Abstract
AIM
To determine the frequency and types of retinal diseases and the extend of the related visual loss in adult patients attending two public eye clinics of Kinshasa, Democratic Republic of Congo.
METHODS
Review of medical records of patients with retinal diseases seen in the major eye clinics in Kinshasa, the University Hospital of Kinshasa (UHK) and Saint Joseph Hospital (SJH), from January 2012 to December 2014. Demographics and diagnoses were retrieved and analyzed. Outcome measures were frequency and prevalence of retinal diseases, blindness and low vision.
RESULTS
A total of 40 965 patients aged 40y or older were examined during this period in both clinics. Of these, 1208 had retinal disease, giving a 3-year and an annual prevalence of 3% and 1%, respectively. Mean age was 61.7±10.7y, and 55.8% of the patients were males. Arterial hypertension (68.1%) and diabetes (43.3%) were the most common systemic comorbidities. Hypertensive retinopathy (41.8%), diabetic retinopathy (37.9%), age-related macular degeneration (AMD; 14.6%), and chorioretinitis and retinal vein occlusion (7.3% each) were the most common retinal diseases, with 3-year prevalence rates of 1.3%, 1.0%, 0.43%, and 0.21% respectively. Bilateral low vision and blindness were present in 26.8% and 8.4% of the patients at presentation. Major causes of low vision and blindness were diabetic retinopathy (14.8%), AMD (4.9%), retinal detachment (2.8%), and retinal vein occlusion (2.5%). The prevalence was significantly higher among males than females, and at the UHK than SJH.
CONCLUSION
Retinal diseases are common among Congolese adult patients attending eye clinics in Kinshasa. They cause a significant proportion of low vision and blindness.
Keywords: retinal disorders; Kinshasa, Democratic Republic of Congo; sub-Saharan Africa; pattern
INTRODUCTION
Retinal diseases such as age-related macular degeneration (AMD) are known to be among the leading causes of blindness in developed countries[1]. In Africa, it was long believed that retinal diseases were uncommon. This was partly due to the lack of data and the fact that many African blindness prevention programs have focused on cataract, refractive errors, and infectious diseases (i.e., trachoma, onchocerciasis) known to be major causes of low vision and blindness[2]. Low- and middle-income countries including those in sub-Saharan Africa (SSA) have been undergoing a demographic change over the past four decades as a result of gradual economic improvement. This had led to an ongoing gradual shift from primarily agrarian to semi-urban and urban populations as well as improvement of heath indices with increased life expectancy. It has been reported that this shift will reflect on the phenotypes of ocular pathologies including retinal disorders[3].
In a recent review on data published in SSA, Bastawrous et al[4] reported that posterior segment eye diseases are already substantial contributors to blindness and visual impairment and account for 13% to 37% of all causes. They also predicted that their contribution as causes of ocular morbidity, visual impairment and blindness will increase as a result of the combined effect of population growth, aging, and shift to urban lifestyle. Available hospital-based data from Nigeria and Ethiopia show that chorioretinal disorders represent 3.9% to 13% of all eye diseases[5]–[9].
Large variations exist in the prevalence, phenotypes, and incidence of retinal disorders in different populations outside Africa[1],[10]–[12], making it difficult to apply findings from other regions of the world to SSA. Within SSA, AMD was found to be the most frequently observed retinal disease in Nigeria[6]–[7],[13]. In Ethiopia, vascular retinal diseases were the most common disorders, among which diabetic retinopathy was the most frequent[8]. Because of the large ethnic and genetic heterogeneity and the difference in lifestyle (i.e., diet) and risk factors among SSA populations, findings from one region may not apply to another region even within the same country. Thus, there is a need for further investigations to provide data on retinal diseases specific to each region and subregions of SSA.
In the Democratic Republic of Congo (DRC), comprehensive reports that provide an overview on retinal diseases as causes of ocular morbidity and blindness are not available. Such reports are important as they may provide the first line of information about hospital utilization and basic epidemiologic measures needed for strategy planning, resource prioritization and allocation, and development of prevention, diagnosis and management programs. As a result of hospitals growing in importance due to increasing medical needs of communities, hospital data have also become more valuable resources for studying epidemiology of diseases. Thus, in resource-limiting settings such as the DRC, where population-based studies (PBS) are still difficult to perform, hospital-based studies (HBS) may be the alternative for providing interim data. This study was designed to assess the epidemiological profile and types of retinal diseases, and to determine the causes of low vision and blindness among adult patients with retinal diseases presenting at two major hospitals in Kinshasa, DRC.
SUBJECTS AND METHODS
Ethical Approval
This was a cross-sectional, retrospective observational study. It received approval from the Institutional Review Board of the University of Kinshasa School of Medicine, and complied with the tenets of the Declaration of Helsinki. Informed consent was waived due to the retrospective nature of the study.
Study Location
The study was conducted in the eye clinics of the University Hospital of Kinshasa (UHK) and Saint Joseph Hospital (SJH), both in Kinshasa, DRC. Kinshasa is a megacity with a population that was recently estimated to be close to 12 million, but with only 71 ophthalmologists as of March 2019. The earlier is a tertiary referral whereas the latter is a second level care facility. Both eye clinics provide care to Kinshasa and its suburbs. They also receive patients from as far as 400 miles. They have basic ophthalmological equipment including slit-lamp biomicroscopes, autorefractors, automated visual field analyzers, fundus camera, spectral domain-optical coherence tomography (OCT), ultrasound, and laser delivery systems.
Study Design, Population and Data Collection
Because electronic medical records is not available, we first manually reviewed the medical charts of all patients who attended the UHK and SJH eye clinics from January 2012 to December 2014 to extract those with the diagnosis of retinal diseases. During this period 3974 and 36 991 patients aged 40y or older were examined in the eye clinics of the UHK and SJH, respectively, making a combined total of 40 965. Charts of patients with retinal disease were identified and included in the study if they had complete information about demographics and clinical diagnoses. If the diagnosis was missing or questionable, or the findings of the fundus examination were not clearly described, those charts were excluded. Glaucoma and non-glaucomatous optic neuropathies were not included in the study.
Age, sex, the affected eye, best corrected visual acuity (BCVA) at presentation, type of retinal disease, and associated systemic conditions such as diabetes, hypertension, and stroke were recorded. Age was divided into five age bands as follows: 40-49, 50-59, 60-69, 70-79, and ≥80y. BCVA was classified as normal (1.0 to 0.4), low vision [0.3 to counting fingers (CF) at 3m] or blindness (CF at 2 m to no light perception, NLP)[14]. The final diagnosis on the type of retinal disease was based on the combination of history, symptoms, and objective slit-lamp and mydriatic fundus examination. OCT was only performed in some of the patients because it was only acquired in the middle of 2014. Ocular ultrasound was performed in case of media opacity. For simplicity in this paper, non-proliferative and proliferative diabetic retinopathies are collapsed into diabetic retinopathy only. The different stages of hypertensive retinopathy are also combined into a single group. Similarly, retinal vein and artery occlusions are not distinguished into branch and central types. Macular pseudohole and full-thickness macular hole are simply presented as macula hole. Anterior segment pathologies, although present in some of the patients, are not reported in this paper.
Statistical Analysis
Analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, USA). Categorical and continuous data were expressed as proportion and mean±standard deviation (SD), respectively. Data obtained were analyzed for frequency and proportions. Comparisons of means and proportions were made using Student t-test and Pearson Chi-squared, respectively. A P-value less than 0.05 was considered statistically significant.
RESULTS
Demographic Features of the Study Population
Of 40 965 patients whose charts were reviewed to extract those with retinal disorders, 1208 were diagnosed as having some type of retinal disorder and therefore included in the study. The demographic characteristics of these patients are shown in Table 1. Retinopathies diagnosed at the UHK accounted for a significantly higher proportion compared to those diagnosed at the SJH (P<0.001). There were significantly more males (55.8%) than females (44.2%), P<0.0001. The mean age was 61.7±10.7y with males significantly older than females (P=0.011). Age comparison between patients from the two medical institutions showed that patients from the UHK were marginally older than those from SJH (P=0.048). Patients in the 60-69 age band (35.3%) significantly outnumbered those in the other age groups (all P<0.0001).
Table 1. Demographics of patients with retinal disorders.
| Characteristics | Total | P1 | UHK | P2 | SJH | P3 | P4 |
| Sex, n (%) | 1208 | <0.001 | 746 | <0.001 | 462 | 0.0004 | <0.001 |
| Male | 674 (55.8) | 416 | 258 | <0.001 | |||
| Female | 534 (44.2) | 330 | 204 | <0.001 | |||
| Male age, y | 62.3±10.4 | 0.011 | 63.1±11.0 | 0.006 | 61.2±9.5 | 0.53 | 0.019 |
| Female age, y | 60.9±11.0 | 60.9±10.8 | 60.6±10.9 | 0.70 | |||
| Mean age, y | 61.7±10.7 | 62.1±11.0 | 60.9±10.2 | 0.048 | |||
| 40-49y | 173 | 110 | 63 | <0.001 | |||
| 50-59y | 341 | 194 | 147 | <0.001 | |||
| 60-69y | 426 | 265 | 161 | <0.001 | |||
| 70-79y | 203 | 133 | 70 | <0.001 | |||
| ≥80y | 65 | 44 | 21 | <0.001 |
UHK: University Hospital of Kinshasa; SJH: Saint Joseph Hospital; P1, P2, and P3: Level of statistical difference of the comparison of male and female proportions and male and female mean ages of the entire study population, patients attending the UHK and SJH, respectively; P4: Statistical significance level of the difference between measures at the UHK and SJH.
Types and Frequency of Retinal Disorders
The most common retinal disorders were hypertensive retinopathy, diabetic retinopathy, AMD, chorioretinitis, vein occlusion, retinal detachment, macular hole, arterial occlusion, and vitreoretinal hemorrhage in proportions of patients ranging between 45% and 1.3% (Table 2). All other retinal disorders were each observed in less than 1% of the patients. Hypertensive retinopathy and AMD were significantly more frequent in females than males whereas retinitis pigmentosa was more frequent in males (all P<0.05). No differences were observed in the proportions of other retinopathies in males vs females. Overall, 61.8% and 38.2% of cases of retinal disorders were diagnosed at the UHK and HSJ, respectively (χ2=134.5, P=0.0001). The UHK had significantly higher numbers of patients diagnosed with hypertensive retinopathy (P<0.0001) and diabetic retinopathy (P<0.0008) than SJH. On the contrary, the number patients diagnosed with retinal vein occlusion (P<0.0001), retinal artery occlusion (P=0.0035) and vitreous hemorrhage (P=0.04) was significantly higher at SJH than UHK. All other pathologies were equally frequent at both centers.
Table 2. Distribution of retinal disorders by sex and medical institution.
| Diagnostic | Total | M/F | χ2 | P1 | UHK/SJH | χ2 | P2 |
| Hypertensive retinopathy | 543 (45.0) | 286/257 | 3.9 | 0.048 | 459/84 | 216 | <0.0001 |
| Diabetic retinopathy | 424 (35.1) | 246/178 | 1.3 | 0.25 | 289/135 | 11.3 | 0.0008 |
| Age-related macular degeneration | 175 (14.5) | 83/92 | 5.8 | 0.02 | 112/63 | 0.45 | 0.5 |
| Chorioretinitis | 85 (7.3) | 44/41 | 0.66 | 0.42 | 52/33 | 0.00 | 0.94 |
| Retinal vein occlusion | 83 (6.9) | 48/35 | 0.12 | 0.73 | 26/57 | 34.5 | <0.0001 |
| Retinal detachment | 64 (5.3) | 41/23 | 1.9 | 0.17 | 32/32 | 3.8 | 0.05 |
| Macular hole | 44 (3.6) | 22/22 | 0.54 | 0.46 | 30/14 | 0.82 | 0.37 |
| Retinal artery occlusion | 20 (1.7) | 12/8 | 0.16 | 0.69 | 6/14 | 8.5 | 0.0035 |
| Vitreous hemorrhage | 16 (1.3) | 12/4 | 2.8 | 0.1 | 6/10 | 4.2 | 0.04 |
| Unspecified macular dystrophy | 11 (0.91) | 7/4 | 0.31 | 0.58 | 5/6 | 1.1 | 0.29 |
| Retinitis pigmentosa | 9 (0.85) | 9/0 | 6.9 | 0.008 | 4/5 | 1.4 | 0.23 |
| Polypoidal choroidal vasculopathy | 9 (0.75) | 6/3 | 0.35 | 0.55 | 8/1 | 3.1 | 0.08 |
| Epiretinal membrane | 5 (0.41) | 2/3 | 0.62 | 0.43 | 4/1 | 0.67 | 0.41 |
| Chorioretinal scar | 3 (0.25) | 2/1 | 0.12 | 0.73 | 3/0 | 1.9 | 0.17 |
| Post-traumatic retinal edema | 3 (0.25) | 3/0 | 2.1 | 0.14 | 3/0 | 1.9 | 0.17 |
| Retinal tear | 2 (0.17) | 2/0 | 1.6 | 0.21 | 0/2 | 2.9 | 0.08 |
| Myopic retinopathy | 2 (0.17) | 1/1 | 0.21 | 0.65 | 1/1 | 0.21 | 0.65 |
| Isolated macular edema | 2 (0.19) | 1/1 | 0.21 | 0.65 | 0/2 | 2.9 | 0.08 |
| Stargardt dystrophy | 1 (0.08) | 0/1 | 1.4 | 0.25 | 0/1 | 1.5 | 0.22 |
| Fundus flavipunctatus | 1 (0.08) | 1/0 | 0.53 | 0.47 | 1/0 | 0.46 | 0.50 |
| Posterior vitreous detachment | 1 (0.08) | 1/0 | 0.53 | 0.47 | 0/1 | 1.5 | 0.22 |
| Sickle cell retinopathy | 1 (0.08) | 1/0 | 0.53 | 0.47 | 1/0 | 0.46 | 0.50 |
| Cone dystrophy | 1 (0.08) | 1/0 | 0.53 | 0.47 | 0/1 | 1.5 | 0.22 |
| Pattern dystrophy | 1 (0.08) | 1/0 | 0.53 | 0.47 | 0/1 | 1.5 | 0.22 |
| Retinal macroaneurysm | 1 (0.08) | 0/1 | 1.4 | 0.25 | 0/1 | 1.5 | 0.22 |
| Unspecified retinal degeneration | 1 (0.08) | 1/0 | 0.53 | 0.47 | 0/1 | 1.5 | 0.22 |
M: Male; F: Female; UHK: University Hospital of Kinshasa; SJH: Saint Joseph Hospital; P1: Significance level of the difference in proportions of retinal disorders between males and females; P2: Significance level of the difference in proportions of retinal disorders between the UHK and SJH.
n (%)
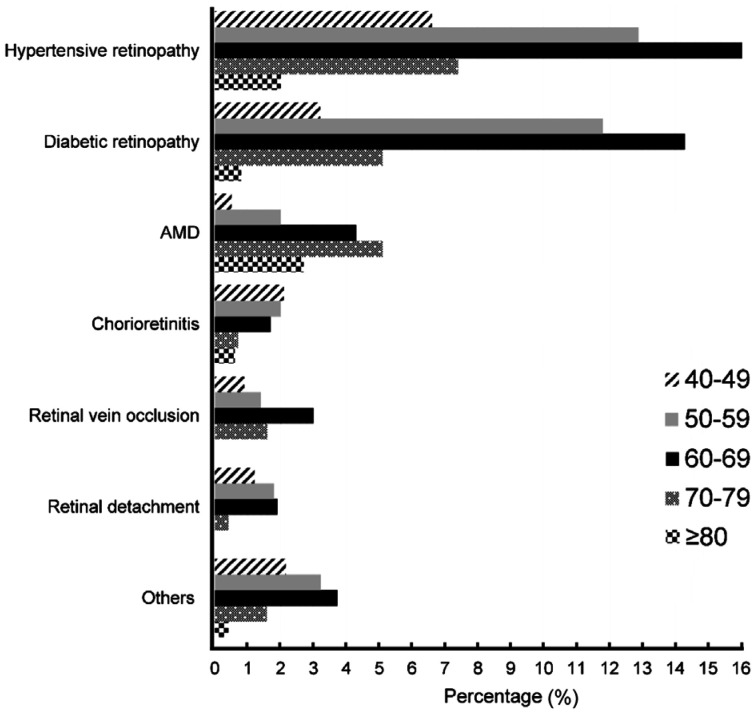
Figure 1 shows that the frequency of hypertensive retinopathy, diabetic retinopathy, retinal vein occlusion, and retinal detachment increased with age to reach the peak in 60-69y people before decreasing afterward. AMD was more frequent in the 70-79 age band (5.1%). The frequency of chorioretinitis was higher and evenly distributed from the 5th to the 7th decade then decreased. All the other retinal disorders combined were more frequent in the 60-69 age group.
Figure 1. Proportion of patients with retinal diseases per age group.
The global, sex- and institution-specific prevalences are presented in Table 3. The 3-year prevalence of retinal diseases regardless of type was 3.0%, or an annual rate of 1%. The global prevalence was 1.3 higher in males than females (P<0.0001) and 15 times higher at the UHK than SJH (P<0.0001). The prevalence was comparable between males and females for all individual disorders (all P>0.05), except for diabetic retinopathy (P=0.002), retinal detachment, vitreous hemorrhage, and retinitis pigmentosa, whose rates were higher in males than females (all P<0.05). Most retinal diseases were more prevalent at the UHK than SJH.
Table 3. Global and sex- and hospital-specific annual prevalence rates of retinal disorders.
| Retinal disease | 3-year rate | Annual rate | Sex-specific rate (male/female) | P1 | Hospital-specific rate (UHK/SJH) | P2 |
| Overall | 3.0 | 1.0 | 3.36/2.56 | <0.0001 | 18.8/1.25 | <0.0001 |
| Hypertensive retinopathy | 1.33 | 0.44 | 1.42/1.23 | 0.09 | 11.6/0.23 | <0.0001 |
| Diabetic retinopathy | 1.04 | 0.35 | 1.22/0.85 | 0.0002 | 7.27/0.36 | <0.0001 |
| AMD | 0.43 | 0.14 | 0.41/0.44 | 0.64 | 2.82/0.17 | <0.0001 |
| Chorioretinitis | 0.21 | 0.07 | 0.22/0.20 | 0.66 | 1.31/0.089 | <0.0001 |
| Retinal vein occlusion | 0.20 | 0.07 | 0.24/0.17 | 0.12 | 0.65/0.154 | <0.0001 |
| Retinal detachment | 0.16 | 0.05 | 0.20/0.11 | 0.02 | 0.81/0.087 | <0.0001 |
| Macular hole | 0.11 | 0.04 | 0.11/0.11 | 1.0 | 0.75/0.038 | <0.0001 |
| Othersa | 0.22 | 0.08 | 0.30/0.12 | 0.005 | 1.08/0.13 | <0.0001 |
P1: Significance level of the difference between prevalence in males and females, P2: Significance level of the difference between prevalence at UHK and SJH. aInclude retinal artery occlusion, vitreous hemorrhage, unspecified macular dystrophy, retinitis pigmentosa, polypoidal choroidal vasculopathy, epiretinal membrane, chorioretinal scar, post-traumatic retinal edema, retinal tear, myopic retinopathy, isolated macular edema, Stargardt's disease, fundus flavipunctatus, posterior vitreous detachment, sickle cell retinopathy, pattern dystrophy, retinal macroaneurysm, unspecified retinal degeneration.
Non-ocular Comorbidities
Seven in 10 patients had systemic arterial hypertension and 4 in 10 patients were diabetic. A history of stroke was reported in 6% of the patients. All the other conditions combined were observed in 5.5% of the patients (Figure 2).
Figure 2. Proportion of systemic comorbidities in patienst with retinal disorders.
aConditions observed in less than 1% each: rheumatism, HIV infection, kidney, failure, sinusitis, gastritis, asthrma, gout, tuterculosis, psychopathy, herniated disc, megacolon, tuberculosis, pituiatary adenoma, facial paralysis, meningoencephalitis, recurrent preeclampsia, and sickle cell anemia.
Retinal Disorders as Causes of Visual Impairment
Among patients with retinal disorders, 94 had no BCVA data due to various reasons such as being bedridden in ICU and lack of cooperation due to stroke. BCVA data was therefore available for 1114 patients. Figure 3 shows the proportion of people with low vision and blindness. Unilateral low vision was observed in approximately 1/3 of all right and all left eyes. About 1/5 of the right and left eyes were blind. When visual impairment was defined based on the BCVA in the best eye, low vision and blindness were present in 26.8% and 8.4% of the patients at presentation, respectively.
Figure 3. Proportion of patients with normal, low vision, and blindness among patients with retinal disorders.
The causes of low vision and blindness are listed in Table 4. Diabetic retinopathy-induced low vision was seen in 13.3% of all patients. In decreasing order, other frequent causes of low vision were AMD, retinal vein occlusion, chorioretinitis, retinal detachmeent, and macular hole, with frequency ranging between 3.9% and 1.2%. Diabetic retinopathy caused half of all cases of low vision and a third of all cases of blindess.
Table 4. Causes and frequency of low vision and blindness.
| Causes | Low visiona | Blindnessb | Totalc |
| Diabetic retinopathy | 148 (13.3/49.5) | 31 (2.8/33.0) | 179 (16.1/45.5) |
| AMD | 43 (3.9/14.4) | 16 (1.4/17.0) | 59 (5.3/15.0) |
| Retinal detachment | 14 (1.3/4.7) | 20 (1.8/21.3) | 34 (3.1/8.7) |
| Retinal vein occlusion | 27 (2.4/9.0) | 4 (0.4/4.3) | 31 (2.8/7.9) |
| Chorioretinitis | 21 (1.9/7.0) | 2 (0.2/2.1) | 23 (2.1/5.9) |
| Macular hole | 14 (1.3/4.7) | 3 (0.3/3.2) | 17 (1.6/4.3) |
| Vitreous hemorrhage | 10 (0.9/3.3) | 1 (0.1/1.1) | 11 (1.0/2.8) |
| Retinitis pigmentosa | 4 (0.4/1.3) | 2 (0.2/2.1) | 6 (0.5/1.5) |
| Retinal arterial occlusion | 4 (0.4/1.3) | 1 (0.1/1.1) | 5 (0.5/1.3) |
| Unspecified macular dystrophy | 5 (0.5/1.7) | 0 | 5 (0.5/1.3) |
| PCV | 0 | 2 (0.2/2.1) | 2 (0.2/0.5) |
| Post-traumatic retinal edema | 0 | 1 (0.1/1.1) | 1 (0.1/0.3) |
| Chorioretinal scar | 0 | 1 (0.1/1.1) | 1 (0.1/0.3) |
| Stargardt's disease | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Myopic retinopathy | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Fundus flavipunctatus | 0 | 1 (0.1/1.1) | 1 (0.1/0.3) |
| Posterior vitreous detachment | 0 | 1 (0.1/1.1) | 1 (0.1/0.3) |
| Retinal tear | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Cone dystrophy | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Unspecified retinal degeneration | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Isolated macular edema | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Pattern dystrophy | 0 | 1 (0.1/1.1) | 1 (0.1/0.3) |
| Sickle cell retinopathy | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
| Retinal macroaneurysm | 1 (0.1/0.3) | 0 | 1 (0.1/0.3) |
aThese two percentages are derived from the number of patients with retinal disorders who had VA data (n=1114) and the number of all patients with low vision (n=299), respectively; bThese proportions are derived from the total number of patients with retinal disorders and the number of people witth blindness (n=94), respectively; cThese percentages are calculated from the number of people with retinal disorders and those with visual impairment (n=393), respectively.
n (%/%)
Overall, 2.8% of the patients were blind as a result of diabetic retinopathy, which was also responsible for 33.0% of all cases of blindness. Blindness resulting from retinal detachment and AMD was recorded in 1.8% and 1.4% of the patients, respectively. Among the 94 people with blindness, retinal detachment accounted for 21.3% and AMD for 17.0% of all cases of blindness.
DISCUSSION
While the types and prevalence of retinal diseases are well known in developed countries, such information is scarce in SSA. In this HBS, we sought to determine the profile of retinal diseases among 40-years and older patients in Kinshasa and the extent to which they induce visual impairment. Unlike all past HBS that have been monocentric, the uniqueness of the present study lies in the fact that it was conducted in the two major public ophthalmological centers of Kinshasa. The difficulty of conducting a PBS makes such a multicenter approach likely to provide more reliable hospital-based data in this setting.
The 3-year prevalence of retinal diseases in the present study was 3.0%, meaning an annual rate of 1.0%. After analyzing the prevalence rates of prior HBS, our annual rate is lower than observed in other settings, except the 0.78% reported by Eze et al[5] as shown in Table 5. However, caution should be exercised when directly comparing our findings to those of the other studies because of differences in study design (i.e., hospital-based vs. population-based) and population sampling (i.e., age of inclusion and inclusion of children). For example, prevalence rates of HBS in SSA were 7.9% in Ethiopia[8] and 0.78%-13.9% in Nigeria[5]–[7],[15]–[16]. Unlike our study that only included people aged at least 40y, these other studies also included children. A study with a similar design that included children in Pakistan found a rate of 13.4%[17]. Other similar investigations in Nigeria[9],[13],[18] and in Brazil[19] did not provide the prevalence rates; they also failed to provide the size of the population from which their study population was drawn from to allow computing prevalence rates. Unlike in Nigeria and elsewhere in other continents where specialized investigational methods for the retina and choroid were used, the diagnosis in the present study was mostly based on clinical grounds. Thus, our prevalence rate may be underestimated. Moving forward, a comprehensive strategy is needed to address issues of vitreoretinal disorders not only in the DRC, but also in most SSA countries. Such a strategy must include equipment provision or upgrade and personnel training.
Table 5. Hospital-based prevalence of retinal disorders across studies.
| Study | Country | Design | Year | n | Mean age | Inclusion of children | Annual prevalence (%) |
| Present study | DRC | HBS | 2019 | 40135 | 61.7 | No | 1.0 |
| Eze et al[5] | Nigeria | HBS | 2010 | 8239 | 49.3 | Yes | 0.78 |
| Nwosu[6] | Nigeria | HBS | 2000 | 610 | 58 | Yes | 13.9 |
| Onakpoya et al[7] | Nigeria | HBS | 2008 | 3130 | 46.3 | Yes | 2.2 |
| Teshome et al[8] | Ethiopia | HBS | 2004 | - | 45.2 | Yes | 7.9 |
| Abiose[15] | Nigeria | HBS | 1979 | 1723 | - | Yes | 5.3 |
| Ajayi et al[16] | Nigeria | HBS | 2016 | 3881 | 57 | Yes | 4.2 |
| Khan et al[17] | Pakistan | HBS | 2011 | 27000 | 46.6 | Yes | 13.4 |
DRC: Democratic Republic of Congo; HBS: Hospital-based study.
We determined that the most common retinal diseases in the study population were hypertensive retinopathy (41.8%). In other studies, hypertensive retinopathy accounted for 2.8% to 13.3% of all retinopathies in various Nigerian settings[5]–[7],[9]. Our high frequency of hypertensive retinopathy is the reflection of the magnitude of hypertension, which has been reported to range between 30.9% and 41.4% among Congolese[20]–[22]. It also corroborates reports that hypertensive retinopathy is common in hypertensive black Africans[23]–[25].
The International Diabetes Federation has estimated that the number of adults with diabetes in Africa will increase by 98%, from 12.1 million in 2010 to 23.9 million in 2045[26]. This will likely increase the rate of diabetic retinopathy, which was the second most common disease in this study. Except the study by Oluyeye and Ajaiyeoba et al[13] where diabetic retinopathy was fifth among retinal disorders and found in 7.6% of the population, diabetic retinopathy was either the leading or second most frequent retinal disorder in other studies. It was found in 10.8% to 24.9% in Nigerian populations[5]–[7],[9], 28.6% in Ethiopia[8], 24.8% in Brazil[19], 39.8% in Pakistan[17]. Though diabetic retinopathy appears to be more frequent in our study, data across studies agree that it is increasing in importance particularly in SSA. It is worth noting that the public health system in Kinshasa has long developed a system for diabetes screening, with prompt referral of patients to a network of ophthalmologists for diabetic retinopathy screening. All diabetic patients attending the diabetes clinic at the UHK are mandatorily referred to the eye clinic for screening. SJH also has a community-based screening strategy in numerous satellite clinics, which also refer patients to their eye clinic for screening. This practice may have contributed to the high frequency of diabetic retinopathy observed in this study.
AMD (14.5%) was the 3rd most common retinal pathology in this study. In five Nigerian settings, AMD was consistently the most common retinal disease, surprisingly, with frequencies comparable to ours and ranging between 14.6% and 19.4%[6]–[7],[9],[13],[16]. In addition, a review of all cases that attended the retina unit of a teaching hospital during a 3-year period in Nigeria, determined that AMD accounted for 14% of all retinal pathologies[27]. In Ethiopia, however, AMD was found only in 2.7%[8]. In line with our finding and contrary to the initial belief that AMD was uncommon in blacks including those in SSA[28], it also emerged from recent investigations in Kenya that AMD is more frequent than previously thought[2],[29]. The combination of increased life expectancy and increased prevalence of risk factors such as diabetes and hypertension may have contributed to heightening AMD frequency in our patients. It is important to mention that unlike AMD, polypoidal choroidal vasculopathy (PCV) only accounted for 0.75% of all retinal disorders. PCV is close to AMD, but differs in its epidemiological and clinical features as well as its less favorable therapeutic response to intravitreal injections of anti-VEGF drugs. Indocyanine green angiography is the gold standard for its diagnosis[30]–[31]. Alternatively, OCT helps diagnose the disease with high sensitivity and specificity[32]. Despite affecting more people of African and Asian descents than those of European ancestry[33], only two studies have described the clinical features of the disease in SSA[34]–[35]. Consequently, the epidemiology and clinical presentation of PCV in SSA remains largely unknown. Contributing reasons included not only the lack of investigational tools, but also shortage of subspecialty trained personnel. The likelihood that the frequency of PCV found in the present study is underestimated is relatively high given the low sensitivity of using ophthalmoscopy and/or fundus photography alone as diagnostic means. Now that OCT devices are becoming available in SSA, studies are needed to determine the true magnitude, to describe the clinical features of PVC, and to assess its impact of the quality of life and its response to treatment.
We found that low vision was present in 26.8% and blindness in 8.4% of the patients. Hospital-based rates of blindness due to retinal disorders in previous studies in SSA were 10.8% and 20.6% in the South and Southeastern of Nigeria with corresponding rates of low vision of 3.6% and 11%[5],[9]. Such rates in Addis Ababa, Ethiopia, were 16.1% and 20.1%, respectively[8]. In this study, diabetic retinopathy was the leading cause of visual impairment with 51.0%. This underlines, once again, the importance of systematic screening for diabetic retinopathy in order to initiate early and adequate treatment where indicated to prevent the occurrence of visual impairment.
It was interesting but not surprising to note that despite the higher volume of patients attending the SJH compared to the UHK, retinopathies taken as a whole were significantly more frequent and prevalent at the UHK than SJH. The prevalence of most retinal disorders also showed the same trend despite their frequencies being similar. It is important to highlight that while the two institutions receive the same types of pathologies, SJH and other clinics end up referring some of the patients to the UHK, particularly complex cases. The UHK also receive referrals from other not only surrounding regions, but also regions as far as 1500 miles. Thus, outside referral play a substantial role in the difference observed in the prevalence of retinal disorders at the two institutions. Hypertensive and diabetic retinopathies are particular because most patients are internally referred from the UHK internal medicine department for assessment.
This is an HBS and, as such, its major limitation is the fact that the findings cannot be extrapolated to the general population due the design. The rates provided herein may have been underestimated for some disorders such as PCV. However, the multicenter approach used to gather information sets this study apart from all other previous studies conducted in SSA and is a more robust methodology to provide a better idea of the features of retinal diseases in Kinshasa. As diagnostic methods for retinal disorders are becoming locally available, more HBS and well-designed PBS will be needed to generate more accurate estimates.
In conclusion, the findings of the present clinic-based study indicate that retinal disorders are frequent in this urban setting. Retinal vascular diseases including hypertensive and diabetic retinopathy are the most common. Diabetic retinopathy is the leading cause of visual impairment among Congolese patients with retinal disorders.
Acknowledgments
We wish to thank Dr. Stella Masengu and Dr. Sabrina Mukash for helping with chart review.
Conflicts of Interest: Kabedi NN, None; Kayembe DL, None; Mwanza JC, None.
REFERENCES
- 1.Li J, Welchowski T, Schmid M, et al. Retinal disease in Europe. Prevalence, incidence and healthcare needs. Euretina. 2017:1–28. [Google Scholar]
- 2.Bastawrous A, Mathenge W, Peto T, Shah N, Wing K, Rono H, Weiss HA, MacLeod D, Foster A, Burton M, Kuper H. Six-year incidence and progression of age-related macular degeneration in Kenya: nakuru eye disease cohort study. JAMA Ophthalmol. 2017;135(6):631–638. doi: 10.1001/jamaophthalmol.2017.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flaxman SR, Bourne RRA, Resnikoff S, Ackland P, Braithwaite T, Cicinelli MV, Das A, Jonas JB, Keeffe J, Kempen JH, Leasher J, Limburg H, Naidoo K, Pesudovs K, Silvester A, Stevens GA, Tahhan N, Wong TY, Taylor HR, Vision Loss Expert Group of the Global Burden of Disease Study Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health. 2017;5(12):e1221–e1234. doi: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 4.Bastawrous A, Burgess PI, Mahdi AM, Kyari F, Burton MJ, Kuper H. Posterior segment eye disease in sub-Saharan Africa: review of recent population-based studies. Trop Med Int Health. 2014;19(5):600–609. doi: 10.1111/tmi.12276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eze BI, Uche JN, Shiweobi JO. The burden and spectrum of vitreo-retinal diseases among ophthalmic outpatients in a resource-deficient tertiary eye care setting in South-eastern Nigeria. Middle East Afr J Ophthalmol. 2010;17(3):246–249. doi: 10.4103/0974-9233.65491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nwosu SN. Prevalence and pattern of retinal diseases at the Guinness eye hospital, onitsha, Nigeria. Ophthalmic Epidemiol. 2000;7(1):41–48. [PubMed] [Google Scholar]
- 7.Onakpoya OH, Olateju SO, Ajayi IA. Retinal diseases in a tertiary hospital: the need for establishment of a vitreo-retinal care unit. J Natl Med Assoc. 2008;100(11):1286–1289. doi: 10.1016/s0027-9684(15)31506-6. [DOI] [PubMed] [Google Scholar]
- 8.Teshome T, Melaku S, Bayu S. Pattern of retinal diseases at a teaching eye department, Addis Ababa, Ethiopia. Ethiop Med J. 2004;42(3):185–193. [PubMed] [Google Scholar]
- 9.Uhumwangho OM, Itina EI. Retinal diseases in a tertiary hospital in southern Nigeria. J West Afr Coll Surg. 2015;5(2):1–16. [PMC free article] [PubMed] [Google Scholar]
- 10.Chan WM. Age-related macular degeneration in Asia. Retina Today. 2009(6):30–34. [Google Scholar]
- 11.Colijn JM, Buitendijk GHS, Prokofyeva E, et al. Prevalence of age-related macular degeneration in Europe: the past and the future. Ophthalmology. 2017;124(12):1753–1763. doi: 10.1016/j.ophtha.2017.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hatef E, Fotouhi A, Hashemi H, Mohammad K, Jalali KH. Prevalence of retinal diseases and their pattern in Tehran: the Tehran eye study. Retina. 2008;28(5):755–762. doi: 10.1097/IAE.0b013e3181613463. [DOI] [PubMed] [Google Scholar]
- 13.Oluleye TS, Ajaiyeoba AI. Retinal diseases in Ibadan. Eye. 2006;20(12):1461–1463. doi: 10.1038/sj.eye.6702343. [DOI] [PubMed] [Google Scholar]
- 14.Brämer GR. International statistical classification of diseases and related health problems. Tenth revision. World Health Stat Q. 1988;41(1):32–36. [PubMed] [Google Scholar]
- 15.Abiose A. Pattern of retinal diseases in Lagos. Ann Ophthalmol. 1979;11(7):1067–1072. [PubMed] [Google Scholar]
- 16.Ajayi IA, Motoye OJ, Ajite KO, Ajogbasile OO. Retinal disorders in a tertiary eye centre in Nigeria. Pak J Ophthalmol. 2016;32(3):152–158. [Google Scholar]
- 17.Khan A, Riaz Q, Soomro F, Qidwai U, Qazi U. Frequency and patterns of eye diseases in retina clinic of a tertiary care hopsital in Karachi. Pak J Ophthalmol. 2011;27(3):155–159. [Google Scholar]
- 18.Bogunjoko TJ, Hassan AO, Ogunro A, Akanbi T, Abudu B. Posterior segment eye diseases in Ijebu, Southwestern Nigeria. Int J Community Med Public Health. 2018;6(1):8. [Google Scholar]
- 19.Malerbi FK, Matsudo NH, Carneiro AB, Lottenberg CL. Retinal diseases in a reference center from a Western Amazon capital City. Einstein (Sao Paulo) 2015;13(4):530–534. doi: 10.1590/S1679-45082015AO3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bayauli MP, M'Buyamba-Kayamba JR, Ngoyi NG, et al. Trends in prevalence of obesity and hypertension in an urban Congolese community. J Epidemiol Res. 2018;4(1):33–40. [Google Scholar]
- 21.Katchunga PB, Mirindi P, Baleke A, Ntaburhe T, Twagirumukiza M, M'buyamba-Kabangu JR. The trend in blood pressure and hypertension prevalence in the general population of South Kivu between 2012 and 2016: Results from two representative cross-sectional surveys-The Bukavu observational study. PLoS One. 2019;14(8):e0219377. doi: 10.1371/journal.pone.0219377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phanzu BK, Mabaka EM, Kintoki EV, et al. Rates of hypertension prevalence, awareness, treatment, and control in a congolese South-West port city. The Influence of gender according to age groups. Global J Med Res. 2015;14(1-8) [Google Scholar]
- 23.Kabedi NN, Mwanza JC, Lepira FB, Kayembe TK, Kayembe DL. Hypertensive retinopathy and its association with cardiovascular, renal and cerebrovascular morbidity in Congolese patients. Cardiovasc J Afr. 2014;25(5):228–232. doi: 10.5830/CVJA-2014-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oluleye ST, Olusanya BA, Adeoye AM. Retinal vascular changes in hypertensive patients in Ibadan, Sub-Saharan Africa. Int J Gen Med. 2016;9:285–290. doi: 10.2147/IJGM.S107241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omotoso AB, Kolo PM, Olanrewaju TO, Owoeye JF, Biliaminu SA, Olatunji VA. Relationship between retinopathy and renal abnormalities in black hypertensive patients. Clin Hypertens. 2016;22:19. doi: 10.1186/s40885-016-0053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho NH, Shaw JE, Karuranga S, et al. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 27.Oluleye TS. Is age-related macular degeneration a problem in Ibadan, Sub-Saharan Africa? Clin Ophthalmol. 2012;6:561–564. doi: 10.2147/OPTH.S27470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedman DS, Katz J, Bressler NM, Rahmani B, Tielsch JM. Racial differences in the prevalence of age-related macular degeneration: the Baltimore Eye Survey. Ophthalmology. 1999;106(6):1049–1055. doi: 10.1016/S0161-6420(99)90267-1. [DOI] [PubMed] [Google Scholar]
- 29.Mathenge W, Bastawrous A, Peto T, Leung I, Foster A, Kuper H. Prevalence of age-related macular degeneration in Nakuru, Kenya: a cross-sectional population-based study. PLoS Med. 2013;10(2):e1001393. doi: 10.1371/journal.pmed.1001393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spaide RF, Yannuzzi LA, Slakter JS, Sorenson J, Orlach DA. Indocyanine green videoangiography of idiopathic polypoidal choroidal vasculopathy. Retina. 1995;15(2):100–110. doi: 10.1097/00006982-199515020-00003. [DOI] [PubMed] [Google Scholar]
- 31.Stanga PE, Lim JI, Hamilton P. Indocyanine green angiography in chorioretinal diseases: indications and interpretation: an evidence-based update. Ophthalmology. 2003;110(1):15–21. doi: 10.1016/s0161-6420(02)01563-4. [DOI] [PubMed] [Google Scholar]
- 32.de Salvo G, Vaz-Pereira S, Keane PA, Tufail A, Liew G. Sensitivity and specificity of spectral-domain optical coherence tomography in detecting idiopathic polypoidal choroidal vasculopathy. Am J Ophthalmol. 2014;158(6):1228–1238. doi: 10.1016/j.ajo.2014.08.025. [DOI] [PubMed] [Google Scholar]
- 33.Kim JB, Nirwan RS, Kuriyan AE. Polypoidal Choroidal Vasculopathy. Curr Ophthalmol Rep. 2017;5(2):176–186. doi: 10.1007/s40135-017-0137-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabedi NN, Kayembe DL, Elongo GM, Mwanza JC. Polypoidal choroidal vasculopathy in Congolese patients. J Ophthalmol. 2020;2020:4103871. doi: 10.1155/2020/4103871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oluleye TS, Babalola Y. Pattern of presentation of idiopathic polypoidal choroidal vasculopathy in Ibadan, Sub-Saharan Africa. Clin Ophthalmol. 2013;7:1373–1376. doi: 10.2147/OPTH.S47511. [DOI] [PMC free article] [PubMed] [Google Scholar]