cells in wound repair. Science 2002, 296, 747–9. [DOI] [PubMed] [Google Scholar]
- 20.Haertel E; Joshi N; Hiebert P; Kopf M; Werner S, Regulatory T cells are required for normal and activin-promoted wound repair in mice. Eur J Immunol 2018, 48, 1001–1013. [DOI] [PubMed] [Google Scholar]
- 21.Frykberg RG; Banks J, Challenges in the Treatment of Chronic Wounds. Adv Wound Care (New Rochelle) 2015, 4, 560–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barrientos S; Brem H; Stojadinovic O; Tomic-Canic M, Clinical application of growth factors and cytokines in wound healing. Wound Repair Regen 2014, 22, 569–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wieman TJ; Smiell JM; Su Y, Efficacy and safety of a topical gel formulation of recombinant human platelet-derived growth factor-BB (becaplermin) in patients with chronic neuropathic diabetic ulcers. A phase III randomized placebo-controlled double-blind study. Diabetes Care 1998, 21, 822–7. [DOI] [PubMed] [Google Scholar]
- 24.Pourmoussa A; Gardner DJ; Johnson MB; Wong AK, An update and review of cell-based wound dressings and their integration into clinical practice. Ann Transl Med 2016, 4, 457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dixit S; Baganizi DR; Sahu R; Dosunmu E; Chaudhari A; Vig K; Pillai SR; Singh SR; Dennis VA, Immunological challenges associated with artificial skin grafts: available solutions and stem cells in future design of synthetic skin. J Biol Eng 2017, 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koehler JB, FP. Goepferich AM, Hydrogel wound dressings for bioactive reatment of acute and chronic wounds. Eur Polym J 2018, 100, 1–11. [Google Scholar]
- 27.Kamoun EA; Kenawy ES; Chen X, A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J Adv Res 2017, 8, 217–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madaghiele M; Demitri C; Sannino A; Ambrosio L, Polymeric hydrogels for burn wound care: Advanced skin wound dressings and regenerative templates. Burns Trauma 2014, 2, 153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Drury JL; Mooney DJ, Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003, 24, 4337–51. [DOI] [PubMed] [Google Scholar]
- 30.Higginson CJ; Kim SY; Pelaez-Fernandez M; Fernandez-Nieves A; Finn MG, Modular degradable hydrogels based on thiol-reactive oxanorbornadiene linkers. J Am Chem Soc 2015, 137, 4984–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kolaczkowska E; Kubes P, Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013, 13, 159–75. [DOI] [PubMed] [Google Scholar]
- 32.Nagaoka T; Kaburagi Y; Hamaguchi Y; Hasegawa M; Takehara K; Steeber DA; Tedder TF; Sato S, Delayed wound healing in the absence of intercellular adhesion molecule-1 or L-selectin expression. Am J Pathol 2000, 157, 237–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gay AN; Mushin OP; Lazar DA; Naik-Mathuria BJ; Yu L; Gobin A; Smith CW; Olutoye OO, Wound healing characteristics of ICAM-1 null mice devoid of all isoforms of ICAM-1. J Surg Res 2011, 171, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Byeseda SE; Burns AR; Dieffenbaugher S; Rumbaut RE; Smith CW; Li Z, ICAM-1 is necessary for epithelial recruitment of gammadelta T cells and efficient corneal wound healing. Am J Pathol 2009, 175, 571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashcroft GS; Horan MA; Ferguson MW, Aging alters the inflammatory and endothelial cell adhesion molecule profiles during human cutaneous wound healing. Lab Invest 1998, 78, 47–58. [PubMed] [Google Scholar]
- 36.Hong V; Kislukhin A; Finn MG, Thiol-Selective Fluorogenic Probes for Labeling and Release. J. Am. Chem. Soc 2009, 131, 9986–9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kislukhin AA; Higginson CJ; Hong VP; Finn MG, Degradable Conjugates from Oxanobornadiene Reagents. J. Am. Chem. Soc 2012, 134, 6491–6497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hiemstra C; Van der Aa LJ; Zhong Z; Dijkstra PJ; Feijen J, Rapidly in Situ-Forming Degradable Hydrogels from Dextran Thiols through Michael Addition. Biomacromolecules 2007, 8, 1548–1556. [DOI] [PubMed] [Google Scholar]
- 39.Higginson CJ; Eno MR; Khan S; Cameron MD; Finn MG, Albumin-Oxanorbornadiene Conjugates Formed ex Vivo for the Extended Circulation of Hydrophilic Cargo. ACS Chem. Biol 2016, 11, 2320–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jansen LE; Negrön-Piñeiro LJ; Galarza S; Peyton SR, Control of thiol-maleimide reaction kinetics in PEG hydrogel networks. Acta Biomater. 2018, Hi 0, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Holland TA; Tabata Y; Mikos AG, In vitro release of transforming growth factor-beta 1 from gelatin microparticles encapsulated in biodegradable, injectable oligo(poly(ethylene glycol) fumarate) hydrogels. J. Control. Release 2003, 91, 299–313. [DOI] [PubMed] [Google Scholar]
- 42.Jeon O; Powell C; Solorio LD; Krebs MD; Alsberg E, Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Control. Release 2011, 154, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Patil NS; Dordick JS; Rethwisch DG, Macroporous poly(sucrose acrylate) hydrogel for controlled release of macromolecules. Biomaterials 1996, 17, 2343–2350. [DOI] [PubMed] [Google Scholar]
- 44.Huang X; Brazel CS, On the Importance and Mechanisms of Burst Release in Matrix-controlled Drug Delivery Systems. J. Control. Release 2001, 73, 121–136. [DOI] [PubMed] [Google Scholar]
- 45.George M; Abraham TE, pH sensitive alginate-guar gum hydrogel for the controlled delivery of protein drugs. Int. J. Pharm 2007, 335, 123–129. [DOI] [PubMed] [Google Scholar]
- 46.Fiedler JD; Brown SD; Lau J; Finn MG, RNA-Directed Packaging of Enzymes within Virus-Like Particles. Angew. Chem. Int. Ed 2010, 49, 9648–9651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sweeney IR; Miraftab M; Collyer G, A critical review of modern and emerging absorbent dressings used to treat exuding wounds. Int Wound J 2012, 9, 601–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hori K; Sotozono C; Hamuro J; Yamasaki K; Kimura Y; Ozeki M; Tabata Y; Kinoshita S, Controlled-release of epidermal growth factor from cationized gelatin hydrogel enhances corneal epithelial wound healing. J Control Release 2007, 118, 169–76. [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro MP; Morgado PI; Miguel SP; Coutinho P; Correia IJ, Dextran-based hydrogel containing chitosan microparticles loaded with growth factors to be used in wound healing. Mater Sci Eng C Mater Biol Appl 2013, 33, 2958–66. [DOI] [PubMed] [Google Scholar]
- 50.Puolakkainen PA; Twardzik DR; Ranchalis JE; Pankey SC; Reed MJ; Gombotz WR, The enhancement in wound healing by transforming growth factor-beta 1 (TGF-beta 1) depends on the topical delivery system. J Surg Res 1995, 58, 321–9. [DOI] [PubMed] [Google Scholar]
- 51.Mohandas A; Anisha BS; Chennazhi KP; Jayakumar R, Chitosan-hyaluronic acid/VEGF loaded fibrin nanoparticles composite sponges for enhancing angiogenesis in wounds. Colloids Surf B Biointerfaces 2015, 127, 105–13. [DOI] [PubMed] [Google Scholar]
- 52.Mokarram N; Bellamkonda RV, A perspective on immunomodulation and tissue repair. Ann Biomed Eng 2014, 42, 338–51. [DOI] [PubMed] [Google Scholar]
- 53.Xie Z; Paras CB; Weng H; Punnakitikashem P; Su LC; Vu K; Tang L; Yang J; Nguyen KT, Dual growth factor releasing multi-functional nanofibers for wound healing. Acta Biomater 2013, 9, 9351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Busilacchi A; Gigante A; Mattioli-Belmonte M; Manzotti S; Muzzarelli RA, Chitosan stabilizes platelet growth factors and modulates stem cell differentiation toward tissue regeneration. Carbohydr Polym 2013, 98, 665–76. [DOI] [PubMed] [Google Scholar]
- 55.Spiller KL; Nassiri S; Witherel CE; Anfang RR; Ng J; Nakazawa KR; Yu T; Vunjak-Novakovic G, Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 2015, 37, 194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dressler J; Bachmann L; Muller E, Enhanced expression of ICAM-1 (CD 54) in human skin wounds: diagnostic value in legal medicine. Inflamm Res 1997, 46, 434–5. [DOI] [PubMed] [Google Scholar]
- 57.Caughman SW; Li LJ; Degitz K, Human intercellular adhesion molecule-1 gene and its expression in the skin. J Invest Dermatol 1992, 98, 61S–65S. [DOI] [PubMed] [Google Scholar]
- 58.Gould L; Abadir P; Brem H; Carter M; Conner-Kerr T; Davidson J; DiPietro L; Falanga V; Fife C; Gardner S; Grice E; Harmon J; Hazzard WR; High KP; Houghton P; Jacobson N; Kirsner RS; Kovacs EJ; Margolis D; McFarland Horne F; Reed MJ; Sullivan DH; Thom S; Tomic-Canic M; Walston J; Whitney JA; Williams J; Zieman S; Schmader K, Chronic wound repair and healing in older adults: current status and future research. J Am Geriatr Soc 2015, 63, 427–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wicke C; Bachinger A; Coerper S; Beckert S; Witte MB; Konigsrainer A, Aging influences wound healing in patients with chronic lower extremity wounds treated in a specialized Wound Care Center. Wound Repair Regen 2009, 17, 25–33. [DOI] [PubMed] [Google Scholar]
- 60.Swift ME; Kleinman HK; DiPietro LA, Impaired wound repair and delayed angiogenesis in aged mice. Lab Invest 1999, 79, 1479–87. [PubMed] [Google Scholar]
- 61.Sgonc R; Gruber J, Age-related aspects of cutaneous wound healing: a mini-review. Gerontology 2013, 59, 159–64. [DOI] [PubMed] [Google Scholar]
- 62.Swift ME; Burns AL; Gray KL; DiPietro LA, Age-related alterations in the inflammatory response to dermal injury. J Invest Dermatol 2001, 117, 1027–35. [DOI] [PubMed] [Google Scholar]
- 63.Hobden JA; Masinick SA; Barrett RP; Hazlett LD, Aged mice fail to upregulate ICAM-1 after Pseudomonas aeruginosa corneal infection. Invest Ophthalmol Vis Sci 1995, 36, 1107–14. [PubMed] [Google Scholar]
- 64.Schneider CA; Rasband WS; Eliceiri KW, NIH Image to ImageJ: 25 years of image analysis. Nat Methods 2012, 9, 671–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witherden DA; Verdino P; Rieder SE; Garijo O; Mills RE; Teyton L; Fischer WH; Wilson IA; Havran WL, The junctional adhesion molecule JAML is a costimulatory receptor for epithelial gammadelta T cell activation. Science 2010, 329, 1205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


