Abstract
AIM
To investigate the alleviation of scutellarein (SN) against inner blood-retinal-barrier (iBRB) dysfunction in microglia cells stimulated by hyperglycemia and to elucidate the engaged mechanism.
METHODS
Microglia BV2 cells were stimulated by using 25 mmol/L D-glucose. The same concentration of mannitol (25 mmol/L) was applied as an isotonic contrast. Real-time PCR, Western-blot assay and immunofluorescence staining assay was performed. The dysfunction of iBRB in vitro was detected by using transendothelial electrical resistance (TEER) assay. Additionally, the leakage of fluorescein isothiocyanate (FITC)-conjugated dextran (70 kDa) was detected.
RESULTS
SN abrogated microglia BV2 cells activation and reduced the phosphorylated activation of extracellular signal-regulated protein kinase (ERK)1/2. SN also decreased the transcriptional activation of nuclear factor κB (NFκB) and the elevated expression of tumor necrosis factor α (TNFα), interleukin (IL)-6 and IL-1β in BV2 cells treated with D-glucose (25 mmol/L). SN attenuated iBRB dysfunction in human retinal endothelial cells (HRECs) or choroid-retinal endothelial RF/6A cells when those cells were treated with TNFα, IL-1β or IL-6, or co-cultured with microglia cells stimulated by D-glucose. Moreover, SN restored the decreased protein expression of tight junctions (TJs) in TNFα-treated HRECs and RF/6A cells.
CONCLUSION
SN not only alleviate iBRB dysfunction via directly inhibiting retinal endothelial injury caused by TNFα, IL-1β or IL-6, but also reduce the release of TNFα, IL-1β and IL-6 from microglia cells by abrogating hyperglycemia-mediated the activation of microglia cells.
Keywords: scutellarein, blood-retinal-barrier, tight junctions, inflammation, tumor necrosis factor α
INTRODUCTION
Blood-retinal barrier (BRB) is composed of an inner BRB (iBRB) and an outer BRB (oBRB). Previous studies have shown that the BRB breakdown occurs in many blinding retinal diseases including diabetic retinopathy (DR), which is a common and serious microvascular complication of diabetes mellitus (DM)[1]. With the development of social economy civilization, science and economy, the living standard of the people has been continuously improved. And the improvement of people's living standard, at the same time, the prevalence rate of diabetes is increasing day by day. DR has become the main cause that leads to the blindness in adults in industrialized countries[1].
Retinal endothelial cells lining micro vessels are critical components that form iBRB[2]–[3]. In iBRB, tight junction (TJ) between neighboring cells forms an exceedingly tight seal and thus prevents potentially noxious materials (toxins, infections, endobiotics, endobiotics etc.) from entering and damaging retina, and it is also critical for the supplement of nutrients from blood[3]–[4]. Additionally, other cells including microglia cells and pericytes also play important roles in maintaining the homeostasis of iBRB[5].
Scutellarein (5,6,7,4′-tetrahydroxy flavone, SN) is the aglycone of scutellarin found in some traditional Chinese medicines like scutellaria barbata (Ban-Zhi-Lian), scutellaria baicalensis (Huang-Qin), oroxylum indicum (Mu-Hu-Die) and erigerontis berba (Deng-Zhan-Xi-Xin). Previous studies have demonstrated that SN has anti-cancer, neuroprotective and anti-inflammatory activities[6]–[10]. SN has already been reported to inhibit human retinal endothelial cells (HRECs) growth and reduce the hypoxia-induced vascular endothelial growth factor (VEGF) expression in HRECs, and all those contributed to its alleviation on DR development[11]. A previous study in our group showed that SN reduced the expression of TNFα in microglia cells through abrogating the activation of ERK1/2-NFκB signaling cascade, and it also directly weakened TNFα-induced BRB breakdown (including iBRB and oBRB) by alleviating oxidative stress injury, and thus attenuated DR development[12]. Whether SN, the aglycone of SN, also alleviates iBRB dysfunction is not known. The alleviation of SN against iBRB disruption mediated by hyperglycemia-stimulated microglia cells and the potential engaged mechanism were observed in this study.
MATERIALS AND METHODS
Reagents
Chemical structure of SN (purity 98%, Shanghai Yihong Bio-Chem Co., Ltd., China) is presented in Figure 1A. Antibodies: ionized calcium-binding adapter molecule1 (Iba1) and claudin-5 [GeneTax (Alton Parkway Irvine, CA, US)]; phospho-inhibitor of NFκB (IKK), NFκBp65, phospho-ERK1/2 (Thr202 and Tyr204), total-ERK1/2, Lamin-B1 and β-actin (Cell Signaling Technology, Danvers, MA, USA); claudin-1, occludin and claudin-19 (Santa Cruz, CA, USA). Peroxidase-conjugated goat anti-rabbit immunoglobulin G (IgG) (H+L) and anti-mouse IgG (H+L) (Jackson ImmunoResearch, West Grove, PA, USA). BCA Protein Assay Kits and NE-PER cytoplasmic and nuclear extraction kits (ThermoFisher Scientific, Waltham, MA, USA). Enzyme-linked immunosorbent assay (ELISA) kits (R&D, Minneapolis, MN, USA). Cell culture mediums, Trizol reagent and 4′, 6-diamidino-2-phenylindole (DAPI; Life Technology Carlsbad, CA, USA). Primescript RT Master Mix and SYBR Premix Ex Taq (Takara, Shiga, Japan). U0126 (Alexis. Biochemicals, San Diego, CA, USA). Recombinant TNFα, IL-1β and IL-6 (PeproTech, Cranbury, NJ, USA). Alexa 568 labeled goat anti-rabbit IgG (BD Biosciences, Franklin Lakes, NJ, USA).
Figure 1. SN reduced microglia cells activation in vitro.
A: The chemical structure of SN; B: Representative immunofluorescence image (n=3); C: Iba1 expression (n=4) aP<0.05 versus control; dP<0.05 versus D-glucose.
Methods
Primary HRECs are cultured in ECM medium (ScienCell, Carlsbad, CA, USA) supplemented with 5% (v/v) fetal bovine serum (FBS). The monkey choroid-retinal endothelial RF/6A cells (ATCC, Manassas, VA, USA) are cultured in RPMI1640 medium supplemented with 10% FBS. Microglia BV2 cells are cultured in DMEM medium with high glucose and 10% FBS (Life Technology, Carlsbad, CA). D-glucose (25 mmol/L) was used to incubate with BV2 cells for the indicated times. At the same time, an isotonic contrast mannitol (25 mmol/L) was used. Cellular RNA was extracted and real-time PCR assay was performed as described in our previous published paper[12]. The results were analyzed by the 2−ΔΔCt method and given as ratio compared with the control. The primer sequences are shown in Table 1. Protein expression was detected and calculated as described in our previous study[12]. Supernatants from cells were used for the ELISA assay. Cells were pre-incubated with SN (20, 50 mmol/L) for 6h, and then further incubated with D-glucose (25 mmol/L) for 24h. Immunofluorescene staining of Iba1 was conducted as described[12]. TEER was measured as described[12]. Permeability of endothelial cells was measured by calculating the leakage of FITC-dextran as describe[12].
Table 1. The list of primers for real-time PCR.
| Target, primer | Sequence |
| TNF-α | |
| FP | 5′-AGGCACTCCCCCAAAAGAT-3′ |
| RP | 5′-CAGTAGACAGAAGAGCGTGGTG-3′ |
| IL-1β | |
| FP | 5′-AGTTGACGGACCCCAAAAG-3′ |
| RP | 5′-CTTCTCCACAGCCACAATGA-3′ |
| IL-6 | |
| FP | 5′-CGGAGAGGAGACTTCACAGAG-3′ |
| RP | 5′-ATTTCCACGATTTCCCAGAG-3′ |
| Actin | |
| FP | 5′-TACAGCTTCACCACCACAGC-3′ |
| RP | 5′-TCTCCAGGGAGGAAGAGGAT-3′ |
FP: Forward primer; RP: Reverse primer.
Statistical Analysis
Data were expressed as mean±standard error of the mean (SEM). The significance of differences within groups was analyzed by using one-way ANOVA with LSD post hoc test. P<0.05 was considered as statistically significant differences.
RESULTS
Scutellarein Attenuated Microglia Cells Activation in vitro
Iba1 is commonly used to identify activated microglia cells[13]. Figure 1B shows that the quantity of activated microglia cells was increased when D-glucose (25 mmol/L) was added into BV2 cells, whereas SN (50 mmol/L) obviously decreased this increase. Meanwhile, SN (50 mmol/L) also decreased the elevated Iba1 expression induced by 25 mmol/L D-glucose in BV2 cells (Figure 1C).
Scutellarein Abrogated the D-glucose-initiated NFκB and ERK1/2 Activation in vitro
Figure 2A-2C shows that D-glucose (25 mmol/L) caused the nuclear translocation of NFκBp65 in BV2 cells, and SN (50 mmol/L) decreased this accumulation. Besides, SN (20, 50 mmol/L) reduced the increased IKK phosphorylation in BV2 cells incubated with D-glucose (25 mmol/L) (Figure 2A-2B). SN (50 µmol/L) and U0126 (20 µmol/L), the inhibitor of MEK1/2, also weakened the increased ERK1/2 phosphorylation stimulated by 25 mmol/L D-glucose in microglia BV2 cells (Figure 2D-2E). Moreover, SN (50 mmol/L) also reduced the up-regulated mRNA expression of IL-1β (Figure 3A) and IL-6 (Figure 3B). TNFα is another important pro-inflammatory cytokine. SN (20, 50 mmol/L) obviously reduced the up-regulated TNFα mRNA expression in D-glucose-treated BV2 cells (Figure 3C). Meanwhile, SN (20, 50 mmol/L) reduced the up-regulated TNFα amount in supernatants isolated from BV2 cells stimulated by D-glucose (Figure 3D).
Figure 2. SN abrogated the D-glucose-initiated NFκB and ERK1/2 activation in BV2 cells.
A: The expression of phosphorylated IKK and NFκBp65 is detected; B: The quantitative analysis of phosphorylated IKK (n=3); C: The quantitative analysis of nuclear and cytosolic NFκBp65 (n=4); D: Elevated ERK1/2 phosphorylation caused by D-glucose (25 mmol/L) was reduced by SN; E: The quantitative analysis of ERK1/2 phosphorylation (n=4) aP<0.05, bP<0.01 versus control; dP<0.05, eP<0.01, fP<0.001 versus D-glucose.
Figure 3. SN inhibited the D-glucose-induced up-regulated expression of TNFα, IL-6 and IL-1β in vitro.
A-C: Cellular mRNA level of IL-1β (n=4), IL-6 (n=3), and TNFα (n=4); D: Content of TNFα in the supernatants from D-glucose (25 mmol/L)-treated BV2 cells (n=3) aP<0.05, bP<0.01 versus control; dP<0.05, eP<0.01 versus D-glucose.
Scutellarein Rescued the D-glucose-stimulated BV2 cells or TNFα-initiated iBRB Injury
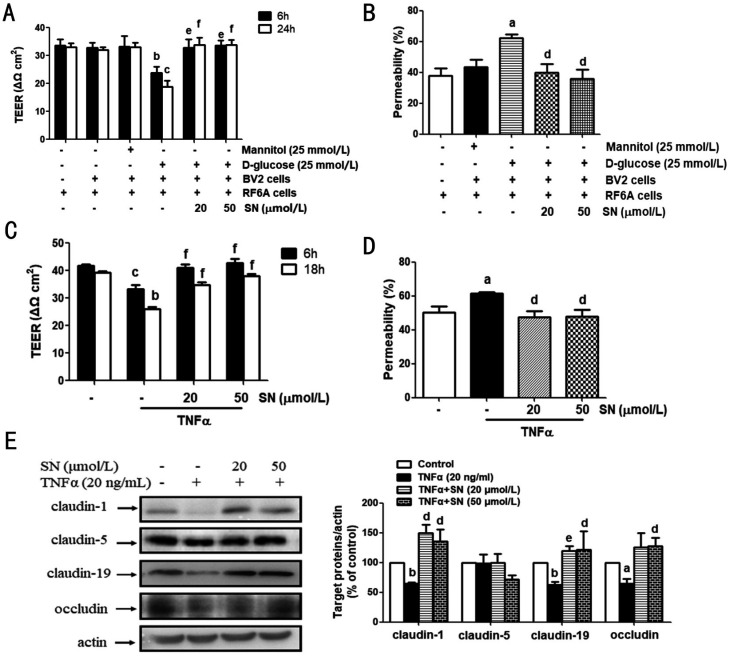
As shown in Figure 4A, when incubating with D-glucose-treated BV2 cells, TEER value was obviously decreased in RF6A cells, and SN (20, 50 mmol/L) rescued this decrease (Figure 4A). When RF/6A cells were incubated with D-glucose-treated BV2 cells for 24h, the FITC-dextran leakage in RF/6A cells was obviously up-regulated. SN (20, 50 mmol/L) reduced this increased leakage (Figure 4B). SN (20, 50 mmol/L) also reversed the TNFα-induced reduction of TEER value and increase of FITC-dextran leakage in RF/6A cells (Figure 4C-4D). Meanwhile, SN (20, 50 mmol/L) restored the TNFα-induced decreased protein expression of claudin-1 and -19 in RF/6A cells (Figure 4E). SN (50 mmol/L) increased the TNFα-induced decreased expression of occludin in RF/6A cells (Figure 4E). SN (20, 50 mmol/L) and TNFα both had no obvious effects on cellular claudin-5 expression (Figure 4E).
Figure 4. SN rescued the D-glucose-stimulated BV2 cells or TNFα-induced iBRB injury in RF/6A cells.
A, B: Upper-chamber seeded with RF/6A cells and lower-chamber seeded with BV2 cells in 24-well plates composed a co-cultured environment, and 6h before the D-glucose (25 mmol/L) stimulation on BV2 cells, SN (20, 50 µmol/L) was added into the bottom of transwells for pre-treatment. TEER (n=3) and FITC-dextran leakage (n=4) were determined. C, D: TNFα (20 ng/mL) was added into the plates under the chambers, and RF/6A cells were cultured in the upper-chamber with the pretreatment with or without SN (20, 50 mmol/L) for 6h. TEER (n=3) and FITC-dextran leakage (n=4) were detected. E: The same treatment as C&D was performed and the cell samples were collected for detecting the content of claudin-1 (n=4), claudin-5 (n=4), claudin-19 (n=3) and occludin (n=4) aP<0.05, bP<0.01, cP<0.001 versus control; dP<0.05, eP<0.01, fP<0.001 versus D-glucose-treated BV2 cells or TNFα.
When incubating with D-glucose-treated BV2 cells, TEER value was obviously decreased in HRECs, and SN (20, 50 mmol/L) enhanced this decrease (Figure 5A). When HRECs cells were incubated with D-glucose-treated BV2 cells for 24h, the FITC-dextran leakage in HRECs cells was obviously up-regulated. SN (20, 50 mmol/L) reduced this increased leakage (Figure 5B). Next, TEER value decreased when HRECs were stimulated with TNFα for 6 or 18h, while SN (20, 50 mmol/L) rescued this decrease (Figure 5C). And TNFa caused the increase of the FITC-dextran leakage in HRECs. However, SN (20, 50 mmol/L) reduced this increase (Figure 5D). Besides, induced by TNFα (20 ng/mL), the protein level of claudin-1, -19 and occludin decreased in HRECs, but SN (50 mmol/L) restored this decrease (Figure 5E). SN (20 mmol/L) also restored the TNFα-induced decreased expression of claudin-19 in HRECs (Figure 5E). SN (20, 50 mmol/L) and TNFα also had no obvious effects on cellular claudin-5 expression (Figure 5E).
Figure 5. SN rescued the D-glucose-stimulated BV2 cells or TNFα-initiated iBRB injury in HRECs.
A, B: Upper-chamber seeded with HREC cells and lower-chamber seeded with BV2 cells in 24-well plates composed a co-cultured environment, and 6h before the D-glucose (25 mmol/L) stimulation on BV2 cells, SN (20, 50 µmol/L) was added into the bottom of transwells for pre-treatment. TEER (A) (n=3) and FITC-dextran leakage (B) (n=4) were determined. C, D: TNFα (20 ng/mL) was added into the plates under the chambers, and HRECs were cultured in the upper-chamber with the pretreatment with or without SN (20, 50 mmol/L) for 6h. TEER (n=3; C) and FITC-dextran leakage (n=4; D) were detected. E: The same treatment as C&D was performed and the cell samples were collected for detecting the content of claudin-1 (n=3), claudin-5 (n=4), claudin-19 (n=3) and occludin (n=3) aP<0.05, bP<0.01, cP<0.001 versus control; dP<0.05, eP<0.01, fP<0.001 versus D-glucose-treated BV2 cells or TNFα.
Scutellarein Rescued the IL-1β or IL-6-mediated iBRB Injury
Both IL-1β and IL-6 down-regulated the TEER value of HRECs. However, SN (20, 50 mmol/L) rescued this decrease (Figure 6A-6B). Similarly, both IL-1β and IL-6 accelerated the leakage of FITC-dextran in HRECs, while SN (20, 50 mmol/L) also reversed this (Figure 6C-6D).
Figure 6. SN rescued the IL-1β or IL-6-mediated iBRB injury in HRECs.
A, B: IL-1β (20 ng/mL) was added into the plates under the chambers, and HRECs were cultured in the upper-chamber with the pretreatment with or without SN (20, 50 mmol/L) for 6h. A: TEER was detected (n=4). B: FITC-dextran leakage was detected (n=3). C, D: IL-6 (20 ng/mL) was added into the plates under the chambers, and HRECs were cultured in the upper-chamber with the pretreatment with or without SN (20, 50 mmol/L) for 6h. C: TEER was detected (n=4). D: FITC-dextran leakage was detected (n=3) aP<0.05, cP<0.001 versus control; dP<0.05, eP<0.01, versus IL-1β or IL-6.
DISCUSSION
The persistent hyperglycemia will destroy the homeostasis of circulatory system, so there are many serious microvascular diabetic complications including DR[14]–[15]. Microglia cells are the long-living resident cells regulating immune response in central nervous system (CNS). Thus it can be seen that the activation of microglia cells is considered to be closely linked to the progression of DR[16]–[18]. SN abrogated the hyperglycemia-induced microglia cells activation in vitro, which may be a useful help for its amelioration of DR.
NFκB is critically involved in regulating inflammatory responses by inducing the expression of various pro-inflammatory mediators, cytokines, etc[19]–[20]. Next, we detected the inhibitory effects provided by SN against hyperglycemia-induced NFκB activation in microglia BV2 cells. Our results showed that SN reduced the enhanced IKK phosphorylation and the subsequent nuclear accumulation of NFκBp65 in BV2 cells incubated with high concentration of D-glucose. Moreover, SN also obviously decreased the elevated expression of TNFα, IL-1β and IL-6. ERK1/2 is a subfamily member of mitogen-activated protein kinases, and its phosphorylation will lead to the activation of microglia cells in both acute and chronic CNS diseases including Alzheimer's disease and stroke[21], and also in DR development[12],[22]–[23]. Additionally, SN also abrogated ERK1/2 activation in BV2 cells induced by high concentration of D-glucose. The above results suggest that SN abrogated microglia cells activation and the subsequent TNFα release from hyperglycemia-stimulated microglia cells, which may contribute to the SN-provided improvement of DR.
Our previous study has shown that hyperglycemia-stimulated microglia BV2 cells caused obvious damage on both iBRB and oBRB[12],[22]. In this study, the hyperglycemia-stimulated microglia BV2 cells caused obvious damage on iBRB in vitro when retinal endothelial cells (including RF/6A cells and HRECs) were co-cultured with BV2 cells stimulated with D-glucose. However, SN rescued this iBRB dysfunction in vitro. TNFα, IL-1β and IL-6 are main pro-inflammatory cytokines released from the activated microglia cells, and previous studies showed that TNFα induced iBRB dysfunction[24]. In this study, TNFα was found to induce obvious injury of iBRB in vitro. Additionally, IL-1β and IL-6 also induced iBRB injury in vitro. However, SN restored the iBRB dysfunction induced by TNFα, IL-1β or IL-6 in vitro. All these results clearly demonstrated that SN rescued the iBRB injury induced by hyperglycemia-stimulated microglia cells or pro-inflammatory cytokines such as TNFα, IL-1β and IL-6.
TJs are mainly composed of junctional adhesion molecules, occludin, claudins, zonula occludens-1 and -2, and TJs integrity is critical for maintaining the normal physiological function of iBRB[3]–[4]. Previous studies have already shown that TNFα and IL-1β caused the reduced expression of TJs and thus led to the breakdown and unbalance of BRB[25]–[26]. Claudin-1, -5 and -19 are classical claudin proteins generally expressed in BRB, and their decreased expression was associated with the development of DR[3]–[4],[27]. We found that SN restored the down-regulated expression of claudin-1 and -19 in TNFα-stimulated retinal endothelial cells. However, the expression of claudin-5 was not changed in TNFα-stimulated retinal endothelial cells. The reduced expression of occludin has already been found in BRB damage during DR development[28]–[29]. TNFα reduced occludin expression in retinal endothelial cells, but SN restored the reduced occludin expression induced by TNFα in retinal endothelial cells. These above results imply that SN maintains BRB integrity by rescuing the TNFα-induced decreased expression of occludin, claudin-1 and -19.
In summary, our results demonstrate that natural product SN alleviated iBRB dysfunction mediated by hyperglycemia-stimulated microglia cells. The proposed engaged mechanism may be that SN abrogates the activation of ERK1/2-NFκB-initiated signaling cascade in hyperglycemia-stimulated microglia cells and decreased the subsequent TNFα, IL-1β or IL-6 expression in microglia cells. SN also directly rescued iBRB damage induced by pro-inflammatory cytokines by maintaining the integrity of TJs. Our study suggests that SN has a good prospect of further research and development for DR treatment.
Acknowledgments
Foundations: Supported by the National Key Research and Development Program of China (No.2018YFC1707302); National Natural Science Foundation of China (No.81960748).
Conflicts of Interest: Li H, None; Mei XY, None; Wang MN, None; Zhang TY, None; Zhang Y, None; Lu B, None; Sheng YC, None.
REFERENCES
- 1.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 2.Kubo Y, Seko N, Usui T, Akanuma SI, Hosoya KI. Lysosomal trapping is present in retinal capillary endothelial cells: insight into its influence on cationic drug transport at the inner blood-retinal barrier. Biol Pharm Bull. 2016;39(8):1319–1324. doi: 10.1248/bpb.b16-00140. [DOI] [PubMed] [Google Scholar]
- 3.Hosoya KI, Tachikawa M. Advances in Experimental Medicine and Biology. New York, NY: Springer New York; 2013. The inner blood-retinal barrier; pp. 85–104. [PubMed] [Google Scholar]
- 4.Campbell M, Humphries P. Advances in Experimental Medicine and Biology. New York, NY: Springer New York; 2013. The blood-retina barrier; pp. 70–84. [PubMed] [Google Scholar]
- 5.Coughlin BA, Feenstra DJ, Mohr S. Müller cells and diabetic retinopathy. Vis Res. 2017;139:93–100. doi: 10.1016/j.visres.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng CY, Hu CC, Yang HJ, Lee MC, Kao ES. Inhibitory effects of scutellarein on proliferation of human lung cancer A549 cells through ERK and NFκB mediated by the EGFR pathway. Chin J Physiol. 2014;57(4):182–187. doi: 10.4077/CJP.2014.BAC200. [DOI] [PubMed] [Google Scholar]
- 7.Tang H, Tang YP, Li NG, Shi QP, Guo JM, Shang EX, Duan JN. Neuroprotective effects of scutellarin and scutellarein on repeatedly cerebral ischemia-reperfusion in rats. Pharmacol Biochem Behav. 2014;118:51–59. doi: 10.1016/j.pbb.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Shi XJ, Chen GF, Liu XQ, Qiu Y, Yang SZ, Zhang Y, Fang XX, Zhang C, Liu XQ. Scutellarein inhibits cancer cell metastasis in vitro and attenuates the development of fibrosarcoma in vivo. Int J Mol Med. 2015;35(1):31–38. doi: 10.3892/ijmm.2014.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sung NY, Kim MY, Cho JY. Scutellarein reduces inflammatory responses by inhibiting src kinase activity. Korean J Physiol Pharmacol. 2015;19(5):441–449. doi: 10.4196/kjpp.2015.19.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo F, Yang F, Zhu YH. Scutellarein from Scutellaria barbata induces apoptosis of human colon cancer HCT116 cells through the ROS-mediated mitochondria-dependent pathway. Nat Prod Res. 2019;33(16):2372–2375. doi: 10.1080/14786419.2018.1440230. [DOI] [PubMed] [Google Scholar]
- 11.Gao R, Zhu BH, Tang SB, Wang JF, Ren J. Scutellarein inhibits hypoxia- and moderately-high glucose-induced proliferation and VEGF expression in human retinal endothelial cells. Acta Pharmacol Sin. 2008;29(6):707. doi: 10.1111/j.1745-7254.2008.00797.x. [DOI] [PubMed] [Google Scholar]
- 12.Mei X, Zhang T, Ouyang H, Lu B, Wang ZT, Ji L. Scutellarin alleviates blood-retina-barrier oxidative stress injury initiated by activated microglia cells during the development of diabetic retinopathy. Biochem Pharmacol. 2019;159:82–95. doi: 10.1016/j.bcp.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Ahmed Z, Shaw G, Sharma VP, Yang C, McGowan E, Dickson DW. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J Histochem Cytochem. 2007;55(7):687–700. doi: 10.1369/jhc.6A7156.2007. [DOI] [PubMed] [Google Scholar]
- 14.Frank RN. Diabetic retinopathy. N Engl J Med. 2004;350(1):48–58. doi: 10.1056/NEJMra021678. [DOI] [PubMed] [Google Scholar]
- 15.Forbes JM, Cooper ME. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 16.Yang XG, Huo FQ, Liu B, Liu J, Chen T, Li JP, Zhu ZQ, Lv B. Crocin inhibits oxidative stress and pro-inflammatory response of microglial cells associated with diabetic retinopathy through the activation of PI3K/Akt signaling pathway. J Mol Neurosci. 2017;61(4):581–589. doi: 10.1007/s12031-017-0899-8. [DOI] [PubMed] [Google Scholar]
- 17.Zhu SH, Liu BQ, Hao MJ, Fan YX, Qian C, Teng P, Zhou XW, Hu L, Liu WT, Yuan ZL, Li QP. Paeoniflorin suppressed high glucose-induced retinal microglia MMP-9 expression and inflammatory response via inhibition of TLR4/NF-κB pathway through upregulation of SOCS3 in diabetic retinopathy. Inflammation. 2017;40(5):1475–1486. doi: 10.1007/s10753-017-0571-z. [DOI] [PubMed] [Google Scholar]
- 18.Grigsby JG, Cardona SM, Pouw CE, Muniz A, Mendiola AS, Tsin ATC, Allen DM, Cardona AE. The role of microglia in diabetic retinopathy. J Ophthalmol. 2014;2014:705783. doi: 10.1155/2014/705783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baeuerle PA, Henkel T. Function and activation of NF-kappa B in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 20.Shih RH, Wang CY, Yang CM. NF-kappaB signaling pathways in neurological inflammation: a mini review. Front Mol Neurosci. 2015;8:77. doi: 10.3389/fnmol.2015.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koistinaho M, Koistinaho J. Role of p38 and p44/42 mitogen-activated protein kinases in microglia. Glia. 2002;40(2):175–183. doi: 10.1002/glia.10151. [DOI] [PubMed] [Google Scholar]
- 22.Zhang T, Ouyang H, Mei X, Lu B, Yu ZY, Chen KX, Wang ZT, Ji L. Erianin alleviates diabetic retinopathy by reducing retinal inflammation initiated by microglial cells via inhibiting hyperglycemia-mediated ERK1/2-NF-κB signaling pathway. FASEB J. 2019;33(11):11776–11790. doi: 10.1096/fj.201802614RRR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang AL, Yu ACH, He QH, Zhu XA, Tso MOM. AGEs mediated expression and secretion of TNF alpha in rat retinal microglia. Exp Eye Res. 2007;84(5):905–913. doi: 10.1016/j.exer.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Huang H, Gandhi JK, Zhong XF, Wei YH, Gong JS, Duh EJ, Vinores SA. TNFalpha is required for late BRB breakdown in diabetic retinopathy, and its inhibition prevents leukostasis and protects vessels and neurons from apoptosis. Investig Ophthalmol Vis Sci. 2011;52(3):1336–1344. doi: 10.1167/iovs.10-5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aveleira CA, Lin CM, Abcouwer SF, Ambrósio AF, Antonetti DA. TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes. 2010;59(11):2872–2882. doi: 10.2337/db09-1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van der Wijk AE, Vogels IMC, van Noorden CJF, Klaassen I, Schlingemann RO. TNFα-induced disruption of the blood-retinal barrier in vitro is regulated by intracellular 3′, 5′-cyclic adenosine monophosphate levels. Investig Ophthalmol Vis Sci. 2017;58(9):3496–3505. doi: 10.1167/iovs.16-21091. [DOI] [PubMed] [Google Scholar]
- 27.Gonçalves A, Ambrósio AF, Fernandes R. Regulation of claudins in blood-tissue barriers under physiological and pathological states. Tissue Barriers. 2013;1(3):e24782. doi: 10.4161/tisb.24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu ZY, Gong CY, Lu B, Yang L, Sheng YC, Ji LL, Wang ZT. Dendrobium chrysotoxum Lindl. alleviates diabetic retinopathy by preventing retinal inflammation and tight junction protein decrease. Exp Diabetes Res. 2015;2015:518317. doi: 10.1155/2015/518317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang YD, Liu L, Steinle JJ. Compound 49b regulates ZO-1 and occludin levels in human retinal endothelial cells and in mouse retinal vasculature. Investig Ophthalmol Vis Sci. 2017;58(1):185–189. doi: 10.1167/iovs.16-20412. [DOI] [PMC free article] [PubMed] [Google Scholar]