Abstract
Background
Apolipoprotein L1 gene (APOL1) G1 and G2 kidney-risk variants (KRVs) cause chronic kidney disease in African Americans, inducing mitochondrial dysfunction. Modifying factors are required, because a minority of individuals with APOL1 high-risk genotypes develop nephropathy. Given that APOL1 function is pH-sensitive and the pH of the kidney interstitium is <7, we hypothesized the acidic kidney interstitium may facilitate APOL1 KRV-induced mitochondrial dysfunction.
Methods
Human embryonic kidney (HEK293) cells conditionally expressing empty vector (EV), APOL1-reference G0, and G1 or G2 KRVs were incubated in media pH=6.8 or 7.4 for 4, 6 or 8 hours. Genotype-specific pH effects on mitochondrial length (μm) were assessed using confocal microscopy in live cells and Fiji derivative of Image J software with MiNA plug-in. Lower mitochondrial length indicated fragmentation and early dysfunction.
Results
After 6 hours doxycycline (Dox)-induction in pH=6.8, G2-expressing cells had shorter mitochondria (6.54±0.40) than cells expressing EV (7.65±0.72, p=0.02) or G0 (7.46±0.31, p=0.003). After 8 hours Dox-induction in pH=6.8, both G1 (6.21±0.26) and G2-expressing cells had shorter mitochondria (6.46±0.34) than cells expressing EV (7.13±0.32, p=0.002 and p=0.008, respectively) or G0 (7.22±0.45, p=0.003 and p=0.01, respectively). Mitochondrial length in cells incubated in pH=7.4 were comparable after 8 hours Dox-induction regardless of genotype. APOL1 mRNA expression and cell viability were comparable regardless of pH or genotype after 8 hours Dox-induction.
Conclusion
Acidic pH facilitates early mitochondrial dysfunction induced by APOL1 G1 and G2 KRVs in HEK293 cells. We propose that the acidic kidney interstitium may play a role in APOL1-mediated mitochondrial pathophysiology and nephropathy.
Keywords: African American, APOL1, chronic kidney disease, FSGS, interstitial pH, mitochondria
Introduction
Approximately 13% of African Americans (AAs) carry two copies of the apolipoprotein L1 gene (APOL1) G1 and/or G2 kidney-risk variants (KRVs), comprising “APOL1 high-risk genotypes” (G1G1, G2G2, G1G2). These three genotypes confer 5–29 fold higher risk[1] of chronic kidney disease (CKD). However, only 20% of those with high-risk genotypes will develop CKD, indicating that additional stressors are required to initiate CKD. Several reports demonstrate that APOL1 KRVs induce mitochondrial dysfunction[2],[3],[4], potentially via mitochondrial fragmentation/fission[5].
APOL1 protein function is pH sensitive and APOL1 induces pH-dependent ion permeability in phospholipid vesicles and forms pH-gated ion channels in lipid bilayers[6],[7]. In this context, it is intriguing that the widely-expressed APOL1 KRVs appear to only cause kidney disease. The kidney is the only organ with an acidic interstitial pH under physiological conditions[8]. The aim of the present study was to investigate whether the acidic kidney interstitium may facilitate mitochondrial dysfunction induced by APOL1 KRVs. We assessed the degree of mitochondrial fragmentation as an early sign of stressed or dysfunctional mitochondria[9], occurring prior to caspase activation and cell death[10]. We found that APOL1 G1 and G2 KRVs induced mitochondrial fragmentation faster in the cells grown in acidic media pH=6.8 than in the cells grown in control pH=7.4 media. This suggests the acidic kidney interstitium may facilitate early mitochondrial dysfunction induced by APOL1 KRVs.
Methods
Study design
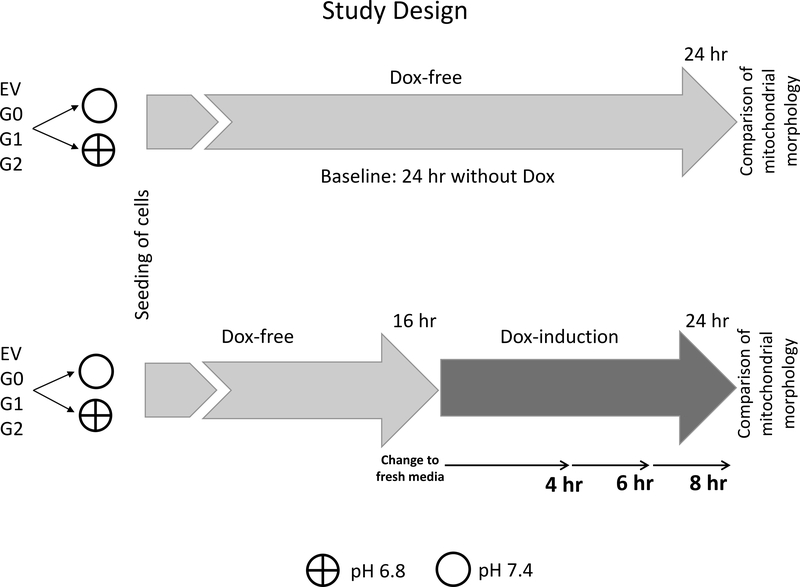
The effects of culture media pH=7.4 or 6.8 on mitochondrial morphology were assessed in human embryonic kidney cells (HEK293) prior to doxyxycline (Dox) exposure (without APOL1 expression), as well as after Dox exposure designed to elicit moderate levels of APOL1 G0, G1 and G2 expression. Effects of culture media pH were assessed after 4, 6 and 8 hours of Dox exposure (Figure 1). HEK293 cells were used because they do not endogenously express APOL1, in contrast to cell cultures created from most other tissues.
Figure 1. Study design.
HEK293 cells were stably transfected with empty vector (EV) or APOL1 reference G0, or G1, or G1 kidney-risk variants in a “Tet-On” system, so that Dox treatment (“Tetracycline-On”) could induce expression of the APOL1 reference or variant gene product. EV G0, G1 and G2 refer to HEK293 Tet-on cells of different APOL1 genotypes.
First, mitochondrial length was evaluated in HEK293 Tet-on cells conditionally expressing EV, APOL1 reference allele G0, and kidney risk variants G1 and G2 in cells cultured in cell growth media pH 6.8 or 7.4 after 24 hours without Dox-induction (without APOL1 expression; upper panel).
Second, mitochondrial length was evaluated in cells incubated in Dox-free cell growth media pH 6.8 or 7.4 for 16 hours and then switched to Dox-induction for 4, 6 and 8 hours in cell growth media pH 6.8 or 7.4 (lower panel).
Transfection and selection for stable clones of HEK293 Tet-on G0, G1, G2 and empty vectors
Complementary DNAs of APOL1 G0 (reference), and the G1 and G2 KRVs were inserted into the NheI- and SalI-digested pTRE2hyg vector (Clontech, Mountain View, CA), as described[11]. Briefly, HEK293 Tet-On 3G cell lines (Clontech, Mountain View, CA) were grown in 100 mm dishes to 30% confluence. Using Lipofectamine transfection reagent (Invitrogen, Waltham, MA), the cells were transfected with 5 μg pTRE2hyg plasmid containing a vector with APOL1 G0, G1, G2 gene variants or an empty vector (EV). Cells were selected 24 hours post-transfection using Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS supplemented with 400 μg/ml G418 (Cellgro, Manassas, VA) and 100 ug/ml hygromycin B (Invitrogen, Waltham, MA). Selection medium was replaced every 48 hours for 20 days. Individual clones were isolated, expanded, and maintained in DMEM media with 10% FBS, 100 μg/ml G418 and 50 ug/ml hygromycin B (referred to as complete DMEM growth media). Eight clones were characterized for each variant; one clone was selected per variant for all experiments. Individual clones with G0, G1 and G2 APOL1 genotypes or empty pTRE2hyg vector were tested for inducible APOL1 expression by RT-PCR, immunoblot analysis and immunofluorescence[2]. HEK293 cells transfected with Tet-on EV do not express APOL1 prior to or after exposure to the tetracycline (Dox)[2]. HEK293 cells transfected with vectors containing Tet-on G0, G1 and G2 APOL1 KRVs express negligible amounts of APOL1 prior to Dox exposure[2].
Cell culture
HEK293 cells were grown in complete DMEM and 5% CO2 atmosphere. It was critical to maintain a stable media pH during all experimental conditions (Figure 1), this was accomplished using established protocols[12],[13],[14],[15]. Media of select pH was created by combining (a) DMEM with (b) Burg’s solution containing bicarbonate (120 mM NaCl, 25 mM NaHCO3, 2.5 mM K2HPO4, 2.0 mM CaCl2, 1.2 mM MgSO4, 4.0 mM Sodium Lactate, 1.0 Mm Sodium Citrate, 6.0 mM L-alanine, and 5.5 mM glucose, pH 7.4 after gassing with 5% CO2), and (c) a solution without bicarbonate (145 mM NaCl, 2.5 mM K2HPO4, 2.0 mM CaC12, 1.2 mM MgSO4, 4.0 mM Sodium Lactate, 1.0 mM Sodium Citrate, 6.0 mM L-alanine, 5.5 mM glucose; pH 7.4). To create media with pH 7.4 (final HCO3− 25 mM), (a) DMEM with 44 mM NaHCO3 was mixed with (b) Burg’s solution and (c) the solution without bicarbonate at a volume ratio 3:5:1, respectively. To create media with pH 6.8 (final HCO3− 6 mM), (a) DMEM without bicarbonate was mixed with (b) Burg’s solution and (c) the solution without bicarbonate at a volume ratio 3:2:4, respectively. Final media also contained 30 u/ml penicillin, 30 pg/ml streptomycin (GIBCO) and 3.3% fetal calf serum (GIBCO). The bicarbonate buffer was chosen because it is a part of an open buffer system in the incubator with 5% CO2 atmosphere. In this case, the buffer is not used up as are most organic buffers under the same experimental conditions. This holds true for up to 24 hours when cells are not proliferating to a great extent and time-averaged buffering is well controlled [16]. Consistent with this rationale and published evidence of the stable pH range of the two media for several hours [12],[13],[14], the phenol red indicator did not change color during the 8 hour period. The pH of the two cell culture media (7.40 and 6.80) was measured with a benchtop pH meter (Fisherbrand accumet AE150) after the media were equilibrated in a cell culture incubator (Sanyo MCO-19AIC) with 5% CO2 at 37°C for 2 hours. This ensured that our solutions and protocols performed as expected[12],[13],[14] and that stable pH value was maintained in the incubator.
Cell viability assay
Stabilized HEK293 Tet-on APOL1 G0, G1 and G2 cells (20,000/well) were seeded on three 96-well plates. After 16 hours of growth in media pH 7.4 or 6.8, the expression of APOL1 gene variants were induced with Dox for 4, 6 or 8 hours. The non-induced cells were also incubated with DMEM media without Dox for an additional 8 hours. Cell viability was measured using the Cytotox 96 lactate dehydrogenase (LDH) viability assay kit (Promega, Madison, WI) per manufacturer instructions. Each cell line (with and without Dox induction) was measured for a minimum of three times in three independent experiments, with values expressed as mean±SD on bar graphs. Complete cell viability was defined as 1.0 (100% viable).
Total RNA isolation, quality control and real-time PCR
Total RNA was isolated from HEK293 Tet-on cells using RNAeasy Mini Kit (Qiagen, Germany). The quantity and quality of isolated RNA were determined by ultraviolet spectrophotometry and electrophoresis, respectively, on the Nanodrop 2000 (Thermo Fisher Scientific, Wilmington, DE) and Agilent 2100 Bioanalyzers (Agilent Technologies, Santa Clara, CA). To determine APOL1 mRNA levels in cells, 200ng of RNA was reverse transcribed with random hexamer primers using the TaqMan RT kit (Applied Biosystems, Foster City, CA). Primers were designed to capture all known APOL1 splice variants. RT-PCR in the presence of SYBRGreen was performed with a Roche 480 Real-Time PCR system (Roche Diagnostics, Germany) using 18S ribosomal RNA for normalization. Primer sequences were described previously[17]. The ΔΔCT method[18] was used to quantify the relative levels of APOL1 mRNA. Fold-changes were normalized to mRNA levels on HEK293 Tet-on G0 cells without Dox induction. All experiments were performed in triplicate and results expressed as mean ± SD.
Confocal microscopy
Confocal imaging was performed in live cells using a sealed, environmentally controlled chamber gassed with 5% CO2 at temperature 37°C, so that cells were continually exposed to stable pH of either 7.4 or 6.8 during imaging. Imaged cells were labeled by MitoTracker Red (Invitrogen), a red-fluorescent dye that stains mitochondria in live cells. Its accumulation in mitochondria depends on the mitochondrial membrane potential, so that staining is accepted as an indicator of mitochondrial functional integrity and a reliable tool to capture mitochondrial morphology. Labeled cells were imaged on a laser, scanning confocal microscope (Olympus FV1200, Waltham, Massachusetts) with 580 nm excitation and 640 nm emission. Olympus super-apochromat oil 100x objective, with numerical aperture of 1.40 was used for imaging. Z-stacks of 0.2 μm thickness were acquired. Three-dimensional imaging allowed morphological evaluation of mitochondria on randomly selected 100μm x 100μm fields. Mitochondrial lengths were scored using Fiji [19], an open-source platform for analysis of biological images and a distribution of the popular open source software of Image J[20] (https://imagej.nih.gov/ij/docs/guide/146-2.html). Fiji was integrated with a plug-in macro toolset Mitochondrial Network Analysis (MiNA). MiNA is freely available at http://github.com/ScienceToolkit/MiNA. MiNA optimizes workflow for analyzing mitochondrial morphology using three-dimensional Z-stacks acquired in Fiji. It uses a binarized copy of the image to estimate the length of mitochondrial structures using a topological skeleton[21]. Mitochondrial length was defined as the length of rods and network branches. During image preprocessing in Fiji, “subtract background” was chosen with a radius of 50. Noise was set at “despeckle”. Enhance Local Contrast (CLAHE) was turned on with a combination of blocksize=9 and slope=4. To match the actual mitochondrial network pattern, “tubeness” was set at “1” for HEK 293 Tet-on cells[22]. A brief description of the principle for mitochondrial length measurement is shown in Supplementary Figure 1. Data are presented as mean±SD and were compared by ANOVA and two-tailed t-test. P<0.05 was considered of nominal statistical significance.
Results
Media pH does not affect mitochondrial morphology in HEK293 without Dox-induced APOL1 expression
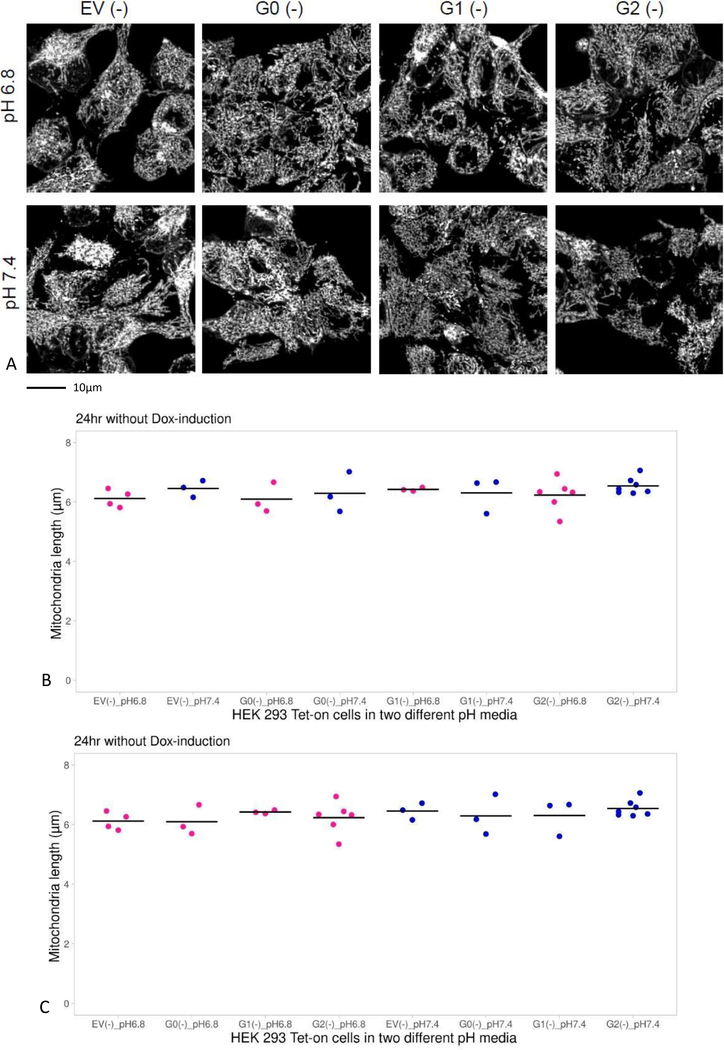
Mitochondrial morphology was examined in HEK293 Tet-on cells with APOL1 G0, G1, or G2 incubated in media pH 7.4 or 6.8 in the absence of Dox (without APOL1 expression). The HEK293 Tet-on cell line with EV incubated in media pH 7.4 or 6.8 in the absence of Dox served as a control. Irrespective of APOL1 genotype or baseline media pH, mitochondrial lengths were comparable for up to 24 hours of incubation (Figures 2A and 2B).
Figure 2. Comparison of mitochondrial length in HEK293 Tet-on cells cultured in different media pH without doxycycline (Dox).
Panel A. Images displaying mitochondrial morphology in cells cultured in growth media pH 6.8 or 7.4 without Dox (absence of APOL1 induction). The cells were labeled with MitoTracker Red, a red-fluorescent dye that labels mitochondria in live cells and imaged on laser scanning confocal microscope with Olympus super-apochromat oil objective at 1000x magnification. The cells were 3D rendered and processed using Fiji software (a derivative of Image J) and MiNA plug in. The cells were kept in a sealed, environmentally controlled chamber gassed with 5% CO2 at temperature 37°C during imaging, so that stable pH of 7.4 or 6.8 was maintained in the same manner as in an incubator.
Panel B. Graphs display a summary of the measurements of mitochondrial length in HEK293 Tet-on cells over 24 hours when APOL1 was not induced. In the absence of Dox, mitochondrial length was comparable in HEK293 cells irrespective of media pH or APOL1 genotype (p=NS, ANOVA); results grouped according to genotype.
Panel C. Same as Panel B, except that data grouped according to media pH (p=NS, ANOVA).
Effect of pH on mitochondrial morphology in HEK293 expressing APOL1
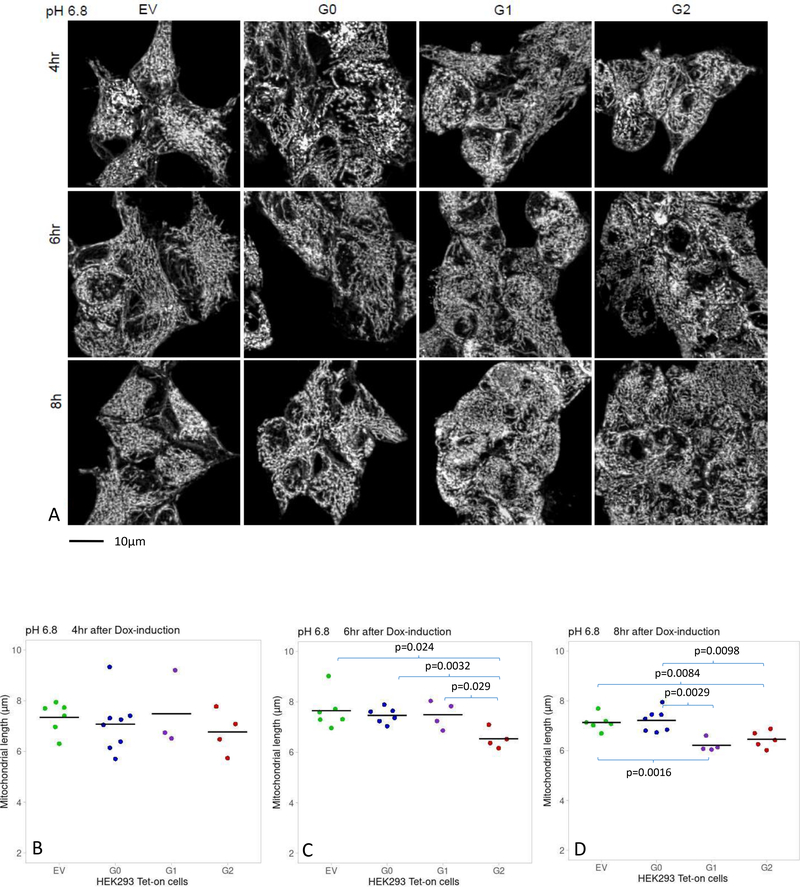
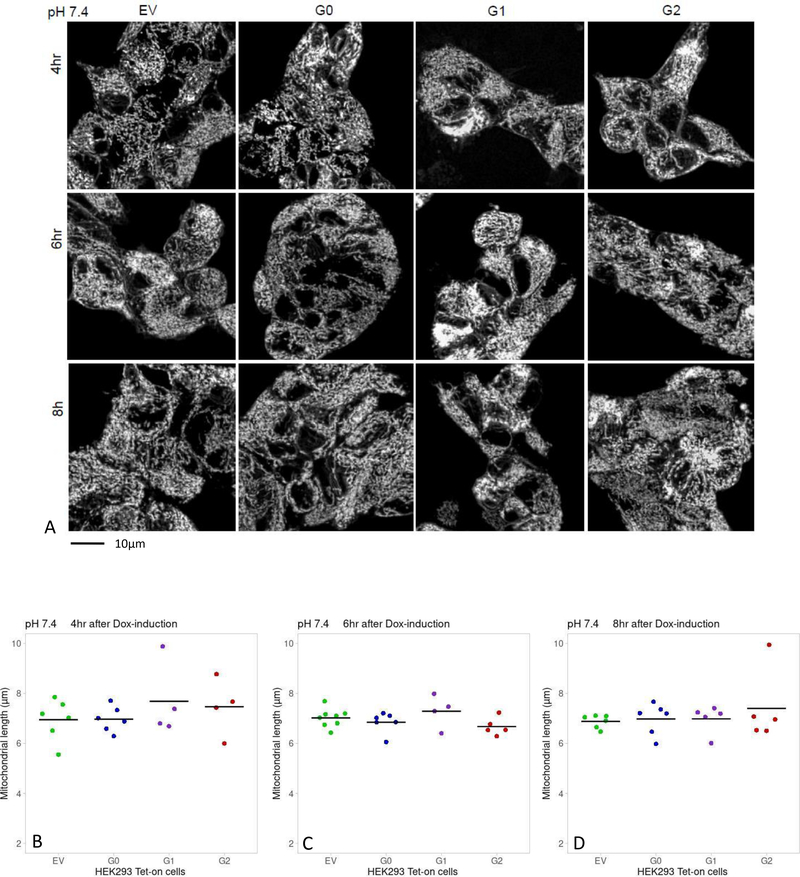
After 16-hour incubation in media pH 6.8 or 7.4, HEK293 Tet-on EV, G0, G1 and G2 cells were placed in fresh media containing Dox for up to another 8 hours. Mitochondrial length was comparable in cells incubated in media pH 6.8 or 7.4 for up to 4 hours of Dox induction regardless of APOL1 genotype (Figure 3A and 3B). After 6 hours of Dox-induction in media pH 6.8, G2-expressing cells had shorter mitochondria (6.54±0.40 μm) than EV-cells (7.65±0.72 μm, p=0.02) and G0-cells (7.46±0. 31 μm, p=0.003) (Figure 3A and 3C). After 8 hours of Dox-induction in media pH 6.8, mitochondrial length in G1-cells (6.21±0.26 μm) and G2-cells (6.46±0.34 μm) were significantly shorter than EV-cells (7.13±0.32 μm, p=0.002 and p=0.008, respectively) and G0-cells (7.22±0.45 μm, p=0.003 and p=0.01, respectively; (Figure 3A and 3D). In media pH 7.4, mitochondrial lengths were comparable for up to 8 hours of Dox-induction regardless of APOL1 genotype (Figure 4 A, B, C, D).
Figure 3. Comparison of mitochondrial length in Dox-induced HEK293 Tet-on cells cultured in media pH=6.8.
Panel A. Images displaying mitochondrial morphology in HEK293 Tet-on cells cultured in media pH 6.8 and Dox-induced for 4, 6 and 8 hours. The cells were imaged and processed as described in Figure 2, Panel A.
Panels B, C, D. Graphs display a summary of measurements of mitochondrial length in HEK293 Tet-on cells cultured in cell growth media pH 6.8 and Dox-induced for 4, 6 or 8 hours, respectively. B) Mitochondrial length was comparable in HEK293 Tet-on EV, G0, G1 and G2 cells after 4 hours of Dox-induction (p=NS, pair-wise t-test and ANOVA). C) Mitochondrial length was lower in HEK293 G2 cells than in EV, G0 or G1 cells after 6 hours of Dox-induction (t-test). D) Mitochondrial length was lower in HEK293 G1 or G2 cells than EV or G0 cells, after 8 hours of Dox-induction (t-test). Mitochondrial length was similar between EV and G0 cells, and between G1 and G2 cells (p=NS, t-test).
Figure 4. Comparison of mitochondrial length in Dox-induced HEK293 Tet-on cells cultured in media pH=7.4.
Panel A. Images displaying mitochondrial morphology in HEK293 Tet-on cells cultured in media pH 7.4 and Dox-induced for 4, 6 and 8 hours. The cells were imaged and processed as explained in Figure 2, Panel A.
Panels B, C, D. Graphs display a summary of measurements of mitochondrial length in HEK293 Tet-on cells cultured in cell growth media pH 7.4 and Dox-induced for 4, 6 or 8 hours, respectively. No differences were detected in mitochondrial length in HEK293 Tet-on EV, G0, G1 and G2 cells irrespective of the genotype or length of Dox-induction (p=NS, pair-wise t-test and ANOVA).
APOL1 expression level by APOL1 genotype and pH in HEK293 Tet-on cells
RT-PCR was employed to assess APOL1 G1 and G2 KRV mRNA levels in Tet-on HEK293 cells expressed as fold change relative to APOL1 G0 mRNA levels prior to Dox induction. As expected, no APOL1 was expressed in HEK293 Tet-on EV cells regardless of media pH. Likewise, media pH did not affect APOL1 expression levels in HEK293 Tet-on G0, G1 or G2 cells given similar Dox induction times (Supplementary Table 1). By design, the expression levels of APOL1 G0 reference transcripts were slightly higher than the expression levels of G1 or G2 KRVs after 6 hours or 8 hours of Dox-induction (Supplementary Table 1). This previously published protocol assured that the effects of APOL1 G1 and G2 KRVs on mitochondrial morphology resulted from their altered function instead of expression levels[5].
Cell viability by APOL1 genotype and pH in HEK293 Tet-on cells
To provide evidence that mitochondrial fragmentation was not a result of impaired cell viability, the LDH assay assessed cell viability for HEK293 Tet-on G0, G1 and G2 cell lines at baseline and after Dox induction. Cell viability was not compromised, irrespective of APOL1 genotype, duration of Dox induction or media pH (Supplementary Figure 2).
Discussion
This report demonstrates that an acidic pH facilitated mitochondrial dysfunction induced by APOL1 G1 and G2 KRVs in HEK293 cells. Mitochondrial dysfunction was based on increasing mitochondrial fragmentation[5]. Results extend prior studies by demonstrating the role of an acidic pH on APOL1 G1 and G2 KRV-induced mitochondrial fragmentation.
In the kidney, APOL1 is synthesized in podocytes, tubule cells, and glomerular endothelial cells[23]. APOL1 is also expressed in the pancreas[24], brain[25] and liver[17],[26]; however, APOL1-associated organ-dysfunction appears to be limited to the kidney. Moreover, recipients of a kidney transplant are more likely to develop early graft failure when receiving kidneys from donors with two APOL1 KRVs[27]. This effect was not seen in recipients of a liver transplant[28]. These data support differences in APOL1 effect on kidney and liver cells. A unique property of the kidney is its acidic interstitial pH[8],[29], whereas liver interstitial pH is neutral[30]. Given the pH-sensitivity of APOL1 protein[6],[7], this study may provide a clue as to why APOL1-associated toxic effects are most prominent in the kidney.
Several reports demonstrate that APOL1 KRVs cause mitochondrial dysfunction [2],[3],[4],[5]. Mitochondrial dysfunction is also implicated in common forms of focal segmental glomerulosclerosis (FSGS)[31], strongly associated with APOL1 in populations with recent African ancestry. Therefore, it is not surprising that APOL1 G1 and G2 variants impact this pathway, because APOL1 is a mitochondrial membrane protein[3],[4]. We reported that APOL1 G1 and G2 KRVs (but not G0) induce mitochondrial fragmentation/fission in HEK293 cells and also in poly IC-stressed human primary tubule cells[5]. This was supported by a recent report using a Trypanosoma rhodesiense model[32]. We postulated that the G0 APOL1 reference allele may protect mitochondria from environmental stressors by stimulating mitochondrial fusion (preventing fragmentation/fission), facilitating autophagy, and inhibiting endoplasmic reticulum (ER) stress[2]; whereas G1 and G2 induce mitochondrial fragmentation[5]; inhibit autophagy[33], and enhance ER stress[34]. If G1 and G2 -induced mitochondrial injury and ER stress cannot be compensated for by appropriate autophagy/mitophagy[35], activation of cell death machinery may ensue[36].
We reported that HEK293 Tet-on G1 and G2 cells developed mitochondrial fragmentation after 16 hours of Dox-induction in standard growth media and that cell viability was fully maintained during this period[5]. In the current study, using the same HEK293 Tet-on G1 and G2 cells, mitochondrial fragmentation was not observed after 8 hours of Dox-induction in media with physiologic pH=7.4. However, mitochondrial length began to significantly decline in acidic media pH=6.8 after only 6 hours of Dox-induction in G2 cells and in both G1 and G2 after 8 hours of Dox-induction (compared to G0 or EV cells; Figure 3). In acidic media pH, the effects of APOL1 G1 and G2 KRVs on mitochondrial fragmentation were similar to those previously observed, but occurred at 6–8 hours (versus 16 hours).
These in vitro experiments modeled kidney interstitial pH according to our previous studies addressing adaptive response of the kidney to increased exogenous acid load[13],[15]. Daily fluctuations in kidney interstitial pH are not only important for acid-base regulation, but are increasingly recognized as a factor facilitating CKD progression[37]. In African Americans from the Jackson Heart Study, higher dietary acid load was associated with an increased prevalence of CKD[38]. Among US adults with CKD, the association of dietary acid load with progression to end-stage kidney disease (ESKD) appears to be stronger in African Americans than in non-Hispanic Whites[39]. Although high dietary acid load showed little evidence of being a “second hit” among individuals with two APOL1 KRVs in the Chronic Renal Insufficiency Cohort (CRIC), the study was not adequately powered nor designed to answer this question[40]. The potential renal acid load trended higher in African Americans in CRIC with two APOL1 KRVs, compared to zero or one KRVs (unadjusted p=0.086; p=0.109 adjusted for CKD progression age, sex, and percentage African ancestry)[40]. It is important to determine whether decreasing dietary acid load may reduce risk of CKD in African Americans with two APOL1 KRVs and whether such intervention could protect allograft function in transplant recipients who received kidneys from donors with two APOL1 KRVs.
Namba et al. demonstrated an important role for mitophagy in maintaining proper mitochondrial functions in vivo, including ammoniagenesis, during adaptive response of the kidney to an increased acid load[41]. However, APOL1 G1 or G2 may impair normal autophagy/mitophagy via defective intracellular trafficking[35],[42], facilitating mitochondrial fragmentation in HEK293 Tet-on APOL1 G1 and G2 cells in acidic media.
Lower interstitial pH increases citrate reabsorption[43], stimulating the tricarboxylic acid cycle (TCA) in mitochondria to provide ATP for acid secretion and secretion of organic acids into the tubule lumen[44]. APOL1 G1 and G2 proteins impair mitochondrial function via fission-mediated collapse of the membrane potential[5] and/or opening of mitochondrial permeability transition pores (mPTP)[4]. These effects may negatively impact mitochondrial membrane complex I (NADH dehydrogenase)[2],[45]. In addition, higher peritubular acidity may strain TCA cycle generation of sufficient ATP for secreting organic acids from proximal tubule cells in individuals with high-risk APOL1 genotypes.
Many believe that APOL1-associated kidney diseases are podocyte-centric glomerular disorders, based on an animal model[33]. Podocyte injury is clearly important in APOL1-associated kidney disease. However, crosstalk occurs between renal proximal tubule cells and podocytes; this could support a role for tubule cell regulation of glomerular function[46]. Potential effects of APOL1 kidney risk variants on renal tubule cells resulting in alterations in glomerular function require additional investigation.
We note limitations in this report. First, alkaline media was not tested in HEK293 Tet-on APOL1 cell lines for effects on mitochondrial morphology. This is important from the standpoint of cell biology; however, its significance in human renal physiology is unclear due to the known acidic environment in the tubulo-interstitial compartment. Second, the mean mitochondrial length of Dox-induced cells after 4-hour induction was longer than non-induced cells. This may have related to culturing non-induced cells for 24 hours to maximize the period for determining whether there were mitochondrial length differences between media with different pH; whereas, Dox induced cells were placed in relatively fresh media (see Figure 1). The longer cells are cultured, the more acidic metabolites accumulate in the media especially during active cell proliferation. This could have impacted mitochondrial length when comparing baseline experiments (non-Dox-induced) with Dox-induction. Over time, extracellular acidification can induce reactive oxygen species- and mPTP-mediated death in HEK293 cells[47], and mitochondrial fragmentation occurs prior to cell death[48].
In conclusion, an acidic pH facilitated early mitochondrial dysfunction induced by APOL1 KRVs in human embryonic kidney cells. It appears likely that progressively lower pH levels in the renal interstitium may trigger APOL1 risk variant effects on mitochondria that may accelerate the decline in kidney function. These findings make it important to determine whether decreasing dietary acid load in African Americans with two APOL1 KRVs can slow nephropathy progression and protect transplant recipients who received kidneys from donors with two APOL1 KRVs.
Supplementary Material
Acknowledgements
We thank Dr. Martin R. Pollak for sharing APOL1 vectors.
Funding Sources
This work was supported by National Institutes of Health grants R01 DK070941 (BIF), R01 DK084149 (BIF), R01 MD009055 (JD, BIF), R21 AG051866 (SP), and a Summer Research Program from the American Physiological Society (DL).
Support: The research was supported by an American Physiological Society (APS) summer undergraduate fellowship (to DL) and grants from National Institutes of Health R01 DK070941 (BIF), R01 DK084149 (BIF) and R01 MD009055 (JD, BIF), R21 AG051866 (SP).
Disclosure Statement
Wake Forest University Health Sciences and Dr. Freedman have rights to an issued United States patent related to APOL1 genetic testing. Dr. Freedman is a consultant for RenalytixAI and AstraZeneca Pharmaceuticals. All other authors have no conflict of interest to declare. Part of the manuscript was presented as an abstract on the Experimental Biology 2019 Meeting, and DengFeng Li was a recipient of the Barbara A. Horwitz and John M. Horowitz Award from the American Physiological Society. This study was approved by the Institutional Review Board at Wake Forest School of Medicine (IRB00006803)
Footnotes
Disclosures: Wake Forest University Health Sciences and Dr. Freedman have rights to an issued U.S. patent related to APOL1 genetic testing. Dr. Freedman is a consultant for AstraZeneca and RenalytixAI.
References
- 1.Foster MC, Coresh J, Fornage M, Astor BC, Grams M, Franceschini N, et al. : APOL1 variants associate with increased risk of CKD among African Americans. J Am Soc Nephrol JASN 2013;24:1484–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ma L, Chou JW, Snipes JA, Bharadwaj MS, Craddock AL, Cheng D, et al. : Renal-Risk Variants Induce Mitochondrial Dysfunction. J Am Soc Nephrol JASN 2017;28:1093–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Granado D, Müller D, Krausel V, Kruzel-Davila E, Schuberth C, Eschborn M, et al. : Intracellular APOL1 Risk Variants Cause Cytotoxicity Accompanied by Energy Depletion. J Am Soc Nephrol 2017;28:3227–3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SS, Lannon H, Dias L, Zhang J-Y, Alper SL, Pollak MR, et al. : APOL1 Kidney Risk Variants Induce Cell Death Mitochondrial Translocation and Opening of the Mitochondrial Permeability Transition Pore. J Am Soc Nephrol JASN 2019; DOI: 10.1681/ASN.2019020114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Ainsworth HC, Snipes JA, Murea M, Choi YA, Langefeld CD, et al. : APOL1 kidney-risk variants induce mitochondrial fission. Kidney Int Reports 2020; DOI: 10.1016/j.ekir.2020.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson R, Finkelstein A: Human trypanolytic factor APOL1 forms pH-gated cation-selective channels in planar lipid bilayers: relevance to trypanosome lysis. Proc Natl Acad Sci United States Am 2015;112:2894–2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bruno J, Pozzi N, Oliva J, Edwards JC: Apolipoprotein L1 confers pH-switchable ion permeability to phospholipid vesicles. J Biol Chem 2017;292:18344–18353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andreev OA, Dupuy AD, Segala M, Sandugu S, Serra DA, Chichester CO, et al. : Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc Natl Acad Sci United States Am 2007;104:7893–7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walczak J, Partyka M, Duszyński J, Szczepanowska J: Implications of mitochondrial network organization in mitochondrial stress signalling in NARP cybrid and Rho0 cells. Sci reports 2017;7:14864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suen D-F, Norris KL, Youle RJ: Mitochondrial dynamics and apoptosis. Genes & Dev 2008;22:1577–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng D, Weckerle A, Yu Y, Ma L, Zhu X, Murea M, et al. : Biogenesis and cytotoxicity of APOL1 renal risk variant proteins in hepatocytes and hepatoma cells. J Lipid Res 2015;56:1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Satlin LM, Schwartz GJ: Cellular remodeling of HCO3(−)-secreting cells in rabbit renal collecting duct in response to an acidic environment. J cell Biol 1989;109:1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schwartz GJ, Tsuruoka S, Vijayakumar S, Petrovic S, Mian A, Al-Awqati Q: Acid incubation reverses the polarity of intercalated cell transporters, an effect mediated by hensin. J Clin Investig 2002;109:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yasoshima K, Satlin LM, Schwartz GJ: Adaptation of rabbit cortical collecting duct to in vitro acid incubation. Am J Physiol 1992;263:F749–F756. [DOI] [PubMed] [Google Scholar]
- 15.Sun X, Stephens L, DuBose TD, Petrovic S: Adaptation by the collecting duct to an exogenous acid load is blunted by deletion of the proton-sensing receptor GPR4. Am J Physiol Ren Physiol 2015;309:F120–F136. [DOI] [PubMed] [Google Scholar]
- 16.Michl J, Park KC, Swietach P: Evidence-based guidelines for controlling pH in mammalian live-cell culture systems. Commun Biol 2019;2:144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma L, Shelness GS, Snipes JA, Murea M, Antinozzi PA, Cheng D, et al. : Localization of APOL1 protein and mRNA in the human kidney: nondiseased tissue, primary cells, and immortalized cell lines. J Am Soc Nephrol JASN 2015;26:339–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001;25:402–408. [DOI] [PubMed] [Google Scholar]
- 19.Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, et al. : Fiji: an open-source platform for biological-image analysis. Nat methods 2012;9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nat methods 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valente AJ, Maddalena LA, Robb EL, Moradi F, Stuart JA: A simple ImageJ macro tool for analyzing mitochondrial network morphology in mammalian cell culture. Acta Histochem 2017;119:315–326. [DOI] [PubMed] [Google Scholar]
- 22.Strack S, Usachev YM (eds): Techniques to Investigate Mitochondrial Function in Neurons. Springer New York, 2015. DOI: 10.1007/978-1-4939-6890-9 [DOI] [Google Scholar]
- 23.Madhavan SM, O’Toole JF, Konieczkowski M, Ganesan S, Bruggeman LA, Sedor JR: APOL1 localization in normal kidney and nondiabetic kidney disease. J Am Soc Nephrol JASN 2011;22:2119–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duchateau PN, Pullinger CR, Orellana RE, Kunitake ST, Naya-Vigne J, O’Connor PM, et al. : Apolipoprotein L, a new human high density lipoprotein apolipoprotein expressed by the pancreas. Identification, cloning, characterization, and plasma distribution of apolipoprotein L. J Biol Chem 1997. [cited 1997 Oct 10];272:25576–25582. [DOI] [PubMed] [Google Scholar]
- 25.Hwang Y, Kim J, Shin JY, Kim JI, Seo JS, Webster MJ, et al. : Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl Psychiatry 2013;3:e321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shukha K, Mueller JL, Chung RT, Curry MP, Friedman DJ, Pollak MR, et al. : Most ApoL1 Is Secreted by the Liver. J Am Soc Nephrol JASN 2017;28:1079–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reeves-Daniel AM, DePalma JA, Bleyer AJ, Rocco MV, Murea M, Adams PL, et al. : The APOL1 gene and allograft survival after kidney transplantation. Am J transplantation: Off J Am Soc Transplant Am Soc Transpl Surg 2011;11:1025–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorr CR, Freedman BI, Hicks PJ, Brown WM, Russell GB, Julian BA, et al. : Deceased-Donor Apolipoprotein L1 Renal-Risk Variants Have Minimal Effects on Liver Transplant Outcomes. PloS one 2016;11:e0152775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goraya N, Wesson DE: Kidney Response to the Spectrum of Diet-Induced Acid Stress. Nutrients 2018;10 DOI: 10.3390/nu10050596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park R, Leach WJ, Arieff AI: Determination of liver intracellular pH in vivo and its homeostasis in acute acidosis and alkalosis. Am J Physiol 1979;236:F240–F245. [DOI] [PubMed] [Google Scholar]
- 31.Müller-Deile J, Schiffer M: The podocyte power-plant disaster and its contribution to glomerulopathy. Front Endocrinol 2014;5:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Uzureau S, Lecordier L, Uzureau P, Hennig D, Graversen JH, Homblé F, et al. : APOL1 C-Terminal Variants May Trigger Kidney Disease through Interference with APOL3 Control of Actomyosin. Cell reports 2020;30:3821–3836.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Beckerman P, Bi-Karchin J, Park ASD, Qiu C, Dummer PD, Soomro I, et al. : Transgenic Expression of Human APOL1 Risk Variants in Podocytes Induces Kidney Disease in Mice. Nat Med 2017;23:429–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wen H, Kumar V, Lan X, Shoshtari SSM, Eng JM, Zhou X, et al. : APOL1 risk variants cause podocytes injury through enhancing endoplasmic reticulum stress. Biosci reports 2018;38 DOI: 10.1042/BSR20171713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Madhavan SM, O’Toole JF, Konieczkowski M, Barisoni L, Thomas DB, Ganesan S, et al. : APOL1 variants change C-terminal conformational dynamics and binding to SNARE protein VAMP8. JCI Insight 2017;2 DOI: 10.1172/jci.insight.92581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boland ML, Chourasia AH, Macleod KF: Mitochondrial Dysfunction in Cancer. Front Oncol 2013;3:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wesson DE, Buysse JM, Bushinsky DA: Mechanisms of Metabolic Acidosis-Induced Kidney Injury in Chronic Kidney Disease. J Am Soc Nephrol : JASN 2020;31:469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banerjee T, Tucker K, Griswold M, Wyatt SB, Harman J, Young B, et al. : Dietary Potential Renal Acid Load and Risk of Albuminuria and Reduced Kidney Function in the Jackson Heart Study. J Ren Nutr Off J Counc Ren Nutr Natl Kidney Found 2018;28:251–258. [DOI] [PubMed] [Google Scholar]
- 39.Crews DC, Banerjee T, Wesson DE, Morgenstern H, Saran R, Burrows NR, et al. : Race/Ethnicity, Dietary Acid Load, and Risk of End-Stage Renal Disease among US Adults with Chronic Kidney Disease. Am J Nephrol 2018;47:174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pike M, Stewart TG, Morse J, Ormsby P, Siew ED, Hung A, et al. : Acid Load, and CKD Progression. Kidney Int reports 2019;4:946–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Namba T, Takabatake Y, Kimura T, Takahashi A, Yamamoto T, Matsuda J, et al. : Autophagic clearance of mitochondria in the kidney copes with metabolic acidosis. J Am Soc Nephrol JASN 2014;25:2254–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kruzel-Davila E, Shemer R, Ofir A, Bavli-Kertselli I, Darlyuk-Saadon I, Oren-Giladi P, et al. : APOL1-Mediated Cell Injury Involves Disruption of Conserved Trafficking Processes. J Am Soc Nephrol JASN 2017;28:1117–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brennan S, Hering-Smith K, Hamm LL: Effect of pH on citrate reabsorption in the proximal convoluted tubule. Am J Physiol 1988;255:F301–F306. [DOI] [PubMed] [Google Scholar]
- 44.Wang K, Kestenbaum B: Proximal Tubular Secretory Clearance: A Neglected Partner of Kidney Function. Clin J Am Soc Nephrol CJASN 2018;13:1291–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Briston T, Roberts M, Lewis S, Powney B, M Staddon J, Szabadkai G, et al. : Mitochondrial permeability transition pore: sensitivity to opening and mechanistic dependence on substrate availability. Sci reports 2017;7:10492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nihalani D, Susztak K: Sirt1-Claudin-1 crosstalk regulates renal function. Nat Med 2013;19:1371–1372. [DOI] [PubMed] [Google Scholar]
- 47.Teixeira J, Basit F, Swarts HG, Forkink M, Oliveira PJ, Willems PHGM, et al. : Extracellular acidification induces ROS- and mPTP-mediated death in HEK293 cells. Redox Biol 2018;15:394–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zorov DB, Vorobjev IA, Popkov VA, Babenko VA, Zorova LD, Pevzner IB, et al. : Lessons from the Discovery of Mitochondrial Fragmentation (Fission): A Review and Update. Cells 2019;8 DOI: 10.3390/cells8020175 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.