Abstract
Background
SARS-CoV-2 infection has noted derangements in coagulation markers along with significant thrombotic complications. Post-mortem examinations show severe endothelial injury and widespread thrombotic microangiopathy in the pulmonary vasculature. Early reports describing the use of prophylactic anticoagulation demonstrated improved survival, leading to the adoption of prophylactic and therapeutic anticoagulation guided by D-dimer levels. The clinical usefulness of D-dimer values, trends, and more intensive anticoagulation remains an area of clinical interest.
Objectives
Assess the outcomes and laboratory trends in COVID-19 patients stratified by intensity of anticoagulation at time of admission.
Patients and methods
Retrospectively review the differences in clinical outcomes and laboratory trends in patients hospitalized with COVID-19 in the Lifespan Health System.
Results
Between 27 February and 24 April 2020, 468 patients were hospitalized. Initial use of high-intensity thromboprophylaxis was associated with improved 30-day mortality (adjusted RR 0.26; 95% confidence interval [CI], 0.07–0.97; p = 0.045) without a significant increased rate of bleeding (p = 0.11).
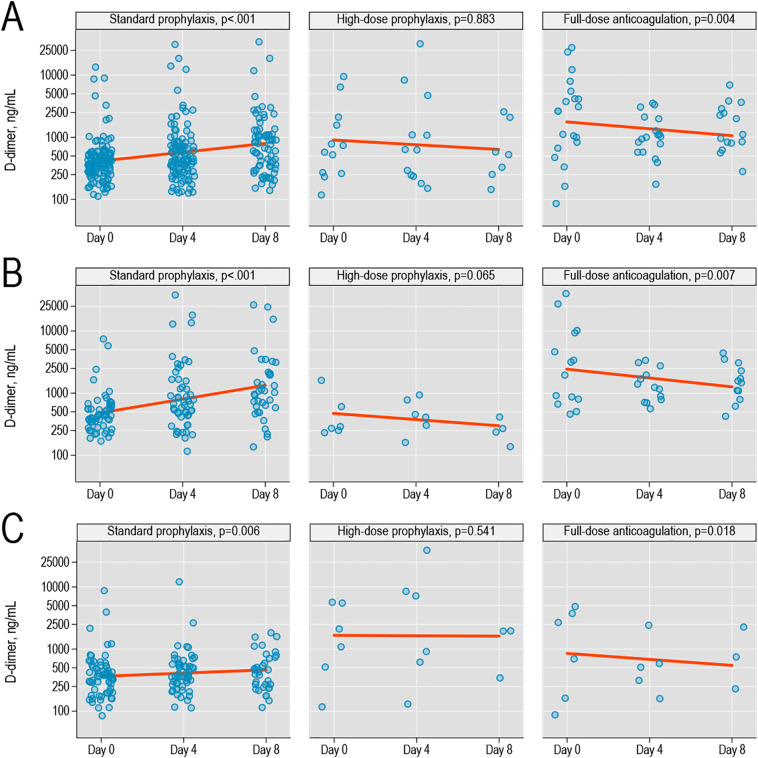
In severe COVID-19, D-dimer significantly increased during hospitalization with standard thromboprophylaxis (p < 0.001) but remained stable or decreased with high-intensity prophylaxis or therapeutic anticoagulation.
Conclusion
Patients who received high-intensity prophylactic anticoagulation had a downtrend in D-dimer levels and improved 30-day mortality. This suggests a role in anticoagulation in mitigating adverse outcomes associated with COVID-19; however, further randomized, prospective studies are needed.
Keywords: SARS-CoV-2, COVID-19, COVID-19 coagulopathy, D-dimer, Anticoagulation
Highlights
-
•
High-intensity prophylaxis was associated with improved 30-day mortality
-
•
High-intensity prophylaxis includes LMWH 40 mg twice daily or unfractionated heparin 7500 units subcutaneous thrice daily.
-
•
In severe COVID-19, D-dimer significantly increased during hospitalization with standard prophylaxis.
-
•
In severe COVID-19, D-dimer was stable or decreased with high-intensity prophylaxis or therapeutic anticoagulation.
-
•
In severe COVID-19, high-intensity prophylaxis or therapeutic anticoagulation did not lead to increased bleeding.
Early publications reported higher rates of coagulopathies in the form of prolonged prothrombin time and elevated D-dimer levels in severe cases of COVID-19 [1]. These findings led to a prospective study by Tang et al. of 183 patients in Wuhan, China that showed derangements in conventional coagulation parameters during COVID-19 were significantly associated with poorer prognosis [2]. Furthermore, patients with COVID-19 coagulopathy who met the International Society of Thrombosis and Haemostasis (ISTH) diagnostic criteria for disseminated intravascular coagulopathy (DIC) had decreased survival; 71% of non-survivors had DIC compared to 0.6% of survivors [2]. Post-mortem examinations show severe endothelial injury with intracellular virus and widespread thrombotic microangiopathy in the pulmonary vasculature [3]. Hence, altered coagulation parameters in COVID-19 are a suspected indicator of thrombotic complications rather than bleeding risk.
One study reported improved 28-day mortality in severe COVID-19 patients with markedly elevated D-dimers who received low-molecular-weight heparin (LMWH) at 40–60 mg daily [4]. This has led to adoption of prophylactic and therapeutic anticoagulation guided by D-dimer levels, although the clinical usefulness of D-dimer values, trends, and more intensive anticoagulation remains an area of research. In this study, we describe patient outcomes and laboratory trends of patients hospitalized with COVID-19 based on receipt of standard venous thromboembolism (VTE) prophylaxis, high-intensity VTE prophylaxis, or full dose/therapeutic anticoagulation.
We retrospectively examined adult patients with PCR-confirmed, COVID-19 in the Lifespan Health System from 27 February to 24 April 2020. Standard VTE prophylaxis was defined as LMWH 40 mg once daily, unfractionated heparin subcutaneous (HSQ) 5000 units three times daily, or apixaban 2.5 mg twice daily while high-intensity prophylaxis was defined as LMWH 40 mg twice daily or HSQ 7500 units three times daily. The use of high-intensity prophylaxis was based upon an institutional algorithm. However, choice of VTE prophylaxis was at the discretion of the admitting provider. As more data was being published on COVID-19, these guidelines were implemented on April 18, 2020 and were based upon D-dimer levels greater than 1000 ng/mL on admission or doubling of D-dimer levels within 48 h. Therapeutic anticoagulation was defined as intravenous heparin, LMWH 1 mg/kg twice daily, dose-adjusted warfarin with a target international normalized ratio (INR) of 2.0 to 3.0, apixaban 5 mg twice daily, or rivaroxaban 20 mg daily. Patients were categorized as having non-severe or severe COVID-19 pneumonia based upon the Infectious Disease Society of America (IDSA)/American Thoracic Society (ATS) criteria [5]. The IDSA/ATS criteria for defining severe pneumonia includes 1 major criterion or at least 3 minor criteria. Major criteria include shock requiring vasopressor; respiratory failure requiring mechanical ventilation. Minor criteria include respiratory rate greater than 30 breaths per minute; PaO2/FIO2 ratio less than 250; multifocal infiltration, altered mental status, uremia, cytopenia secondary to infection, hypothermia, hypotension requiring aggressive fluid resuscitation.
Primary endpoints were 30-day mortality, rates of symptomatic and asymptomatic VTE (defined as any thrombus in the venous system including deep venous thrombosis [DVT] and pulmonary embolism [PE]) and cerebrovascular accident (CVA) at 30-days after diagnosis. Secondary endpoints were rates of bleeding while on anticoagulation and changes in D-dimer levels from admission throughout hospitalization. Categorical variables were compared with x2 or Fisher's exact tests and continuous variables using Mann-Whitney test as appropriate. We examined the association between binary outcomes (30-day mortality, occurrence of thrombosis) and exposure in a multivariable generalized linear model (GLM, with Poisson distribution and robust standard error) adjusting for age, sex, indicators of COVID-19 severity, baseline comorbidities, and baseline anticoagulant use. Changes in the D-dimer levels were examined in a hierarchical GLM with a random intercept for each individual patient.
Severe COVID-19 pneumonia was associated with increased risk of VTE (9% vs 4%, p = 0.026), CVA (5% vs 1%, respectively, p = 0.004), and higher 30-day mortality (45% vs 5%, p < 0.001). Patient in the non-severe cohort developed VTE localized to the lower extremities. In the severe cohorts, the majority of VTE were localized to the lower extremities with 1 in the brachial vein, 1 in the axillary vein, and 1 in the internal jugular vein thrombus that was catheter-related. Of the lower extremity VTEs, 1 was in the bilateral peroneal veins and the remainder were proximally located. Patients were only evaluated for VTEs if there was clinical suspicion. There was no statistical difference in rate of PE (2% vs 1%, p = 0.279) or DVT (6% vs 2%, p = 0.054) in severe versus non-severe COVID-19. Furthermore, when compared to patients with non-severe COVID-19 pneumonia, those with severe COVID-19 had significantly increased risk of developing acute kidney injury (AKI) (68% vs 9%, p < 0.001) and AKI requiring the use of hemodialysis (16% vs 0%, p < 0.001). Patients with severe COVID-19 pneumonia were also more likely to receive high-intensity thromboprophylaxis or therapeutic anticoagulation than patients with non-severe COVID-19 pneumonia, without a significant difference in overall rates of bleeding (p = 0.11), including World Health Organization grade 3 (p = 0.41) or grade 4 bleeding rates (p = 0.65). A D-dimer >1000 ng/mL on admission was associated with a higher risk of VTE (23% vs 3%, p < 0.001) and 30-day mortality (39% vs 12%, p < 0.001).
In a multivariable model (Table 1 in Supplementary Appendix), 30-day mortality was significantly lower among all patients who received high-intensity thromboprophylaxis (adjusted relative risk [RR] versus standard-intensity, 0.26; 95% confidence interval [CI], 0.07–0.97, p = 0.045) and a non-significant association was observed among patients with severe COVID-19 (adjusted RR = 0.32; 95% CI, 0.07–1.56, p = 0.15). Of note, patients who received therapeutic anticoagulation had statistically higher 30-day mortality when compared to the standard and high-intensity prophylaxis cohorts (40% vs 15% vs 6%, respectively, p < 0.001). Of note, patients who received therapeutic anticoagulation had statistically higher rates of acute respiratory distress syndrome (27% vs 16% v 13%, respectively, p = 0.011) and AKI (54% vs 33% vs 31%, respectively, p = 0.024), suggesting that the higher 30-day mortality is likely due to the selection of a more critically ill population. Patients who received therapeutic anticoagulation had non-statistically higher rates of VTE when compared to the standard prophylaxis cohort (10% vs 5%, p = 0.19); however, 80% of the VTEs in this cohort was found on admission, leading to therapeutic anticoagulation as the initial strategy. Additional patient characteristics and outcomes stratified by intensity of anticoagulation are reported in Table 1. In severe COVID-19, D-dimer significantly increased during hospitalization with standard thromboprophylaxis (p < 0.001). However, D-dimer levels remained stable with high-intensity prophylaxis and decreased with therapeutic anticoagulation (Fig. 1).
Table 1.
Patient characteristics stratified by no prophylaxis, standard prophylaxis, high-intensity prophylaxis, or therapeutic anticoagulation.
| Factor | None | Standard prophylaxis | High-intensity prophylaxis | Therapeutic anticoagulation | p-Value |
|---|---|---|---|---|---|
| N | 27 | 377 | 16 | 48 | |
| Median age at presentation, years [IQR] | 70 [45–82] | 60 [49–73] | 61.5 [53–67.5] | 69 [61–79.5] | 0.005 |
| Male, n (%) | 15 (55.6%) | 210 (55.7%) | 7 (43.8%) | 25 (52.1%) | 0.79 |
| Charlson Comorbidity Index, median [IQR] | 4 [0–6] | 3 [1–5] | 3 [2–4.5] | 5 [3–7] | <0.001 |
| CAD, n (%) | 5 (18.5%) | 46 (12.2%) | 2 (12.5%) | 18 (37.5%) | <0.001 |
| Diabetes, n (%) | 4 (14.8%) | 131 (34.7%) | 7 (43.8%) | 26 (54.2%) | 0.004 |
| COPD, n (%) | 3 (11.1%) | 35 (9.3%) | 4 (25.0%) | 7 (14.6%) | 0.14 |
| Severe COVID-19 pneumonia, n (%) | 5 (18.5%) | 113 (30.0%) | 7 (43.8%) | 26 (54.2%) | 0.002 |
| ICU Admission, n (%) | 1 (3.7%) | 103 (27.3%) | 8 (50%) | 22 (45.8%) | <0.001 |
| ARDS, n (%) | 0 | 59 (15.6%) | 2 (12.5%) | 13 (27.1%) | 0.011 |
| AKI, n (%) | 7 (25.9%) | 123 (32.6%) | 5 (31.3%) | 26 (54.2%) | 0.024 |
| Disseminated intravascular coagulation, n (%) | 0 | 2 (0.5%) | 0 | 1 (2.1%) | 0.201 |
| VTE, n (%) | 0 | 18 (4.8%) | 1 (6.3%) | 5 (10.4%) | 0.19 |
| DVT, n (%) | 0 | 14 (3.7%) | 1 (6.3%) | 1 (2.1%) | 0.701 |
| Pulmonary embolism, n (%) | 0 | 4 (1.1%) | 0 (0%) | 4 (8.3%) | 0.021 |
| Cerebrovascular accidents, n (%) | 2 (7.4%) | 8 (2.1%) | 0 | 1 (2.1%) | 0.24 |
| WHO any grade bleeding events, n (%) | 0 | 10 (13%) | 0 | 7 (15%) | 0.88 |
| WHO grade 3 bleeding events, n (%) | 0 | 5 (6%) | 0 | 1 (2%) | 0.62 |
| WHO grade 4 bleeding events, n (%) | 0 | 3 (4%) | 0 | 2 (4%) | 1.0 |
| 30-day mortality, n (%) | 8 (29.6%) | 56 (14.9%) | 1 (6.3%) | 19 (39.6%) | <0.001 |
Fig. 1.
(A) D-dimer values on admission (day 0), day 4, and day 8 of hospital admission among patients with COVID-19 infection, stratified by type of anticoagulation received; p-values for trends were obtained from univariate log-gamma models accounting for within-patient correlation; lines show linearized trends fit on a logarithmic scale; (B) trends in the subgroup with severe COVID-19; (C) trends in the subgroup with non-severe COVID-19.
In a multivariable model (Table 1 in Supplementary Appendix), 30-day mortality was significantly lower among all patients who received high-intensity thromboprophylaxis (adjusted relative risk [RR] versus standard-intensity, 0.26; 95% confidence interval [CI], 0.07–0.97, p = 0.045) and a non-significant association was observed among patients with severe COVID-19 (adjusted RR = 0.32; 95% CI, 0.07–1.56, p = 0.15). Of note, patients who received therapeutic anticoagulation had statistically higher 30-day mortality when compared to the standard and high-intensity prophylaxis cohorts (40% vs 15% vs 6%, respectively, p < 0.001). Of note, patients who received therapeutic anticoagulation had statistically higher rates of acute respiratory distress syndrome (27% vs 16% v 13%, respectively, p = 0.011) and AKI (54% vs 33% vs 31%, respectively, p = 0.024), suggesting that the higher 30-day mortality is likely due to the selection of a more critically ill population. Patients who received therapeutic anticoagulation had non-statistically higher rates of VTE when compared to the standard prophylaxis cohort (10% vs 5%, p = 0.19); however, 80% of the VTEs in this cohort was found on admission, leading to therapeutic anticoagulation as the initial strategy. Additional patient characteristics and outcomes stratified by intensity of anticoagulation are reported in Table 1. In severe COVID-19, D-dimer significantly increased during hospitalization with standard thromboprophylaxis (p < 0.001). However, D-dimer levels remained stable with high-intensity prophylaxis and decreased with therapeutic anticoagulation (Fig. 1).
It has been widely recognized that patients with COVID-19 have dramatically altered coagulation parameters and are prone to develop thrombotic complications in more severe cases with markedly elevated D-dimer levels associated with higher mortality [5]. Thus far the adoption of prophylactic LMWH in the management of patients hospitalized with COVID-19 is based upon prospective data.
The question remains on whether standard VTE prophylaxis is adequate for VTE prevention. In our retrospective study, we found that patients who initially received high-intensity prophylaxis or therapeutic anticoagulation had improved 30-day mortality without increased rates of bleeding. Of note is that patients in our study with severe COVID-19 showed only a non-significant improvement in 30-day mortality with initial high-intensity prophylaxis. Further examination of the risks and benefits of high-intensity prophylaxis in COVID-19 is therefore warranted.
The early initiation of therapeutic anticoagulation with unfractionated heparin to prevent clinical deterioration in patients with severe forms of COVID-19 is based upon the observation that higher fibrinogen levels in COVID-19 patients can lead to heparin resistance thereby reducing the efficacy of standard thromboembolic prophylaxis [6]. Higher levels of fibrinogen are also associated with increased risk of thrombosis [7]. As result, it has proposed that patients with COVID-19 may require more aggressive anticoagulation regimens, such as high-intensity prophylactic or therapeutic anticoagulation, to prevent clinical deterioration and multi-organ failure from thrombotic microangiopathy.
It has been recognized that elevations in D-dimer levels in COVID-19 patients is indicative of a hypercoagulable state and has been prognostic of in-hospital mortality [2]. In our study population, elevated D-dimer levels was associated with higher rates of VTE along with 30-day mortality. The hypercoagulable state has been thought to be attributed to dysfunction of endothelial cells through direct injury leading to excess thrombin generation leading to the thrombotic microangiopathy found on post-mortem examinations [3]. In addition, COVID-19 infection has been associated with an aggressive pro-inflammatory response which further contributes to hypercoagulability as described by Virchow's triad. Prior publications have detailed the anti-inflammatory properties of heparin and heparin-related products through the binding of inflammatory cytokines, inhibition of neutrophil chemotaxis and leukocyte migration, and the sequestration of acute phase reactants and complement [8]. Other properties unrelated to direct anticoagulation effects that have been described include the interaction with the S1 spike protein of COVID-19, preventing viral adhesion, attachment, and cellular entry leading to direct endothelial injury, thrombin generation, and downstream thrombotic microangiopathy [9,10]. In our study, the decrease in D-dimer levels during hospitalization in patients who received high-intensity prophylactic or therapeutic anticoagulation underscores both its potential efficacy in primary prevention of thrombosis and its anti-inflammatory effects in mitigating further thrombosis. This effect on D-dimer trends was not seen with standard prophylaxis in severe or non-severe COVID-19 which suggests that more intensive anticoagulation regimens are required for effective prevention of thrombosis in COVID-19.
Our study is limited by its single-institution, retrospective case-control design with a small sample sizes of patients initially treated with high-intensity thromboprophylaxis. Nonetheless, in hospitalized patients with mild or moderate forms of COVID-19, the use of high-intensity prophylactic anticoagulation may be an acceptable strategy for more aggressive anticoagulation, while in severe forms of COVID-19 high-intensity prophylactic or even therapeutic anticoagulation in the absence of thrombosis may be acceptable regimens. Although ultimately the utility of high-intensity prophylactic anticoagulation requires confirmation in an adequately powered clinical study, our results, when coupled with the wide availability and clinical familiarity of VTE prophylaxis, warrants consideration of high-intensity prophylaxis in the current standard of care treatment of COVID-19 patients.
The following are the supplementary data related to this article.
Univariate (unadjusted) and multivariable (adjusted) models for the association between various factors and 30-day mortality.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.thromres.2020.09.030.
Consent
This study was approved by the Institutional Review Board at Lifespan Health System.
CRediT authorship contribution statement
Andrew Hsu: Data curation, Investigation, Writing - original draft, Writing - review & editing, Validation. Yuchen Liu: Data curation, Investigation, Writing - original draft, Writing - review & editing, Validation. Adam S. Zayac: Data curation, Investigation, Writing - original draft, Writing - review & editing, Validation. Adam J. Olszewski: Formal analysis, Writing - original draft, Writing - review & editing, Validation. John L. Reagan: Formal analysis, Writing - original draft, Writing - review & editing, Validation.
Declaration of competing interest
The authors declare no conflicts of interest.
References
- 1.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Xang X. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. Apr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackermann M., Verleden S.E., Kuechnel M. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2015432. May 21. (Online ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang N., Bai H., Chen X. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Metlay J.P., Waterer G.W., Long A.C. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am. J. Respir. Crit. Care Med. 2019;200(7):e45–e67. doi: 10.1164/rccm.201908-1581ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harr J.N., Moore E.E., Chin T.L. Postinjury hyperfibrinogenemia compromises efficacy of heparin-based venous thromboembolism prophylaxis. Shock. 2014;41:33–39. doi: 10.1097/SHK.0000000000000067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klovaite J., Nordestgaard B.G., Tybjaerg-Hansen A. Elevated fibrinogen levels are associated with risk of pulmonary embolism, but not with deep venous thrombosis. Am. J. Respir. Crit. Care Med. 2013;187:286–293. doi: 10.1164/rccm.201207-1232OC. [DOI] [PubMed] [Google Scholar]
- 8.Li J.P., Vlodavsky I. Heparin, heparan sulfate and heparanase in inflammatory reactions. Thromb. Haemost. 2009;102(5):823–828. doi: 10.1160/TH09-02-0091. [DOI] [PubMed] [Google Scholar]
- 9.Mycroft-West C., Su D., Elli S. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. bioRxiv. 2020 doi: 10.1101/2020.02.29.971093. [DOI] [Google Scholar]
- 10.Ma J., Bai J. Protective effects of heparin on endothelial cells in sepsis. Int. J. Clin. Exp. Med. 2015;8(4):5547–5552. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Univariate (unadjusted) and multivariable (adjusted) models for the association between various factors and 30-day mortality.