Abstract
Intracerebral hemorrhage (ICH) can be a devastating complication of coronavirus disease (COVID-19). We aimed to assess risk factors associated with ICH in this population. We performed a retrospective cohort study of adult patients admitted to NYU Langone Health system between March 1 and April 27 2020 with a positive nasopharyngeal swab polymerase chain reaction test result and presence of primary nontraumatic intracranial hemorrhage or hemorrhagic conversion of ischemic stroke on neuroimaging. Patients with intracranial procedures, malignancy, or vascular malformation were excluded. We used regression models to estimate odds ratios and 95% confidence intervals (OR, 95% CI) of the association between ICH and covariates. We also used regression models to determine association between ICH and mortality. Among 3824 patients admitted with COVID-19, 755 patients had neuroimaging and 416 patients were identified after exclusion criteria were applied. The mean (standard deviation) age was 69.3 (16.2), 35.8% were women, and 34.9% were on therapeutic anticoagulation. ICH occurred in 33 (7.9%) patients. Older age, non-Caucasian race, respiratory failure requiring mechanical ventilation, and therapeutic anticoagulation were associated with ICH on univariate analysis (p < 0.01 for each variable). In adjusted regression models, anticoagulation use was associated with a five-fold increased risk of ICH (OR 5.26, 95% CI 2.33–12.24, p < 0.001). ICH was associated with increased mortality (adjusted OR 2.6, 95 % CI 1.2–5.9). Anticoagulation use is associated with increased risk of ICH in patients with COVID-19. Further investigation is required to elucidate underlying mechanisms and prevention strategies in this population.
Electronic supplementary material
The online version of this article (10.1007/s11239-020-02288-0) contains supplementary material, which is available to authorized users.
Keywords: Anticoagulation, COVID-19, Hemorrhagic stroke, Intracerebral hemorrhage, Mechanical ventilation
Highlights
Intracerebral hemorrhage (ICH) occurs in patients with coronavirus disease (COVID-19).
Older age and respiratory failure are associated with ICH.
Anticoagulation use is associated with a five-fold increased risk of ICH.
ICH is associated with mortality in patients with COVID-19.
Further studies are warranted for improved prevention of ICH in this population.
Introduction
Intracerebral hemorrhage (ICH) is a devastating complication of coronavirus disease (COVID-19) and is associated with significant mortality [1–3]. In the general population, ICH portends a high mortality rate of 24–40%, [4, 5] and in anticoagulated patients, ICH is associated with an even higher mortality rate of 60% [6]. Older age, non-Caucasian ethnicity, prolonged activated partial thromboplastin time (aPTT) and therapeutic anticoagulation use are known risk factors for ICH [4–12].
We have previously examined the radiographic and clinical characteristics of 33 patients with COVID-19 who presented with ICH or developed ICH during hospitalization [1, 2, 13]. We have further studied a population of primary ICH and non-aneurysmal subarachnoid hemorrhage patients with COVID-19 in comparison with contemporary and historical controls [3]. Here, our aim is to examine all patients with COVID-19 with concomitant neuroimaging and compare patients with COVID-19 and ICH to those without ICH to assess risk factors associated with ICH and to further elucidate the association between anticoagulation use and ICH in this patient population.
Materials and methods
The study was approved by the NYU Grossman School of Medicine Institutional Review Board, IRB # i20-00567, who has granted the waiver for informed consent. Data from this study will be shared upon reasonable request by emailing the corresponding author.
Study population
We performed a retrospective cohort study of adult (age ≥ 18 years) patients admitted to the NYU Langone Health System (NYU Langone Medical Center, NYU Brooklyn, NYU Winthrop, or NYU Langone Orthopedic Hospital) who had both a positive severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) polymerase chain reaction (PCR) test result and neuroimaging performed between March 1 and April 27, 2020. Patients without neuroimaging were excluded due to the potential for unidentified ICH in this cohort.
Primary predictor—anticoagulation use
We classified anticoagulation use as therapeutic or prophylactic. Therapeutic anticoagulation was defined based on treatment with (1) therapeutic dose oral anticoagulation, (2) any dose of intravenous heparin or argatroban, (3) subcutaneous heparin at a total daily dose greater than 15,000 units (5000 units three times a day or 7500 units twice a day), or 4) enoxaparin at greater than 40 mg twice a day for BMI ≥ 40, and either 30 mg twice a day or 40 mg once a day for BMI < 40. All other anticoagulation use was considered prophylactic. At our institution, therapeutic anticoagulation was started for COVID-19 patients when there was a known or highly suspected thrombotic event [14–17] or if the patient was considered to be extremely high risk for thrombosis based on elevated D-dimer using the following paradigm: possible therapeutic anticoagulation for D-dimer 2000–10,000 ng/mL and strong consideration of therapeutic anticoagulation for D-dimer >10,000 ng/mL.
Primary outcome
The primary outcome was ICH defined as acute hemorrhage (primary intraparenchymal bleed or hemorrhagic conversion of ischemic stroke) diagnosed on neuroimaing obtained during the index hospitalization. Patients with only subarachnoid hemorrhage or subdural hemorrhage were excluded.
Covariates
Through chart review, we obtained data on demographics, comorbid diseases, mechanical ventilation use, timing of intubation, intensive care unit (ICU) admission, laboratory values, imaging results, and discharge disposition as of May 30, 2020 for all patients. We obtained peak or nadir lab values for a period of 72 h prior to imaging. We calculated the Sequential Organ Failure Assessment (SOFA) [18] score was calculated at admission and the maximum during admission. Glasgow Coma Scale (GCS) was calculated at the time of imaging. A board certified neuroradiologist identified radiographic ICH characteristics including ICH location and etiological adjudication on the first neuroimaging study with evidence of ICH. ICH score [7] was calculated based on initial head CT by a board certified neurointensivist.
Statistical methods
Discrete variables are presented as frequency and percentage, and continuous variables are presented as means and standard deviation (SD) or median with the interquartile range (IQR). Unadjusted differences in variables comparing patients with ICH to those without ICH were evaluated using Pearson’s χ2 test, Students t test, and Wilcoxon rank sum test for categorical, parametric continuous, and non-parametric continuous variables respectively. We also compared unadjusted differences in patients on therapeutic anticoagulation to patients without. We further evaluated independent predictive values of coagulation function on ICH using a receiver operating characteristic (ROC) curve. The associations between lab parameters and the risk of ICH in COVID-19 patients were expressed as the area under the ROC curve (AUC) [19]. Discharge mortality rate was determined for the ICH and non-ICH cohort.
We used binary logistic regression models to estimate the odds ratios and 95% confidence intervals (OR, 95% CI) of therapeutic anticoagulation use and ICH in patients hospitalized with COVID-19 adjusting for previously identified factors associated with ICH (age, gender, ethnicity, hypertension, systolic blood pressure) and might plausibly be associated with ICH in COVID-19 patients. Binary logistic regression models were used to assess for mortality in both ICH and non-ICH patients adjusting for previously reported risk factors, age [7] and maximum hospital SOFA score, [18] as a marker for disease severity.
As a sensitivity analysis, we performed binary logistic regression to determine the association between anticoagulation use and ICH adjusting for exposure variables associated with ICH on univariate analysis (excluding variables with co-linearity). We used p values that were two-tailed and we considered p < 0.05 significant. Analyses were performed using SPSS version 25.0 (Chicago, IL).
Results
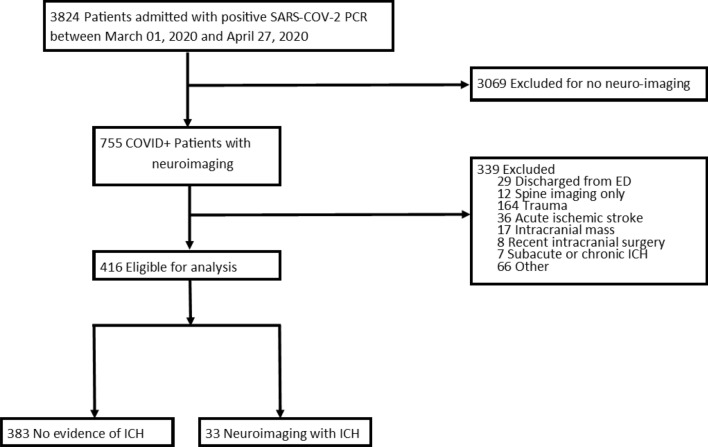
Among 3824 patients admitted with positive SARS-CoV-2 PCR during the study period, 755 had brain imaging available for review, 416 (55%) of whom were identified after exclusion criteria were applied. Of those, 33 patients had ICH and 383 patients did not (Fig. 1). In our entire cohort, patient mean age (standard deviation) was 69.3 (16.2) and 35.8% of patients were women (Table 1). Median (interquartile range) BMI was 27.4 (24.2–31.6). Comorbidities included atrial fibrillation in 31.5% of patients, coronary artery disease in 31.0% of patients, and hypertension in 78.8% of patients. Therapeutic anticoagulation was started in 145 (34.9%) patients. The most common indication for therapeutic anticoagulation was an elevated D-dimer in 18% of patients, and 8.7% of patients were diagnosed with an arterial or venous thrombosis. The most frequent indication for neuroimaging was encephalopathy (65% of non-ICH patients, and 51.5% of ICH patients).
Fig. 1.
3824 patients were admitted with COVID-19. Of these, 755 had brain imaging available for review, of which 416 met our inclusion criteria. We identified 37 patients with ICH, and further excluded 4 patients for recent trauma (2), intracranial neoplasm (2) and thus 33 were included for analysis.
Table 1.
Characteristics of both in ICH and non-ICH patients.
| TOTAL | ICH | No ICH | p value | |
|---|---|---|---|---|
| (N = 416)α | (N = 33) | (N = 383) | ||
| Admission Demographics | ||||
| Age; mean (SD) years | 69.3 (16.2) | 61.6 (11.2) | 70.0 (16.4) | <0.01 |
| Female | 149 (35.8) | 7 (21.1) | 142 (37.1) | 0.08 |
| Caucasian | 207 (50.1) | 9 (22.3) | 198 (52.1) | <0.01 |
| BMI; median (IQR), kg/m2 | 27.4 (24.2-31.6) | 29.5 (25.7-31.6) | 27.1 (23.8-31.6) | 0.4 |
| Medical Comorbidities | ||||
| Atrial Fibrillation | 131 (31.5) | 9 (27.3) | 122 (31.9) | 0.7 |
| Coronary Artery Disease | 129 (31.0) | 4 (12.1) | 125 (32.6) | 0.01 |
| Hypertension | 328 (78.8) | 22 (66.7) | 306 (79.9) | 0.08 |
| Diabetes | 200 (48.1) | 12 (36.4) | 188 (49.1) | 0.2 |
| Clinical variables at time of neuroimaging | ||||
| Admission to neuroimaging, mean (SD) days | 7.0 (10.5) | 13.9 (9.3) | 6.4 (10.4) | 0.7 |
| Indication for neuroimaging | ||||
| Encephalopathy | 266 (63.9) | 17 (51.5) | 249 (65.0) | 0.1 |
| Focal Neurologic Deficit | 67 (16.1) | 12 (36.4) | 55 (14.3) | <0.01 |
| Focal Weakness | 49 (11.8) | 7 (21.2) | 42 (11.0) | 0.09 |
| Brainstem Abnormality | 10 (2.4) | 4 (12.1) | 6 (1.6) | <0.01 |
| Aphasia | 6 (1.4) | 1 (3.0) | 5 (1.3) | 0.4 |
| Dysarthria | 2 (0.5) | 0 (0) | 2 (0.5) | 1 |
| Syncope | 18 (4.3) | 0 (0) | 18(4.7) | 0.4 |
| Seizure | 17 (4.1) | 2 (6.1) | 15 (3.9) | 0.6 |
| Headache | 13 (3.1) | 0 (0) | 13 (3.4) | 0.6 |
| Vertigo | 10 (2.4) | 0 (0) | 10 (2.6) | 1 |
| Fall | 5 (1.2) | 0 (0) | 5 (1.3) | 1 |
| Cardiac Arrest | 4 (1.0) | 0 (0) | 4 (1.0) | 1 |
| Other | 16 (3.8) | 2 (6.1) | 14 (3.7) | 0.4 |
| Peak SBP; mean (SD) mm HG | 130.3 (33.3) | 121 (33.1) | 130.8 (33.3) | 0.8 |
| D-dimer; mean (SD) ng/ul | 3085.1 (6008.4) | 3895.7 (4127.6) | 2984.2 (6202.4) | 0.4 |
| INR; median (IQR) | 1.2 (1.1-1.5) | 1.3 (1.2-1.8) | 1.2 (1.1-1.4) | 0.03 |
| aPTT; mean (SD) seconds | 48.3 (48.0) | 73.2 (58.1) | 43.6 (44.4) | <0.01 |
| Platelet nadir, mean (SD)103/uL | 215.6 (113.0) | 204.1 (119.1) | 216 (119.5) | 0.6 |
| Peak, mean (SD) IU/mL | 0.5 (0.3) | 0.5 (0.2) | 0.5 (0.3) | 0.7 |
| Therapeutic Anticoagulation | 145 (34.9) | 24 (72.7) | 121 (31.6) | < 0.001 |
| DOAC | 3 (0.7) | 1 (3.0) | 2 (0.5) | 0.2 |
| Warfarin | 3 (0.7) | 1 (3.0) | 2 (0.5) | 0.2 |
| Heparin Full Dose | 104 (25.0) | 21 (63.6) | 83 (21.7) | < 0.001 |
| Enoxaparin Full Dose | 35 (8.4) | 1 (3.0) | 34 (8.9) | 0.3 |
| Indication for Therapeutic Anticoagulation Use | ||||
| Elevated D-dimer | 75 (18.0) | 18 (54.5) | 57 (14.9) | < 0.001 |
| Arterial or Venous Thrombus | 36 (8.7) | 4 (12.1) | 23 (8.4) | 0.5 |
| Atrial Fibrillation | 29 (7.0) | 1 (3.0) | 28 (7.3) | 0.7 |
| Otherβ | 3 (0.7) | 1 (3.0) | 2 (0.5) | 0.2 |
| Clinical severity | ||||
| Admission SOFA score | 0 (0-0) | 0 (0-0) | 0 (0-0) | 0.8 |
| Maximum SOFA Score | 10 (4-15) | 11 (7-16) | 8.5 (4-14) | < 0.001 |
| Mechanical Ventilation prior to neuroimaging | 136 (32.7) | 26 (78.8) | 110 (28.7) | < 0.001 |
| Mortality | 126 (30.3) | 17 (51.5) | 109 (28.5) | 0.01 |
aPTT activated partial thromboplastin time, BMI body mass index, ICH intracerebral hemorrhage, INR international normalized ratio, SOFA sequential organ failure assessment
αAll numbers are n (%) unless otherwise specified.
βOther indications for anticoagulation use include mechanical valve (n = 2) and antiphospholipid syndrome (n = 1)
Lab values are peak (or nadir) within 72 h prior neuroimaging
Univariate analysis
Among patients with ICH, the mean (SD) age was 61.6 (11.2), 21.2% were women, 27.3% had atrial fibrillation, 12.1% had coronary artery disease, and 66.7 % had hypertension. In univariate analysis, we found 24 (72.7%) ICH patients had been on home anticoagulation or started on therapeutic anticoagulation during hospitalization, compared to 31.6% of non-ICH patients (OR 6.3, 95% CI 2.8–14.5, p < 0.001) (Table 1). There were six patients with ICH identified on admission scan and two of these patients were on anticoagulation prior to presentation. In ICH patients, the indication for therapeutic anticoagulation was elevated D-dimer in 18 (54.5%) patients, while this was the indication in only 14.9% of patients without ICH (p < 0.001). Patients with ICH underwent neuroimaging for an indication of focal neurologic deficit more commonly that patients without ICH (p < 0.01). There were no significant differences in hematoma size, location or ICH for patients who were or were not on therapeutic anticoagulation (Supplemental Table 1). Other variables associated with ICH were older age (p < 0.01), prolonged peak aPTT (p < 0.01) and higher INR (p = 0.03), while being Caucasian was associated with decreased likelihood of ICH (p < 0.01). Patients with ICH were more likely to have been admitted to the ICU, have respiratory failure requiring mechanical ventilation, and had higher SOFA scores during their hospitalization (p < 0.001 for all variables).
Patients on anticoagulation had a higher median BMI (IQR) of 29.5 (26.5–31.5) compared to those in whom anticoagulation was not started (25.9 (22.3–28.6), p = 0.05). They were more likely to have a history of atrial fibrillation (40% vs 26.9%, p < 0.01). Otherwise, there were no significant differences identified in admission demographics or ICH characteristics in patients who were and were not on therapeutic anticoagulation prior to neuroimaging (Supplemental Table 1). The indication for neuroimaging was most frequently encephalopathy in both groups; however, the indication was more frequently seizure or cardiac arrest in patients on anticoagulation, and was more commonly syncope or headache in patients not on anticoagulation (p < 0.04 for all). D-dimer and markers of coagulation were significantly higher in patients on anticoagulation (p < 0.001 for all), however there was no difference in peak D-dimer (p = 0.5), platelet nadir (p = 0.6), or peak anti-factor Xa levels (p = 0.7) at the time of neuroimaging in patients with ICH compared to those without.
Association between anticoagulation use and ICH
In unadjusted models, there was an association between anticoagulation use and ICH (OR 5.77 95% CI 2.61–12.80 p < 0.001). This association persists in fully adjusted models (OR 5.26 95% CI 2.33–11.85, p < 0.001) that includes age, ethnicity, sex, hypertension history, and peak systolic blood pressure prior to neuroimaging (see Table 2). Sensitivity analyses indicated the association between anticoagulation use and ICH persisted (adjusted OR 2.71, 95% CI 1.02–7.17, p = 0.05) after adjusting for factors associated with ICH in univariate analysis with p < 0.01 (Supplemental Table 2). In this model, we also found mechanical ventilation (OR 5.03, 95% CI 1.82–13.81, p < 0.01) and non-Caucasian ethnicity (OR 2.77, 95% CI 1.20–6.41, p = 0.02) were factors associated with an increased risk of ICH.
Table 2.
Regression models for risk factors associated with ICH in COVID-19 patients
| Covariates | Model 1 | Model 2 | Model 3 |
|---|---|---|---|
| Therapeutic anticoagulation | 5.77 (2.61–12.80), p < 0.001 | 5.45 (2.42–12.24), p < 0.001 | 5.26 (2.33–11.85), p < 0.001 |
| Age | 0.98 (0.96–1.00), p = 0.1 | 0.99 (0.96–1.01), p = 0.2 | |
| Caucasian | 0.38 (0.17–0.87), p = 0.02 | 0.38 (0.16–0.87), p = 0.02 | |
| Male | 1.90 (0.78–4.67), p = 0.2 | 1.86 (0.76–4.58), p = 0.2 | |
| Hypertension | 0.74 (0.31–1.75), p = 0.5 | ||
| Peak systolic blood pressure | 1.00 (0.99–1.01), p = 0.5 |
Values are represented as OR (95% CI), p value
Association between ICH and mortality
As of May 30th, 126 (30.3%) patients expired in the hospital or were discharged to hospice, including 17 (51.5%) patients with ICH and 109 (28.5%) patients without ICH. Patients with ICH who were on therapeutic anticoagulation had a non-statistically significant higher mortality than ICH patients who were not on anticoagulation (14 vs 3 patients with ICH, OR 2.8, 95% CI 0.56–13.59). ICH was associated with mortality in univariate analysis (OR 2.67, 95 % CI 1.30–5.47), p < 0.01. The remaining patients were discharged to home (140; 33.7%), a nursing facility (106; 25.5%), acute rehab (19; 4.6%), or another acute care facility 7 (1.7%). A small portion (4.3%) remained hospitalized as of May 30, 2020. After adjusting for age and maximum SOFA score during hospitalization, the odds of mortality following ICH was 2.65 (95 % CI 1.18–5.92), p = 0.02.
Additional analyses
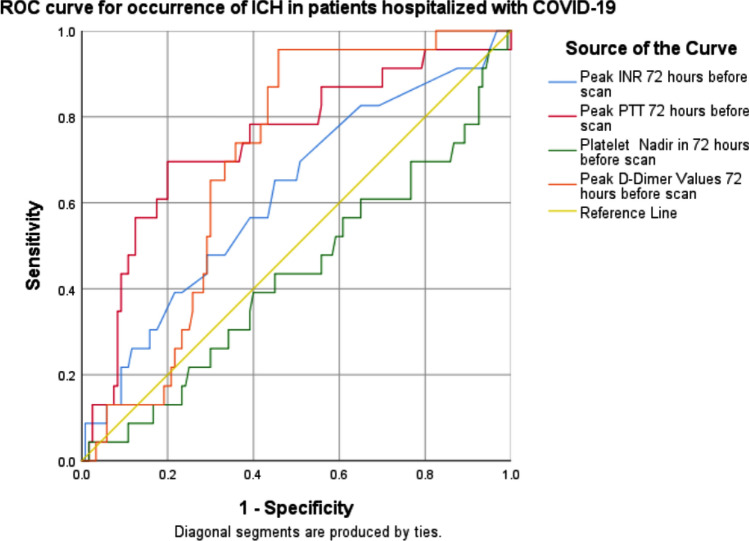
To further determine the predictive value of markers of coagulation for ICH, the ROC curve for coagulation abnormalities was plotted (Fig. 2). The AUC of peak aPTT for ICH in patients with COVID-19 was 0.73 (95% CI 0.62-0.86); the AUC of D-dimer peak was 0.67 (95% CI 0.57–0.76); the AUC for peak INR was 0.64 (95% CI 0.52–0.76); and the AUC of the platelet nadir was 0.45 (95% CI 0.32–0.57).
Fig. 2.
ROC curve for occurrence of ICH predicted by coagulation parameters. ROC curve analysis indicated that the area under the curve of INR, aPTT, platelet nadir and peak D-dimer in patients with ICH was 0.64, 0.73, 0.45 and 0.67, respectively (p = 0.01, p < 0.01, p = 0.4 and p < 0.01; respectively). aPTT: activated partial thromboplastin time; ICH: intracerebral hemorrhage cerebral microbleeds; ROC: receiver operating characteristic
Discussion
Bleeding diathesis seen in COVID-19 can have devastating outcomes, particularly when occurring in the brain [1, 3, 20]. We have previously explored the clinical and radiographic features seen with ICH in COVID-19 patients [1, 2, 13] and here we evaluate the risk associated with ICH in all COVID-19 patients with neuroimaging. We found anticoagulation use was strongly associated with ICH in patients with COVID-19. We found ICH is significantly associated with mortality in our cohort after adjusting for age and SOFA as a marker of illness severity.
Pathophysiology underlying ICH in COVID-19 patients is uncertain and may be variable. Literature from related coronavirus types has demonstrated evidence of the neuroinvasive potential of coronavirus, [21–23] autopsy proven vasculitis in SARS-CoV-1, [24] and reports of ICH as a result of thrombocytopenia, disseminated intravascular coagulation (DIC), and platelet dysfunction in patients with Middle East Respiratory Syndrome Coronavirus [25].
Proposed theories regarding etiology of ICH in SARS-CoV-2 similarly include a DIC like response [20] in which a consumptive coagulopathy makes the cerebral tissue more prone to bleeding. However, elevated D-dimer is also an acute phase reactant and a marker of increased thrombogenesis and propensity for clot formation. Despite the proposed association between ICH and DIC, D-dimer peak levels and platelet nadir at the time of neuroimaging are not statistically different in our cohort.
The significant association between respiratory failure requiring mechanical ventilation and ICH in patients with COVID-19 deserves further attention. These hypoxic patients might have hypoxia induced blood brain barrier disruption (similar what occurs in high altitude cerebral edema) leaving them more susceptible to ICH [26]. Alternatively, the downstream effects of the hypoxic, inflammatory milieu might contribute to endothelial dysfunction and cerebral injury [27]. Endothelial damage has been shown to occur in SARS-CoV-2 [28, 29] and microscopic disruption of the endothelium of cerebral veins could lead to microbleeds and eventual ICH [27]. Another proposed hypothesis is the microvascular thrombosis suggested to result in hypoxemic respiratory failure in some patients with COVID-19 might also result in direct neural injury and/or the microbleeds observed in patients with COVID-19 [30–32] Microbleeds could portend further risks for cerebral hemorrhage, particularly in the setting of anticoagulation use [11, 32, 33].
The common use of anticoagulation in patients with COVID-19 [16, 34] is not surprising given these patients have been shown to be predisposed to thrombotic events [15–17, 35–38]. In non-COVID-19 patients with atrial fibrillation, anticoagulation use increases the risk of ICH by a 2–10 fold increase [6, 10, 11, 39–42]. The indication for therapeutic anticoagulation in our cohort was elevated D-dimers in over half of the patients with ICH. In contrast, less than a quarter of patients without ICH were on anticoagulation for this indication. It is possible that patients with higher D-dimers requiring anticoagulation might represent a sicker patient more likely to have ICH. There was no difference in admission D-dimer levels nor in the 72 hours prior to neuroimaging. There was a significant difference in anticoagulation type in patients who did and did not experience ICH. Again, the fact that a greater percentage of ICH patients were on full dose heparin might be a marker of disease severity rather than represent a difference in ICH risk when on full dose heparin vs full dose enoxaparin [43]. Only patients with a clear indication and no contraindication should receive anticoagulation and the benefit versus risk of different doses of anticoagulation in hospitalized COVID-19 patients is currently being investigated. (URL: https://www.clinicaltrials.gov. Unique identifier: NCT04359277).
There are several limitations to our study, particularly the retrospective nature of the study of a somewhat heterogeneous population. We found it necessary to include patients with hemorrhagic conversion of ischemic stroke primarily because of the difficulty in distinguishing this entity from primary ICH on CT scan alone, and it was not always feasible to obtain an MRI given constraints of the pandemic. Furthermore, it is possible the CT findings considered to represent ischemic stroke might be consistent with viral associated subcortical microvascular inflammation and/or thrombosis. Excluding patients without neuroimaging may limit the generalization of these conclusions to all COVID-19 patients, but we felt it was important to limit the comparison of ICH patients to patients we could be certain did not have ICH. It is plausible we missed the diagnosis of ICH in a small number of patients who did not have head imaging. Finally, other factors that we did not evaluate may also be associated with ICH and outcome.
Conclusion
Anticoagulation use is independently associated with subsequent risk of ICH among patients with COVID-19. Further investigation is required to elucidate mechanisms of ICH and to explore means to prevent ICH in this patient population.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
KRM, SY, AL, and JF contributed to the study conception and design. Material preparation and data collection were performed by KRM, MC, SD, RZ, RJ, JC, and SB. Analysis were performed by KRM and SY. The first draft of the manuscript was written by KRM. MC, SD, RZ, RJ, SY, AL, BMC, ER, AL, JSB and JF commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
JAF reports grant funding from NIH/NIA grant 3P30AG066512-01S1 for investigation into impact of age on COVID related neurologic complications.
Data availability
Data from this study will be shared upon reasonable request by emailing the corresponding author.
Compliance with ethical standards
Conflicts of interest
RJ is a consultant for Cancer Panels Inc., receives royalties from Thieme Inc., and is on the advisory board for Nuevozen Inc. The remaining authors report no conflicts.
Ethics approval/Consent to participate
The study was approved by the NYU Grossman School of Medicine Institutional Review Board, IRB # i20-00567, who has granted the waiver for informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dogra S, Jain R, Cao M, Bilaloglu S, Zagzag D, Hochman S, Lewis A, Melmed K, Hochman K, Horwitz L, et al. Hemorrhagic stroke and anticoagulation in COVID-19. J Stroke Cerebrovasc Dis. 2020;29:104984. doi: 10.1016/j.jstrokecerebrovasdis.2020.104984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jain R, Young M, Dogra S, Kennedy H, Nguyen V, Jones S, Bilaloglu S, Hochman K, Raz E, Galetta S, Horwtiz L. COVID-19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci. 2020;414:116923. doi: 10.1016/j.jns.2020.116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kvernland A, Kumar A, Yaghi S, Raz E, Frontera J, Lewis A, Czeisler B, Kahn DE, Zhou T, Ishida K, et al (2020) Anticoagulation use and hemorrhagic stroke in SARS-CoV-2 patients treated at a new york healthcare system. Neurocrit Care [DOI] [PMC free article] [PubMed]
- 4.An SJ, Kim TJ, Yoon BW. Epidemiology, risk factors, and clinical features of intracerebral hemorrhage: an update. J Stroke. 2017;19:3–10. doi: 10.5853/jos.2016.00864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Javalkar V, Kuybu O, Davis D, Kelley RE. factors associated with inpatient mortality after intracerebral hemorrhage: updated information from the united states nationwide inpatient sample. J Stroke Cerebrovasc Dis. 2020;29:104583. doi: 10.1016/j.jstrokecerebrovasdis.2019.104583. [DOI] [PubMed] [Google Scholar]
- 6.Rosand J, Eckman MH, Knudsen KA, Singer DE, Greenberg SM. The effect of warfarin and intensity of anticoagulation on outcome of intracerebral hemorrhage. Arch Intern Med. 2004;164:880–884. doi: 10.1001/archinte.164.8.880. [DOI] [PubMed] [Google Scholar]
- 7.Hemphill JC, 3rd, Bonovich DC, Besmertis L, Manley GT, Johnston SC. The ICH score: a simple, reliable grading scale for intracerebral hemorrhage. Stroke. 2001;32:891–897. doi: 10.1161/01.STR.32.4.891. [DOI] [PubMed] [Google Scholar]
- 8.Koch S, Elkind MS, Testai FD, Brown WM, Martini S, Sheth KN, Chong JY, Osborne J, Moomaw CJ, Langefeld CD, et al. Racial-ethnic disparities in acute blood pressure after intracerebral hemorrhage. Neurology. 2016;87:786–791. doi: 10.1212/WNL.0000000000002962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shen AY, Yao JF, Brar SS, Jorgensen MB, Chen W. Racial/ethnic differences in the risk of intracranial hemorrhage among patients with atrial fibrillation. J Am Coll Cardiol. 2007;50:309–315. doi: 10.1016/j.jacc.2007.01.098. [DOI] [PubMed] [Google Scholar]
- 10.da Silva IRF, Frontera JA. Resumption of anticoagulation after intracranial hemorrhage. Curr Treat Options Neurol. 2017;19:39. doi: 10.1007/s11940-017-0477-y. [DOI] [PubMed] [Google Scholar]
- 11.Gurol ME. Nonpharmacological management of atrial fibrillation in patients at high intracranial hemorrhage risk. Stroke. 2018;49:247–254. doi: 10.1161/STROKEAHA.117.017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Essien UR, Holmes DN, Jackson LR, 2nd, Fonarow GC, Mahaffey KW, Reiffel JA, Steinberg BA, Allen LA, Chan PS, Freeman JV, et al. Association of race/ethnicity with oral anticoagulant use in patients with atrial fibrillation: findings from the outcomes registry for better informed treatment of atrial fibrillation II. JAMA Cardiol. 2018;3:1174–1182. doi: 10.1001/jamacardio.2018.3945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carroll E, Lewis A (2020) catastrophic intracranial hemorrhage in two critically ill patients with COVID-19. Neurocrit Care [DOI] [PMC free article] [PubMed]
- 14.Tang N, Bai H, Chen X, Gong J, Li D, Sun Z, Lindahl U, Li JP, Klok FA, Kruip M, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui S, Chen S, Li X, Liu S, Wang F (2020) Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost [DOI] [PMC free article] [PubMed]
- 16.Connors JM, Levy JH, Wang T, Chen R, Liu C, Liang W, Guan W, Tang R, Tang C, Zhang N, et al (2020) COVID-19 and its implications for thrombosis and anticoagulation. In: Blood. Volume 7. United States: © 2020 American Society of Hematology e362–e363
- 17.Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54–67. doi: 10.1007/s11239-020-02134-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 19.Hanley JA, Hajian-Tilaki KO. Sampling variability of nonparametric estimates of the areas under receiver operating characteristic curves: an update. Acad Radiol. 1997;4:49–58. doi: 10.1016/S1076-6332(97)80161-4. [DOI] [PubMed] [Google Scholar]
- 20.Sharifi-Razavi A, Karimi N, Rouhani N. COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect. 2020;35:100669. doi: 10.1016/j.nmni.2020.100669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arbour N, Côté G, Lachance C, Tardieu M, Cashman NR, Talbot PJ. Acute and persistent infection of human neural cell lines by human coronavirus OC43. J Virol. 1999;73:3338–3350. doi: 10.1128/JVI.73.4.3338-3350.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tsai LK, Hsieh ST, Chang YC. Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan. 2005;14:113–119. [PubMed] [Google Scholar]
- 23.Arabi YM, Harthi A, Hussein J, Bouchama A, Johani S, Hajeer AH, Saeed BT, Wahbi A, Saedy A, AlDabbagh T, et al. Severe neurologic syndrome associated with Middle East respiratory syndrome corona virus (MERS-CoV) Infection. 2015;43:495–501. doi: 10.1007/s15010-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding Y, Wang H, Shen H, Li Z, Geng J, Han H, Cai J, Li X, Kang W, Weng D, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol. 2003;200:282–289. doi: 10.1002/path.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiménez-Ruiz A, García-Grimshaw M, Ruiz-Sandoval JL, Algahtani H, Subahi A, Shirah B (2020) Neurological Complications of Middle East Respiratory Syndrome Coronavirus: A Report of Two Cases and Review of the Literature. In: Gac Med Mex. Volume 156. Mexico 3502683 [DOI] [PMC free article] [PubMed]
- 26.Riech S, Kallenberg K, Moerer O, Hellen P, Bärtsch P, Quintel M, Knauth M. The pattern of brain microhemorrhages after severe lung failure resembles the one seen in high-altitude cerebral edema. Crit Care Med. 2015;43:e386–389. doi: 10.1097/CCM.0000000000001150. [DOI] [PubMed] [Google Scholar]
- 27.Benger M, Williams O, Siddiqui J, Sztriha L. Intracerebral haemorrhage and COVID-19: Clinical characteristics from a case series. Brain Behav Immun. 2020;88:940–944. doi: 10.1016/j.bbi.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, et al (2020) Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med [DOI] [PMC free article] [PubMed]
- 30.Kandemirli SG, Dogan L, Sarikaya ZT, Kara S, Akinci C, Kaya D, Kaya Y, Yildirim D, Tuzuner F, Yildirim MS, et al (2020) Brain MRI findings in patients in the intensive care unit with COVID-19 Infection. Radiology 201697. [DOI] [PMC free article] [PubMed]
- 31.Radmanesh A, Derman A, Lui YW, Raz E, Loh JP, Hagiwara M, Borja MJ, Zan E, Fatterpekar GM (2020) COVID-19 -associated diffuse leukoencephalopathy and microhemorrhages. Radiology 202040. [DOI] [PMC free article] [PubMed]
- 32.Agarwal S, Jain R, Dogra S, Krieger P, Lewis A, Nguyen V, Melmed K, Galetta S. Cerebral microbleeds and leukoencephalopathy in critically ill patients With COVID-19. Stroke. 2020;51:2649–2655. doi: 10.1161/STROKEAHA.120.030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charidimou A, Boulouis G, Shams S, Calvet D, Shoamanesh A. Intracerebral haemorrhage risk in microbleed-positive ischaemic stroke patients with atrial fibrillation: preliminary meta-analysis of cohorts and anticoagulation decision schema. J Neurol Sci. 2017;378:102–109. doi: 10.1016/j.jns.2017.04.042. [DOI] [PubMed] [Google Scholar]
- 34.Thachil J, Tang N, Gando S, Falanga A, Cattaneo M, Levi M, Clark C, Iba T. ISTH interim guidance on recognition and management of coagulopathy in COVID-19. J Thromb Haemost. 2020;18:1023–1026. doi: 10.1111/jth.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carod-Artal FJ (2020) Neurological complications of coronavirus and COVID-19. In: Rev Neurol. Volume 70. Spain 311-322 [DOI] [PubMed]
- 36.Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, Henninger N, Trivedi T, Lillemoe K, Alam S, et al (2020) SARS2-CoV-2 and Stroke in a New York Healthcare System. Stroke Strokeaha120030335 [DOI] [PMC free article] [PubMed]
- 37.Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H (2020) Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res [DOI] [PMC free article] [PubMed]
- 38.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS (2020) Thrombosis in hospitalized patients With COVID-19 in a New York City Health System. JAMA [DOI] [PMC free article] [PubMed]
- 39.Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral anticoagulant therapy prescription in patients with atrial fibrillation across the spectrum of stroke risk: insights from the NCDR PINNACLE registry. JAMA Cardiol. 2016;1:55–62. doi: 10.1001/jamacardio.2015.0374. [DOI] [PubMed] [Google Scholar]
- 40.Steiner T, Rosand J, Diringer M. Intracerebral hemorrhage associated with oral anticoagulant therapy: current practices and unresolved questions. Stroke. 2006;37:256–262. doi: 10.1161/01.STR.0000196989.09900.f8. [DOI] [PubMed] [Google Scholar]
- 41.Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146:857–867. doi: 10.7326/0003-4819-146-12-200706190-00007. [DOI] [PubMed] [Google Scholar]
- 42.Tawfik A, Bielecki JM, Krahn M, Dorian P, Hoch JS, Boon H, Husereau D, Pechlivanoglou P. Systematic review and network meta-analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol. 2016;8:93–107. doi: 10.2147/CPAA.S105165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turshudzhyan A. Anticoagulation options for coronavirus disease 2019 (COVID-19)-induced coagulopathy. Cureus. 2020;12:e8150. doi: 10.7759/cureus.8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from this study will be shared upon reasonable request by emailing the corresponding author.