Abstract
Studies in experimental ischemia models by permanent bilateral common carotid artery occlusion (BCCAO) have reported reduced retinal electrophysiological function, coupled with inner retinal degeneration and gliosis. In the current study, we tested the hypothesis that long-term (up to 14 days) BCCAO impairs oxygen delivery (DO2), which affects oxygen metabolism (MO2) and extraction fraction (OEF), electrophysiological function, morphology, and biochemical pathways. Twenty-one rats underwent BCCAO (N=12) or sham surgery (N=9) and were evaluated in separate groups after 3-, 7-, or 14-days. Electroretinography (ERG), optical coherence tomography, blood flow and vascular oxygen tension imaging, morphological and biochemical evaluations were performed in both eyes. Reduced ERG b-wave amplitudes and delayed implicit times were reported at 3-, 7-, and 14-days following BCCAO. Total retinal blood flow, MO2, and DO2 were reduced in all BCCAO groups. OEF was increased in both 3- and 7-day groups, while no significant difference was observed in OEF at 14-days compared to the sham group. At 14-days following BCCAO, total and inner retinal layer thickness were reduced, while the outer nuclear layer thickness and gliosis were increased. There was an increase in nuclei containing fragmented DNA at 3-days following BCCAO. The compensatory elevation in OEF following BCCAO did not meet the tissue demand, resulting in the subsequent reduction of MO2. The associations between retinal MO2, DO2, and retinal function were shown to be significant in the sequalae of persistent ischemia. In sum, measurements of DO2, MO2, and OEF may become useful for characterizing salvageable tissue in vision threatening pathologies.
Keywords: retinal ischemia, chronic hypoperfusion, oxygen metabolism, oxygen delivery, oxygen extraction fraction, penumbra
Introduction:
Retinal ischemia has been implicated to play a significant role in vision-threatening diseases, including retinal vascular occlusions, ocular ischemic syndrome, retinopathy of prematurity, diabetic retinopathy, and glaucoma [1]. Specifically, the high metabolic demands of light processing events in the retina are sustained by both oxygen and glucose delivered from two arterial systems: the choroid which predominately supplies the outer retina, and the central retinal artery which predominately supplies the inner retina [2]. If the metabolic demands are not met due to reduced retinal circulation caused by stenosis or occlusion, the retina undergoes ischemic injury. Due to the heterogeneity of comorbidities associated with retinal ischemia, and since there are no current available treatments for retinal ischemia, prevention requires an interdisciplinary approach from clinicians: ophthalmologists, vascular surgeons, cardiologists, and neurologists [3]. However, further research is merited to understand the pathophysiological sequelae of retinal ischemia in hopes of finding effective interventional targets and improving the clinical management of retinal ischemia and other nonretinal ischemic pathologies [4].
If hypoperfusion levels reach ischemic thresholds, neurons and other retinal cell-types become starved for oxygen (hypoxia) and activate anaerobic glycolysis pathways, eventually activating apoptotic and inflammatory pathways [3]. Therefore, retinal hypoperfusion by experimental permanent bilateral common carotid artery occlusion (BCCAO) has been used to study impairment of retina due to ischemia [5-9]. Slakter et al. were among the pioneers to study retinal pathology in the BCCAO model for incomplete retinal ischemia [10]. Other investigators later showed BCCAO causes a reduction in retinal function measured by electroretinography (ERG) [11, 7, 12, 13, 9], alterations in retinal oxygen delivery (DO2), oxygen extraction fraction (OEF), and oxygen metabolism (MO2) immediately following BCCAO [14, 15]. Furthermore, with prolonged BCCAO, retinal degeneration [5, 16-19, 8, 20], Muller cell gliosis [16, 4, 8, 21], as well as cerebral degeneration [22-24, 7] have been reported. In the retinal tissue, regulation of the blood flow under both physiologic and pathologic conditions has been well-established [25, 26]. Nevertheless, once vascular compensation fails to maintain homeostasis of retinal MO2, ischemic injury progresses to cellular death [27]. In ischemic brain pathologies, the cerebral metabolic rate of oxygen, which is similar to retinal MO2, has been shown to be superior in predicting cellular-death and reperfusion-dependent survival of the penumbra compared to other parameters such as OEF [28, 29]. Therefore, it is imperative to measure MO2 as a means to monitor retinal viability and potentially predict ischemia outcome.
We have recently reported decreased DO2, increased OEF, and subsequent reduction in MO2 immediately following BCCAO [15] and evaluated these parameters up to 2 weeks after BCCAO (unpublished data). In the current study, we tested the hypothesis that long-term (up to 14 days) BCCAO impairs DO2, which affects MO2, OEF, electrophysiological function, morphology, and biochemical pathways. The findings may bring further insight into associations of impaired MO2 with visual dysfunction, morphologic and biochemical abnormalities, and potentially help establish prognostic parameters to assess the severity of damage in retinal ischemia.
Materials and Methods:
Animals
All experiments were approved by the University of Southern California Institutional Animal Care and Use Committee, complied with the guidelines of the statement of Use of Animals in Ophthalmic and Vision research by the Association for Research in Vision and Ophthalmology, and reported according to the Animal Research: Reporting of In Vivo Experiments guidelines. The rats were housed in clear, air-filtered cages with 12-hour light/dark cycle and ad lib feeding. A total of 21 adult male Long-Evans rats (age: 12.6±1.7 weeks; mean±SD) (weight: 369±59 g) (Charles River, Wilmington, MA) were studied.
During the BCCAO procedure, rats were anesthetized by exposure to a gaseous mixture consisting of 97.5% oxygen and 2.5% isoflurane. As previously described [15], an anterior midline cervical incision was made, and the right and left common carotid arteries were exposed and ligated with 5-0 silk sutures. The skin was sutured, and the animal was allowed to recover. Surgery in rats from the sham group included exposure of the common carotid arteries without ligature. Rats underwent BCCAO (12 rats) or sham (9 rats) surgery. Separate groups of rats were evaluated at 3-days (4 BCCAO and 4 sham), 7-days (4 BCCAO and 5 sham), or 14-days (4 BCCAO).
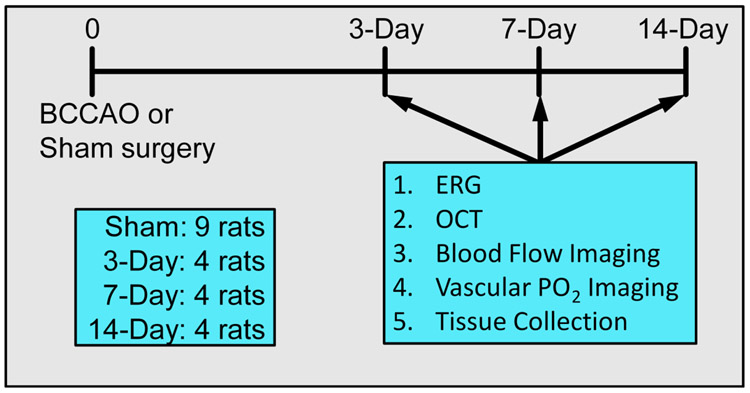
At the time of evaluation, rats were anesthetized intraperitoneally with a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg) and were maintained by supplementary doses during all procedures. The femoral artery was cannulated to administer 2-μm polystyrene fluorescent microspheres (107 particles/mL 0.9% saline) (Life Technologies, Eugene, OR) for blood flow imaging and an oxygen-sensitive molecular probe and Pd-Porphine (20 mg/kg) (Frontier Scientific, Logan, UT) for oxygen tension imaging. Pupils were dilated using phenylephrine 2.5% (Paragon, Portland, OR) and tropicamide 1% (Bausch and Lomb, Tampa, FL). ERG, optical coherence tomography (OCT), blood flow, and oxygen tension imaging were performed. The timeline of experimental procedures is presented in Fig. 1.
Figure 1. Timeline for experimental procedures.
Diagram showing imaging sequence, outcome variables, and then tissue collection post-BCCAO. Rats were divided into four cohorts: sham, 3-day, 7-day, or 14-day BCCAO groups.
Electroretinography
After a two-hour dark adaption period, scotopic ERG was performed using a commercially available device (Handheld Multi-species-ElectroRetinoGraph (HMsERG), Ocuscience, Kansas City, MO, USA). A ground electrode was placed in the proximal portion of the tail skin. The reference needle electrodes were placed approximately 2 cm below each eye and contact lenses with gold-embedded electrodes (MAYO Corporation, Japan) were placed on the corneas using a conductance medium (GenTeal Lubricant Eye Gel (hypromellose 0.3%). From the ERG waveforms, b-wave amplitude and implicit time were obtained at multiple flash intensities. Data from the flash intensity of 1 cd s/m2 were used for analysis.
Spectral-domain optical coherence tomography
Spectral-domain OCT imaging was performed using a commercially available instrument (Spectralis, Heidelberg Engineering, Heidelberg, Germany), as described previously [30]. OCT images were analyzed using the instrument’s commercial software (Heidelberg Eye Explorer 1.9.10.0; Heidelberg Engineering) to measure the total retinal thickness (TRT) as the depth separation between the inner limiting membrane and Bruch’s membrane. Measurements were obtained in regions nasal and temporal to the optic nerve head and averaged.
Blood flow imaging
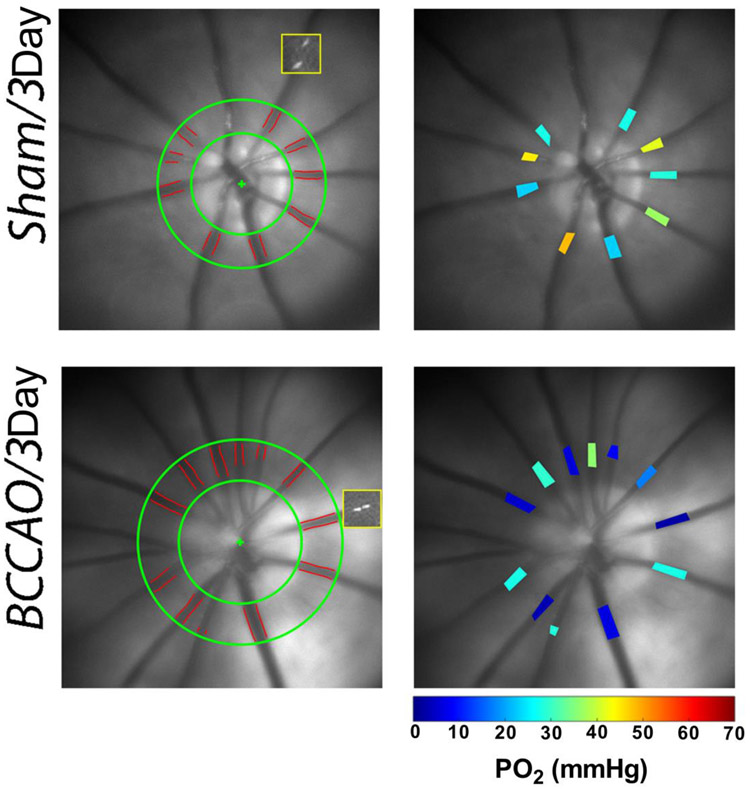
Blood flow imaging was performed as previously described [14, 31-33]. Briefly, venous blood velocity (V) was measured by imaging intravascular fluorescent microspheres and analyzing a sequence of images to measure the displacement of microspheres over time. Venous blood vessel diameter (D) was measured from red-free retinal images. Blood flow was calculated as VπD2/4 for each vein and summed over all veins to calculate the total retinal blood flow (TRBF). Representative examples of blood flow imaging can be seen in Figure 2.
Figure 2. Methodology of blood flow and PO2 imaging.
Left Column: For blood flow imaging, retinal vessel boundaries are outlined (red) in a region of interest (between green circles), the positions of one microsphere at two time points (37 msec apart) are indicated in yellow squares. Right Column: Retinal vascular PO2 is displayed in pseudo-color for all major arteries and veins according to color bar.
Vascular oxygen tension imaging
Retinal vascular oxygen tension (PO2) was measured using our phosphorescence lifetime imaging system as described previously [14, 31-33]. Briefly, phosphorescence lifetime of Pd-porphine in the retinal vessels was determined using a frequency-domain approach and converted to PO2 measurements using the Stern-Volmer equation. The vascular O2 content was calculated as the sum of oxygen bound to hemoglobin and dissolved in blood. Arterial (O2A) and venous (O2V) oxygen contents were calculated by averaging values in retinal arteries and veins, respectively. Arteriovenous oxygen content difference (O2AV) was calculated as O2A-O2V. Representative examples of PO2 imaging can be seen in Figure 2.
Oxygen delivery, metabolism, extraction fraction
Retinal DO2, MO2, and OEF were calculated using the following equations: DO2 = TRBF x O2A, MO2 = TRBF x O2AV, OEF = MO2/DO2.
Histology and ex vivo imaging
After imaging, the rats were euthanized and eyes were enucleated, post-fixed in Davidson’s fixative, and paraffin embedded. Globes were opened such that the central section included the 3 and 9 o’clock plane, and 5-micron sections were cut to include the pupil, optic nerve, and the retina directly temporal and nasal to the nerve. After staining of sections, digital images of the retinal sections were generated using a Zeiss Axio Imager 2 microscope (Zeiss, Jena, Germany).
Cell layer thickness
For thickness measurements, retinal sections were stained with Hematoxylin and Eosin (H&E) using a standard procedure. Digital images in regions both nasal and temporal to the optic nerve head were acquired. Images were analyzed in ImageJ software by manually identifying retinal layer boundaries at 48μm intervals to provide 10 measurements per region. The software automatically recorded distances between retinal layer boundaries and calculated thickness of inner retina (IRL), inner plexiform Layer (IPL), inner nuclear layer (INL), outer plexiform layer (OPL), outer nuclear layer (ONL), photoreceptor layer (PR), and total retinal thickness (TRT). The thickness of combined nerve fiber layer (NFL) and retinal ganglion cell layer (RGCL) was calculated as: IR-(IPL+INL). Finally, measurements for each layer were averaged over the 2 regions.
Cellular apoptosis and immunoreactivity for GFAP
Immunofluorescence staining was performed using a previously described method [34]. Briefly, the slides were deparaffinized, boiled for 20 min in a citrate antigen retrieval buffer (10 mM Sodium Citrate, 0.05% Tween 20, pH 6.0), blocked for 30 min with 10% normal donkey serum, incubated with the primary antibodies rabbit anti-GFAP (1:200; ab7260, RRID:AB_305808), and incubated with a secondary antibody conjugated with FITC and then 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI) (nuclear marker, color blue) (Jackson ImmunoResearch). Negative control staining was performed by omitting the primary antibody, and no staining was observed in the imaging of this control.
Retinal cellular apoptosis was evaluated by using the terminal deoxynucleotidyl transferase dUTP nick and labeling (TUNEL) assay (Roche, USA). For quantitative analysis, the number of TUNEL positive cells (green) colocalized with DAPI-stained throughout one retinal section was manually counted, without knowledge of group allocation.
Relative immunoreactivities for GFAP were estimated using a previously reported grading criteria [16]: 0=no signal, 1=few positive glial endfeet in GCL or scattered processes in other layers, 2=few labelled processes reaching from GCL to OLM, and 3=most labelled processes reaching from RGL to the OLM. In both GFAP and TUNEL analysis, data were obtained from 4 rats from each of the following groups: sham, 3-, 7-, and 14-day BCCAO.
Statistical analysis
Since there were no statistically significant differences in MO2, DO2, OEF, or TRBF between the sham groups (3 days and 7 days), data in both sham groups were combined to generate a single sham group. Compiled data in both eyes were classified into 4 groups: sham, 3-day BCCAO, 7-day BCCAO, and 14-day BCCAO. Compiled O2A, O2V, and TRBF data were evaluated by group, and 3 outliers (values more than three times the interquartile range) were removed, leaving data in a total of 39 eyes. Linear mixed model analysis was performed with group (sham, 3-day, 7-day, 14-day) and eye (right, left) as fixed factors and animal as a random factor. Linear mixed model analysis was also used to determine the slope estimates relating parameters. After normality of the data distribution was confirmed, one-way ANOVA with post-hoc Tukey HSD test was used to determine differences in GFAP and TUNEL data among groups. SSPS Statistics, Version 24 (IBM Armonk, New York), was used for linear mixed model analysis. GraphPad Prism 8 (La Jolla, CA, USA) was used for ANOVA testing and generating the figures. All statistical tests were 2-sided, and significance was accepted at P≤0.05.
Data availability
The authors confirm that the data are available on request to the corresponding author.
Results.
Retinal electrophysiology
Under BCCAO, previous studies have established retinal dysfunction as measured by reductions in both a-wave and b-wave amplitudes and increased implicit times [7, 11]. The b-wave amplitude depends in part on the function of retinal Muller and bipolar cells. [35, 36, 9, 37, 19]. Also, the latency of the b-wave amplitude (implicit time) is a measure of cellular dysfunctions in the inner nuclear layer [38] and has been shown to be altered in glaucoma and early diabetic retinopathy [39, 40]. Therefore, to evaluate the function of inner retinal neurons, ERG waveforms were analyzed to measure the b-wave amplitude and implicit time.
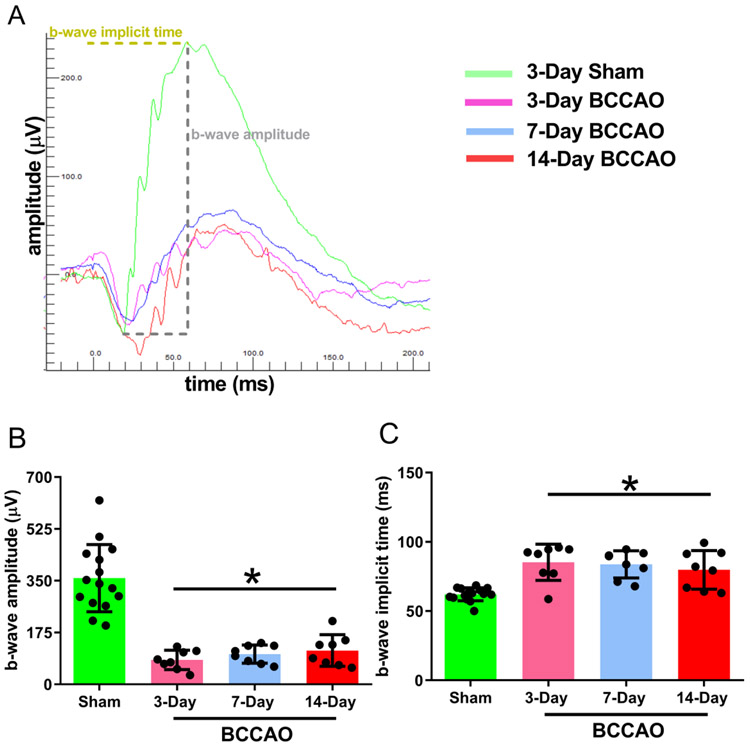
Figure 3A shows a representative example of an ERG waveform and demonstrates the method for measurements of the b-wave amplitude and implicit time. Mean and standard deviation of ERG b-wave amplitudes stratified by group are shown in Figure 3B. In the sham group, the ERG b-wave amplitude was 359±114 μV. Compared to the sham group, ERG b-wave amplitude was reduced in 3-day (β=−277 μV; t34=−8.0; 95% confident interval (CI):−347, −206), 7-day (β=−257 μV; t34=−7.4; 95% CI:−328, −186), and 14-day (β=−245 μV; t34=−7.1; 95% CI: −316, −174) BCCAO groups (Linear mixed model analysis; P<0.001). No significant differences were detected in b-wave amplitudes between 3-, 7-, and 14-day BCCAO groups (P>0.05).
Figure 3. Retinal electrophysiological function following long-term BCCAO.
(A) Representative scotopic ERG responses from sham (green curve), 3-day (pink curve), 7-day (blue curve), and 14-day (red curve) groups. The b-wave amplitude was measured from the a-wave peak to the b-wave peak as indicated. To measure implicit time, the time interval from the flash onset to the b-wave peaks was used. Quantification of the average (B) amplitudes and (C) implicit times from sham (N=15), 3-day (N=8), 7-day (N=8), and 14-day (N=8) groups. The data are presented as mean ± SD. *p<0.05 vs Sham.
Mean and standard deviation of ERG b-wave implicit times stratified by group are shown in Figure 3C. In the sham group, the implicit time was 62.1±4.7 ms. The implicit time was increased in 3-day (β=23.3 ms; t16=4.4; 95% CI: 12.0, 34.7), 7-day (β=21.8 ms; t16=4.1; 95% CI: 10.5, 33.2), and 14-day (β=17.8 ms; t16=3.3; 95% CI:6.5, 29.2) BCCAO groups (Linear mixed model analysis; P<0.01). No significant differences were detected in implicit times between 3-, 7-, and 14-day BCCAO groups (P>0.05).
Retinal blood flow, oxygen delivery, metabolism, and extraction fraction
Given that the main arterial blood supply to the retina in rats comes from the ophthalmic artery, which is downstream to the common carotid artery, BCCAO reduces the blood supply to the retina. Indeed, reduced but not completely extinguished retinal blood flow resulting from BCCAO has been reported [14, 6, 15]. Furthermore, immediately after BCCAO, reductions in MO2 and DO2 have been previously demonstrated [15]. However, the effect of prolonged BCCAO on DO2, MO2, and OEF has not been completely investigated. Since retinal metabolism is mostly oxidative [32], assessment of MO2and DO2may offer insight into the physiological state of retinal tissue in BCCAO.
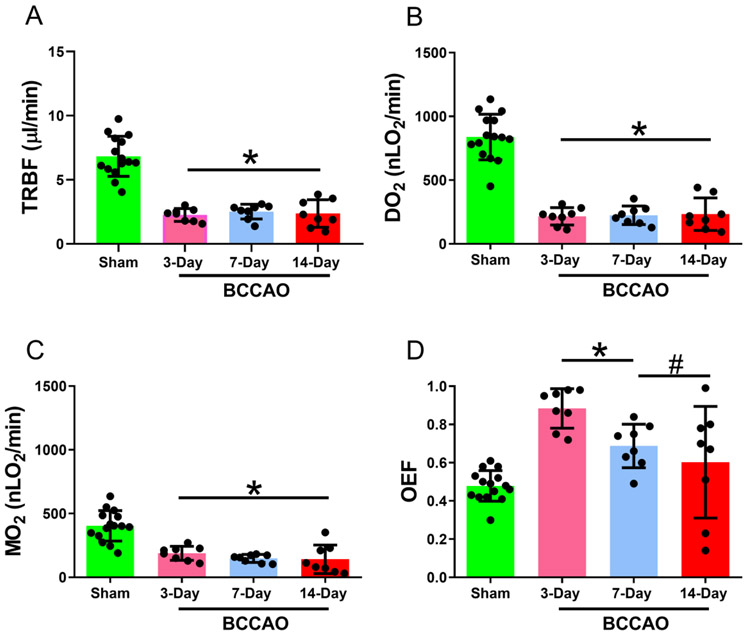
Mean and standard deviation of TRBF stratified by group are shown in Figure 4A. In the sham group, TRBF was 6.83±1.6 μl/min. Compared to the sham group, TRBF was reduced in all BCCAO groups: 3-day (β= −4.57 μl/min; t16=−8.4; 95% CI: −5.7, −3.4), 7-day (β= −4.30 μl/min; t16=−7.9; 95% CI: −5.5, −3.2), and 14-day (β= −4.46 μl/min; t16=−8.2; 95% CI:−5.6, −3.3) (Linear mixed model analysis; P<0.001). No significant differences were detected in TRBF between 3-, 7-, and 14-day BCCAO groups (P>0.05).
Figure 4. Retinal oxygen delivery and metabolism following long-term BCCAO.
(A) Total retinal blood flow (TRBF), (B) oxygen delivery (DO2), (C) oxygen metabolism (MO2), and (D) oxygen extraction fraction (OEF) in sham (N=15), 3-day (N=8), 7-day (N=8), and 14-day (N=8) BCCAO groups. The data are presented as mean ± SD. *p<0.05 vs Sham. #p<0.05 vs 3-day BCCAO.
Mean and standard deviation of DO2, MO2, and OEF stratified by group are shown in Figure 4B-D, respectively. In the sham group, DO2 MO2, and OEF were 838±179 nLO2/min, 404±119 nLO2/min, and 0.48±0.08, respectively.
Compared to the sham group, DO2 was reduced in 3-day (β=−622 nLO2/min; t34=−10.4; 95% CI:−743, −501), 7-day (β=−614 nLO2/min; t34=−10.3; 95% CI:−735, −493), and 14-day (β=−605 nLO2/min; t34=−10.1; 95% CI:−726, −484) BCCAO groups (Linear mixed model analysis; P<0.001). No significant difference was detected in DO2 between 3-, 7-, and 14-day BCCAO groups (P>0.05).
Similarly, MO2 was reduced in 3-day (β=−216 nLO2/min; t16=−4.5; 95% CI: −317, −115), 7-day (β=−256 nLO2/min; t16=−5.4; 95% CI: −357, −155), and 14-day (β=−262 nLO2/min; t16=−5.5; 95% CI:−363, −161) BCCAO groups (Linear mixed model analysis; P<0.001). No significant differences were detected in MO2 between 3-, 7-, and 14-day BCCAO groups (P>0.05).
OEF was increased in both the 3-day (β=0.405; t34=5.85; 95% CI: 0.26, 0.55; P<0.001) and 7-day (β=0.209; t34=3.02; 95% CI: 0.07, 0.35; P<0.01) BCCAO groups, while no significant difference was observed between OEF at 14-days compared to the sham group (P>0.05). OEF was higher in the 3-day BCCAO group compared to the 7-day and 14-day BCCAO groups (Linear mixed model analysis; P<0.01). No significant differences were detected in OEF between 7- and 14-day BCCAO groups (P>0.05).
Retinal thickness
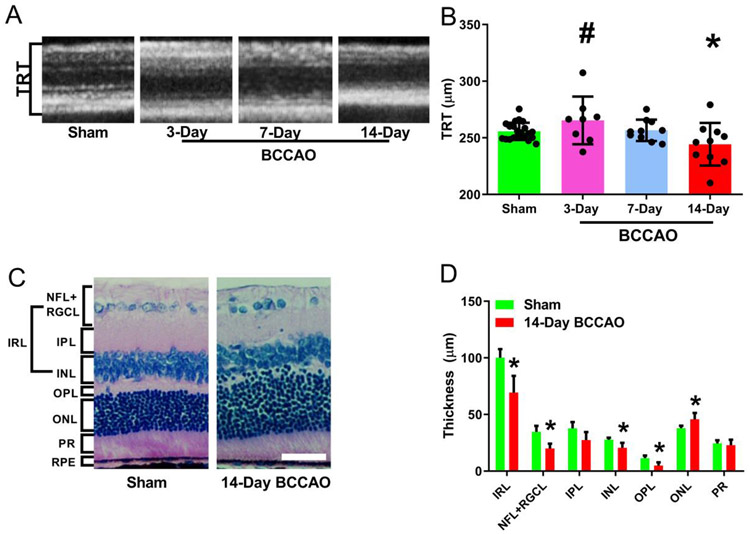
Investigators have used OCT imaging to report thinning of the mouse retina after 6 weeks of BCCAO [9]. Also, studies using H&E staining reported retinal degeneration and thinning following BCCAO in rats [5, 16-19, 8, 20]. Therefore, to detect abnormalities in the retinal structure and quantify the extent of retinal anatomical damage, OCT was used to measure TRT in vivo, and histology was used to measure thickness of individual cell layers ex vivo.
Representative examples of OCT images acquired in rats from sham and 14-day BCCAO groups are shown in in Figure 5A. Mean and standard deviation of TRT stratified by group are shown in Figure 5B. TRT was 255±7 μm in the sham group and was reduced in the 14-day group (β=−16.3 μm; t16=−2.3; 95% CI: −31.2, −1.4; p<0.05). Compared to the sham group, TRT in 3-day and 7-day BCCAO groups were not significantly different (P>0.05). TRT was higher in the 3-day BCCAO group compared to the 14-day BCCAO group (Linear mixed model analysis; P<0.05). No significant differences were detected in TRT between 3- and 7-day BCCAO groups, nor between 7- and 14-day BCCAO groups (P>0.05).
Figure 5. Morphological changes and thickness measurements in the retinal layers following long-term BCCAO.
(A) Representative optical coherence tomography images of the retina. (B) The resulting images were analyzed using the Heidelberg Spectralis system software to measure the total thickness. (C) Example Hematoxylin and Eosin (H&E) sections were used to compare layer thickness (original magnification 400X). (D) H&E thickness quantification of each layer in different groups. The data were presented as mean ± SD. *p<0.05 vs Sham. Layer name abbreviations: NFL: nerve fiber layer; RGCL: retinal ganglion cell layer; IPL: inner plexiform layer; INL: inner nuclear layer; IRL: inner retinal layer; OPL: outer plexiform layer; ONL: outer nuclear layer; PR: photoreceptor layer; RPE: retinal pigment epithelium. Scale bar= 50 μm.
Representative examples of H&E images obtained in rats from the sham and 14-day BCCAO groups are shown in Figure 5C. Both images revealed organized and demarcated retinal cell layers. Mean and standard deviation of retinal layer thicknesses stratified by group is shown in Figure 5D. In the sham group, the thickness for each retinal layer was as follows: ONL: 37.8±2.2 μm, NFL/RGCL: 34.6±5.4 μm, INL: 27.7±1.7 μm, OPL: 11.5±2.2 μm, IR 100±7.61 μm, IPL: 37.7±5.6 μm, and PR: 24.4±2.8 μm. Compared to the sham group, there was a reduction in the thickness of NFL/RGCL (β=−14.6 μm; t11=−5.5; 95% CI: −20, −8.8), INL (β=−7.21 μm; t5=−2.6; 95% CI: −14.4, −0.02), OPL (β=−6.70 μm; t5=−3.18; 95% CI: −12.1, −1.3), and IR (β=−30.7 μm; t5=−3.4; 95% CI: −54, −7.7), while the ONL thickness was increased (β=7.88 μm; t5=2.7; 95% CI: 0.4, 15.3) in the 14-day BCCAO group (Linear mixed model analysis; P<0.05). No significant differences were observed in the IPL and PRL thicknesses of BCCAO groups compared to the sham group (P>0.05).
Retinal cellular gliosis and DNA fragmentation
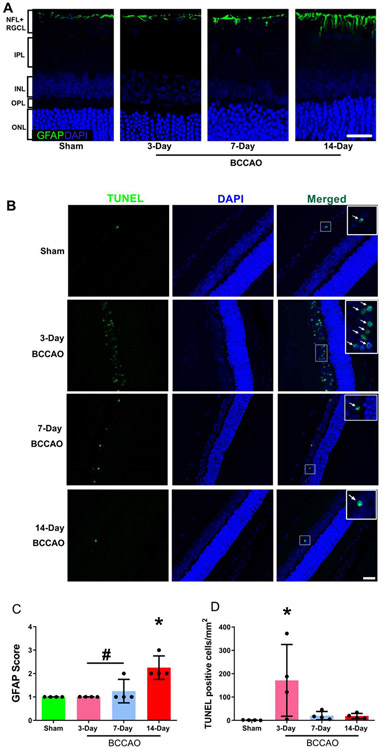
GFAP localization, representing reactive astrocytes and Mueller cell processes, is normally expressed in the nerve fiber layer (NFL) and the GCL, and it is a sensitive, ubiquitous marker that increases expression in response to retinal disease and injury [11, 16, 41]. Also, since activation of extrinsic and intrinsic pathways of caspase-mediated cell death occur in events of ischemia [42, 43, 3], we evaluated cellular apoptosis in the retina via labeling with TUNEL following chronic hypoperfusion.
Representative examples of GFAP images are shown in Figure 6A. Mean and standard deviation of GFAP expression stratified by group are shown in Figure 6C. GFAP expression had a score of 1.0±0.0, 1.0±0.0, 1.25±0.5, and 2.25±0.5 in the sham, 3-, 7-, and 14-day BCCAO groups, respectively. Gliosis was increased in the 14-day BCCAO group compared to 3-day, 7-day BCCAO, and sham groups (One-way ANOVA; Tukey’s test; n=4; F(3, 12)=11.3; p<0.01). No significant differences were detected in GFAP expression between 3-day BCCAO, 7-day BCCAO, and sham groups (P>0.05).
Figure 6. Gliosis and DNA damage following long-term BCCAO.
(A) Representative glial fibrillary acidic protein (GFAP) in sham, 3-day, 7-day, and 14-day BCCAO groups. GFAP is presented as the green color and cell nuclei are presented as blue color. (B) Fragmented DNA in the nuclei was evaluated by TUNEL staining in the retinal tissue. TUNEL-positive cells were presented as the green color, while cell nuclei were presented as blue color. The white arrows indicate co-expression in the same cell. (C) Semi-quantitative analysis of GFAP was performed on the highest most severe region of the retina. (D) Semi-quantitative analysis of TUNEL-positive cells was performed across the whole retina of each animal. Sham (N=4), 3-day (N=4), 7-day (N=4), and 14-day (N=4) BCCAO groups. The data are presented as mean ± SD. *p<0.05 vs Sham. #p<0.05 vs 14-day. Scale bar= 20 μm.
Representative examples of TUNEL images are shown in Figure 6B. Mean and standard deviation of TUNEL-positive cells stratified by group are shown in Figure 6D. The numbers of TUNEL-positive cells per section were 1.0±0.8, 171±154, 20.2±18, and 18.7±11 in the sham, 3-, 7-, and 14-day BCCAO groups, respectively. Compared to the sham group, TUNEL-positive cells were increased in the 3-day BCCAO group (One-way ANOVA; Tukey’s test; n=4; F(3, 12)=4.2; p<0.05), but not significantly different in 7-day and 14-day BCCAO groups (P>0.05). TUNEL-positive cells were marginally increased in the 3-day BCCAO group compared to 7-day and 14-day BCCAO groups (p=0.07). No significant differences were detected in TUNEL-positive cells between 7-day and 14-day BCCAO groups (P>0.05).
Relations of retinal electrophysiological with DO2 and MO2
To date, the role of reductions in DO2 and MO2 on retinal functional due to BCCAO have not been clarified. For this reason, we investigated if DO2, MO2, OEF were related to retinal function following BCCAO.
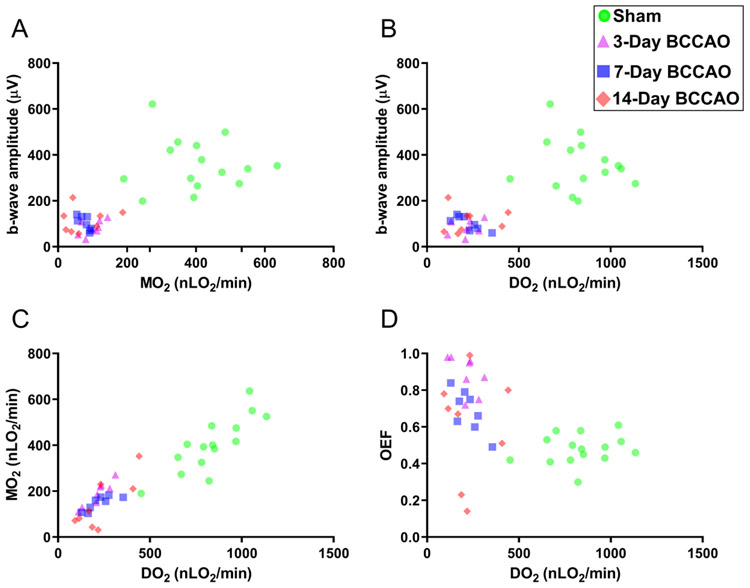
Based on compiled data from all groups, the relationships of ERG b-wave amplitude with MO2 and DO2 are depicted in Figures 7A-B. ERG b-wave amplitude was related to MO2 (β=0.6 μV/100nLO2/min; t24=4.77; 95% CI: 0.34, 0.87) and DO2 (β=0.35 μV/100nLO2/min; t36=7.2; 95% CI: 0.25, 0.45) (Linear mixed model analysis; P<0.01).
Figure 7. Relations of retinal electrophysiological function with oxygen delivery and metabolism following long-term BCCAO.
Based on compiled data from all groups, the relationships of the ERG b-wave amplitude with oxygen metabolism (MO2) and oxygen (DO2) are displayed in panels A and B, respectively. The relationships of MO2 and oxygen extraction fraction (OEF) with DO2 are presented in panels C and D, respectively. Sham (circle), 3-day (triangle), 7-day (square), and 14-day (diamond) BCCAO groups.
The relationships of MO2 and OEF with DO2 are depicted in Figures 7C-D. MO2 was related to DO2 (β=0.43; t23=13.3; 95% CI: 0.36, 0.50), whereas OEF was inversely related to DO2 (β= −0.35 unit/μLO2/min; t22= −3.78; 95% CI: −0.54, −0.16) (Linear mixed model analysis; P<0.01). Although we assumed linearity, a more exact non-linear mathematical model that demonstrates these relationships has been previously reported [15, 14].
Discussion
In the present study, we report: (1) reduced MO2, decreased ERG b-wave amplitudes, and delayed b-wave implicit times at 3-, 7-, and 14-days following BCCAO; (2) direct linear relationships between ERG b-wave amplitude and both MO2 and DO2; (3) increased OEF at 3- and 7-days following BCCAO, but similar to values in the sham group after 14 days of BCCAO, concurrent with reduced DO2 and MO2; (4) reduced TRT and inner retinal layer thickness and increased ONL thickness at 14-days following BCCAO; (5) increased nuclei containing fragmented DNA at 3-days following BCCAO; and (6) increased gliosis at 14-day following BCCAO. Thus, we confirmed our hypothesis that long-term (up to 14 days) BCCAO impairs DO2, which affects MO2, OEF, electrophysiological function, morphology, and biochemical pathways.
Functional outcome and correlations
In the present study, we demonstrated decreased MO2 up to 14 days post BCCAO. The finding of reduced MO2 is consistent with our previously published studies on the immediate response of retinal tissue to BCCAO [15, 14]. MO2 depends on both the oxygen supplied by the retinal circulation and the fraction of this oxygen extracted from the blood. Therefore, under conditions of decreased TRBF, MO2 may be sustained by increased OEF in efforts to maintain metabolic function and prevent tissue damage. However, in our study, MO2 was reduced in 3-, 7-, and 14-days post BCCAO which suggests reduced oxygen consumption by surviving cells and/or a lower number of consuming cells due to cellular death.
Reduced ERG b-wave amplitudes and delayed implicit times were demonstrated up to 14-days following BCCAO. In agreement with our findings, previous studies have reported reduced b-wave amplitude at 3 days [12], 7 days [11, 13, 7], and 14-days [7], and increased implicit times [7, 44, 11] following BCCAO. For the first time in the literature, we showed that the reduced ERG b-wave amplitude was linearly associated with impaired MO2 and DO2. This finding demonstrates a dependence of visual function on MO2 and DO2.
OEF following BCCAO
The high metabolic rate of the retina requires a specific oxygen availability threshold be met by the retinal circulations to maintain ATP synthesis and function. Therefore, a reduction in TRBF is expected to result in a compensatory increase in OEF to maintain retinal metabolic function. However, under conditions of misery perfusion, when maximum OEF is achieved and O2V becomes negligible and all available oxygen is extracted, oxidative metabolism may no longer be supported, resulting in cellular dysfunction or injury [45]. Similarly in the brain, after middle cerebral artery occlusion in patients, increased OEF was observed as cerebral blood flow was decreased to maintain the cerebral metabolic rate of oxygen, but when severe occlusions occurred, the metabolic rate of oxygen became reduced since OEF could not offset the decreased blood flow [46]. Thus, elevated OEF may also represent threatened but potentially salvageable tissue and may sustain tissue MO2. However, in our study, compensatory response in OEF at both 3- and 7-days post BCCAO did not meet the tissue demand, such that increased OEF near the maximum value was concurrent with reduced MO2. With the downward trending MO2, OEF was no longer elevated at 14-days following BCCAO and returned to sham levels, suggesting a new state for the remaining cells with equal reductions in DO2 and MO2.
Cellular degeneration and death
Since BCCAO has been shown to severely diminish but incompletely extinguish blood flow, Osborne et al. reported significant photoreceptor loss was only detectable at 3-9 months following BCCAO [13]. In our current study, limited measurable morphological damage resulted from ischemia from 3- to 7-days. However, at 14-days following BCCAO, we reported a significant reduction in total retinal thickness, specifically in the inner retinal layers, in agreement with reports by others [11, 16, 19, 8, 21]. Comparably, in patients with an inferotemporal branch retinal artery occlusion, OCT images acquired over time showed an acute increase in TRT due to edema, but retinal thinning rapidly occurred as cellular loss supervened, to the point where differentiation was lost between the IPL and INL/OPL after 2 months (Ritter, Sacu et al. 2012).
Paralleling previous BCCAO reports that investigated cell-death after 5-, 10- and 15-days of BCCAO [3], we found an increase in nuclei containing fragmented DNA at 3-days following BCCAO. Thus, interventions prior to 3-days are needed to mitigate DNA damage and cell death. Additionally, investigating chronic hypoperfusion induced cerebral degeneration, clinicians have shown that stroke lesion volumes may continue to increase up to 60 days from symptom onset if no recanalization occurs [47]. Taken together, future studies are needed to characterize reperfusion interventions which may prevent subsequent retinal degeneration following hypoperfusion and minimize permanent visual dysfunction.
Delayed onset of gliosis
At 14 days following BCCAO, Muller cells, the principal glial cells of the retina, were activated to undergo gliosis. This resulted in hypertrophy and increased expression of intermediate filaments, e.g., increased GFAP expression: a non-specific, sensitive, and ubiquitous response to retinal disease and injury [48]. Whereas Muller cells typically establish both the blood-retinal barrier and the regulation of water movement across the glio-vascular interface, in retinal ischemia they divergently increase vascular permeability through the secretion of factors such as vascular endothelial growth factor (VEGF) and matrix metalloproteinases that proteolytically degrade tight junctions in retinal endothelial cells [49]. Therefore, the blood-retinal barrier degeneration contributes to the eventual degeneration of retinal neurons in ischemia. Although Muller cell responses may be neuroprotective, when the pathological insult has overcome endogenous defenses, reactive gliosis is triggered and exacerbates disease progression-- directly and indirectly damaging neurons and surrounding vasculature, inhibiting tissue repair, and increasing vascular permeability of toxic compounds and pro-inflammatory molecules [50, 51]. While the effect of retinal gliosis on visual function has not been investigated in BCCAO models, Noailles et al. reported that lipopolysaccharide induced activation of reactive gliosis resulted in reduced ERG b-wave amplitude and accelerated disease progression in P23H transgenic rats [52]. In our study, impaired oxygen delivery and metabolism were the primary factors affecting ERG responses following BCCAO, though gliosis may have also contributed to the observed reduced ERG responses at 14-days following BCCAO. Previous studies have reported gliosis to further increase beyond 14-days of BCCAO [11, 16], but we did not investigate these time points in this study. Therefore, gliosis may also be potentially considered as a target to prevent further scaring and subsequent cellular degeneration.
The sequalae of “tissue at risk”
With low levels of blood flow, it is possible that some of the retinal tissue retained structural integrity but not function, and was in fact in an “ischemic penumbra” state, as originally described by Astrup [53]. In BCCAO, hypoperfusion does not equally deprive all retinal cells of oxygen and nutrients, e.g., the relative degree of hypoxia and/or anoxia may depend on the distance of cells from the nearest capillary as well as the distance along the vessel from the arterial end to the venous end [54]. Illustrated by the Krogh cylinder oxygen transport model, as oxygen molecules leave the capillary through the capillary wall and enter surrounding tissue in ischemia, the oxygen demand of proximal tissue would first be satisfied, whereas distant tissue may suffer from a want of oxygen [55]. In our study, the cells furthest from the nearest capillary would therefore be most vulnerable to reduced DO2. In ischemic pathologies, McLeod et al. proposed three cellular states to exist in the retina: normoxic, hypoxic, and anoxic [56]. Based on McLeod’s retinal classification of tissue, we outline these three potential categories: i) anoxic tissue that does not receive enough nourishment to survive and rapidly becomes irreversibly committed to progressive degeneration and death; ii) hypoxic tissue that has a marginal oxygen and nutrient supply and loses function, but remains viable (penumbral tissue at risk for death); and iii) normoxic tissue that has an adequate supply of oxygen and nutrients and retains function (not at risk for death). Without intervention, hypoxic retinal tissue at risk has a marginal oxygen and nutrient supply and usually progresses to anoxic death via apoptotic pathways. However, when some cells die or reperfusion occurs, oxygen and nutrients may then become available to the hypoxic tissue, so that it can recover and no longer be at risk for cellular death. In the brain, it has been shown that the “tissue at risk” may undergo irreversible cellular damage and subsequent necrosis in the first hours, and if reperfusion does not occur, irreversible apoptosis may propagate across the ischemic penumbra after several hours or days [57]. Additionally, apoptosis prototypically recruits penumbral tissue to the ischemic core in the brain [58]. Our finding of increased apoptotic cells at 3-days following BCCAO implies the highest loss of cells likely occurred prior to 3 days of retinal hypoperfusion.
In the brain, total cerebral blood flow levels from 30-40% of normal can sustain the penumbra for 30 to 48 hours [59]. In the current study, total retinal blood flow levels were between 34 and 37% of the sham level in all BCCAO groups. Therefore, it is plausible that some of the retinal tissue may have been at a threshold to sustain a state of penumbra for up to 48 hours, given that apoptosis can occur within 2-24 hours, and peaks 24-48 hours following hypoperfusion [60, 61]. Moreover, under low cerebral blood flow conditions, McBride et al. reported that delayed recanalization at either 3-, 7-, or 14-day post occlusion of the middle cerebral artery improved function of the penumbra compared to the function of the penumbra in non-reperfused rats-- 3-day reperfusion reporting the highest efficacy [62]. Future studies are warranted to characterize salvageable retinal tissue and determine the optimal reperfusion treatment window to reduce subsequent cellular degeneration in retinal ischemic related pathologies, where perfusion, though reduced, would be sufficient to facilitate survival.
Relating the brain to the retina
As an extension of the diencephalon, the retina is considered part of the central nervous system, and, therefore, the retina and brain share many physiological similarities [63]. For example, the blood-retinal barrier is composed of microvascular endothelial cells that are connected by tight junctions and encompassed by astroglial and Muller cells, resembling the blood-brain barrier, mediating selective diffusion of molecules, and maintaining homeostasis. Furthermore, studies have reported associations between retinal microvascular abnormalities (such as arteriovenous nicking [64, 65], retinal hemorrhage [64], and arteriolar narrowing [66]) and brain infarcts and/or white matter lesions [63]. In different rat models of cerebral ischemia or hypoperfusion, Kalesnykas et al. reported increased retinal gliosis, functional impairment, retinal layer thinning, and increased protein markers of ischemia-related stress responses [42]. Taken together, the retina may serve as a window to the brain for advancing knowledge of the pathophysiology of cerebral ischemic injury and development of strategies for prevention and therapeutic intervention. Nevertheless, further investigations are merited to understand the association of ischemic damage between the retina and brain following permanent BCCAO.
Potential treatments
In ischemic neural pathologies, there is a heterogeneity of pathways involved in injury progression, and to achieve neuroprotection, therapies must be developed to target multiple pathways. One such treatment option is endogenously expressed selenoproteins. For example, in a middle cerebral artery occlusion mouse model (MCAO), Amani et al. reported that intraperitoneal pretreatment with selenium nanoparticles, modified with OX26 monoclonal antibodies (mAB) to increase delivery to the brain via transferrin receptor-mediated endocytosis, improved functional outcome via activation/inhibition of several pathways [67]. In detail, selenoprotein therapy activated metabolic proteins, oxidative defense systems, and functional properties of the hippocampal neurons, while inhibiting inflammatory, autophagy, and apoptotic cell death pathways. Other investigators have argued that gold nanoparticles, ranging from 20-40 nm in size, may also show promise as a therapeutic option [67]. However, in a MCAO mouse model, one-hour post treatment with gold nano particles (20-40 nm) modified with OX26 mAB increased reactive oxygen species stress and neuronal loss [68]. Given the potential of endogenous nanoparticles, as well as many other treatments, a variety of animal models are needed to more accurately represent the many subgroups of patients with retinal or cerebral ischemia/stroke and to possibly identify ideal, novel applications for promising neuroprotective agents. Taken together, early reperfusion in combination with “magic-bullet” treatments may attenuate the adverse effects associated with ischemia in the brain and retina.
Limitations
We describe several limitations in the current study. First, we are limited in generalizing our results because we only investigated the effects of BBCAO in male Long-Evan rats. For instance, there are variations in injury threshold and collateralization capacity [69] according to species/strains, sex, and age. Second, duration of anesthesia and age of animals may influence the response of the retinal tissue to ischemia. Grinberg et al. have shown blood flow in the forebrain to be reduced by 25-65% in rats under ketamine-xylazine anesthesia [70]. Therefore, measurements of DO2 and vascular compensatory response may be affected by duration of anesthesia, such that values in 7-day BCCAO group in the current study were lower compared to our measurements in the same groups studied under a shorter anesthesia duration and different age group (unpublished data). However, the anesthesia duration did not impact the relative changes induced by BCCAO on MO2 and biochemical protein expressions. Third, technical factors may have limited provision of absolute value of measurements. Specifically, constants in the Stern-Volmer equation, hemoglobin concentrations, oxygen-hemoglobin dissociation curve, and pH of the arterial blood (and assuming the arterial and venous blood have the same pH) may be different within the retinal tissue environment. Nevertheless, these constants would not significantly impact the relative changes induced by BCCAO. Fourth, it was assumed that the same retinal region was supplied by retinal circulation at different durations of BCCAO with no contribution from the choroidal circulation. When oxygen consumption by photoreceptor cells is reduced, more oxygen may be supplied to inner retinal neurons thereby lowering MO2 measurements, which is based on the rate that oxygen is extracted from the retinal circulation. Finally, all the oxygen extracted from the retinal vasculature may not have been used for energy metabolism. Although the majority of oxygen is used for metabolism, some oxygen may be used for the synthesis of hormones, proteins, water, and etc.
Summary
In conclusion, associations between retinal MO2, DO2, and retinal function were shown to be significant in the sequalae of persistent ischemia. The compensatory elevation in OEF following BCCAO did not meet the tissue demand, resulting in the subsequent reduction of MO2. Permanent reduction in DO2 resulted in cell apoptosis after 3-days of BCCAO, followed by increased gliosis, retinal thinning, reduction in OEF, and sustained MO2 impairment of the surviving cells after 14-days of BCCAO. Measurements of DO2, MO2, and OEF may become useful for characterizing salvageable tissue in vision threatening pathologies; thus, improving clinical management of disparate ischemic pathologies by therapeutically targeting an optimal temporal window as improved therapeutic modalities become available.
Acknowledgements
The authors thank James Burford for performing all animal procedures.
Sources of Funding
This work was supported by NEI grants, EY017918 and EY029220, and Research to Prevent Blindness Foundation.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
MS holds a patent for the oxygen imaging technology. The authors have no conflict of interest.
Research Involving Animals
All applicable international, national, and/or institutional guidelines for animal care and use were followed.
References
- 1.Szabo A, Danyadi B, Bognar E, Szabadfi K, Fabian E, Kiss P et al. Effect of PACAP on MAP kinases, Akt and cytokine expressions in rat retinal hypoperfusion. Neuroscience Letters. 2012;523(2):93–8. doi: 10.1016/j.neulet.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 2.Szabadfi K, Mester L, Reglodi D, Kiss P, Babai N, Racz B et al. Novel Neuroprotective Strategies in Ischemic Retinal Lesions. Int J Mol Sci. 2010;11(2):544–61. doi: 10.3390/ijms11020544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du R, Meng Z-Y, Wang J-L, Wang Y-L. Efficacy of Osthole in Management of Hypoperfused Retina. J Ophthalmol. 2018;2018:6178347. doi: 10.1155/2018/6178347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guo XJ, Tian XS, Ruan Z, Chen YT, Wu L, Gong Q et al. Dysregulation of neurotrophic and inflammatory systems accompanied by decreased CREB signaling in ischemic rat retina. Exp Eye Res. 2014;125:156–63. doi: 10.1016/j.exer.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Stevens WD, Fortin T, Pappas Bruce A. Retinal and Optic Nerve Degeneration After Chronic Carotid Ligation. Stroke. 2002;33(4):1107–12. doi: 10.1161/01.STR.0000014204.05597.0C. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y, Fan S, Li J, Wang Y-l. Bilateral Common Carotid Artery Occlusion in the Rat as a Model of Retinal Ischaemia. Neuroophthalmology. 2014;38(4):180–8. doi: 10.3109/01658107.2014.908928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qin Y, Ji M, Deng T, Luo D, Zi Y, Pan L et al. Functional and morphologic study of retinal hypoperfusion injury induced by bilateral common carotid artery occlusion in rats. Scientific Reports. 2019;9(1):80. doi: 10.1038/s41598-018-36400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Werling D, Reglodi D, Banks WA, Salameh TS, Kovacs K, Kvarik T et al. Ocular Delivery of PACAP1–27 Protects the Retina From Ischemic Damage in Rodents. Invest Ophthalmol Vis Sci. 2016;57(15):6683–91. doi: 10.1167/iovs.16-20630. [DOI] [PubMed] [Google Scholar]
- 9.Crespo-Garcia S, Reichhart N, Skosyrski S, Foddis M, Wu J, Figura A et al. Individual and temporal variability of the retina after chronic bilateral common carotid artery occlusion (BCCAO). PLoS One. 2018;13(3). doi: 10.1371/journal.pone.0193961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Slakter JS, Spertus AD, Weissman SS, Henkind P. An Experimental Model of Carotid Artery Occlusive Disease. American Journal of Ophthalmology. 1984;97(2):168–72. doi: 10.1016/S0002-9394(14)76086-6. [DOI] [PubMed] [Google Scholar]
- 11.Barnett NL, Osborne NN. Prolonged bilateral carotid artery occlusion induces electrophysiological and immunohistochemical changes to the rat retina without causing histological damage. Exp Eye Res. 1995;61(1): 83–90. [DOI] [PubMed] [Google Scholar]
- 12.Blixt FW, Johansson SE, Johnson L, Haanes KA, Warfvinge K, Edvinsson L. Enhanced Endothelin-1 Mediated Vasoconstriction of the Ophthalmic Artery May Exacerbate Retinal Damage after Transient Global Cerebral Ischemia in Rat. PLoS One. 2016;11(6):e0157669. doi: 10.1371/journal.pone.0157669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osborne NN, Safa R, Nash MS. Photoreceptors are preferentially affected in the rat retina following permanent occlusion of the carotid arteries. Vision Research. 1999;39(24):3995–4002. doi: 10.1016/S0042-6989(99)00127-3. [DOI] [PubMed] [Google Scholar]
- 14.Blair NP, Felder AE, Tan MR, Shahidi M. A Model for Graded Retinal Ischemia in Rats. Transl Vis Sci Technol. 2018;7(3). doi: 10.1167/tvst.7.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karamian P, Burford J, Farzad S, Blair NP, Shahidi M. Alterations in Retinal Oxygen Delivery, Metabolism, and Extraction Fraction During Bilateral Common Carotid Artery Occlusion in Rats. Invest Ophthalmol Vis Sci. 2019;60(8):3247–53. doi: 10.1167/iovs.19-27227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yamamoto H, Schmidt-Kastner R, Hamasaki DI, Yamamoto H, Parel J-M. Complex neurodegeneration in retina following moderate ischemia induced by bilateral common carotid artery occlusion in Wistar rats. Exp Eye Res. 2006;82(5):767–79. doi: 10.1016/j.exer.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Mester L, Szabo A, Atlasz T, Szabadfi K, Reglodi D, Kiss P et al. Protection against chronic hypoperfusion-induced retinal neurodegeneration by PARP inhibition via activation of PI-3-kinase Akt pathway and suppression of JNK and p38 MAP kinases. Neurotox Res. 2009;16(1):68–76. doi: 10.1007/s12640-009-9049-6. [DOI] [PubMed] [Google Scholar]
- 18.Szabadfi K, Danyadi B, Kiss P, Tamas A, Fabian E, Gabriel R et al. Protective effects of vasoactive intestinal peptide (VIP) in ischemic retinal degeneration. J Mol Neurosci. 2012;48(3):501–7. doi: 10.1007/s12031-012-9774-9. [DOI] [PubMed] [Google Scholar]
- 19.Danyadi B, Szabadfi K, Reglodi D, Mihalik A, Danyadi T, Kovacs Z et al. PACAP application improves functional outcome of chronic retinal ischemic injury in rats-evidence from electroretinographic measurements. J Mol Neurosci. 2014;54(3):293–9. doi: 10.1007/s12031-014-0296-5. [DOI] [PubMed] [Google Scholar]
- 20.Joachim SC, Renner M, Reinhard J, Theiss C, May C, Lohmann S et al. Protective effects on the retina after ranibizumab treatment in an ischemia model. PLoS One. 2017;12(8):e0182407. doi: 10.1371/journal.pone.0182407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Werling D, Banks WA, Salameh TS, Kvarik T, Kovacs LA, Vaczy A et al. Passage through the Ocular Barriers and Beneficial Effects in Retinal Ischemia of Topical Application of PACAP1–38 in Rodents. Int J Mol Sci. 2017;18(3). doi: 10.3390/ijms18030675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tasdemiroglu E Mild hypothermia fails to protect late hippocampal neuronal loss following forebrain cerebral ischaemia in rats. Acta Neurochir (Wien). 1996; 138(5):570–8; discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 23.Nanri M, Miyake H, Murakami Y, Matsumoto K, Watanabe H. GTS-21, a nicotinic agonist, attenuates multiple infarctions and cognitive deficit caused by permanent occlusion of bilateral common carotid arteries in rats. Jpn J Pharmacol. 1998;78(4):463–9. [DOI] [PubMed] [Google Scholar]
- 24.Antonawich FJ, Federoff HJ, Davis JN. BCL-2 transduction, using a herpes simplex virus amplicon, protects hippocampal neurons from transient global ischemia. Exp Neurol. 1999;156(1):130–7. doi : 10.1006/exnr.1998.7004. [DOI] [PubMed] [Google Scholar]
- 25.Kur J, Newman EA, Chan-Ling T. Cellular and physiological mechanisms underlying blood flow regulation in the retina and choroid in health and disease. Prog Retin Eye Res. 2012;31(5):377–406. doi : 10.1016/j.preteyeres.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pournaras CJ, Rungger-Brandle E, Riva CE, Hardarson SH, Stefansson E. Regulation of retinal blood flow in health and disease. Prog Retin Eye Res. 2008;27(3):284–330. doi: 10.1016/j.preteyeres.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 27.Country MW. Retinal metabolism: A comparative look at energetics in the retina. Brain Research. 2017;1672:50–7. doi: 10.1016/j.brainres.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 28.Touzani O, Young AR, Derlon JM, Baron JC, MacKenzie ET. Progressive impairment of brain oxidative metabolism reversed by reperfusion following middle cerebral artery occlusion in anaesthetized baboons. Brain Res. 1997;767(1): 17–25. doi: 10.1016/s0006-8993(97)00515-5. [DOI] [PubMed] [Google Scholar]
- 29.An H, Ford AL, Chen Y, Zhu H, Ponisio R, Kumar G et al. Defining the Ischemic Penumbra using Magnetic Resonance Oxygen Metabolic Index. Stroke. 2015;46(4):982–8. doi: 10.1161/strokeaha.114.008154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair NP, Tan MR, Felder AE, Shahidi M. Retinal Oxygen Delivery, Metabolism and Extraction Fraction and Retinal Thickness Immediately Following an Interval of Ophthalmic Vessel Occlusion in Rats. Sci Rep. 2019;9(1):8092. doi: 10.1038/s41598-019-44250-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wanek J, Teng PY, Blair NP, Shahidi M. Inner retinal oxygen delivery and metabolism in streptozotocin diabetic rats. Invest Ophthalmol Vis Sci. 2014;55(3): 1588–93. doi: 10.1167/iovs.13-13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wanek J, Teng PY, Blair NP, Shahidi M. Inner retinal oxygen delivery and metabolism under normoxia and hypoxia in rat. Invest Ophthalmol Vis Sci. 2013;54(7):5012–9. doi: 10.1167/iovs.13-11887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wanek J, Teng PY, Albers J, Blair NP, Shahidi M. Inner retinal metabolic rate of oxygen by oxygen tension and blood flow imaging in rat. Biomed Opt Express. 2011;2(9):2562–8. doi: 10.1364/boe.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zheng W, Matei N, Pang J, Luo X, Song Z, Tang J et al. Delayed recanalization at 3 days after permanent MCAO attenuates neuronal apoptosis through FGF21/FGFR1/PI3K/Caspase-3 pathway in rats. Exp Neurol. 2019;320:113007. doi: 10.1016/j.expneurol.2019.113007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlman I The Electroretinogram: ERG. In: Kolb H, Fernandez E, Nelson R, editors. Webvision: The Organization of the Retina and Visual System. Salt Lake City (UT)1995. [PubMed] [Google Scholar]
- 36.Wen R, Oakley B. K(+)-evoked Müller cell depolarization generates b-wave of electroretinogram in toad retina. Proc Natl Acad Sci U S A. 1990;87(6):2117–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Y, Yu B, Xiang YH, Han XJ, Xu Y, So KF et al. Changes in retinal morphology, electroretinogram and visual behavior after transient global ischemia in adult rats. PLoS One. 2013;8(6):e65555. doi: 10.1371/journal.pone.0065555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992;8(2):107–26. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- 39.Holopigian K, Seiple W, Lorenzo M, Carr R. A comparison of photopic and scotopic electroretinographic changes in early diabetic retinopathy. Invest Ophthalmol Vis Sci. 1992;33(10):2773–80. [PubMed] [Google Scholar]
- 40.Velten IM, Horn FK, Korth M, Velten K. The b-wave of the dark adapted flash electroretinogram in patients with advanced asymmetrical glaucoma and normal subjects. Br J Ophthalmol. 2001;85(4):403–9. doi: 10.1136/bjo.85.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin F-L, Lin C-H, Ho J-D, Yen J-L, Chang H-M, Chiou GCY et al. The natural retinoprotectant chrysophanol attenuated photoreceptor cell apoptosis in an N-methyl-N-nitrosourea-induced mouse model of retinal degenaration. Scientific Reports. 2017;7:41086. doi: 10.1038/srep41086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalesnykas G, Tuulos T, Uusitalo H, Jolkkonen J. Neurodegeneration and cellular stress in the retina and optic nerve in rat cerebral ischemia and hypoperfusion models. Neuroscience. 2008;155(3):937–47. doi: 10.1016/j.neuroscience.2008.06.038. [DOI] [PubMed] [Google Scholar]
- 43.Chidlow G, Holman MC, Wood JPM, Casson RJ. Spatiotemporal Characterization of Optic Nerve Degeneration after Chronic Hypoperfusion in the Rat. Invest Ophthalmol Vis Sci. 2010;51(3): 1483–97. doi: 10.1167/iovs.09-4603. [DOI] [PubMed] [Google Scholar]
- 44.Block F, Sontag KH. Differential effects of transient occlusion of common carotid arteries in normotensive rats on the somatosensory and visual system. Brain Res Bull. 1994;33(5):589–93. doi: 10.1016/0361-9230(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 45.McLeod D Ischemic penumbra in retina endures: vascular neuropathology is reconciled. Neural Regen Res. 2016;11(5):737–9. doi: 10.4103/1673-5374.181367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Z, Li Y. Cortical Cerebral Blood Flow, Oxygen Extraction Fraction, and Metabolic Rate in Patients with Middle Cerebral Artery Stenosis or Acute Stroke. AJNR Am J Neuroradiol. 2016;37(4):607–14. doi: 10.3174/ajnr.A4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pialat JB, Wiart M, Nighoghossian N, Adeleine P, Derex L, Hermier M et al. Evolution of lesion volume in acute stroke treated by intravenous t-PA. J Magn Reson Imaging. 2005;22(1):23–8. doi: 10.1002/jmri.20363. [DOI] [PubMed] [Google Scholar]
- 48.Jünemann AGM, Rejdak R, Huchzermeyer C, Maciejewski R, Grieb P, Kruse FE et al. Elevated vitreous body glial fibrillary acidic protein in retinal diseases. Graefes Arch Clin Exp Ophthalmol. 2015;253(12):2181–6. doi: 10.1007/s00417-015-3127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichenbach A, Wurm A, Pannicke T, Iandiev I, Wiedemann P, Bringmann A. Muller cells as players in retinal degeneration and edema. Graefes Arch Clin Exp Ophthalmol. 2007;245(5):627–36. doi: 10.1007/s00417-006-0516-y. [DOI] [PubMed] [Google Scholar]
- 50.de Hoz R, Rojas B, Ramírez AI, Salazar JJ, Gallego BI, Triviño A et al. Retinal Macroglial Responses in Health and Disease. Biomed Res Int. 2016;2016. doi: 10.1155/2016/2954721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ganesh BS, Chintala SK. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS One. 2011;6(3):e18305. doi: 10.1371/journal.pone.0018305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Noailles A, Maneu V, Campello L, Lax P, Cuenca N. Systemic inflammation induced by lipopolysaccharide aggravates inherited retinal dystrophy. Cell Death Dis. 2018;9(3):350. doi: 10.1038/s41419-018-0355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Astrup J, Siesjo BK, Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981; 12(6):723–5. [DOI] [PubMed] [Google Scholar]
- 54.Teng PY, Blair NP, Wanek J, Shahidi M. Oxygen tension and gradient measurements in the retinal microvasculature of rats. Graefes Arch Clin Exp Ophthalmol. 2012;250(3):361–7. doi: 10.1007/s00417-011-1859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krogh A The number and distribution of capillaries in muscles with calculations of the oxygen pressure head necessary for supplying the tissue. J Physiol. 1919;52(6):409–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McLeod D, Beatty S. Evidence for an enduring ischaemic penumbra following central retinal artery occlusion, with implications for fibrinolytic therapy. Prog Retin Eye Res. 2015;49:82–119. doi: 10.1016/j.preteyeres.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 57.Paciaroni M, Caso V, Agnelli G. The concept of ischemic penumbra in acute stroke and therapeutic opportunities. Eur Neurol. 2009;61(6):321–30. doi: 10.1159/000210544. [DOI] [PubMed] [Google Scholar]
- 58.Ramos-Cabrer P, Campos F, Sobrino T, Castillo J. Targeting the ischemic penumbra. Stroke. 2011;42( 1 Suppl):S7–11. doi: 10.1161/strokeaha.110.596684. [DOI] [PubMed] [Google Scholar]
- 59.Lee DH, Kang DW, Ahn JS, Choi CG, Kim SJ, Suh DC. Imaging of the Ischemic Penumbra in Acute Stroke. Korean J Radiol. 2005;6(2):64–74. doi: 10.3348/kjr.2005.6.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elmore S Apoptosis: A Review of Programmed Cell Death. Toxicol Pathol. 2007;35(4):495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40(5):e331–9. doi: 10.1161/strokeaha.108.531632. [DOI] [PubMed] [Google Scholar]
- 62.McBride DW, Wu G, Nowrangi D, Flores JJ, Hui L, Krafft PR et al. Delayed Recanalization Promotes Functional Recovery in Rats Following Permanent Middle Cerebral Artery Occlusion. Transl Stroke Res. 2018;9(2):185–98. doi: 10.1007/s12975-018-0610-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.London A, Benhar I, Schwartz M. The retina as a window to the brain-from eye research to CNS disorders. Nat Rev Neurol. 2013;9(1):44–53. doi: 10.1038/nrneurol.2012.227. [DOI] [PubMed] [Google Scholar]
- 64.Wong TY, Klein R, Couper DJ, Cooper LS, Shahar E, Hubbard LD et al. Retinal microvascular abnormalities and incident stroke: the Atherosclerosis Risk in Communities Study. The Lancet. 2001;358(9288):1134–40. doi: 10.1016/S0140-6736(01)06253-5. [DOI] [PubMed] [Google Scholar]
- 65.Cheung N, Mosley T, Islam A, Kawasaki R, Sharrett AR, Klein R et al. Retinal microvascular abnormalities and subclinical magnetic resonance imaging brain infarct: a prospective study. Brain. 2010; 133(7): 1987–93. doi: 10.1093/brain/awq127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wong TY, Klein R, Sharrett AR, Couper DJ, Klein BEK, Liao D-P et al. Cerebral White Matter Lesions, Retinopathy, and Incident Clinical Stroke. JAMA. 2002;288(1):67–74. doi: 10.1001/jama.288.1.67. [DOI] [PubMed] [Google Scholar]
- 67.Amani H, Habibey R, Shokri F, Hajmiresmail SJ, Akhavan O, Mashaghi A et al. Selenium nanoparticles for targeted stroke therapy through modulation of inflammatory and metabolic signaling. Sci Rep. 2019;9(1):6044. doi: 10.1038/s41598-019-42633-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Amani H, Mostafavi E, Alebouyeh MR, Arzaghi H, Akbarzadeh A, Pazoki-Toroudi H et al. Would Colloidal Gold Nanocarriers Present An Effective Diagnosis Or Treatment For Ischemic Stroke? Int J Nanomedicine. 2019;14:8013–31. doi: 10.2147/ijn.s210035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sommer CJ. Ischemic stroke: experimental models and reality. Acta Neuropathol. 2017;133(2):245–61. doi: 10.1007/s00401-017-1667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lei H, Grinberg O, Nwaigwe CI, Hou HG, Williams H, Swartz HM et al. The effects of ketamine-xylazine anesthesia on cerebral blood flow and oxygenation observed using nuclear magnetic resonance perfusion imaging and electron paramagnetic resonance oximetry. Brain Res. 2001;913(2): 174–9. doi: 10.1016/s0006-8993(01)02786-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data are available on request to the corresponding author.