Abstract
Background
Population-based data on COVID-19 are essential for guiding policies. There are few such studies, particularly from low or middle-income countries. Brazil is currently a hotspot for COVID-19 globally. We aimed to investigate severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody prevalence by city and according to sex, age, ethnicity group, and socioeconomic status, and compare seroprevalence estimates with official statistics on deaths and cases.
Methods
In this repeated cross-sectional study, we did two seroprevalence surveys in 133 sentinel cities in all Brazilian states. We randomly selected households and randomly selected one individual from all household members. We excluded children younger than 1 year. Presence of antibodies against SARS-CoV-2 was assessed using a lateral flow point-of-care test, the WONDFO SARS-CoV-2 Antibody Test (Wondfo Biotech, Guangzhou, China), using two drops of blood from finger prick samples. This lateral-flow assay detects IgG and IgM isotypes that are specific to the SARS-CoV-2 receptor binding domain of the spike protein. Participants also answered short questionnaires on sociodemographic information (sex, age, education, ethnicity, household size, and household assets) and compliance with physical distancing measures.
Findings
We included 25 025 participants in the first survey (May 14–21) and 31 165 in the second (June 4–7). For the 83 (62%) cities with sample sizes of more than 200 participants in both surveys, the pooled seroprevalence increased from 1·9% (95% CI 1·7–2·1) to 3·1% (2·8–3·4). City-level prevalence ranged from 0% to 25·4% in both surveys. 11 (69%) of 16 cities with prevalence above 2·0% in the first survey were located in a stretch along a 2000 km of the Amazon river in the northern region. In the second survey, we found 34 cities with prevalence above 2·0%, which included the same 11 Amazon cities plus 14 from the northeast region, where prevalence was increasing rapidly. Prevalence levels were lower in the south and centre-west, and intermediate in the southeast, where the highest level was found in Rio de Janeiro (7·5% [4·2–12·2]). In the second survey, prevalence was similar in men and women, but an increased prevalence was observed in participants aged 20–59 years and those living in crowded conditions (4·4% [3·5–5·6] for those living with households with six or more people). Prevalence among Indigenous people was 6·4% (4·1–9·4) compared with 1·4% (1·2–1·7) among White people. Prevalence in the poorest socioeconomic quintile was 3·7% (3·2–4·3) compared with 1·7% (1·4–2·2) in the wealthiest quintile.
Interpretation
Antibody prevalence was highly heterogeneous by country region, with rapid initial escalation in Brazil's north and northeast. Prevalence is strongly associated with Indigenous ancestry and low socioeconomic status. These population subgroups are unlikely to be protected if the policy response to the pandemic by the national government continues to downplay scientific evidence.
Funding
Brazilian Ministry of Health, Instituto Serrapilheira, Brazilian Collective Health Association, and the JBS Fazer o Bem Faz Bem.
Introduction
Although the need for population-based data on COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is widely recognised,1, 2 few nationwide surveys are available.3, 4, 5, 6, 7, 8 The first COVID-19 case in Brazil was reported on Feb 26, 2020, in the city of São Paulo, and as of Sept 4, approximately 125 000 deaths have been reported.9 Three population-based antibody surveys done in the south and southeast regions of Brazil showed prevalence ranging from 0·05% to 2·1%.10, 11, 12
The government's response to the pandemic has been marked by controversy, with the country's president, Jair Bolsonaro, opposing physical distancing measures and downplaying the importance of COVID-19.13 However, physical distancing policies vary widely across the country and the implementation of such policies depends primarily on city and state governments.14 Testing is limited to patients with severe illnesses and evidence suggests that COVID-19 deaths are undercounted.15 Thus, periodic, population-based data on the pandemic are urgently needed.
Research in context.
Evidence before this study
Brazil has become a global hotspot for the COVID-19 pandemic in terms of reported cases and deaths. We searched PubMed, Web of Science and Scielo for papers in any language, published from Jan 1, 2019 onwards. We used the search terms: ((“severe acute respiratory syndrome coronavirus 2”[All Fields] OR “severe acute respiratory syndrome coronavirus 2”[All Fields] OR “ncov”[All Fields] OR “2019-nCoV”[All Fields] OR “COVID-19”[All Fields] OR “SARS-CoV-2”[All Fields] AND (Brasil OR Brazil)). Globally, few nationwide population-based studies on the prevalence of antibodies against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are available, and none from low-income or middle-income countries. Existing studies in Brazil have focused on the more developed parts of the country, represented by the southern and southeastern regions.
Added value of this study
We did two household surveys in the most populous cities in all 133 mesoregions of Brazil, covering 26 states and the Federal District. We included more than 25 000 participants in the period May 14–21 and over 32 000 in June 4–7. We documented an increase in prevalence during this time interval, with strong concentration in 11 cities along the Amazon River, where prevalence was as high as 25% in both surveys. In the second survey, rapid increases in prevalence were also observed in the northeast. High-prevalence areas are poorer and less well-served by health and other services than areas in the rest of the country are. Prevalence among Indígena (Indigenous) individuals was over four times higher than among Branco (White) people, and prevalence in the poorest socioeconomic quintile was over twice as high as in the richest quintile.
Implications of all the available evidence
The poorest areas of Brazil, particularly the Amazon River basin, were the first to present high prevalence of antibodies against SARS-CoV-2, by contrast with the initially low prevalence observed in the southern and centre-western regions. Our geographical-level and individual-level analyses showed remarkable inequality in the prevalence of infection, with poverty and Indígeno ethnicity driving the progression of the pandemic in the country. The controversial handling of the epidemic by the federal government is likely to have contributed to the rapid spread of COVID-19 in the country's most susceptible populations.
We aimed to investigate antibody prevalence by city and according to sex, age, ethnicity group, and socioeconomic status, and compare seroprevalence estimates with official statistics on deaths and cases.
Methods
Study design and sampling
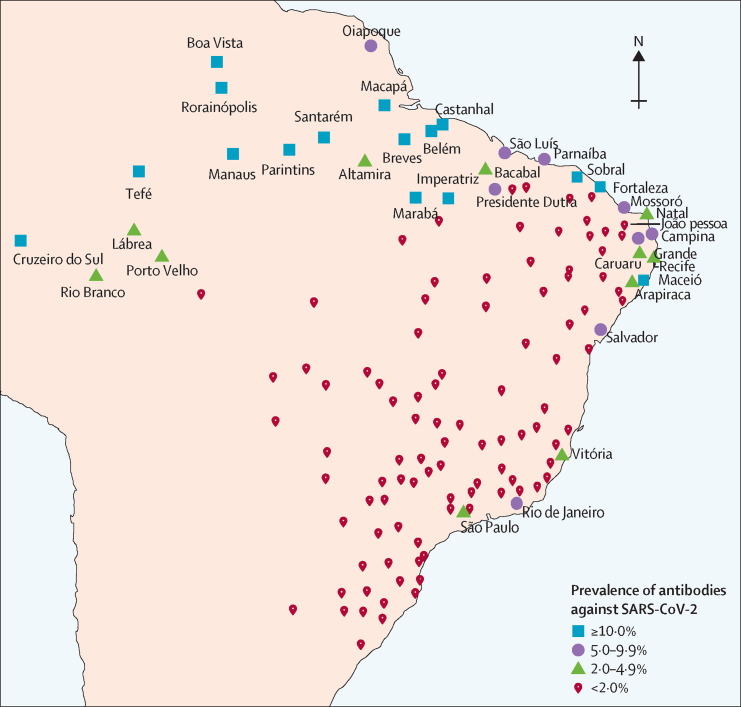
We did nationwide seroprevalence surveys on May 14–21, and June 4–7, 2020, in 133 sentinel cities from the 26 Brazilian states and the Federal District (figure 1 ). Brazil's 27 federation units are divided by the Brazilian Institute of Geography and Statistics into 133 intermediary regions. The most populous city in each region was selected.
Figure 1.
Location of the 133 sentinel cities in Brazil
Figures shows cities with prevalence of 5% or higher for antibodies against SARS-CoV-2 in the second survey. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
We selected 25 urban census tracts with probability proportionate to size in each sentinel city, and ten households at random in each tract, using maps and household listings made available by the Brazilian Institute of Geography and Statistics. One individual was randomly selected from a listing of all household members. Children younger than 1 year were excluded because parents or guardians were not likely to have consented to the collection of blood. If the selected individual did not provide a sample, another household member was randomly selected. If this person also refused, the interviewers moved on to the next household on the right, which was also selected in the case of absent residents.
Interviewers were tested on the day before fieldwork using the antibody test and only participated in the study if the result was negative, and were provided with personal protective equipment (aprons, gloves, surgical face masks, and shoe and hair covers) that were discarded as hospital waste after each interview. Ethics approval was obtained from the Brazilian's National Ethics Committee (CAAE 30721520.7.1001.5313), with written informed consent from all participants or by parents for minors. Positive cases were reported to the municipal COVID-19 surveillance systems; participants agreed to the disclosure in the consent form.
Procedures
Prevalence of antibodies was assessed with a rapid point-of-care test, the WONDFO SARS-CoV-2 Antibody Test (Wondfo Biotech, Guangzhou, China), using two drops of blood from finger prick samples. This lateral-flow assay detects IgG and IgM isotypes that are specific to the SARS-CoV-2 receptor binding domain of the spike protein. By pooling the results from four validation studies, weighted by sample sizes, sensitivity was estimated at 84·8% (95% CI 81·4–87·8%) and specificity at 99·0% (97·8–99·7).10 Specificity estimates were obtained with frozen sera and might have been underestimated.16 In early April 2020, we did a household probability survey in nine cities in the state of Rio Grande do Sul,17 when the pandemic was at an early stage in the state. Of 4188 participants we found only two (<1%) positive results. Assuming that all cases in that survey were false positives leads to a specificity of 99·95%. We therefore used as correction parameters in the main analyses a sensitivity of 84·8% and the 99·95% specificity derived from our previous population-based survey.17 Analyses using the same sensitivity level and a specificity of 99·0% are presented in the appendix (pp 1, 2).
Data collection
A smartphone app for data collection was used for listing household members, selecting one at random, recording answers to the questionnaire, photographing test results, and obtaining the geographic coordinates of each home. For quality control purposes, each interview was also voice recorded, and 10% of all recordings were listened to by a supervisor.
Participants answered short questionnaires on sociodemographic information (sex, age, education, ethnicity, household size, and household assets) and compliance with physical distancing measures. Fieldworkers used tablet computers to voice record the full interviews, register answers, and photograph the test results.
The official Brazilian classification of ethnicity recognises five groups: Branco (White), Pardo (Brown), Preto (Black), Amarelo (East Asian), and Indígena (Indigenous).
Interviewers were instructed to select Indígena when any of the multiple first nations were given as ethnicity. Pardo reflects mixed ancestry including European, African, and Indígena backgrounds. This system is endorsed by the Afro-descendants movement.18
All positive or inconclusive tests were read by a second observer, as well as 20% of the negative tests. If a participant in a household had a positive result, all other household members were invited to be tested. Results from household members were not included in the analyses, except for results on family clustering.
Data analysis
We compared seroprevalence results with official numbers and rates of reported cases and deaths in 133 cities.9 We multiplied the corrected antibody prevalence in each city by the city's population to obtain an estimate of the number of people infected, and used this number to calculate under-reporting of cases and infection-fatality rates.
With 250 individuals per city, the margin of error (1·96 standard errors) for estimating prevalence at the city level is 1·92 percentage points at 2% prevalence, 2·93 at 5%, and 4·12 at 10%. At the national level the margin of error is 0·15 percentage points at 2% prevalence, 0·24 at 5%, and 0·33 for a design effect of 1·18 derived from the study results.
Socioeconomic position was assessed using a wealth index derived through principal component analyses of household assets (appendix p 3). Data analyses took into account the sampling design of the survey and corrected for the test validity (appendix pp 4–6).
Hypothesis testing was done using Cochran's Q heterogeneity test implemented as a fixed effects meta-regression. Logistic regression was used to adjust for confounding variables in the analyses by ethnic groups. All analyses were done using R (version 3.6.1). The “survey” package19, 20 was used to account for the sampling design. Meta-regression was implemented using the “metafor” package.21
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
We interviewed 25 025 individuals in the first survey in 132 cities. During the first survey, we could not do any interviews in Picos, in the northeast region, which was under lockdown. 250 individuals were tested in 46 cities, 200–249 in 44 cities, 100–199 in 14 cities, and 1–99 in 28. The sample fell short of the planned number because of lockdown measures imposed in several cities with restrictions to mobility for the interviewers, and because of poor coordination between the Ministry of Health and the city and state governments. These difficulties were compounded by the rapid spread of disinformation through social media characterising the interviewers as swindlers, or of even being part of a plot to spread the virus. Interviewers were arrested in 27 cities and in eight cities tests were destroyed by local police forces.
In the second survey we overcame some of these problems because of repeated contact with the city health and police authorities and local media before data collection. The research team was not allowed to enter one city that was under lockdown. 31 165 individuals were tested in 132 cities. 250 individuals were tested in 98 cities, 200–249 in 22 cities, 100–199 in 11 cities, and 1–99 in one city. Overall, 31 165 (93·7%) of the planned 33 250 tests were carried out.
In the first survey, residents from 10 655 (23%) households refused to participate, and in 10 331 (22%) households reisdents were not present at the time of visit, resulting in 25 025 (54%) interviews from 46 011 households contacted (appendix p 7). In the second survey, 12 432 (21%) of the contacted households did not participate and in 15 621 (26%) households residents were not present at the time of the visit, resulting in 31 165 (53%) interviews from 59 218 households contacted (appendix p 7). Combining both surveys, response rates were 66% in the north, 57% in the northeast, 45% in the southeast, 47% in the south, and 61% in the centre-west region. A comparison of socioeconomic indicators of included cities with those of cities that were not included in the study and the national average is available in the (appendix p 8). Compared with the overall Brazilian population, our sample included more individuals from the north, and fewer from the southeast. Men and children and adolescents (aged 1–19 years) were under-represented, as were those older than 50 years. The distribution in terms of ethnicity showed fewer individuals who reported being Branco, and larger percentages of Pretos, Pardos, and Indígenas (appendix p 9).
We found 347 (1·39%) positive results in 24 995 individuals with valid test results in the first survey, and 746 (2·40%) in 31 128 in the second survey. The corrected prevalence estimates were 1·6% (95% CI 1·4–1·8) in the first survey and 2·8% (2·5–3·1) in the second.
The number of tests done in each round, by city, with prevalence results are shown in the appendix (pp 10–15). Among the 90 cities with more than 200 tests in the first survey, 16 (18%) cities had a prevalence above 2·0%, of which 11 were in the north, three in the northeast, and two in the southeast region (appendix pp 10–15). The six cities with the highest prevalence were all located along a 2000 km stretch of the Amazon river (appendix p 16).
In the appendix (pp 17–22) we also present estimates for all cities corrected for 99·0% specificity as in validation studies using frozen sera.10 The largest difference in city prevalence levels using the two methods was 1·1 percentage point.
In the first survey, 54 (60%) of 90 cities with at least 200 participants had no positive tests, and 13 (14%) had only one positive test. All nine cities had zero cases in the centre-west region, 16 (80%) of 20 in the south, 11 (55%) of 20 in the southeast, 12 (55%) of 22 in the northeast, and only six (32%) of 19 in the north. In the second survey, 49 (41%) of 120 cities with 200 or more tests had zero cases, and 18 (15%) had only one positive result. 34 (28%) cities had a prevalence of 2% or higher, of which 11 were in the north, 14 in the northeast and, three in the southeast (Rio de Janeiro with 7·5%, Vitória with 3·2%, and São Paulo with 2·3%). Prevalence in the second survey is shown in figure 1.
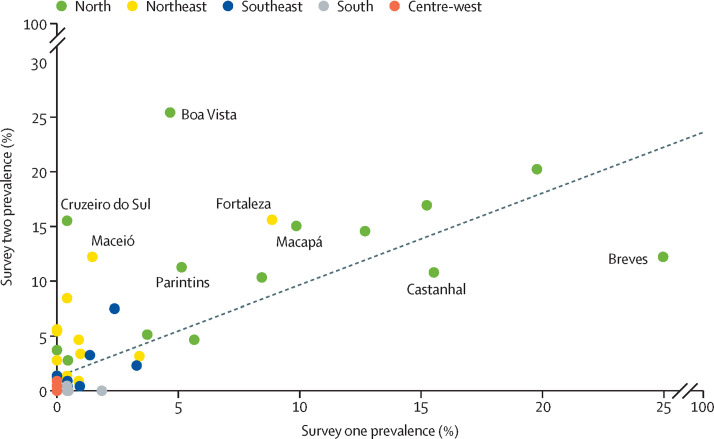
Among cities with 200 or more tests in both surveys, pooled prevalence increased from 1·9% (95% CI 1·7–2·1%) to 3·1% (2·8–3·4%) over time. The largest increase was observed in Boa Vista, from 4·7% (2·2–9·5) in the first survey to 25·4% (19·5–32·2) in the second (figure 2 ). Few cities showed reductions over time—most notably, Breves, with reduction from 25·4% (17·5–33·7%) to 12·2% (6·6 to 20·2%). Pearson's correlation coefficient between the two consecutive prevalence estimates was 0·728 (p<0·0001).
Figure 2.
Comparison on antibody prevalence by survey
Figures shows 83 cities with at least 200 participants in both surveys. The solid line indicates the diagonal (equal prevalence in both surveys) and the dashed line shows the linear regression slope.
When a participant tested positive, other household members were also tested. 21·6% of positive participants had at least one other positive household member in the first survey, and 33·0% in the second survey.
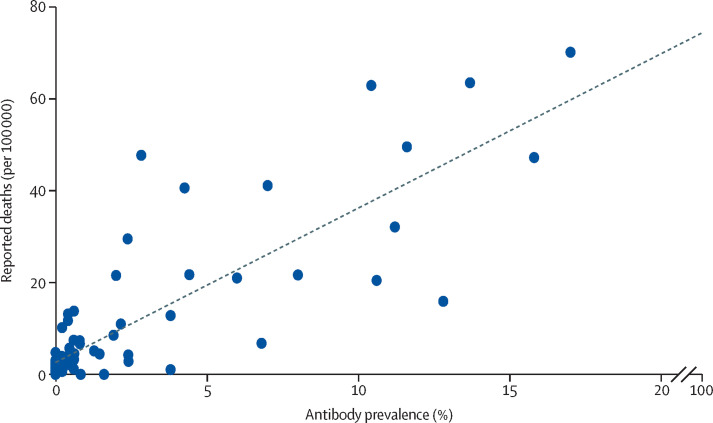
Figure 3 shows prevalence estimates by pooling the two rounds of data in each city and plotting these values against the reported mortality rates.9 We used the mean number of deaths on May 13, and June 3, before the two rounds of testing. We found a correlation between prevalence estimated by the survey and the number of reported deaths per population, with a correlation coefficient of 0·826 (p<0·0001). The infection-fatality rate was estimated at 0·71%. Our results by region of the country were also consistent with officially reported deaths (appendix p 23).
Figure 3.
Scatter diagram for survey-based seroprevalence versus reported cases and deaths per population
We calculated the ratio of estimated infections to reported cases in 83 cities with 200 or more tests in both surveys. Taken together, these cities reported 172 420 cases by May 23, compared with our estimate of 1 778 401 infected individuals. The ratio of estimated infections to reported cases was equal to 10·3.
The table shows a breakdown of prevalence findings, resulting from analyses based on all individuals tested. In the north region, prevalence was 6·3% (95% CI 5·4–7·2) in the first survey and 9·0% (8·0–10·1) in the second (p=0·0001). An increase from 0·8% (0·5–1·1) to 3·2% (2·8–3·7) prevalence was also observed in the northeast (p<0·0001), but in the other three regions change over time was not significant.
Table.
Seroprevalence according to sociodemographic characteristics
|
First survey |
Second survey |
p values for comparisons over time |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Positive tests | Adjusted for sampling design (estimate [95% CI]) | Adjusted for sampling design and test validity (estimate [95% CI]) | Positive tests | Adjusted for sampling design (estimate [95% CI]) | Adjusted for sampling design and test validity (estimate [95% CI]) | Unadjusted | Adjusted | ||
| Region | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | |||||
| Northeast | 46/6552 | 0·7% (0·5– 1·0) | 0·8% (0·5–1·1) | 273/9801 | 2·8% (2·4–3·2) | 3·2% (2·8–3·7) | p<0·001 | p<0·001 | |
| North | 272/5064 | 5·4% (4·7– 6·1) | 6·3% (5·4–7·2) | 419/5449 | 7·7% (6·9–8·5) | 9·0% (8·0–10·1) | p<0·001 | p<0·001 | |
| Centre-west | 0/2477 | 0% (0·0–0·1) | 0% | 13/3565 | 0·4% (0·2–0·6) | 0·4% (0·2–0·7) | * | * | |
| Southeast | 22/5833 | 0·4% (0·2–0·6) | 0·4% (0·2–0·7) | 45/7778 | 0·6% (0·4– 0·8) | 0·6% (0·4–0·9) | p=0·099 | p=0·177 | |
| South | 7/5069 | 0·1% (0·0– 0·4) | 0·1% (0·0–0·6) | 3/4569 | 0·1% (0·0– 0·2) | 0% (0·0– 0·3) | p=0·281 | p=0·476 | |
| Sex | p=0·16 | p=0·27 | p=0·095 | p=0·16 | |||||
| Female | 189/14464 | 1·3% (1·1–1·5) | 1·5% (1·2–1·8) | 461/18155 | 2·5% (2·3–2·8) | 2·9% (2·6–3·3) | p<0·001 | p<0·001 | |
| Male | 158/10531 | 1·5% (1·3–1·8) | 1·7% (1·4–2·0) | 292/13007 | 2·2% (2·0–2·5) | 2·6% (2·3–3·0) | p<0·001 | p<0·001 | |
| Age, years | p=0·22 | p=0·42 | p=0·0015 | p=0·0056 | |||||
| 0–4 | 6/432 | 1·4% (0·5– 3·0) | 1·6% (0·6–3·5) | 11/573 | 1·9% (1·0–3·4) | 2·2% (1·1–4·0) | p=0·520 | p=0·563 | |
| 5–9 | 8/681 | 1·2% (0·5–2·5) | 1·3% (0·5–2·9) | 16/983 | 1·6% (0·9–2·6) | 1·9% (1·0–3·1) | p=0·448 | p=0·522 | |
| 10–19 | 31/2287 | 1·4% (0·9–1·9) | 1·5% (1·0–2·2) | 52/2856 | 1·8 (1·4–2·4) | 2·1% (1·5–2·8) | p=0·190 | p=0·225 | |
| 20–29 | 55/3866 | 1·4% (1·1–1·9) | 1·6% (1·2–2·2) | 118/4761 | 2·5% (2·0–3·0) | 2·9% (2·3–3·5) | p<0·001 | p=0·002 | |
| 30–39 | 58/3834 | 1·5% (1·2–1·9) | 1·7% (1·3–2·3) | 122/4668 | 2·6% (2·2–3·1) | 3·0% (2·5–3·6) | p<0·001 | p=0·001 | |
| 40–49 | 63/3972 | 1·6 (1·2–2·0) | 1·8% (1·4–2·4) | 155/4885 | 3·2% (2·7–3·7) | 3·7% (3·1–4·4) | p<0·001 | p<0·001 | |
| 50–59 | 67/4016 | 1·7% (1·3– 2·1) | 1·9% (1·4– 2·5) | 133/5019 | 2·6% (2·2–3·1) | 3·1% (2·5–3·7) | p=0·002 | p=0·005 | |
| 60–69 | 33/3382 | 1·0% (0·7–1·4) | 1·1% (0·7–1·6) | 79/4264 | 1·9% (1·5–2·3) | 2·1% (1·7–2·7) | p=0·002 | p=0·005 | |
| 70–79 | 22/1797 | 1·2% (0·8–1·8) | 1·4% (0·8–2·1) | 49/2262 | 2·2% (1·6–2·9) | 2·5% (1·8–3·4) | p=0·025 | p=0·040 | |
| ≥80 | 4/728 | 0·5% (0·1–1·4%) | 0·6% (0·1–1·6) | 18/891 | 2·0% (1·2–3·2) | 2·3% (1·4–3·7) | p=0·018 | p=0·045 | |
| Ethnicity | p<0·0001 | p<0·0001 | p<0·0001 | p<0·0001 | |||||
| Amarelo (East Asian) | 8/685 | 1·2% (0·5–2·4) | 1·3% (0·5–2·8) | 15/827 | 1·8% (1·0–2·9) | 2·1% (1·1–3·5) | p=0·311 | p=0·392 | |
| Branco (White) | 61/9493 | 0·6% (0·5–0·8) | 0·7% (0·5–1·0) | 138/11011 | 1·3% (1·1–1·5) | 1·4% (1·2–1·7) | p<0·001 | p<0·001 | |
| Indígena (Indigenous) | 12/327 | 3·7% (1·9–6·4) | 4·2% (2·2–7·2) | 24/440 | 5·5% (3·6–7·9) | 6·3% (4·2–9·2) | p=0·251 | p=0·298 | |
| Pardo (Brown) | 229/11042 | 2·1% (1·8–2·4) | 2·4% (2·0–2·8) | 444/14282 | 3·1% (2·8–3·4) | 3·6% (3·2–4·0) | p<0·001 | p<0·001 | |
| Preto (Black) | 32/2961 | 1·1% (0·7–1·5) | 1·2% (0·8–1·8) | 114/3923 | 2·9% (2·4–3·5) | 3·4% (2·7–4·1) | p<0·001 | p<0·001 | |
| Household size | p<0·0001 | p<0·0001 | p=0·0003 | p=0·0023 | |||||
| 1 | 62/5074 | 1·2% (0·9–1·6) | 1·4% (1·0–1·8) | 150/5888 | 2·5% (2·1–3·0) | 3·0% (2·5–3·5) | p<0·001 | p<0·001 | |
| 2 | 79/7012 | 1·1% (0·9–1·4) | 1·3% (1·0–1·6) | 192/9148 | 2·1% (1·8–2·4) | 2·4% (2·0–2·8) | p<0·001 | p<0·001 | |
| 3 | 54/5429 | 1·0% (0·7–1·3) | 1·1% (0·8–1·5) | 153/6807 | 2·2% (1·9–2·6) | 2·6% (2·2–3·1) | p<0·001 | p<0·001 | |
| 4–5 | 102/5835 | 1·7% (1·4–2·1) | 2·0% (1·6–2·5) | 189/7483 | 2·5% (2·2–2·9) | 2·9% (2·5–3·4) | p=0·002 | p=0·006 | |
| ≥6 | 50/1645 | 3·0% (2·1–4·2 | 3·5% (2·5–4·9) | 69/1836 | 3·8% (3·0–4·7) | 4·4% (3·4–5·6) | p=0·245 | p=0·317 | |
| Wealth quintiles | p=0·0052 | p=0·012 | p<0·0001 | p<0·0001 | |||||
| Poorest | 101/5590 | 1·8% (1·5–2·2) | 2·1% (1·6–2·6) | 237/7369 | 3·2% (2·8–3·7) | 3·7% (3·2–4·3) | p<0·001 | p<0·001 | |
| 2nd | 73/4728 | 1·5% (1·2–2·0) | 1·8% (1·3–2·3) | 168/5774 | 2·9% (2·5–3·4 | 3·4% (2·9–4·0) | p<0·001 | p<0·001 | |
| 3rd | 63/4798 | 1·3% (1·0–1·7) | 1·5% (1·1–2·0) | 127/5950 | 2·1% (1·8–2·6) | 2·5% (2·0–3·0) | p=0·001 | p=0·005 | |
| 4th | 64/4852 | 1·3% (1·0–1·7) | 1·5% (1·1–1·9) | 127/5927 | 2·1% (1·8–2·6) | 2·5% (2·0–3·0) | p=0·001 | p=0·004 | |
| Richest | 46/5018 | 0·9% (0·7–1·2) | 1·0% (0·7–1·4) | 94/6142 | 1·5% (1·2–1·9) | 1·8% (1·4–2·2) | p=0·004 | p=0·009 | |
| Total | |||||||||
| Cities with ≥200 participants in both surveys | 333/20 084 | 1·7% (1·5–1·9) | 1·9% (1·7–2·2) | 551/20 414 | 2·7% (2·5–3·0) | 3·1% (2·8–3·4) | p<0·001 | p<0·001 | |
| All cities in each phase | 347/24 995 | 1·4% (1·2–1·6) | 1·6% (1·4–1·8) | 753/31 162 | 2·4% (2·2–2·6) | 2·8% (2·5–3·1) | p<0·001 | p<0·001 | |
Missing values represented 2·1% of the responses on ethnicity, and less than 0·1% for the remaining variables.
p values for trends over time could not be calculated because prevalence was equal to 0 in the first survey.
Prevalence was similar among men and women (table). We found no significant difference according to age in the first survey, but in the second survey prevalence was higher for individuals aged 20–59 years than for younger or older individuals (table). Children and adolescents showed similar prevalence within each survey (table). The increased prevalence in individuals aged 20–59 years was associated with an increase in the proportion of individuals who left home on a daily basis, from 27·2% (95% CI 26·5–28·8) to 31·6% (30·8–32·4; p<0·0001).
We found marked differences in prevalence according to ethnic group, ranging from 0·7% (95% CI 0·5–1·0) among Branco individuals to 4·2% in Indígenas in the first wave, and from 1·4% (1·2–1·7) for Branco individuals to 6·3% in the second wave. We did further analyses to verify whether the higher prevalence in Indígena individuals was due to confounding by region, number of family members, and household wealth (appendix p 24). To increase sample sizes for Indígena participants, we pooled the results from both surveys of the study for all ethnic groups. The prevalence odds ratio (OR) for Indígena relative to Branco participants was 4·72 (95% CI 2·91–7·67) in the model without confounding factors. After adjustment, the OR was reduced to 1·87 (1·18–2·96). When analyses were restricted to the north region, the prevalence OR was 1·64 (0·87–3·10). In terms of ethnicity, the second highest prevalence (2·4% [2·0–2·8] in the first survey and 3·6% [3·2–4·0] in the second) was found in Pardo participants.
Prevalence was directly associated with household size in both surveys (table). Results for socioeconomic status show clear social gradients in both surveys, with greater than two-times higher prevalence in the poorest than in the wealthiest quintile (table). We found no evidence of greater increases in a particular quintile (p for interaction=0·96).
Discussion
To our knowledge, this is the largest population-based study of prevalence of antibodies to SARS-CoV-2 in geographical scope. Our most remarkable finding was the cluster of high prevalence in 11 cities along the Amazon River, with levels that were among the highest ever reported in population-based studies.22 This finding of high prevalence in a tropical region contradicts common wisdom that continents such as Africa might be protected against COVID-19 because of high ambient temperature.23
The first COVID-19 case in Brazil's north region was reported in Manaus on March 13, 2020, and the spread in the pandemic was consistent with the main river-boat routes along the Amazon.24 Long boat trips—eg, 36 h from Manaus to Tefé—offer the possibility of intense contagion in overcrowded boats, where most passengers use hammocks for sleeping or resting on the decks. One study25 showed an inverse association (Pearson's r −0·78; p<0·0001) between the daily number of boats leaving the capital to a given city and the number of days elapsed until the first reported case in each city.
Another possibility is that Indígena individuals have an increased genetic susceptibility to SARS-CoV-2 infection because several outbreaks have been reported in Indigenous territories.26 Our analyses suggest that the excess risk of Indígena individuals is largely explained by geographical region, household size, and socioeconomic status. Nevertheless, the adjusted results do not negate the finding that Indígena people are at higher risk than people of other ethnicities are, if not for genetic reasons then because of Indígena people's exposure to poverty and to crowded living conditions. Historically, mortality rates among Indígena peoples have been substantially higher than those in other ethnic groups,27, 28 and Indígena populations were left behind when Brazil made rapid progress in health during the 1990s.29 High seroprevalence, combined with comorbidity with metabolic and cardiovascular diseases that are also increasing rapidly among Indigenous Brazilians30 will probably place those people at increased risk of death due to COVID-19.
Pardo people are mostly descended from native Americans and Europeans in the north region, and from Africans and Europeans in the rest of the country.31 Baqui and colleagues32 reported that hospital case-fatality from COVID-19 was higher among individuals with Preto or mixed ethnicity, compared with Branco people. The number of Indígena participants in that study was not sufficient for analyses.
Antibody prevalence increased by over 50% in the period of 2–3 weeks between the two surveys, showing increases in most cities studied. The notable exception was Breves, where prevalence declined from the highest observed prevalence in the first round by almost 50% in the second survey. Only one other city (Castanhal, near Breves) showed a reduction greater than 2 percent points. Serum titres in previously positive individuals might have fallen below the detection threshold for the test between the first and second surveys. Indeed, a report showed evidence of rapid declines in serum IgG titres among COVID-19-infected patients over time, leading to loss of ELISA positivity. This decline was especially prevalent among seropositive individuals who were asymptomatic, 40% of whom became seronegative after an 8-week period.33 A 14% decline in positive results in the same participants was observed in the Spanish national surveys between the first to the third survey (approximately 40 days).34 These findings might have important implications for the interpretation of seroprevalence studies, which are likely to underestimate true prevalence because previously positive individuals become negative over a short time. Longitudinal or repeated panel studies are required to confirm these findings.
Another possible explanation for high prevalence in the Amazon is cross-reactivity with other coronaviruses endemic to the region. We regard this explanation as unlikely. First, positivity correlated well geographically with reported mortality due to COVID-19, which would not occur for past infections with related coronaviruses. Second, we found rapid increases in prevalence in several Amazon cities between the two surveys. Third, the rapid test detects antibodies specific to the SARS-CoV-2 receptor binding domain of the spike protein, which is poorly conserved across coronaviruses.35
We were only able to identify one population-based study of antibodies to SARS-CoV-2 in children. In Spain, prevalence was less than half in children younger than 5 years than in adults.7, 8 We found that young children displayed similar prevalence to that observed in older age groups in the first survey, but in the second survey prevalence was lower in those aged up to 19 years, compared with adults aged 20–59 years.
The limitations of our analyses include the restriction of the sample to sentinel cities that constitute regional hubs, which are larger, more developed, and better equipped with health services than the country as a whole. Rural areas, where approximately 15% of all Brazilians live, were excluded.36 Our response rate of 53–54% is similar to that in the Spanish survey (60%) and higher than that achieved in national surveys in Iceland and Austria.4, 5 We found that many families have been moving away from large cities to towns or rural areas since physical distancing was recommended. We could not collect information from household members who were either away at the time of visit or refused to participate.
Our sample had fewer children than was expected, which was probably due to children's reluctance to undergo a finger prick when randomly selected within the household; also, infants were excluded from our sample. In terms of ethnicity, Branco people were under-represented in the sample compared with the national population, possibly because of low response rates in apartment buildings and gated communities, where many Branco people live.
Concerns have been raised regarding the use of point-of-contact antibody tests for clinical decision making and for so-called immune passports. However, use of such tests for large-scale, population-based, seroprevalence studies is less controversial, provided that sensitivity and specificity are sufficiently high and appropriately corrected for.37, 38 The test we used is one of the most precise lateral-flow tests.16 We used two sets of sensitivity and specificity parameters for correction, and the largest prevalence difference between the two approaches was 1·1 percentage point. Additionally, the possibility of spectrum bias should be considered because sensitivity assessments are usually limited to samples from patients with severe disease and thus with higher antibody levels;39 this limitation applies to all antibody tests.
Given the scale of our study, point-of-contact tests were the most viable alternative. Our results have strong face validity, showing a high correlation with reported death rates, an increase over time as the pandemic progressed, and distribution by age, socioeconomic status, and household size that would be expected. The comparison with officially reported cases and deaths showed that only one in ten infections were reported as cases, and our estimate of infection-fatality rate was lower than the official figure for case-fatality.9
Our results must be interpreted in terms of the controversial management of the pandemic by the national government.13 Testing was restricted to individuals with severe symptoms during the early stages of the pandemic and contact tracing was virtually non-existent. Two consecutive health ministers were either dismissed or resigned in less than 1 month because of opposition to the president's stance regarding physical distancing and the use of hydroxychloroquine to treat COVID-19, and since May 15, the country has not had a health minister. By contrast with the federal government, most state governors and city mayors enforced closure of schools, shops, and non-essential services, and recommended the use of face masks. Nevertheless, hospital services have been at the brink of collapse due to the high numbers of patients requiring intensive care. Several mayors and governors have relaxed physical distancing policies throughout the country, despite the persisting high incidence of new cases and deaths. The effect of these measures is still too early to assess, but further waves of serological surveys will allow monitoring of the progression of the pandemic and help assess the effectiveness of policy changes.
Data sharing
Data will become publicly available upon request from the corresponding author 30 days after publication. The EPICOVID19 datasets are freely available online.
Acknowledgments
Acknowledgments
The study was funded by the Brazilian Ministry of Health, Instituto Serrapilheira, Brazilian Collective Health Association and JBS Fazer o Bem Faz Bem.
Editorial note: the Lancet Group takes a neutral position with respect to territorial claims in published maps and institutional affiliations.
Contributors
PCH, FPH, BLH, MFS, CJS, LPV, LCP, OAD, MNB, GDV, AMBM, FCB, AJDB, and CGV contributed to the conception and design of the work, to the acquisition, analysis, and interpretation of data, and the draft of the manuscript. NAN contributed to the acquisition of data. All authors have approved the submitted version.
Declaration of interests
We declare no competing interests.
Supplementary Material
References
- 1.Pearce N, Vandenbroucke JP, VanderWeele TJ, Greenland S. Accurate statistics on COVID-19 are essential for policy guidance and decisions. Am J Public Health. 2020;110:949–951. doi: 10.2105/AJPH.2020.305708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barreto ML, Barros AJD, Carvalho MS. What is urgent and necessary to inform policies to deal with the COVID-19 pandemic in Brazil? Revista Brasileira de Epidemiologia. 2020;23 doi: 10.1590/1980-549720200032. [DOI] [PubMed] [Google Scholar]
- 3.Wise J. Covid-19: surveys indicate low infection level in community. BMJ. 2020;369 doi: 10.1136/bmj.m1992. [DOI] [PubMed] [Google Scholar]
- 4.Gudbjartsson DF, Helgason A, Jonsson H. Spread of SARS-CoV-2 in the Icelandic Population. N Engl J Med. 2020;382:2302–2315. doi: 10.1056/NEJMoa2006100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Institute for Social Research and Consulting Ogris & Hofinger GmbH (SORA) COVID-19 Prevalence. 2020. https://www.sora.at/fileadmin/downloads/projekte/Austria_COVID-19_Prevalence_BMBWF_SORA_20200410_EN_Version.pdf
- 6.Public Health Agency of Sweden Nya resultat från undersökning av förekomsten av covid-19 i Sverige. 2020. folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2020/maj/nya-resultat-fran-undersokning-av-forekomsten-av-covid-19-i-sverige/
- 7.Ministerio de Ciencia e Innovacion and Ministerio de Salud Spain. Estudio ENE-COVID19: primera ronda estudio nacional de sero-epidemiologia de la infección por SARS-CoV-2 en España. Informe preliminar 13 mayo de 2020. Madrid, Spain. 2020. https://www.mscbs.gob.es/ciudadanos/ene-covid/docs/estudio_ene-covid19_primera_ronda_informe_preliminar.pdf
- 8.Ministerio de Ciencia e Innovacion and Ministerio de Salud Spain. Estudio ENE-Covid-19: Segunda Ronda. Estudio Nacional de Sero-Epdemiologia de la Infección por SARS-CoV-2 en España. Madrid, España. 2020. https://www.mscbs.gob.es/ciudadanos/ene-covid/docs/estudio_ene-covid19_segunda_ronda_informe_preliminar.pdf
- 9.Saude BMd Coronavírus Brasil. 2020. https://covid.saude.gov.br
- 10.Pellanda LC, Wendland EM, McBride AJA. Sensitivity and specificity of a rapid test for assessment of exposure to SARS-CoV-2 in a community-based setting in Brazil. medRxiv. 2020 doi: 10.1101/2020.05.06.20093476. published online May 10. (preprint) [DOI] [Google Scholar]
- 11.Comitê Técnico do Inquérito Epidemiológico SARS-CoV-2 . Universidade de São Paulo; Ribeirão Preto: 2020. Avaliação da prevalência de marcadores virológicos e sorológicos do SARS-CoV-2 na população de Ribeirão Preto: um inquérito epidemiológico. Resultados preliminares. [Google Scholar]
- 12.Gomes CC, Cerutti C, Zandonade E. A population-based study of the prevalence of COVID-19 infection in Espirito Santo, Brazil: methodology and results of the first stage. medRxiv. 2020 doi: 10.1101/2020.06.13.20130559. published online June 16. (preprint) [DOI] [Google Scholar]
- 13.The Lancet COVID-19 in Brazil: “So what?”. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.University of Miami COVID-19 observatory: public polity adoption index in Brazil. 2020. http://observcovid.miami.edu/brazil/
- 15.França EB, Ishitani LH, Teixeira RA. Deaths due to COVID-19 in Brazil: how many are there and which are being identified? Revista Brasileira de Epidemiologia. 2020;23 doi: 10.1590/1980-549720200053. [DOI] [PubMed] [Google Scholar]
- 16.Whitman JD, Hiatt J, Mowery CT. Test performance evaluation of SARS-CoV-2 serological assays. medRxiv. 2020 doi: 10.1101/2020.04.25.20074856. published online May 17. (preprint) [DOI] [Google Scholar]
- 17.Silveira MF, Barros AJD, Horta BL. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26:1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 18.Petrucelli JL, Saboia AL, editors. Características étnico-raciais da população: classificação e identidades. Instituto Brasileiro de Geografia e Estatística; Rio de Janeiro, Brazil: 2013. [Google Scholar]
- 19.Lumley T. Analysis of complex survey samples. J Stat Softw. 2004;9:1–19. [Google Scholar]
- 20.Lumley T. Survey: analysis of complex survey samples. https://cran.r-project.org/web/packages/survey/citation.html
- 21.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36:1–48. [Google Scholar]
- 22.Popovich N, Sanger-Katz M. The world is still far from herd immuity for coronavirus. New York Times. May 28, 2020. https://www.nytimes.com/interactive/2020/05/28/upshot/coronavirus-herd-immunity.html
- 23.The Lancet COVID-19 in Africa: no room for complacency. Lancet. 2020;395 doi: 10.1016/S0140-6736(20)31237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pereira HS, Barbosa DES, Lorenzi BC, Vasconcelos ARM. Fases da pandemia em 6 capitais da Amazônia brasileira. ODS Atlas Amazonas. 2020;2:1–2. [Google Scholar]
- 25.Aleixo N, Silva Neto J, Pereira H, Barbosa D, Lorenzi B. Along waterways: mobility and evolution of COVID-19 in the state of Amazonas. Confins. 2020;45 [Google Scholar]
- 26.Articulacao de Povos Indigenas do Brasil Vidas indígenas e o COVID-19. 2020. http://apib.info/2020/05/14/1-vidas-indigenas-e-covid-19/
- 27.Campos MB, Borges GM, Queiroz BL, Santos RV. Differences in mortality between indigenous and non-indigenous persons in Brazil based on the 2010 Population Census. Cad Saude Publica. 2017;33 doi: 10.1590/10.1590/0102-311X00015017. [DOI] [PubMed] [Google Scholar]
- 28.Santos RV, Borges GM, Campos MB, Queiroz BL, Coimbra CEA, Jr, Welch JR. Indigenous children and adolescent mortality inequity in Brazil: what can we learn from the 2010 National Demographic Census? SSM Popul Health. 2020;10 doi: 10.1016/j.ssmph.2020.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coimbra CEA, Santos RV. Emerging health needs and epidemiological research in indigenous peoples in Brazil. In: Salzano FM, Hurtado AM, editors. Lost paradises and the ethics of research and publication. Oxford University Press; Oxford: 2004. pp. 89–109. [Google Scholar]
- 30.Schmidt MI, Duncan BB, Azevedo e Silva G. Chronic non-communicable diseases in Brazil: burden and current challenges. Lancet. 2011;377:1949–1961. doi: 10.1016/S0140-6736(11)60135-9. [DOI] [PubMed] [Google Scholar]
- 31.Pena SD, Di Pietro G, Fuchshuber-Moraes M. The genomic ancestry of individuals from different geographical regions of Brazil is more uniform than expected. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baqui P, Bica I, Marra V, Ercole A, van der Schaar M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: a cross-sectional observational study. Lancet Global Health. 2020;8:e1018–e1026. doi: 10.1016/S2214-109X(20)30285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Long Q-X, Tang X-J, Shi Q-L. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26:1200–1204. doi: 10.1038/s41591-020-0965-6. [DOI] [PubMed] [Google Scholar]
- 34.Ministerio de Ciencia e Innovacion and Ministerio de Salud Spain. Estudio ENE-Covid-19: Informe Final Estudio Nacional de Sero-Epdemiologia de la Infección por SARS-CoV-2 en España. Madrid, Spain. 2020. https://www.mscbs.gob.es/ciudadanos/ene-covid/docs/ESTUDIO_ENE-COVID19_INFORME_FINAL.pdf
- 35.Premkumar L, Segovia-Chumbez B, Jadi R. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Instituto Brasileiro de Geografia e Estatística Projeções da População. 2019. https://www.ibge.gov.br/estatisticas/sociais/populacao/9109-projecao-da-populacao.html?=&t=resultados
- 37.Theel ES, Slev P, Wheeler S, Couturier MR, Wong SJ, Kadkhoda K. The role of antibody testing for SARS-CoV-2: is there one? J Clin Microbiol. 2020;58:e00797–e00820. doi: 10.1128/JCM.00797-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bryant JE, Azman AS, Ferrari MJ. Serology for SARS-CoV-2: apprehensions, opportunities, and the path forward. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abc6347. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi S, Greenhouse B, Rodríguez-Barraquer I. Are SARS-CoV-2 seroprevalence estimates biased? OSF Preprints. 2020 doi: 10.31219/osf.io/y3fxt. published online May 30. (preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will become publicly available upon request from the corresponding author 30 days after publication. The EPICOVID19 datasets are freely available online.