Abstract
Objectives
The SARS-CoV-2 epidemic presents a poorly understood epidemiological cycle. We aimed to compare the age and weekly distributions of the five human coronaviruses, including SARS-CoV-2, that circulated in southeastern France.
Methods
We analyzed all available diagnoses of respiratory viruses, including SARS-CoV-2, performed between 09/2013 and 05/2020 at the University Hospital Institute Méditerranée Infection in Marseille, southeastern France.
Results
For SARS-CoV-2, positive children <15 years of age represented 3.4% (228/6,735) of all positive cases, which is significantly less than for endemic coronaviruses (46.1%; 533/1,156; p < 0.001). Among 10,026 patients tested for SARS-CoV-2 and endemic coronaviruses in 2020, children <15 years represented a significantly lower proportion of all positive cases for SARS-CoV-2 than for endemic coronaviruses [2.2% (24/1,067) vs. 33.5% (149/445), respectively; p < 0.001]. Epidemic curves for endemic coronaviruses and SARS-CoV-2 in 91,722 patients showed comparable bell-shaped distributions with a slight time lag. In contrast, the age distribution of endemic coronaviruses and 14 other respiratory viruses differed significantly compared to that of SARS-CoV-2, which was the only virus to relatively spare children.
Conclusions
We observed for SARS-CoV-2 a temporal distribution resembling that of endemic coronaviruses but an age distribution that relatively spares the youngest subjects, who are those the most exposed to endemic coronaviruses.
Keywords: SARS-CoV-2, Endemic coronavirus, Age, Children, Cross-immunity, Southeastern France
Introduction
The SARS-CoV-2 epidemic, which apparently started in December 2019 in China (Wu and McGoogan, 2020), currently presents a poorly understood epidemiological cycle. In China, Korea, and now in Europe, it seems to have had a bell-shaped distribution (https://coronavirus.jhu.edu/data/new-cases; https://www.mediterranee-infection.com/covid-19/) as is typical for viral respiratory infections. Furthermore, we and others have shown that detection of SARS-CoV-2 in children is rare, as are clinical cases (Colson et al., 2020, Gudbjartsson et al., 2020, Jones et al., 2020, Li et al., 2020, Wu and McGoogan, 2020). In three extensive studies, children under ten years of age accounted for <1%, 0%, and 1.3% of SARS-CoV-2 cases in China (Wu and McGoogan, 2020), Iceland (Gudbjartsson et al., 2020), and Germany (Jones et al., 2020), respectively. The fate of this epidemic remains unknown, but we found it interesting to compare the age and weekly distribution of the five human coronaviruses, including SARS-CoV-2, that circulated in southeastern France in order to compare the temporal and age distribution of these different viruses.
Methods
We analyzed all available diagnoses of respiratory viruses, including SARS-CoV-2, performed between September 2013 and May 2020 at the clinical microbiology and virology laboratory of the University Hospital Institute Méditerranée Infection (https://www.mediterranee-infection.com/) and the University Hospitals of Marseille, the second-largest French city, southeastern France. Testing of respiratory samples was performed using the FTD Respiratory pathogens 21 (Fast Track Diagnosis, Luxembourg), the Biofire FilmArray Respiratory panel 2 plus (Biomérieux, Marcy-l'Etoile, France), the Respiratory Multi-Well System r-gene (Argene, BioMérieux), or the GeneXpert Xpert Flu/RSV (Cepheid, Sunnyvale, CA) assays, or by one-step simplex real-time quantitative RT-PCR amplifications as previously reported (Hoang et al., 2019). Diagnosis by reverse transcription-PCR of SARS-CoV-2 infection was performed as previously described (Amrane et al., 2020). This study retrospectively analyzed patients' data from the hospital information system (RGPD/APHM 2019-73). Statistics were performed using OpenEpi version 3.01 software (https://www.openepi.com/Menu/OE_Menu.htm); a p-value < 0.05 was considered significant. Moreover, epidemic curves were analyzed by Markov Chain Monte Carlo fitting of five commonly used distributions with different skewnesses (Normal, Log-normal, Gamma, Weibull, Gompertz) using R-4.0.1 (https://www.r-project.org/). Distributions with the best goodness-of-fit criteria [Akaike's Information Criterion, (AIC)] were chosen, and their parameters bootstrapped.
Results
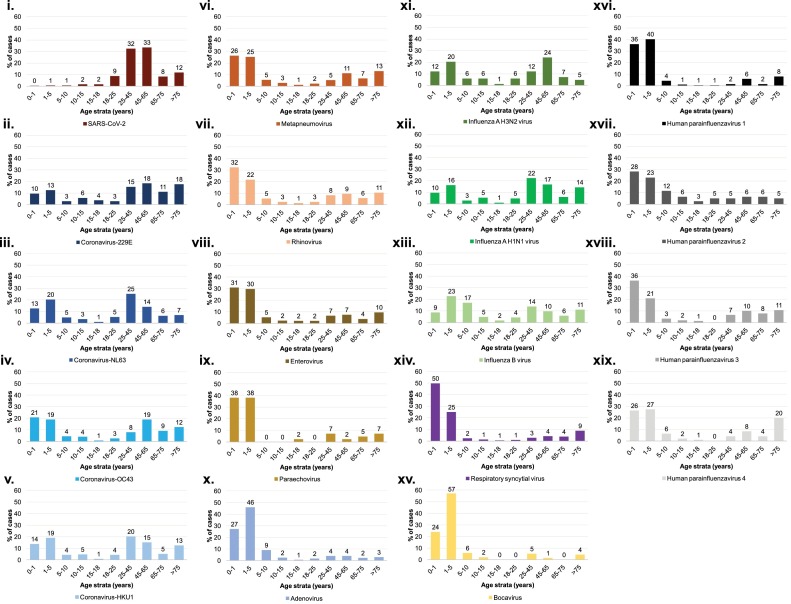
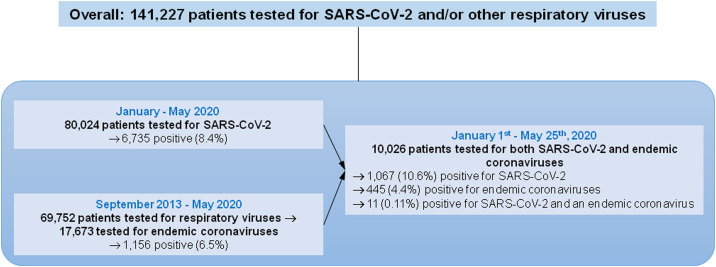
First, we analyzed all available diagnoses of SARS-CoV-2 and other respiratory viruses for 141,227 patients. Between January and May 2020, we tested respiratory samples from 80,024 patients for SARS-CoV-2 and found 6,735 (8.4%) positive (Figure 1 ). In addition, between September 2013 and May 2020, we tested respiratory samples from 69,752 patients for respiratory viruses. Of these, 17,673 were tested for endemic coronaviruses (HCoV-229E, HCoV-NL63, HCoV-OC43, HCoV-HKU1) and 1,156 (6.5%) were positive. For SARS-CoV-2, positive children under 15 years represented 3.4% (228/6,735) of all positive patients. This proportion was significantly lower than that for endemic coronaviruses (46.1%; 533/1,156; p < 0.001, Chi–square test). In fact, positive patients in each group 0−1 year, 1−5 years, 5−10 years, and 10−15 years represented significantly lower proportions of all positive patients when comparing SARS-CoV-2 to other endemic coronavirus infections (Table 1 ). Compared to SARS-CoV-2-positive patients, those infected with endemic coronaviruses or other respiratory viruses were significantly more likely to be <10 years of age (Figure 1). Therefore, this age group accounted for 1.8% of SARS-CoV-2 cases compared to between 25.0% (for HCoV-229E) and 87.0% (for bocavirus) of infections with other respiratory viruses (p < 0.05 for all comparisons).
Figure 1.
Age distribution of the proportions of patients diagnosed with coronaviruses and other respiratory viruses compared to the total population tested.
(i) SARS-CoV-2; (ii) Coronavirus-229E; (iii) Coronavirus-NL63; (iv) Coronavirus-OC43; (v) Coronavirus-HKU1; (vi) Metapneumovirus; (vii) Rhinovirus; (viii) Enterovirus; (ix) Paraechovirus; (x) Adenovirus; (xi) Influenza A H3N2 virus; (xii) Influenza A H1N1 virus; (xiii) Influenza B virus; (xiv) Respiratory syncytial virus; (xv) Bocavirus; (xvi) Human parainfluenzavirus 1; (xvii) Human parainfluenzavirus 2; (xviii) Human parainfluenzavirus 3; (xix) Human parainfluenzavirus 4.
Table 1.
Number of cases per age group for all patients tested for SARS-CoV-2 or for endemic coronaviruses, and proportion of all tested patients per age group.
| Age group | SARS-CoV-2 |
Endemic CoV |
P* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (years) | Tested |
Positive |
Tested |
Positive |
|||||
| N | %** | N | %** | N | %** | N | %** | ||
| 0−1 | 796 | 1.0 | 32 | 0.5 | 2 412 | 14.6 | 207 | 17.9 | <0.001 |
| 1−5 | 1 453 | 1.8 | 40 | 0.6 | 1 661 | 11.3 | 217 | 18.8 | <0.001 |
| 5−10 | 1 231 | 1.5 | 50 | 0.7 | 628 | 4.9 | 65 | 5.6 | <0.001 |
| 10−15 | 1 197 | 1.5 | 106 | 1.6 | 366 | 3.0 | 44 | 3.8 | <0.001 |
| 15−18 | 1 090 | 1.4 | 118 | 1.8 | 202 | 1.6 | 12 | 1 | 0.051 |
| 18−25 | 6 680 | 8.3 | 594 | 8.8 | 409 | 3.9 | 43 | 3.7 | <0.001 |
| 25−45 | 27 059 | 33.6 | 2 184 | 32.4 | 1 502 | 14.9 | 165 | 14.3 | <0.001 |
| 45−65 | 24 487 | 30.4 | 2 257 | 33.5 | 2 250 | 18.3 | 176 | 15.2 | <0.001 |
| 65−75 | 6 545 | 8.1 | 560 | 8.3 | 1 419 | 10.3 | 79 | 6.8 | 0.050 |
| >75 | 9 986 | 12.4 | 794 | 11.8 | 2 528 | 17.2 | 148 | 12.8 | 0.175 |
| Total | 80 524 | 100.0 | 6 735 | 100.0 | 17 673 | 100.0 | 1 156 | 100.0 | – |
Yates-corrected Chi–square test.
Proportion of cases in the age group compared to the total number of cases.
Second, we analyzed 10,026 patients who were tested for both SARS-CoV-2 and endemic coronaviruses between January 1st and May 25th, 2020. A total of 1,067 patients (10.6%) were SARS-CoV-2- positive, and 445 (4.4%) were diagnosed with endemic coronaviruses. Children under 15 years of age accounted for a significantly lower proportion of all positive cases for SARS-CoV-2 than for endemic coronaviruses [2.2% (24/1,067) vs. 33.5% (149/445), respectively; p < 0.001] as was the case in each age group: 0−1 year, 1−5 years, 5−10 years and 10−15 years (Figure 1i–v, Table 2 ). Only eleven (0.11%) patients were infected with SARS-CoV-2 and an endemic coronavirus. They represented a significantly lower proportion than the proportion of SARS-CoV-2-positive patients among those negative for endemic coronaviruses [11/445 (2.5%) vs 1,056/9,581 (11.0%); p < 0.001]. None of these eleven patients was under 18 years of age.
Table 2.
Number of cases per age group for SARS-CoV-2 or endemic coronaviruses for patients tested for all five coronaviruses, and proportion of all tested patients per age group.
| Age group (years) | Tested |
SARS-CoV-2-positive |
Endemic CoV-positive |
P* | |||
|---|---|---|---|---|---|---|---|
| N | %*** | N | %*** | N | %*** | ||
| 0−1 | 477 | 4,8 | 11 | 1.0 | 41 | 9.2 | <0.001 |
| 1−5 | 715 | 7,1 | 5 | 0.5 | 68 | 15.3 | <0.001 |
| 5−10 | 402 | 4,0 | 4 | 0.4 | 18 | 4.0 | <0.001** |
| 10−15 | 270 | 2,7 | 4 | 0.4 | 22 | 4.9 | <0.001** |
| 15−18 | 160 | 1,6 | 11 | 1.0 | 5 | 1.1 | 0.454 |
| 18−25 | 590 | 5,9 | 75 | 7.0 | 25 | 5.6 | 0.186 |
| 25−45 | 2321 | 23,1 | 245 | 23.0 | 109 | 24.5 | 0.283 |
| 45−65 | 2491 | 24,8 | 385 | 36.1 | 83 | 18.7 | <0.001 |
| 65−75 | 1002 | 10,0 | 128 | 12.0 | 26 | 5.8 | <0.001 |
| >75 | 1598 | 15,9 | 199 | 18.7 | 48 | 10.8 | <0.001 |
| Total | 10026 | 100,0 | 1067 | 100.0 | 445 | 100.0 | – |
Yates-corrected Chi–square test.
Fischer exact test.
Proportion of cases in the age group compared to the total number of cases.
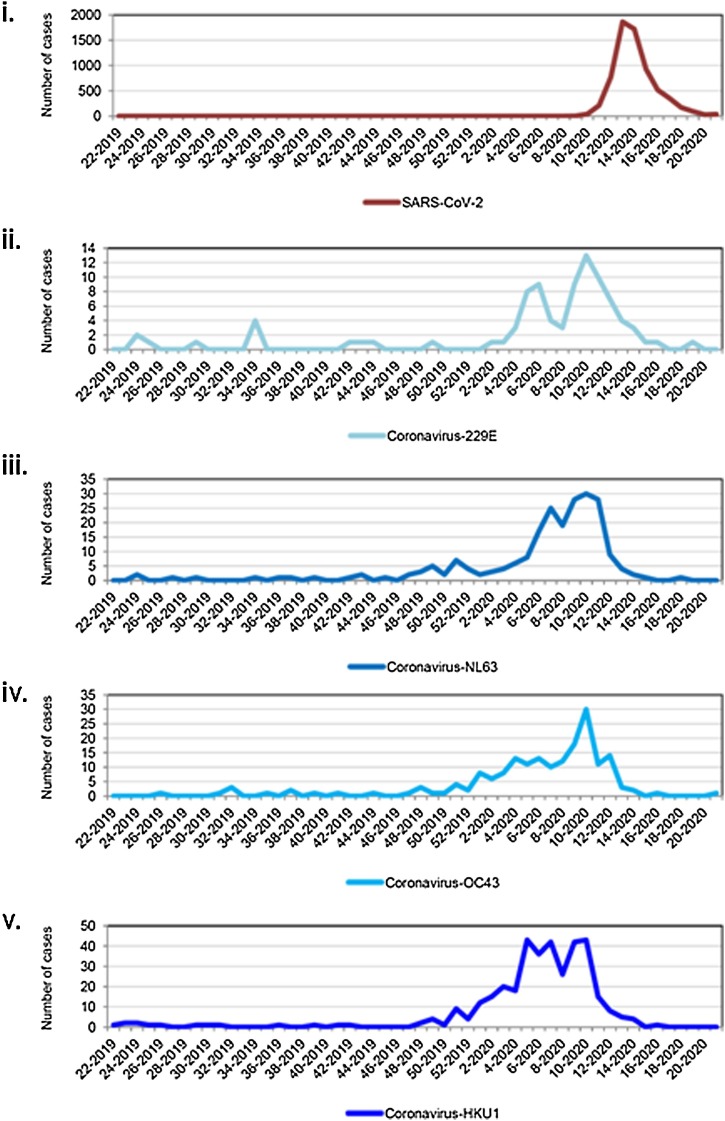
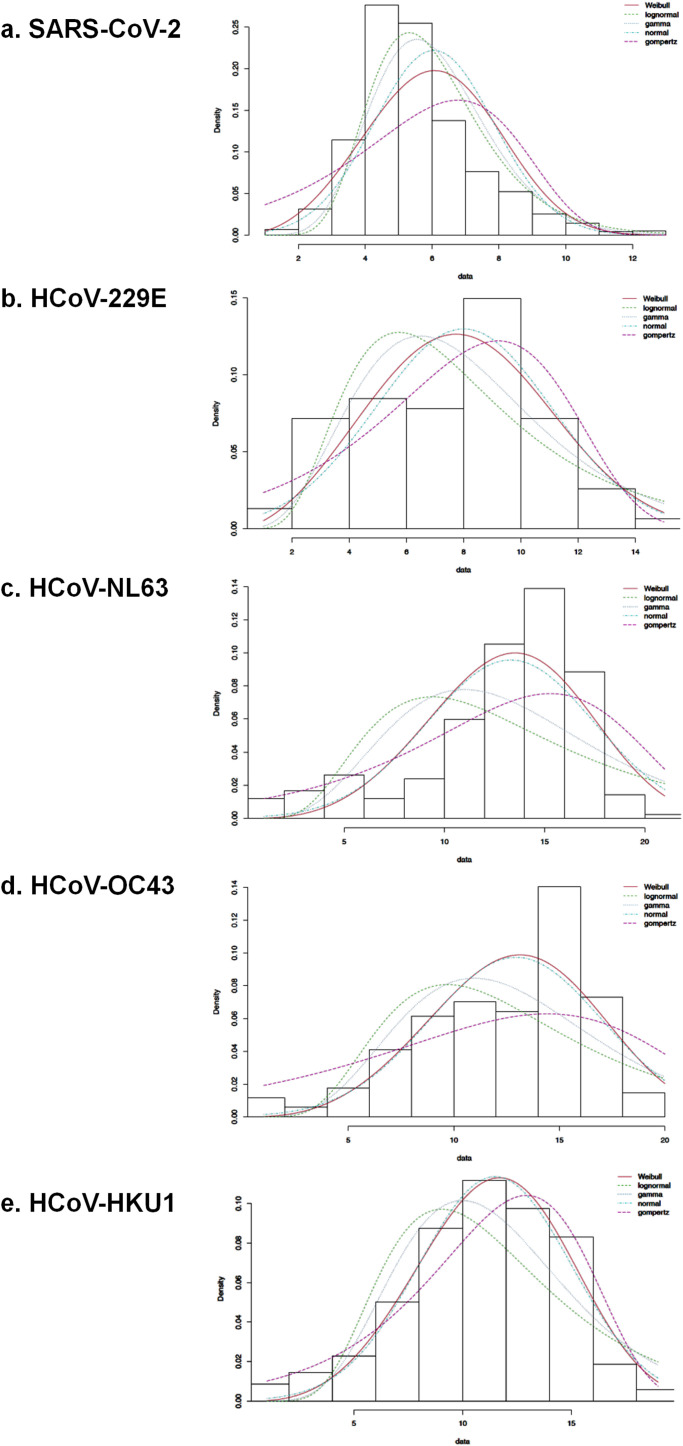
Moreover, over one year (from June 2019 to May 2020), we observed that epidemic curves were comparable for the four endemic coronaviruses and SARS-CoV-2 (Figure 2B). Cases of endemic coronavirus increased in December 2019, peaked in mid-March 2020, and ended in early April, while cases of SARS-CoV-2 increased in early March, peaked in late March, and nearly ended in mid-May. The fitted distributions reflected three kinds of epidemic curves (Supplementary Figure 1). SARS-CoV-2 fitted with a left-skewed Gamma distribution (AIC = 26345.6). HCoV-OC43 fitted with a quasi-symmetric curve and Normal distribution (AIC = 971.4). Epidemic curves of HCoV-229E, HCoV-NL63, and HCoV-HKU1 were right-skewed and fitted with a Gompertz distribution (AIC = 394.5, 1191.2, and 1861.2, respectively).
Figure 2.
Number of patients positive for coronaviruses over one year from June 2019 through May 2020.
(i) SARS-CoV-2; (ii) HCoV-229E; (iii) HCoV-NL63; (iv) HCoV-OC43; (v) HCoV-HKU1.
The X-axis corresponds to weeks and years (week-year).
Discussion
In this large study, two elements are particularly noteworthy. First, in our geographical area the temporal distributions of infections by all coronaviruses are comparable. Thus, all five viruses exhibit a bell-shaped incidence curve, and their circulation stopped in the spring, suggesting that this is the natural SARS-CoV-2 epidemic pattern. Hence, we can speculate for temperate countries, including Europe, that SARS-CoV-2 could reappear seasonally during winter and circulate epidemically until spring. Alternatively, SARS-CoV-2 might disappear in the absence of an asymptomatic human chronic carriage, like SARS-CoV-1 (Raoult et al., 2020). Second, the age distribution of SARS-CoV-2 cases largely spares children, which is radically different from other coronavirus and respiratory virus infections. Thus, SARS-CoV-2 is the only one we analyzed that does not significantly affect children. Therefore, its epidemiology cannot be predicted based on previous knowledge of viral respiratory diseases. The simplest explanation for this difference is that a substantial proportion of children, particularly those under five years of age, may have acquired immunity to endemic coronaviruses that infect young children with high frequencies (Raoult et al., 2020, Zhou et al., 2013). Indeed, there is evidence that part of the population exhibited immune responses against SARS-CoV-2 before the epidemic, supporting the hypothesis of cross-immunity between endemic coronaviruses and the new coronavirus. Thus, in the US, circulating SARS-CoV-2-specific CD4+ and CD8+ T cells were detected in ≈20−60% of unexposed individuals sampled in 2015–2018 (Grifoni et al., 2020). In the UK, IgG to SARS-CoV-2 were detected in 15% of SARS-CoV-2-uninfected patients with recent HCoV infection and in 10% of SARS-CoV-2-uninfected pregnant women (Ng et al., 2020). Also, we detected IgM to SARS-CoV-2 at titers ≥1:100 in 9/50 patients with endemic coronaviruses (Edouard et al., 2020). It is also worth noting that the coinfection rate observed here with SARS-CoV-2 and another coronavirus was very low (0.1%) and that SARS-CoV-2-positivity was significantly lower among patients positive than negative for an endemic coronavirus, which supports the hypothesis of a protective cross-immunity.
Overall, we believe that this work contributes to the understanding of the epidemiology of SARS-CoV-2, which has a temporal distribution resembling that of endemic coronaviruses but an age distribution that largely spares the youngest subjects, who are precisely those the most frequently exposed to endemic coronaviruses and may have consequently acquired protective immunity. Susceptibility to SARS-CoV-2 in the elderly perhaps reflects the loss of immunity acquired during childhood or changes in social organization that occurred during recent decades. Indeed, a small proportion of people over the age of 50 lived in communities with very young children, whereas women’s work evolution has led to much earlier socialization of children. Finally, the fact that age distributions for infections by SARS-CoV-2 and other respiratory viruses differ, underscores that real data collection and real-time analysis are critical in the event of an outbreak to decipher the epidemiology of emerging pathogens.
Ethics
All data have been generated as part of the routine work at Assistance Publique-Hôpitaux de Marseille (Marseille university hospitals), and this study results from routine standard clinical management. Access to the patients’ biological and registry data from the hospital information system was approved by the data protection committee of Assistance Publique-Hôpitaux de Marseille (APHM). It was recorded in the European General Data Protection Regulation registry under number RGPD/APHM 2019-73. This study has been approved by our institution’s ethics committee (N°: 2020-029). The authors have no conflicts of interest to declare. Funding sources had no role in the study's design and conduct, collection, management, analysis and interpretation of the data, and preparation, review, or approval of the manuscript.
Conflict of interest
None declared.
Acknowledgments
This work was supported by the French Government under the “Investments for the Future” program managed by the National Agency for Research (ANR), Méditerranée-Infection 10-IAHU-03 and was also supported by Région Provence Alpes Côte d’Azur and European funding FEDER PRIMMI (Fonds Européen de Développement Régional-Plateformes de Recherche et d'Innovation Mutualisées Méditerranée Infection), FEDER PA 0000320 PRIMMI. We are very grateful to Laurence Thomas, Elsa Prudent and Céline Gazin for their technical help.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ijid.2020.09.1417.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- Amrane S., Tissot-Dupont H., Doudier B., Eldin C., Hocquart M., Mailhe M. Rapid viral diagnosis and ambulatory management of suspected COVID-19 cases presenting at the infectious diseases referral hospital in Marseille, France, -January 31st to March 1st, 2020: a respiratory virus snapshot. Travel Med Infect Dis. 2020:101632. doi: 10.1016/j.tmaid.2020.101632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Tissot-Dupont H., Morand A., Boschi C., Ninove L., Esteves-Vieira V. Children account for a small proportion of diagnoses of SARS-CoV-2 infection and do not exhibit greater viral loads than adults. Eur J Infect Dis Clin Microbiol. 2020;39:1983–1987. doi: 10.1007/s10096-020-03900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edouard S., Colson P., Melenotte C., De Pinto F., Thomas L., La Scola B. Preprint MedRxiv; 2020. Evaluating the serological status of COVID-19 patients using an indirect immunofluorescent assay, France. URL: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. S0092-8674(20)30610-30613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson D.F., Helgason A., Jonsson H., Magnusson O.T., Melsted P., Norddahl G.L. Spread of SARS-CoV-2 in the icelandic population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang V.T., Goumballa N., Dao T.L., Ly T.D.A., Ninove L., Ranque S. Respiratory and gastrointestinal infections at the 2017 Grand Magal de Touba, Senegal: a prospective cohort survey. Travel Med Infect Dis. 2019;32:101410. doi: 10.1016/j.tmaid.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones Tc, Muhlemann B., Veith T., Zuchowski M., Hofman J., Stein A. An analysis of SARS-CoV-2 viral load by patient age. medRxiv preprint. 2020 doi: 10.1101/2020.06.08.20125484. [DOI] [Google Scholar]
- Li X., Xu W., Dozier M., He Y., Kirolos A., Theodoratou E. The role of children in transmission of SARS-CoV-2: a rapid review. J Glob Health. 2020;10:011101. doi: 10.7189/jogh.10.011101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng K., Faulkner N., Cornish G., Rosa A., Earl C., Wrobel A. Pre-existing and de novo humoral immunity to SARS-CoV-2 in humans. bioRxiv preprint. 2020 doi: 10.1101/2020.05.14.095414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020:2762130. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zhou W., Wang W., Wang H., Lu R., Tan W. First infection by all four non-severe acute respiratory syndrome human coronaviruses takes place during childhood. BMC Infect Dis. 2013;13:433. doi: 10.1186/1471-2334-13-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.