Abstract
Epithelial tissues provide tissue barriers and specialize in organs and glands. When epithelial homeostasis is physiologically or pathologically stimulated, epithelial cells produce mesenchymal cells through the epithelial-mesenchymal transition, forming new tissues, promoting the cure of diseases or leading to illness. A variety of cytokines are involved in the regulation of epithelial cell differentiation. Bone morphogenetic proteins (BMPs), especially the bone morphogenetic protein 4 (BMP4) has a variety of biological functions and plays a prominent role in the regulation of epithelial cell differentiation. BMP4 is an important regulatory factor of a series of life activities in vertebrates, which is also related to cell proliferation, differentiation and mobility, it also has relation with tumor development. This paper mainly reviews the mechanism of BMP4’s regulation on epithelial tissues, as well as its effect on the growth, differentiation, benign lesions and malignant lesions of epithelial tissues, and expounds the function of BMP4 in epithelial tissues, to provide theoretical support for the research on reducing epithelial diseases.
Keywords: Bone morphogenetic protein 4, Epithelial tissue, Growth and differentiation, Benign lesions, Cancer
Epithelial tissues and classification
Epithelial tissues consist of a large number of cells with regular shape and few intercellular stroma, referred to as epithelium. Epithelial cells have two polarities, one is called the free surface, facing the surface of the body or cavity organs, and the other is called the basal surface, facing deep connective tissues. There are no blood vessels in epithelial tissues, and they are rich in nerve endings that can sense various stimuli(Honda 2017) (Table 1).
Table 1.
Characteristics and representative of different kinds of epithelium
| Kinds of epithelium | Characteristics of epithelium | Typical epithelial tissues |
|---|---|---|
| Covering epithelium |
1.Composed of regular dense epithelial cells and a small amount of intercellular stroma 2. Can evolve into glandular epithelium and sensory epithelium during the development of embryos. |
Palatal epithelium Alveolar epithelium Corneal epithelium Airway epithelium Gastric epithelium Ovarian epithelium ... |
| Glandular epithelium | Specialized for secretory function |
Urogenital sinus epithelium Breast epithelium Thymic epithelium... |
| Metastatic epithelium | The shape and level may vary with the contraction or expansion of the host organ |
Ureteral epithelium Urinary epithelium Bladder epithelium... |
| Sensory epithelium | Specialized for special sensory functions | Sensory epithelium... |
The regulatory mechanism of BMP4 in epithelial tissues
Uptream signal pathways and sources of BMP4
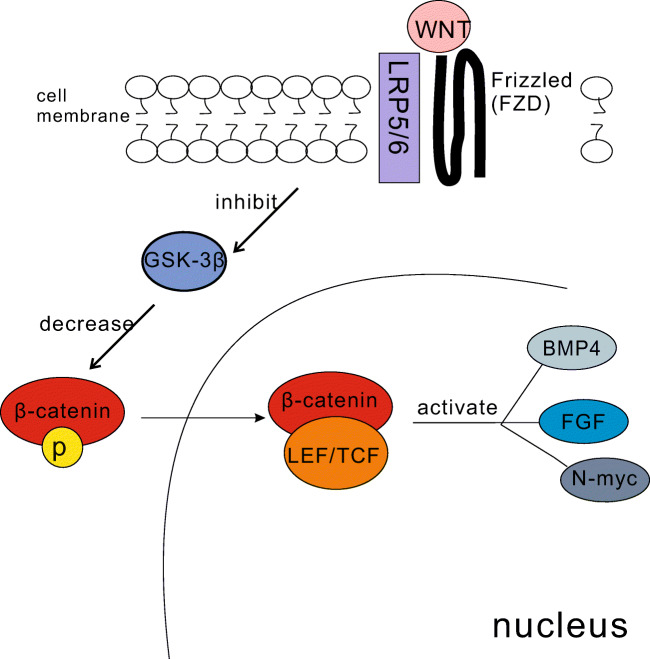
BMP4 plays an important role in mesoderm differentiation and is regulated by its upstream pathways. Wnt/β-catenin signal (Fig. 1) is one of its upstream signal pathways(Kim et al. 2002),which may activate or inhibit BMP4 in different tissues or functions, and the correlation between them remains to be studied. BMP4 exists in many kinds of cells, and when the BMP4 in epithelial cells is activated, it migrates to mesenchymal cells, activates the downstream signaling pathway of BMP4 in mesenchymal cells, undergoes epithelial-mesenchymal transition(Li et al. 2019; Seppo Valnio et al. 1993), and regulates the growth and differentiation of epithelial cells through the paracrine sequence between mesenchymal and adjacent epithelium provided by BMP4 ligands(Kahata et al. 2018).
Fig. 1.
This figure introduces the basic mechanism of WNT pathway. When the WNT pathway is opened, three proteins on the cell membrane bind, inhibiting the activity of corresponding kinases, reducing the phosphorylation of β-catenin. And β-catenin aggregates in the nucleus, BMP4 is its downstream pathway. In the formation and differentiation of lung, β-catenin plays an active role in BMP4, and then activates exogenous mesenchymal stem cells to differentiate into epithelial cells, regulating lung injury and repair. In corneal stratification, β-catenin inhibits BMP4
BMPs ligands and receptors
BMP ligands bind to BMP type I and II receptors (BMPRI, BMPRII), which show kinase activity and send signals that activate intracellular effectors, such as drosophila mothers against decapentaplegic protein (Smad) and signal branches of protein kinases, lipid kinases and small molecule kinases(Kahata et al. 2018). BMP4 binds to BMPRII and phosphorylates BMPRI to form a complex that activates its downstream pathway (Rahman et al. 2015).
Downstream signal pathways of BMP4
BMP4 is a member of the TGF-β family. TGF-β ligands express and secrete at sites where epithelial cells interact with mesenchymal cells, contributing to regulate the growth and differentiation of epithelial cells and regulate differentiated epithelial cells(Kahata et al. 2018).
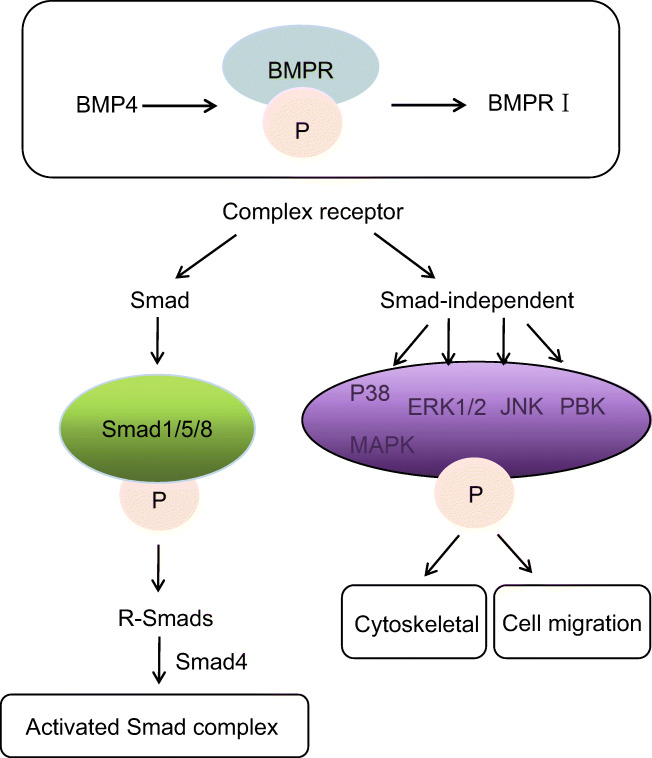
BMPs have a series of downstream signaling pathways, Smad is one of the most important. Smad includes receptor-regulated Smads (r-smads), common partner Smads (coSmads) and path-restricted Smads(Miyazawa and Miyazono 2017). Activation of BMPRI regulates the phosphorylation of r-smads, among which the phosphorylation of Smad1, Smad5 and Smad8 has BMP signal specificity. Phosphorylated r-smads protein forms a complex with Smad4, a coSmad protein with BMP signal specificity. This complex enters the nucleus and binds to a variety of co-activators or co-inhibitors to regulate the transcription of target genes (Liu et al. 2017).
Smad-independent downstream signaling pathway is also an important pathway of BMP4. P38 mitogen-activated protein kinase (P38 MAPK), extracellular regulated protein kinase 1/2 (ERK1/2), c-Jun amino terminal kinase (JNK), phosphatidylinositol-3-kinase (PI3-K), and serine / threonine kinase B (PKB) are all downstream signals independent of Smad (Chen et al. 2004). BMP4 binding to receptor induces phosphorylation of downstream signals and up-regulates or down-regulates the expression of related factors (Li et al. 2013). Furthermore, it is involved in the regulation of cytoskeletal change, migration and cell survival, and can affect the transcription of target genes either alone or in coordination with the Smad pathway (Fig. 2).
Fig. 2.
BMP4 and its downstream pathways.
Regulation of BMP4 on epithelium
Regulation of BMP4 on epithelial tissue growth
Regulation of BMP4 on the growth of covering epithelium
In the process of palatal development, if the integrity of the palatal epithelium is destroyed, it usually leads to abnormal adhesion or fusion between the palatal framework connected with the adjacent structure, resulting in cleft palate defect (He et al. 2010). BMP4 is expressed in the prepalatal stroma to maintain the expression of Msx1 and promote the proliferation of anterior palatal epithelial cells and prepalatal stromal cells through the msx1-bmp4 positive feedback loop(Hilliard et al. 2005).
BMP4 also regulates the growth of upper and lower molars. BMP4 signals in the epithelium can activate BMP4 signals in the stroma, thereby promoting odontogenic proliferation of mesenchymal cells in the process of tooth morphogenesis and tooth formation (Seppo Valnio et al. 1993). Studies have shown that BMP4 can inhibit the activity of Dkk2, an antagonist of its upstream Wnt pathway, and synergize with Msx1 to promote the growth of epithelial cells and mesenchymal cells in the upper and lower molars (Jia et al. 2013).
There are type I alveolar cells (AT1s) and type II alveolar cells (AT2s) in alveolar epithelium (Fiaturi et al. 2018). After pneumonectomy, the compensatory regeneration of alveoli promotes the proliferation of AT2s, and new AT1s are formed to restore alveolar surface area and pulmonary function.BMP4 is active in most AT1s and AT2s in quiet adult lungs.BMP4 in AT2 helps to maintain its stability.However, at the early stage of pneumonectomy, the phosphorylated smad1/5/8 in AT2 decreases, making it sensitive to proliferation and differentiation signals, thus promoting the proliferation of AT2 cells and their differentiation into AT1 cells, thus promoting alveolar compensatory regeneration(Chung et al. 2018). Therefore, BMP4 can promote the growth of covering epithelium of multiple organs.
Regulation of BMP4 on the growth of glandular epithelium
BMP4 can inhibit the growth of glandular epithelium in some organs. The prostate gland is a male-specific exocrine gland developed from the urogenital sinus of the lower urethra (Marker et al. 2003). Activation of androgen receptors in the urogenital sinus mesenchyma will regulate the formation of prostatic buds from the epithelial cells of the urogenital sinus (Cunha 1985). However, bud formation and branch morphogenesis of prostate are regulated by BMPs, among which BMP4 is an inhibitor of prostatic bud formation and branch morphogenesis (Keil et al. 2015). Studies have shown that in urogenital sinus, exogenous BMP4 inhibits testosterone-induced prostatic duct germination and epithelial cell proliferation. In the development of many organs, the regulation of heparan sulfate sulfonation is an important determinant of growth factor signal transduction. Extracellular sulfatase sulf-1 (Sulf1) inhibits prostate development (Kleinschmit et al. 2010), and BMP4 inhibits prostate development by inducing the expression of Sulf1 (Buresh-Stiemke et al. 2012). Therefore, BMP4 can inhibit the proliferation of the urogenital sinus epithelium and thus inhibit the development of prostate.
BMP4 can also inhibit the growth of breast epithelium. The formation of dorsal ventral boundary is the key to determine body plan during embryonic development. When Tbx3 gene is overexpressed in the mammary gland formation region, it promotes prolongation of the dorsal ventral boundary and exhibits epithelial thickening of the characteristics of the mammary ectoderm. Bmp4 negatively regulates Tbx3 and inhibits the growth of breast epithelium, thereby limiting the growth of breast epithelium to a specific location (Cho et al. 2006). Therefore, BMP4 has a certain inhibitory effect on the growth of glandular epithelium.
Regulation of BMP4 on the growth of sensory epithelium
BMP4 promotes the apoptosis of cochlear sensory epithelial cells and inhibits the growth of cochlear sensory epithelium. Cochlear tissues and a sensory epithelial cell line (OC1) are continuously growing sensory epithelial cells of the cochlea. BMP4 and its receptors are highly expressed in the sensory area of the developing rat cochlea and are involved in the regulation of hair cell development (Zheng et al. 2008). Studies have shown that excessive intracellular calcium ion loading can lead to the disorder of calcium channels leading to cell apoptosis (Huber et al. 2018). BMP4 can increase intracellular calcium ion concentration, thereby inhibiting DNA synthesis and promoting the apoptosis of OC1 cells. In addition, aspartic acid receptor subunit gene (NR2B gene) is a part of the brain N-methyl-D-aspartate nerve endings. It is highly expressed in the sensory epithelial cells of the cochlea, which can promote the apoptosis of OC1. BMP4 promotes the apoptosis of cochlear sensory epithelial cells and inhibits the growth of cochlear sensory epithelium by increasing the expression of NR2B gene (Chen et al. 2014).
Regulation of BMP4 on epithelial tissue differentiation
Regulation of BMP4 on the differentiation of covering epithelium
BMP4 can promote the differentiation of corneal epithelial cells into layers. The cornea is a transparent tissue located on the anterior surface of the eye, consisting of layered non-keratinized epithelium, thick matrix scattered over keratinocytes, and monolayer endothelium (Zieske 2004). The activity of Wnt/β-catenin signal in corneal stromal cells was negatively correlated with the stratification and proliferation of corneal epithelial cells. When the Wnt/β-catenin signal is removed, its inhibition on BMP4 is removed, and the expression of BMP4 is increased as a paracrine factor to enhance the expression of P63 in corneal epithelial stromal cells. P63 is necessary to initiate epithelial stratification and maintain the proliferation potential of basal keratinocytes during corneal and skin development (Zhu et al. 2014). Therefore, BMP4 can promote the stratification and proliferation of corneal epithelial cells (Zhang et al. 2015).
Regulation of BMP4 on the differentiation of glandular epithelial
The embryonic epithelial thymus gland is originated from the endoderm of the third pharyngeal sac. Under the influence of forkhead transcription factor 1 (Foxn1), it eventually differentiates into functional thymic epithelial cells. Foxn1 is a key transcriptional regulator for thymic epithelial differentiation (Swann et al. 2017). At the early stage of embryonic development, the primitive endoderm signals the adjacent mesenchyma to induce BMP4 expression, which in turn activate the expression of Foxn1 and induce the differentiation of thymic epithelium (Neves et al. 2012). When BMP signal in thymic embryo is suppressed, thymus will be dysplastic(Soza-Ried et al. 2008), which further proves that BMP4 can promote the differentiation of thymic epithelium.
Regulation of BMP4 on the differentiation of metastatic epithelium
BMP4 can promote the proliferation and differentiation of ureteral epithelial cells and induce the formation of mesenchymal cells. The ureter is an important part of the urinary system, which ensures the effective discharge of urine from the renal pelvis to the bladder (Bohnenpoll et al. 2017a). Sonic hedgehog factor is an important signal for growth and differentiation of ureteral epithelial cells. Secreted by ureteral epithelium, sonic hedgehog factor activates its downstream pathway and induces the expression of Forkhead transcription factor 1 (Foxf1) in mesenchymal tissue. Foxf1 activates and maintains the expression of BMP4, which regulates the proliferation and differentiation of ureteral mesenchymal and epithelial cells (Bohnenpoll et al. 2017b; Haraguchi et al. 2012). Therefore, BMP4 plays a crucial role in the differentiation of ureteral epithelium as a downstream signal. BMP4 plays a role in promoting epithelial differentiation in different types of epithelial tissues of different organs.
Regulation of BMP4 on benign lesions of epithelium
Regulation of BMP4 on benign lesions of covering epithelium
Inflammatory response can remove harmful factors in tissues, but uncontrolled inflammatory response will lead to tissue damage. If inflammatory factors can not be removed in time, acute inflammation will turn into chronic inflammation, causing serious consequences (Khedoe et al. 2013). Gastric epithelial cells are monolayer columnar epithelium, which will release a large amount of cytokines and chemokines when stimulated by inflammation. Peptides such as tumor necrosis factor (TNF-α), Interferon-γ (IFN-γ), and active interleukin 8 (IL-8) can promote and maintain the injury of gastric mucosa (Obonyo et al. 2002). BMP4 was not expressed in normal human gastric antrum tissues, but was abundantly expressed in inflammatory gastric antrum tissues infected by Helicobacter pylori (Bleuming et al. 2006). In the gastric mucosa, the signals are generated by BMP target gastric epithelial cells, and BMP4 in gastric mucosa cells can inhibit the expression of IL-8, thereby slowing down gastric inflammation. In addition, BMP4 is also expressed in the respiratory epithelium, and TNF-αincreases the expression of BMP4 in the respiratory tract, thereby activating the downstream Smad pathway to reduce the IL-8 induced by TNF-αand alleviate the inflammatory response (Li et al. 2014). Therefore, activation of BMP signals, including BMP4, can inhibit the expression and release of inflammatory cytokines in covering epithelial cells (Takabayashi et al. 2014).
Barrett’s esophagus (BE) is a dermal metaplasia in which the normal squamous epithelium in the distal esophagus is replaced by columnar epithelium. The most abundant bile acids in the patients’ reflux fluid are deoxycholic acid and deoxycholic acid (Taddei et al. 2014). When exposed to deoxycholic acid, the expression of BMP4 in esophageal squamous epithelium increases, activating the expression of kruppel-like factor (KLF4) in its downstream pathway and inducing the metaplasia transformation in the process of BE formation (Yan et al. 2016).
Human airway epithelium is pseudostratified columnar epithelium, and basal cells are stem cells of human airway epithelium, which can differentiate into ciliated cells and secretory cells (Rock et al. 2010). Smoking may lead to chronic obstructive pulmonary disease (COPD), which is characterized by morphological changes in airway epithelium, including basal and intermediate cells proliferation, reduction of ciliated cells, goblet cell proliferation and squamous epithelial metaplasia (Shaykhiev et al. 2013). Studies have shown that BMP4 expression is very low in normal airway epithelium. However, in the airway epithelium of smokers with copd, BMP4 type I receptors increase and play a role in regulating BMP4. BMP4 inhibits normal basal cells from differentiating into mucociliary epithelial cells and secretory cells, and induces squamous metaplasia by activating the downstream Smad pathway (Zuo et al. 2019). Therefore, BMP4 promotes morphological changes of airway epithelium and induces the occurrence of airway epithelial diseases.
In summary, BMP4 can both slow down the inflammatory response of the covering epithelium and induce corresponding benign lesions by inducing epithelial metaplasia or differentiation.
Regulation of BMP4 on benign lesions of metastatic epithelium
Urinary epithelium is a kind of metastatic epithelium. When the urethra is infected or injured by inflammation, the expression of Shh signal in basal cells and Wnt signal in stromal cells are up-regulated, and the expression of BMP4 is promoted, thus promoting the regeneration and injury repair of urinary epithelium and restoring the barrier function of urinary epithelium (Wang et al. 2017). Urinary tract infection is caused by urinary pathogenic Escherichia coli infection, and urinary tract infection will reduce the expression of BMP4 and the activity of downstream pathway Smad1, thereby leading to dysdifferentiation of urinary epithelial cells (Mysorekar et al. 2009). Therefore, BMP4 plays an important regulatory role in the repair of urethral epithelial injury.
Reconstruction of the bladder is also associated with structural restoration of the bladder epithelium and detrusor. When damaged, under the stimulation of mesenchymal stem cells, Hh ligands (mainly Shh) were increased in bladder epithelial basal stem cells, thereby promoting the production of Hh target gene product BMP4. BMP4 transmits paracrine stimulation to bladder epithelium to promote the repair of bladder epithelium and smooth muscle layer (Pokrywczynska et al. 2019). Therefore, BMP4 plays a repair role in the lesion of transitional epithelium.
Regulation of BMP4 on benign lesions of glandular epithelium
BMP plays an important role in the formation of submandibular glands by regulating the synthesis of extracellular matrix. During the development of submandibular gland, epithelial cells grow and germinate. The initial bud divides and elongates to form a network of epithelial stems with buds at the top and mesenchymal substances derived from neural crest around it (Miao et al. 2019). The development of submandibular gland will be affected if there are obstacles in the formation of branches. BMP4 inhibits the size and number of buds and their further branches, thus inhibiting the development of submandibular glands (Hoffman et al. 2002). It can be speculated that BMP4 inhibits the development of glands by inhibiting the growth of glandular epithelium, thus leading to diseases..
Regulation of BMP4 on malignant lesions of epithelium
Regulation of BMP4 on malignant lesions of covering epithelium
Ovarian cancer is the most fatal cancer among gynecological malignancies. Most ovarian cancer originates from ovarian epithelium, which forms a continuous monolayer of cells and attaches to the surface of the ovary through the basement membrane composed of fibronectin, collagen type IV and laminin. Ovarian epithelium secretes vitreous binding protein and integrin to enhance cell adhesion (Auersperg et al. 2001; Shepherd and Nachtigal 2003). Studies have shown that Hedgehog signaling produced in ovarian cancer cells can regulate the production of BMP4 by mesenchymal stem cells associated with ovarian cancer, which in turn enhances Hedgehog signaling (Coffman et al. 2016). BMP4 can not only regulate the expression of extracellular matrix components and integrin, but also promote the expression of inhibitor of differentiation 3 (ID3), thus promoting the migration and morphogenesis of ovarian cancer cells (Shepherd et al. 2008; Theriault et al. 2007).
In addition, odontogenic tumors are a group of tumors that derived from the tooth formator or its remnants and can only be found in the jaw or associated soft tissue. Increased expression of BMP4 in odontogenic tumors can promote its occurrence (Swarup et al. 2018).
Rgulation of BMP4 on malignant lesions of metastatic epithelium
Bladder epithelium is a metastatic epithelium in which BMPs plays an important role in regulating homeostasis through fine regulation of Wnt and Hedgehog signals. In this process, BMPs induce differentiation of urothelial cells (Shin et al. 2014). Data shows that BMP4 signals generated by bladder cancer cells can induce the polarization of monocytes/macrophages to the M2 phenotype (Martinez et al. 2017). Macrophages can be divided into two polarization types according to their phenotypes and the polarization factors they secrete, namely, classical activation (M1) and selective activation (M2) of macrophages. The macrophages secreting pro-inflammatory factors are mainly M1 macrophages, while M2 macrophages play a major role in reducing inflammation and tissue repair. Among them, M2 macrophages are beneficial to the growth of cancer cells (Zaki et al. 2011). Therefore, BMP4 can promote the development of bladder cancer by inducing macrophage polarization to the M2 phenotype.
Regulation of BMP4 on malignant lesions of glandular epithelial
BMP4 promotes the development of breast cancer. BMP4 and BMP7 are overexpressed in breast cancer and enhance the migration and invasiveness of breast cancer cells. If BMPR2 is inhibited, the phosphorylation of the downstream signaling pathway smad1/5/8 of BMP4 is also inhibited (Owens et al. 2012), further demonstrating the promoting effect of BMP4 on the progress of breast cancer.
In addition, BMP4 has an inhibitory effect on lung adenocarcinoma. Adriamycin can induce premature senescence of lung cancer cells. Adriamycin induces up-regulation of BMP4 expression, thereby activating the Smad pathway, acetylating histone acetylase p300 in the promoter regions of P16 and P21, and mediating senescence of lung cancer cells (Su et al. 2009).
It is worth noting that BMPs are major regulators of development and play an important role in carcinogenesis. BMP4 plays a dual role in the regulation of cancer cells, either inhibiting or stimulating the occurrence and metastasis of cancer cells, depending on the tissue and the type of cancer. BMP4 can induce the development of ovarian cancer, odontogenic tumor, bladder cancer, breast cancer, urethral cancer and prostate cancer, as well as inhibit the occurrence of lung adenocarcinoma, liver cancer (Zhang et al. 2012)and gastric cancer (Shirai et al. 2011). Therefore, the role of BMP4 in the development of cancer in different organs remains to be studied.
Conclusion
Since the regulation of BMP4 on epithelial tissues has been discovered, the research on BMP4 has become more and more extensive. This article reviews the regulation mechanism of BMP4 on epithelial tissue, and summarizes the regulation of BMP4 on epithelial growth, differentiation, benign lesions and malignant lesions according to different epithelial types (Table 2)
Table 2.
Different roles and corresponding pathways of BMP4 in different epithelial tissues
| Epithelial tissue and related phenomena | Involved pathways and genes | The function of BMP4 | |
|---|---|---|---|
| Growth | Covering epithelium | Msx1-BMP4 | Promote the growth of multiple organ epithelium |
| Glandular epithelium | BMP4-Sulf1/Tbx3 | Inhibit the growth of glandular epithelium | |
| Sensory epithelium | BMP4-Ca2 + NR2B | Inhibit the growth of cochlear sensory epithelium | |
| Differentiation | Covering epithelium | Wnt /β-catenin | Promote epithelial differentiation |
| Glandular epithelium | Foxn1-BMP4 | ||
| Transitional epithelium | Foxf1-BMP4 | ||
| Benign lesions | Covering epithelium | Smad, Inhibition of IL-8 expression | Slow down the inflammatory response and induce morphological transformation of epithelium |
| Glandular epithelium | Unknown | Inhibiting glandular development | |
| Transitional epithelium | Smad | Promote repair of epithelial injury | |
| Malignant lesions | Covering epithelial | Wnt、Hedgehog | Promote cancer development |
| Glandular epithelium | Smad | Promote/inhibit cancer development | |
| Transitional epithelium | Wnt、Hedgehog | Promote cancer development | |
The upstream and downstream pathways of BMP4 and their roles in different diseases remain to be studied. At present, studies have shown that myofibroblasts can increase the expression of BMP4 in the esophagus and promote epithelial growth in the form of esophageal myofibroblast - paratrophic secretion (Zhang et al. 2018). Thymic endothelial cells can also promote the expression of BMP4 after injury and promote thymic regeneration through Foxn1 and its downstream target (Wertheimer et al. 2018).Despite the pathways mentioned in this review, BMP4 also has differentiation inhibitor 2(ID2) and other downstream pathways. In liver cancer cells, BMP4 promotes cell proliferation, inhibits cell differentiation, and promotes cell proliferation by affecting the expression of ID2, a downstream pathway protein that binds to helical transcription factors (Ma et al. 2017). Studying the BMP4 pathway is helpful to enhance or inhibit its function and fully exert its physiological effects.
BMP4 may promote or inhibit cancer in different tissues, and the study on the mechanism of BMP4 action may help us further clarify the relationship between BMP4 and cancer. In addition, BMP4 can be used to produce more real organ-like organs, which can be used in induced pluripotent stem cells, embryonic cells and other organ-like organs to promote the formation of organ-like organs (Mills et al. 2017), which may be helpful for organ culture and transplantation. Therefore, the role of BMP4 in different epithelial tissues and its corresponding mechanisms have broad research prospects.
Acknowledgments
This work was supported by the Outstanding Young Teacher Training Program of Jilin University[419080520314]; the Science and Technology Department [20180101146JC]; the Science and Technology Project of Jilin Traditional Chinese Medicine[2019134]; and the International Cooperation Project of Jilin Provincial Science and Technology Department in the charge of Zhang Yan.
Abbreviations
- BMPs
bone morphogenetic proteins
- BMP4
bone morphogenetic protein 4
- BMPRI, BMPRII
BMP type I and II receptors
- Smad
drosophila mothers against decapentaplegic protein
- r-smads
receptor-regulated Smads
- coSmads
common partner Smads
- P38 MAPK
P38 mitogen-activated protein kinase
- ERK1/2
extracellular regulated protein kinase 1/2
- JNK
c-Jun amino terminal kinase
- PI3-K
phosphatidylinositol-3-kinase
- PKB
serine/threonine kinase B
- AT1s
type I alveolar cells
- AT2s
type II alveolar cells
- Sulf1
sulfatase sulf-1
- OC1
cochlear tissues and a sensory epithelial cell line
- NR2B gene
aspartic acid receptor subunit gene
- Foxn1
forkhead transcription factor 1
- TNF-α
tumor necrosis factor
- IFN-γ
interferon-γ
- IL-8
active interleukin 8
- BE
Barrett’s esophagus
- KLF4
kruppel-like factor
- COPD
chronic obstructive pulmonary disease
- ID3
inhibitor of differentiation 3
- M1
classical activation of macrophages
- M2
selective activation of macrophages
- ID2
differentiation inhibitor 2
Compliance with ethical standard
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Conflict of interest
The authors declared that they have no conflicts of interest to this work.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Auersperg N, Wong AS, Choi KC, Kang SK, Leung PC. Ovarian surface epithelium: biology, endocrinology, and pathology. Endocr. Rev. 2001;22:255–288. doi: 10.1210/edrv.22.2.0422. [DOI] [PubMed] [Google Scholar]
- Bleuming SA, et al. Altered bone morphogenetic protein signalling in the helicobacter pylori-infected stomach. J. Pathol. 2006;209:190–197. doi: 10.1002/path.1976. [DOI] [PubMed] [Google Scholar]
- Bohnenpoll T, Feraric S, Nattkemper M, Weiss AC, Rudat C, Meuser M, Trowe MO, Kispert A. Diversification of Cell Lineages in Ureter Development. J Am Soc Nephrol. 2017;28:1792–1801. doi: 10.1681/asn.2016080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnenpoll T, et al. A SHH-FOXF1-BMP4 signaling axis regulating growth and differentiation of epithelial and mesenchymal tissues in ureter development. PLoS Genet. 2017;13:e1006951. doi: 10.1371/journal.pgen.1006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buresh-Stiemke RA, Malinowski RL, Keil KP, Vezina CM, Oosterhof A, Van Kuppevelt TH, Marker PC. Distinct expression patterns of Sulf1 and Hs6st1 spatially regulate heparan sulfate sulfation during prostate development. Developmental dynamics: an official publication of the American Association of Anatomists. 2012;241:2005–2013. doi: 10.1002/dvdy.23886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins growth factors (Chur, Switzerland) 22:233-241 10.1080/08977190412331279890 [DOI] [PubMed]
- Chen J, Zheng Y, Xiong H, Ou Y. NMDA receptors are involved in the regulation of BMP4-mediated survival in rat cochlear epithelial cells. Neurosci. Lett. 2014;566:275–279. doi: 10.1016/j.neulet.2014.02.067. [DOI] [PubMed] [Google Scholar]
- Cho KW, et al. Molecular interactions between Tbx3 and Bmp4 and a model for dorsoventral positioning of mammary gland development. Proc. Natl. Acad. Sci. U. S. A. 2006;103:16788–16793. doi: 10.1073/pnas.0604645103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung MI BM, Barkauskas CE, Kobayashi Y, Hogan BLM. (2018) Niche-mediated BMP/SMAD signaling regulates lung alveolar stem cell proliferation and differentiation Biologists Ltd 145 [DOI] [PMC free article] [PubMed]
- Coffman LG, Choi YJ, McLean K, Allen BL, di Magliano MP, Buckanovich RJ. Human carcinoma-associated mesenchymal stem cells promote ovarian cancer chemotherapy resistance via a BMP4/HH signaling loop. Oncotarget. 2016;7:6916–6932. doi: 10.18632/oncotarget.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR. Mesenchymal-epithelial interactions during androgen-induced development of the prostate. Prog. Clin. Biol. Res. 1985;171:15–24. [PubMed] [Google Scholar]
- Fiaturi NRJ, Nielsen HC, Castellot JJ., Jr CCN5 in alveolar epithelial proliferation and differentiation during neonatal lung oxygen injury. J Cell Commun Signal. 2018;12:217–229. doi: 10.1007/s12079-017-0443-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haraguchi R, Matsumaru D, Nakagata N, Miyagawa S, Suzuki K, Kitazawa S, Yamada G. The hedgehog signal induced modulation of bone morphogenetic protein signaling: an essential signaling relay for urinary tract morphogenesis. PLoS One. 2012;7:e42245. doi: 10.1371/journal.pone.0042245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He F, et al. Modulation of BMP signaling by noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev. Biol. 2010;347:109–121. doi: 10.1016/j.ydbio.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard SA, Yu L, Gu S, Zhang Z, Chen YP. Regional regulation of palatal growth and patterning along the anterior-posterior axis in mice. J. Anat. 2005;207:655–667. doi: 10.1111/j.1469-7580.2005.00474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman MP, Kidder BL, Steinberg ZL, Lakhani S, Ho S, Kleinman HK, Larsen M. Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development (Cambridge, England) 2002;129:5767–5778. doi: 10.1242/dev.00172. [DOI] [PubMed] [Google Scholar]
- Honda H. The world of epithelial sheets. Develop. Growth Differ. 2017;59:306–316. doi: 10.1111/dgd.12350. [DOI] [PubMed] [Google Scholar]
- Huber GA, Priest SM, Geisbuhler TP. Cardioprotective Effect of Hydroxysafflor Yellow A via the Cardiac Permeability Transition Pore. Planta Med. 2018;84:507–518. doi: 10.1055/s-0043-122501. [DOI] [PubMed] [Google Scholar]
- Jia S, Zhou J, Gao Y, Baek JA, Martin JF, Lan Y, Jiang R. Roles of Bmp4 during tooth morphogenesis and sequential tooth formation. Development (Cambridge, England) 2013;140:423–432. doi: 10.1242/dev.081927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahata K, Dadras MS, Moustakas A (2018) TGF-beta Family Signaling in Epithelial Differentiation and Epithelial-Mesenchymal Transition. Cold Spring Harb. Perspect. Biol.:10. 10.1101/cshperspect.a022194 [DOI] [PMC free article] [PubMed]
- Keil KP, Altmann HM, Abler LL, Hernandez LL, Vezina CM. Histone acetylation regulates prostate ductal morphogenesis through a bone morphogenetic protein-dependent mechanism. Developmental dynamics : an official publication of the American Association of Anatomists. 2015;244:1404–1414. doi: 10.1002/dvdy.24321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedoe PP, et al. The effect of PPE-induced emphysema and chronic LPS-induced pulmonary inflammation on atherosclerosis development in APOE*3-LEIDEN mice. PLoS One. 2013;8:e80196. doi: 10.1371/journal.pone.0080196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JS, Crooks H, Dracheva T, Nishanian TG, Singh B, Jen J, Waldman T. Oncogenic beta-catenin is required for bone morphogenetic protein 4 expression in human cancer cells. Cancer Res. 2002;62:2744–2748. [PubMed] [Google Scholar]
- Kleinschmit A, Koyama T, Dejima K, Hayashi Y, Kamimura K, Nakato H. Drosophila heparan sulfate 6-O endosulfatase regulates wingless morphogen gradient formation. Dev. Biol. 2010;345:204–214. doi: 10.1016/j.ydbio.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Zhang S, Sui Y, Fu X, Li Y, Wei S (2019) Sequential stimulation with different concentrations of BMP4 promotes the differentiation of human embryonic stem cells into dental epithelium with potential for tooth formation. Stem Cell Res Ther 10. 10.1186/s13287-019-1378-7 [DOI] [PMC free article] [PubMed]
- Li X, et al. BMP4 increases canonical transient receptor potential protein expression by activating p38 MAPK and ERK1/2 signaling pathways in pulmonary arterial smooth muscle cells. Am. J. Respir. Cell Mol. Biol. 2013;49:212–220. doi: 10.1165/rcmb.2012-0051OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. Bone morphogenetic protein 4 inhibits liposaccharide-induced inflammation in the airway. Eur. J. Immunol. 2014;44:3283–3294. doi: 10.1002/eji.201344287. [DOI] [PubMed] [Google Scholar]
- Liu Y, et al. The BMP4-Smad signaling pathway regulates hyperandrogenism development in a female mouse model. J. Biol. Chem. 2017;292:11740–11750. doi: 10.1074/jbc.M117.781369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J, et al. BMP4 enhances hepatocellular carcinoma proliferation by promoting cell cycle progression via ID2/CDKN1B signaling. Mol. Carcinog. 2017;56:2279–2289. doi: 10.1002/mc.22681. [DOI] [PubMed] [Google Scholar]
- Marker PC, Donjacour AA, Dahiya R, Cunha GR. Hormonal, cellular, and molecular control of prostatic development. Dev. Biol. 2003;253:165–174. doi: 10.1016/S0012-1606(02)00031-3. [DOI] [PubMed] [Google Scholar]
- Martinez VG, et al. BMP4 induces M2 macrophage polarization and favors tumor progression in bladder. Cancer clinical cancer research: an official journal of the American Association for Cancer Research. 2017;23:7388–7399. doi: 10.1158/1078-0432.Ccr-17-1004. [DOI] [PubMed] [Google Scholar]
- Miao N, et al. Loss of Fam20c causes defects in the acinar and duct structure of salivary glands in mice. Int. J. Mol. Med. 2019;43:2103–2117. doi: 10.3892/ijmm.2019.4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CG, Lawrence ML, Munro DAD, Elhendawi M, Mullins JJ, Davies JA. Asymmetric BMP4 signalling improves the realism of kidney organoids. Sci. Rep. 2017;7:14824. doi: 10.1038/s41598-017-14809-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K, Miyazono K (2017) Regulation of TGF-beta Family Signaling by Inhibitory Smads. Cold Spring Harb. Perspect. Biol.:9. 10.1101/cshperspect.a022095 [DOI] [PMC free article] [PubMed]
- Mysorekar IU, Isaacson-Schmid M, Walker JN, Mills JC, Hultgren SJ. Bone morphogenetic protein 4 signaling regulates epithelial renewal in the urinary tract in response to uropathogenic infection. Cell Host Microbe. 2009;5:463–475. doi: 10.1016/j.chom.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neves H, Dupin E, Parreira L, Le Douarin NM. Modulation of Bmp4 signalling in the epithelial-mesenchymal interactions that take place in early thymus and parathyroid development in avian embryos. Dev. Biol. 2012;361:208–219. doi: 10.1016/j.ydbio.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Obonyo M, Guiney DG, Harwood J, Fierer J, Cole SP. Role of gamma interferon in Helicobacter pylori induction of inflammatory mediators during murine infection. Infect. Immun. 2002;70:3295–3299. doi: 10.1128/IAI.70.6.3295-3299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens P, et al. Disruption of bone morphogenetic protein receptor 2 (BMPR2) in mammary tumors promotes metastases through cell autonomous and paracrine mediators. Proc. Natl. Acad. Sci. U. S. A. 2012;109:2814–2819. doi: 10.1073/pnas.1101139108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrywczynska M, Rasmus M, Jundzill A, Balcerczyk D, Adamowicz J, Warda K, Buchholz L, Drewa T (2019) Mesenchymal stromal cells modulate the molecular pattern of healing process in tissue-engineered urinary bladder: the microarray data. Stem Cell Res Ther 10:–17. 10.1186/s13287-019-1266-1 [DOI] [PMC free article] [PubMed]
- Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM (2015) TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Research 3(1). 10.1038/boneres.2015.5 [DOI] [PMC free article] [PubMed]
- Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis. Model. Mech. 2010;3:545–556. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppo Valnio IK, Adrian Jowett, Irma Thesleff (1993) Identification of BMP-4 as a Signal Mediating Secondary Induction between Epithelial and Mesenchymal Tissues during Early Tooth Development Cell 75 [PubMed]
- Shaykhiev R, Zuo WL, Chao I, Fukui T, Witover B, Brekman A, Crystal RG. EGF shifts human airway basal cell fate toward a smoking-associated airway epithelial phenotype. Proc. Natl. Acad. Sci. U. S. A. 2013;110:12102–12107. doi: 10.1073/pnas.1303058110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd TG, Nachtigal MW. Identification of a putative autocrine bone morphogenetic protein-signaling pathway in human ovarian surface epithelium and ovarian cancer cells. Endocrinology. 2003;144:3306–3314. doi: 10.1210/en.2003-0185. [DOI] [PubMed] [Google Scholar]
- Shepherd TG, Theriault BL, Nachtigal MW. Autocrine BMP4 signalling regulates ID3 proto-oncogene expression in human ovarian cancer cells. Gene. 2008;414:95–105. doi: 10.1016/j.gene.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Shin K, et al. Hedgehog signaling restrains bladder cancer progression by eliciting stromal production of urothelial differentiation factors. Cancer Cell. 2014;26:521–533. doi: 10.1016/j.ccell.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirai YT, Ehata S, Yashiro M, Yanagihara K, Hirakawa K, Miyazono K. Bone morphogenetic protein-2 and -4 play tumor suppressive roles in human diffuse-type gastric carcinoma. Am. J. Pathol. 2011;179:2920–2930. doi: 10.1016/j.ajpath.2011.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soza-Ried C, Bleul CC, Schorpp M, Boehm T (2008) maintenance of thymic epithelial phenotype requires extrinsic signals in mouse and zebrafish journal of immunology (Baltimore, Md : 1950) 181:5272–5277 [DOI] [PubMed]
- Su D et al. (2009) BMP4-Smad signaling pathway mediates Adriamycin-induced premature senescence in lung Cancer cells*S the journal of biological chemistry 284:12153-12164 10.1074/jbc.M807930200 [DOI] [PMC free article] [PubMed]
- Swann JB, Krauth B, Happe C, Boehm T. Cooperative interaction of BMP signalling and Foxn1 gene dosage determines the size of the functionally active thymic epithelial compartment. Sci. Rep. 2017;7:8492. doi: 10.1038/s41598-017-09213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup N, Nayak MT, Chowdhary Z, Mehendiratta M, Khatana S, Choi SJ, Sagolsem C (2018) evaluation and Immunolocalization of BMP4 and FGF8 in Odontogenic cyst and tumors analytical cellular pathology (Amsterdam) 2018 10.1155/2018/1204549 [DOI] [PMC free article] [PubMed]
- Taddei A, et al. Cyclooxygenase-2 and inflammation mediators have a crucial role in reflux-related esophageal histological changes and Barrett's esophagus. Dig. Dis. Sci. 2014;59:949–957. doi: 10.1007/s10620-013-2975-4. [DOI] [PubMed] [Google Scholar]
- Takabayashi H, et al. Anti-inflammatory activity of bone morphogenetic protein signaling pathways in stomachs of mice. Gastroenterology. 2014;147:396–406.e397. doi: 10.1053/j.gastro.2014.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theriault BL, Shepherd TG, Mujoomdar ML, Nachtigal MW. BMP4 induces EMT and rho GTPase activation in human ovarian cancer cells. Carcinogenesis. 2007;28:1153–1162. doi: 10.1093/carcin/bgm015. [DOI] [PubMed] [Google Scholar]
- Wang C, Ross WT, Mysorekar IU. Urothelial generation and regeneration in development, injury, and cancer. Developmental dynamics: an official publication of the American Association of Anatomists. 2017;246:336–343. doi: 10.1002/dvdy.24487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer T et al (2018) Production of BMP4 by endothelial cells is crucial for endogenous thymic regeneration. Science immunology 3. 10.1126/sciimmunol.aal2736 [DOI] [PMC free article] [PubMed]
- Yan W, et al. BMP4 promotes a phenotype change of an esophageal squamous epithelium via up-regulation of KLF4. Exp. Mol. Pathol. 2016;101:259–266. doi: 10.1016/j.yexmp.2016.09.007. [DOI] [PubMed] [Google Scholar]
- Zaki MA, et al. Prognostic implication of types of tumor-associated macrophages in Hodgkin lymphoma. Virchows Archiv: an international journal of pathology. 2011;459:361–366. doi: 10.1007/s00428-011-1140-8. [DOI] [PubMed] [Google Scholar]
- Zhang C, Niu C, Yang K, Shaker A (2018) Human esophageal myofibroblast secretion of bone morphogenetic proteins and GREMLIN1 and paracrine regulation of squamous epithelial growth. Sci. Rep. 8. 10.1038/s41598-018-30799-7 [DOI] [PMC free article] [PubMed]
- Zhang L, et al. BMP4 administration induces differentiation of CD133+ hepatic cancer stem cells, blocking their contributions to hepatocellular carcinoma. Cancer Res. 2012;72:4276–4285. doi: 10.1158/0008-5472.Can-12-1013. [DOI] [PubMed] [Google Scholar]
- Zhang Y et al. (2015) Wnt/beta-catenin signaling modulates corneal epithelium stratification via inhibition of Bmp4 during mouse development development (Cambridge, England) 142:3383-3393 10.1242/dev.125393 [DOI] [PMC free article] [PubMed]
- Zheng Y, Rayner M, Feng L, Hu X, Zheng X, Bearth E, Lin J. EGF Mediates Survival of Rat Cochlear Sensory Cells via an NF-kappaB Dependent Mechanism In Vitro. The open neuroscience journal. 2008;2:9–15. doi: 10.2174/1874082000802010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XJ, Liu Y, Dai ZM, Zhang X, Yang X, Li Y, Qiu M, Fu J, Hsu W, Chen Y, Zhang Z. BMP-FGF signaling axis mediates Wnt-induced epidermal stratification in developing mammalian skin. PLoS Genet. 2014;10:e1004687. doi: 10.1371/journal.pgen.1004687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zieske JD. Corneal development associated with eyelid opening. The international journal of developmental biology. 2004;48:903–911. doi: 10.1387/ijdb.041860jz. [DOI] [PubMed] [Google Scholar]
- Zuo WL et al (2019) Exaggerated BMP4 Signalling alters human airway basal progenitor cell differentiation to cigarette smoking-related phenotypes. Eur. Respir. J. 10.1183/13993003.02553-2017 [DOI] [PMC free article] [PubMed]