Abstract
Specific recommendations on surfactant administration in late preterm (LPT) infants with pulmonary disease are lacking. We performed an online-based, nationwide survey amongst all (n = 102) Belgian neonatologists to identify the use of surfactant in LPT infants suffering from several respiratory pathologies. The survey used clearly defined clinical cases and resulted in a 86% response rate. Neonatologists adhere to the 200 mg/kg initial surfactant dosing scheme. Surfactant is widely used in respiratory distress syndrome (70.1%), but there is less unanimity on its use in meconium aspiration syndrome (58.0%), transient tachypnoea of the newborn (30.6%), congenital pneumonia (27.2%) and congenital diaphragmatic hernia (8.6%). Respondents adhere to the European guideline of a timely referral to a newborn intensive care unit (non-invasive ventilation and FiO2 > 0.30 at 12 h of age), in order to minimise the risk of deterioration.
Conclusion: We demonstrate a wide variety in the use of surfactant within LPT infants. The majority of Belgian neonatologists therefore urge for an investment in multi-centre trials on surfactant administration in LPT infants, in order to create an evidence-based practice as well as to reduce the strain on health care budgets.
Trial registration: https://clinicaltrials.gov
|
What is Known: • Any late preterm (LPT) infant with respiratory distress needs a timely referral to a neonatal intensive care unit in case of non-invasive ventilation and FiO2 > 0.30 at 12 h of life, in order to minimise the risk of acute deterioration as well as chronic lung disease. • Any modest increase in morbidity in the sizeable group of LPT infants exerts a significant strain on health care budgets. | |
|
What is New: • We report the attitudes and opinions of Belgian neonatologists about the use of surfactant in LPT infants suffering from several respiratory diseases. • Our survey demonstrates a significant variability in practice between neonatologists during treatment of respiratory pathologies in LPT infants. This highlights an urgent need for univocal therapeutic lines. |
Keywords: Late preterm infant, Surfactant therapy, Respiratory distress syndrome, Nationwide survey
Introduction
Late preterm (LPT) infants (i.e. infants born between 34 and 36 + 6 weeks GA) account for up to 75% of all preterm births. Historically these babies were classified as ‘near term’, since they have weights similar to term infants at birth, making them appear deceivingly healthy [1, 2]. However, LPT infants have structurally immature lungs and may have a suboptimal surfactant production. Compared with infants born at term, LPT infants are more likely to suffer poorer short-term outcomes such as neonatal respiratory distress syndrome (nRDS) (relative risk [RR], 17.3), intraventricular haemorrhage (RR, 4.9) and death < 28 days (RR, 5.9) [3].
The fourth update of the European Consensus Guidelines on the Management of Respiratory Distress Syndrome summarises the evidence regarding indications, administration and outcomes for surfactant replacement therapy [4]. Within the group of LPT infants, many simultaneously present characteristics of nRDS, as well as transient tachypnoea of the newborn (TTN) and persistent pulmonary hypertension of the newborn (PPHN) in the course of the same respiratory disease. Such complicates any neonatologist’s decision whether or not to give surfactant. With respect to TTN or PPHN in the LPT infant, neonatologists base their decisions to administer surfactant on small single-centre studies, as there are no large prospectively organised trials available [5]. Also, a distinction between surfactant deficiency nRDS and lung inflammation/infection due to chorio-amnionitis is sometimes difficult to make during routine clinical practice [6].
Hence, although surfactant is commonly used in the management of respiratory failure in LPT infants, many questions remain unanswered:
What is the optimal timing and dosage of surfactant administration in LPT infants?
What is the correct FiO2 threshold to administer surfactant?
Should we administer surfactant in the context of TTN, meconium aspiration syndrome (MAS), congenital pneumonia (CP) or congenital diaphragmatic hernia (CDH)?
We therefore need to better understand why and how surfactant is used for different respiratory pathologies seen in LPT infants. In order to create a Belgian consensus statement on the use of surfactant within these infants, we needed a clear idea of individual practices across all newborn intensive care units (NICUs). As such, we conducted a nationwide survey, i.e. a collection of data in a standardised way.
Materials and methods
This survey research has been conducted based on a good practice checklist [7]. Seven Belgian neonatologists with a specific interest in neonatal respiratory diseases were selected based on their previous clinical and scientific experience. Consensus on the development of 5 cases as well as several survey questions was obtained during two face-to-face meetings in which a mini-Delphi technique was used, i.e. an interactive and structured discussion forum. The meetings were expanded with email exchange, i.e. a process of continuous interaction, enabling the seven panellists to exchange their final comments. LC acted as the facilitator (sending out questions, collecting responses, identifying common and conflicting viewpoints) to achieve consensus and to converge to the final questionnaire, i.e. an online survey tool (SurveyMonkey, Portland, OR).
The survey was then conducted using a non-random sampling technique, i.e. the structured questionnaire was sent to the unique group of Belgian neonatologists (n = 102), working in one of 19 NICUs, covering 118,000 deliveries per year. The survey was not sent to junior doctors in training, but only to certified neonatologists that are active within a clinical setting. All neonatologists were invited by email (neonatology.belgium@gmail.com) to participate. One reminder was sent 2 months after the initial invitation, in order to increase the response rate. The survey has been approved by the Ethical Comité of AZ St-Jan Bruges-Ostend AV. All participants had to read through a covering letter explaining the rationale of the survey, as well as what will happen with the information provided. Prior to starting the survey, they needed to tick a box in order to consent. The study was registered onto https://clinicaltrials.gov.
Each person completed the survey in a semi-anonymous way: no personal names were asked. However, in order to ensure there were a sufficiently high number of neonatologists responding from each unit, we asked for the hospital’s name each person was working at.
Five written clinical cases were presented to the neonatologists (Table 1), together with the same set of structured questions for each clinical case. The survey ended with open-ended questions regarding patient selection and experiences of surfactant efficacy within this LPT patient group.
Table 1.
Description of 5 clinical cases
|
Case 1 A 34-week 2/7 GA infant is born (2.750 g); the mother is GBS negative. There is no PPROM. The infant is tachypnoeic at birth (80/min) and is in need of FiO2 0.25. IV access is obtained, blood culture taken and IV antibiotics are started. The infant is transferred to the NICU. At the age of 1 h, the chest X-ray shows an air bronchogram. The infant remains tachypnoeic and needs FiO2 0.35. nCPAP is started. At the age of 4 h, the infant still needs FiO2 0.38. | |
|
Case 2 A 38-week GA infant is born (3.800 g); the mother is GBS negative. The patient presents with foetal tachycardia after delivery by emergency C-Section. Amniotic fluid is meconium stained. The infant needs intubation and ventilation at the age of 1 min. At the age of 3 h, the infant saturates 86% with invasive ventilation (SIMV), pressures 24/5 cm H2O, frequency 45/min, FiO2 0.60. Chest X-ray is patchy. The following parameters are available on an arterial blood gas: pH 7.10, PaCO2 55 mmHg and PaO2 45 mmHg. Cardiac ultrasound demonstrates evidence of pulmonary hypertension. | |
|
Case 3 A 36-week1/7 GA infant is born (2.900 g) after an urgent Caesarean section within the context of acute maternal blood loss (abruption of the placenta). The infant is born in good condition, but needs FiO2 0.30 during the first golden minutes of life. Intravenous access is obtained, blood culture taken. The infant is transferred to the NICU with nCPAP and FiO2 0.30. At the age of 2 h, the chest X-ray shows extra fluid in the fissures separating the long lobes. At the age of 4 h, having inserted umbilical lines, the infant remains tachypnoeic and needs FiO2 0.40. | |
|
Case 4 A 35-week GA infant is born (2.750 g); the mother is GBS positive. There is no PPROM. The infant needs 5 insufflations, remains tachypnoeic (90/min) and is in need of FiO2 0.35. IV access is obtained, blood culture taken and IV antibiotics are started. The infant is transferred with nCPAP to the NICU. At the age of 2 h, chest X-ray shows patchy lung fields. CRP level is 42 mg/L on D0. The infant remains tachypnoeic and needs FiO2 between 0.30 and 0.40. | |
|
Case 5 A 36-week GA infant is born (2.900 g); the mother is GBS negative. The infant presents with severe respiratory distress at birth, requiring intubation and ventilation. A chest X-ray demonstrates the presence of bowel in the left hemi-thorax. At the age of 3 h, SIMV ventilation is switched to high-frequency oscillation ventilation with the following settings: mean airway pressure 11 cm H2O, Delta P 28 cm H2O, FiO2 0.45. An arterial blood gas shows pH 7.15, PaCO2 52 mmHg, PaO2 49 mmHg. The infant continues to saturate at best 82%. |
Data analysis is descriptive. Categorical variables are presented in absolute numbers and percentages.
Results
All participants were given a 6 months’ time window to respond to the survey (September 2019 to February 2020). Two reminders (month 3 and month 5) were sent.
Responses were received from n = 90/102 neonatologists (n = 65 females, n = 25 males). All Belgian NICUs were represented (response rate varying between 33 and 100% per unit). Two participants did not fully complete the survey; hence, their responses were excluded. We performed all analyses on n = 88/102 responses, i.e. a response rate of 86%.
Clinical experience is spread across four age groups as follows (the number of years one has been working as a neonatologist): 40.2% (< or = 10 years), 37.9% (11–20 years), 18.4% (21–30 years), 3.4% (31–40 years). All units exclusively use poractant alfa.
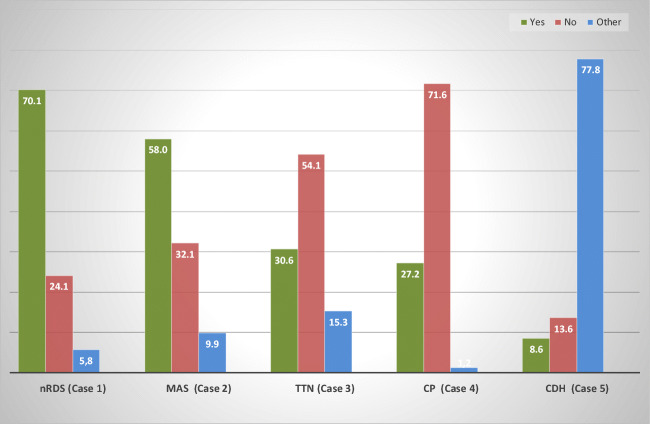
Administration of surfactant (case 1–case 5)
Figure 1 indicates the % of respondents that will administer (or not) surfactant to the 5 clinical cases. Surfactant in LPT infants is mostly given for nRDS (70.1%) and MAS (58.0%). In TTN and CP, many respondents prefer not to give surfactant, quoting reasons such as preference to optimise the infant’s position and non-invasive ventilator support, together with cardiac ultrasound to exclude PPHN. In CDH, surfactant is rarely used, as most respondents remain indecisive. Respondents prefer the administration of iNO, as well as an optimisation of blood pressure management. Those respondents that give surfactant in CDH note clearly that it is mostly given as a ‘rescue’ therapy, especially when ventilation remains difficult.
Fig. 1.
Administration of surfactant (case 1–case 5). Expressed in % of neonatologists. ‘Other’ refers to neonatologists that are indecisive and may include careful blood pressure management or a trial of Inhaled nitric oxygen (iNO), prior to ‘possibly’ reverting to the administration of surfactant (as a final rescue). nRDS, neonatal respiratory distress; MAS, meconium aspiration syndrome; TTN, transient tachypnoea of the newborn; CP, congenital pneumonia; CDH, congenital diaphragmatic hernia
Method of administration of surfactant (case 1–case 4 )
Regarding the method of surfactant administration, the majority prefers the less invasive approach in the case of RDS, TTN and CP, i.e. less invasive surfactant administration (LISA) or intubation, surfactant administration, extubation (INSURE) (Fig. 2). It is however worth noting that, when an LPT infant needs a second dose of surfactant in these cases, 45.6% of the respondents administer the second dose after intubation and ventilation only. In the case of MAS, the majority of the respondents (n = 88.0%) will preferentially administer surfactant using intubation and ventilation.
Fig. 2.
Method of surfactant administration (case 1–case 4)
Expressed in % of neonatologists. LISA, less invasive surfactant administration; INSURE, intubation, surfactant administration, extubation; nRDS, neonatal respiratory distress syndrome; MAS, meconium aspiration syndrome; TTN, transient tachypnoea of the newborn; CP, congenital pneumonia
Threshold FiO2 to administer surfactant (cases 1, 2 and 4 )
In case 1 (nRDS), threshold FiO2 for transfer to a high care neonatal centre that can provide the appropriate level of care (and thus the administration of surfactant) is ≥ 0.30 in 76.3% (n = 54) and ≥ 0.40 in 23.7% (n = 15) (Fig. 3). Of those respondents who consider surfactant administration in case 1 (i.e. below 24 h of age), only n = 38 still consider surfactant when the infant would be between 24 and 48 h of age. The 24.1% of neonatologists (n = 21) who would not give surfactant to case 1 were asked at which FiO2 they would give surfactant if the infant further deteriorates: the majority (n = 17) will give surfactant as soon as FiO2 ≥ 0.40 is needed, whilst the other n = 4 will wait until an FiO2 ≥ 0.50 is needed. The majority (66%) of the latter group prefers to use a clinical nRDS score in order to help them to decide.
Fig. 3.
LPT infant with nRDS—age and FiO2 threshold for transfer to NICU. Expressed in % of neonatologists. LPT, late preterm; nRDS, neonatal respiratory distress syndrome; NICU, neonatal intensive care unit
32.1% of the respondents (n = 29) will not give surfactant in MAS whereas n = 9 envisages to do so as soon as FiO2 ≥ 0.50. 54.1% of the respondents (n = 47) will not give surfactant in TTN whereas n = 21 is prepared to do so as soon as FiO2 ≥ 0.40. A larger group of n = 25 respondents prefers to even wait until FiO2 ≥ 0.40. 71.6% of the respondents (n = 63) will not administer surfactant in CP, and two third (65.5%) within that group would not consider surfactant even in the case of FiO2 ≥ 0.40.
Dosing of surfactant (case 1–case 4 )
In nRDS, the majority (95%) agree with a theoretical dose of 200 mg/kg. However, depending on the weight of the infant, 51% of the respondents admit they prefer to downscale the dosage of poractant alfa to 160 mg/kg (e.g. 480 mg in case 1 or 2 vials of 240 mg) whilst in reality, 44% will administer the full 200 mg/kg (e.g. 600 mg in case 1 or 2 vials of 240 mg and 1 vial of 120 mg). The majority of neonatologists accept a difference of maximally 50 mg/kg between the recommended (200 mg/kg) and practical dosages (150–200 mg/kg).
In TTN, the majority (92%) of the respondents agree with a theoretical dose of 200 mg/kg and accept a difference of maximally 50 mg/kg between the recommended (200 mg/kg) and practical dosage (150–200 mg/kg). iNO is not seen as an alternative ‘first line’ treatment in this case.
In CP and MAS, those who will give surfactant dose at 200 mg/kg.
Discussion
When using surfactant for respiratory diseases in LPT infants, we observe a great deal of variability, both across Belgian NICU centres as well as among individual neonatologists. Such variability in care can be explained by a lack of evidence-based practice and may contribute to the currently observed morbidity among LPT infants. Using a survey on surfactant use in LPT infants across all Belgian neonatologists, a high response rate (86%) was obtained which is significantly above a recommended 65% level [7]. Such makes this survey representative and thus less open to bias.
There are some limitations to creating a case-based survey. Firstly, as with any survey, data that are being produced are always likely to lack details or depth on the topic being investigated [7]. Secondly, at the outset of severe pulmonary disease in LPT infants the underlying aetiology is frequently unclear: respiratory disease often starts as a delayed respiratory transition and its subsequent course can be unpredictable. Thirdly, many LPT infants present mixed characteristics of nRDS, TTN and PPHN in the course of the same respiratory disease. In order to avoid ambiguous answers, we therefore opted to ‘simplify’ our survey using clearly delineated case presentations. A survey based on such well-described clinical cases is therefore somewhat different from the reality, as when confronted with an infant in respiratory distress, an exact diagnosis can only be obtained through the integration of anamnestic, clinical, imaging (especially lung ultrasound) and biological data (e.g. CRP). Such requires time and a significant effort, but it is the only way to understand what is the actual situation of that patient in that particular moment. This is particularly important as surfactant is expensive and may not always be effective. Fourthly, by looking at LPT infants (and thus a group of infants with a specific GA), we do not imply that surfactant administration should be based on GA. Clearly, the underlying pathophysiology of respiratory illnesses in these infants is a major reason why not all LPT infants may benefit from surfactant administration. Fifthly, a case-based survey remains a theoretical exercise on ‘intended care’, so caution is needed during the interpretation of answers. Finally, given the relatively small local newborn population (i.e. a total of approximately 6000 admissions annually across all Belgian NICUs), results need to be interpreted with caution. Indeed, some answers may have been influenced by the way neonatal health care is organised in Belgium and as such might be less relevant in different settings.
Respiratory distress syndrome
It is widely accepted that prophylactic use of surfactant has no advantage over the initiation of CPAP alone [8]. However, it remains important to determine when CPAP alone will not be effective. Whilst earlier studies recommend that surfactant should be administered as soon as FiO2 > 0.30 for very immature babies and FiO2 > 0.40 for more mature infants, the 2019 European directive recommends a threshold of FiO2 > 0.30 to be used for all infants with a clinical diagnosis of neonatal respiratory distress syndrome (nRDS) regardless of their GA [4]. The majority (76%) of the Belgian neonatologists agrees with the principle that FiO2 > 0.30 in the first hours of life is a reasonable predictor of CPAP failure [9] and supports referral to a level III NICU as soon as FiO2 > 0.30 at the age of 12 h.
Intubation and ventilation are rarely used in nRDS (6.6%), whilst such is more common in MAS, CP and severe TTN. A reduction of the percentage of invasive mechanical ventilation during the first 3 days of respiratory disease in LPT infants may thus be feasible in our country and should be aimed for, in line with the 2019 European directives [4].
Transient tachypnoea of the neonate
Whilst literature is scarce on surfactant administration within the clinical entity of transient tachypnoea of the newborn (TTN), it is somehow surprising to observe a fairly high percentage of neonatologists (30.6%) is willing to administer surfactant in the case of TTN. Belgian neonatologists are not relying their final decision on chest X-ray appearance, but they rather take into account the level of FiO2. Some centres use pulmonary ultrasound, which is a promising bedside tool in order to differentiate between TTN and nRDS [10]. Pulmonary ultrasound has no adverse effects and allows neonatologists to detect those patients that may benefit from surfactant or mechanical ventilation, even prior to reaching oxygenation criteria [11, 12].
Congenital pneumonia
It is interesting to see that more than half of the neonatologists do not consider surfactant administration in congenital pneumonia (CP). From this group, 65% would still not administer surfactant if the FiO2 increases to 0.40. Whilst the clinical picture of nRDS and TTN is very similar, CP is a clinically more heterogeneous entity, and probably therefore neonatologists are less inclined to consider the use of surfactant.
An interim analysis of a large ongoing international study suggests however that more and more neonatologists do consider surfactant in the case of CP [13] since CP often leads to surfactant deficiency or dysfunction [14, 15]. However, in the absence of randomised controlled trials in LPT infants with proven or suspected pneumonia [16], one cannot state with certainty that any improvement in oxygenation upon surfactant therapy is either due to the surfactant itself or due to a natural recovery related to supportive treatment. Hence, the question of whether or not we should administer surfactant in CP remains unanswered, as reflected in our survey.
Meconium aspiration syndrome
In our survey, a significantly higher number of neonatologists opted to administer surfactant in meconium aspiration syndrome (MAS) compared with TTN and CP. The surfactant dysfunction in MAS seems to result in more from secondary inactivation than from primary deficiency [17]. Indeed, the aspiration of meconium induces pulmonary inflammation with subsequent type II cell dysfunction. Surfactant administration reduces the number of infants with progressive respiratory failure requiring support with ECMO [18, 19].
Our respondents state that surfactant in MAS should be given at high doses (200 mg/kg) and preferably as soon as possible when a trial of iNO with aggressive blood pressure management appears unsuccessful. Some studies suggest the opposite, i.e. that surfactant administration at a very early stage of MAS and prior to starting iNO may result in a better oxygenation due to optimised iNO diffusion [20]. Overall, the literature on MAS is more abundant than on CP [21]. The higher rate of surfactant administration observed in this survey reflects not only on the fact that patients with MAS are likely to present with a more severe respiratory failure than other disease groups but also on the fact that neonatologists base their decisions on available evidence.
Congenital diaphragmatic hernia
Pulmonary hypertension accounts for a significant morbidity and mortality in neonates with congenital diaphragmatic hernia (CDH). Whether CDH is associated with alterations in alveolar surfactant composition and function remains controversial, as animal data are limited and sometimes difficult to confirm in clinical practice. For instance, antenatal treatment with vitamin A restores foetal rat lung maturation in an animal-model of CDH, whilst most clinical studies have shown no significant benefit of vitamin A associated with surfactant therapy for (near) term infants with CDH [22]. There is however one study suggesting that partial alveolar SP-B deficiency could be a major contributor to respiratory insufficiency in newborn infants with CDH [23].
Notwithstanding this limited evidence for surfactant administration in CDH, we were surprised to see that the majority of the respondents still use surfactant, predominantly as a rescue therapy post-surgery. 94% of the respondents administer 200 mg/kg even if the patient may have only one lung. Given the low incidence of CDH (1/4.000 infants = 30 infants/year in Belgium), exposure to this type of pathology is rare, and most likely, surfactant is being given as a ‘last resort’. Nevertheless, if new-generation synthetic surfactant-containing recombinant SP-B were available on the market, then it would be interesting to investigate whether alveolar SP-B correction might represent a therapeutic goal in CDH [23].
Dosage issues during surfactant replacement therapy
It is interesting to observe that a correct surfactant dosage of 200 mg/kg is not always followed; i.e. Belgian clinicians are tempted to administrate a rounded dose (to the vial content). As such, there exists a risk of under- and overtreating infants. For instance, a tendency to overtreat extremely low birth weight neonates and a trend to undertreat neonates > 28 weeks GA has been reported in France and in Italy [24, 25]. A specific action to avoid under- or overtreating is to carefully weigh each infant prior to surfactant administration [26]. If however surfactant is required before the baby’s birth weight is known (surfactant for early rescue therapy), it is acceptable to use whole vial dosing based on an estimated weight [9]. Undertreating small infants for financial reasons must be abandoned as such increases the odds for a retreatment with a second dose.
Conclusion
We demonstrated that a large fraction of Belgian neonatologists uses surfactant in LPT infants that present with pulmonary problems other than nRDS, such as MAS, TTN and CP. Respiratory care provided to this sizeable group of LPT infants remains an area with significant variability in practice. The high response rate to our survey initiative demonstrates a clear need to identify the best practice regarding surfactant therapy in LPT infants. Until large randomised controlled multi-centre trials on the use of surfactant in LPT infants are being organised, we will develop a national consensus statement, in order to reduce the current variability in respiratory care practice. Such quality-improvement initiative may result in a more rational use of surfactant and possibly a reduction in strain on health care budgets.
Acknowledgements
The authors would like to thank all neonatologists that participated in this study. Furthermore, they would like to thank the Bureau of the ‘Belgian Association for Neonatologists’ for their help in developing the questionnaire. Finally, they would like to thank Zarha Vermeulen, Nathalie Dirix and Pauline Capiau (Medical Department Chiesi) for providing logistic support during both Advisory Board meetings.
Abbreviations
- CDH
Congenital diaphragmatic hernia
- CP
Congenital pneumonia
- CPAP
Continuous positive airway pressure
- GA
Gestational age
- iNO
Inhaled nitric oxygen
- INSURE
Intubation, surfactant administration, extubation
- LISA
Less invasive surfactant administration
- LPT
Late preterm
- MAS
Meconium aspiration syndrome
- NICU
Newborn intensive care unit
- PPHN
Persistent pulmonary hypertension of the newborn
- nRDS
Neonatal respiratory distress syndrome
- TTN
Transient tachypnoea of the newborn
Authors’ contributions
All authors participated in the conceptualisation and design of the survey. LC carried out the data analysis and interpretation of the data and drafted the initial paper. All authors participated in discussing the survey (mini-Delphi technique). They also reviewed and revised the paper. All authors approved the final paper as submitted and agree to be accountable for all aspects of the work.
Compliance with ethical standards
Conflict of interest
Chiesi provided logistic support (meeting room) for both advisory board meetings. All authors received honorarium from Chiesi as a reimbursement for travel costs. The authors maintained complete control over all content and phrasing within the manuscript and accept full responsibility for the content.
Ethical approval
The survey has been approved by the Ethical Comité of AZ St-Jan Bruges-Ostend AV.
Informed consent
Informed consent was obtained from all individual participants that completed the survey.
Disclaimer
Neither Chiesi nor any other corporate entity contributed to data analysis/interpretation and preparation of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
L. Cornette, Email: luc.cornette@azsintjan.be
A. Mulder, Email: antonius.mulder@uza.be
A. Debeer, Email: anne.debeer@uzleuven.be
G. Malfilâtre, Email: malfilatre.genevieve@gmail.com
V. Rigo, Email: vincent.rigo@chu.ulg.ac.be
F. Cools, Email: filip.cools@uzbrussel.be
O. Danhaive, Email: olivier.danhaive@uclouvain.be
References
- 1.Escobar GJ, Clark RH, Greene JD. Short-term outcomes of infants born at 35 and 36 weeks gestation: we need to ask more questions. Semin Perinatol. 2006;30(1):28–33. doi: 10.1053/j.semperi.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 2.Boyle JD, Boyle EM. Born just a few weeks early: does it matter? Arch Dis Child Fetal Neonatal Ed. 2013;98(1):F85–F88. doi: 10.1136/archdischild-2011-300535. [DOI] [PubMed] [Google Scholar]
- 3.Teune MJ, Bakhuizen S, Gyamfi Bannerman C, et al. A systematic review of severe morbidity in infants born late preterm. Am J Obstet Gynecol. 2011;205(4):374.e1–374.e9. doi: 10.1016/j.ajog.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 4.Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, te Pas A, Plavka R, Roehr CC, Saugstad OD, Simeoni U, Speer CP, Vento M, Visser GHA, Halliday HL. European consensus guidelines on the management of respiratory distress syndrome - 2019 update. Neonatology. 2019;115(4):432–450. doi: 10.1159/000499361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dani C, Mosca F, Vento G, Tagliabue P, Picone S, Lista G, Fanos V, Pratesi S, Boni L. Effects of surfactant treatment in late preterm infants with respiratory distress syndrome. J Matern Fetal Neonatal Med. 2018;31(10):1259–1266. doi: 10.1080/14767058.2017.1313828. [DOI] [PubMed] [Google Scholar]
- 6.Bancalari EH, Jobe AH. The respiratory course of extremely preterm infants: a dilemma for diagnosis and terminology. J Pediatr. 2012;161(4):585–588. doi: 10.1016/j.jpeds.2012.05.054. [DOI] [PubMed] [Google Scholar]
- 7.Kelley K, Clark B, Brown V, Sitzia J. Good practice in the conduct and reporting of survey research. Int J Qual Health Care. 2003;15(3):261–266. doi: 10.1093/intqhc/mzg031. [DOI] [PubMed] [Google Scholar]
- 8.Isayama T, Chai-Adisaksopha C, McDonald SD. Noninvasive ventilation with vs without early surfactant to prevent chronic lung disease in preterm infants: a systematic review and meta-analysis. JAMA Pediatr. 2015;169(8):731–739. doi: 10.1001/jamapediatrics.2015.0510. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee S, Fernandez R, Fox GF, Goss KCW, Mactier H, Reynolds P, Sweet DG, Roehr CC. Surfactant replacement therapy for respiratory distress syndrome in preterm infants: United Kingdom national consensus. Pediatr Res. 2019;86(1):12–14. doi: 10.1038/s41390-019-0344-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brat R, Yousef N, Klifa R, Reynaud S, Shankar Aguilera S, de Luca D. Lung ultrasonography score to evaluate oxygenation and surfactant need in neonates treated with continuous positive airway pressure. JAMA Pediatr. 2015;169(8):e151797. doi: 10.1001/jamapediatrics.2015.1797. [DOI] [PubMed] [Google Scholar]
- 11.Raimondi F, Yousef N, Rodriguez Fanjul J, de Luca D, Corsini I, Shankar-Aguilera S, Dani C, di Guardo V, Lama S, Mosca F, Migliaro F, Sodano A, Vallone G, Capasso L. A multicenter lung ultrasound study on transient tachypnea of the neonate. Neonatology. 2019;115(3):263–268. doi: 10.1159/000495911. [DOI] [PubMed] [Google Scholar]
- 12.Gregorio-Hernández R, Arriaga-Redondo M, Pérez-Pérez A, Ramos-Navarro C, Sánchez-Luna M. Lung ultrasound in preterm infants with respiratory distress: experience in a neonatal intensive care unit. Eur J Pediatr. 2020;179(1):81–89. doi: 10.1007/s00431-019-03470-0. [DOI] [PubMed] [Google Scholar]
- 13.Crocker C, Courtney SE, van Kaam A, et al. The Neonatal ARDS Worldwide Network: summary on patient data on respiratory support, course and outcomes. Pediatr Acad Soc 2019 Meeting, abstract 3164737.
- 14.De Luca D, van Kaam AH, Tingay DG, et al. The Montreux definition of neonatal ARDS: biological and clinical background behind the description of a new entity. Lancet Respir Med. 2017;5(8):657–666. doi: 10.1016/S2213-2600(17)30214-X. [DOI] [PubMed] [Google Scholar]
- 15.Herting E, Gefeller O, Land M, van Sonderen L, Harms K, Robertson B, Members of the Collaborative European Multicenter Study Group Surfactant treatment of neonates with respiratory failure and group B streptococcal infection. Pediatrics. 2000;106:957–964. doi: 10.1542/peds.106.5.957. [DOI] [PubMed] [Google Scholar]
- 16.Tan K, Lai NM, Sharma A. Surfactant for bacterial pneumonia in late preterm and term infants. Cochrane Database Syst Rev. 2012;2:CD008155. doi: 10.1002/14651858.CD008155.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Findlay RD, Taeusch HW, Walther FJ. Surfactant replacement therapy for meconium aspiration syndrome. Pediatrics. 1996;97(1):48–52. [PubMed] [Google Scholar]
- 18.Lotze A, Knight GR, Martin GR, Bulas DI, Hull WM, O'Donnell RM, Whitsett JA, Short BL. Improved pulmonary outcome after exogenous surfactant therapy for respiratory failure in term infants requiring extracorporeal membrane oxygenation. J Pediatr. 1993;122(2):261–268. doi: 10.1016/S0022-3476(06)80131-9. [DOI] [PubMed] [Google Scholar]
- 19.Natarajan CK, Sankar MJ, Jain K, Agarwal R, Paul VK. Surfactant therapy and antibiotics in neonates with meconium aspiration syndrome: a systematic review and meta-analysis. J Perinatol. 2016;36(Suppl 1):S49–S54. doi: 10.1038/jp.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rais-Bahrami K, Rivera O, Seale WR, Short BL. Effect of nitric oxide in meconium aspiration syndrome after treatment with surfactant. Crit Care Med. 1997;25(10):1744–1747. doi: 10.1097/00003246-199710000-00027. [DOI] [PubMed] [Google Scholar]
- 21.El Shahed AI, Dargaville PA, Ohlsson A, et al. Surfactant for meconium aspiration syndrome in term and late preterm infants. Cochrane Database Syst Rev. 2014;12:CD002054. doi: 10.1002/14651858.CD002054.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lally KP, Lally PA, Langham MR, Hirschl R, Moya FR, Tibboel D, van Meurs K, Congenital Diaphragmatic Hernia Study Group Congenital Diaphragmatic Hernia Study Group. Surfactant does not improve survival rate in preterm infants with congenital diaphragmatic hernia. J Pediatr Surg. 2004;39(6):829–833. doi: 10.1016/j.jpedsurg.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Cogo PE, Simonato M, Danhaive O, Verlato G, Cobellis G, Savignoni F, Peca D, Baritussio A, Carnielli VP. Impaired surfactant protein B synthesis in infants with congenital diaphragmatic hernia. Eur Respir J. 2013;41(3):677–682. doi: 10.1183/09031936.00032212. [DOI] [PubMed] [Google Scholar]
- 24.Jourdain G, Zacaria F, Ammar F, De Luca D. Appropriateness of surfactant dosing for preterm babies with respiratory distress syndrome: retrospective cohort study. Arch Dis Child Fetal Neonatal Ed. 2016;101(2):F182–F183. doi: 10.1136/archdischild-2015-310195. [DOI] [PubMed] [Google Scholar]
- 25.Boix H, Rite S, Arruza L, Fernandez C, Serrano I, Baquedano I, Sanchez A, Ferreira E, Fernandez P, Gonzalez R, Elorza MD. Underdosing of surfactant for preterm babies with respiratory distress syndrome in clinical practice: a retrospective cohort study. Am J Perinatol. 2019;36(9):943–948. doi: 10.1055/s-0038-1675645. [DOI] [PubMed] [Google Scholar]
- 26.Foligno S, De Luca D. Porcine versus bovine surfactant therapy for RDS in preterm neonates: pragmatic meta-analysis and review of physiopathological plausibility of the effects on extra-pulmonary outcomes. Respir Res. 2020;21(1):8. doi: 10.1186/s12931-019-1267-8. [DOI] [PMC free article] [PubMed] [Google Scholar]