Abstract
LDL receptor–related proteins 6 (LRP6) is a type I transmembrane receptor (C-terminus in cytosol), which appears to be essential in numerous biological processes, since it is an essential co-receptor of Wnt ligands for canonical β-catenin dependent signal transduction. It was shown that tissue plasminogen activator (tPA), physically interacting with LRP6, induces protein phosphorylation, which may have large implication in the regulation of neural processes. In this investigation we analyzed whether LRP6 is associated with lipid rafts following tPA triggering in neuroblastoma cells and the role of raft integrity in LRP6 cell signaling. Sucrose gradient separation revealed that phosphorylated LRP6 was mainly, but not exclusively present in lipid rafts; this enrichment became more evident after triggering with tPA. In these microdomains LRP6 is strictly associated with ganglioside GM1, a paradigmatic component of these plasma membrane compartments, as revealed by coimmunoprecipitation experiments. As expected, tPA triggering induced LRP6 phosphorylation, which was independent of LRP1, as revealed by knockdown experiments by siRNA, but strictly dependent on raft integrity. Moreover, tPA induced β-catenin phosphorylation was also significantly prevented by previous pretreatment with methyl-β-cyclodextrin. Our results demonstrate that LRP6 mediated signal transduction pathway triggered by tPA acts through lipid rafts in neuroblastoma cells. These findings introduce an additional task for identifying new molecular target(s) of pharmacological agents. Indeed, these data, pointing to the key role of lipid rafts in tPA triggered signaling involving β-catenin, may have pharmacological implications, suggesting that cyclodextrins, and potentially other drugs, such as statins, may represent an useful tool.
Keywords: LRP6, Lipid rafts, tPA, β-Catenin, Neuroblastoma cells
Introduction
LRPs (LDL receptor–related proteins) are transmembrane proteins, belonging to the LDL receptor gene family which are known to be involved in lipoprotein metabolism. Thus, since LRPs structural similarity to the LDL receptor, a role in lipoprotein metabolism and cholesterol homeostasis was hypothesized. They play a role in the regulation of development and cell movements, polarity, axon guidance and synapse formation, as well as in different pathological conditions, including cancer and osteoporosis (Herz and Strickland 2001; He et al. 2004). LRPs, like all the members of the LDL receptor gene family, consist of common structural features: in the extracellular domains they contain ligand binding repeats, β-propeller motifs and epidermal growth factor-like repeats; in the intracellular domains they have several domains that are responsible for downstream signaling events by interacting with cytoplasmic adaptors and scaffolds (He et al. 2004; Lara-Castillo and Johnson 2015). In particular, LRP6 is a type I transmembrane receptor (C-terminus in cytosol), characterized at the extracellular domain by four YWTD β-propellers, four EGF-like domains, and LDLR type A domains and at the intracellular domain by five PPPSP motifs (Lara-Castillo and Johnson 2015). This receptor appears to be essential in numerous biological processes, indeed LRP6 deficiency contributes to synaptic anomalies and amyloid pathology (Song et al. 2016). LRP6 is an essential co-receptor of Wnt ligands for canonical β-catenin dependent signal transduction, together with seven motif transmembrane protein Frizzled (Huelsken and Behrens 2002). LRP6 with a large extracellular domain binds several Wnt ligands in vitro. In addition to Wnt proteins, the extracellular region of LRP6 also interacts with other agonists and antagonists of the Wnt pathway, including members of the Dkk family, Sclerostin and Wise (Goel et al. 2012). It was shown that tPA, physically interacting with LRP6, induces protein phosphorylation, which may have large implication in the regulation of neural processes from differentiation to the regulation of synaptic plasticity in neural progenitors cells (Lee et al. 2014). Activation of the evolutionarily conserved Wnt/β-catenin signaling pathway, also called canonical Wnt pathway, involves stabilization of β-catenin through binding of Wnt ligands (the canonical WNTs are WNT1, WNT3a and WNT8) to Frezzled cell surface receptors and low-density LRP5/6 coreceptors. In the absence of Wnt, β-catenin, which represents the key effector of this pathway, is continuously degraded by the “degradation complex”, including Axin, adenomatis polyposis coli (APC), glycogen synthase kinase3β (GSK3β), casein kinase1alpha (CK1α) and the E3 ubiquitin ligase subunit β-TrCP1. Wnt stimulation through LRP6, dismantles the degradation complex, thereby leading to the accumulation of unphosphorylated β-catenin. The stabilized β-catenin translocates to the cell nucleus and through transcription factors, induces expression of an array of genes downstream (Ring et al. 2014; Nag et al. 2017). Activated β-catenin can be oncogenic, driving the onset of a wide spectrum of carcinomas like hepatocellular carcinoma (Tao et al. 2014). Haack et al. (2015) suggested an involvement of lipid rafts in the WNT/β-catenin pathway. Lipid rafts are sphingolipid and cholesterol-enriched microdomains, which impose an increased degree of rigidity on the plasma membrane and play an essential role in assembling protein complexes involved in cell-signaling (Brown and London 1998, 2000; Simons and Toomre 2000) and moreover lipid rafts were revealed to be involved in cell apoptosis (Malorni et al. 2007; Sorice et al. 2012a, b). We previously demonstrated that LRP1 resides transiently in rafts and transfers to clathrin-coated pits, where it undergoes endocytosis and the raft disruption selectively affects the signaling activity of LRP1 (Laudati et al. 2016). A few number of papers suggested that lipid rafts may play a role in LRP6 initiated cell signaling (Sakane et al. 2010; Özhan et al. 2013). In this study we analyzed whether LRP6 is associated with lipid rafts following tPA triggering in neuroblastoma cells and investigated the role of raft integrity in the LRP6 cell signaling.
Materials and methods
Cell culture and treatments
Human SK-N-BE2 neuroblastoma cells (ATCC, LGC Standards S.r.l., Milan, Italy) were grown in RPMI 1640 medium (Sigma-Aldrich, Milan, Italy) supplemented with 10% fetal calf serum (FCS), 100 units/ml penicillin and 10 mg/ml streptomycin, at 37 °C in humified 5% CO2 atmosphere. Cells were cultured until 80% confluent and then treated for 10 min at 37 °C with 10 nM tPA (Molecular Innovations, Novi, MI, USA) (Mantuano et al. 2016; Mattei et al. 2019). To analyze the effect of disruption of lipid rafts, where indicated, cells were treated for 30 min at 37 °C with 5 mM methyl-β-cyclodextrin (Sigma-Aldrich, Milan, Italy) (Garofalo et al. 2003, 2018; Mattei et al. 2019), a compound which is known to induce cholesterol efflux from the membrane (Whitehead et al. 2012). Preliminary experiments demonstrated that cell viability after MβCD treatment was about 95%. After treatment, cells were collected and prepared for experimental procedures.
Sucrose-gradient fractionation
Lipid raft fractions were isolated as previously described (Garofalo et al. 2005). Briefly, 1 × 108 SK-N-BE2 cells, untreated and treated as indicated, were lysed in 1 ml of homogenization buffer, containing 1% Triton X-100, 10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4 and 75 U of aprotinin for 20 min at 4 °C. The lysate was first mechanically homogenized (10 strokes) and then centrifuged for 5 min at 1300×g. After mixing with an equal volume of 85% sucrose (w/v) in the same buffer and centrifugation in a SW41 rotor (Beckman Institute, U.S.A.) at 200000×g for 16–18 h at 4 °C, the supernatant fraction was placed at the bottom of a linear sucrose gradient (5–30%) in lysis buffer (10 mM Tris-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA). Starting from the top of the tube 11 fractions were collected from the gradient. The fraction samples were loaded by volume and analyzed by Western blot. All steps were carried out at 0–4 °C.
Western blot analysis of sucrose-gradient fractions
Fraction samples were subjected to 7.5% sodium-dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The proteins were electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). Membranes were blocked with 1% albumin in TBS (Bio-Rad, Hercules, CA, USA), containing 0.05% Tween 20 (Bio-Rad, Hercules, CA, USA) and probed with goat anti-LRP6 antibody (R&D Systems, Minneapolis, MI, USA), with rabbit anti-phospho-LRP6 pAb (R&D Systems, Minneapolis, MI, USA), with anti-Phospho-β catenin pAb (Cell Signaling Technology, Danvers, MA, USA) with anti-Transferrin receptor, also known as Cluster of Differentiation 71 (CD71) mAb or with anti-flotillin polyclonal Ab (Abcam, Cambridge, MA, USA). The reaction of bounding was visualized with horseradish peroxidase (HRP)-conjugated anti-goat IgG (Sigma-Aldrich, Milan, Italy), anti-mouse IgG (Sigma-Aldrich, Milan, Italy) or anti-rabbit IgG (Sigma-Aldrich, Milan, Italy). Immunoreactivity was assessed by chemiluminescence reaction, using the ECL Western detection system (Amersham, Buckinghamshire, UK). Densitometric scanning analysis was performed with Mac OS X (Apple Computer International), using NIH Image J 1.62 software.
Immunoprecipitation experiments
SK-N-BE2 cell-free lysates, untreated or treated with 10 nM tPA (Molecular Innovations, Novi, MI, USA) for 10 min at 37 °C were then incubated at 4 °C for 2 h on a rotary shaker with protein G-acrylic beads (Sigma-Aldrich, Milan, Italy). The beads were removed by centrifugation (500×g for 1 min) and the supernatants were incubated with goat anti-LRP6 Ab (R&D Systems, Minneapolis, MI, USA) at 4 °C overnight. The antibody complexes were captured by addition of protein G-acrylic beads for additional 1 h at 4 °C. As a negative control, immunoprecipitation was performed with an irrelevant polyclonal IgG. The immunoprecipitates were washed once with PBS, splitted into two aliquots and analyzed by dot blot analysis for GM1 detection and, as a control, by Western blot for LRP6.
Dot-blot analysis
The presence of GM1 in the immunoprecipitates was verified by immuno-dot-blot analysis. Briefly, immunoprecipitates were spotted onto nitrocellulose strips. The strips were blocked with 1% albumin in TBS, containing 0.05% Tween 20 for 1 h to block the residual binding sites on the paper. The strips were rinsed for 10 min in TBS/Tween and then incubated with Cholera Toxin B Subunit-Peroxidase from Vibrio Cholerae (Sigma-Aldrich, Milan, Italy) for 1 h at room temperature, or with anti-LRP6 mAb (Abcam, Cambridge, UK). Immunoreactivity was assessed by chemiluminescence reaction, using the ECL Western detection system.
Western blotting analysis
Cells, untreated and treated with tPA (Molecular Innovations, Novi, MI, USA), as above, in the presence or in the absence of 5 mM MβCD for 30 min at 37 °C, were subjected to sodium-dodecyl sulphate polyacrilamide gel electrophoresis (SDS-PAGE). The proteins were electrophoretically transferred onto polyvinilidene difluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA) and probed with rabbit anti-phospho-LRP6 Ab (R&D Systems, Minneapolis, MI, USA), goat anti-LRP6 Ab (R&D Systems, Minneapolis, MI, USA), anti-Phospho-β catenin Ab (Cell Signaling Technology, Danvers, MA, USA), anti-β catenin mAb (Cell Signaling Technology, Danvers, MA, USA). Horseradish peroxidase (HRP)-conjugated anti-rabbit IgG, anti-goat IgG or anti-mouse IgG (Sigma-Aldrich, Milan, Italy) were used to visualize the reaction of bounding. Immunoreactivity was assessed by chemiluminescence reaction, using the ECL Western detection system (Amersham). Densitometric scanning analysis was performed by Chemidoc (Bio-Rad, Hercules, CA, USA).
Knockdown LRP1 by siRNA
Cells were seeded (2 × 105 cells/ml) in 6-well plate, with DMEM containing serum and antibiotics. Twenty-four hours after seeding, cells were transfected with 5 nM siRNA LRP1 (Qiagen, Valencia, CA), obtained from humans, using HiPerFect Transfection Reagent (Qiagen, Valencia, CA), according to the manufacturer’s instructions. As experimental control, cells were also transfected with 5 nM scrambled siRNA (AllStars Negative Control Qiagen Valencia, CA). After 72 h, cells were incubated with 10 nM tPA (Molecular Innovations, Novi, MI, USA) for 10 min at 37 °C and analyzed by Western Blot, as specified above. The expression of LRP1 was evaluated by Western Blot using goat anti-LRP1 Ab (R&D Systems, Minneapolis, MI, USA). Loading control was evaluated using anti-ACTB (actin, β) mAb (Sigma-Aldrich, Milan, Italy).
High-performance thin layer chromatography (HPTLC) analysis of cholesterol
Cells, untreated and treated with tPA, as above, in the presence or in the absence of 5 mM MβCD were lysed in lysis buffer containing 1% Triton X-100, 10 mM TRIS-HCl (pH 7.5), 150 mM NaCl, 5 mM EDTA, 1 mM Na3VO4, and 75 U of aprotinin and allowed to stand for 20 min at 4 °C. The cell suspension was disrupted using Dounce homogenization (10 strokes). Lysate was centrifuged at 1300×g to for 5 min in order to remove nuclei and large cellular debris. After evaluation of the protein concentration the lysate was subjected to cholesterol analysis. Neutral lipid extracts were separated by HPTLC, using a solvent system of hexane/diethyl ether/acetic acid (70: 30:1, v/v/v) and were detected by staining with 2% copper acetate solution in 8% phosphoric acid and subsequent heating at 120 °C for 15 min. Quantitative analysis was performed using NIH Image1.62 (Mac OS X, Apple Computer International).
Data analysis and statistics
Data were analyzed using one-way analysis of variance (ANOVA) after Bartlett’s test for the homogeneity of variances and Kolmogorov–Smirnov’s test for the Gaussian distribution and followed by Newman-Keuls multiple-comparison test or, when appropriate, with Student’st-test. All data reported were verified in at least three different experiments and reported as mean ± SD. Only p values of <0.05 were considered as statistically significant.
Results
LRP6 enrichment in lipid rafts of SK-N-BE2 neuroblastoma cells
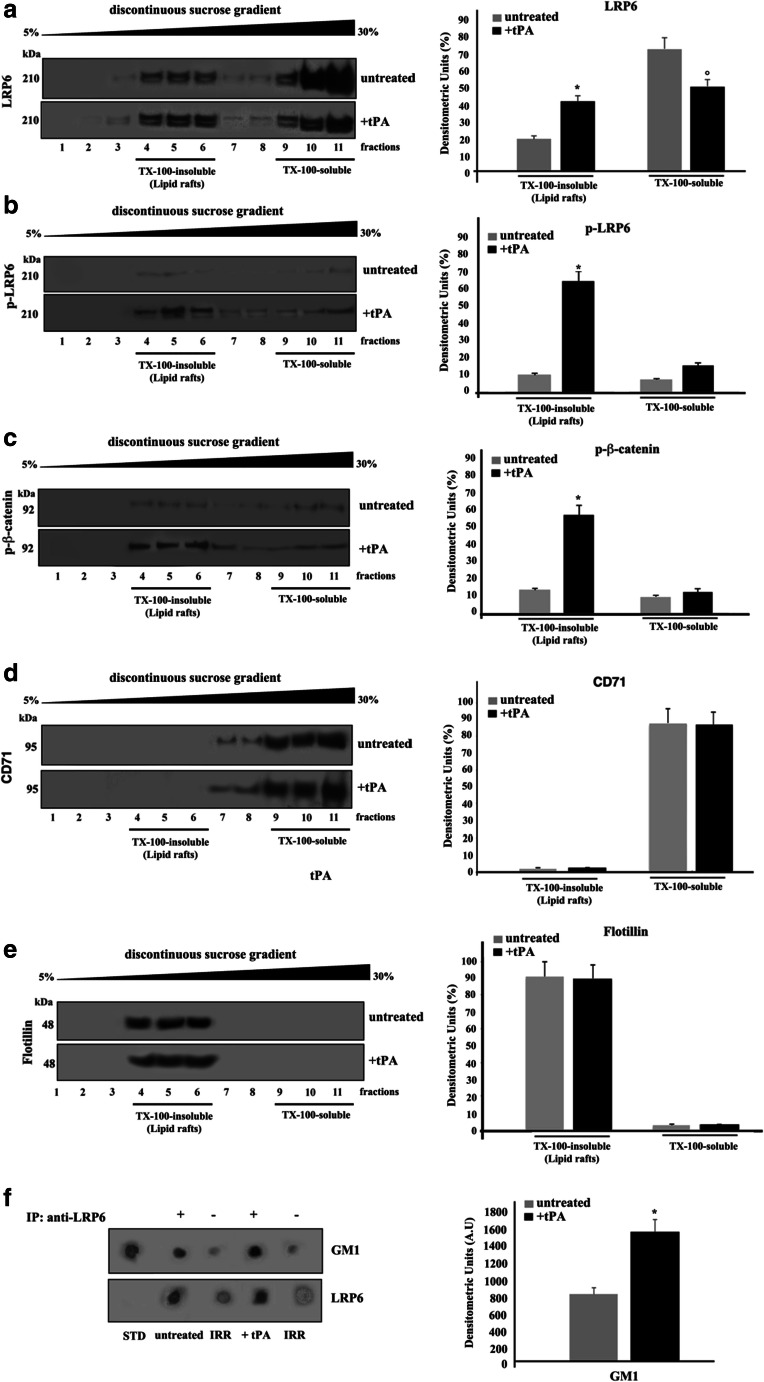
We analyzed the possible association of LRP6 in both Triton X-100 insoluble and soluble fractions, following tPA triggering. As shown in Fig. 1a, left panel, the analysis of the distribution of LRP6 in fractions obtained by a 5–30% linear sucrose gradient revealed that the protein was highly enriched in fractions 4–6, corresponding to lipid rafts. The protein was also detected in Triton-soluble fractions (fractions 10–11), confirming that LRP6 is mainly, but not exclusively present in lipid rafts. This enrichment became more evident after triggering with tPA, as revealed by densitometric analysis (Fig. 1a, right panel). In untreated cells the signal of p-LRP6 was very low in both TX-100 unsoluble and soluble fractions, as expected (Fig. 1b, right panel); upon tPA triggering, p-LRP6 preferentially, but not exclusively, locates in lipid rafts fractions (Fig. 1b, left panel). Similar behaviour was observed for β-catenin (Fig. 1c), It suggests that p-LRP6 may facilitate the phosphorylation of β-catenin. As controls, the distribution of the transmembrane transferrin receptor (CD71), as a Triton X-100 soluble protein, and of flotillin as a marker of lipid rafts, were also tested. As expected, CD71 appeared to be highly enriched in the Triton X-100-soluble fractions (9–11) in both control and tPA-treated cells (Fig. 1d). In contrast, the majority of flotillin appeared in Triton X-100-insoluble fractions (4–6) in both control and tPA-treated cells (Fig. 1e). These results were quantified by densitometric analyses (Fig. 1d-e, right panels).
Fig. 1.
Lipid rafts localization of LRP6 in SK-N-BE2 cells. a-e SK-N-BE2 cells, either untreated or treated with 10 nM tPA for 10 min at 37 °C, were lysed and the supernatant fraction was subjected to sucrose density gradient. After centrifugation, the gradient was fractionated and each fraction was recovered and analyzed by western blot using anti-LRP6 pAb (a), anti-phospho LRP6 pAb (p-LRP6) (b), anti-phospho β-catenin (p-β-catenin) pAb (c), anti-CD71 mAb (d) or an anti-flotillin pAb (e). Right panel: Densitometric analysis of sucrose gradient fractions. The columns indicate the percent distribution across the gel of raft-fractions 4–5 and 6 (Triton X-100-insoluble fractions) and 9–10 and 11 (Triton X-100-soluble fractions), as detected by densitometric scanning analysis. Results represent the mean ± SD from three independent experiments (*) p < 0.01 TX-100-insoluble fractions from tPA-treated cells vs TX-100-insoluble from control cells; (°) p < 0.01 TX-100-soluble fractions from tPA-treated cells vs TX-100-soluble from control cells. f SK-N-BE2 cells, untreated or treated with 10 nM tPA, were lysed in lysis buffer, followed by immunoprecipitation with goat anti-LRP6 pAb. An IgG isotypic control was employed. The immunoprecipitates were spotted onto nitrocellulose strips and incubated with Cholera Toxin B Subunit-Peroxidase (from Vibrio Cholerae), as described in Materials and Methods. As a control, the immunoprecipitates were assessed by Dot-blot with anti-LRP6 mAb. A representative experiment among three is shown. Bar graph in the right panel shows densitometric analysis. Results represent the mean ± SD from three independent experiments. (*) p < 0.01 tPA vs control cells
Coimmunoprecipitation of LRP6 and GM1 in SK-N-BE2 neuroblastoma cells
To verify whether LRP6 may bind directly to gangliosides, cell-free lysates from SK-N-BE2 neuroblastoma cells treated and untreated with tPA were immunoprecipitated with anti-LRP6 pAb, followed by protein G-acrylic beads. Acidic glycosphingolipids were then extracted from the LRP6 immunoprecipitates and labeled with cholera toxin. Dot blot analysis showed that a GM1 spot was selectively detectable in the immunoprecipitates (Fig. 1f, left panel). The amount of GM1 associated with LRP6 became more evident after triggering with tPA, as revealed by densitometric analysis (Fig. 1f, right panel).
In control samples, as well as in the immunoprecipitate with IgG with irrelevant specificity, under the same condition, did not result in detectable levels of ganglioside.
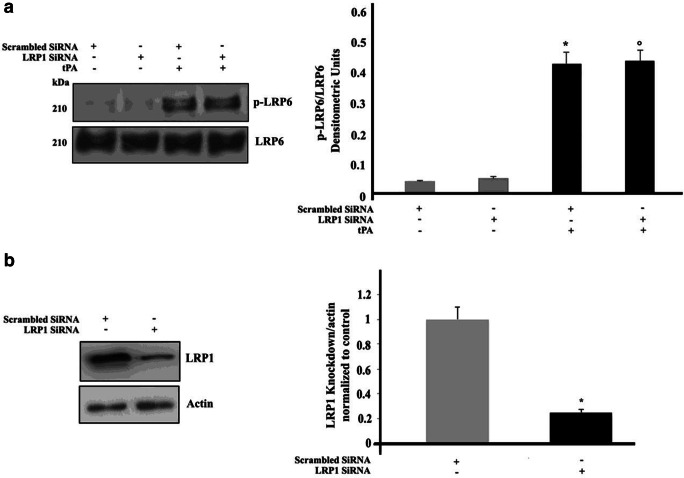
Effect of LRP1 silencing on LRP6 phosphorylation induced by tPA
Since it is well known that tPA is able to bind LRP1, in order to exclude the possibility that tPA-induced LRP6 phosphorylation may be dependent on LRP1 activation, LRP1 was knocked down by siRNA. LRP1 siRNA-treated cells were incubated with tPA for 10 min at 37 °C and analyzed by Western blot using an anti-phospho-LRP6 Ab. It revealed that the phosphorylation of LRP6 was not affected by LRP1 silencing (Fig. 2a). The efficiency of LRP1 silencing is shown in Fig. 2b.
Fig. 2.
Effect of LRP1 silencing on LRP6 phosphorylation induced by tPA in SK- N BE2 cells. a SK-N-BE2 cells, untreated or treated with 10 nM tPA, in the presence or in the absence of pre-treatment with siRNA LRP1, were analyzed by Western blot, using anti-phospho LRP6 pAb. PVDF was stripped and analyzed with anti-total LRP6 pAb. Densitometric analysis is shown. Results represent the mean ± SD from 3 independent experiments. (*) p < 0.01 scrambled SiRNA +tPA treated cells vs scrambled SiRNA, (°) p < 0.01 SiRNA LRP1+ tPA treated cells vs SiRNA LRP1. b Evaluation of LRP1 expression after 72 h siRNA transfection by Western blot analysis using anti-LRP1 pAb. PVDF was stripped and analyzed with anti-actin mAb. A scrambled siRNA was used as control. Bar graph to the right shows densitometric analysis. Results represent the mean ± SD from three independent experiments. (*) p < 0.01 SiRNA LRP1 vs scrambled siRNA
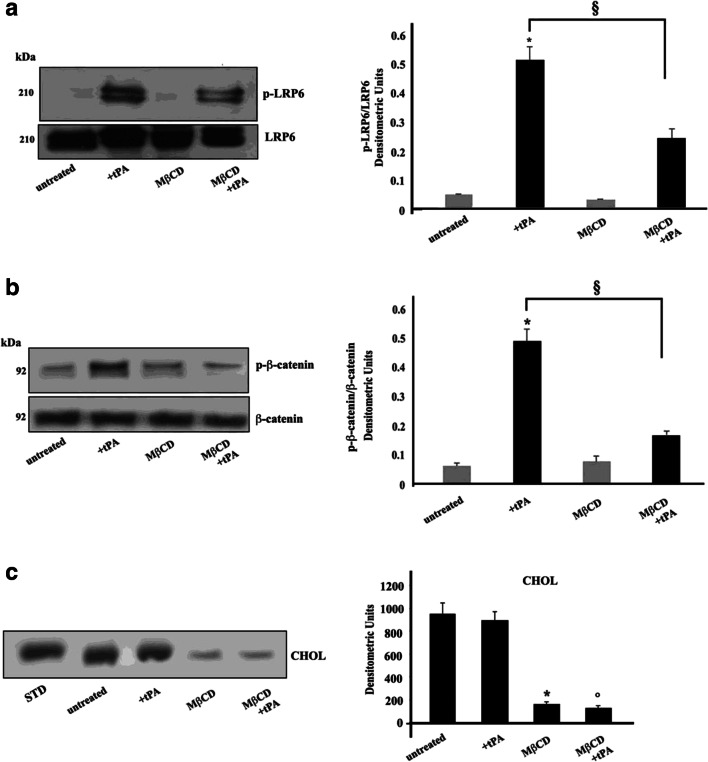
Functional role of lipid rafts on LRP6 phosphorylation
Next, since gangliosides are structural components of lipid rafts, we investigated whether the integrity of these microdomains may play a role in tPA-induced LRP6 phosphorylation. With this aim, cells were first incubated with methyl-β-cyclodextrin, since this compound is known to induce cholesterol efflux from the membrane and, consequently, lipid rafts disruption, and then stimulated with tPA. Interestingly, methyl-β-cyclodextrin significantly prevented LRP6 phosphorylation, as confirmed by Western blot analysis using an anti phospho-LRP6 Ab (Fig. 3a), indicating that lipid rafts integrity is essential for LRP6 activity.
Fig. 3.
Involvement of “lipid rafts” on LRP6 phosphorylation and β-catenin phosphorylation induced by tPA in SK-N BE2 cells. a SK-N-BE2 cells, untreated or treated with 10 nM tPA, in the presence or in the absence of pre-treatment with 5 mM MβCD, were analyzed by Western blot, using anti-phospho LRP-6 pAb. PVDF was stripped and analyzed with anti-total LRP6 pAb. Densitometric analysis is shown. Results represent the mean ± SD from 3 independent experiments. (*) p < 0.01 tPA treated cells vs untreated cells; (§) p < 0.01 MβCD +tPA treated cells vs tPA treated cells. b SK-N-BE2 cells, untreated or treated with 10 nM tPA, in the presence or in the absence of pre-treatment with 5 mM MβCD were analyzed by Western blot, using anti-phospho β-catenin pAb PVDF was stripped and analyzed with anti-β-catenin mAb. Densitometric analysis is shown. Results represent the mean ± SD from 3 independent experiments. (*) p < 0.01 tPA treated cells vs untreated cells; (§) p < 0.01 MβCD +tPA treated cells vs tPA treated cells; c SK-N-BE2 cells, untreated or treated with 10 nM tPA, in the presence or in the absence of pre-treatment with 5 mM MβCD were lysed in lysis buffer and subjected to cholesterol (CHOL) analysis. Neutral lipid extracts were separated by high-performance thin layer chromatography (HPTLC) using a solvent system of hexane/diethyl ether/acetic acid (70: 30: 1, v/v/v) and detected by staining with 2% copper acetate solution in 8% phosphoric acid and subsequent heating at 120 °C for 15 min. (*) p < 0.01 MβCD treated cells vs untreated cells; (°) p < 0.01 MβCD +tPA treated cells vs tPA treated cells
To further confirm the effect of lipid rafts on LRP6-mediated signaling, cells, pre-incubated with methyl-β-cyclodextrin, and then stimulated with tPA, were analyzed by Western blot for activated β-catenin, which may be consequent to stimulation through LRP6. Our results showed that tPA induced β-catenin phosphorylation, which was significantly prevented by previous pretreatment with methyl-β-cyclodextrin (Fig. 3b).
The effect of methyl-β-cyclodextrin on cholesterol content was checked by HPTLC (Fig. 3c).
Discussion
In this investigation, we analyzed LRP6 interaction with lipid rafts following tPA triggering, since this compound induces protein phosphorylation by directly interacting with LRP6, thus regulating neural differentiation, as well as synaptic plasticity (Lee et al. 2014).
Our results demonstrate that LRP6 mediated signal transduction pathway triggered by tPA acts through lipid rafts in neuroblastoma cells. In particular, we observed that LRP6 was enriched in gradient insoluble fractions, corresponding to raft microdomains and coimmunoprecipitates with ganglioside GM1, a paradigmatic component of these plasma membrane compartments. These findings are not surprising, since it has been already shown that Wnt-dependent phosphorylation of LRP6 occurs within lipid rafts, where it may be removed by the inhibition factor Dickkopf (Dkk)1 (Yamamoto et al. 2008). However, we observed that this enrichment became more evident after triggering with tPA, whereas Wnt3a did not affect the distribution of LRP6 between the two fractions (Sakane et al. 2010). This finding supports the view that the phosphorylation of LRP6 triggered by tPA occurs in the lipid raft.
In addition, our results further extended the primary data obtained in cells activated by Wnt3a (Sakane et al. 2010). Indeed, we demonstrated that cell pretreatment with a compound, methyl-β-cyclodextrin, able to induce cholesterol efflux from the membrane and consequently lipid rafts disruption, prevented LRP6 phosphorylation. Interestingly, the raft-mediated effect of tPA on LRP6 phosphorylation was independent of LRP1, although this molecule is known to be a receptor of tPA and this binding is able to initiate cell signaling (Mantuano et al. 2016) through lipid rafts (Laudati et al. 2016; Mattei et al. 2019). Indeed, we demonstrated that LRP1 knockdown did not significantly affect raft-mediated LRP6 phosphorylation. This phosphorylation is essential for the accumulation of β-catenin (Zilberberg et al. 2004). Of note, in the present study we observed that methyl-β-cyclodextrin also prevented tPA induced β-catenin phosphorylation. It may have several physiopathological implications, since, once β-catenin is stabilized, it translocates to the cell nucleus, where alters the activity of members of the lymphoid enhancer factor (Lef)/T cell factor (Tcf), Lef/Tcf family of HMG-box transcription factors acting as transcriptional switches, recruiting various chromatin modifiers and remodelers to Lef/Tcf target genes, inducing expression of an array of genes downstream (Ring et al. 2014; Nag et al. 2017). Activated β-catenin can be oncogenic, driving the onset of a wide spectrum of carcinomas (Mao et al. 2019; Yao et al. 2017).
In conclusion, we provide evidence that tPA triggered signaling involving β-catenin acts through lipid rafts. These microdomains are small and highly dynamic structures which can play a role in signal transduction by concentrating molecules involved in signaling pathways (Simons and Toomre 2000), by allowing their molecular interaction, and/or by initiating and modulating cell signaling (Mollinedo and Gajate 2015). In this way they facilitate the transport and regulate the release of the neurotransmitters, recruit the receptor molecules to the synapse and the associated signal transduction molecules (Díaz et al. 2018).
Thus, these findings introduce an additional task for identifying new molecular target(s) of pharmacological agents. Indeed, these findings may have several pharmacological implications. In fact, cyclodextrins that exert their effects via the formation of noncovalent inclusion complexes are being used in an ever-increasing way to improve therapeutic indices and site-targeted delivery of different drugs. Hence, our results, pointing to the key role of lipid rafts in tPA triggered signaling involving β-catenin, could provide further insight in this field, and suggest that cyclodextrins, and potentially other drugs, such as statins, may represent an useful tool and the combined effect of β -cyclodextrins with “classical” anti-neoplastic drugs has to be carefully evaluated and tested.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, and not-for-profit sectors.
Author contributions statement
Gloria Riitano, Valeria Manganelli, Antonella Capozzi, Vincenzo Mattei, Serena Recalchi, and Stefano Martellucci gave substantial contributions to the acquisition, analysis and interpretation of data; Agostina Longo, Roberta Misasi, Tina Garofalo and Maurizio Sorice gave substantial contributions to the conception and design of the work.
Abbreviations
- LRPs
LDL receptor–related proteins
- tPA
Tissue plasminogen activator
- MβCD
Methyl-β-cyclodextrin
- mAb
Monoclonal antibody
- SDS-PAGE
Sodium-dodecyl sulfate polyacrylamide gel electrophoresis
- PVDF
Polyvinylidene difluoride
- HRP
Horseradish peroxidase
Compliance with ethical standards
This study does not contain any studies with human participants or animals performed by any of the authors.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gloria Riitano, Valeria Manganelli, Tina Garofalo and Maurizio Sorice contributed equally to this work.
References
- Brown DA, London E. Functions of lipid rafts in biological membranes. Annu Rev Cell Dev Biol. 1998;14:111–136. doi: 10.1146/annurev.cellbio.14.1.111. [DOI] [PubMed] [Google Scholar]
- Brown DA, London E. Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J Biol Chem. 2000;275:17221–17224. doi: 10.1074/jbc.R000005200. [DOI] [PubMed] [Google Scholar]
- Díaz M, Fabelo N, Ferrer I, Marín R. "Lipid raft aging" in the human frontal cortex during nonpathological aging: gender influences and potential implications in Alzheimer's disease. Neurobiol Aging. 2018;67:42–52. doi: 10.1016/j.neurobiolaging.2018.02.022. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Misasi R, Mattei V, Giammarioli AM, Malorni W, Pontieri GM, Pavan A, Sorice M. Association of the death-inducing signaling complex with microdomains after triggering through CD95/Fas. Evidence for caspase-8-ganglioside interaction in T cells. J Biol Chem. 2003;278:8309–8315. doi: 10.1074/jbc.M207618200. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Giammarioli AM, Misasi R, Tinari A, Manganelli V, Gambardella L, Pavan A, Malorni W, Sorice M. Lipid microdomains contribute to apoptosis-associated modifications of mitochondria in T cells. Cell Death Differ. 2005;12(11):1378–1389. doi: 10.1038/sj.cdd.4401672. [DOI] [PubMed] [Google Scholar]
- Garofalo T, Ferri A, Sorice M, Azmoon P, Grasso M, Mattei V, Capozzi A, Manganelli V, Misasi R. Neuroglobin overexpression plays a pivotal role in neuroprotection through mitochondrial raft-like microdomains in neuroblastoma SK-N-BE2 cells. Mol Cell Neurosci. 2018;88:167–176. doi: 10.1016/j.mcn.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Goel S, Chin EN, Fakhraldeen SA, Berry SM, Beebe DJ, Alexander CM. Both LRP5 and LRP6 receptors are required to respond to physiological Wnt ligands in mammary epithelial cells and fibroblasts. J Biol Chem. 2012;287:16454–16466. doi: 10.1074/jbc.M112.362137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haack F, Lemcke H, Ewald R, Rharass T, Uhrmacher AM. Spatio-temporal model of endogenous ROS and Raft-dependent WNT/Beta-catenin signaling driving cell fate commitment in human neural progenitor cells. PLoS Comput Biol. 2015;11(3):e1004106. doi: 10.1371/journal.pcbi.1004106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Semenov M, Tamai K, Zeng X. LDL receptor-related proteins 5 and 6 in Wnt/β-catenin signaling: arrows point the way. Development. 2004;131:1663–1677. doi: 10.1242/dev.01117. [DOI] [PubMed] [Google Scholar]
- Herz J, Strickland DK. LRP: a multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI200113992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huelsken J, Behrens J. The Wnt signalling pathway. J Cell Sci. 2002;115:3977–3978. doi: 10.1242/jcs.00089. [DOI] [PubMed] [Google Scholar]
- Lara-Castillo N, Johnson ML. LRP receptor family member associated bone disease. N Rev Endocr Metab Disord. 2015;16:141–148. doi: 10.1007/s11154-015-9315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laudati E, Gilder AS, Lam MS, Misasi R, Sorice M, Gonias SL, Mantuano E. The activities of LDL receptor-related Protein-1 (LRP1) compartmentalize into distinct plasma membrane microdomains. Mol Cell Neurosci. 2016;76:42–51. doi: 10.1016/j.mcn.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Ko HM, Kwon KJ, Lee J, Han SH, Han DW, Cheong JH, Ryu JH, Shin CY. tPA regulates Neurite outgrowth by phosphorylation of LRP5/6 in neural progenitor cells. Mol Neurobiol. 2014;49:199–215. doi: 10.1007/s12035-013-8511-x. [DOI] [PubMed] [Google Scholar]
- Malorni W, Giammarioli A, Garofalo T, Sorice M. Dynamics of lipid raft components during lymphocyte apoptosis: the paradigmatic role of GD3. Apoptosis. 2007;12:941–949. doi: 10.1007/s10495-007-0757-1. [DOI] [PubMed] [Google Scholar]
- Mantuano E, Brifault C, Lam MS, Azmoon P, Gilder AS, Gonias SL. LDL receptor-related protein-1 regulates NFκB and microRNA-155 in macrophages to control the inflammatory response. Proc Natl Acad Sci U S A. 2016;113:1369–1374. doi: 10.1073/pnas.1515480113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao X, Tey SK, Ko FCF, Kwong EML, Gao Y, Ng IO, Cheung ST, Guan XY, Yam JWP. C-terminal truncated HBx protein activates caveolin-1/LRP6/β-catenin/FRMD5 q1axis in promoting hepatocarcinogenesis. Cancer Lett. 2019;444:60–69. doi: 10.1016/j.canlet.2018.12.003. [DOI] [PubMed] [Google Scholar]
- Mattei V, Manganelli V, Martellucci S, Capozzi A, Mantuano E, Longo A, Ferri A, Garofalo T, Sorice M, Misasi R (2019) A multimolecular signaling complex including PrPC and LRP1 is strictly dependent on lipid rafts and is essential for the function of tissue plasminogen activator. J Neurochem. 10.1111/jnc.14891 [DOI] [PubMed]
- Mollinedo F, Gajate C. Lipid rafts as major platforms for signaling regulation in cancer. Adv Biol Regul. 2015;57:130–146. doi: 10.1016/j.jbior.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Nag JK, Kancharla A, Maoz M, Turm H, Agranovich D, Gupta CL, Uziely B, Bar-Shavit R. Low-density lipoprotein receptor-related protein 6 is a novel coreceptor of protease-activated receptor-2 in the dynamics of cancer-associated β-catenin stabilization. Oncotarget. 2017;8:38650–38667. doi: 10.18632/oncotarget.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özhan G, Sezgin E, Wehner D, Pfister AS, Kühl SJ, Kagermeier-Schenk B, Kühl M, Schwille P, Weidinger G. Lypd6 enhances Wnt/β-catenin signaling by promoting Lrp6 phosphorylation in raft plasma membrane domains. Dev Cell. 2013;26:331–345. doi: 10.1016/j.devcel.2013.07.020. [DOI] [PubMed] [Google Scholar]
- Ring L, Neth P, Weber C, Steffens S, Faussner A. β-Catenin-dependent pathway activation by both promiscuous “canonical” WNT3a–, and specific “noncanonical” WNT4– and WNT5a–FZD receptor combinations with strong differences in LRP5 and LRP6 dependency. Cell Signal. 2014;26:260–267. doi: 10.1016/j.cellsig.2013.11.021. [DOI] [PubMed] [Google Scholar]
- Sakane H, Yamamoto H, Kikuchi A. Lrp6 is internalized by dkk1 to suppress its phosphorylation in the lipid raft and is recycled for reuse. J Cell Sci. 2010;123:360–368. doi: 10.1242/jcs.058008. [DOI] [PubMed] [Google Scholar]
- Simons K, Toomre D. Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000;1:31–39. doi: 10.1038/35036052. [DOI] [PubMed] [Google Scholar]
- Song Z, Zhu T, Zhou X, Barrow P, Yang W, Cui Y, Yang L, Zhao D. Rest alleviates neurotoxic prion peptide-induced synaptic abnormalities, neurofibrillary degeneration and neuronal death partially via LRP6-mediated Wnt-β-catenin signaling. Oncotarget. 2016;7:12035–12052. doi: 10.18632/oncotarget.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorice M, Garofalo T, Misasi R, Manganelli V, Vona R, Malorni W. Ganglioside GD3 as a raft component in cell death regulation. Anti Cancer Agents Med Chem. 2012;12:376–382. doi: 10.2174/187152012800228670. [DOI] [PubMed] [Google Scholar]
- Sorice M, Mattei V, Matarrese P, Garofalo T, Tinari A, Gambardella L, Ciarlo L, Manganelli V, Tasciotti V, Misasi R, Malorni W. Dynamics of mitochondrial raft-like microdomains in cell life and death. Commun Integr Biol. 2012;5:217–219. doi: 10.4161/cib.19145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao J, Calvisi DF, Ranganathan S, Cigliano A, Zhou L, Singh S, Jiang L, Fan B, Terracciano L, Armeanu-Ebinger S, Ribback S, Dombrowski F, Evert M, Chen X, Monga SPS. Activation of β catenin and Yap1 in human Hepatoblastoma and induction of Hepatocarcinogenesis in mice. Gastroenterology. 2014;147:690–701. doi: 10.1053/j.gastro.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead SN, Gangaraju S, Aylsworth A, Hou ST. Membrane raft disruption results in neuritic retraction prior to neuronal death in cortical neurons. Bioscience Trends. 2012;6:183–191. doi: 10.5582/bst.2012.v6.4.183. [DOI] [PubMed] [Google Scholar]
- Yamamoto H, Sakane H, Yamamoto H, Michiue T, Kikuchi A. Wnt3a and Dkk1 regulate distinct internalization pathways of LRP6 to tune the activation of beta-catenin signaling. Dev Cell. 2008;15:37–48. doi: 10.1016/j.devcel.2008.04.015. [DOI] [PubMed] [Google Scholar]
- Yao Q, An Y, Hou W, Cao YN, Yao MF, Ma NN, Hou L, Zhang H, Liu HJ, Zhang B. LRP6 promotes invasion and metastasis of colorectal cancer through cytoskeleton dynamics. Oncotarget. 2017;8:109632–109645. doi: 10.18632/oncotarget.22759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberberg A, Yaniv A, Gazit A. The low density lipoprotein receptor-1, LRP1, interacts with the human frizzled-1 (HFz1) and down-regulates the canonical Wnt signaling pathway. J Biol Chem. 2004;279:17535–17542. doi: 10.1074/jbc.M311292200. [DOI] [PubMed] [Google Scholar]