SUMMARY
Stable representations of past experience are thought to depend on processes that unfold after events are initially encoded into memory. Post-encoding reactivation and hippocampal-cortical interactions are leading candidate mechanisms thought to support memory retention and stabilization across hippocampal-cortical networks. While putative consolidation mechanisms have been observed during sleep and periods of awake rest, the direct causal contribution of awake consolidation mechanisms to later behavior are unclear, especially in humans. Moreover, it has been argued that observations of putative consolidation processes are epiphenomenal and not causally important, yet there are few tools to test the functional contribution of these mechanisms in humans. Here, we combined Transcranial Magnetic Stimulation (TMS) and fMRI to test the role of awake consolidation processes by targeting hippocampal interactions with lateral occipital cortex (LOC). We applied theta-burst TMS to LOC (and a Control site) to interfere with an extended time window (approx. 30–50 minutes) after memory encoding. Behaviorally, post-encoding TMS to LOC selectively impaired associative memory retention compared to multiple control conditions. In our Control TMS condition, we replicated prior reports of post-encoding reactivation and memory-related hippocampal-LOC interactions during periods of awake rest using fMRI. However, post-encoding LOC TMS reduced these processes, such that post-encoding reactivation in LOC and memory-related hippocampal-LOC functional connectivity were no longer present. By targeting and manipulating post-encoding neural processes, these findings highlight the direct contribution of awake time periods to episodic memory consolidation. This combined TMS-fMRI approach provides an opportunity for causal manipulations of human memory consolidation.
Keywords: resting state, memory consolidation, memory reactivation, hippocampal-cortical interactions, transcranial magnetic stimulation, causal manipulations
Graphical Abstract
eTOC blurb:
Tambini and D’Esposito test the role of awake post-encoding processes in episodic memory consolidation by performing Theta-burst Transcranial Magnetic Stimulation after memory encoding. Stimulation to Lateral Occipital Cortex impairs associative memory retention and disrupts post-encoding reactivation and hippocampal-cortical interactions.
INTRODUCTION
After information is initially encoded into memory, post-encoding processes are thought to stabilize representations of past experience in the service of enduring memory formation [1]. While the hippocampus plays a critical role in the initial encoding of episodic memories, over time, memory representations are reorganized across hippocampal-cortical networks [2–5]. Memory reorganization, or systems-level consolidation, is thought to be mediated by post-encoding mechanisms: the ‘replay’ or reactivation of memory representations in conjunction with coordinated hippocampal-cortical interactions [6–9]. These processes are thought to occur in an off-line fashion, or during time periods when the hippocampus is not engaged in encoding new information into memory, such as sleep or awake, quiet restful periods [10,11].
To examine the contribution of post-encoding reactivation and hippocampal-cortical interactions to memory consolidation, many studies have established correlational links between the relative strength of post-encoding processes and subsequent memory. Specifically, higher levels of spontaneous reactivation and experience-dependent changes in hippocampal-cortical interactions have been positively related to subsequent memory [12–20] (for a review see [21]) and are modulated by factors that enhance memory, such as reward or novelty [22–24]. Although correlational relationships are helpful for assessing the potential contribution of these processes, they do not alone demonstrate that post-encoding mechanisms directly support memory. For example, it is possible that post-encoding consolidation measures reflect encoding-related activity that is itself predictive of later memory and simply persists in time (as suggested by [25]). One approach to addressing this issue is to assess correlations between post-encoding activity and later memory that are statistically independent from encoding activity [15,17]. However, targeted manipulations of post-encoding processes provide a powerful method to test their direct contribution to later behavior.
Few approaches exist to target and manipulate post-encoding reactivation and hippocampal-cortical interactions in humans. In rodent studies, the causal role of reactivation has been tested by influencing neural activity during sharp-wave ripple (SWR) events, when hippocampal reactivation typically occurs. Disrupting SWRs impairs learning [26–28] and degrades subsequent hippocampal reinstatement [29,30], while prolonging SWRs improves memory [31], highlighting the importance of post-encoding SWR events. Prior work seeking to causally influence human consolidation has primarily targeted electrophysiological activity during non-rapid-eye-movement sleep, implicating slow-wave activity and spindles in memory retention (e.g. [32–34]). However, such studies primarily record scalp electroencephalography and cannot typically target or measure hippocampal-cortical interactions or reactivation (but see [35]). While other approaches seek to induce or bias reactivation during sleep via the presentation of external stimuli (targeted memory reactivation, [36,37]), this method typically cannot target neural processing in particular brain structures. Moreover, it has become apparent that post-encoding consolidation mechanisms are not limited to sleep but are also present during awake rest periods immediately after learning [21,38–40]. Yet, the causal contribution of awake hippocampal-cortical interactions to human memory consolidation has not been tested.
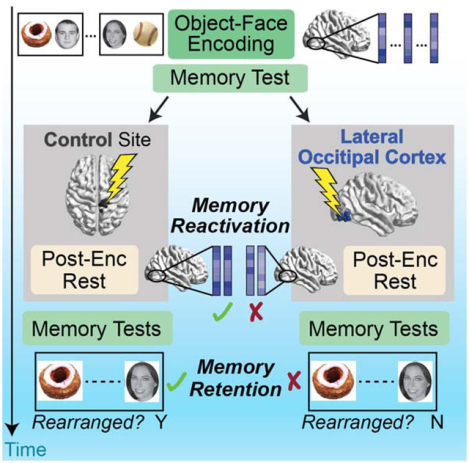
Here, we sought to target and test the role of post-encoding processes in human memory consolidation using a combined transcranial magnetic stimulation (TMS) and fMRI approach. We took advantage of prior work showing that representational regions in occipitotemporal cortex spontaneously reactivate representations of recently encoded stimuli and display post-encoding interactions with the hippocampus that are related to later memory [13,15,20,41–43]. To interfere with these processes, we applied inhibitory continuous theta-burst TMS (cTBS, [44]) to lateral occipital cortex (LOC) after participants incidentally encoded object-face pairs and before an extended post-encoding awake rest period (Figure 1). cTBS influences neural function for up to approximately 30–50 minutes after application [44–47], allowing us to interfere with an extended time window of tens of minutes after encoding. We predicted that interference with post-encoding processes, via TMS to LOC, would impair memory retention and disrupt fMRI evidence of memory consolidation during post-encoding rest (reactivation and hippocampal-cortical interactions). To test these predictions, post-encoding cTBS was applied to either right LOC or a Control site, surprise memory tests were given before TMS and after the influence of TMS subsided (Figure 1), and fMRI scanning was performed to assess consolidation measures. Lastly, we tested the hypothesis that if post-encoding interactions specifically support information encoded by the hippocampus, their disruption should preferentially impair the retention of associative versus item memories, as associative memory is most strongly supported by the hippocampus whereas item memory can be supported by extra-hippocampal structures [48–50].
Figure 1. Experimental Design.
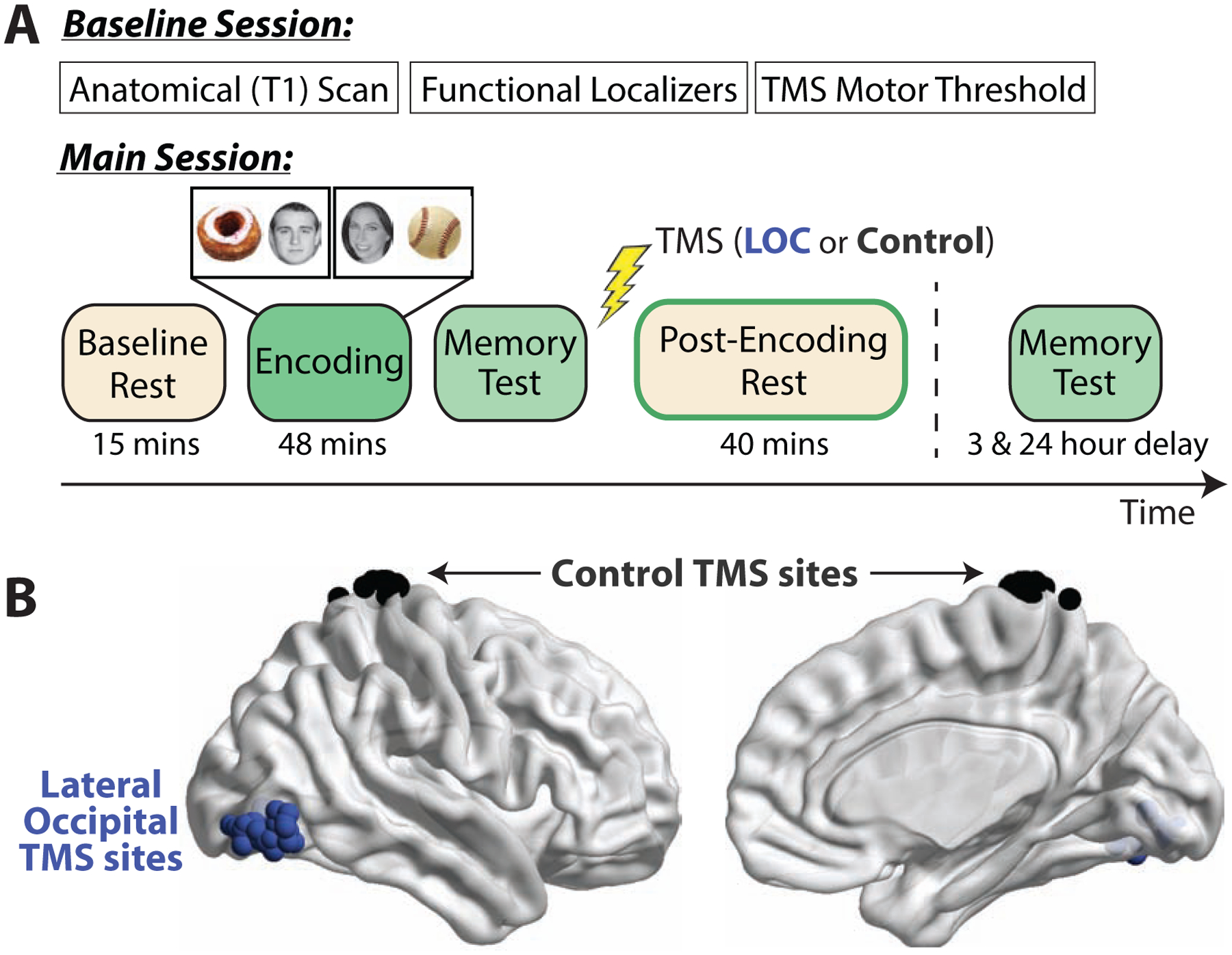
(A) Participants completed a Baseline Session and Main TMS-fMRI Session. Theta-burst TMS was administered after the Immediate memory test. Participants returned approximately three and 24 hours after TMS for surprise Delayed memory tests. A unique subset of stimuli were tested in each memory test. Memory tests were performed outside of the MRI scanner. (B) TMS sites (LOC, blue; Control, black) are shown in MNI space.
RESULTS
Memory Retention
To assess whether post-encoding TMS influenced memory retention, memory tests were administered before and after TMS (Immediate and Delayed tests, respectively). Associative and item memory were estimated separately to test whether TMS preferentially influenced associative versus item memory retention. We first established that Immediate (pre-TMS) associative and item memory did not differ between groups (Figure S1, Tables S1–2).
After verifying comparable pre-TMS memory, we assessed whether memory retention (Delayed relative to Immediate memory) was influenced by TMS and whether this differed based on the time of memory testing. A main effect of TMS site was found on associative memory retention (F1,56 = 8.06, P = .006, ηp2 = .13), reflecting reduced associative memory retention after post-encoding TMS to LOC versus Control TMS (Figure 2A). No significant interaction was found between TMS and the time of Delayed memory testing (same-day versus next-day; F1,56 = 2.25, P = .14, ηp2 = .04). As shown in Figure 2A (left bars), LOC TMS significantly impaired associative memory retention during same-day memory testing compared to Control TMS (t56 = 3.11, P = .003, Cohen’s d = .82) and the No TMS group (t53 = 2.61, P = .012, Cohen’s d = .71). Qualitatively, associative memory retention (.45 ± .12) three hours after LOC TMS decayed to levels of memory retention 24 hours later in the control groups (Control TMS, .45 ± .11; No TMS, .43 ± .07), which highlights the enhanced rate of forgetting associated with post-encoding LOC TMS. This reduction was also present when examining unnormalized corrected memory (Figure S1), rather than retention (Delayed normalized by Immediate memory).
Figure 2. Memory retention (delayed divided by immediate memory accuracy).
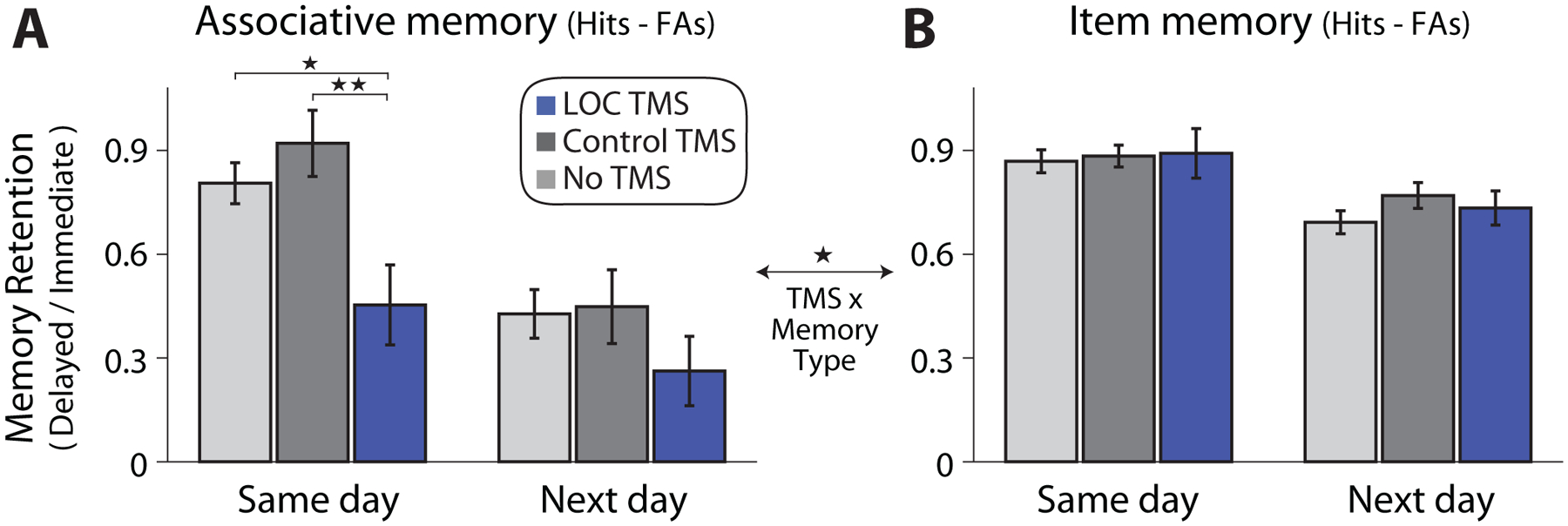
(A) Associative memory (associative hits minus associative false alarms) retention differed across groups, with reduced retention after LOC TMS. (B) No reliable influence of TMS was found on Item memory (item hits minus false alarms) retention. A significant TMS site by memory-type (associative versus item retention) interaction was found, indicated by the comparison across plots. Error bars show standard error of the mean. *P < .05, **P < .005. See also Figures S1, S2, Tables S1, S2.
Although LOC TMS numerically reduced associative memory retention during next-day testing (Figure 2A, right bars), this was not significantly different from Control TMS (t56 = 1.27, P = .21), the No TMS group (t53 = 1.32, P = .19), or when compared to both control conditions (t82 = 1.53, P = .13). To better understand whether reductions in associative memory retention due to LOC TMS might have persisted during next-day testing, we asked whether participants with the lowest memory retention during same-day testing also showed reduced retention during next-day testing. Next-day associative memory (both retention and unnormalized memory accuracy) were reduced after LOC TMS for participants with the lowest associative memory retention during same-day testing, compared to both control groups (Figure S2). These results suggest that while LOC TMS did not, at the group-level, reduce associative memory one day later, participants with the strongest initial impairments in associative memory retention continued to show decreased associative memory 24 hours later. As expected, no differences in associative memory retention were found between Control TMS and No TMS groups (same-day testing, t53 = 1.00, P = .32; next-day testing, t53 = .16, P = .87).
In contrast to the influence of post-encoding LOC TMS on associative memory, item memory was not reliably affected by TMS (Figure 2B and S1). A two-way ANOVA examining item memory retention showed no main effect of TMS site (F1,56 = .046, P = .83) or interaction between TMS site and memory test (F1,56 = .68, P = .41). Item memory retention also did not differ between either TMS group and the No TMS condition (LOC vs. No TMS, same-day, t53 = .28, P = .78, next-day, t53 = .50, P = .68; Control vs. No TMS, same-day, t53 = .33, P = .74, next-day, t53 = 1.53, P = .13). A lack of influence of post-encoding TMS on item memory was also found when examining object and face memory separately (face retention, main effect of TMS: F1,56 = .33, P = .56, TMS by memory test interaction: F1,56 = 1.40, P = .24; object retention, main effect of TMS: F1,56 = 1.55, P = .22, TMS by memory test interaction: F1,56 = 1.37, P = .25).
We directly compared associative and item retention to test whether TMS preferentially influenced associative memory. A three-way mixed-effects ANOVA showed a significant interaction between memory-type (associative vs. item retention) and TMS site (F1,56 = 5.37, P = .024, ηp2 = .09), indicating a differential influence of post-encoding TMS on associative versus item retention. In conjunction with the prior findings, these results indicate that post-encoding TMS to LOC selectively impaired associative memory retention.
Reactivation Evidence in LOC
We next tested the prediction that post-encoding LOC TMS would impair reactivation of recently encoded stimuli, based on prior reports of multi-voxel reactivation of patterns corresponding to recently encoded visual stimuli in occipitotemporal cortex [19,41,42,51,52]. We first focused on object and face patterns within LOC, using a classifier approach and pattern similarity (see below for the similarity approach). Classifiers were trained in each participant to distinguish viewing faces and objects (versus scrambled images) using activation patterns from functional localizer scans and applied to individual time-points of the rest scans (Figure 3A). Before considering reactivation, we ensured that classifiers reliably detected face and object information content. Average cross-validated accuracy within the functional localizer scans was high (face-classifier accuracy = 94 ± 2.1%, object-classifier accuracy = 88 ± 1.7%) and well above chance (face: t51 = 20.7, P = 1.7 × 10−26, object: t51 = 22.8, P = 2.1 × 10−28). When applied to the encoding data, classifier outputs were modulated in a reliable, trial-evoked manner (Figure 3B, mean trial-evoked change in face and object classification = .46 ± .02, t51 = 20.3, P = 4.6 × 10−26). Similar trial-evoked modulation of classifier outputs during encoding was present across TMS groups (comparison between LOC versus Control TMS, t50 = 1.30, P = .20).
Figure 3. Post-encoding reactivation in LOC.
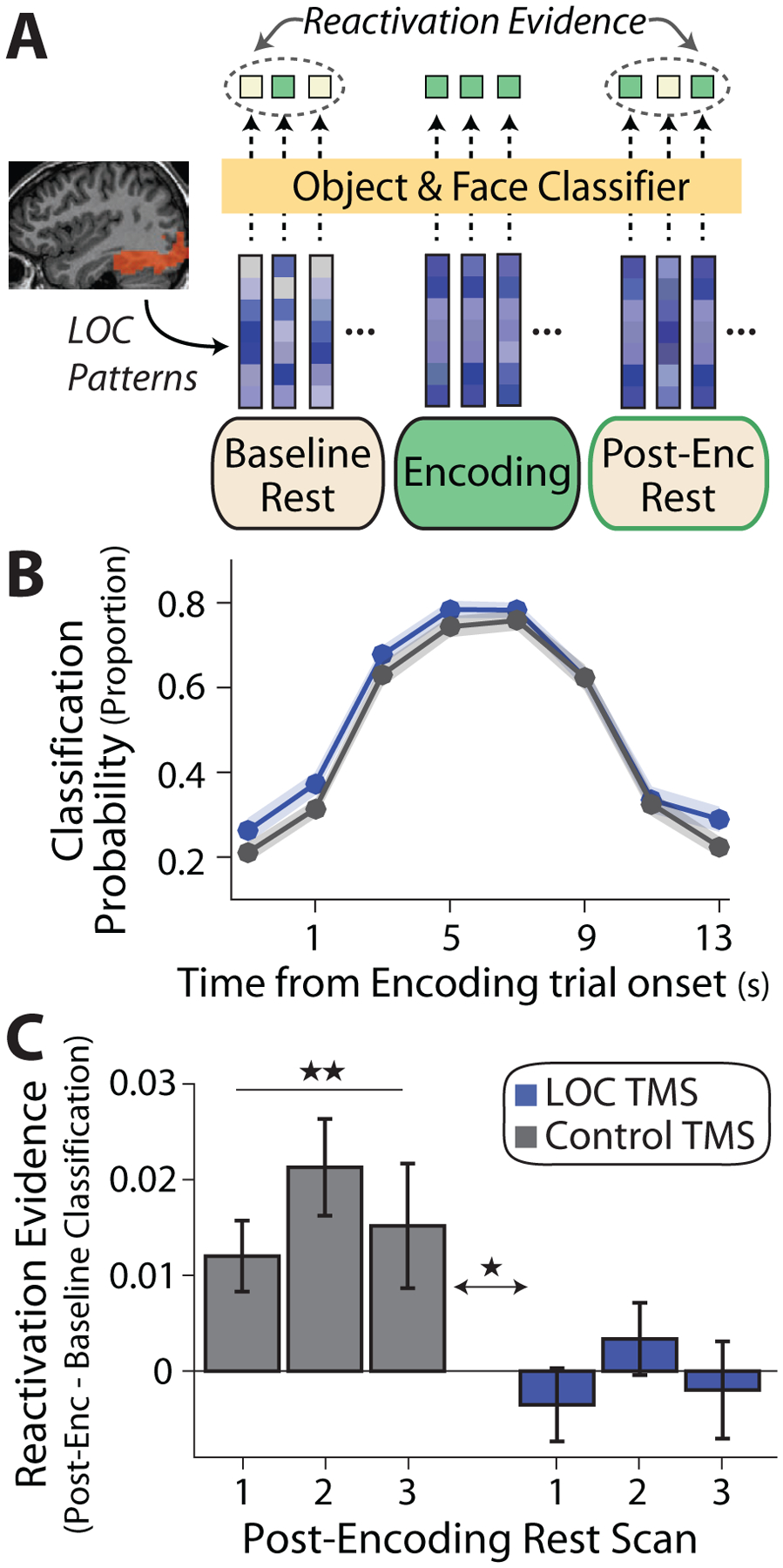
(A) Analysis approach: object and face classifiers (yellow box) were trained in each participant (using data from functional localizer scans) and LOC patterns were extracted from volumes during rest and encoding were fed into the classifiers (depicted as vectors). Reactivation evidence was operationalized as an increase in the proportion of volumes classified as a face or object from Baseline to Post-Encoding Rest scans (increase in green squares). (B) As expected, a stimulus-evoked increase in classifier evidence (proportion of volumes classified as a face or object) was found during object-face encoding in both TMS groups (LOC, blue; Control, gray). Shaded region represents standard error of the mean across participants. (C) Reactivation evidence or change in the proportion of time-points classified as a face or object from Baseline to each Post-Encoding Rest scan. An increase was found in the Control TMS group (gray bars) but not the LOC TMS group, with a significant difference between TMS groups. *P < .05, **P < .005. See also Figures S3, S4.
Classifiers were then applied to the rest scans and reactivation evidence was operationalized as an experience-dependent increase in the proportion of volumes classified as an object (or face) from Baseline to Post-Encoding Rest (Figure 3A). Note that nuisance signals were previously removed and time-points surrounding high motion events were excluded. In the Control TMS group, an increase in the proportion of time-points classified as a face or object from Baseline to Post-Encoding Rest was found (Figure 3C, grey bars, mean proportion across Post-Encoding Rest scans, t25 = 3.54, P = .0016, Cohen’s d = .69). This reactivation evidence after Control TMS did not reliably vary across the three Post-Encoding scans, as indicated by a non-significant main effect of rest scan (F2,50 = 1.88, P = .16).
In contrast to the Control TMS group, no reliable difference was found in the proportion of time-points classified as a face or object from Baseline to Post-Encoding Rest following LOC TMS (Figure 3C, blue bars, mean across Post-Encoding scans, t25 = .16, P = .87), and this did not vary across the three Post-Encoding scans (no main effect of rest scan, F2,50 = 1.09, P = .34). A mixed-effects ANOVA comparing reactivation evidence between TMS groups showed a main effect of TMS site (F1,50 = 6.90, P = .011, ηp2 = .12) and no interaction between TMS site and Post-Encoding Rest scan (F2,100 = .31, P = .73). These results demonstrate that LOC TMS impaired the reactivation of face and object representations in LOC (relative to Control TMS), paralleling the TMS-related impairment in associative memory retention.
We also examined reactivation using a similarity-based approach to ensure that the results were not driven by the particular classification method. Multi-voxel template patterns associated with viewing faces or objects (relative to fixation) were created for each participant and compared to individual time-points during the rest scans to identify a subset of time-points showing high similarity (correlation). An increase in the proportion of high similarity time-points was found between the Baseline and Post-Encoding Rest scans in the Control TMS group, while no reliable increase was found after LOC TMS across all Post-Encoding Rest scans, and reactivation evidence significantly differed between TMS groups (Figure S3). These findings further support the notion that post-encoding LOC TMS impaired the reactivation of object and face patterns within LOC.
Given reliable reactivation after Control TMS, we asked whether object and face patterns were reinstated during adjacent time-points, suggesting reactivation of object-face representations. As shown in Figure S4, temporally clustered object-face reactivation in LOC was present in the Control TMS group, which was significantly greater than in the LOC TMS group. These results suggest that object and face representations tended to be reactivated in a temporally clustered fashion, which was reduced by LOC TMS.
Reactivation Evidence in the Hippocampus
Beyond LOC, the direct site of stimulation, we examined whether TMS impaired post-encoding reactivation in the hippocampus using a previously developed PCA-based approach [14]. PCA was performed to derive the strongest multi-voxel hippocampal patterns (PCs) present during encoding for each participant (Figure 4A) and reactivation was operationalized as an increase in the variance explained by encoding patterns from Baseline to Post-Encoding Rest. We first confirmed that the strongest PCs captured reliable activation during encoding (Figure 4A; PC-1, t51 = 7.10, P = 3.7 × 10−9; PC-2, t51 = 3.43, P = .0012; PC-3, t51 = 1.21, P = .23; PC-4, t51 = 1.41, P = .16), as shown previously [13, Figure 3].
Figure 4. Post-encoding reactivation in the hippocampus.
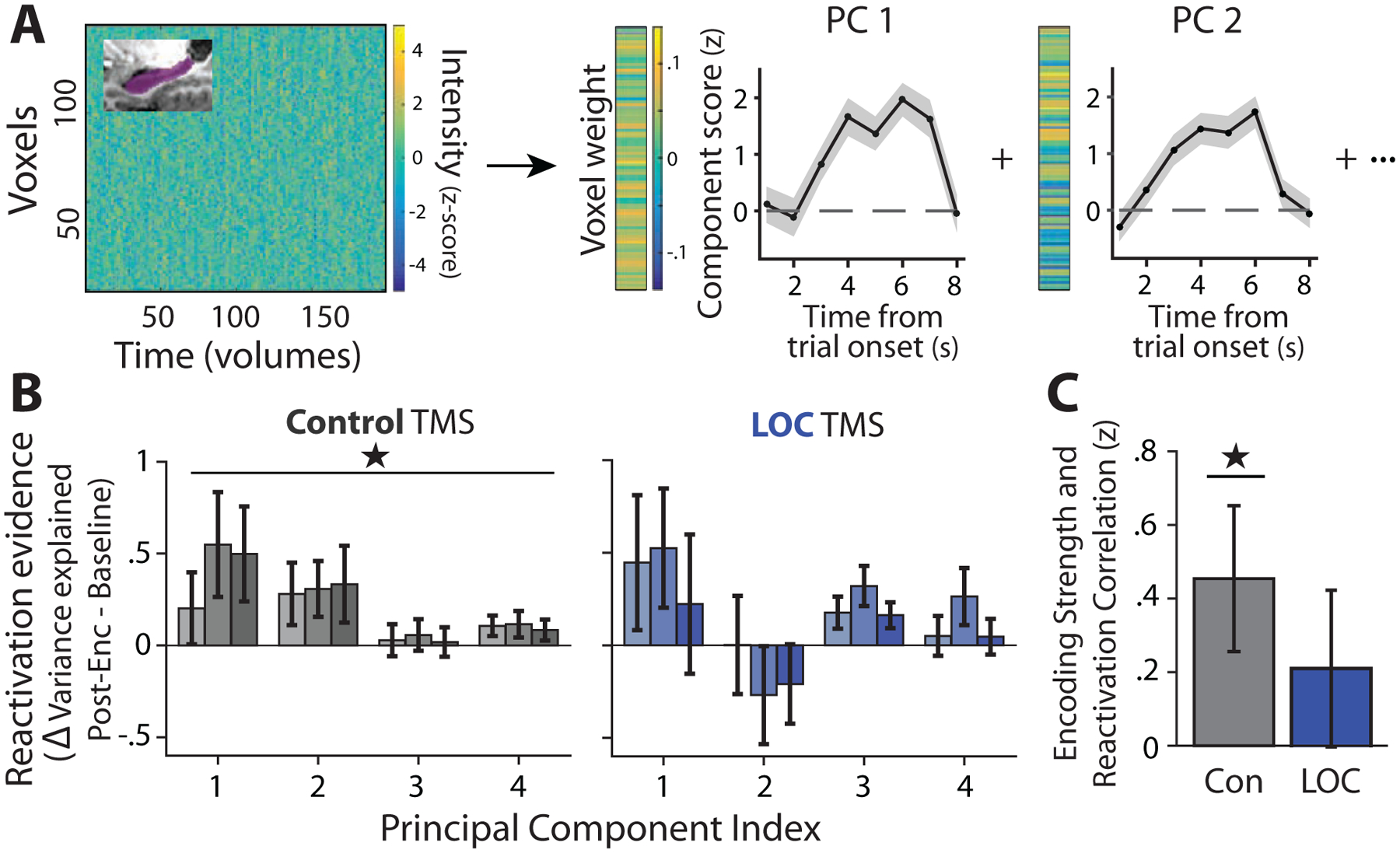
(A) Analysis approach: denoised hippocampal encoding patterns were extracted (matrix on the left) and PCA was performed on each participant’s data, resulting in principal component (PC) encoding patterns (vectors on the right). Trial-triggered averages of the temporal projection of encoding PCs showing stimulus-evoked activity are displayed to the right of PC patterns. Shaded region depicts standard error of the mean across trials. (B) Reactivation evidence (change in the proportion of variance explained by each encoding PC from Baseline to Post-Encoding Rest) is shown for the top four PCs for each TMS group and scan (lightest bar = first Post-Encoding scan, second bar = second Post-Encoding scan, darkest bar = third Post-Encoding scan). Reactivation evidence is present in the Control TMS group, but not the LOC TMS group. (C) Correlation (Fisher Z-transformed) between the strength of each hippocampal encoding PC (eigenvalue) and its reactivation evidence (change in variance explained from Baseline to the average across Post-Encoding Rest scans) was assessed for each participant. A significant correlation is found in the Control TMS group, but not in the LOC TMS group. *P < .05
When examining reactivation evidence for the top hippocampal PCs (1–4), an increase in the amount of variance explained by the top four encoding PCs was found from Baseline to Post-Encoding Rest in the Control TMS group (Figure 4B; average across Post-Encoding Rest scans; t25 = 2.81, P = .0095, Cohen’s d = .55). A repeated-measures ANOVA examining the change in variance explained (within-subjects factors of Post-Encoding scan and PC index) showed a significant intercept (F1,25 = 7.9, P = .0095), consistent with reactivation across all scans, and no reliable difference across the Post-Encoding scans (F2,50 = .72, P = .49). Similar to prior work, in the Control TMS group, the strength of each encoding PC, within each participant, was correlated with its persistence from Baseline to Post-Encoding Rest (Figure 4C, t25 = 2.20, P = .031). In the LOC TMS group, no change in the amount of variance explained by the top encoding PCs was found from Baseline to Post-Encoding Rest (Figure 4B; average across Post-Encoding scans; t25 = 1.04, P = .309), with no differences across the Post-Encoding scans (main effect of rest scan, F2,50 = 1.15, P = .33). Similarly, no consistent correlation between PC strength and Post-Encoding persistence was found after LOC TMS (Figure 4C, t25 = .98, P = .33). However, when we directly contrasted hippocampal reactivation evidence between TMS sites, no main effect of TMS (F1,25 = .194, P = .66) or interaction between TMS and other factors was found (TMS by rest scan, F2,100 = 1.004, P = .37; triple interaction, F6,300 = .90, P = .49; TMS by PC index, F3,150 = 1.72, P = .166). Likewise, correlations between encoding component strength and persistence did not differ between TMS groups (t50 = .84, P = .41). These results indicate that the Control TMS group showed expected hippocampal reactivation evidence during Post-Encoding Rest. Although no reliable hippocampal reactivation was found after LOC TMS, differences between TMS groups were not significant, making it unclear whether LOC TMS impaired hippocampal reactivation.
Hippocampal-LOC Interactions and Memory Retention
We next tested the prediction that post-encoding LOC TMS would disrupt experience-dependent changes in hippocampal interactions with representational regions in occipitotemporal cortex, which have been related to associative memory [13,15,20,41]. We isolated hippocampal regions that we expected to show memory-related changes in resting functional connectivity (FC) from subsequent memory contrasts. A region in the left posterior hippocampus (Figure 5) showed the strongest subsequent associative memory effect across both TMS groups (P < .001, uncorrected). Although no group-level changes in hippocampal-LOC FC were found in the Control TMS group across all Post-Encoding scans (Baseline vs. average Post-Encoding, t25 = 1.13, P = .27), an increase was found when comparing the second Post-Encoding scan to Baseline Rest (t25 = 2.36, P = .026). Furthermore, average changes in hippocampal-LOC FC across Post-Encoding Rest scans were positively related to same-day associative memory retention in the Control TMS group (Figure 5A, r = .40, t24 = 2.14, P = .042, robust regression used for all across-participant correlations). In contrast, no reliable relationship was found between hippocampal-LOC FC changes and associative memory retention after LOC TMS (Figure 5A, r = −.18, t24 = −.87, P = .39). Additionally, correlations between hippocampal-LOC FC changes and same-day associative memory retention were significantly different between TMS sites (Figure 5A, permutation test, P = .021).
Figure 5. Relationships between Post-Encoding measures and memory retention.
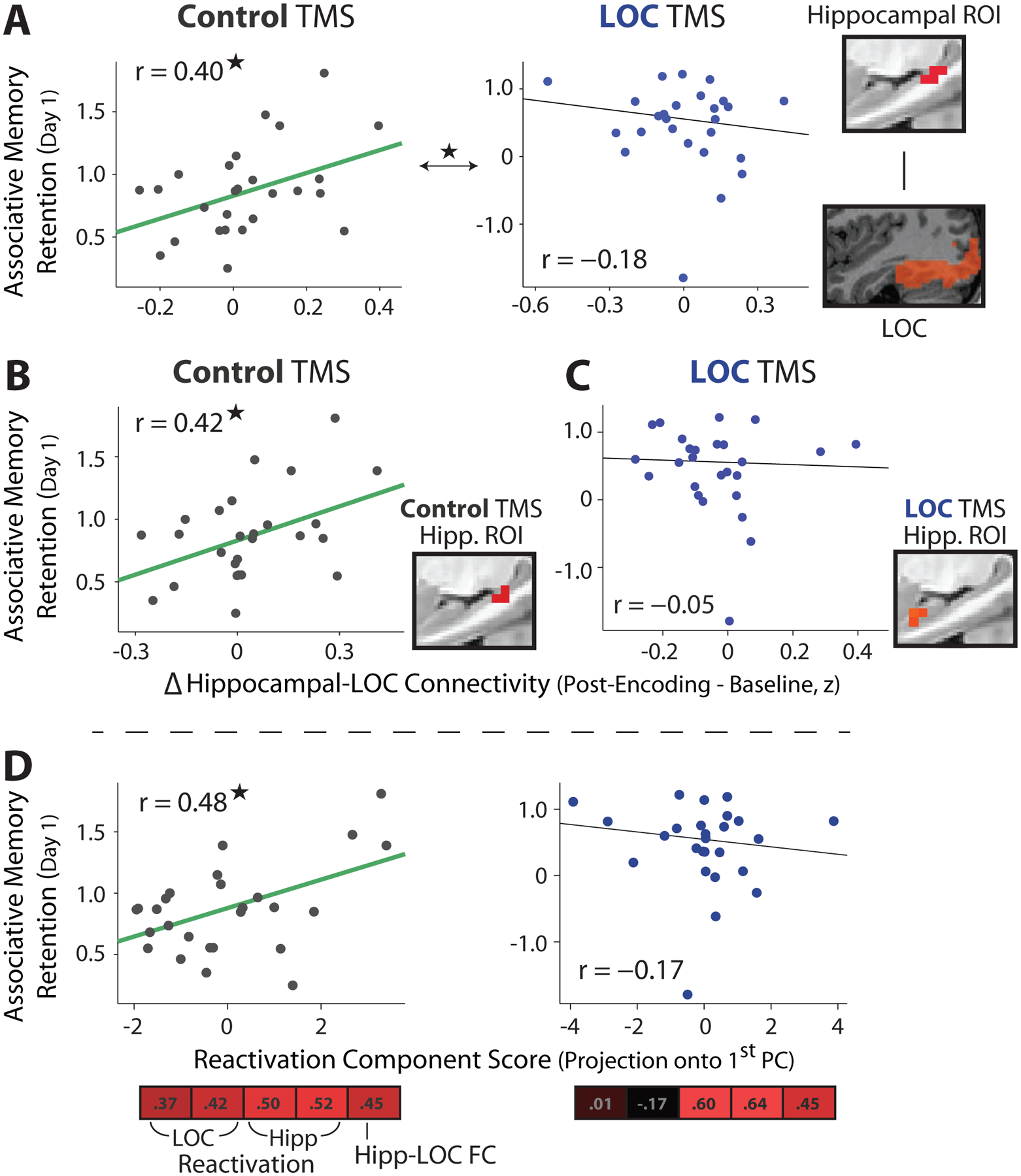
(A) Hippocampal ROI (right) defined from a subsequent associative memory contrast across all participants. Experience-dependent changes in hippocampal-LOC FC (from Baseline to the average across Post-Encoding Rest scans, Fisher Z-transformed) were related to same-day associative memory retention across participants in the Control TMS group (left), but not in the LOC TMS group (right). A significant difference was found between these correlations. Robust regression was used for all across-participant correlations. (B) The same as (A), using a hippocampal ROI defined from Control TMS participants. (C) The same as (A), using a hippocampal ROI defined from LOC TMS participants. (D) Summary score of reactivation measures was positively correlated with same-day associative memory retention in the Control TMS group, but not in the LOC TMS group. Summary reactivation score was derived from PCA on five measures: LOC reactivation (two measures), hippocampal reactivation (two measures), and changes in hippocampal-LOC FC. Vector below scatterplots depicts weighting of these measures to construct summary score (first PC). *P < .05
To assess whether this pattern of results was driven by the specific hippocampal ROI used (isolated from participants in both TMS groups), we defined hippocampal regions showing the strongest subsequent associative memory effects separately for each TMS group (Figure 5B,C). Similar to the prior results, changes in hippocampal-LOC FC from Baseline to Post-Encoding Rest in the Control TMS group were related to same-day associative memory retention (using the hippocampal ROI from the Control TMS group, 5B, r = .42, t24 = 2.26, P = .033). No reliable relationships were found in the LOC TMS group, when using a left anterior hippocampal region (which displayed the strongest subsequent associative memory effect, Figure 5C, r = −.05, t24 = −.24, P = .81) or other hippocampal ROIs isolated from the LOC TMS group (middle left hippocampus, r = −.12, t24 = −.55, P = .59; right anterior hippocampus, r = .14, t24 = .68, P = .50). Together with the prior results, these findings suggest that post-encoding LOC TMS disrupted relationships between hippocampal-LOC connectivity and associative memory retention.
Beyond relationships between hippocampal-LOC connectivity changes and same-day memory retention, we asked whether FC changes were related to overnight (next-day) memory retention. No significant relationships were found between changes in hippocampal-LOC FC and next-day associative memory retention in either TMS group (control: r = −.33, t24 = −1.67, P = .11; LOC: r = .06, t24 = .31, P = .76).
Relationships between Reactivation and Memory Retention
Outside of hippocampal-LOC interactions, we also assessed whether memory retention was related to the set of reactivation measures examined and whether TMS influenced these relationships. To do so, we extracted a dominant pattern that captured the most variance in reactivation measures across participants (using PCA). The strongest component positively weighted most measures in both TMS groups (Figure 5D) and accounted for substantial variance in the data (Control: 48%; LOC: 44%). Each participant’s reactivation summary or component score (projection onto the first PC) was positively correlated with same-day associative memory retention in the Control TMS group (Figure 5D, r =.48, t24 = 2.70, P = .012), but not in the LOC TMS group (r =−.17, t24 = −.80, P = .43), and these correlations were significantly different from each other (P = .014, permutation test). Similar to the hippocampal-LOC results, next-day associative memory retention was not reliably correlated with the reactivation score in either TMS group (Control: r = −.21, t24 = −1.04, P = .31; LOC: r = .21, t24 = .96, P = .35).
Given that the reactivation measures analyzed thus far were related to same-day, but not next-day memory retention, we performed exploratory analyses to ask whether post-encoding hippocampal interactions were related to next-day memory retention, and whether these were similar or distinct across TMS sites. In a whole-brain analysis, increases in hippocampal interactions with retrosplenial cortex (Figure 6A), from Baseline to Post-Encoding Rest, were positively related to next-day associative memory retention across participants in the Control TMS group (FWE-corrected). In the LOC TMS group, a region in right anterior temporal cortex survived FWE-correction (Figure 6A). These exploratory analyses suggest that post-encoding hippocampal interactions were related to next-day memory retention, with differing hippocampal interactions predicting memory in each TMS condition.
Figure 6. Exploratory analyses of whole-brain changes in hippocampal interactions related to next-day memory retention.
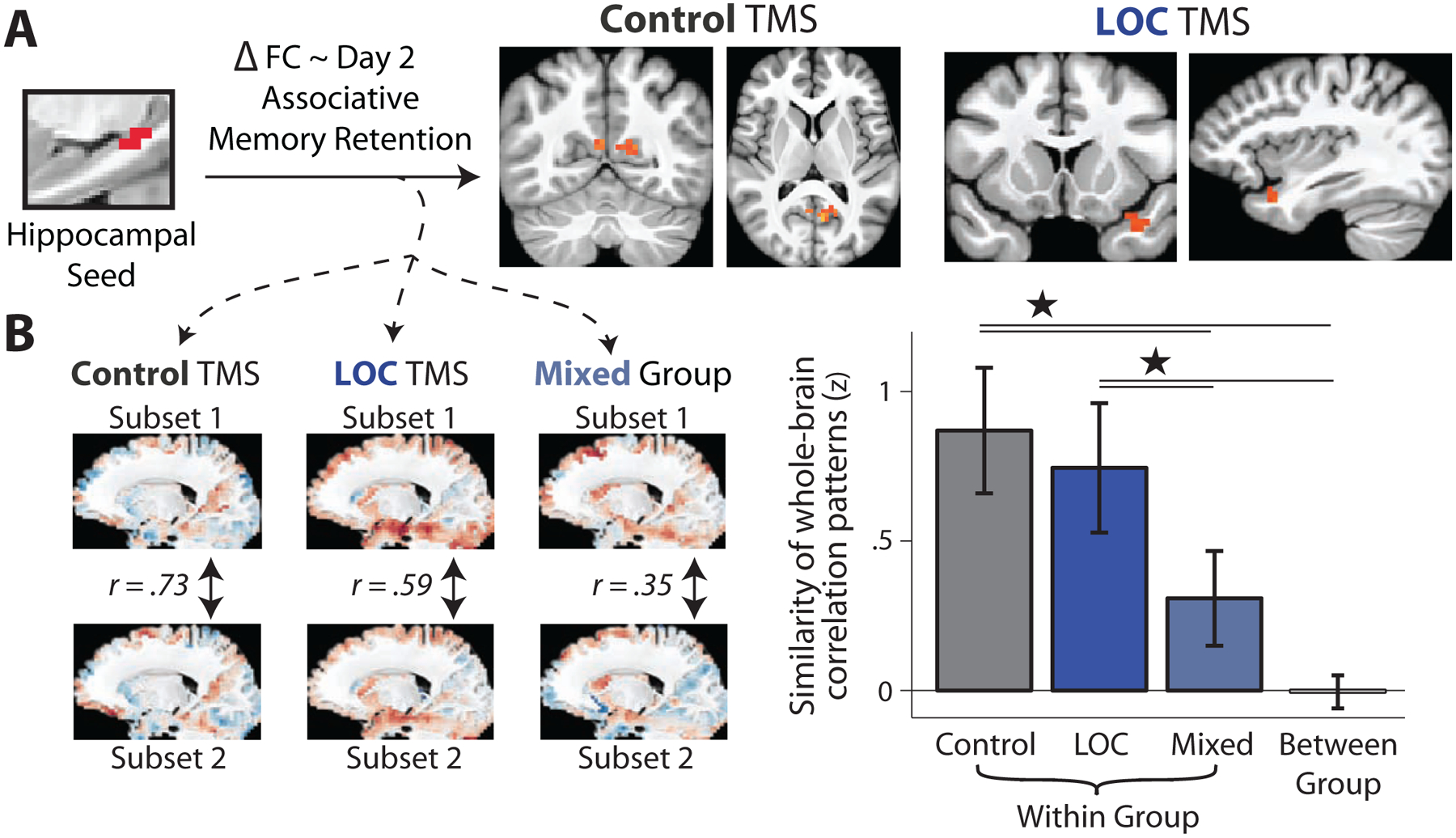
(A) Correlations between hippocampal (Figure 5A) FC changes across the whole brain and next-day associative memory retention (FWE-corrected using permutation testing). The only regions surviving FWE-correction are shown: retrosplenial cortex in the Control TMS group and right anterior temporal cortex in the LOC TMS group. Maps are shown on study-specific group-level template brain. (B) Patterns of correlations between hippocampal FC changes and next-day associative memory retention were repeatedly estimated for random subsets of each TMS group (left and middle) and a mixed set of participants across TMS groups (right) using subsampling. Plot shows similarity or correlation of these patterns across subsamples within each TMS group, within the mixed group, and the between-group similarity. Bars show mean Fisher Z-transformed correlation (similarity) and error bars show the standard deviation of Fisher Z-transformed correlations across iterations. *P < .001. See also Figure S5
The above findings suggest that TMS may modulate the sets of post-encoding hippocampal interactions that are related to memory retention. However, it is difficult to infer this using thresholded statistical maps. To better address this possibility, we repeatedly estimated whole-brain patterns of correlations between hippocampal FC changes and memory retention in each TMS group (Figure 6B). This allowed us to test whether connectivity patterns associated with memory retention differed based on TMS site, by comparing the similarity of connectivity patterns related to memory within each TMS group to the similarity between TMS groups and from a mixed set of participants across groups. As shown in Figure 6B, the similarity of hippocampal FC patterns related to next-day associative memory retention was greater within each TMS group compared to the between-group similarity (Control TMS vs. between-group, t36 = 18.4, P < 10−10; LOC TMS versus between-group, t36 = 14.9, P < 10−10) and to a mixed set of participants across TMS groups (Control versus mixed-group, t36 = 9.5, P < .001; LOC TMS versus mixed-group, t36 = 7.0, P < .001). The same pattern of results was found when examining patterns of hippocampal interactions related to same-day memory retention (Figure S5). These results suggest that distinct patterns of hippocampal post-encoding interactions support memory retention across TMS sites.
Questionnaires
To assess whether Post-Encoding fMRI measures may be driven by active rehearsal, questionnaires were given to assess participants’ thought content during Post-Encoding Rest. Many participants reported thinking about the stimuli encountered during encoding during rest (47/58; Table S3). However, the vast majority of stimulus-related mentation was reported to be spontaneous in nature (90.1%) while the minority (9.9%) was reported as intentional in nature (retrieval in preparation for an upcoming memory test, Table S3). These findings suggest that it is unlikely that fMRI Post-Encoding measures were driven by explicit rehearsal of stimuli.
DISCUSSION
Long-term memory retention is thought to depend, in part, on processes that unfold after events are initially encoded into memory. Post-encoding reactivation and hippocampal-cortical interactions are leading candidate mechanisms thought to support memory retention and the stabilization of representations across hippocampal-cortical networks. However, most evidence for these mechanisms in humans have relied on correlational links between reactivation and later memory, making it unclear whether post-encoding reactivation plays a unique role supporting memory. Here, we causally tested the role of reactivation during awake rest periods by performing post-encoding theta-burst TMS to a region involved in consolidation (LOC). Post-encoding TMS to LOC, compared to Control TMS and a no TMS condition, impaired associative (object-face) memory retention. The influence of post-encoding TMS to LOC was selective to associative memory as it did not influence item memory retention. By combining TMS with fMRI, we found that LOC TMS reduced multi-voxel pattern reactivation during post-encoding rest and disrupted relationships between hippocampal-LOC interactions and associative memory retention. Together, these findings provide important evidence that awake post-encoding reactivation and hippocampal-cortical interactions play an important role in episodic memory.
We specifically targeted LOC in this study because of prior work indicating that post-encoding hippocampal interactions with occipitotemporal cortex were related to later visual memory [13,15,20,41,43] and reports of pattern reactivation in occipitotemporal cortex [19,41,42,51,52]. Here, we found evidence for these mechanisms after Control TMS which conceptually replicates prior work: multi-voxel pattern reactivation was present during post-encoding rest, both in the hippocampus and LOC, and post-encoding hippocampal-LOC interactions were positively related to associative memory retention assessed during same-day memory testing. However, theta-burst TMS to LOC disrupted several of these measures: reactivation of object and face patterns in LOC (Figure 3) and relationships between hippocampal-LOC interactions and same-day associative memory retention (Figure 5), although it did not reliably influence hippocampal reactivation (Figure 4). Thus, while reactivation and hippocampal-LOC interactions were positively related to same-day associative memory retention after Control TMS, they were no longer related to later memory after LOC TMS (Figure 5). Additionally, we found that distinct patterns of post-encoding hippocampal interactions were related to associative memory retention between Control and LOC TMS conditions (Figure 6). These findings suggest that post-encoding TMS to LOC disrupted ongoing cortical reactivation and hippocampal interactions, possibly causing a change in the nature of the hippocampal interactions that support later memory.
The hippocampus is thought to play a critical role in orchestrating memory reactivation and driving hippocampal-cortical interactions [2,6,53–55]. This suggests that reactivation during rest should be preferentially related to memory representations that are most supported by the hippocampus. We tested this by contrasting the influence of post-encoding TMS on associative versus item memory and found that LOC TMS selectively impaired associative (object-face) memory retention without influencing memory retention for individual items (Figure 2). This suggests that post-encoding reactivation and hippocampal-cortical interactions specifically promote the retention of memories that are supported by the hippocampus. This resonates with behavioral work demonstrating that the presence of post-encoding rest periods preferentially enhances later recollection as opposed to familiarity [56] and prior work showing a dissociation in the neural structures that support later associative versus item memory during post-encoding rest [57]. This dissociation also reinforces the notion that distinct processes underlie forgetting in a manner that is dependent on the nature of the memory representation [58,59]. Our findings add to this literature by showing that awake post-encoding processes preferentially influence memories that are most supported by the hippocampus.
Prior work has shown that levels of reactivation or hippocampal interactions during immediate post-encoding awake periods are related to later memory, both when memory is tested minutes to hours later [13,14,16,17] and after one or more nights of sleep [15,57,60,61]. Here, we tested memory at multiple time-points after TMS (several hours after encoding, and approximately 24 hours later) to understand how post-encoding TMS influenced memory retention over time. Our findings are ambiguous as to whether post-encoding LOC TMS showed a greater influence on same-day versus next-day memory retention; although a significant effect of LOC TMS was found on same-day associative memory retention, while no significant group-level difference was present for next-day associative memory retention, no reliable interaction was found across these time-points. Additionally, a subset of LOC TMS participants (who showed the greatest impairment in same-day memory retention) exhibited a persistent reduction in next-day associative memory, suggesting that some individuals may show lasting memory deficits. It is thus unclear whether the impairment in associative memory seen here extends to memory tested at longer delays; future studies are needed to investigate this issue.
Considering the lack of a clear influence of LOC TMS on next-day memory retention, it is possible that other mechanisms, beyond the post-encoding processes that were targeted with LOC TMS, contributed to memory assessed 24 hours later. First, increased post-encoding hippocampal interactions with anterior temporal cortex were positively related to next-day associative memory retention in participants who received LOC TMS. This finding, coupled with the presence of distinct post-encoding hippocampal interactions that were related to memory retention after Control TMS (Figure 6), suggests that LOC TMS may have resulted in a reorganization of the interactions or processes that support later memory during the immediate post-encoding time period. Second, it is likely that consolidation mechanisms during intervening sleep (and other awake periods) contributed to next-day memory retention, which were not measured or manipulated in this study. These opportunities for subsequent consolidation (beyond the initial post-encoding time period influenced by TMS) may have resulted in a weak and non-significant TMS-related impairment in memory retention during next-day memory testing at the group-level.
The cTBS protocol used in this study is thought to be inhibitory and influence neural function for up to 50 minutes [44–46], with the strongest changes seen around 30 minutes post-stimulation. The properties of this protocol allowed us to interfere with ongoing reactivation processes for an extended time period (tens of minutes) after encoding and test the influence of TMS both on reactivation immediately after stimulation as well as on subsequent behavior. This approach is similar to prior work that has used external manipulations to influence memory consolidation. Specifically, repetitive TMS after reminder cues has been shown to impair reconsolidation [62,63] and repetitive TMS applied after learning influences changes in motor memory [64–66], visual skill learning [67], and competition between perceptual [68] or motor memories [69]. Here, we targeted immediate post-encoding time periods to interfere with hippocampal-cortical interactions and reactivation thought to underlie episodic memory. Additionally, behavioral studies have shown that the presence of 10 minute post-encoding rest periods, which may promote reactivation compared to active tasks, benefit memory retention [70–72]. Our findings extend prior approaches by targeting a brain region (LOC) that showed prior evidence of post-encoding reactivation and memory-related hippocampal interactions, and by combining TMS and fMRI, demonstrating that targeting LOC both reduced associative memory retention and fMRI measures of post-encoding reactivation and hippocampal interactions.
Given the timescale of cTBS, which has been investigated in prior studies and summarized in meta-analyses [38–40], we think it is unlikely that the behavioral impairment found here was due to an influence of TMS on retrieval processes three hours later. Although an influence of TMS on retrieval is consistent with a qualitatively stronger impairment on same-day versus next-day memory, it is incompatible with prior data showing that neural function returns to baseline 60 minutes after cTBS and beyond [38–40]. Moreover, an influence of TMS on retrieval three hours later would not explain why a subset of participants showed persistent impairments in next-day memory retention (24 hours later). Our results showing altered reactivation and memory-related hippocampal-cortical interactions during immediate post-encoding rest further support the notion that LOC TMS influenced neural function during an initial consolidation period (rather than during retrieval).
The TMS protocol used here was intended to disrupt and test the role of systems-level processes during post-encoding rest (hippocampal-LOC interactions and reactivation). By combining TMS, fMRI, and memory testing, we demonstrate that LOC TMS disrupted associative memory retention, LOC reactivation, and memory-related hippocampal-cortical interactions. Yet, it is important to highlight that TMS likely did not selectively influence these processes, which is difficult to achieve using non-invasive procedures alone but can be accomplished in animal models [30,31,73,74]. It is likely that other underlying mechanisms which we cannot readily measure (e.g. changes in synaptic plasticity) contributed to our findings. For example, cTBS reduces cortical excitability in an NMDA receptor-dependent fashion [75] and BDNF, a regulator of plasticity, is related to the efficacy of cTBS [76], suggesting that cTBS may be mediated by LTD-like plasticity mechanisms [77,78]. It is thus possible that cTBS in this study may have induced synaptic changes and depotentiation within LOC, which may have in turn contributed to reduced multi-voxel pattern reactivation in LOC and other TMS-related changes (reduced associative memory retention and memory-related hippocampal-LOC interactions). Regardless of the potential underlying mechanisms, we think it is important to demonstrate that external manipulations which modulate post-encoding processes of interest also result in changes in memory, providing tighter links between these processes and later behavior.
In summary, by combining fMRI and post-encoding theta-burst TMS, this study demonstrates that processes unfolding during awake post-encoding time periods make an important contribution to episodic memory. This work adds to a growing body of literature highlighting the role of awake rest periods and reactivation in supporting memory [21,30,39,79,80] and provides a tool for modulating post-encoding hippocampal-cortical interactions and reactivation in humans. While it has been argued that correlations between post-encoding reactivation and later memory may be epiphenomenal and reflect encoding-related activity that simply persists in time [25], these findings show that post-encoding processes make a unique contribution to subsequent memory that is separable from encoding activity. Although the current work examined the influence of post-encoding TMS on a simple measure of how memory changes over time (retention), this approach can be used to more broadly understand the contribution of post-encoding processes to other ways in which memory changes over time, such as the neural re-organization of memory representations [60] or the preferential retention of salient information [81], as well as other changes in behavior and to modify memory retention in disease states.
STAR METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact, Arielle Tambini (atambini@uci.edu).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The datasets and code used in this study are available from the corresponding author.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Healthy young adults were recruited to participate in the main experiment (referred to as the fMRI-TMS study) or a behavioral control study. Informed consent was obtained from all participants in accordance with the Committee for Protection of Human Subjects at the University of California, Berkeley.
fMRI-TMS Study
A total of 62 participants completed all sessions in the fMRI-TMS experiment (fMRI, TMS, and behavioral sessions shown in Figure 1). Of those, four participants were excluded due to chance initial memory performance (prior to TMS), resulting in data from 58 participants for behavioral analyses (29 per TMS site, with 16 females and 13 males per TMS site). An additional six participants were excluded from fMRI analyses due to excessive head motion (see definition below) in five participants and a large number of scanner artifacts in one participant, resulting in fMRI data from 52 participants (26 per TMS site, with 14 females and 12 males per TMS site). The average age of all participants was 20.6 years (LOC TMS participants: average 20.4 years, range 18 – 25, Control TMS participants: average 20.7, range 18 – 26). Excessive head motion was defined as more than 25% of volumes flagged to be removed (volumes surrounding motion spikes > .5mm Framewise Displacement, FD, see definition below) across more than one scan.
Behavioral Study
A control group (N=26 participants) was used to examine memory retention without an influence of TMS (referred to as the No TMS group). The average age of the participants was 20.3 years (range: 18 – 25) and included 11 male and 15 female participants.
METHOD DETAILS
Experimental Procedures
A between-subjects design was used to assess the influence of LOC TMS on memory. Specifically, the LOC TMS group was compared to an active TMS condition (Control TMS) as well as a No TMS control group. This allowed us to assess whether any modulation of memory was due to a general influence of TMS (by comparing the TMS groups to the No TMS condition) or was specific to targeting post-encoding processes involving LOC (by comparing LOC TMS and the Control TMS group). We tested memory before and after TMS in order to compare a normalized measure of memory (retention, or delayed relative to immediate memory) across groups, which explicitly controls for variability in pre-TMS memory across participants. Each memory test contained randomly chosen subsets of stimuli seen during encoding such that each stimulus was only tested once.
Participants in the main fMRI-TMS study first completed a Baseline Session to localize TMS targets (from anatomical and functional localizer scans) and measure each participant’s TMS motor threshold. The main session (depicted in Figure 1) consisted of Baseline Rest scans (one blood-oxygen-level-dependent or BOLD scan followed by one arterial spin labeling or ASL scan), a brief practice of the imagery task performed during encoding (outside of the MRI scanner after Baseline Rest), incidental object-face encoding (an imagery task performed across five BOLD scans), an immediate memory test, cTBS to either right LOC or a Control site, and Post-Encoding Rest scans (three BOLD and two ASL scans, collected in an interleaved fashion). Participants were randomly assigned to each TMS site (LOC or Control). Delayed memory tests were administered on the same day (approximately three hours after TMS) and the next day (20–28 hours after TMS). Participants left the laboratory and went about normal activities in between the scanning session and the delayed memory test. All memory tests and TMS sessions took place outside of the MRI scanner. Participants completed questionnaires after the last memory test to assess their thought content during the rest scans. Results from ASL rest scans are beyond the scope of the current manuscript and are not reported here.
The time of day of the procedures was not fixed but was permitted to vary across participants for practical considerations to accommodate participant scheduling. On average, TMS was administered in the main session between 3 and 4pm (15.6 ± .5 hours for LOC TMS, 15.2 ± .5 hours for Control TMS), with no systematic differences in the time of day of the procedures across TMS groups (comparison of the time of TMS, t56 = .46, P = .65). No significant interactions were found between the time of day and the influence of TMS on memory retention.
The TMS protocol used in this study (cTBS) influences neural function for up to approximately 50 minutes after stimulation, such that cortical excitability and BOLD responses are most strongly decreased from 0–30 minutes after stimulation, with smaller but reliable changes in excitability present 40–50 minutes post-stimulation [44–47]. Levels of cortical excitability and BOLD responses return to pre-TMS baseline levels after 1 hour and beyond [38,44; for meta-analyses see 39, 40]. Thus, the 3-hour delay between TMS and delayed memory testing allowed us to influence the post-encoding time period without explicitly manipulating retrieval processes.
Participants in the behavioral, No TMS study performed the same encoding and memory tests as in the fMRI study, but all procedures took place in behavioral testing conditions.
Encoding
Participants incidentally encoded object-face pairs while performing an imagery task; they were not informed that their memory would be later tested. The object-face encoding procedure used here was chosen based on prior work which found correlations between post-encoding hippocampal-LOC interactions and subsequent associative memory [13]. Stimulus pairs (180) were viewed over the course of five 9.6-minute blocks or scans (36 pairs per block). Participants were instructed to vividly imagine the specific person pictured interacting with the specific object shown and to rate the vividness of their mental imagery. Each trial consisted of a fixation cross (.25 seconds), followed by an object-face pair (5.75 seconds), a response period (1 second), and an active baseline arrows task (9 seconds). Each object-face pair contained one stimulus to the right and left of a central cross. Trials were counterbalanced with respect to face gender and left/right screen position of object and face stimuli. During the response period, the words “High Medium Low” were presented on the screen and participants were instructed to rate the vividness of their mental imagery via button press (index, middle, and ring finger, respectively). An active baseline arrows task was used to reduce hippocampal BOLD activity during the inter-trial interval [82]. The arrows task was self-paced and consisted of the presentation of a series of arrows pointing to the left, up, or the right. Participants responded via button press to indicate the direction of the arrow (index, middle, and ring finger, respectively); a new arrow was presented after each correct response. A brief practice session was performed before the first encoding block (after the Baseline Rest scan).
Memory Assessment
All participants performed three memory tests: one immediately after encoding and before TMS (referred to as Immediate memory test) and two Delayed tests (approx. three hours and 20 – 28 hours after TMS). Object-face pairs were presented during all memory tests. Participants first rated each stimulus pair as ‘Intact’ (meaning the stimuli were presented together during encoding), ‘Rearranged’ (meaning both stimuli were seen during encoding but were presented with other stimuli) or ‘New’ (either one or both stimuli were not seen during encoding). If participants chose ‘New’ they were asked which stimulus was new: the object, face, or both stimuli. Memory testing was self-paced and participants were instructed to be as accurate as possible. Participants were not informed that their memory for stimuli seen during encoding would be tested during the Immediate or Delayed tests; they were only instructed about each memory test immediately before it was administered.
A subset of the encoded stimuli was presented on each memory test. The Immediate memory test contained a total of 60 stimulus pairs (32 intact, 8 rearranged, 8 one new/one old, 12 both new stimuli). Each Delayed memory test contained 92 pairs (48 intact, 12 rearranged, 16 one new/one old, 16 both new stimuli). The presentation of Intact pairs during memory testing was prioritized to perform subsequent associative memory analysis of the fMRI data, which require an adequate number of Intact trials to obtain both associative hit and miss trials.
Functional Localizer Scans and Analysis
Functional localizer scans were collected during the Baseline Session. Participants performed a one-back task while viewing blocks of object, face, scene, and scrambled images. Each block lasted for 16 seconds and contained 20 stimuli per block (300 ms stimulus presentation followed by a blank 500 ms inter-stimulus interval). Two stimuli were repeated in each block and participants were instructed to respond to stimulus repetitions via button press. Each scan contained six blocks of each stimulus class, which were interleaved with seven blocks of passive fixation. Each participant performed two localizer scans and a brief practice was performed before the scanning session.
Stimuli
A total of 244 object and face stimuli were presented across encoding and memory testing (180 pairs seen during encoding, with the remaining stimuli serving as new images during memory testing). The pairing of face and object stimuli and the assignment of stimuli to be seen during encoding or serve as new images during memory testing was randomly determined for each participant. Face stimuli consisted of frontal views of faces that were cropped in an oval shape. Face gender was counterbalanced during encoding and memory testing. Object stimuli consisted of common everyday objects. Separate stimuli were used for the functional localizer scans (containing faces, objects, scenes, and phase scrambled images) such that there was no overlap in the stimuli between encoding and localizer scans. To equate visual content across stimulus categories, all images were presented on phase scrambled backgrounds. All stimuli were sized 350 × 350 pixels displayed on a screen of resolution 1024 × 768 pixels.
Questionnaire
A questionnaire was given to participants after the final memory test to assess their thought content during rest scans in the fMRI-TMS study. We sought to estimate how often participants thought about stimuli encoded during the imagery task during Post-Encoding Rest and whether stimulus-related mentation was intentional or spontaneous in nature. Participants were asked to estimate the proportion of time (of the entire Post-Encoding Rest period) spent thinking about object or face stimuli seen during the imagery task. If participants reported thinking about the stimuli at all during Post-Encoding Rest, they were further asked to estimate whether stimulus-related thoughts that were intentional in nature (“you thought that your memory for the images would be tested and that trying to think about/remember them during the rest period might improve your memory”) or spontaneous in nature (“they just popped into your mind somewhat randomly/you were not trying to strategically recall them to prepare yourself for a potential upcoming memory test”). This allowed us to assess both the occurrence and nature of stimulus-related mentation during Post-Encoding Rest.
TMS Sites
TMS sites were defined in a participant-specific manner. The LOC TMS site was defined as a 6mm region around the site of maximal responsiveness to objects (objects > scrambled objects contrast from the functional localizer scans) in right lateral occipital cortex using a threshold of P < .0001 (as in prior TMS work, 46). Right LOC (as opposed to left LOC) was targeted as prior work found post-encoding hippocampal-LOC interactions that were greater for right versus left LOC [13] and other studies have successfully interfered with object processing by targeting right LOC [83]. The control TMS site (dorsomedial somatosensory cortex, S1) was defined anatomically (the most superior portion of the right postcentral gyrus that fell within a gray matter mask). The TMS sites for all participants are shown in normalized (MNI) space in Figure 1B. An active control condition was used to ensure that any influence of TMS was not due to non-specific effects of stimulation or potential changes induced by the acoustic or tactile scalp sensations associated with TMS. As in prior work [84–86], dorsomedial S1 was chosen as the active control site since it demonstrates relatively local connectivity within somatosensory cortex [87] and corresponds to the leg somatosensory representation, making it unlikely that S1 cTBS would influence hippocampal function or interactions related to memory consolidation.
TMS Procedures
TMS was applied using a MagStim Super Rapid 2 stimulator with a figure-eight double air film coil with a 70mm diameter. Active Motor Threshold (AMT) was measured during the Baseline Session. Electromyography (EMG) was recorded using electrodes placed on the first dorsal interosseus (FDI) muscle on the left hand. To localize the hand area of right motor cortex, single TMS pulses were delivered to find the region that elicited a visible finger twitch when the participant’s hand was at rest. AMT was then defined as the minimum intensity required to produce a motor-evoked potential (of at least 200 μV) on 5 out of 10 trials while the participant maintained 20% of their maximum contraction of the FDI muscle. Visual feedback of the EMG signal was presented to the participant to aid in the maintenance of the appropriate level of muscle contraction.
cTBS was applied after the immediate memory test during the Main Session (to either LOC or the Control site). TMS targets were localized using a computerized frameless stereotaxic system (Brainsight, Rogue Research). cTBS was applied at 80% of AMT and comprised of 50 Hz triplets (three single pulses separated by 20ms) repeated at a frequency of 5 Hz (every 200ms) for a duration of 40s or 600 pulses (using parameters from Huang et al., 2005). This TMS protocol is considered to be inhibitory, as it reduces cortical excitability [44–46] and BOLD responses for up to 50 minutes after application [47], such that excitability and BOLD responses are most strongly impacted from 0–30 post-stimulation with measures of neural function returning to pre-TMS baseline levels approximately 60 minutes after stimulation [44–47].
MRI Data Acquisition
Scanning was performed using a 3T Siemens TIM/Trio MRI system with a 12-channel whole-head coil located at the UC Berkeley Henry H. Wheeler Jr. Brain Imaging Center. High-resolution T1-weighted (magnetization-prepared rapid-acquisition gradient echo) images were acquired during the Baseline Session (240 × 256 × 160 matrix of 1 mm isotropic voxels; TR = 2300 ms; TE = 2.98 ms; flip angle = 9). Functional T2*-weighted BOLD data were collected using a gradient-echo planar pulse (EPI) sequence (repetition time [TR] = 2 s, echo time [TE] = 28 ms, field of view = 210 mm, 32 slices, 3 × 3 × 3 mm voxel size, .6 mm interslice gap, flip angle = 78°). EPI scans lasted for 9 minutes/270 volumes (rest scans), 9.6 minutes/288 volumes (encoding scans), or 8.27 minutes/248 volumes (functional localizer scans) after discarding the first five volumes to allow for T1 equilibration. During the rest scans, participants were instructed to close their eyes and simply rest and think about anything that they wanted to while remaining awake.
QUANTIFICATION AND STATISTICAL ANALYSES
Memory Analyses
A key question of this study was to assess how TMS to LOC influenced memory retention from before to after TMS. Corrected memory (hits minus false alarms) was computed for each test for both associative and item information (shown in Figure S1, S2, Tables S1 and S2). Memory retention was computed as delayed divided by immediate corrected memory (shown in Figure 2, S2). Associative memory was measured as the proportion of associative hits (proportion of Intact trials that were labeled Intact) minus associative false alarms (proportion of Rearranged trials labeled Intact plus proportion of New trials labeled Intact). Item memory was computed for each individual stimulus regardless of associative memory accuracy; each old item was considered a hit if it was endorsed as old regardless of the status of the item it was presented with, and new items were considered to be false alarms if they were labeled as old via any response option (e.g. labeled as Intact, Rearranged, or labeled as old with the other stimulus labeled as new). Item memory was primarily assessed across both faces and objects (shown in Figure 2), but we also examined whether results were consistent across stimulus categories (separately for faces and objects).
Memory retention was compared across TMS groups (LOC versus Control) using mixed-effects ANOVAs implemented in R. Two-way ANOVAs were performed to assess whether memory retention was influenced by TMS, using a between-subjects factor of TMS site (LOC or Control) and a within-subjects factor of time of Delayed memory testing (same-day and next-day). Separate ANOVAs were performed for associative and item memory retention. To compare TMS-related changes between associative and item memory, a three-way mixed-effects ANOVA was performed with a between-subjects factor of TMS (LOC or Control) and within-subjects factors of time of memory test (same and next day tests) and memory-type (associative and item memory retention). Unpaired t-tests were used to perform direct comparisons between TMS groups, as well as between each TMS group and the no TMS group.
Exploratory analyses were also performed to assess whether next-day memory retention was reduced by LOC TMS, based on levels of memory retention during same-day memory testing. To do so, a split half analysis was performed on levels of same-day LOC TMS associative memory retention, such that 14/29 participants with the lowest retention after LOC TMS were placed into one group (‘Low’ group in Figure S2), with the other 15 participants with the highest retention after LOC TMS placed into another group (‘High’ group in Figure S2). Next-day associative memory retention (and corrected next-day associative memory) was then examined for these sets of participants and compared with the control groups using unpaired t-tests.
MRI Preprocessing
The imaging data were preprocessed using SPM8 (Wellcome Department of Cognitive Neuroscience, University College London, London, UK). Functional data were first corrected for differences in slice timing acquisition followed by motion correction across all runs and removal of low frequency trends (< .009 Hz, using spm_filter). Each participant’s functional data was co-registered to their own T1-weighted anatomical image using the mean functional image from that session and spatially smoothed with a 6-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. A group-level anatomical template was created with ANTs [88] using brain extracted anatomical scans of the fMRI-TMS study participants. Most analyses were performed in each participant’s native space (multi-voxel reactivation analyses) and data were transformed to the group-level template space when needed (for group-level subsequent memory analyses and group-level functional connectivity analyses).
Noise was removed from all functional EPI scans by performing nuisance signal regression followed by the exclusion of volumes surrounding motion events (motion scrubbing, 52). Nuisance signal regression was performed using a modified version of aCompCor [90] as previously implemented [14]. After brain tissue segmentation, white matter (WM) and cerebrospinal fluid (CSF) probability maps were resampled in functional resolution and converted to binary masks. The top principal components (PCs) from WM and CSF voxels, in addition to six motion parameters and their 1st temporal derivatives, were removed from the BOLD signal in each scan a voxel-wise manner using linear regression. As in (53), a variable number of WM/CSF PCs was used for each participant based on null simulations. For functional connectivity analyses, a low-pass filter was applied to data from rest scans (< .1 Hz, 4th order Butterworth filter) after nuisance signals were removed. Motion scrubbing was performed as a final step for all scans by discarding volumes surrounding sudden motion events (one volume before and two volumes after all events of FD > .5 mm, computed according to 52).
Functional Localizer Analysis
Localizer scans were modeled by convolving each 16 second block with a standard double gamma hemodynamic response function (HRF). One regressor was used to model each stimulus category (faces, objects, scenes, and scrambled images). The lateral occipital complex region of interest (ROI) used for reactivation and hippocampal functional connectivity analyses was individually defined for each participant by performing a standard contrast to isolate object-responsive voxels (objects > scrambled images). All voxels reaching a threshold of P < .0001 within lateral occipital cortex and the posterior fusiform gyrus were included in the ROI [91]. Anatomical masks of these regions were created using Freesurfer (Desikan-Killiany atlas, 55). The LOC TMS site was defined as described above (see TMS Sites).
LOC Reactivation Analysis
Multi-voxel pattern classification was performed to assess object and face reactivation in LOC during Post-Encoding Rest in addition to a similarity-based approach (described below). The approaches used for assessing reactivation both in LOC and the hippocampus (see below) assume that: 1) patterns of activation that are present during the object-face encoding task are similarly present again during rest scans after encoding (as is typical in memory reactivation studies, e.g. [93]) and 2) reactivation evidence during Post-Encoding Rest may reflect the re-processing of specific information or memories from prior encoding (shown in a similar tasks in [14]). To examine reactivation in LOC, support vector machine (SVM) classifiers were trained based on data from the functional localizer scans and then applied to the main session (rest and encoding scans; Figure 3A). Two primary classifiers were trained for each participant: an object classifier (trained to discriminate objects vs. scrambled images) and face classifier (trained to discriminate faces from scrambled images). Classification was performed using MATLAB (R2015a) with a binary linear SVM classifier (via the “fitcsvm” and “predict” functions). The default parameters were used (including a default parameter of C = 1) with the exception of setting standardization to true (which z-scores individual samples or patterns across voxels). Time-series from the functional localizer runs were extracted for all voxels in LOC and z-scored over time for each scan. Each classifier was trained on data from blocks of interest (face, object, and scrambled object blocks); the average patterns across TRs 4–7 after block onset (7 – 13s) were used for classification. Voxels were excluded if they contained excessive noise (variance > four standard deviations above the median variance) during either the functional localizer or the rest scans. The primary classifiers (which were applied to data from the main session) were trained on all samples from the functional localizer scans.
Before applying the classifiers to examine LOC reactivation evidence during rest (Figure 3A), we ensured that they accurately decoded face and object information content. To do so, we first performed cross-validation within the functional localizer scans (while objects and faces were viewed during separate blocks), and secondly confirmed that object and face content was accurately detected during the object-face encoding task (when object-face pairs were viewed). Cross-validation was performed across the two scans of the functional localizer task, such that a classifier was trained on the data from the first scan and applied to the second scan, and vice versa. This resulted in a measure of cross-validated accuracy for each scan and participant which was compared to chance levels (50%).
To apply the classifiers to the encoding and rest data, time-series from the LOC voxels were extracted, z-scored over time, and patterns corresponding to individual TRs were fed into the classifier (see schematic in Figure 3A; note that nuisance signals were already removed the time-series and volumes surrounding high motion events were excluded from all analyses). Trial-evoked changes in object and face classification during encoding (shown in Figure 3B) were estimated by averaging classifier output across trials for the eight TRs corresponding to each trial. A summary measure of trial-evoked decoding was computed by averaging classifier output 5 – 7s after trial onset (corresponding to TRs 4–5) and subtracting the average classifier output at trial onset. This summary measure of trial-evoked classification was compared across TMS conditions in order to ensure that similar object and face information was present during encoding between TMS conditions. Reactivation of object and face information during rest (‘reactivation evidence’) was measured by computing the proportion of volumes in each Post-Encoding Rest scan classified as a face (or object) and subtracting the proportion of Baseline Rest volumes classified as a face (or object). Reactivation evidence was averaged across the object and face classifiers for each participant and then compared across TMS conditions (Figure 3C).
A similarity-based approach was also used to measure LOC reactivation, to ensure that the above results were driven by the specific classifiers used. Here, two multivoxel patterns (associated with viewing faces and objects during the localizer scans) were constructed for each participant by calculating the average, z-scored BOLD signal across TRs 4–8 (7 – 13s) after block onset relative to an implicit fixation baseline. Noisy voxels were excluded from this analysis in the same manner as above. We then measured the similarity (correlation) of each (face or object) template pattern from the localizer scans with each time-point during rest. High similarity events were defined for each Post-Encoding Rest scan by examining the distribution of correlation values across the Baseline Rest and the current Post-Encoding scan and isolating time-points with the highest correlation values (> 90% of all correlations). The proportion of volumes labeled as a high similarity events were then contrasted between the Baseline and Post-Encoding scans (shown in Figure S3). This approach yielded similar results as using a standard deviation cutoff for isolating high similarity events, which has been used in prior work [16,18].
Hippocampus Reactivation Analysis
Given that the hippocampus does not typically show category-specific multi-voxel activation patterns [94], hippocampal reactivation evidence was assessed using a prior approach [14]. Briefly, we first characterized multi-voxel hippocampal patterns present during encoding using principal components analysis (PCA) on denoised, unsmoothed data from the hippocampus (anatomically defined) and then measured the amount of variance explained by these encoding patterns during rest. Anatomical hippocampal ROIs were created using FSL’s FIRST [95] and altered when necessary to conform to the definitions of [96]. Voxels with low signal (< 2 standard deviations below the mean of a whole brain mask) or excessive variance (> four standard deviations above the median variance across hippocampal voxels) in any scan were excluded from the analysis. PCA was performed on denoised hippocampal multi-voxel data concatenated across all encoding scans (each voxel was z-scored before the data was concatenated). Based on [14], which found reactivation of the top 4 hippocampal encoding PCs, we focused our analyses on PCs 1–4.
We verified that the top hippocampal PCs captured task-evoked activity by analyzing the time course or temporal projection of each PC on the encoding data. Average time courses (trial triggered averages) of the z-scored temporal projection are shown in Figure 4A from an example participant (for the top PCs). Summary measures of trial-evoked activity were computed for each participant by averaging the temporal projection 5 – 7 seconds after trial onset (corresponding to TRs 4 – 5) and subtracting the average value at trial onset (the same metric used for the LOC classifier analyses). The amount of variance explained by each encoding PC during each rest scan was calculated as in [14]. Reactivation was operationalized as an increase in the amount of variance explained from Baseline Rest to Post-Encoding Rest. Paired t-tests were used to assess average reactivation evidence across the top four PCs and all three Post-Encoding Rest scans (vs. Baseline Rest; Figure 4B). One-way repeated measures ANOVAs assessed whether reactivation varied across scans within each TMS group (using a within-subjects factor of rest scan number). We also assessed whether reactivation evidence across individual PCs within each participant was correlated with the strength of each hippocampal PC during encoding (eigenvalue of each PC) across the top ten PCs (Figure 4C); this measure has previously been related to individual differences in later memory [14]. Average reactivation evidence (across the top four PCs) was compared across TMS groups using mixed effects ANOVA with a within-subjects factor of rest scan number and a between-subjects factor of TMS condition (LOC and Control). Correlations between encoding strength and reactivation evidence were Fisher Z-transformed and compared to zero for each TMS group (paired t-test) and across TMS groups (un-paired t-test).
fMRI Encoding Analysis
Subsequent memory analyses were performed on the encoding scans to localize group-level hippocampal ROIs likely to show memory-related resting functional connectivity changes. Encoding trials were modeled as a 6 second boxcar (from fixation onset through to stimulus offset) which was convolved with a standard double gamma HRF. Trials were sorted according to subsequent associative memory, with separate regressors modeling associative hits (trials that were presented intact during memory testing and labeled as intact), associative misses (trials presented as intact and labeled as rearranged), and item misses (trials presented intact and labeled as one item being new or both items labeled as new). To isolate regions that contributed to delayed memory, separate regressors were included for stimulus pairs assessed during immediate and delayed memory testing. This resulted in a total of 7 regressors: delayed associative hits, delayed associative misses, delayed item misses, immediate associative hits, immediate misses (immediate associative and item misses were combined into one regressor due to low counts across these trial types), all other trials (those not presented as intact during memory testing), and a regressor corresponding to the one second response period. Nuisance signals (motion and WM/CSF signals) were removed prior to model estimation. High motion time-points were removed or temporally censored at the level of model estimation by removing flagged time-points from both the functional data and design matrices.
To isolate group-level hippocampal ROIs that track associative memory formation commonly across both TMS groups, a general linear model (GLM) was first estimated using the data from all participants. Separate GLMs were also estimated for participants in each TMS group to isolate ROIs specific to each group. Participants were included in subsequent memory analyses if they had at least 10 trials in each relevant trial type (i.e. delayed associative hits and delayed associative misses). Given our a priori focus on defining hippocampal ROIs, group-level contrasts were masked with a group-level hippocampal ROI (when isolating hippocampal ROIs).
fMRI Resting Functional Connectivity Analyses
Functional connectivity was estimated to measure hippocampal post-encoding interactions and their relationship with memory retention. As in prior work, we focused on experience-dependent changes in functional connectivity, or changes from Baseline to Post-Encoding Rest [13,15,57]. Hippocampal-LOC interactions were measured for each rest scan by computing Fisher Z-transformed Pearson’s correlation values between the relevant time-series (after nuisance signal removal, low-pass filtering, and temporal censoring or removal of high motion time-points). Post-encoding functional connectivity was estimated by averaging connectivity values across the three Post-Encoding Rest scans. Robust regression was used to assess relationships between functional connectivity changes and memory retention (shown in Figure 5 and 6), separately for memory retention measured during each delayed memory test (same-day and next-day memory).
Exploratory analyses were used to ask whether post-encoding hippocampal interactions were related to next-day associative memory retention, and whether distinct patterns of hippocampal interactions are related to memory retention for Control versus LOC TMS. First, a whole-brain analysis was performed separately for each TMS group to isolate regions that showed increases in functional connectivity with the hippocampus (from Baseline to the average connectivity across Post-Encoding Rest scans) that were positively correlated with next-day associative memory retention (using robust regression in a voxel-wise manner, P < .001, one-tailed test; shown in Figure 6A). Family-wise error (FWE) correction was performed on whole-brain maps using permutation testing. Specifically, on each permutation (N=1000), the pairings between memory retention and changes in connectivity were randomized, correlations were computed, and the size of clusters remaining that exceeded P <.001 significance (one-tailed, showing positively correlations with memory) were stored. The size of the clusters from the true analysis was then compared to the null distribution to estimate significance.
In addition to examining whether individual brain regions survive whole-brain correction in the prior analysis, we also asked whether distinct hippocampal connectivity patterns were related to memory retention across TMS groups. To do this, we used subsampling to repeatedly estimate patterns of hippocampal connectivity (across gray matter voxels) that were related to memory retention for three groups of participants: Control TMS, LOC TMS, and a mixed group of participants (from both TMS groups). This allowed us to test the prediction that hippocampal connectivity patterns related to memory within each group are distinct by comparing the reliability or similarity of these patterns within each TMS group (across subsampling iterations) vs. the similarity of the patterns between TMS groups and the similarity of the mixed TMS group patterns (shown in Figure 6B). This analysis was performed separately to relate hippocampal connectivity to next-day (Figure 6B) and same-day associative memory retention (Figure S4).
On each iteration of the subsampling procedure, a subset of 20 participants was randomly chosen (for Control and LOC TMS groups, 20 out of 26 participants were randomly chosen, and for the mixed TMS group, a random set of 10 participants from each TMS group was chosen to create a group of N=20 participants) and correlations between hippocampal functional connectivity changes and next-day associative memory retention were computed in gray matter voxels to estimate a pattern. A total of 1,000 subsampling estimates were created for each group, and the similarity or correlation between patterns within and between groups was computed. Since this procedure generates a large and arbitrary number of patterns to compare (which would artificially inflate the degrees of freedom in statistical testing), we randomly chose a small number (N=19, the degrees of freedom in the initial correlation) of similarity values from each condition to compare across conditions when performing statistical testing. This was done in a repeated fashion to ensure that the results were reliable; average statistics (unpaired t-tests comparing similarity across conditions) are reported in the results.
fMRI Summary Reactivation Measure
Beyond examining relationships between specific measures (changes in functional connectivity) and memory retention, we used PCA to create a summary measure of reactivation (shown in Figure 5D). PCA was performed for each group (Control and LOC TMS) across five main reactivation measures: 1) LOC reactivation using the classifier approach (change in proportion of volumes classified as a face/object from Baseline to Post-Encoding Rest, averaged across scans and face and object classifiers, shown in Figure 3C), 2) LOC reactivation using the similarity approach (same as classifier approach, but change in the proportion of volumes labeled as high similarity events, shown in Figure S3), 3) Hippocampal reactivation (change in variance explained by top hippocampal encoding PCs from Baseline to Post-Encoding Rest scans, averaged across scans and top four PCs, shown in Figure 4B), 4) correlation between Hippocampal reactivation and encoding strength (correlation between encoding PC strength and Hippocampal reactivation for each participant, shown in Figure 4C), and 5) change in hippocampal-LOC FC from Baseline to Post-Encoding Rest scans (Fisher Z-transformed and averaged across scans). Before performing PCA on each group, these values were z-scored across participants. The top (first) PC is shown in Figure 5D as a vector below each scatterplot, which indicates the weighting of the five reactivation measures. The resulting reactivation summary or component score (shown on x-axis in Figure 5D) is the projection of each participant’s data onto the first PC, which was correlated with associative memory retention using robust regression.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and Algorithms | ||
| MATLAB R2015a | www.mathworks.com | RRID: SCR_001622 |
| SPM8 | www.fil.ion.ucl.ac.uk/spm | RRID: SCR_007037 |
| FSL | www.fmrib.ox.ac.uk/fsl | RRID: SCR_002823 |
| BrainNet Viewer | www.nitrc.org/projects/bnv | RRID: SCR_009446 |
| RStudio | www.rstudio.com | RRID: SCR_000432 |
| ANTs | www.picsl.upenn.edu/ANTS | RRID: SCR_004757 |
| Psychtoolbox | www.psychtoolbox.org | RRID: SCR_002881 |
| Freesurfer | surfer.nmr.mgh.harvard.edu | RRID: SCR_001847 |
| Brainsight | rogue-research.com/tms/brainsight-tms | RRID: SCR_009539 |
| NeuroElf | www.nitrc.org/projects/bvqxtools | RRID: SCR_014147 |
Highlights:
Post-encoding TMS was applied to manipulate awake memory consolidation in humans
Associative memory retention was selectively reduced by TMS to LOC
TMS impaired cortical memory reactivation and hippocampal-cortical interactions
TMS modified patterns of post-encoding hippocampal interactions related to memory
ACKNOWLEDGEMENTS
This work was supported by the National Institutes of Health (MH63901 and MH106280). We thank Matthew Fain for assistance with data analysis, Justin Riddle for help with TMS, and Nima Hejazi for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- 1.McGaugh JL (2000). Memory--a Century of Consolidation. Science (80-.) 287, 248–251. Available at: http://www.sciencemag.org/cgi/doi/10.1126/science.287.5451.248 [Accessed August 6, 2013]. [DOI] [PubMed] [Google Scholar]
- 2.McClelland JL, McNaughton BL, and O’Reilly RC (1995). Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev 102, 419–457. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=7624455. [DOI] [PubMed] [Google Scholar]
- 3.Winocur G, Sekeres MJ, Binns MA, and Moscovitch M (2013). Hippocampal lesions produce both nongraded and temporally graded retrograde amnesia in the same rat. Hippocampus 23, 330–341. [DOI] [PubMed] [Google Scholar]
- 4.Squire LR, Genzel L, Wixted JT, and Morris RG (2015). Memory Consolidation. Cold Spring Harb. Perspect. Biol, 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frankland PW, and Bontempi B (2005). The organization of recent and remote memories. Nat Rev Neurosci 6, 119–30. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15685217 [Accessed August 11, 2013]. [DOI] [PubMed] [Google Scholar]
- 6.Diekelmann S, and Born J (2010). The memory function of sleep. Nat Rev Neurosci 11, 114–26. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20046194 [Accessed August 9, 2013]. [DOI] [PubMed] [Google Scholar]
- 7.Sutherland GR, and McNaughton BL (2000). Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr Opin Neurobiol 10, 180–186. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10753801. [DOI] [PubMed] [Google Scholar]
- 8.O’Neill J, Pleydell-Bouverie B, Dupret D, and Csicsvari J (2010). Play it again: reactivation of waking experience and memory. Trends Neurosci. 33, 220–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20207025 [Accessed August 14, 2013]. [DOI] [PubMed] [Google Scholar]
- 9.Alvarez P, and Squire LR (1994). Memory consolidation and the medial temporal lobe: a simple network model. Proc Natl Acad Sci U S A 91, 7041–5. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=44334&tool=pmcentrez&rendertype=abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mednick SC, Cai DJ, Shuman T, Anagnostaras S, and Wixted JT (2011). An opportunistic theory of cellular and systems consolidation. Trends Neurosci. 34, 504–14. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3183157&tool=pmcentrez&rendertype=abstract [Accessed March 29, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buzsáki G (1989). Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience 31, 551–570. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=2687720. [DOI] [PubMed] [Google Scholar]
- 12.Dupret D, O’Neill J, Pleydell-Bouverie B, and Csicsvari J (2010). The reorganization and reactivation of hippocampal maps predict spatial memory performance. Nat Neurosci 13, 995–1002. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=20639874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tambini A, Ketz N. a., and Davachi L (2010). Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65, 280–290. Available at: http://www.ncbi.nlm.nih.gov/pubmed/20152133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tambini A, and Davachi L (2013). Persistence of hippocampal multivoxel patterns into postencoding rest is related to memory. Proc. Natl. Acad. Sci. U. S. A 110, 19591–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24218550 [Accessed January 23, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murty VP, Tompary A, Adcock RA, and Davachi L (2017). Selectivity in post-encoding connectivity with high-level visual cortex is associated with reward-motivated memory. J. Neurosci 37, 537–545. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.4032-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staresina BP, Alink A, Kriegeskorte N, and Henson RN (2013). Awake reactivation predicts memory in humans. Proc. Natl. Acad. Sci 110, 21159–21164. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24324174 [Accessed December 11, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gruber MJ, Ritchey M, Wang S, Doss MK, and Ranganath C (2016). Post-learning Hippocampal Dynamics Promote Preferential Retention of Rewarding Events. Neuron 89, 1110–1120. Available at: 10.1016/j.neuron.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schapiro AC, McDevitt EA, Rogers TT, Mednick SC, and Norman KA (2018). Human hippocampal replay during rest prioritizes weakly-learned information and predicts memory performance. Nat. Commun, 3920 Available at: https://www.biorxiv.org/content/early/2017/08/06/173021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deuker L, Olligs J, Fell J, Kranz T. a., Mormann F, Montag C, Reuter M, Elger CE, and Axmacher N (2013). Memory Consolidation by Replay of Stimulus-Specific Neural Activity. J. Neurosci 33, 19373–19383. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.0414-13.2013 [Accessed December 4, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z-X, Grady CL, and Moscovitch M (2017). The effect of prior knowledge on post-encoding brain connectivity and its relation to subsequent memory. Neuroimage 167, 211–223. Available at: http://linkinghub.elsevier.com/retrieve/pii/S105381191730959X. [DOI] [PubMed] [Google Scholar]
- 21.Tambini A, and Davachi L (2019). Awake Reactivation of Prior Experiences Consolidates Memories and Biases Cognition. Trends Cogn. Sci 23, 876–890. Available at: https://linkinghub.elsevier.com/retrieve/pii/S1364661319301834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O’Neill J, Senior TJ, Allen K, Huxter JR, and Csicsvari J (2008). Reactivation of experience-dependent cell assembly patterns in the hippocampus. Nat. Neurosci 11, 209–15. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18193040 [Accessed August 8, 2013]. [DOI] [PubMed] [Google Scholar]
- 23.Singer AC, and Frank LM (2009). Rewarded outcomes enhance reactivation of experience in the hippocampus. Neuron 64, 910–21. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2807414&tool=pmcentrez&rendertype=abstract [Accessed August 18, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomperts SN, Kloosterman F, and Wilson MA (2015). VTA neurons coordinate with the hippocampal reactivation of spatial experience. Elife 4, 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yonelinas AP, Ranganath C, Ekstrom AD, and Wiltgen BJ (2019). A contextual binding theory of episodic memory: systems consolidation reconsidered. Nat. Rev. Neurosci 20, 364–375. Available at: 10.1038/s41583-019-0150-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ego-Stengel V, and Wilson MA (2010). Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19816984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, and Zugaro MB (2009). Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci 12, 1222–1223. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=19749750. [DOI] [PubMed] [Google Scholar]
- 28.Nokia MS, Mikkonen JE, Penttonen M, and Wikgren J (2012). Disrupting neural activity related to awake-state sharp wave-ripple complexes prevents hippocampal learning. Front. Behav. Neurosci 6, 84 Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3540934&tool=pmcentrez&rendertype=abstract [Accessed September 24, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van de Ven GM, Trouche S, McNamara CG, Allen K, and Dupret D (2016). Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples. Neuron 92, 968–974. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0896627316307218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roux L, Hu B, Eichler R, Stark E, and Buzsáki G (2017). Sharp wave ripples during learning stabilize the hippocampal spatial map. Nat. Neurosci 20, 845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fernández-Ruiz A, Oliva A, Fermino de Oliveira E, Rocha-Almeida F, Tingley D, and Buzsáki G (2019). Long-duration hippocampal sharp wave ripples improve memory. Science (80-.) 364, 1082–1086. Available at: http://www.sciencemag.org/lookup/doi/10.1126/science.aax0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lustenberger C, Boyle MR, Alagapan S, Mellin JM, Vaughn BV, and Frohlich F (2016). Feedback-Controlled Transcranial Alternating Current Stimulation Reveals a Functional Role of Sleep Spindles in Motor Memory Consolidation. Curr Biol, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ngo H-VV, Martinetz T, Born J, and Mölle M (2013). Auditory closed-loop stimulation of the sleep slow oscillation enhances memory. Neuron 78, 545–53. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23583623 [Accessed September 23, 2013]. [DOI] [PubMed] [Google Scholar]
- 34.Ketz N, Jones A, Bryant N, Clark VP, and Pilly PK (2018). Closed-loop slow-wave tACS improves sleep dependent long-term memory generalization by modulating endogenous oscillations. J. Neurosci [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cairney SA, Guttesen A. á. V., El Marj N, and Staresina BP (2018). Memory Consolidation Is Linked to Spindle-Mediated Information Processing during Sleep. Curr. Biol 28, 948–954.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oudiette D, and Paller KA (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends Cogn. Sci 17, 142–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/23433937 [Accessed August 14, 2013]. [DOI] [PubMed] [Google Scholar]
- 37.Rasch B, Buchel C, Gais S, and Born J (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science (80-.) 315, 1426–1429. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17347444. [DOI] [PubMed] [Google Scholar]
- 38.Foster DJ, and Wilson MA (2006). Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16474382. [DOI] [PubMed] [Google Scholar]
- 39.Ólafsdóttir HF, Bush D, and Barry C (2018). The Role of Hippocampal Replay in Memory and Planning. Curr. Biol 28, R37–R50. Available at: http://linkinghub.elsevier.com/retrieve/pii/S0960982217314410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joo HR, and Frank LM (2018). The hippocampal sharp wave–ripple in memory retrieval for immediate use and consolidation. Nat. Rev. Neurosci 19, 744–757. Available at: 10.1038/s41583-018-0077-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schlichting ML, and Preston AR (2014). Memory reactivation during rest supports upcoming learning of related content. Proc. Natl. Acad. Sci 111, 15845–15850. Available at: http://www.pnas.org/cgi/doi/10.1073/pnas.1404396111 [Accessed October 21, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Voogd LD, Fernández G, and Hermans EJ (2016). Awake reactivation of emotional memory traces through hippocampal–neocortical interactions. Neuroimage 134, 563–572. Available at: http://linkinghub.elsevier.com/retrieve/pii/S105381191630060X. [DOI] [PubMed] [Google Scholar]
- 43.Kark SM, and Kensinger EA (2019). Post-encoding Amygdala-Visuosensory Coupling Is Associated with Negative Memory Bias in Healthy Young Adults. J. Neurosci, 2834–18. Available at: http://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.2834-18.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, and Rothwell JC (2005). Theta burst stimulation of the human motor cortex. Neuron 45, 201–6. Available at: http://www.ncbi.nlm.nih.gov/pubmed/15664172 [Accessed December 12, 2013]. [DOI] [PubMed] [Google Scholar]
- 45.Wischnewski M, and Schutter DJLG (2015). Efficacy and time course of theta burst stimulation in healthy humans. Brain Stimul. 8, 685–692. Available at: 10.1016/j.brs.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 46.Chung SW, Hill AT, Rogasch NC, Hoy KE, and Fitzgerald PB (2016). Use of theta-burst stimulation in changing excitability of motor cortex: A systematic review and meta-analysis. Neurosci. Biobehav. Rev 63, 43–64. Available at: 10.1016/j.neubiorev.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 47.Hubl D, Nyffeler T, Wurtz P, Chaves S, Pflugshaupt T, Lüthi M, von Wartburg R, Wiest R, Dierks T, Strik WK, et al. (2008). Time course of blood oxygenation level-dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience 151, 921–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/18160225 [Accessed December 8, 2014]. [DOI] [PubMed] [Google Scholar]
- 48.Davachi L (2006). Item, context and relational episodic encoding in humans. Curr Opin Neurobiol 16, 693–700. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=17097284. [DOI] [PubMed] [Google Scholar]
- 49.Diana R. a, Yonelinas AP, and Ranganath C (2007). Imaging recollection and familiarity in the medial temporal lobe: a three-component model. Trends Cogn. Sci 11, 379–86. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17707683 [Accessed January 13, 2014]. [DOI] [PubMed] [Google Scholar]
- 50.Mayes A, Montaldi D, and Migo E (2007). Associative memory and the medial temporal lobes. Trends Cogn. Sci 11, 126–135. [DOI] [PubMed] [Google Scholar]
- 51.Guidotti R, Del Gratta C, Baldassarre A, Romani GL, and Corbetta M (2015). Visual Learning Induces Changes in Resting-State fMRI Multivariate Pattern of Information. J. Neurosci 35, 9786–9798. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3920-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bang JW, Sasaki Y, Watanabe T, and Rahnev D (2018). Feature-Specific Awake Reactivation in Human V1 after Visual Training. J. Neurosci 38, 9648–9657. Available at: http://www.jneurosci.org/lookup/doi/10.1523/JNEUROSCI.0884-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Battaglia FP, Benchenane K, Sirota A, Pennartz CMA, and Wiener SI (2011). The hippocampus: hub of brain network communication for memory. Trends Cogn. Sci 15, 310–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21696996 [Accessed September 20, 2013]. [DOI] [PubMed] [Google Scholar]
- 54.Feld GB, and Born J (2017). Sculpting memory during sleep: concurrent consolidation and forgetting. Curr. Opin. Neurobiol 44, 20–27. Available at: 10.1016/j.conb.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 55.Fuentemilla L, Miró J, Ripollés P, Vilà-Balló A, Juncadella M, Castañer S, Salord N, Monasterio C, Falip M, and Rodríguez-Fornells A (2013). Hippocampus-dependent strengthening of targeted memories via reactivation during sleep in humans. Curr. Biol 23, 1769–75. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24012316 [Accessed November 13, 2013]. [DOI] [PubMed] [Google Scholar]
- 56.Dewar MT, Alber J, Cowan N, and Della Sala S (2014). Boosting Long-Term Memory via Wakeful Rest: Intentional Rehearsal Is Not Necessary, Consolidation Is Sufficient. PLoS One 9, e109542 Available at: http://dx.plos.org/10.1371/journal.pone.0109542 [Accessed October 21, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tompary A, Duncan KD, and Davachi L (2015). Consolidation of Associative and Item Memory Is Related to Post-Encoding Functional Connectivity between the Ventral Tegmental Area and Different Medial Temporal Lobe Subregions during an Unrelated Task. J. Neurosci 35, 7326–31. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25972163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sadeh T, Ozubko JD, Winocur G, and Moscovitch M (2014). How we forget may depend on how we remember. Trends Cogn. Sci 18, 26–36. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24246135 [Accessed December 12, 2013]. [DOI] [PubMed] [Google Scholar]
- 59.Sadeh T, Ozubko JD, Winocur G, and Moscovitch M (2016). Forgetting Patterns Differentiate Between Two Forms of Memory Representation. Psychol. Sci 27, 810–820. Available at: http://journals.sagepub.com/doi/10.1177/0956797616638307. [DOI] [PubMed] [Google Scholar]
- 60.Tompary A, and Davachi L (2017). Consolidation Promotes the Emergence of Representational Overlap in the Hippocampus and Medial Prefrontal Cortex. Neuron 96, 228–241.e5. Available at: 10.1016/j.neuron.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Murty VP, DuBrow S, and Davachi L (2018). Decision-making Increases Episodic Memory via Postencoding Consolidation. J. Cogn. Neurosci 26, 1–10. Available at: https://www.mitpressjournals.org/doi/abs/10.1162/jocn_a_01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sandrini M, Censor N, Mishoe J, and Cohen LG (2013). Causal Role of Prefrontal Cortex in Strengthening of Episodic Memories through Reconsolidation. Curr. Biol 23, 2181–4. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24206845 [Accessed December 13, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Censor N, Horovitz SG, and Cohen LG (2014). Interference with Existing Memories Alters Offline Intrinsic Functional Brain Connectivity. Neuron 81, 69–76. Available at: http://linkinghub.elsevier.com/retrieve/pii/S089662731300994X [Accessed January 9, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tunovic S, Press DZ, and Robertson EM (2014). A Physiological Signal That Prevents Motor Skill Improvements during Consolidation. J. Neurosci 34, 5302–5310. Available at: http://www.jneurosci.org/cgi/doi/10.1523/JNEUROSCI.3497-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Galea JM, Albert NB, Ditye T, and Miall RC (2010). Disruption of the dorsolateral prefrontal cortex facilitates the consolidation of procedural skills. J. Cogn. Neurosci 22, 1158–64. Available at: http://www.ncbi.nlm.nih.gov/pubmed/19413472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Robertson EM, Press DZ, and Pascual-Leone A (2005). Off-line learning and the primary motor cortex. J. Neurosci 25, 6372–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16000627 [Accessed September 24, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Weerd P, Reithler J, van de Ven V, Been M, Jacobs C, and Sack AT (2012). Posttraining transcranial magnetic stimulation of striate cortex disrupts consolidation early in visual skill learning. J. Neurosci 32, 1981–8. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22323712 [Accessed October 5, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bang JW, Milton D, Sasaki Y, Watanabe T, and Rahnev D (2019). Post-training TMS abolishes performance improvement and releases future learning from interference. Commun. Biol 2, 1–7. Available at: 10.1038/s42003-019-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cohen D. a, and Robertson EM (2011). Preventing interference between different memory tasks. Nat. Neurosci 14, 953–5. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=3144999&tool=pmcentrez&rendertype=abstract [Accessed September 19, 2013]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Craig M, Dewar MT, Harris MA, Della Sala S, and Wolbers T (2016). Wakeful rest promotes the integration of spatial memories into accurate cognitive maps. Hippocampus 26, 185–193. [DOI] [PubMed] [Google Scholar]
- 71.Craig M, Dewar MT, Della Sala S, and Wolbers T (2015). Rest boosts the long-term retention of spatial associative and temporal order information. Hippocampus 25, 1017–1027. Available at: http://www.ncbi.nlm.nih.gov/pubmed/25620400. [DOI] [PubMed] [Google Scholar]
- 72.Brokaw K, Tishler W, Manceor S, Hamilton K, Gaulden A, Parr E, and Wamsley EJ (2016). Resting state EEG correlates of memory consolidation. Neurobiol. Learn. Mem 130, 17–25. Available at: 10.1016/j.nlm.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 73.de Sousa AF, Cowansage KK, Zutshi I, Cardozo LM, Yoo EJ, Leutgeb S, and Mayford M (2019). Optogenetic reactivation of memory ensembles in the retrosplenial cortex induces systems consolidation. Proc. Natl. Acad. Sci, 201818432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Novitskaya Y, Sara SJ, Logothetis NK, and Eschenko O (2016). Ripple-triggered stimulation of the locus coeruleus during post-learning sleep disrupts ripple/spindle coupling and impairs memory consolidation. Learn. Mem 23, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang YZ, Chen R-S, Rothwell JC, and Wen H-Y (2007). The after-effect of human theta burst stimulation is NMDA receptor dependent. Clin. Neurophysiol 118, 1028–32. [DOI] [PubMed] [Google Scholar]
- 76.Cheeran B, Talelli P, Mori F, Koch G, Suppa A, Edwards M, Houlden H, Bhatia K, Greenwood R, and Rothwell JC (2008). A common polymorphism in the brain-derived neurotrophic factor gene (BDNF) modulates human cortical plasticity and the response to rTMS. J. Physiol 586, 5717–5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hoogendam JM, Ramakers GMJ, and Di Lazzaro V (2010). Physiology of repetitive transcranial magnetic stimulation of the human brain. Brain Stimul. 3, 95–118. [DOI] [PubMed] [Google Scholar]
- 78.Huang YZ, Lu MK, Antal A, Classen J, Nitsche M, Ziemann U, Ridding M, Hamada M, Ugawa Y, Jaberzadeh S, et al. (2017). Plasticity induced by non-invasive transcranial brain stimulation: A position paper. Clin. Neurophysiol 128, 2318–2329. Available at: 10.1016/j.clinph.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 79.Wamsley EJ (2019). Memory Consolidation during Waking Rest. Trends Cogn. Sci 23, 171–173. Available at: 10.1016/j.tics.2018.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tambini A, Berners-Lee A, and Davachi L (2017). Brief targeted memory reactivation during the awake state enhances memory stability and benefits the weakest memories. Sci. Rep 7, 1–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stickgold R, and Walker MP (2013). Sleep-dependent memory triage: evolving generalization through selective processing. Nat. Neurosci 16, 139–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stark CEL, and Squire LR (2001). When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A 98, 12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pitcher D, Duchaine B, Walsh V, Yovel G, and Kanwisher NG (2011). The role of lateral occipital face and object areas in the face inversion effect. Neuropsychologia 49, 3448–53. [DOI] [PubMed] [Google Scholar]
- 84.Gratton C, Lee TG, Nomura EM, and D’Esposito M (2013). The effect of theta-burst TMS on cognitive control networks measured with resting state fMRI. Front. Syst. Neurosci 7, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nee DE, and D’Esposito M (2017). Causal evidence for lateral prefrontal cortex dynamics supporting cognitive control. Elife 6, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tambini A, Nee DE, and D’Esposito M (2018). Hippocampal-targeted Theta-burst Stimulation Enhances Associative Memory Formation. J. Cogn. Neurosci 26, 194–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yeo BTT, Krienen FM, Chee MWL, and Buckner RL (2013). Estimates of segregation and overlap of functional connectivity networks in the human cerebral cortex. Neuroimage 88C, 212–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Avants BB, Epstein CL, Grossman M, and Gee JC (2008). Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal 12, 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Power JD, Barnes K. a, Snyder AZ, Schlaggar BL, and Petersen SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59, 2142–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Behzadi Y, Restom K, Liau J, and Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. Neuroimage 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schwarzlose RF, Swisher JD, Dang S, and Kanwisher NG (2008). The distribution of category and location information across object-selective regions in human visual cortex. Proc Natl Acad Sci U S A 105, 4447–4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, et al. (2006). An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31, 968–80. [DOI] [PubMed] [Google Scholar]
- 93.Wilson MA, and McNaughton BL (1994). Reactivation of hippocampal ensemble memories during sleep. Science. 265, 676–679. Available at: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8036517. [DOI] [PubMed] [Google Scholar]
- 94.Huffman DJ, and Stark CEL (2014). Multivariate pattern analysis of the human medial temporal lobe revealed representationally categorical cortex and representationally agnostic hippocampus. Hippocampus 24, 1394–403. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24976498 [Accessed October 22, 2014]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Patenaude B, Smith SM, Kennedy DN, and Jenkinson M (2011). A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N, Lupien S, and Evans a C. (2000). Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb. cortex 10, 433–42. Available at: http://www.ncbi.nlm.nih.gov/pubmed/10769253. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets and code used in this study are available from the corresponding author.


