Abstract
With the ongoing development of the COVID-19 pandemic, research continues to emerge regarding the pathophysiology, characteristics, and treatment considerations for patients with COVID-19. No reports have highlighted the specific challenges posed in the management of pediatric patients with COVID-19 who present with complicated rhinosinusitis. In this report, we discuss our preoperative, intraoperative, and postoperative multidisciplinary treatment strategy for these cases and provide two examples of complicated rhinosinusitis cases in COVID-19 patients, treated with two different approaches. Pearls, insights, and a brief review of the literature are discussed.
Keywords: COVID-19, Coronavirus, Sars-CoV-2, Pediatric rhinosinusitis, Pediatric COVID-19, Complicated sinusitis
1. Introduction
Complicated rhinosinusitis is defined as the sequelae of direct extension of acute rhinosinusitis to adjacent structures [1,2]. Sequelae of complicated rhinosinusitis include orbital complications, such as preseptal cellulitis, subperiosteal abscess, orbital cellulitis, orbital abscess, and cavernous sinus thrombosis [[1], [2], [3], [4]]. Intracranial complications also can occur, resulting in meningitis, epidural abscess, subdural abscess, intracerebral abscess, and osteomyelitis of the frontal bone [5]. Viral infections are the most common cause of rhinosinusitis and have been found to be a nidus for bacterial superinfection [6].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which on March 11, 2020 was declared a worldwide pandemic by the World Health Organization (WHO) known as Coronavirus disease 2019 (COVID-19), has not previously been associated with complicated rhinosinusitis. Since the WHO declaration, there has been evidence reporting high viral loads of SARS-CoV-2 in the sinonasal cavity and nasopharynx [7]. As of July 18, 2020, over 14 million cases of COVID-19 have been reported worldwide with over 600,000 global deaths [8]. In the Unites States (US), COVID-19 cases have continued to demonstrate an upward trend since March 2020, now with over 3.6 million documented cases [8]. Despite vast research being performed to elucidate the pathophysiology, patient characteristics, and treatment considerations, no case of complicated rhinosinusitis in COVID-19 patients to our knowledge has been described in the current otolaryngology literature.
2. Illustrative Case I
An obese 15-year-old African American male with recent travel history presented to an outside emergency department (ED) with a three-day history of headaches, subjective fevers, nasal congestion, diarrhea, nausea, emesis, right periorbital swelling, pain, and blurred vision. Computed tomography (CT) revealed right-sided rhinosinusitis and an epidural collection posterior to the right frontal sinus (Fig. 1 ). He was transferred to our facility. The patient was febrile, tachycardic, and tachypneic, with a right-sided orbital cellulitis on exam. Nasal endoscopy revealed right middle meatal purulence and mucosal inflammation. He was neurologically intact. Table 1 highlights his laboratory values. SARS-CoV-2 ribonucleic acid (RNA) testing was positive.
Fig. 1.
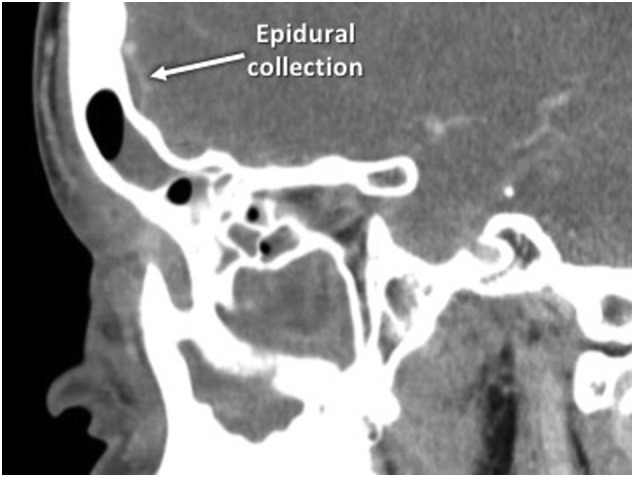
Preoperative sagittal computed tomography scan of the paranasal sinuses of the patient in Case I depicting rhinosinusitis with a partially opacified right frontal sinus and a small epidural collection posterior to the right frontal sinus posterior table.
Table 1.
Laboratory values of pediatric patients presenting with complicated rhinosinusitis.
| Lab value | Case I | Case II | RV | Lab value | Case I | Case II | RV |
|---|---|---|---|---|---|---|---|
| Hemoglobin | 13.2 | 14.1 | 13.0–16.0 g/dL | Urea | 11 | 10 | 4–19 mg/dL |
| Hematocrit | 40.1 | 41.7 | 37.0–49.0% | Creatinine | 0.9 | 0.55 | 0.7–1.2 mg/dL |
| Leukocytes | 7.5 | 6.5 | 4.5–13.0 × 103/μL | CRP | 169 | 22 | 0–5 mg/L |
| Lymphocytes | 10.7 | 38.0 | 20.0–50.0% | ESR | 34 | NA | 0–5 mm/h |
| Monocytes | 13.1 | 7.0 | 2.0–12.0% | Ferritin | 1216 | NA | 30–400 ng/mL |
| Platelets | 122 | 281 | 150-450 × 103/μL | Blood culture | Negative | Negative | Negative |
RV = reference value. CRP = C-reactive protein. ESR = erythrocyte sedimentation rate. Bold represents an abnormal value.
Multidisciplinary management approach with otolaryngology, ophthalmology, neurosurgery, infectious disease, and pediatrics was initiated. Treatment consisted of vancomycin and ceftriaxone, tobramycin eye drops, nasal saline irrigation, intranasal oxymetazoline and fluticasone, and prophylactic levetiracetam. After no clinical improvement for 24 h, magnetic resonance imaging (MRI) revealed persistent rhinosinusitis, epidural collection, and superior ophthalmic vein thrombosis (SOVT) (Fig. 2 ).
Fig. 2.
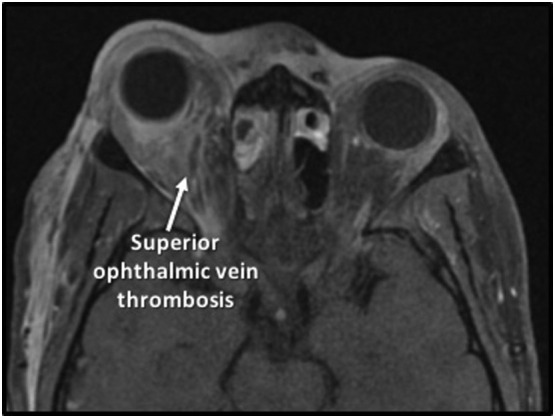
Preoperative T1-weighted gadolinium enhanced magnetic resonance imaging scan of the brain and orbits of the patient in Case I demonstrating mucosal thickening of the ethmoid sinuses, enhancement of the periorbita, intraconal and extraconal fat infiltration and enhancement, and thrombosis of the right superior ophthalmic vein.
On hospitalization day 3, he developed worsening chemosis, proptosis, and visual acuity. He was taken to the operating room (OR) for endoscopic right maxillary antrostomy, total ethmoidectomy, and frontal sinusotomy (Fig. 3 ). Only non-powered instrumentation was utilized. Intraoperative cultures grew coagulase-negative Staphylococcus. Postoperatively, he developed hypoxia to 89% on room air. Chest x-ray revealed bilateral, patchy opacities. He was also started on metronidazole, hydroxychloroquine, enoxaparin, zinc, vitamin C, and thiamine. He necessitated transfer to the pediatric intensive care unit (PICU) for bilevel positive airway pressure (BiPAP) therapy. His respiratory status improved with further uncomplicated hospital course. He was continued to metronidazole and ceftriaxone for five weeks after discharge. After completion of his antibiotic regimen, he was subsequently lost to follow-up.
Fig. 3.
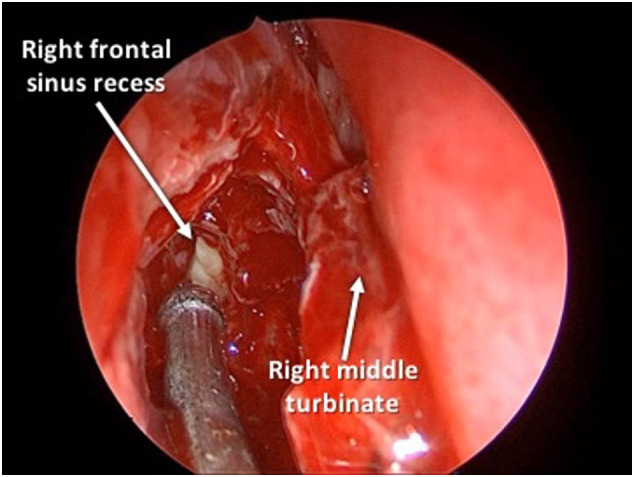
Intraoperative photograph of Case I after total ethmoidectomy and during Draf2A frontal sinusotomy showing purulence emanating from the right frontal sinus recess.
3. Illustrative Case II
A 12-year-old Egyptian male with seasonal allergies and remote history of adenotonsillectomy presented to an outside ED with nasal congestion and progressive right eye swelling for three days. He was evaluated by his pediatrician the day prior and started on oral amoxicillin/clavulanate and ofloxacin eye drops. CT revealed right-sided rhinosinusitis and subperiosteal abscess (Fig. 4 ). He was transferred to our facility. Table 1 highlights his laboratory values. SARS-CoV-2 RNA testing was positive. Vancomycin and ceftriaxone were administered, and he was taken to the OR expediently by the ophthalmology team for right orbitotomy and drainage of abscess. Intraoperatively, an extraconal collection with mucopurulence and hemorrhagic contents was evacuated. Intraoperative cultures grew no organisms. His postoperative course was uncomplicated. He was continued on vancomycin and ceftriaxone, intranasal oxymetazoline and fluticasone, nasal saline irrigations, and topical ocular tobramycin. His clinical status continued to improve, and he was discharged home with two-week course of amoxicillin/clavulanate and nasal irrigations. He had resolution of his symptoms upon outpatient follow-up. Interval imaging six weeks post discharge did reveal persistent right-sided rhinosinusitis with opacification of the maxillary, ethmoid, and frontal sinuses. He ultimately underwent right-sided endoscopic sinus surgery (ESS) for persistent sinus disease after further testing was negative for SARS-CoV-2 RNA.
Fig. 4.
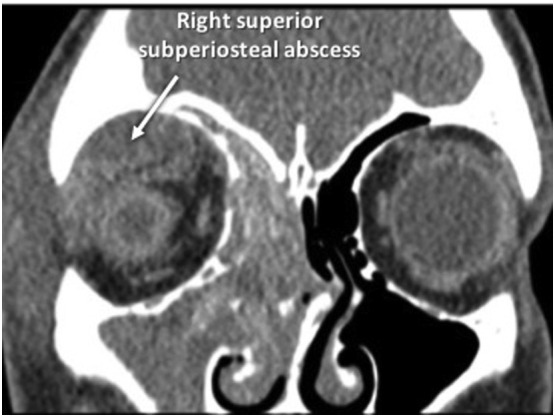
Preoperative coronal computed tomography scan of the paranasal sinuses of the patient in Case II depicting right-sided rhinosinusitis with a large rim-enhancing complex extraconal superior subperiosteal fluid collection with trace peripheral enhancement, and orbital cellulitis.
4. Discussion
Viral upper respiratory infection (URI) has been linked to the development of acute bacterial rhinosinusitis [6]. This had previously been described by Marom et al., demonstrating the correlation between the presence of rhinovirus at initial URI and subsequent development of bacterial sinusitis in children [6]. Our cases highlight this issue in the context of the COVID-19 viral pandemic, and the unique challenges presented in their perioperative management. To our knowledge, despite multiple novel viral pandemics over the past decade, no cases of complicated rhinosinusitis due to Middle East respiratory syndrome (MERS), SARS, or the current COVID-19 have been reported in the otolaryngology literature. This report highlights the challenges presented in the management of acute, complicated rhinosinusitis with both orbital and intracranial complications in pediatric patients with concurrent COVID-19.
COVID-19 as a viral illness in the pediatric population is still not well understood. Xu et al. found that pediatric patients were 2.7 times less likely than adults to contract the illness [9]. Research has suggested less severe disease manifestations in the pediatric population, however pediatric mortalities have been documented in the US [10]. The nasal cavity and nasopharynx are now well known reservoirs for the virus [7]. Additionally, it has previously been hypothesized that the presence of angiotensin-converting enzyme 2 (ACE2) receptors in the nasal epithelium is the underlying pathophysiological mechanism of the high incidence of anosmia reported in COVID-19 infection [11]. Consistent with prior epidemiological analyses regarding complicated rhinosinusitis, adolescents are at increased risk for intracranial complications due to the increased vascularity of the diploic bones [4]. Previous reports of severe COVID-19 infection in pediatric patients have been associated with lymphopenia and elevated c-reactive protein (CRP) [12]. These above findings are consistent with our patients presentation in Case I. While elevated CRP has been found in multiple case series of complicated sinusitis, our patient's presentation CRP of 169 mg/dL is well above the average elevation previously documented in these reports (18 mg/dL) [3].
Previously published COVID-19 guidelines have recommended deferment of operative intervention and initiation of maximal medical therapy for 48–72 h in patients with “complicated acute rhinosinusitis with orbital extension without vision or globe compromise” [13]. This is consistent with a recent systematic review by Wong et al. which recommended 48 h of parenteral antibiotic therapy for postseptal cellulitis in the pre-COVID-19 era [2]. Despite optimizing medical care, the clinical condition of the patient in Case I continued to worsen with the development of SOVT. Derangements in coagulation profiles and the resulting sequalae in patients with COVID-19 have been previously reported throughout the pandemic, including myocardial infarction, ischemic stroke, limb ischemia, and cerebral venous thrombosis among others [[14], [15], [16], [17]]. Urgent ESS in Case I was felt necessary to decrease the risk of developing cavernous sinus thrombosis and further neurologic complications [1]. In contrast, Case II responded well to initial orbital abscess drainage and improved clinically without immediate need for ESS. ESS was eventually required, although this was able to be performed on an outpatient, non-emergent basis after the patient tested negative for SARS-CoV-2 RNA.
In Case I, our patient was noted to experience new onset respiratory deterioration following operative intervention. A retrospective review from Wuhan, China examined 34 patients who underwent surgery during the asymptomatic incubation period [18]. 44% of the patients subsequently required ICU admission for respiratory deterioration with a mortality rate of 20.5%. A more recent and extensive review from 235 hospitals in 24 countries revealed postoperative pulmonary complications occur in half of patients with perioperative SARS-CoV-2 infection. The 30-day mortality in this patient cohort was noted to be 23.8% [19]. Worsening postoperative pulmonary status is consistent with our patient in Case I, highlighted by the necessity of PICU care and maximum non-invasive oxygen supplementation. Possible pulmonary complications from undergoing general anesthesia (up to 10%) is a known risk although patients with concomitant SARS-CoV-2 infection seem to have increased risk, likely due to the pro-inflammatory cytokine and immunosuppressive responses to surgery and mechanical ventilation [[20], [21], [22]]. This provides further evidence to possibly raise the threshold for operative intervention in COVID-19 patients, with need for close postoperative monitoring.
5. Conclusion
COVID-19 remains a novel threat to both the adult and pediatric populations. The cases provided in this report highlight the unique challenge of caring for pediatric patients presenting with complicated rhinosinusitis and concomitant COVID-19 infection. We recommend utilizing a multi-disciplinary approach in optimizing care of these patients when at all feasible. Further research is still needed to further elucidate the complex pathophysiology and optimal treatment algorithms of SARS-CoV-2 patients with complicated rhinosinusitis.
Financial disclosures
None.
Declaration of competing interest
None.
References
- 1.Kou Y.-F., Killeen D., Whittemore B. Intracranial complications of acute sinusitis in children: the role of endoscopic sinus surgery. Int J Pediatr Otorhinolaryngol. 2018;110:147–151. doi: 10.1016/j.ijporl.2018.05.015. [DOI] [PubMed] [Google Scholar]
- 2.Wong S.J., Levi J. Management of pediatric orbital cellulitis: a systematic review. Int J Pediatr Otorhinolaryngol. 2018;110:123–129. doi: 10.1016/j.ijporl.2018.05.006. [DOI] [PubMed] [Google Scholar]
- 3.Din-Lovinescu C., Mir G., Blanco C. Intracranial complications of pediatric rhinosinusitis: identifying risk factors and interventions affecting length of hospitalization. Int J Pediatr Otorhinolaryngol. 2020;131 doi: 10.1016/j.ijporl.2019.109841. [DOI] [PubMed] [Google Scholar]
- 4.Kaplan R. Neurological complications of infections of head and neck. Otolaryngol Clin North Am. 1976;9:729–749. [PubMed] [Google Scholar]
- 5.Magit A. Pediatric rhinosinusitis. Otolaryngol Clin North Am. 2014;47:733–746. doi: 10.1016/j.otc.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Marom T., Alvarez-Fernandez P.E., Jennings K., Patel J.A., McCormick D.P., Chonmaitree T. Acute bacterial sinusitis complicating viral upper respiratory tract infection in young children. Pediatr Infect Dis J. 2014;33:803. doi: 10.1097/INF.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zou L., Ruan F., Huang M. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. New England Journal of Medicine. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.John'’s Hopkins Coronavirus Center https://coronavirus.jhu.edu/map.html
- 9.Xu Y., Li X., Zhu B. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020:1–4. doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bialek S., Gierke R., Hughes M., McNamara L.A., Pilishvili T., Skoff T. 2020. Coronavirus disease 2019 in children—United States, February 12–April 2, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brann D., Tsukahara T., Weinreb C., Logan D.W., Datta S.R. Non-neural expression of SARS-CoV-2 entry genes in the olfactory epithelium suggests mechanisms underlying anosmia in COVID-19 patients. BioRxiv. 2020 doi: 10.1101/2020.03.25.009084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henry B.M., Lippi G., Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Chem Lab Med. 2020;58(7):1135–1138. doi: 10.1515/cclm-2020-0272. [DOI] [PubMed] [Google Scholar]
- 13.Bann D.V., Patel V.A., Saadi R.A. Best practice recommendations for pediatric otolaryngology during the COVID-1 19 pandemic. Otolaryngol. Head Neck Surg. 2020;162(6):783–794. doi: 10.1177/0194599820921393. [DOI] [PubMed] [Google Scholar]
- 14.Han H., Yang L., Liu R. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clinical Chemistry and Laboratory Medicine (CCLM) 2020:1. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 15.Violi F., Pastori D., Cangemi R., Pignatelli P., Loffredo L. Hypercoagulation and antithrombotic treatment in coronavirus 2019: a new challenge. Thromb Haemost. 2020;120(6):949–956. doi: 10.1055/s-0040-1710317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes C., Nichols T., Pike M., Subbe C., Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. European Journal of Case Reports in Internal Medicine. 2020;7 doi: 10.12890/2020_001691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bikdeli B., Madhavan M.V., Jimenez D. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei S., Jiang F., Su W. Clinical characteristics and outcomes of patients undergoing surgeries during the incubation period of COVID-19 infection. EClinicalMedicine. 2020;21 doi: 10.1016/j.eclinm.2020.100331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Collaborative C Mortality and pulmonary complications in patients undergoing surgery with perioperative SARS-CoV-2 infection: an international cohort study. Lancet. 2020;396:27–38. doi: 10.1016/S0140-6736(20)31182-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirmeier E., Eriksson L.I., Lewald H. Post-anaesthesia pulmonary complications after use of muscle relaxants (POPULAR): a multicentre, prospective observational study. Lancet Respir Med. 2019;7:129–140. doi: 10.1016/S2213-2600(18)30294-7. [DOI] [PubMed] [Google Scholar]
- 22.Neto A.S., da Costa L.G.V., Hemmes S.N. The LAS VEGAS risk score for prediction of postoperative pulmonary complications: an observational study. European Journal of Anaesthesiology (EJA) 2018;35:691–701. doi: 10.1097/EJA.0000000000000845. [DOI] [PMC free article] [PubMed] [Google Scholar]


