Although various pharmacologic agents are under active study [1], there are currently no effective and evidence-based antiviral drugs or drug combinations against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) infection, namely coronavirus disease 2019 (COVID-19). Therefore, supportive therapy and active treatment of COVID-19 clinical manifestations by using feasible and currently available agents, especially Food and Drug Administration (FDA)-approved drugs, still remains an essential therapeutic approach [[2], [3], [4]].
To this regard, great interest has risen with respect to the potential beneficial effects against COVID-19 of drugs that are currently used for cardiovascular prevention [3,5]. Indeed, although the main clinical manifestations of COVID-19 involve the respiratory system, an increased risk of cardiovascular complications, including myocarditis, cardiac arrhythmias, and arterial and venous thrombosis, has been reported in COVID-19 patients [[6], [7], [8]]. In addition, underlying cardiovascular diseases (CVDs) and/or cardiovascular risk factors (e.g., smoking, diabetes, obesity) have been associated with an increased risk of severe clinical complications and death in COVID-19 patients [[6], [7], [8], [9], [10], [11], [12]].
Whether statins may be re-purposed to treat COVID-19 patients is a matter of debate. There are several points that are worthy of being considered in support of possible beneficial effects of statins against COVID-19. First, statins are widespread, available, low-cost, and safe cholesterol-lowering drugs, that have been extensively demonstrated to reduce significantly CVD risk [13,14]. Each mmol/l reduction in LDL-cholesterol (LDL-C) reduces the risk of major cardiovascular events by about one quarter for each year statin therapy is continued [13,14]. Therefore, it is likely that statins may also mitigate CVD risk in COVID-19 patients. Second, due to their cholesterol-lowering activity and their pleiotropic effects, statins can inhibit inflammation, immune response, and oxidative stress [[15], [16], [17]], exert direct antiviral effects [18], improve endothelial function [[19], [20], [21]], and regulate hemostasis [22,23], potentially reducing the incidence of severe clinical manifestations and improving prognosis in COVID-19 patients. This latter point will be discussed in more depth in the following text.
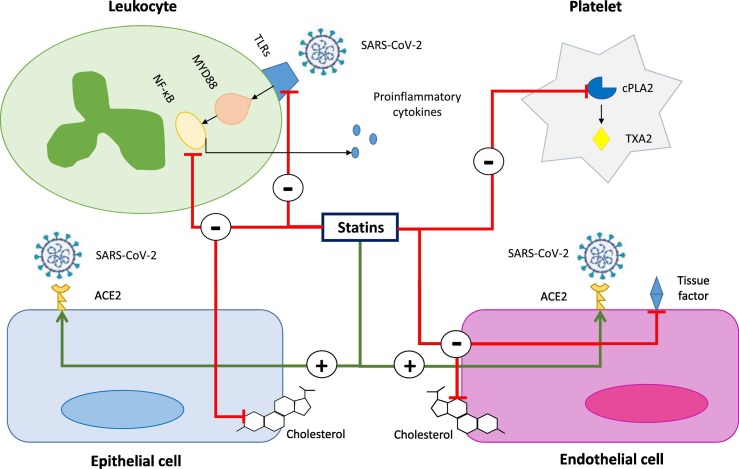
Statins may conceivably protect against inflammation by controlling cytokine overexpression and modulating immune responses [15,16], thereby potentially preventing the development of acute distress respiratory syndrome (ARDS) and reducing the incidence of cardiovascular complications in COVID-19 patients. Indeed, statins have been shown to directly inhibit nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kB) (Fig. 1 ), which is a crucial mediator of inflammatory responses during infections, including those caused by coronaviruses [24]. In addition, they have been reported to negatively regulate the expression of toll-like receptor-4 (TLR4) and consequent activation of the TLR4-myeloid differentiation primary response (MYD)88-NF-kB signaling pathway, which plays a critical role in the recognition of pathogens and induction of the innate immune response against viral infections [25,26]. By counteracting cytokine storm statins may reduce the risk of myocardial injury and myocarditis in acute phases of COVID-19 [27]. In addition, statin-mediated anti-inflammatory effects may also promote stabilization of atherosclerotic plaques, thereby protecting from the occurrence of plaque rupture and cardiovascular events [8]. These effects provide an argument in support of statin continuation, even at high doses, for mitigating cardiovascular risk in the acute phases of COVID-19, especially in patients with manifest atherosclerotic CVD or CVD risk factors. Indeed, COVID-19-driven inflammation might promote atherosclerotic plaque instability [27]. In addition, although statin-mediated stabilization of atherosclerotic plaques is known to be time-dependent, there are reports showing that some modulation of inflammation and some reduction of plaque remodelling may be obtained also after a short period of treatment with high doses of statins [28,29]. However, it should be emphasized that they are currently lacking clinical studies showing a significant impact of statin therapy in reduction of incident cardiovascular events in COVID-19 patients.
Fig. 1.
Potential cellular pathways modulated by statins in SARS-CoV-2 infection. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interacts strongly with the human angiotensin-converting enzyme 2 (ACE2), allowing the virus to enter host cells, including endothelial and epithelial cells. Statins can upregulate the expression of ACE2 on the surface of endothelial and epithelial cells. Recognition of SARS-CoV-2 by Toll-like receptors (TLRs) on the surface of leukocytes activates the MYD88–NF-κB pathway and the innate immune response of the host, with the release of proinflammatory mediators. Statins can inhibit the MYD88–NF-κB proinflammatory pathway and the production of inflammatory cytokines. Statins can exert anti-thrombotic effects by reducing the expression of tissue factor on activated endothelial cells and decreasing the cytosolic phospholipase A2 (cPLA2)-induced thromboxane A2 (TXA2) synthesis by platelets. Statins could reduce cholesterol content in the plasma membrane of SARS-CoV-2 host cells, thereby destabilizing viral replication phases. Through all these mechanisms, statins might prove beneficial effects in COVID-19 patients.
Statins can exert some direct antiviral activity [18,30]. By inhibiting cholesterol synthesis, they can reduce the intracellular availability of a crucial compound for the viral cell cycle [31]. Indeed, the presence of cholesterol-rich subdomains on the plasma membrane of host cells, namely lipid rafts, is crucial for viral fusion and entry [18,30]. Furthermore, through cholesterol-dependent mechanisms, statins can inhibit the isoprenylation of different proteins (e.g., RhoA, Rac, and Cdc42), which are critical downstream molecules regulating viral cell cycle [18,30]. To date, a number of observational studies have suggested that statin therapy can reduce the risk of various severe complications and mortality in patients admitted with Middle East respiratory syndrome coronavirus (MERS-CoV) infection and influenza [[32], [33], [34], [35]]. Therefore, it is possible that the beneficial effects of statin therapy as an add-on treatment in viral infections could be extended to COVID-19. To this regard, it should be considered that a peculiar antiviral mechanism of action of statins against COVID-19 might consist of inhibition of SARS-CoV-2 entry into host cells due to the binding of its main protease [36]. Nonetheless, whether statin therapy may reduce the risk of SARS-CoV-2 infection still needs to be investigated.
Statins have well-known protective effects against endothelial dysfunction and injury [[19], [20], [21]]. Due to their ability to upregulate ACE2 signaling pathways via epigenetic histone modifications, statins might exert some beneficial effects in terms of endothelial protection in the supportive therapy of COVID-19 patients [37]. Indeed, high levels of ACE2 on pulmonary endothelium have been associated with reduced severity of ARDS [38]. In addition, by promoting endothelial repair statins might counteract SARS-CoV-2-induced endothelitis in lungs as a direct consequence of both infection of endothelial cells and the host inflammatory response [39,40], thereby potentially accelerating recovery from ARDS.
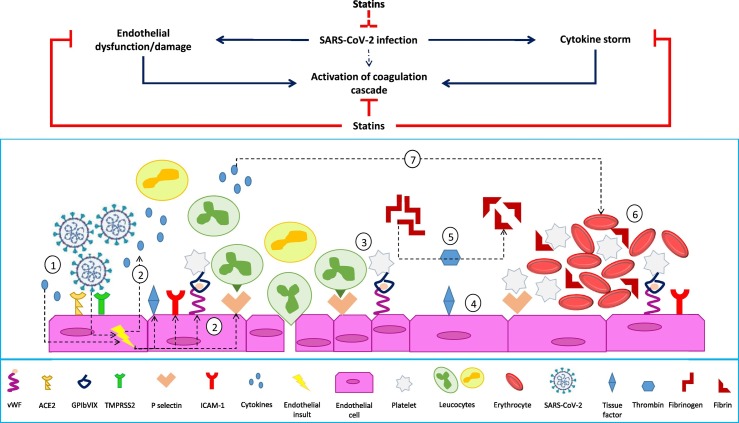
By inhibiting the activation of coagulation cascade and platelet function as well as increasing fibrinolytic activity, statins can exert antithrombotic effects [22] (Fig. 2 ). Specifically, different statins have been shown to directly interfere with tissue factor expression, thrombin generation, fibrinogen cleavage, factor V and factor XIII activation, endothelial thrombomodulin expression, and platelet activation [22]. In addition, some inhibitory activity of statins on thrombus formation has been ascribed to their ability to modulate endothelial function and inflammatory response [22] (Fig. 2). Arterial and venous thromboembolic events have been described as typical clinical features of severe COVID-19 [[6], [7], [8]]. Therefore, albeit being still not proved, it is plausible that statins might be useful to improve clinical outcomes of COVID-19 by reducing the incidence of COVID-19-related coagulopathy.
Fig. 2.
The possible statin-mediated modulation of SARS-CoV-2 infection pro-thrombotic profile. Endothelial activation and release of pro-inflammatory cytokines induced by SARS-CoV-2 (1) lead to increased expression of adhesion molecules (ICAM-1, P-selectins, von Willebrand factor, αvβ3) and further release of proinflammatory cytokines (2), promoting the recruitment of platelets and leukocytes (3). Activated endothelial cells also express tissue factor, which promotes the activation of factor VII, factor Xa, and the generation of thrombin (4). Thrombin cleaves fibrinogen into fibrin (5), which is crucial for thrombus formation (6). Pro-inflammatory mediators may also activate the coagulation cascade and influence platelet activation, promoting accelerated thrombus formation (7). Statins can inhibit the release of proinflammatory cytokines by endothelial cells, as well as the activation of coagulation cascade. Also, statins might inhibit SARS-CoV-2 entry into endothelial cells expressing ACE2 and subsequent endothelial activation.
To date, most of available data from clinical studies support the protective effect of statins against SARS-CoV-2 infection. Indeed, a number of retrospective studies have shown lower inflammatory parameters, decreased incidence of severe clinical manifestations or reduced mortality rates in COVID-19 patients under statin treatment as compared to those not taking statins [41,42]. However, consistent evidence from prospective studies is not currently available. In addition, two recent meta-analyses of observational studies exploring the impact of statin therapy on COVID-19 outcomes have reported contrasting results [43,44]. Therefore, clinical trials investigating this issue are eagerly awaited.
Against the hypothesis of a clinical benefit of statin use in COVID-19 patients, some safety concerns need to be taken into account. The main doubt about the beneficial effects of statins as add-on therapy in COVID-19 may be raised when considering the possible detrimental impact of reduced low-density lipoprotein (LDL) cholesterol levels on COVID-19 prognosis, as suggested by some retrospective studies [45,46]. However, reverse causality (i.e., viral infection as a cause of LDL cholesterol reduction) instead of causality (i.e., LDL cholesterol reduction as a factor promoting viral infection) might explain the association between LDL cholesterol and severe COVID-19 manifestations [47]. This could distract from refuting the potential benefits of statin treatment in this clinical setting.
Additional safety concerns may be raised considering the ability of statins to upregulate the expression of angiotensin-converting enzyme 2 (ACE2), which mediates SARS-CoV-2 entry into host cells [37,48]. However, such an effect may be clinically not significant if we assume that viral load is not necessarily related to the disease severity. Moreover, soluble ACE2 may bind to SARS-CoV-2, preventing it from fusion with the membrane of host cells and, therefore, inhibiting viral replication [48].
Another issue is represented by the risk of statin-related myotoxicity and hepatotoxity, which may be increased by drug-to-drug interactions between statins and antiviral, antiretroviral, antiparasitic, and antirheumatic drugs as well as antibiotics (mainly macrolides) that may be concomitantly administered to COVID-19 patients [49]. In some cases, either discontinuation of statin therapy or continuation with caution and at lower doses is possible options [49]. Nonetheless, when statin discontinuation is required, other lipid-lowering therapies could be considered, especially in patients at high CVD risk, which are more prone to undergo COVID-19 complications [49,50].
An additional reason for caution about statin therapy in COVID-19 patients may be the possible statin-mediated increase of lipoprotein(a), which is known to exert an anti-fibrinolytic activity by controlling the activity of plasminogen activators [51]. However, it should be emphasized that statin-mediated increasing effect on lipoprotein(a) is not consistent and in some cases may be not significant [52,53]. Therefore, it remains uncertain as to whether an increased thrombotic risk may be expected by statin impact on lipoprotein(a) levels in COVID-19 patients.
Further concerns are related to the observation that treatment with high-potency statins may be associated with an increased risk of incident type 2 diabetes, especially in obese patients [54]. Indeed, diabetes is a significant predictor of COVID-19 severe clinical manifestations. However, the risk of developing insulin resistance with initiation of statin therapy is relatively low according to available data [55]. Therefore, whether statin-induced diabetes may be a reason to discourage statin use in COVID-19 patients is questionable.
Finally, the benefit-risk balance of statin therapy should be carefully evaluated in older patients with COVID-19. Indeed, beyond being at higher risk of poor prognosis, these patients are also at higher risk of statin-related adverse effects [56].
Overall, available data and hypotheses based on biological plausibility do not support the notion that statin therapy may worsen the prognosis of patients with COVID-19. Conversely, they suggest that some clinical benefits in COVID-19 patients may derive from statin-mediated cholesterol-lowering and pleiotropic effects. To this regard, although no clinical studies are currently available clearly showing that statins can reduce the incidence of cardiovascular events in COVID-19 patients, evidence is emerging showing a possible benefit from statin therapy in reduction of COVID-19 severity and mortality. Therefore, the continuation of statin therapy in COVID-19 patients should be considered [49,57]. Further, despite a current lack of direct information in COVID-19 subjects, observational and interventional studies appear warranted to establish both the efficacy and safety of de novo initiation of statin therapy as add-on treatment for the management of COVID-19.
CRediT authorship contribution statement
AS, SG and VB conceived the study and designed the review. SG and VB wrote the first draft. AS, PEP, MP, MB and GFW critically revised the text. All authors approved the final version.
Declaration of competing interest
MB has served on the speakers bureau of Abbott/Mylan, Abbott Vascular, Actavis, Akcea, Amgen, Biofarm, KRKA, MSD, Sanofi-Aventis, Servier and Valeant, and has served as a consultant to Abbott Vascular, Akcea, Amgen, Daichii Sankyo, Esperion, Lilly, MSD, Resverlogix, Sanofi-Aventis; Grants from Sanofi and Valeant; GFW has received honoraria for lectures and advisory boards for Sanofi, Regeneron, Kowa, and Amgen; PEP owns four shares in Astra Zeneca PLC and has received travel/speaker's fees from Amgen Inc. The other authors have no competing interests to declare.
References
- 1.Mahase E. Covid-19: what treatments are being investigated? BMJ. 2020;368:m1252. doi: 10.1136/bmj.m1252. [DOI] [PubMed] [Google Scholar]
- 2.Fajgenbaum D.C., Khor J.S., Gorzewski A., Tamakloe M.A., Powers V., Kakkis J.J. Treatments administered to the first 9152 reported cases of COVID-19: a systematic review. Infect Dis Ther. 2020:1–15. doi: 10.1007/s40121-020-00303-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianconi V., Violi F., Fallarino F., Pignatelli P., Sahebkar A., Pirro M. Is acetylsalicylic acid a safe and potentially useful choice for adult patients with COVID-19? Drugs. 2020;23:1–14. doi: 10.1007/s40265-020-01365-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zahedipour F., Hosseini S.A., Sathyapalan T., Majeed M., Jamialahmadi T., Al-Rasadi K. Potential effects of curcumin in the treatment of COVID-19 infection. Phytother Res. 2020;10 doi: 10.1002/ptr.6738. 1002/ptr.6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bikdeli B., Madhavan M.V., Jimenez D., Chuich T., Dreyfus I., Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–6. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1–8. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 9.American College of Cardiology COVID-19 clinical guidance for the cardiovascular care team. 2020. https://www.acc.org/latest-in-cardiology/features/~/media/Non-Clinical/Files-PDFs-Excel-MS-Word-etc/2020/02/S20028-ACC-Clinical-Bulletin-Coronavirus.pdf
- 10.Palaiodimos L., Kokkinidis D.G., Li W., Karamanis D., Ognibene J., Arora S. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hill M.A., Mantzoros C., Sowers J.R. Commentary: COVID-19 in patients with diabetes. Metabolism. 2020;107:154217. doi: 10.1016/j.metabol.2020.154217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Society of Cardiology ESC guidance for the diagnosis and management of CV disease during the COVID-19 pandemic. 2020. https://www.escardio.org/Education/COVID-19-and-Cardiology/ESC-COVID-19-Guidance
- 13.Johnston T.P., Korolenko T.A., Pirro M., Sahebkar A. Preventing cardiovascular heart disease: promising nutraceutical and non-nutraceutical treatments for cholesterol management. Pharmacol Res. 2017;120:219–225. doi: 10.1016/j.phrs.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 14.Borén J., Chapman M.J., Krauss R.M., Packard C.J., Bentzon J.F., Binder C.J. Low-density lipoproteins cause atherosclerotic cardiovascular disease: pathophysiological, genetic, and therapeutic insights: a consensus statement from the European atherosclerosis society consensus panel. Eur Heart J. 2020;41:2313–2330. doi: 10.1093/eurheartj/ehz962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liberale L., Carbone F., Montecucco F., Sahebkar A. Statins reduce vascular inflammation in atherogenesis: a review of underlying molecular mechanisms. Int J Biochem Cell Biol. 2020;122 doi: 10.1016/j.biocel.2020.105735. [DOI] [PubMed] [Google Scholar]
- 16.Chruściel P., Sahebkar A., Rembek-Wieliczko M. Impact of statin therapy on plasma adiponectin concentrations: a systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis. 2016;253:194–208. doi: 10.1016/j.atherosclerosis.2016.07.897. [DOI] [PubMed] [Google Scholar]
- 17.Parizadeh S.M.R., Azarpazhooh M.R., Moohebati M., Nematy M., Ghayour-Mobarhan M., Tavallaie S. Simvastatin therapy reduces prooxidant-antioxidant balance: results of a placebo-controlled cross-over trial. Lipids. 2011;46:333–340. doi: 10.1007/s11745-010-3517-x. [DOI] [PubMed] [Google Scholar]
- 18.Gorabi A.M., Kiaie N., Bianconi V., Jamialahmadi T., Al-Rasadi K., Johnston T.P. Antiviral effects of statins. Prog Lipid Res. 2020;101054 doi: 10.1016/j.plipres.2020.101054. [DOI] [PubMed] [Google Scholar]
- 19.Gorabi A.M., Kiaie N., Pirro M., Bianconi V., Jamialahmadi T., Sahebkar A. Effects of statins on the biological features of mesenchymal stem cells and therapeutic implications. Heart Fail Rev. 2020 doi: 10.1007/s10741-020-09929-9. [DOI] [PubMed] [Google Scholar]
- 20.Ii M., Losordo D.W. Statins and the endothelium. Vascul Pharmacol. 2007;46:1–9. doi: 10.1016/j.vph.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 21.Bianconi V., Sahebkar A., Kovanen P., Bagaglia F., Ricciuti B., Calabrò P. Endothelial and cardiac progenitor cells for cardiovascular repair: a controversial paradigm in cell therapy. Pharmacol Ther. 2018;181:156–168. doi: 10.1016/j.pharmthera.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Bianconi V., Sahebkar A., Banach M., Pirro M. Statins, haemostatic factors and thrombotic risk. Curr Opin Cardiol. 2017;32:460–466. doi: 10.1097/HCO.0000000000000397. [DOI] [PubMed] [Google Scholar]
- 23.Sahebkar A., Serban C., Mikhailidis D.P., Undas A., Lip G.Y.H., Muntner P. Association between statin use and plasma d-dimer levels: a systematic review and meta-analysis of randomised controlled trials. Thromb Haemost. 2015;114:546–557. doi: 10.1160/TH14-11-0937. [DOI] [PubMed] [Google Scholar]
- 24.DeDiego M.L., Nieto-Torres J.L., Regla-Nava J.A., Jimenez-Guardeño J.M., Fernandez-Delgado R., Fett C. Inhibition of NF-κB-mediated inflammation in severe acute respiratory syndrome coronavirus-infected mice increases survival. J Virol. 2014;88:913–924. doi: 10.1128/JVI.02576-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang S.S., Li R., Qu X., Fang W., Quan Z. Atorvastatin decreases toll-like receptor 4 expression and downstream signaling in human monocytic leukemia cells. Cell Immunol. 2012;279:96–102. doi: 10.1016/j.cellimm.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 26.Sheahan T., Morrison T.E., Funkhouser W., Uematsu S., Akira S., Baric R.S. MyD88 is required for protection from lethal infection with a mouse-adapted SARS-CoV. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radenkovic D., Chawla S., Pirro M., Sahebkar A., Banach M. Cholesterol in relation to COVID-19: should we care about it? J Clin Med. 2020;9:1909. doi: 10.3390/jcm9061909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sposito A.C., Chapman M.J. Statin therapy in acute coronary syndromes: mechanistic insight into clinical benefit. Arterioscler Thromb Vasc Biol. 2002;22:1524–1534. doi: 10.1161/01.atv.0000032033.39301.6a. [DOI] [PubMed] [Google Scholar]
- 29.Della-Morte D., Moussa I., Elkind M.S., Sacco R.L., Rundek T. The short-term effect of atorvastatin on carotid plaque morphology assessed by computer-assisted gray-scale densitometry: a pilot study. Neurol Res. 2011;33:991–994. doi: 10.1179/1743132811Y.0000000039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mehrbod P., Omar A.R., Hair-Bejo M., Haghani A., Ideris A. Mechanisms of action and efficacy of statins against influenza. Biomed Res Int. 2014;2014:872370. doi: 10.1155/2014/872370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gordon D. Statins may be a key therapeutic for Covid-19. Med Hypotheses. 2020;144:110001. doi: 10.1016/j.mehy.2020.110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frost F.J., Petersen H., Tollestrup K., Skipper B. Influenza and COPD mortality protection as pleiotropic, dose-dependent effects of statins. Chest. 2007;131:1006–1012. doi: 10.1378/chest.06-1997. [DOI] [PubMed] [Google Scholar]
- 33.Vandermeer M.L., Thomas A.R., Kamimoto L., Reingold A., Gershman K., Meek J. Association between use of statins and mortality among patients hospitalized with laboratory-confirmed influenza virus infections: a multistate study. J Infect Dis. 2012;205:13–19. doi: 10.1093/infdis/jir695. [DOI] [PubMed] [Google Scholar]
- 34.Yuan S. Statins may decrease the fatality rate of Middle East respiratory syndrome infection. mBio. 2015;6:e01120. doi: 10.1128/mBio.01120-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fedson D.S. Statin protection against influenza and COPD mortality. Chest. 2007;132:1406–1407. doi: 10.1378/chest.07-0992. [DOI] [PubMed] [Google Scholar]
- 36.Reiner Ž., Hatamipour M., Banach M., Pirro M., Al-Rasadi K., Jamialahmadi T. Statins and the COVID-19 main protease: in silico evidence on direct interaction. Arch Med Sci. 2020;16:490–496. doi: 10.5114/aoms.2020.94655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tikoo K., Patel G., Kumar S., Karpe P.A., Sanghavi M., Malek V. Tissue specific up regulation of ACE2 in rabbit model of atherosclerosis by atorvastatin: role of epigenetic histone modifications. Biochem Pharmacol. 2015;93:343–351. doi: 10.1016/j.bcp.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 38.Wösten-van Asperen R.M., Bos A.P., Bem R.A., Dierdorp B.S., Dekker T., van Goor H. Imbalance between pulmonary angiotensin-converting enzyme and angiotensin-converting enzyme 2 activity in acute respiratory distress syndrome. Pediatr Crit Care Med. 2013;14:e438. doi: 10.1097/PCC.0b013e3182a55735. e41. [DOI] [PubMed] [Google Scholar]
- 39.Varga Z., Flammer A.J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A.S. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ackermann M., Verleden S.E., Kuehnel M., Haverich A., Welte T., Laenger F. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang X.J., Qin J.J., Cheng X., Shen L., Zhao Y.C., Yuan Y. In-hospital use of statins is associated with a reduced risk of mortality among individuals with COVID-19. Cell Metab. 2020;32:176–187. doi: 10.1016/j.cmet.2020.06.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez-Nava G., Trelles-Garcia D.P., Yanez-Bello M.A., Chung C.W., Trelles-Garcia V.P., Friedman H.J. Atorvastatin associated with decreased hazard for death in COVID-19 patients admitted to an ICU: a retrospective cohort study. Crit Care. 2020;24:429. doi: 10.1186/s13054-020-03154-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kow C.S., Hasan S.S. Meta-analysis of effect of statins in patients with COVID-19. Am J Cardiol. 2020;S0002–9149(20):30823–30827. doi: 10.1016/j.amjcard.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hariyanto T.I., Kurniawan A. Statin therapy did not improve the in-hospital outcome of coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020 doi: 10.1016/j.dsx.2020.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fan J., Wang H., Ye G., Cao X., Xu X., Tan W. Letter to the editor: low-density lipoprotein is a potential predictor of poor prognosis in patients with coronavirus disease 2019. Metabolism. 2020;107:154243. doi: 10.1016/j.metabol.2020.154243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X., Chen D., Wu L., He G., Ye W. China; Lancet: 2020. Low serum cholesterol level among patients with COVID-19 infection in Wenzhou. [DOI] [Google Scholar]
- 47.Pirro M, Bianconi V, Sahebkar A. Cholesterol and cholesterol-lowering in COVID-19: why we should not let our guard down. BMJ, https://www.bmj.com/content/368/bmj.m1182/rr-18, 2020 [accessed 15 August 2020].
- 48.Lima Martínez M.M., Contreras M.A., Marín W., D’Marco L. Statins in COVID-19: is there any foundation? Clin Investig Arterioscler. 2020;S0214-9168(20):30063. doi: 10.1016/j.arteri.2020.06.003. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banach M., Penson P.E., Fras Z., Vrablik M., Pella D., Reiner Ž. Brief recommendations on the management of adult patients with familial hypercholesterolemia during the COVID-19 pandemic. Pharmacol Res. 2020;158:104891. doi: 10.1016/j.phrs.2020.104891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katsiki N., Banach M., Mikhailidis D.P. Lipid-lowering therapy and renin-angiotensin-aldosterone system inhibitors in the era of the COVID-19 pandemic. Arch Med Sci. 2020;16:485–489. doi: 10.5114/aoms.2020.94503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Edelberg J.M., Pizzo S.V. Lipoprotein (a) in the regulation of fibrinolysis. J Atheroscler Thromb. 1995;2(Suppl. 1):S5–S7. doi: 10.5551/jat1994.2.supplement1_s5. [DOI] [PubMed] [Google Scholar]
- 52.Willeit P., Ridker P.M., Nestel P.J., Simes J., Tonkin A.M., Pedersen T.R. Baseline and on-statin treatment lipoprotein (a) levels for prediction of cardiovascular events: individual patient-data meta-analysis of statin outcome trials. Lancet. 2018;392:1311–1320. doi: 10.1016/S0140-6736(18)31652-0. [DOI] [PubMed] [Google Scholar]
- 53.Yahya R., Berk K., Verhoeven A., Bos S., Van Der Zee L., Touw J. Statin treatment increases lipoprotein (a) levels in subjects with low molecular weight apolipoprotein (a) phenotype. Atherosclerosis. 2019;289:201–205. doi: 10.1016/j.atherosclerosis.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Ahmadizar F., Ochoa-Rosales C., Glisic M., Franco O.H., Muka T., Stricker B.H. Associations of statin use with glycaemic traits and incident type 2 diabetes. Br J Clin Pharmacol. 2019;85:993–1002. doi: 10.1111/bcp.13898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Casula M., Mozzanica F., Scotti L., Tragni E., Pirillo A., Corrao G. Statin use and risk of new-onset diabetes: a meta-analysis of observational studies. Nutr Metab Cardiovasc Dis. 2017;27:396–406. doi: 10.1016/j.numecd.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 56.Cholesterol Treatment Trialists’ Collaboration Efficacy and safety of statin therapy in older people: a meta-analysis of individual participant data from 28 randomised controlled trials. Lancet. 2019;393:407–415. doi: 10.1016/S0140-6736(18)31942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khera A., Baum S.J., Gluckman T.J., Gulati M., Martin S.S., Michos E.D. Continuity of care and outpatient management for patients with and at high risk for cardiovascular disease during the COVID-19 pandemic: a scientific statement from the American Society for Preventive Cardiology. Am J Prev Cardiol. 2020;1:100009. doi: 10.1016/j.ajpc.2020.100009. [DOI] [PMC free article] [PubMed] [Google Scholar]