Abstract
Prescription opioid abuse during and after pregnancy is a rising public health concern. While earlier studies have documented that offspring exposed to opioids in utero have impaired neurodevelopment, a significant knowledge gap remains in comparing the overall development between offspring exposed in utero and postnatally. Adding a layer of complexity is the role of heredity in the overall development of these exposed offspring. To fill in these important knowledge gaps, the current study uses a preclinical rat model mimicking oxycodone (oxy) exposure in utero (IUO) and postnatally (PNO) to investigate comparative and intergenerational effects in the two different treatment groups. While significant phenotypic attributes were observed with the two treatments and across the two generations, RNA sequencing revealed alterations in the expression of key synaptic genes in the two exposed groups in both generations. RNA sequencing and post validation of genes using RT-PCR highlighted the differential expression of several neuropeptides associated with the hypocretin system, a system recently implicated in addiction. Further, behavior studies revealed anxiety-like behaviors and social deficits that persisted even in the subsequent generations in the two treatment groups. To summarize, our study for the first time reveals a new line of investigation on the potential risks associated with oxy use during and after pregnancy, specifically the disruption of neurodevelopment and intergenerational impact on behavior.
Subject terms: Molecular neuroscience, Neuroscience
Introduction
The widespread abuse of prescription opioids and a dramatic increase in the availability of illicit opioids have created what is commonly referred to as the opioid epidemic1. As a particularly vulnerable group, pregnant women are prescribed opioids such as morphine, buprenorphine, and methadone to alleviate pregnancy and postpartum pain, all of which have been shown to cross the placenta2–4. Data collected by the Centers for Disease Control and Prevention from a 2008 to 2012 study found that more than a third of reproductive-aged women enrolled in Medicaid (39%) and more than a quarter of those with private insurance (28%) filled a prescription for an opioid pain medication each year5. Additionally, postpartum women, regardless of delivery method or pain measurement, commonly receive similar amounts of opioids upon discharge from the hospital6,7. The lack of standardization in prescribing patterns of these opioids contributes to a sizable amount of leftover medication, which can lead to nonmedical use of the prescriptions6,8. Among the prescription opioids, oxycodone (oxy) has recently emerged as a serious contender for widespread abuse. A postoperative analgesic, oxy has been reported in the literature for postpartum pain or caesarian sections in lieu of morphine drips9,10, potentially exposing neonates to this powerful opioid.
Several studies11 have been conducted with rodent models to investigate the detrimental effects of gestational opioid use on neurodevelopment of the offspring, but few of these studies consider oxy. Additionally, these prior studies relied on self-administration or continuous release pumps for drug administration, and very few have used oral delivery to mimic the usual route of administering pain medication. Orally-administered analgesics are the most common form of pain relief prescribed after caesarian sections12,13, and oral oxy administration has been shown to be safer than and as effective as intravenous administration of other opioids9,14,15. Previous oxy studies have shown deficits such as behavioral impairments and disruption in both OPRM1 and endothelin receptor expression during development in offspring exposed to oxy in utero16–19. However, these studies focused primarily on prenatal oxy exposure, leaving a large gap in the knowledge regarding postnatal oxy exposure.
Opiates have been shown to pass into the placenta and act on fetal opioid receptors2–4. Opioids also accumulate in the breastmilk20. The degree of exposure of an infant to a drug passed through the breastmilk depends on the concentration of the drug in the milk, the amount of milk ingested, and the rate of elimination from the infant21. A human study by Seaton et al. showed that oxy is concentrated in the breastmilk, and offspring exposed via the breastmilk may receive <10% of a typical oral therapeutic infant dose (0.1–0.2 mg/kg)22. Despite this low dose, infant exposure to oxy via the breastmilk has been associated with sedation, central nervous system depression, and neonatal toxicity23–26, and a number of animal studies have also revealed deficits in behavior and development associated with perinatal opioid exposure17–19,27. The full extent of postnatal oxy exposure effects is not yet known particularly in regard to central neural synaptic function and gene expression. Further, pre- and postnatal opioid abuse can result in several phenotypic consequences across multiple generations of offspring, despite no previous exposure to drugs28. Currently, a gap in knowledge exists regarding the long-term, intergenerational effects of oxy exposure during the perinatal period on future generations.
The present study was performed to determine the intergenerational effects of in utero (IUO) and postnatal oxy (PNO) exposure on development in both the F1 and F2 generations. This study was conducted using a Sprague Dawley rat model our labs have previously established29. This model consists of two groups of pregnant F0 dams that had been orally-administered oxy. The first group was treated daily with an oral administration of oxy before, during, and after pregnancy, thereby exposing the F1 pups to IUO throughout fetal and postnatal development. The second group was treated daily with an oral administration of oxy only after giving birth, exposing the F1 pups to oxy postnatally. The F2 generation was descended from the germlines of F1 dams exposed to oxy via the breastmilk or in utero, allowing us to elucidate intergenerational effects. Importantly, both the PNO and IUO groups are clinically relevant29. The IUO dams represent women who exhibit chronic opioid use before, during, and after pregnancy, and the PNO group represents children exposed to opioids after their mothers are prescribed medication post-caesarian section. Additionally, the PNO group represents neonates in the neonatal intensive care unit (NICU) that may be exposed to high-dose opiates through infusions. Infants born with heart defects and persistent pulmonary hypertension are exposed to opiates for sedation and analgesia while being supported by extracorporeal membrane oxygenation (ECMO) and mechanical ventilation30. Infants may be supported by ECMO for 3 weeks or longer, exposing the newborns to potent opiates for an extended period of time and contributing to the increase in diagnoses of neonatal abstinence syndrome31,32. Little is known regarding the implications of these opiate drips and how they may impact neurodevelopment in newborns. Our PNO group provides a high-dose opiate exposure to the pups that is comparable to the high-dose opiate exposure neonates in the NICU experience, establishing clinical relevance for the inclusion of this group in future studies.
The present study uses a holistic integrated systems biology approach to determine the intergenerational effects of pre- and postnatal oxy exposure on development in both the F1 and F2 generations descended from oxy-exposed mothers. Our overall hypothesis was that F0 maternal oxy use and F1 in utero and postnatal oxy exposure result in developmental impairments (physical, molecular, and behavioral) in the F1 offspring that persist in the F2 generation. The comprehensive and systematic approach used in this study allows for thorough research into intergenerational effects of pre- and postnatal oxy abuse, a critical step in closing the knowledge gap surrounding this commonly used opioid analgesic.
Methods
Animals
Male and female Sprague Dawley rats were obtained from Charles River Laboratories Inc. (Wilmington, MA, USA) and group housed in a 12 h light–dark cycle and fed ad libitum. The complete number of animals used per group for each experiment can be found in Supplementary Table 1. All procedures and protocols were approved by the Institutional Animal Care and Use Committee of the University of Nebraska Medical Center and conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Oxycodone treatment
The treatment paradigm was adapted from our lab’s previous work to include the F2 generation29. To elucidate intergenerational effects in the IUO and PNO groups, untreated F1 females (P70) from each condition were mated with male breeders naïve to the experiment (Supplementary Fig. 1). F2 pups were housed with their mothers until weaning (P21). All data presented are from complete litters per condition and include both male and female offspring.
Phenotypic measurements
Body measurements included weight, body length, and head size circumference and were obtained from the complete litters at P1, P7, P14, and P30. Body mass index (BMI) and Lee’s Obesity Index (LOI) were calculated as described by Novelli et al.33.
RNA-seq and post validation via RT-PCR
Total RNA from nucleus accumbens (NAc) tissue was isolated from the randomly selected male or female pups of each treatment group at P14 using the Direct-Zol RNA kit (Zymo Research, CA, USA). RNA samples were sent on dry ice to LC Sciences (Houston, TX, USA) for sequencing. Transcriptomes from all samples were merged to reconstruct a comprehensive transcriptome using a proprietary Perl script of LC Sciences (Houston, Texas, U.S.A.). Following transcriptome reconstruction, FPKM (Fragments Per Kilobase Million) reads were evaluated by StringTie, and differentially expressed genes (DEGs) were evaluated by edgeR. Raw data has been deposited to NCBI’s Gene Expression Omnibus (GEO; accession number GSE157348). Potential hits having ±1.5-fold expression and p < 0.05 were validated using relevant TaqMan probes by real-time PCR (RT-PCR). Further analysis into the Hcrt system was similarly done using RT-PCR TaqMan probes. Delta-delta Ct method34 was used to calculate fold change and statistical significance.
Bioinformatic data analyses
Functional relevance of DEGs (up- and downregulated) was evaluated using the Cytoscape plug-in ClueGO35. A kappa score level threshold of 0.4 was used to restrict the GO network connectivity with three as minimum and eight as maximum level of the genes in each GO term.
Behavioral studies
Social testing
Social testing consisting of social novelty and social preference were carried out in P60–65 F1 and F2 male and female rats from the different treatment groups using an in-house built chamber. Briefly, a 90 × 40 × 40 cm acrylic chamber was divided into three 30 × 40 × 40 cm compartments. Left and right compartments contained 15 × 15 × 30 cm isolation cubes with evenly-drilled holes spaced 1 cm apart along the entirety of the cube.
To evaluate social novelty, a naive animal of the same sex and of similar age and size was placed into the left isolation cube. A cagemate of the test animal’s housing cage was placed into the right isolation cube. The test animal was placed into the central chamber. For assessing social preference, a new naive rat (not used in social novelty) was placed into the left isolation cube, a rubber toy in the right isolation cube, and the test animal into the central chamber. After 5 min of acclimation, the two doors were lifted and the test animal was allowed to freely explore the entirety of the social chamber for 15 min. Animals were then returned to their housing cages, and the social chamber was cleaned and sterilized.
Scoring for both social tests consisted of the time the animal spent in each chamber, the number of entries into each chamber, and the number of active contacts toward one of the isolation cubes. Entry into a chamber was scored if an animal’s head and all four paws were within the compartment. An active contact was defined as any attempt to sniff, paw, scratch, touch, or stretch toward any of the isolation cubes when inside the compartment containing an isolation cube. Testing was recorded, and recordings were scored manually by scorers blinded to the conditions.
Marble burying
Marble burying was tested on all three groups on male and female pups between ages P65 and P70. A rat cage (929 cm2, 43.18 × 21.59 × 20.32 cm) contained a leveled 5 cm layer of ¼-inch corncob bedding (Envigo #7097), and 20 standard glass marbles (15 mm diameter, 5.2 g) were lightly placed in a 5 × 4 arrangement along the bedding. The subject was placed into the cage, and the cage was covered for 30 min. The animal was removed, and the marbles were imaged and scored by a scorer blinded to the conditions. A marble was considered buried if more than 2/3 of a marble was under the bedding.
Data and statistical analyses
All data represented in the paper are reported as mean ± SEM. Data in each analysis were normally distributed. Significant intergenerational differences were computed using two-way ANOVA followed by Tukey’s test with a significance criterion of p ≤ 0.05. Main interactions were further analyzed independently within generations using one-way ANOVA followed by Tukey’s test with a significance criterion of p ≤ 0.05. Two-way ANOVA was also used for social testing analysis (row factor: chamber; column factor: treatment), followed by Tukey’s test. All variances are listed in Supplementary Statistical Data, sorted by figure number. Data were analyzed using the Graph Pad Prism software (La Jolla, CA, USA).
Results
Oxy induces intergenerational developmental differences in exposed pups
To determine whether oxy exposure affects physical development in the PNO and IUO pups, we measured head size, body weight, and body length on postnatal days (P) 1, 7, 14, and 30 in both generations (Fig. 1). Additionally, we calculated the BMI and LOI to estimate obesity and body fat, respectively (Fig. 2). By measuring at these time points, we can assess perinatal development through periadolescence36. Additionally, these time points correspond with human aging from infant, young child, childhood, and preadolescence37.
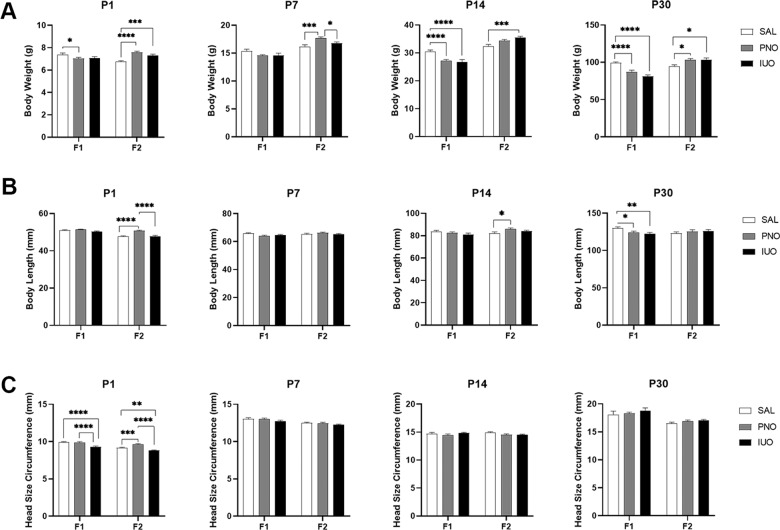
Fig. 1. Phenotypic measurements.
a–c Alterations in physical development patterns in both the IUO and PNO offspring as observed through body weight, body length, and head size circumference. *p < 0.05; ***p < 0.001; ****p < 0.0001 as determined by two-way ANOVA followed by a post hoc Tukey’s test.
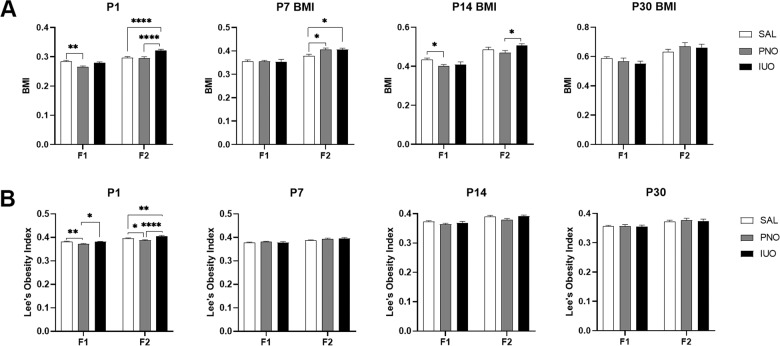
Fig. 2. Phenotypic measurements.
a, b Alterations in physical development patterns in both the IUO and PNO offspring as observed through body mass index (BMI) and Lee’s Obesity Index (LOI). *p < 0.05; **p < 0.01; ****p < 0.0001 as determined by two-way ANOVA followed by a post hoc Tukey’s test.
At P1 in the F1 animals, PNO pups weighed less than controls. While there were no differences in F1 weights at P7, a dramatic decline was observed in both PNO and IUO groups at P14 that persisted through P30. Interestingly, F2 PNO and IUO animals showed a significant increase in body weights compared to the saline controls at each time point (Fig. 1a). While no significant changes in body length were seen in the F1 pups at P1, P7, and P14, there was a marked reduction in the body length at P30. In the F2 pups, the PNO offspring had longer body lengths than both the saline and IUO groups at P1 and longer body lengths than controls at P14 (Fig. 1b). Head size circumference was markedly affected in the F1 and F2 IUO offspring at birth while F2 PNO pups had larger head size circumference than both IUO and controls at P1 (Fig. 1c).
BMI and LOI measurements were significantly different in the oxy-exposed groups of both generations. The F1 PNO group had both lower BMI (Fig. 2a) and a lower score on the LOI (Fig. 2b) than the saline and IUO groups at P1, suggesting this group may be underweight. Intriguingly, in the F2 animals, both the BMI (Fig. 2a) and LOI (Fig. 2b) indicated obesity and greater body fat in the IUO at P1. Additionally, while there were no differences in BMI in the F1 P7 animals, the F2 PNO and IUO groups had larger BMI than the controls at P7, indicating obesity (Fig. 2a). At P14, F1 PNO had a lower BMI than the saline controls while F2 IUO had a larger BMI than PNO (Fig. 2a). We did not observe any differences in BMI (Fig. 2a) in either generation at P30.
RNA-seq identified distinct gene signatures and molecular pathways in the two generations
Based on the significant physical attributes observed at P14 in the F1 and F2 generations, we performed RNA-Seq analysis on the NAc. The NAc was chosen given its association with the reward pathway. RNA-Seq analysis showed several up- and downregulated genes among the IUO, PNO, and saline groups in both the F1 and F2 generations (Supplementary Table 2). Using ClueGO analysis (Supplementary Fig. 2; Supplementary Table 3), we found that pathways involved in the regulation of neurological system process, forebrain and neural crest development, and synaptic transmission were affected by the DEGs in the F1 animals. In the F2 animals, we identified pathways involved in fear response, neurodevelopment, cell signaling, digestive processes, and heart rate regulation that were affected by the DEGs. Further, when comparing the same F1 group comparisons with the F2 comparisons, we found that a number of genes were consistently differentially regulated in both generations (Fig. 3a; Supplementary Tables 4–6). Of the differentially regulated genes in both the F1 and F2 generations of saline compared to IUO, 23 genes were common. For saline compared to PNO, 15 genes were commonly differentially regulated between the generations. Lastly, when comparing PNO and IUO, 10 genes were consistently differentially regulated in the F1 and F2 generations.
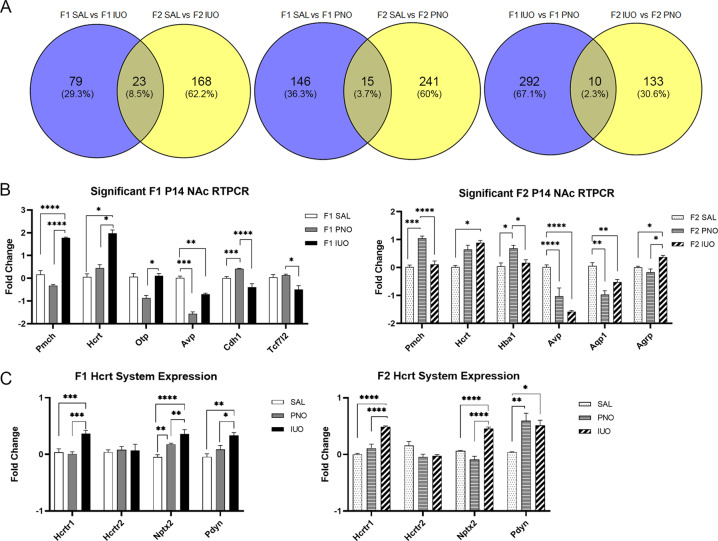
Fig. 3. RNA-seq analysis on P14 nucleus accumbens (NAc) of F1 and F2 animals.
a Venn diagram depicting the total number of genes affected in the comparison of treatment groups of each generation. b Of the genes post validated in the F1 NAc samples, Pmch, Hcrt, Otp, Avp, Cdh1, Oxt, and Tcf7l2 were significant. Of the genes post validated in the F2 NAc samples, Pmch, Hcrt, Hba1, Avp, Aqp1, and Agrp were significant. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 as determined by one-way ANOVA followed by a post hoc Tukey’s test. c Further investigation of the hypocretin system neuropeptide expression levels found Hcrtr1, Nptx2, and Pdyn were upregulated in the IUO group of both generations. *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001 as determined by two-way ANOVA followed by a post hoc Tukey’s test.
Potential hits based on a fold change value of ±1.5 that were involved in behavior and development included a total of 18 and 12 genes in the F1 and F2 generations, respectively (Supplementary Fig. 3). Post validation of these hits using RT-PCR resulted in six genes successfully post validated in the F1 and F2 generation (Fig. 3b). Interestingly, of the genes that were post validated in both F1 and F2, Pmch, Hcrt, and Avp were significant in both generations. Hcrt and Avp also had similar expression trends among the experimental groups in both F1 and F2. Intriguingly, the F1 IUO had significantly higher expression of Pmch compared to both groups, while the PNO had significantly higher expression in the same F2 comparison. These three genes are important in behavior and development and, based on their differential expression, may contribute to the differences observed between the F1 and F2 offspring.
Because the hypocretin (Hcrt) expression trends were the same in both generations, we sought to further investigate this system (Fig. 3c). We found that Hcrtr1 expression was significantly higher in the IUO group in both generations. Nptx2, a protein expressed in orexin neurons, was also highly expressed in IUO compared to PNO and controls in both generations. In the F1 generation, the PNO also high a higher expression of Nptx2 compared to controls. Further, Pdyn, the precursor for dynorphin (an inhibitory neuropeptide co-released with hypocretin), showed higher expression levels in IUO than both PNO and controls in the F1 generation; both IUO and PNO offspring had higher levels of Pdyn than controls in the F2 generation.
Behavioral deficits continue in the next generation of oxy-exposed pups
Early life insults can significantly impact developmental and behavioral outcomes exhibited during adulthood. Based on our RNA-seq data that highlighted several genes associated with behavioral responses, we examined if oxy exposure induces intergenerational behavioral deficits during adulthood in both IUO and PNO pups. Accordingly, we performed social interaction and marble burying tests in adult animals (P60–70).
In the F1 generation, social novelty tests revealed no significant differences in social interactions of the PNO and IUO groups (Supplementary Fig. 4). However, in the F2 generation, IUO offspring spent more time with the cagemate than the PNO group and less time with the naïve animal than both the control and PNO groups (Fig. 4a). Although not significant, the IUO demonstrated a higher tendency to enter the cagemate chamber than the other two groups (Fig. 4a). Additionally, when considering the number of contacts with the naïve animal, the IUO displayed fewer contacts with the naive animal (Fig. 4a), suggesting a social deficit and hesitancy to spend more time with or interact with the unknown animal. Similar to the F1 social novelty test results, F1 social preference tests revealed no significant differences in social interactions exhibited by the PNO and IUO groups (Supplementary Fig. 4). In the F2 social preference task, IUO animals spent less time with the naive animal than did either of the other groups (Fig. 4b), and IUO had more contacts with the toy than had either the PNO or control animals, further suggesting social deficits in the F2 generation.
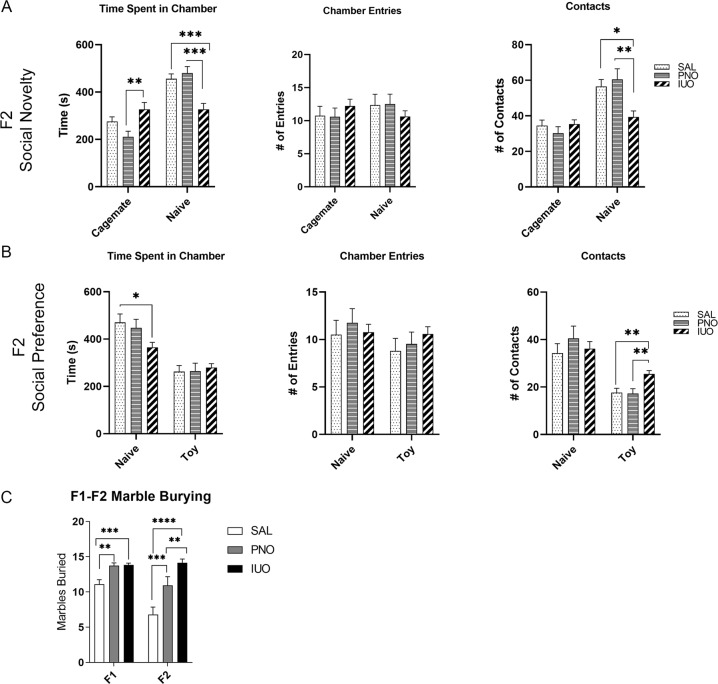
Fig. 4. Behavior tests.
a F2 social novelty testing revealed that IUO offspring spent less time with naive animals and more time with the cagemate than the other groups. IUO also had fewer contacts with the naïve animal. b F2 social preference testing showed IUO spent less time with the naive animal than the other groups, and IUO had more contacts with the toy than did the other groups. *p < 0.05; **p < 0.01; ***p < 0.001 as determined by two-way ANOVA followed by a post hoc Tukey’s test. c Marble burying tests in both generations showed increased burying activity in the PNO and IUO groups. **p < 0.01; ***p < 0.001; ****p < 0.0001 as determined by two-way ANOVA followed by a post hoc Tukey’s test.
Marble burying tests, which measure repetitive stereotypy, compulsive behaviors, and anxiety-like behavior38, showed that both the PNO and IUO groups in both generations buried more marbles than the controls, suggesting heightened anxiety and compulsivity in these animals (Fig. 4c). Interestingly, IUO animals in the F2 group buried more marbles than the PNO, suggesting that the F1 IUO exposure may result in more pronounced intergenerational anxiety and compulsive behaviors than F1 PNO exposure. In summary, in utero and postnatal oxy exposure can significantly alter behavioral outcomes that persist into the next generation.
Discussion
The model system and dose of oxy (15 mg/kg) used in our study mimicked a chronic prescription opiate-dependent woman during gestation and parturition. This dose has been shown to be well-tolerated in animals and mimic development of chronic analgesia in human subjects experiencing breakthrough pain39,40. A gap in knowledge exists regarding the implications of how perinatal oxy exposure may impact neurodevelopment in newborns and affect long-term adult behaviors in an intergenerational manner. Intergenerational transmission of traits and phenotypes results from direct exposure of the F0 parent and F1 offspring to a stressor41, such as oxy. When the oxy exposure occurs in utero, as in the IUO group, the F1 fetus and its germlines are directly exposed. Additionally, when exposed via the breastmilk, which occurs in both the PNO and IUO groups, the germlines are also exposed. The exposure of the germ line within the F1 offspring results in an intergenerational transmission to the F2 generation. Studying the intergenerational effects of oxy exposure in utero and postnatally can highlight the long-term adverse consequences that extend beyond the mother into future generations.
Opioid use during pregnancy has been associated with smaller head sizes, lighter birthweights, and shorter body lengths in newborns42–46. Our data dovetail with this trend, showing that in utero oxy exposure affects birthweight, body length, and head development. A rat study using prenatal exposure to morphine yielded similar results, with offspring during the preweaning period having a lower body weight as well as a reduction in brain and cerebellar weights and widths47. Similarly, a study using buprenorphine showed delayed offspring development, decreased body weight, decreased body length, and lower pain sensitivity48. In our F1 generation, body length appeared to be delayed at P30 when compared to controls. Interestingly, the F2 PNO pups, specifically, had longer bodies at P1 and P14, but all F2 body lengths were comparable at P30. As for head size, decreased head size and body weight have been reported in a study of NICU neonates49. In our study, IUO head size circumference in both generations was smaller compared to controls at P1; head size circumference was comparable to controls at the remaining time points, however.
With regard to weight, in utero morphine exposure has also been shown to produce weight deficits that persisted in rats through adulthood50, much like what we observed in the F1 PNO and IUO groups. Eriksson and Ronnback showed that rats exposed to prenatal morphine treatment not only had lower birthweights than controls but also gained less weight than controls until P1951. In our study, F1 PNO and IUO pups appeared to maintain the weight deficit through P30 and did not approach weight levels comparable to saline controls. Our study also identified differences in BMI and LOI during early development. Intriguingly, IUO and PNO in the F2 generation had heavier body weights at all time points compared to controls. Additionally, while the F1 generation trended toward lower BMIs early in development, the F2 generation had higher BMI scores. In other studies considering underweight mothers and the weight gain of their offspring, maternal undernutrition improves the metabolic health of the next generation52 and can lead to weight gain and obesity in the next generation53, much like what we observed in our overweight F2 generation compared to their underweight F1 mothers.
After post validation of our RNA-seq results, the upregulation of Hcrt in the IUO groups of both generations led us to investigate the expression of key genes in the hypocretin system. Hypocretins are involved in arousal, but they are also involved in drug addiction and reward-related behaviors54. Hypocretin activation of Hcrtr1 is critical for morphine withdrawal, and NAc activation during withdrawal is dependent on Hcrtr1 function55. Not only do our data show an increase in Hcrt expression in IUO pups of both generations, which is associated with acute opiate withdrawal56,57, but the expression of Hcrtr1 in the NAc is also increased significantly in the IUO, further suggesting these IUO pups may be experiencing acute withdrawal. Additionally, Nptx2, which regulates the clustering of AMPA receptors at the synapse, has been shown to increase following opiate withdrawal58. The IUO animals in both generations exhibited an increase in Nptx2 expression, again suggesting withdrawal in these animals. Lastly, our IUO animals had higher levels of Pdyn expression in both generations. Chronic exposure to drugs of abuse results in the upregulation of the dynorphin (Dyn)/KOR system, and this system has been shown to contribute to psychiatric disorders such as anxiety, depression, and addiction59,60.
In addition to highlighting the hypocretin system, our RNA-seq data also highlighted several genes associated with behavioral responses; therefore we sought to investigate the impact of IUO and PNO exposure on social behaviors. In human studies, prenatal opioid exposure has been associated with increased social problems and difficulty with sociability49,61. In our social novelty and preference tasks, we did not observe any significant differences in social behaviors among the F1 groups; however, the F2 IUO group appeared to exhibit deficits in social interaction behaviors. In the literature, conflicting results exist regarding the impact of in utero and postnatal opioid exposure on social behaviors in animal models. In one study of prenatal exposure to buprenorphine, methadone, and morphine, social interactions in exposed rats were impaired, similar to what has been reported in human studies62. In contrast, other studies have shown that prenatal morphine exposure increased social behaviors and resulted in less social avoidance63,64. Differences in results may be due to different experimental designs, such as behavior chambers and scoring or the dosing schedule of the animals. For example, many reported behavioral tests for prenatal opioid exposure studies have tested social interaction and play behavior in open chambers where the animals can interact freely62–64. Our social preference and novelty tests used a three-chambered apparatus that did not allow for free interaction or play between the test rat and the cagemate or naive animal. As for treatment differences, both Hol et al. and Niesink et al. treated rats with morphine during the last week of gestation and showed increased play behavior in the offspring63,64. Najam and Panksepp, however, showed that early postnatal treatment causes a delay in achieving control levels of play behavior in rats65. Our treatment paradigms started prior to mating and continued until weaning (IUO) or started only after giving birth and continued until weaning (PNO). Alterations in rodent behavior post treatment may depend on the stage of development at which the offspring are exposed to treatment, and social problems resulting from intrauterine49 or adolescent36 exposure may resolve with age. Our social behavioral tests occurred during young adulthood (P60–65), so perhaps any existing social deficits returned to baseline by the time of testing. The antisocial behavior exhibited by the F2 IUO animals may be attributed to the downregulation of Avp highlighted in our NAc RNA-seq and post-validation data. AVP systems have been shown to modulate social behaviors in rats66, and blocking AVP receptors resulted in significantly decreased investigation of novel objects67. Therefore, the regulation of Avp and its effects on social behavior in the context of perinatal opioid exposure may be an important avenue of study for future work.
In addition to social behaviors, we investigated compulsive and anxiety-like behaviors using marble burying tests in both generations. In our study, both the PNO and IUO groups in F1 buried more marbles than the control group, suggesting the presence of anxiety-like and obsessive-compulsive phenotypes. This pattern persisted into the F2 generation, demonstrating intergenerational effects of maternal opioid exposure on subsequent generations. Importantly, the hypocretin system has been implicated in anxiety disorders68. Hcrtr1 antagonists have been shown to attenuate anxiety-like behaviors69. Further, Hcrtr1 activity is anxiogenic, while Hcrtr2 activity is anxiolytic70. The increased expression of Hcrt and Hcrtr1 may contribute to the anxiety-like behaviors exhibited by both generations. Further, prenatal exposure to morphine and methadone has been shown to increase anxiety-like behaviors in light–dark transition and elevated plus-maze tests in both male and female rats62. Additionally, Rohbani et al. found that parental morphine exposure affected compulsive grooming and anxiety-like behaviors through marble burying in the offspring71. In the case of the F2 IUO, the F1 germ line that would produce F2 was directly exposed, contributing to the intergenerational effect we saw on anxiety-like behavior in this group. In a study using marble burying to assess autism-like and anxiety-like behaviors, increased marble burying occurred in F1, F2, and F3, suggesting that these behaviors may exist through transgenerational epigenetic inheritance72. Our F2 animals exhibited the same anxiety-like behaviors as the F1 animals; thus, extension of this study to F3 may elucidate whether transgenerational inheritance is involved in this behavioral phenotype. It is important to note that, while the number of marbles buried in the F1 oxy-exposed groups was roughly similar, the F2 IUO buried significantly more marbles than the F2 PNO, suggesting the IUO may be the more severely impacted of the two groups.
Overall, our study is the first to identify intergenerational effects of pre- and postnatal oxy exposure on development and behavior, thus prompting caution, as both routes of exposure can lead to developmental deficiencies that may persist into the next generation.
Supplementary information
Acknowledgements
This work was supported by NIH grants DA049577 (G.P.), DA046284 (G.P.), DA042379 (S.V.Y.), DA046852 (G.P. and S.V.Y.), and departmental startup funds to G.P. and S.V.Y. The funding entity played no role in study design or its conduction. The Bioinformatics and Systems Biology Core at UNMC received support from Nebraska Research Initiative and NIH (5P20GM103427; 5P30CA036727; 5P30MH062261) for the bioinformatics analysis performed in this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors jointly supervised this work: Sowmya V. Yelamanchili, Gurudutt Pendyala
Contributor Information
Sowmya V. Yelamanchili, Email: syelamanchili@unmc.edu
Gurudutt Pendyala, Email: gpendyala@unmc.edu.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41398-020-01012-z).
References
- 1.Volkow ND, McLellan AT. Opioid abuse in chronic pain—misconceptions and mitigation strategies. N. Engl. J. Med. 2016;374:1253–1263. doi: 10.1056/NEJMra1507771. [DOI] [PubMed] [Google Scholar]
- 2.Gerdin E, Rane A, Lindberg B. Transplacental transfer of morphine in man. J. Perinat. Med. 1990;18:305–312. doi: 10.1515/jpme.1990.18.4.305. [DOI] [PubMed] [Google Scholar]
- 3.Nanovskaya T, Deshmukh S, Brooks M, Ahmed MS. Transplacental transfer and metabolism of buprenorphine. J. Pharmacol. Exp. Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Nanovskaya TN, Nekhayeva IA, Hankins GD, Ahmed MS. Transfer of methadone across the dually perfused preterm human placental lobule. Am. J. Obstet. Gynecol. 2008;198:126.e121–124. doi: 10.1016/j.ajog.2007.06.073. [DOI] [PubMed] [Google Scholar]
- 5.Ailes EC, et al. Opioid prescription claims among women of reproductive age—United States, 2008–2012. MMWR Morb. Mortal. Wkly. Rep. 2015;64:37–41. [PMC free article] [PubMed] [Google Scholar]
- 6.Bianchi DW, Gillman MW. Addressing the impact of opioids on women and children. Am. J. Obstet. Gynecol. 2019;221:123.e121–123.e124. doi: 10.1016/j.ajog.2019.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badreldin N, Grobman WA, Chang KT, Yee LM. Opioid prescribing patterns among postpartum women. Am. J. Obstet. Gynecol. 2018;219:103.e101–103.e108. doi: 10.1016/j.ajog.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bateman BT, et al. Persistent opioid use following cesarean delivery: patterns and predictors among opioid-naïve women. Am. J. Obstet. Gynecol. 2016;215:353.e351–353.e318. doi: 10.1016/j.ajog.2016.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niklasson B, Arnelo C, Ohman SG, Segerdahl M, Blanck A. Oral oxycodone for pain after caesarean section: A randomized comparison with nurse-administered IV morphine in a pragmatic study. Scand. J. Pain. 2015;7:17–24. doi: 10.1016/j.sjpain.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Nie JJ, Sun S, Huang SQ. Effect of oxycodone patient-controlled intravenous analgesia after cesarean section: a randomized controlled study. J. Pain. Res. 2017;10:2649–2655. doi: 10.2147/JPR.S142896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byrnes EM, Vassoler FM. Modeling prenatal opioid exposure in animals: current findings and future directions. Front. Neuroendocrinol. 2018;51:1–13. doi: 10.1016/j.yfrne.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mkontwana, N. & Novikova, N. Oral analgesia for relieving post-caesarean pain. Cochrane Database Syst. Rev. CD010450. 10.1002/14651858.CD010450.pub2 (2015). [DOI] [PMC free article] [PubMed]
- 13.Butwick A. Improving post-caesarean analgesia: where to next? BJOG. 2017;124:1071. doi: 10.1111/1471-0528.14597. [DOI] [PubMed] [Google Scholar]
- 14.Eizadi P, Jalili M, Dehpour A. Oral oxycodone compared with intravenous morphine sulfate for pain management of isolated limb trauma; a randomized clinical trial. Emergency (Tehran) 2018;6:e59. [PMC free article] [PubMed] [Google Scholar]
- 15.Cheung CW, Ching Wong SS, Qiu Q, Wang X. Oral oxycodone for acute postoperative pain: a review of clinical trials. Pain Phys. 2017;20:SE33–SE52. doi: 10.36076/ppj.2017.sE52. [DOI] [PubMed] [Google Scholar]
- 16.Vassoler FM, Oranges ML, Toorie AM, Byrnes EM. Oxycodone self-administration during pregnancy disrupts the maternal-infant dyad and decreases midbrain OPRM1 expression during early postnatal development in rats. Pharmacol. Biochem. Behav. 2018;173:74–83. doi: 10.1016/j.pbb.2018.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sithisarn T, et al. The effects of perinatal oxycodone exposure on behavioral outcome in a rodent model. Front. Pediatr. 2017;5:180. doi: 10.3389/fped.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis CP, Franklin LM, Johnson GS, Schrott LM. Prenatal oxycodone exposure impairs spatial learning and/or memory in rats. Behav. Brain Res. 2010;212:27–34. doi: 10.1016/j.bbr.2010.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Devarapalli M, et al. Prenatal oxycodone exposure alters CNS endothelin receptor expression in neonatal rats. Drug Res. (Stuttg.) 2016;66:246–250. doi: 10.1055/s-0035-1569279. [DOI] [PubMed] [Google Scholar]
- 20.Ito S. Opioids in breast milk: pharmacokinetic principles and clinical implications. J. Clin. Pharmacol. 2018;58:S151–S163. doi: 10.1002/jcph.1113. [DOI] [PubMed] [Google Scholar]
- 21.Atkinson HC, Begg EJ, Darlow BA. Drugs in human milk. Clinical pharmacokinetic considerations. Clin. Pharmacokinet. 1988;14:217–240. doi: 10.2165/00003088-198814040-00003. [DOI] [PubMed] [Google Scholar]
- 22.Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after caesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust. N. Z. J. Obstet. Gynaecol. 2007;47:181–185. doi: 10.1111/j.1479-828X.2007.00715.x. [DOI] [PubMed] [Google Scholar]
- 23.Wachman EM, et al. Epigenetic variation in OPRM1 gene in opioid-exposed mother-infant dyads. Genes Brain Behav. 2018;17:e12476. doi: 10.1111/gbb.12476. [DOI] [PubMed] [Google Scholar]
- 24.Lam J, et al. Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J. Pediatr. 2012;160:33–37.e32. doi: 10.1016/j.jpeds.2011.06.050. [DOI] [PubMed] [Google Scholar]
- 25.Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatr. Drugs. 2008;10:399–404. doi: 10.2165/0148581-200810060-00007. [DOI] [PubMed] [Google Scholar]
- 26.Madadi P, et al. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin. Pharmacol. Ther. 2009;85:31–35. doi: 10.1038/clpt.2008.157. [DOI] [PubMed] [Google Scholar]
- 27.Fan R, et al. Chronic oxycodone induces axonal degeneration in rat brain. BMC Neurosci. 2018;19:15. doi: 10.1186/s12868-018-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod. Toxicol. 2008;25:2–F6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahjin, F. et al. Brain-derived extracellular vesicle microRNA signatures associated with in utero and postnatal oxycodone exposure. Cells9, 10.3390/cells9010021 (2019). [DOI] [PMC free article] [PubMed]
- 30.Nair J, Lakshminrusimha S. Update on PPHN: mechanisms and treatment. Semin. Perinatol. 2014;38:78–91. doi: 10.1053/j.semperi.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lakshminrusimha S, Keszler M. Persistent pulmonary hypertension of the newborn. Neoreviews. 2015;16:e680–e692. doi: 10.1542/neo.16-12-e680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lewis T, Erfe BL, Ezell T, Gauda E. Pharmacoepidemiology of opiate use in the neonatal ICU: Increasing cumulative doses and iatrogenic opiate withdrawal. J. Opioid Manag. 2015;11:305–312. doi: 10.5055/jom.2015.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novelli EL, et al. Anthropometrical parameters and markers of obesity in rats. Lab. Anim. 2007;41:111–119. doi: 10.1258/002367707779399518. [DOI] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Bindea G, et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Counotte DS, Smit AB, Pattij T, Spijker S. Development of the motivational system during adolescence, and its sensitivity to disruption by nicotine. Dev. Cogn. Neurosci. 2011;1:430–443. doi: 10.1016/j.dcn.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andersen SL. Trajectories of brain development: point of vulnerability or window of opportunity? Neurosci. Biobehav. Rev. 2003;27:3–18. doi: 10.1016/S0149-7634(03)00005-8. [DOI] [PubMed] [Google Scholar]
- 38.Angoa-Pérez, M., Kane, M. J., Briggs, D. I., Francescutti, D. M. & Kuhn, D. M. Marble burying and nestlet shredding as tests of repetitive, compulsive-like behaviors in mice. J. Vis. Exp. 50978, 10.3791/50978 (2013). [DOI] [PMC free article] [PubMed]
- 39.FDA PI. Oxycodone. https://www.drugs.com/pro/oxycodone.html (2017).
- 40.Zhang Y, et al. Alterations of expression of inflammation/immune-related genes in the dorsal and ventral striatum of adult C57BL/6J mice following chronic oxycodone self-administration: a RNA sequencing study. Psychopharmacology (Berl) 2017;234:2259–2275. doi: 10.1007/s00213-017-4657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klengel T, Dias BG, Ressler KJ. Models of intergenerational and transgenerational transmission of risk for psychopathology in mice. Neuropsychopharmacology. 2016;41:219–231. doi: 10.1038/npp.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finnegan LP. Effects of maternal opiate abuse on the newborn. Fed. Proc. 1985;44:2314–2317. [PubMed] [Google Scholar]
- 43.Towers, C. V. et al. Neonatal head circumference in newborns with neonatal abstinence syndrome. Pediatrics143, 10.1542/peds.2018-0541 (2019). [DOI] [PubMed]
- 44.Attarian S, et al. The neurodevelopmental impact of neonatal morphine administration. Brain Sci. 2014;4:321–334. doi: 10.3390/brainsci4020321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chiang YC, Hung TW, Lee CW, Yan JY, Ho IK. Enhancement of tolerance development to morphine in rats prenatally exposed to morphine, methadone, and buprenorphine. J. Biomed. Sci. 2010;17:46. doi: 10.1186/1423-0127-17-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lacroix I, et al. Buprenorphine versus methadone in pregnant opioid-dependent women: a prospective multicenter study. Eur. J. Clin. Pharmacol. 2011;67:1053–1059. doi: 10.1007/s00228-011-1049-9. [DOI] [PubMed] [Google Scholar]
- 47.Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacology. 1977;15:276–282. doi: 10.1159/000136699. [DOI] [PubMed] [Google Scholar]
- 48.Wallin CM, Bowen SE, Roberge CL, Richardson LM, Brummelte S. Gestational buprenorphine exposure: effects on pregnancy, development, neonatal opioid withdrawal syndrome, and behavior in a translational rodent model. Drug Alcohol Depend. 2019;205:107625. doi: 10.1016/j.drugalcdep.2019.107625. [DOI] [PubMed] [Google Scholar]
- 49.Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicol. Teratol. 2012;34:47–55. doi: 10.1016/j.ntt.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 50.Zagon IS, McLaughlin PJ. Effects of chronic morphine administration on pregnant rats and their offspring. Pharmacology. 1977;15:302–310. doi: 10.1159/000136703. [DOI] [PubMed] [Google Scholar]
- 51.Eriksson PS, Rönnbäck L. Effects of prenatal morphine treatment of rats on mortality, bodyweight and analgesic response in the offspring. Drug Alcohol Depend. 1989;24:187–194. doi: 10.1016/0376-8716(89)90055-0. [DOI] [PubMed] [Google Scholar]
- 52.Cissé O, et al. Effect of diet in females (F1) from prenatally undernourished mothers on metabolism and liver function in the F2 progeny is sex-specific. Eur. J. Nutr. 2019;58:2411–2423. doi: 10.1007/s00394-018-1794-y. [DOI] [PubMed] [Google Scholar]
- 53.Joaquim AO, et al. Maternal food restriction in rats of the F. Reprod. Fertil. Dev. 2017;29:1340–1348. doi: 10.1071/RD15309. [DOI] [PubMed] [Google Scholar]
- 54.Mahler SV, Smith RJ, Moorman DE, Sartor GC, Aston-Jones G. Multiple roles for orexin/hypocretin in addiction. Prog. Brain Res. 2012;198:79–121. doi: 10.1016/B978-0-444-59489-1.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sharf R, Sarhan M, Dileone RJ. Orexin mediates the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol. Psychiatry. 2008;64:175–183. doi: 10.1016/j.biopsych.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Georgescu D, et al. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J. Neurosci. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhou Y, et al. Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J. Endocrinol. 2006;191:137–145. doi: 10.1677/joe.1.06960. [DOI] [PubMed] [Google Scholar]
- 58.Reti IM, Baraban JM. Opiate withdrawal induces Narp in the extended amygdala. Neuropsychopharmacology. 2003;28:1606–1613. doi: 10.1038/sj.npp.1300205. [DOI] [PubMed] [Google Scholar]
- 59.Chartoff EH, Mavrikaki M. Sex differences in kappa opioid receptor function and their potential impact on addiction. Front. Neurosci. 2015;9:466. doi: 10.3389/fnins.2015.00466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Anderson RI, Becker HC. Role of the dynorphin/kappa opioid receptor system in the motivational effects of ethanol. Alcohol Clin. Exp. Res. 2017;41:1402–1418. doi: 10.1111/acer.13406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Anand KJ, Campbell-Yeo M. Consequences of prenatal opioid use for newborns. Acta Paediatr. 2015;104:1066–1069. doi: 10.1111/apa.13121. [DOI] [PubMed] [Google Scholar]
- 62.Chen HH, et al. Buprenorphine, methadone, and morphine treatment during pregnancy: behavioral effects on the offspring in rats. Neuropsychiatr. Dis. Treat. 2015;11:609–618. doi: 10.2147/NDT.S70585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hol T, Niesink M, van Ree JM, Spruijt BM. Prenatal exposure to morphine affects juvenile play behavior and adult social behavior in rats. Pharmacol. Biochem. Behav. 1996;55:615–618. doi: 10.1016/S0091-3057(96)00274-2. [DOI] [PubMed] [Google Scholar]
- 64.Niesink RJ, van Buren-van Duinkerken L, van Ree JM. Social behavior of juvenile rats after in utero exposure to morphine: dose-time-effect relationship. Neuropharmacology. 1999;38:1207–1223. doi: 10.1016/S0028-3908(99)00050-7. [DOI] [PubMed] [Google Scholar]
- 65.Najam N, Panksepp J. Effect of chronic neonatal morphine and naloxone on sensorimotor and social development of young rats. Pharmacol. Biochem Behav. 1989;33:539–544. doi: 10.1016/0091-3057(89)90383-3. [DOI] [PubMed] [Google Scholar]
- 66.Smith CJ, et al. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct. Funct. 2017;222:981–1006. doi: 10.1007/s00429-016-1260-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veenema AH, Bredewold R, De Vries GJ. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav. 2012;61:50–56. doi: 10.1016/j.yhbeh.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Johnson, P. L., Molosh, A., Fitz, S. D., Truitt, W. A. & Shekhar, A. in Orexin/Hypocretin System, Vol. 198, 133–161 (Elsevier, 2012). [DOI] [PMC free article] [PubMed]
- 69.Johnson PL, et al. Orexin 1 receptors are a novel target to modulate panic responses and the panic brain network. Physiol. Behav. 2012;107:733–742. doi: 10.1016/j.physbeh.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Summers CH, Yaeger JDW, Staton CD, Arendt DH, Summers TR. Orexin/hypocretin receptor modulation of anxiolytic and antidepressive responses during social stress and decision-making: potential for therapy. Brain Res. 2020;1731:146085. doi: 10.1016/j.brainres.2018.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rohbani K, et al. Parental morphine exposure affects repetitive grooming actions and marble burying behavior in the offspring: Potential relevance for obsessive-compulsive like behavior. Eur. J. Pharmacol. 2019;865:172757. doi: 10.1016/j.ejphar.2019.172757. [DOI] [PubMed] [Google Scholar]
- 72.Choi CS, et al. The transgenerational inheritance of autism-like phenotypes in mice exposed to valproic acid during pregnancy. Sci. Rep. 2016;6:36250. doi: 10.1038/srep36250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.