Abstract
Na+/H+ exchanger NHE1, a major determinant of intracellular pH (pHi) in mammalian central neurons, promotes neurite outgrowth under both basal and netrin-1-stimulated conditions. The small GTP binding proteins and their effectors have a dominant role in netrin-1-stimulated neurite outgrowth. Since NHE1 has been shown previously to work downstream of the Rho GTPases-mediated polarized membrane protrusion in non-neuronal cells, we examined whether NHE1 has a similar relationship with Cdc42, Rac1 and RhoA in neuronal morphogenesis. Interestingly, our results suggest the possibility that NHE1 acting upstream of Rho GTPases to promote neurite outgrowth induced by netrin-1. First, we found that netrin-1-induced increases in the activities of Rho GTPases using FRET (Forster Resonance Energy Transfer) analyses in individual growth cones; furthermore, their increased activities were abolished by cariporide, a specific NHE1 inhibitor. Second, NHE1 inhibition had no effect on neurite retraction induced by L-α-Lysophosphatidic acid (LPA), a potent RhoA activator. The regulation of Rho GTPases by NHE1 was further evidenced by reduced Rac1, Cdc42 and RhoA activities in NHE1-null neurons. Taken together, our findings suggest that NHE1-dependent neuronal morphogenesis involves the activation of Rho-family of small GTPases.
Keywords: NHE1, Rho GTPases, RhoA, Rac1, Cdc42, Neurite outgrowth, Netrin-1
Introduction
One of the principal characteristics of neuronal differentiation is the induction of membrane protrusions that develop into neurites, which become discernable as axons and dendrites as the neuron becomes polarized (da Silva and Dotti 2002; Luo 2002). The critical roles of the Rho-family GTPases Cdc42, Rac1 and RhoA in neuronal morphogenesis have been especially well-studied (Govek et al. 2005; Heasman and Ridley 2008; Luo 2002). Cdc42 and Rac1 promote neurite outgrowth and the formation of actin-based structures such as filopodia and lamellipodia at growth cones and along neurites. In contrast, global activation of RhoA and RhoA-associated kinase/ROK/ROCK in neurons lead to contraction of cortical acto-myosin and neurite retraction (Govek et al. 2005; Heasman and Ridley 2008; Luo 2002). On the other hand selective activation of RhoA at the peripheral domain of neuronal growth cones promotes the initial events of membrane protrusion and the formation of focal adhesive complexes leading to neurite outgrowth (Kurokawa and Matsuda 2005; Kurokawa et al. 2005; Nakamura et al. 2005; Pertz et al. 2006).
We have previously identified Na+/H+ exchanger isoform 1 (NHE1), a major acid extrusion mechanism in mammalian central neurons (Baxter and Church 1996; Chesler 2003; Luo and Sun 2007), as a novel regulator of early neurite morphogenesis in basal and netrin-1-stimulated conditions (Sin et al. 2009). NHE1 regulates pHi by catalyzing the electroneutral exchange of extracellular Na+ for cytosolic H+ (Casey et al. 2010; Orlowski and Grinstein 2004; Orlowski and Grinstein 2007). In addition, its C-terminal domain not only contains many phosphorylation motifs and protein binding sites which regulate N-terminal ion transport activity (Malo and Fliegel 2006; Slepkov et al. 2007) but also serves as an anchor for the actin cytoskeleton and as a scaffold for the assembly of macromolecular signaling complexes (Denker and Barber 2002a; Denker and Barber 2002b; Denker et al. 2000). In migrating non-neuronal cells, NHE1 accumulates at the leading edge where it regulates reorganization of the actin cytoskeleton (Cardone et al. 2005a; Denker et al. 2000; Meima et al. 2007), and reductions in ion translocation activity or mislocalization of NHE1 away from the leading edge impair leading edge protrusion and cell migration (Cardone et al. 2005b; Denker and Barber 2002a; Denker and Barber 2002b; Grinstein et al. 1993; Lagana et al. 2000; Paradiso et al. 2004; Stock and Schwab 2006). In an analogous fashion, NHE1 is highly expressed in neuronal growth cones which, like leading edges are regions with active actin dynamics (Sin et al. 2009), and both ion translocation and actin cytoskeletal anchoring by the protein contribute to the regulation of neurite outgrowth (Sin et al. 2009).
A number of mechanisms have been suggested to contribute to the effects of NHE1 membrane protrusion in migrating cells (Meima et al. 2007; Stock and Schwab 2006), including regulation of the activation states of Rho-family GTPases. In this aspect, NHE1 has been shown mainly to work downstream of RhoA and Rac1 in migrating non-neuronal and cancer cells to mediate their effects on actin cytoskeleton (Cardone et al. 2005a; Lagana et al. 2000; Paradiso et al. 2004; Tominaga and Barber 1998; Tominaga et al. 1998), However, there is also a report demonstrating NHE1 working upstream to promote the activation of Cdc42 at the leading edge of migrating fibroblasts (Frantz et al. 2007).
In our previous study, we observed neurite outgrowth mediated by netrin-1, but not IGF1 or BDNF, is extremely sensitive to NHE1 inhibition (Sin et al. 2009). The small GTP binding proteins and their effectors are featured prominently in the signal transduction mechanisms engaged by netrin-1 binding to Deleted in Colorectal Cancer (DCC) (Barallobre et al. 2005; Causeret et al. 2004; Lai Wing Sun et al. 2011; Round and Stein 2007). Netrin-1 primarily mediates neurite outgrowth by binding to DCC receptor which recruits Cdc42 and Rac1 in the DCC signaling complex leading to Cdc42/Rac1-dependent neurite morphogenesis (Causeret et al. 2004; Li et al. 2002b; Moore et al. 2008; Shekarabi and Kennedy 2002; Shekarabi et al. 2005). We therefore seek to determine whether NHE1 affects neurite outgrowth by regulating the activation of Rho GTPases.
Materials and methods
Reagents
NHE1 inhibitor cariporide (HOE642), a gift from Sanofi-Aventis, was dissolved in DMSO and applied at 1 μM, a NHE1-selective concentration (Masereel et al. 2003). L-α-Lysophosphatidic acid (LPA) (Sigma-Aldrich) was dissolved in methanol and used at 10 μM. Y-27632 dihydrochloride monohydrate, a ROCK inhibitor (Sigma-RBI), was dissolved in PBS and used at 25 μM. Recombinant mouse netrin-1 (R&D Systems) was diluted in 1% BSA and applied at 100 ng/ml, a maximally-effective concentration for the promotion of neurite outgrowth.
Cell culture and Tranfections
Primary neocortical neurons were isolated from wild type (WT) embryonic day 16 (E16) C57BL/6 mice (Charles River Laboratories, Inc.) or postnatal day 0.5 (P0.5) Nhe1 homozygous mutant (NHE1−/−) mice as detailed in our previous publication (Sin et al. 2009). Briefly, mouse cortices were triturated and the resultant cell suspension was filtered with a 70 μm cell strainer (BD Biosciences) and resuspended in a 1:1 mixture of HBSS and plating medium (Neurobasal medium and DMEM/F12 (3:2) supplemented with 10% FBS and penicillin/streptomycin). Neurons were plated at low density (1 x 105cells/cm2) and maintained for 48 h at 37 °C under a 5% CO2 atmosphere in Neurobasal medium and DMEM/F12 (3:2) containing 2% B27 supplement. All procedures involving mice conformed to guidelines established by the Canadian Council on Animal Care and were approved by The University of British Columbia Animal Care Committee.
Neocortical neurons were transfected with HA-tagged cDNA encoding full length NHE1 (pCMV-NHE1-HA) (Denker et al. 2000; Orlowski 1993) using Fugene 6 (Roche) in serum-free medium according to the manufacturers’ instructions.
Immunofluorescence cell staining
Neocortical neurons were fixed for 10 min at room temperature in 4% paraformaldehyde in PBS, washed twice with PBS, permeabilized with 0.2% Triton X-100 for 5 min and blocked with 2% BSA in PBS for 30 min. Mouse anti-hemagglutinin (HA) antibody (Covance) was used at 1:1000 for at 4 °C overnight. After three washes in PBS, cells were incubated with Alexa Fluor 488-conjugated secondary antibody (Molecular Probes/Invitrogen) for 60 min at room temperature, followed by three further washes in PBS. Actin was visualized by staining with Alexa 568-conjugated phalloidin (Molecular Probes/Invitrogen) at 1:40 dilution. Coverslips were mounted onto glass slides using ProLong Gold antifade reagent with DAPI (Molecular Probes/Invitrogen) and viewed with either a Zeiss Axioskop epifluorescence microscope or an Olympus Fluoview FV1000 confocal microscope.
Rho GTPase activation assay and Western blotting
The level of activated Rho GTPases in total cell lysates was determined with activation assay kits purchased from Cytoskeleleton Inc. Neurons were treated with netrin for 10 min, harvested and processed according to the manufacturer’s instructions. Cell lysates were incubated with glutathione-S-transferase (GST) agarose beads coupled to Rhothekin RBD recombinant protein that binds to activated GTP-bound RhoA and GST-PAK-CRIB recombinant protein binds to Rac-GTP and Cdc42-GTP. Activated Rho GTPases precipitated by GST beads was released by boiling for 2 min in Laemmli sample buffer, separated on a 12% polyacrylamide gel and transferred onto PVDF membrane (Bio-Rad Laboratories). Bound RhoA, Rac and Cdc42 were detected by western analysis with mouse anti-RhoA, Rac and Cdc42 supplied in the kit, followed by anti-mouse-HRPO (Sigma-Aldrich) as secondary antibody. Protein bands were detected with ECL Plus Western Blotting reagents (Amersham).
Forster resonance energy transfer (FRET) analysis
The detection of activated Rho GTPases in neuronal growth cones was carried out with pRaichu-1026X (Rac1), pRaichu-1069X (Cdc42) and pRaichu-1298X (RhoA) biosensors originally described by Matsuda and coworkers (Aoki and Matsuda 2009; Yoshizaki et al. 2003). The presence of activated GTP-bound Rac1, Cdc42 or RhoA brings YFP and CFP in close proximity allowing FRET to occur. pRaichu plasmids were introduced into primary mouse neocortical neurons by standard lipofectamine 2000 (Invitrogen) transfection. FRET images were collected after 48 h using an Olympus FV1000 laser-scanning confocal microscope. The occurrence of FRET was determined by the Acceptor Photobleaching method where FRET efficiency (E%) = 1 – (quenched donor / unquenched donor). The image of the donor CFP was captured before and after photobleaching the acceptor YFP at 515 nm. FRET efficiencies were calculated using an algorithm developed by Periasamy and coworkers (Chen et al. 2005) as a plug-in for Image J software (Abramoff et al. 2004). Only pixels showing >80% bleaching of the YFP acceptor were included in the final analyses. One way ANOVA was used to determine whether there are differences in the levels of activated Cdc42, Rac1 and RhoA in 3 conditions: untreated, netrin+cariporide and netrin-1 treatment. This was followed by pairwise comparison using Bonferroni t-test. Each condition was performed on at least three different batches of cultured neurons.
Morphometric analyses
Images of individual neocortical pyramidal neurons at 2 days in vitro (DIV) unless otherwise noted were captured from randomly chosen fields on a Zeiss Axioplan 2 imaging microscope, blinded to treatment group, and traced using Zeiss AxioVision software (v4.2) as described in detail previously (Sin et al. 2009). Each experiment was performed on at least three different batches of cultured neurons. Data are presented as means ± S.E. as a bar graph, and also as scatter plot with the accompanying n value referring to the number of cells from which data were obtained. The dataset was checked for normality and equal variance with SigmaPlot software (Systat Software). Kruskal-Wallis one-way ANOVA on ranks and pairwise comparison by Dunn’s method were performed when morphometric data failed the equal variance test.
Results
Activation of rho GTPases by netrin-1 is sensitive to NHE1 inhibition
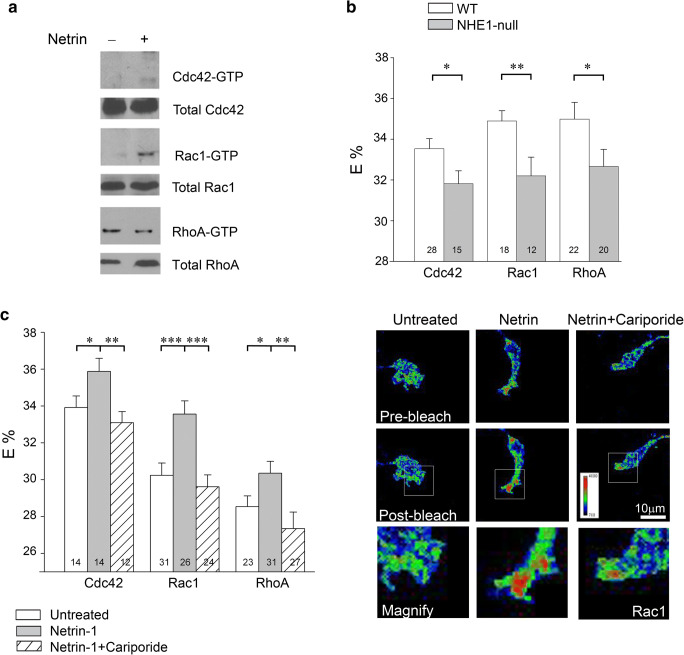
We previously reported that netrin-1-induced increases in neurite branching and elongation (Barallobre et al. 2005; Causeret et al. 2004; Round and Stein 2007) are highly sensitive to the NHE1 inhibitor cariporide and are effectively abolished in NHE1-null neurons (Sin et al. 2009). The critical role of Rho GTPases in netrin-1 signaling is well known (Li et al. 2002b; Picard et al. 2009). Furthermore, a potential link between NHE1 activity and netrin-1-stimulated neurite morphogenesis is suggested by a previous report that H+ efflux by NHE1 promotes the activation of Cdc42 at the leading edge of migrating fibroblasts (Frantz et al. 2007). Indeed, using a well-established GST pull-down activation assay that makes use of the increased affinity between GTP-bound GTPases with the Rho-binding domain of their respective effectors (Benard et al. 1999) to measure the level of GTP-bound Cdc42, Rac1 and RhoA in the total lysates of mouse neurons after netrin-1 treatment, we observed an increase in the levels of Cdc42-GTP and Rac1-GTP, and a corresponding reduction of RhoA-GTP, (Fig. 1a) in agreement with previous studies (Causeret et al. 2004; Li et al. 2002a; Li et al. 2002b; Moore et al. 2008). However, when we used Forster Resonance Energy Transfer (FRET) analysis with Raichu probes (Aoki and Matsuda 2009; Yoshizaki et al. 2003) to determine the activation states of Cdc42, Rac1 and RhoA in individual neuronal growth cones of NHE1-null neurons, the activities of Cdc42, Rac1 and RhoA were significantly reduced in growth cones of NHE1-null compared to WT neurons (Fig. 1b). We next examined whether the activation of endogenous Rho GTPases by netrin-1 in mouse cortical neuronal growth cones were sensitive to NHE1 activity using FRET. Consistent with previous findings, we observed that netrin-1 increased the activities of Cdc42 and Rac1 in individual WT neuronal growth cones, and also found that these increases were abolished by cariporide (Fig. 1c). Interestingly, we also found that netrin-1 activated RhoA in WT growth cones, an effect that was also sensitive to cariporide (Fig. 1c), which was in agreement with a previous report (Picard et al. 2009).
Fig. 1.
Inhibition of NHE1 activity reduces the activation of Rho GTPases by netrin-1 in growth cones. a. Netrin-1 activates Cdc42 and Rac1 and down-regulates RhoA in total cell lysates of mouse neocortical neurons. Neurons were treated with 100 ng/ml netrin-1 for 10 min. GST-PAK-CRIB and GST-rhotekin-RBD fusion proteins were used to pull-down activated Rho GTPases (which were then identified by their respective antibodies). RhoA-GTP/total RhoA ratio, as determined by gel module of Image J analysis software, decreased to 0.07 from 0.7 after netrin treatment. b. Acceptor photobleaching FRET of Rho GTPase activities in mouse neocortical neuron growth cones transfected with YFP-GTPase/Effector-binding domain-CFP fusion probes (i.e. Raichu probes). FRET efficiencies (E%) were quantified on a pixel-by-pixel basis. Compared to growth cones of WT neurons, Rho GTPase activities are reduced in growth cones of NHE1-null neurons-. *P < 0.05, **P < 0.01. Numbers of growth cones analyzed under each condition are shown in columns. c. Left panel: Quantification of FRET efficiencies (E%) in WT neuronal growth cones, in the absence or presence of netrin-1 (100 ng/ml for 10 min). Netrin-1 increased Rho GTPase activities above control values that in turn were abolished by cariporide (1 μM). *P < 0.05, **P < 0.01, ***P < 0.001. Numbers of growth cones analyzed under each condition are shown in the columns. Right panel: Representative pre- and post-bleach images of Rac1 FRET under the conditions indicated
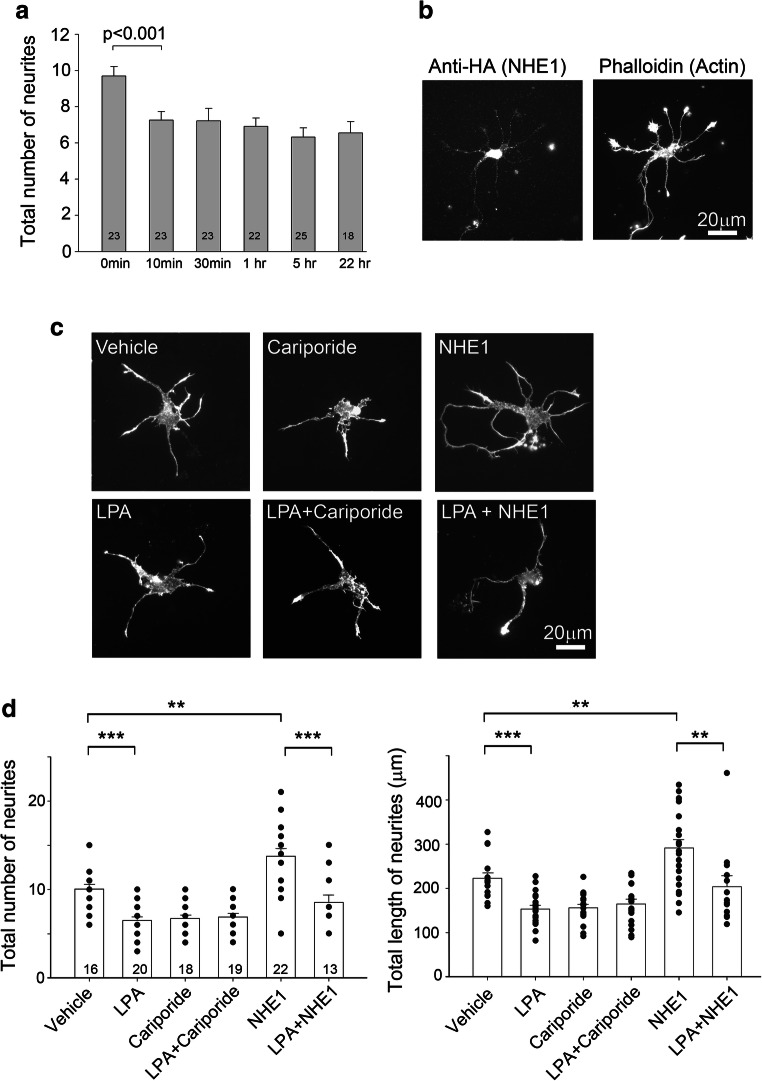
Activated RhoA does not require NHE1 activity to mediate its effects
The above findings suggest that NHE1 works upstream from Rho GTPases to regulate netrin-1-stimulated neurite outgrowth. To determine whether NHE1 regulates RhoA-dependent actin remodeling in neurons, we made use of lysophosphatidic acid (LPA), a potent activator of RhoA that causes neurite retraction (Kranenburg et al. 1999; Tigyi et al. 1996; Yamaguchi et al. 2001). Treatment of WT neurons with 10 μM LPA for 10 min was sufficient to reduce the total number of neurites during this period (Fig. 2a). For this series of experiment, we used cariporide and NHE1 overexpression (Fig. 2b) to reduce and increase Na/H exchange activity, respectively. LPA-induced neurite retraction in WT neurons was not affected by cariporide (Fig. 2c, d), suggesting inhibition of NHE1 activity did not affect RhoA-dependent signaling. In agreement with our previous study, overexpression of NHE1 enhanced neurite outgrowth (Sin et al. 2009), an effect that was abolished by LPA treatment (Fig. 2c, d), strengthening that RhoA works downstream of NHE1 to mediate its effect on morphogenesis.
Fig. 2.
Morphological changes induced by activated Rho GTPases is not affected by NHE1 inhibition. a. Wild type mouse neocortical neurons at 2 DIV were treated with 10 μm of lysophosphatidic acid (LPA), a well-known RhoA activator. Time course of the effect of LPA treatment on the total number of neurites. Numbers of WT neurons analyzed under each condition are shown in the columns. b. A mouse neuron showing the expression of HA-tagged NHE1. The neurites were visualized with phalloidin that binds actin. c. LPA-induced neurite retraction is neither sensitive to cariporide nor rescued by NHE1 overexpression. Representative examples of WT neurons that were either untransfected (WT) or transiently transfected with HA-tagged full-length NHE1 (NHE1) and cultured for 48 h, LPA (10 μM) and/or cariporide (1 μM) were present for 1 h before fixation. NHE1 transfected neurons were identified by anti-HA antibody. d. Quantification of neurite outgrowth in untransfected WT or NHE1-transfected neurons, in the absence or presence of LPA. **P < 0.01, ***P < 0.001
NHE1 activity is not required for neurite outgrowth due to inhibition of RhoA effector ROCK
The preceding results suggest that NHE1 acts upstream of Rho GTPases to regulate neurite outgrowth. However, neuronal morphogenesis is a process that is previously shown to be associated with activation of Cdc42 and Rac1 but inhibition of RhoA activity and its effector (Bito et al. 2000; Hirose et al. 1998; Yamaguchi et al. 2001). Indeed, downregulation of ROCK has a role in netrin-1 simulated outgrowth (Li et al. 2002b; Moore et al. 2008). Therefore, we took advantage of Y-27632 (Riento and Ridley 2003), a widely used inhibitor for ROCK that promotes neurite elaboration and cariporide, which we have demonstrated to abolish NHE1-mediated increased in intracellular pH at the neuronal growth cones (Sin et al. 2009). As expected, treatment of WT mouse neurons with Y-27632 led to an increase in the total number and cumulative length of neurites (Fig. 3a, b). However, these effects were not inhibited by cariporide (Fig. 3a, b), suggest ROCK-mediated cellular effects are not dependent on NHE1 activity. In contrast, consistent with our earlier report (Sin et al. 2009), the increased neurite outgrowth observed in neurons over-expressing full-length NHE1 was cariporide-sensitive (Fig. 3a, b).
Fig. 3.
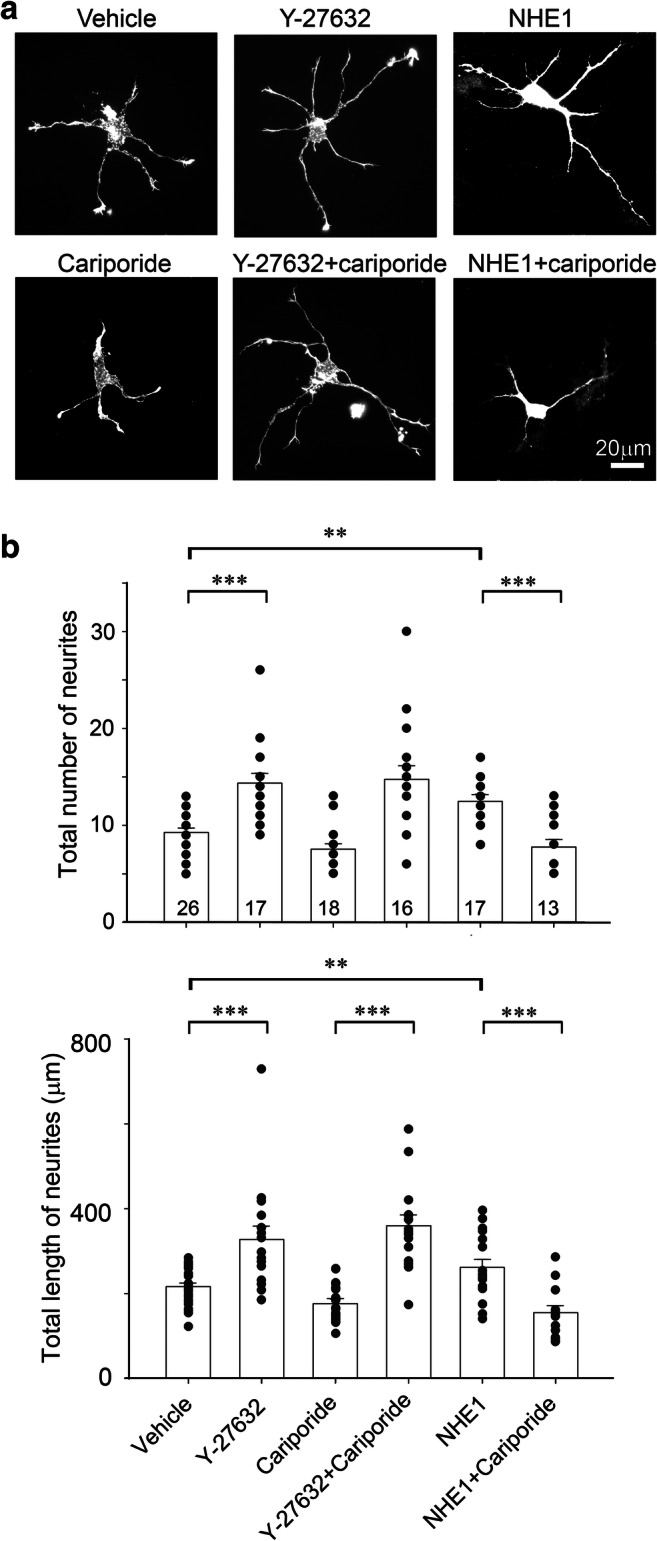
Overexpression of NHE1 does not further enhance neurite outgrowth following RhoA inhibition. a. Representative examples of WT neurons that were either untransfected (WT) or transiently transfected with HA-tagged full-length NHE1 (NHE1) and cultured for 48 h, in the absence or presence of the ROCK inhibitor Y-27632 (25 μM) and/or cariporide (1 μM). NHE1 transfected neurons were identified by anti-HA antibody. b. Neurons treated with Y-27632 exhibited increased neurite outgrowth which was insensitive to cariporide. Quantification of neurite outgrowth in WT and NHE1 transfected neurons, in the absence or presence of Y-27632 and in the absence or presence of cariporide. **P < 0.01, ***P < 0.001
Discussion
We first report the presence of NHE1 in growth cones of extending neurites, and that the alterations in the activity and expression levels of NHE1 under basal and netrin-1 stimulated conditions affect optimal branching and elongation of mouse neocortical neurons (Sin et al. 2009). Rho GTPases are key regulators of actin cytoskeletal rearrangement in neuronal morphogenesis (Govek et al. 2005; Heasman and Ridley 2008; Luo 2002), which is a process that is typically associated with the activation of Cdc42 and Rac1, with a reciprocal global down-regulation of RhoA activity (Causeret et al. 2004; Moore et al. 2008; Sander et al. 1999).
In contrast to findings in non-neuronal cells that stimulation of NHE1 acts downstream of RhoA in formation of actin stress fibers (Cardone et al. 2005a; Paradiso et al. 2004; Tominaga and Barber 1998; Tominaga et al. 1998; Vexler et al. 1996), our results demonstrate that NHE1 activity is not required for activated RhoA to mediate its effect in cortical neurons. First, NHE1 overexpression fails to rescue the reduction in neurite outgrowth evoked by LPA, which maximally activates RhoA and causes neurite retraction. Second, the promotion of neurite outgrowth by the ROCK inhibitor Y-27632 is not affected by NHE1 inhibition. It is however, entirely possible that NHE1 function is dependent on the cell type in which it is expressed (Wakabayashi et al. 1997). Intriguingly, however, but in complete agreement with previous reports (Ohnami et al. 2008; Pertz et al. 2006; Picard et al. 2009; Woo and Gomez 2006) we find that netrin-1 activates RhoA, which is also blocked by cariporide, in neuronal growth cones; this effect is consistent with a role for the selective activation of RhoA at the peripheral domain of neuronal growth cones in promoting the initial events of membrane protrusions leading to neurite outgrowth (Kurokawa and Matsuda 2005; Nakamura et al. 2005; Pertz et al. 2006; Rajasekharan et al. 2009; Rajasekharan et al. 2010).
A number of mechanisms have been proposed to underlie the role of NHE1 in actin rearrangement, including the formation subplasmalemmal alkaline microdomains that modulate the activities of actin-regulating proteins that are sensitive to changes in intracellular pH and affect actin polymerization directly (Bernstein et al. 2000; Hawkins et al. 1993). The activation of Rac and Cdc42 is extremely sensitive to changes in pH and is impaired by NHE1 inhibition (Frantz et al. 2007; Koivusalo et al. 2010). In this regard, we have previously demonstrated that pH is consistently higher in the growth cones of actively extending neurites (Sin et al. 2009). NHE1 inhibition has been shown to lower pH thereby preventing Rac1 and Cdc42 signaling (Koivusalo et al. 2010). We speculate that the proton efflux mediated by NHE1 stimulate guanine nucleotide exchange factors (GEFs) that are sensitive to subplasmalemmal pH changes, which in turn activate Rac1 and Cdc42 in extending neurites. GEFs that have been demonstrated to link netrin-1 signaling to Rho GTPases includes DOCK180 and Trio (Briancon-Marjollet et al. 2008; Li et al. 2008; Li et al. 2002a; Li et al. 2002b). Moreover, a number of reports have highlighted the modulation of actin polymerization by NHE1 acting as a scaffold through recruiting ERM (Ezrin/Radixin/Moesin) and other actin-regulating proteins (Baumgartner et al. 2004; Denker et al. 2000; Meima et al. 2007; Wu et al. 2004).
Because netrin-1 induces cytoskeletal rearrangements by activating Rac1/Cdc42 and inhibiting RhoA and its effector, ROCK (Causeret et al. 2004; Govek et al. 2005; Lai Wing Sun et al. 2011), our findings suggest that NHE1 can act through RhoA/ROCK to mediate neurite outgrowth. Indeed, a recent paper has demonstrated overexpression of NHE1 induces the sustained activation of ROCK (Wakabayashi et al. 2019). Our results are consistent with a role for NHE1 in local activation of RhoA because its activity in the growth cone is reduced in both cariporide-treated and NHE1-null neurons. In contrast to earlier findings in non-neuronal cells (Tominaga and Barber 1998; Tominaga et al. 1998), overexpression of NHE1 does not reverse neurite retraction mediated by activated RhoA, suggesting that NHE1 activity is not an immediate downstream target of RhoA in neurons. Rho GTPases are primarily activated by GEFs (Cook et al. 2014), our present findings reveal NHE1 as a novel RhoA activator in neuronal morphogenesis.
Footnotes
Grant Sponsor: Canadian Institutes of Health Research
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abramoff MD, Magelhaes PJ, Ram SJ. Image Processing with Image. J Biophotonics International. 2004;11:36–42. [Google Scholar]
- Aoki K, Matsuda M. Visualization of small GTPase activity with fluorescence resonance energy transfer-based biosensors. Nat Protoc. 2009;4:1623–1631. doi: 10.1038/nprot.2009.175. [DOI] [PubMed] [Google Scholar]
- Barallobre MJ, Pascual M, Del Rio JA, Soriano E. The Netrin family of guidance factors: emphasis on Netrin-1 signalling. Brain Res Brain Res Rev. 2005;49:22–47. doi: 10.1016/j.brainresrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Baumgartner M, Patel H, Barber DL. Na(+)/H(+) exchanger NHE1 as plasma membrane scaffold in the assembly of signaling complexes am. J Physiol Cell Physiol. 2004;287:C844–C850. doi: 10.1152/ajpcell.00094.2004. [DOI] [PubMed] [Google Scholar]
- Baxter KA, Church J. Characterization of acid extrusion mechanisms in cultured fetal rat hippocampal neurones. J Physiol. 1996;493(Pt 2):457–470. doi: 10.1113/jphysiol.1996.sp021396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard V, Bohl BP, Bokoch GM. Characterization of rac and cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J Biol Chem. 1999;274:13198–13204. doi: 10.1074/jbc.274.19.13198. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Painter WB, Chen H, Minamide LS, Abe H, Bamburg JR. Intracellular pH modulation of ADF/cofilin proteins. Cell Motil Cytoskeleton. 2000;47:319–336. doi: 10.1002/1097-0169(200012)47:4<319::AID-CM6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bito H et al. (2000) A critical role for a rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons Neuron 26:431-441 [DOI] [PubMed]
- Briancon-Marjollet A, et al. Trio mediates netrin-1-induced Rac1 activation in axon outgrowth and guidance. Mol Cell Biol. 2008;28:2314–2323. doi: 10.1128/MCB.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone RA, et al. Protein kinase a gating of a pseudopodial-located RhoA/ROCK/p38/NHE1 signal module regulates invasion in breast cancer cell lines. Mol Biol Cell. 2005;16:3117–3127. doi: 10.1091/mbc.E04-10-0945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardone RA, Casavola V, Reshkin SJ. The role of disturbed pH dynamics and the Na+/H+ exchanger in metastasis. Nat Rev Cancer. 2005;5:786–795. doi: 10.1038/nrc1713. [DOI] [PubMed] [Google Scholar]
- Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol. 2010;11:50–61. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- Causeret F, Hidalgo-Sanchez M, Fort P, Backer S, Popoff MR, Gauthier-Rouviere C, Bloch-Gallego E. Distinct roles of Rac1/Cdc42 and rho/Rock for axon outgrowth and nucleokinesis of precerebellar neurons toward netrin 1. Development. 2004;131:2841–2852. doi: 10.1242/dev.01162. [DOI] [PubMed] [Google Scholar]
- Chen Y, Elangovan M, Periasamy A. FRET data analysis: the algorithm. In: Periasamy AD, R.N., editors. Molecular imaging - FRET microscopy and spectrocopy. New York: Oxford University Press; 2005. pp. 126–145. [Google Scholar]
- Chesler M. Regulation and modulation of pH in the brain. Physiol Rev. 2003;83:1183–1221. doi: 10.1152/physrev.00010.2003. [DOI] [PubMed] [Google Scholar]
- Cook DR, Rossman KL, Der CJ. Rho guanine nucleotide exchange factors: regulators of Rho GTPase activity in development and disease. Oncogene. 2014;33:4021–4035. doi: 10.1038/onc.2013.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- Denker SP, Barber DL. Cell migration requires both ion translocation and cytoskeletal anchoring by the Na-H exchanger NHE1. J Cell Biol. 2002;159:1087–1096. doi: 10.1083/jcb.200208050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denker SP, Barber DL. Ion transport proteins anchor and regulate the cytoskeleton. Curr Opin Cell Biol. 2002;14:214–220. doi: 10.1016/s0955-0674(02)00304-6. [DOI] [PubMed] [Google Scholar]
- Denker SP, Huang DC, Orlowski J, Furthmayr H, Barber DL. Direct binding of the Na--H exchanger NHE1 to ERM proteins regulates the cortical cytoskeleton and cell shape independently of H(+) translocation. Mol Cell. 2000;6:1425–1436. doi: 10.1016/s1097-2765(00)00139-8. [DOI] [PubMed] [Google Scholar]
- Frantz C, Karydis A, Nalbant P, Hahn KM, Barber DL. Positive feedback between Cdc42 activity and H+ efflux by the Na-H exchanger NHE1 for polarity of migrating cells. J Cell Biol. 2007;179:403–410. doi: 10.1083/jcb.200704169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govek EE, Newey SE, Van Aelst L. The role of the Rho GTPases in neuronal development. Genes Dev. 2005;19:1–49. doi: 10.1101/gad.1256405. [DOI] [PubMed] [Google Scholar]
- Grinstein S, Woodside M, Waddell TK, Downey GP, Orlowski J, Pouyssegur J, Wong DC, Foskett JK. Focal localization of the NHE-1 isoform of the Na+/H+ antiport: assessment of effects on intracellular pH. EMBO J. 1993;12:5209–5218. doi: 10.1002/j.1460-2075.1993.tb06216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins M, Pope B, Maciver SK, Weeds AG. Human actin depolymerizing factor mediates a pH-sensitive destruction of actin filaments. Biochemistry. 1993;32:9985–9993. doi: 10.1021/bi00089a014. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nat Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Hirose M, et al. Molecular dissection of the rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141:1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koivusalo M, et al. Amiloride inhibits macropinocytosis by lowering submembranous pH and preventing Rac1 and Cdc42 signaling. J Cell Biol. 2010;188:547–563. doi: 10.1083/jcb.200908086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranenburg O, Poland M, van Horck FP, Drechsel D, Hall A, Moolenaar WH. Activation of RhoA by lysophosphatidic acid and Galpha12/13 subunits in neuronal cells: induction of neurite retraction. Mol Biol Cell. 1999;10:1851–1857. doi: 10.1091/mbc.10.6.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Matsuda M. Localized RhoA activation as a requirement for the induction of membrane ruffling. Mol Biol Cell. 2005;16:4294–4303. doi: 10.1091/mbc.E04-12-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurokawa K, Nakamura T, Aoki K, Matsuda M. Mechanism and role of localized activation of Rho-family GTPases in growth factor-stimulated fibroblasts and neuronal cells. Biochem Soc Trans. 2005;33:631–634. doi: 10.1042/BST0330631. [DOI] [PubMed] [Google Scholar]
- Lagana A, Vadnais J, Le PU, Nguyen TN, Laprade R, Nabi IR, Noel J. Regulation of the formation of tumor cell pseudopodia by the Na(+)/H(+) exchanger NHE1. J Cell Sci. 2000;113(Pt 20):3649–3662. doi: 10.1242/jcs.113.20.3649. [DOI] [PubMed] [Google Scholar]
- Lai Wing Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138:2153–2169. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- Li X, Meriane M, Triki I, Shekarabi M, Kennedy TE, Larose L, Lamarche-Vane N. The adaptor protein Nck-1 couples the netrin-1 receptor DCC (deleted in colorectal cancer) to the activation of the small GTPase Rac1 through an atypical mechanism. J Biol Chem. 2002;277:37788–37797. doi: 10.1074/jbc.M205428200. [DOI] [PubMed] [Google Scholar]
- Li X, Saint-Cyr-Proulx E, Aktories K, Lamarche-Vane N. Rac1 and Cdc42 but not RhoA or rho kinase activities are required for neurite outgrowth induced by the Netrin-1 receptor DCC (deleted in colorectal cancer) in N1E-115 neuroblastoma cells. J Biol Chem. 2002;277:15207–15214. doi: 10.1074/jbc.M109913200. [DOI] [PubMed] [Google Scholar]
- Li X, Gao X, Liu G, Xiong W, Wu J, Rao Y. Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat Neurosci. 2008;11:28–35. doi: 10.1038/nn2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L. Actin cytoskeleton regulation in neuronal morphogenesis and structural plasticity. Annu Rev Cell Dev Biol. 2002;18:601–635. doi: 10.1146/annurev.cellbio.18.031802.150501. [DOI] [PubMed] [Google Scholar]
- Luo J, Sun D. Physiology and pathophysiology of Na(+)/H(+) exchange isoform 1 in the central nervous system. Curr Neurovasc Res. 2007;4:205–215. doi: 10.2174/156720207781387178. [DOI] [PubMed] [Google Scholar]
- Malo ME, Fliegel L. Physiological role and regulation of the Na+/H+ exchanger. Can J Physiol Pharmacol. 2006;84:1081–1095. doi: 10.1139/y06-065. [DOI] [PubMed] [Google Scholar]
- Masereel B, Pochet L, Laeckmann D. An overview of inhibitors of Na(+)/H(+) exchanger. Eur J Med Chem. 2003;38:547–554. doi: 10.1016/s0223-5234(03)00100-4. [DOI] [PubMed] [Google Scholar]
- Meima ME, Mackley JR, Barber DL. Beyond ion translocation: structural functions of the sodium-hydrogen exchanger isoform-1. Curr Opin Nephrol Hypertens. 2007;16:365–372. doi: 10.1097/MNH.0b013e3281bd888d. [DOI] [PubMed] [Google Scholar]
- Moore SW, Correia JP, Lai Wing Sun K, Pool M, Fournier AE, Kennedy TE. Rho inhibition recruits DCC to the neuronal plasma membrane and enhances axon chemoattraction to netrin 1. Development. 2008;135:2855–2864. doi: 10.1242/dev.024133. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Aoki K, Matsuda M. FRET imaging in nerve growth cones reveals a high level of RhoA activity within the peripheral domain. Brain Res Mol Brain Res. 2005;139:277–287. doi: 10.1016/j.molbrainres.2005.05.030. [DOI] [PubMed] [Google Scholar]
- Ohnami S, Endo M, Hirai S, Uesaka N, Hatanaka Y, Yamashita T, Yamamoto N. Role of RhoA in activity-dependent cortical axon branching. J Neurosci. 2008;28:9117–9121. doi: 10.1523/JNEUROSCI.1731-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlowski J. Heterologous expression and functional properties of amiloride high affinity (NHE-1) and low affinity (NHE-3) isoforms of the rat Na/H exchanger. J Biol Chem. 1993;268:16369–16377. [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. 2004;447:549–565. doi: 10.1007/s00424-003-1110-3. [DOI] [PubMed] [Google Scholar]
- Orlowski J, Grinstein S. Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr Opin Cell Biol. 2007;19:483–492. doi: 10.1016/j.ceb.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso A, et al. The Na+-H+ exchanger-1 induces cytoskeletal changes involving reciprocal RhoA and Rac1 signaling, resulting in motility and invasion in MDA-MB-435 cells. Breast Cancer Res. 2004;6:R616–R628. doi: 10.1186/bcr922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertz O, Hodgson L, Klemke RL, Hahn KM. Spatiotemporal dynamics of RhoA activity in migrating cells. Nature. 2006;440:1069–1072. doi: 10.1038/nature04665. [DOI] [PubMed] [Google Scholar]
- Picard M, Petrie RJ, Antoine-Bertrand J, Saint-Cyr-Proulx E, Villemure JF, Lamarche-Vane N. Spatial and temporal activation of the small GTPases RhoA and Rac1 by the netrin-1 receptor UNC5a during neurite outgrowth. Cell Signal. 2009;21:1961–1973. doi: 10.1016/j.cellsig.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE. Netrin 1 and dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136:415–426. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Bin JM, Antel JP, Kennedy TE. A central role for RhoA during oligodendroglial maturation in the switch from netrin-1-mediated chemorepulsion to process elaboration. J Neurochem. 2010;113:1589–1597. doi: 10.1111/j.1471-4159.2010.06717.x. [DOI] [PubMed] [Google Scholar]
- Riento K, Ridley AJ. Rocks: multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4:446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- Round J, Stein E. Netrin signaling leading to directed growth cone steering. Curr Opin Neurobiol. 2007;17:15–21. doi: 10.1016/j.conb.2007.01.003. [DOI] [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekarabi M, Kennedy TE. The netrin-1 receptor DCC promotes filopodia formation and cell spreading by activating Cdc42 and Rac1 Mol cell. Neurosci. 2002;19:1–17. doi: 10.1006/mcne.2001.1075. [DOI] [PubMed] [Google Scholar]
- Shekarabi M, Moore SW, Tritsch NX, Morris SJ, Bouchard JF, Kennedy TE. Deleted in colorectal cancer binding netrin-1 mediates cell substrate adhesion and recruits Cdc42, Rac1, Pak1, and N-WASP into an intracellular signaling complex that promotes growth cone expansion. J Neurosci. 2005;25:3132–3141. doi: 10.1523/JNEUROSCI.1920-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin WC, Moniz DM, Ozog MA, Tyler JE, Numata M, Church J. Regulation of early neurite morphogenesis by the Na+/H+ exchanger NHE1. J Neurosci. 2009;29:8946–8959. doi: 10.1523/JNEUROSCI.2030-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepkov ER, Rainey JK, Sykes BD, Fliegel L. Structural and functional analysis of the Na+/H+ exchanger. Biochem J. 2007;401:623–633. doi: 10.1042/BJ20061062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock C, Schwab A. Role of the Na/H exchanger NHE1 in cell migration. Acta Physiol (Oxf) 2006;187:149–157. doi: 10.1111/j.1748-1716.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- Tigyi G, Fischer DJ, Sebok A, Yang C, Dyer DL, Miledi R. Lysophosphatidic acid-induced neurite retraction in PC12 cells: control by phosphoinositide-Ca2+ signaling and rho. J Neurochem. 1996;66:537–548. doi: 10.1046/j.1471-4159.1996.66020537.x. [DOI] [PubMed] [Google Scholar]
- Tominaga T, Barber DL. Na-H exchange acts downstream of RhoA to regulate integrin-induced cell adhesion and spreading. Mol Biol Cell. 1998;9:2287–2303. doi: 10.1091/mbc.9.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga T, Ishizaki T, Narumiya S, Barber DL. p160ROCK mediates RhoA activation of Na-H exchange. EMBO J. 1998;17:4712–4722. doi: 10.1093/emboj/17.16.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vexler ZS, Symons M, Barber DL. Activation of Na+-H+ exchange is necessary for RhoA-induced stress fiber formation. J Biol Chem. 1996;271:22281–22284. doi: 10.1074/jbc.271.37.22281. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S, Shigekawa M, Pouyssegur J. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol Rev. 1997;77:51–74. doi: 10.1152/physrev.1997.77.1.51. [DOI] [PubMed] [Google Scholar]
- Wakabayashi S, Morihara H, Yokoe S, Nakagawa T, Moriwaki K, Tomoda K, Asahi M. Overexpression of Na(+)/H(+) exchanger 1 specifically induces cell death in human iPS cells via sustained activation of the rho kinase ROCK. J Biol Chem. 2019;294:19577–19588. doi: 10.1074/jbc.RA119.010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo S, Gomez TM. Rac1 and RhoA promote neurite outgrowth through formation and stabilization of growth cone point contacts. J Neurosci. 2006;26:1418–1428. doi: 10.1523/JNEUROSCI.4209-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KL, Khan S, Lakhe-Reddy S, Jarad G, Mukherjee A, Obejero-Paz CA, Konieczkowski M, Sedor JR, Schelling JR. The NHE1 Na+/H+ exchanger recruits ezrin/radixin/moesin proteins to regulate Akt-dependent cell survival. J Biol Chem. 2004;279:26280–26286. doi: 10.1074/jbc.M400814200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Katoh H, Yasui H, Mori K, Negishi M. RhoA inhibits the nerve growth factor-induced Rac1 activation through rho-associated kinase-dependent pathway. J Biol Chem. 2001;276:18977–18983. doi: 10.1074/jbc.M100254200. [DOI] [PubMed] [Google Scholar]
- Yoshizaki H, et al. Activity of rho-family GTPases during cell division as visualized with FRET-based probes. J Cell Biol. 2003;162:223–232. doi: 10.1083/jcb.200212049. [DOI] [PMC free article] [PubMed] [Google Scholar]