Abstract
The present study was designed to propose a simple, cost-effective, and efficient method for the preparation of a biocompatible composite made from magnetic diatomaceous earth (mDE) coated by aminopropyltriethoxysilane (APTES) and its application for immobilization of porcine pancreatic lipase (PPL). The produced mDE-APTES was instrumentally characterized and the obtained results of FTIR analysis and scanning electron microscopy equipped by energy-dispersive X-ray spectroscopy (SEM–EDS) showed successful coating of APTES on mDE surface. PPL was then immobilized onto mDE to obtain the biocatalyst of PPL@mDE (immobilization yield and efficiency of 78.0 ± 0.3% and 80.1 ± 0.6, respectively) and the presence of enzyme was confirmed by EDS method. The attained results of the reusability of PPL@mDE revealed that 57% of the initial activity was retained after 11 cycles of biocatalyst application. PPL@mDE demonstrated higher storage stability than the free enzyme at 4 °C, 25 °C, and 37 °C. The apparent Km (2.35 ± 0.12 mM) and Vmax (13.01 ± 0.64 µmol/min) values for the immobilized enzyme were considerably altered compared to those of the free enzyme (p > 0.05). PPL@mDE was subsequently employed for the synthesis of banana flavor (isoamyl acetate) in n-hexane, which yields an esterification percentage of 100 at 37 °C after 3 h. However, it merits further investigations to find out about large-scale application of the as-synthesized biocatalyst for esterification.
Keywords: Porcine pancreatic lipase, Diatomaceous earth, Magnetic, Esterification, Banana flavor
Introduction
The majority of flavor and fragrance ingredients are prepared using traditional techniques including extraction from natural resources or chemical synthesis (Poornima and Preetha 2017). Extracted esters from the natural origin are generally considered too scarce and expensive to be applied at commercial scales. In the case of chemical synthesis, creation of polluting liquid acids due to the post-treatment of applied catalysts is the main drawback (Kiss et al. 2004). Apparently, the use of enzymes and other biocatalysts are promising alternatives for ester preparation with the milder conditions and the final product could be labeled as natural (Kapoor and Gupta 2012).
Lipases (triacylglycerol hydrolase, EC 3.1.1.3) are one of the valuable enzymes among biocatalysts which naturally catalyze the hydrolysis of fats (Imanparast et al. 2018). However, with a proper working environment, lipases can be highly active biocatalysts in esterification, transesterification, and alcoholysis reactions (Kapoor and Gupta 2012). These biocatalysts have been widely applied in different industries such as chemical, detergent, food (e.g. flavor and fragrance synthesis), pharmaceutical, cosmetic, and agro-chemical manufacturing (Imanparast et al. 2018). In general, lipases are featured with a “lid” domain helping the enzyme to alter its conformation from “close” (less active) to “open” (more active) form (Imanparast et al. 2018). These changes, known as “interfacial activation” might have a negative effect on the activity of lipases (Fernandez-Lafuente 2010). It has been shown that immobilization of lipases on hydrophobic supports makes the open form of the enzyme more stable (Palomo et al. 2007). Furthermore, immobilization of the enzymes onto organic or inorganic solid supports has been identified as a reliable way to improve the stability of enzymes and operational lifetime (Fernandez-Lafuente 2010; Palomo et al. 2007). Efficient and ease of recovery and reusing of the immobilized biocatalyst, convenient work-up, and lower protein contamination of the final product are among other advantages of enzyme immobilization (Rodrigues et al. 2019).
Diatomaceous earth (DE) is an inorganic lightweight mineral clay mostly composed of amorphous SiO2.nH2O (hydrated silica) in addition to a considerable amount of Al2O3 (alumina) and Fe2O3 (ferric oxide) (Cabrera et al. 2017). This inorganic material has attracted special attention because of several properties such as high cation exchange capacity, large surface area, porous structure, chemical inertness, low thermal conductivity, low density, reasonable price, and high availability (Cabrera et al. 2017; Tsai et al. 2006). Natural or modified DE has been employed in manufacturing adsorbents, filter media, catalyst supports, and natural insecticides, and also examined for ecological procedures such as the removal of heavy metals and organic pollutants (Cabrera et al. 2017; Mohamad et al. 2015). DE also showed potential applications as functional drug carrier and as adjuvant in poultry vaccine formulation (Cabrera et al. 2018; Mohamad et al. 2015). Furthermore, some researchers have produced electrically conducting diatomite by coating it with polyaniline (PANI) (Cabrera et al. 2018). However, the modified DE as a matrix for immobilization of biomolecules has been rarely studied (Cabrera et al. 2017, 2018).
In the present study, magnetic diatomaceous earth (mDE) was prepared and subsequently modified with the bifunctional molecule of APTES to improve the adsorption capacity for lipase. The porcine pancreatic lipase (PPL) was then immobilized onto mDE coated with APTES by covalent binding. The immobilized lipase was then used for the synthesis of isoamyl acetate, known as banana flavor. Based on the literature, there is no report on the immobilization of lipase on APTES-functionalized mDE as a simple, low cost, and recyclable biocatalyst and its application in the esterification reaction to produce banana flavor.
Materials and methods
Chemicals
Raw diatomaceous earth (DE) was provided by Dicalite Company (Pennsylvania, USA). A process of water washing and repeated sedimentation was applied to purify the raw DE. Porcine pancreatic lipase (PPL; 20 U/mg) was supplied by AppliChem (Darmstadt, Germany). Glutaraldehyde, bovine serum albumin (BSA), aminopropyltriethoxysilane (APTES), and p-nitrophenyl palmitate (p-NPP) were purchased from Sigma-Aldrich (St. Louis, USA). Other chemicals were of high purity commercially available.
Preparation of magnetic DE
The magnetic DE were prepared according to the method of Cabrera et al. (2018). In brief, 5 mL of an iron solution containing 0.6 M FeCl2.4H2O and 1.1 M FeCl3.6H2O were mixed with DE (2% w/v). The final pH of the reaction mixture was adjusted to 11 using ammonium hydroxide (7.6 M). Afterward, the magnetic solution was stirred for 30 min at 100 °C. The magnetic diatomaceous earth (mDE) was then rinsed by distilled water to reach the pH 7.0 and separated using a 0.6 T magnetic field (Ciba Corning). The mDE particles were kept aside at 25 ± 2 °C for later use.
Surface functionalization and activation of magnetic DE
To get higher stability of the immobilized enzyme, mDE was functionalized with APTES, which yields an immense volume of amino groups for covalent cross-linking to immobilize the PPL. The process was contained suspending of 5 mg mDE in 100 mL of anhydrous acetone, followed by addition of 5 mL APTES for each gram of support and refluxing the prepared mixture for 2 h. The product was amino-functionalized materials that collected using filtration and rinsed by distilled water to eliminate the unreacted reagents. To dry the dark sediment, it was placed in a vacuum at 40 °C and the sample was marked as mDE-APTES. Glutaraldehyde (2% v/v) was used to activate mDE-APTES by stirring at 25 °C for 12 h and then rinsing by distilled water for 15 times.
Characterization of mDE-APTES
Dried supports were analyzed using scanning electron microscopy (SEM, VEGA/TESCAN, VEGA3, Czech Republic) with an acceleration voltage of 15 kV before and after the immobilization procedure (Taghizadeh et al. 2020). Brunauer–Emmett–Teller (BET, BEL JAPAN Inc., Japan) analysis was applied to determine the surface area (Taghizadeh et al. 2020). To estimate the porosity and surface area, nitrogen (N2) adsorption/desorption analysis was performed at – 196 °C. The Vmicro and DP were measured using Barrett-Joyner-Halenda (BJH) and t-plot methods, respectively (Taghizadeh et al. 2020). In addition, Fourier transform infrared (FTIR) spectra (4000–400 cm−1 range) were recorded by a model IFS 66 spectrometer (Bruker Optics, Germany). To examine the magnetic property of the samples, different magnetic fields ranging from 0 to 20 kOe (2 T) were employed using a SQUID VSM magnetometer (Quantum Design Model MPMS-5S, Germany).
Enzyme immobilization procedure
A solution containing PPL (20 U/mL) was prepared in phosphate buffer (100 mM, pH 7.5), incubated together with the activated mDE-APTES (5 mg) at 20 °C, and mildly stirred for 2 h. Afterward, the immobilized lipase on the support (PPL@mDE) was collected using an external magnetic field (0.6 T) for at least 1 min. The supernatants of the first two washings steps were used to determine protein concentration based on the Bradford (1976) method while BSA was applied as standard. The immobilized enzyme was kept in phosphate buffer (100 mM, pH 7.5) at 4 °C for further use.
Lipase assay and the protein estimation
The activity of the immobilized lipase was measured using an earlier mentioned method (Khoobi et al. 2014). The reaction mixture was prepared by addition of p-NPP in ethanol (3 mM) to 0.9 mL phosphate buffer (100 mM, pH 7.5). To start the enzymatic reaction, 0.1 mL of free lipase solution or 0.2 mg of the immobilized lipase was added to the reaction mixture. The mixture was incubated at 37 °C under mild shaking (120 rpm) for 15 min. The reaction was terminated by adding acetone/ethanol (1:1, v/v) followed by centrifugation (10,000 rpm) for 10 min. The absorbance of the obtained supernatant was then measured at 420 nm by a UV–vis spectrophotometer (Pharmacia LKB Ultrospec III, USA) in comparison with a blank without the enzyme that treated in parallel. One unit (U) of the lipase activity was defined as the amount of enzyme caused the release of 1 μmol of p-NP per min from p-NPP substrate under the standard assay conditions. The concentration of protein was demonstrated according to the Bradford (1976) method using BSA as standard.
The immobilization yield and efficiency
The following equation was applied to calculate the immobilization yield (IY) and efficiency (IE): IY (%) = [(Y0 − Y1)/Y0] × 100; and IE (%) = [E1/E0] × 100; where Y0 and Y1 stand for the amount of total protein and protein that are not attached to mDE, respectively. In addition, E0 and E1 are the total and immobilized enzyme activity, respectively.
Activity profiles and storage stability of the immobilized lipase
The combined effects of pH and temperature on the relative activity of the free and immobilized enzyme were measured in 100 mM citrate–phosphate buffer (pH 4–5) or 100 mM phosphate buffer (pH 6–8) or 100 mM glycine–NaOH buffer (pH 9–11) for 15 min at temperatures ranging from 25 °C to 45 °C. To examine the storage stability, the immobilized enzyme was incubated for 20 days at 4 °C, 25 °C, and 37 °C, and then the remained activity in collected samples was analyzed.
Reusability of PPL@mDE
Reusability of the immobilized enzyme was measured by magnetically collecting the immobilized lipase after each reaction run, followed by washing the immobilized biocatalyst three times with phosphate buffer (100 mM, pH 7.5). It was then added to a fresh reaction medium and the enzymatic activity was determined at optimal conditions. In the first run, the activity was considered as 100% and over the following runs; it was determined as a relative activity.
Kinetic study
Kinetic parameters of the free and immobilized enzyme were examined by changing the concentrations of p-NPP as the substrate in the range of 0.12–8 mM. To estimate the Michaelis constant (Km) and the maximum rate of the enzyme-catalyzed reaction (Vmax), Lineweaver–Burk plots were employed. The enzyme activity assays were repeated three times and the boiled lipase was used as a control to determine the volume of p-NPP reacted with the support without the enzyme.
Flavor ester synthesis
Enzymatic synthesis of isoamyl acetate was carried out by the immobilized lipase in n-hexane medium containing acetic acid and isoamyl alcohol under shaking at 120 rpm and 37 °C against heat-inactivated free lipase as control. To evaluate the effect of different factors on esterification percent, PPL@mDE (0–10 mg) was treated with acetic acid/isoamyl alcohol (molar ratio of 0.5:4, 1:4, 2:4, 3:4, 4:4, 5:4, and 6:4) in an organic solvent containing medium (n-hexane) under mild shaking (100 rpm) for 3 h. The proper amount of PPL@mDE and acid/alcohol ratio were then applied to evaluate the process during 7 h (with 1-h intervals). At specified time intervals, unused acid was extracted twice by 10 mL of NaOH (25 mM) and n-hexane was then washed twice by distilled water. The ester synthesis was estimated based on the volume of acetic acid consumed. Extraction and washing solutions were collected in an Erlenmeyer flask and the concentration of remaining NaOH was calculated by back titration using a solution of HCl (25 mM) and phenolphthalein as an indicator (Khoobi et al. 2014). The whole experiments were repeated three times along with controls (boiled enzyme solution) that indicated an ester yield below 2%. To examine the reusability of the immobilized lipase for the synthesis of isoamyl acetate, the biocatalyst was recovered and rinsed with phosphate buffer (100 mM, pH 7.5) after each run and used for 13 cycles of ester synthesis. The relative activity was then calculated after each reusing cycle compared to that of the blank.
GC–MS analysis of the esterification reaction
To analyze the esterification process, a sample from esterification reaction was withdrawn, extracted using n-hexane, and subjected to GC–MS analysis using an Agilent mass spectrometer (model 5973 N MSD) with a 7683 autosampler and a model 6890 gas chromatography apparatus equipped with a 30 m × 0.25 mm i.d. HP-5 column with 0.25 mm film thickness (Agilent, Palo Alto, CA). The initial oven temperature was kept at 40 °C for 6 min, which was then increased at 2.5 °C/min to 150 °C and finally at 90 °C/ min to 250 °C. The injection port and ionizing source were held at 250 °C and 280 °C, respectively. The split ratio was 10:1 with 2 µL of sample injected. There was a solvent delay of 2 min, after which time the mass spectrum was collected from m/z 35 to 300, generating 5.27 scans/s. Compound identifications were made by comparison of the mass spectra and retention times with those of corresponding standards (Aldrich Chemical Co., St. Louis, MO; Bedoukian Research, Inc., Danbury, CT).
Statistical analyses
All of the above-mentioned experiments were repeated three times and the obtained results were displayed as mean ± SD. The difference between the kinetic constants of the free and immobilized lipase was examined based on Student’s t test (p-value 0.05). In this study, Sigmaplot version 14.0 (Systat Software, San Jose, CA) was employed for all statistical analysis.
Results and discussion
Synthesis and characterization of mDE-APTES
Magnetization measurement
The magnetic DE were successfully synthesized and then functionalized with APTES and glutaraldehyde. To measure the magnetic properties of the support, an external magnetic field (ranging from 0 to 20 kOe) was applied. The superparamagnetic behavior of mDE and mDE-APTES is shown in Fig. 1a. It was notable that mDE forms a significant hysteresis loop because of the high values of remnant magnetization (Mr) and coercivity (Hc). In addition, saturation magnetization values (Ms) were 16.6 emu/g and 4.3 emu/g for mDE and mDE-APTES, respectively (Fig. 1a). According to the obtained results, a remarkable change occurred in the magnetic properties of mDE after functionalization with APTES which could be ascribed to the non-magnetic properties of inserted APTES layer (Fig. 1a). Similar results were also reported by Xie and Huang (2020) who immobilized Candida rugosa lipase on magnetic Fe3O4-poly(glycidyl methacrylate-co-methacrylic acid) composite. They observed that the value of saturation magnetization of the bared Fe3O4 nanoparticle decreased from 68.61 emu/g to 54.54 emu/g after coating process which might be attributed to the loading of a magnetically inactive layer on the surface of the Fe3O4 nanoparticle (Xie and Huang 2020). In contrast, Cabrera et al. (2018) who functionalized magnetic diatomite nanoparticles with polyaniline and applied it for immobilization of invertase, β-galactosidase, and trypsin reported no substantial change in the magnetic properties of mDE after coating by polyaniline. In addition, mDE-APTES particles demonstrated a magnetic response following the application of an external magnetic field which let the as-synthesized particles be easily separated from the reaction medium (Cabrera et al. 2018; Kahraman et al. 2007). A summary of the corresponding textural properties of BET surface area (as), pore volume (Vp), and pore diameter (Dp) is presented in Table 1. “as, BET” considerably decreased after coating mDE with APTES. However, the pore size measured for mDE was similar to that of mDE-APTES (p > 0.05). These results indicated that the pores of the magnetic nanoparticles surface were partially filled with APTES after the coating process. These findings were in agreement with the attained results of Cabrera et al. (2017) who determined a decrease in specific surface area of invertase@mDE-APTES (56.6 m2/g) compared to mDE (41.2 m2/g). They ascribed such decrease to the covering of the mDE pores by APTES and immobilization of enzymes (Cabrera et al. 2017). Table 1 also showed that “as, BET” of PPL@mDE was more considerably declined (11.67 ± 0.06) compared with that of mDE-APTES (12.40 ± 0.13) that might be ascribed to the filling of the pores of mDE-APTES surface by PPL.
Fig. 1.

a Magnetic properties of magnetic diatomaceous earth (mDE) before (black balls) and after (red balls) coating by aminopropyltriethoxysilane (APTES) determined by SQUID VSM magnetometer in the range of 0–20 kOe. b The FTIR spectra of DE before (1) and after (2) magnetization by 0.6 M FeCl2.4H2O and 1.1 M FeCl3.6H2O, and after (3) coating of mDE by APTES
Table 1.
Specific surface area (as, BET), pore volume (Vp), and pore diameter (Dp) measured for mDE, mDE-APTES, and PPL@mDE
| Sample | as, BET (m2 g–1) | Vp (cm3 g–1) | Dp (nm) |
|---|---|---|---|
| mDE | 24.21 ± 0.18 | 0.06 ± 0.00 | 13.60 ± 0.04 |
| mDE-APTES | 12.40 ± 0.13 | 0.04 ± 0.00 | 10.11 ± 0.02 |
| PPL@mDE | 11.67 ± 0.06 | 0.04 ± 0.00 | 10.00 ± 0.07 |
FTIR spectra of the support
The FTIR spectra of DE, mDE, and mDE-APTES consist of the remarkable peaks for asymmetric stretching vibration of Si–O–Si (1090 cm–1), symmetric stretching vibration of Si–O (798 cm–1), and bending vibration of Si–O–Si (468 cm–1) (Fig. 1b) (de los Ángeles Calixto-Romo et al. 2008; Janićijević et al. 2015). The band of 3600 cm–1 is also due to stretching vibration frequency of SiO–H. It assists to characterize the diatom cell wall that is mainly composed of hydrous amorphous silica (SiO2.nH2O) (Fig. 1b). In addition, the identical bands of Fe–O (around 580 cm–1) are found in the FTIR spectra of mDE and mDE-APTES particles that supports the magnetic properties of mDE and mDE-APTES (Fig. 1b(2) and Fig. 1b(3)). The vibration frequency of the N–H terminal group (1540 cm–1) is also observed in the spectrum of mDE-APTES (Fig. 1b(3)) that is associated to the N–H groups of APTES. Cabrera et al. (2017) applied the mDE-APTES for the immobilization of invertase and observed similar FTIR pattern before and after magnetization of DE.
SEM–EDS analysis and surface area parameters
Figure 2a shows the SEM image of DE frustules before the magnetization process. After functionalization of DE with APTES in combination with glutaraldehyde, physical changes of the mDE were observed (Fig. 2b). Thus, mDE-APTES were morphologically different with an appearance of irregular and rougher surfaces compared to DE. Moreover, Fig. 2b demonstrates the pores of mDE-APTES partially filled with APTES after the coating process. Similar findings for magnetic DE were reported by Cabrera et al. (2018) who observed that polyaniline polymerization process occurred at the pore surface of DE particles. Furthermore, the pore volume and mean pore size values decreased compared to DE (Cabrera et al. 2018). Structural characterization of APTES modified mDE was investigated by scanning electron microscope equipped by an energy-dispersion spectrometer (SEM–EDS). The result of EDS for the DE displayed the expected peaks of Si, O, and other elements (Al, Ca, and Fe) (Fig. 2c and e). The EDS analysis of PPL@mDE-APTES (Fig. 2d and f) revealed an enhancement in the signal corresponding to Fe (Fig. 2f), supporting the existence of magnetite on mDE. Figure 2d and f also illustrated the appearance of a signal corresponding to the sulfur atom after immobilization of the enzyme on mDE-APTES. Disulfide bonds and amino acid residues including sulfur group are responsible for the presence of sulfur peak (Fig. 2d and f). Based on these results, it can be concluded that the immobilization of lipase was successfully achieved.
Fig. 2.
SEM images of diatomaceous earth (DE) a before and b after surface modification with APTES. Energy-dispersive X-ray spectroscopy (EDS) map images with quantitative analysis results of mDE (c and e) before and (d and f) after lipase immobilization
Preparation of PPL@mDE
A magnetic biocatalyst was developed through covalent binding between mDE-APTES and PPL. At first, mDE was functionalized using APTES as a source of amine groups (–NH2) and was surrounded by a hydrophilic environment. The covalent binding was then formed between lipase and mDE-APTES by adding glutaraldehyde as a chemical arm that led to immobilization of the enzyme. According to the study of Barbosa et al. (2014), protein was first adsorbed by the physical forces between the charged amino acids and the functional groups on the surface of the support. Then, covalent bonds are established between the nucleophilic groups of the enzyme and the support. Immobilization yield and efficiency were presented in Table 2. The immobilization yield (%) for PPL@mDE-APTES was 78.0 ± 0.3%. The mentioned percentage depended on the enzyme concentration. As the enzyme concentration was enhanced, the amount of enzyme adsorbed on the support increased and then reached a saturation point (Fig. 3a). The apparent activity of the immobilized lipase gradually decreased when the added lipase exceeded 3 mg (Fig. 3b). As shown in Fig. 3c, similar results were also observed in the immobilization time optimization that the enzyme loading improved with the time extension at the beginning 3 h, while the enzyme loading was fixed after 3 h. This phenomenon was similar to the previous reports (Bindu and Mohanan 2017). Increasing in relative activity could be attributed to the increase in lipase accumulation on the support surface while decreasing in the relative activity could be ascribed to the enzyme overcrowding that delay the substrate diffusion into the active site (Zhang et al. 2019). As a result, 3 mg of added lipase with 3 h of conjugation time was the best combination for the enzyme immobilization on mDE-APTES. Bindu and Mohanan (2017) believed that less α-amylase activity after the saturation point occurred due to the conformational changes of the enzyme or the steric hindrance caused by chitosan-TiO2 nanocomposite as support at the active site. As shown by the obtained results (Table 2), lipase activity recovery on mDE-APTES support (13.2 U/mg) corresponded to 66% of the free enzyme activity (20 U/mg). In general, covalent immobilization imparts rigidification of the enzyme conformation, which is beneficial with respect to the stability of the immobilized enzyme, but often lead to a reduction of the retained activity after immobilization (Prlainović et al. 2016). In this study, immobilization yield and efficacy values were higher than those reported for most immobilized lipases on different supports. For example, the lipase of Thermomyces lanuginose was immobilized on MCM-41@PEI-SUC and MCM-41 with 58% and 52% efficiency, respectively (Khoobi et al. 2014).
Table 2.
Immobilization yield, efficiency, and specific activity of free and lipase immobilized on the surface of mDE-APTES
| The catalyst | Immobilization yield (%) | Immobilization efficiency (%) | Enzyme activity (U mg–1) |
|---|---|---|---|
| The free lipase | – | – | 20.0 ± 0.1 |
| PPL@mDE | 78.0 ± 0.3 | 80.1 ± 0.6 | 13.2 ± 0.2 |
Fig. 3.
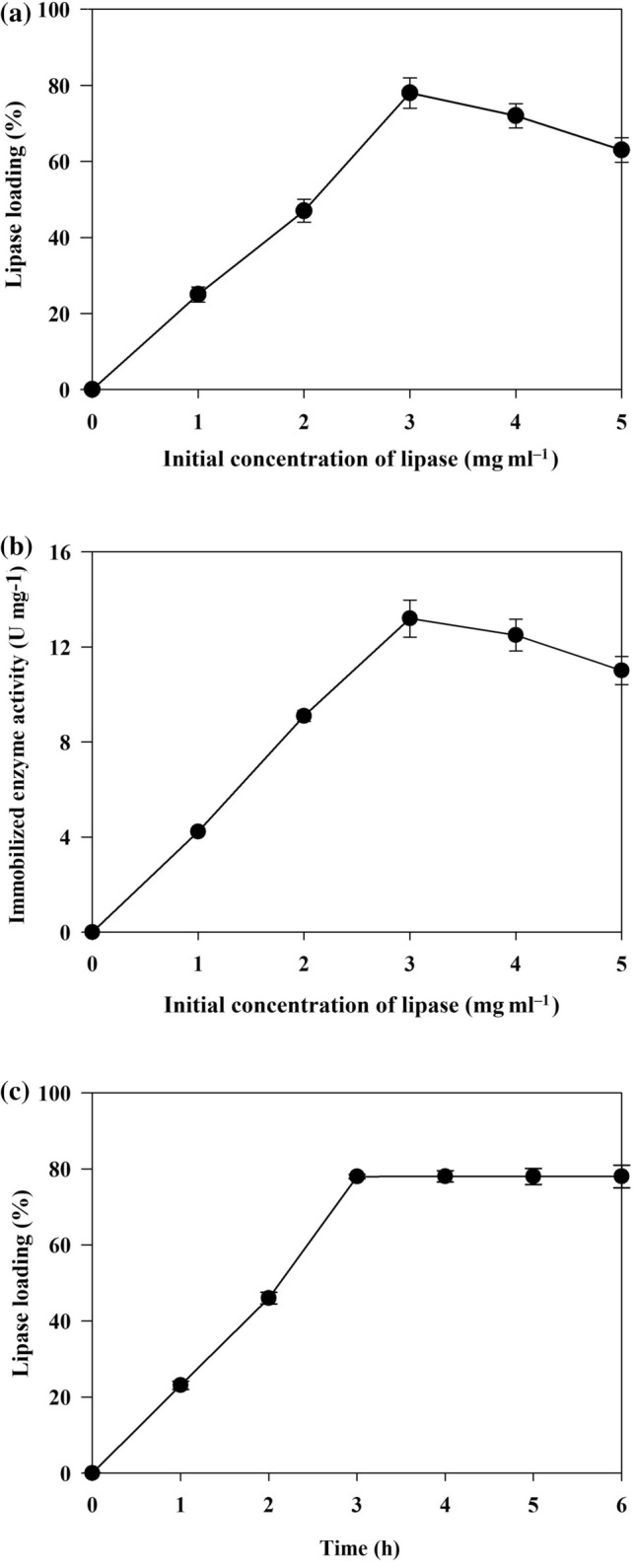
a Effect of initial lipase concentration on the enzyme loading (%) and b immobilized lipase activity (U/mg) in phosphate buffer (100 mM, pH 7.5) after shaking for 2 h at 20 °C. c The effect of immobilization time (h) on lipase loading (%) on the mDE-APTES surface in the presence of 3 mg/mL lipase in phosphate buffer (100 mM, pH 7.5) at 20 °C. Each value is mean ± SD of three replicates
Literature review revealed that researchers employed different strategies for lipase immobilization to reach the most effective and efficient biocatalysts. For example, Mendes et al. (2011) applied different materials such as hydrophobic supports (poly(hydroxybutyrate), octyl-agarose, and Amberlite resin XAD-4), ionic exchange resins (MANAE-agarose), and covalent attachment inducer composites (glyoxyl-agarose cross-linked with glutaraldehyde and epoxysilica- polyvinyl alcohol composite) for the immobilization of Penicillium camembertii lipase. They concluded that epoxy-SiO2-PVA using hexane as coupling medium was the most efficient protocol to achieve the highest lipolytic activity (128.2 ± 8.10 U/g of support). Ozyilmaz and Gezer (2010) immobilized Candida rugosa lipase (CRL) and PPL on calcium alginate gel via encapsulation procedure and introduced the entrapped PPL in alginate gel as a more efficient biocatalyst compared to the free counterpart. The study by Jegannathan et al. (2009) revealed that Burkholderia cepacia lipase encapsulated in κ-carrageenan was more stable than the free lipase in organic solvents containing ethanol, methanol, n-hexane, iso-propanol, and n-heptane.
Temperature and pH profiles of the free and immobilized lipase
The optimum activity temperature of free and immobilized lipase was evaluated over a range of 25 °C to 45 °C (Fig. 4). Similar optimum temperature was found for free and immobilized lipase. In contrast, some researchers found that immobilization often increased the optimum temperature of enzymes activity (Cabrera et al. 2018; Khoobi et al. 2014). de Lima et al. (2018) observed that the temperature of 40 °C was the optimum point for maximum activity of immobilized tannase (on mDE-PANI support) while free tannase exhibited its maximum activity at 30 °C. Figure 4 also shows the effect of pH on the activity of free or immobilized lipase. The highest activity of both appeared at pH of 7.5. Generally, a slight increase/decrease often occurs in an optimal pH of enzymes following immobilization (Fernandez-Lafuente 2010). For example, Cabrera et al. (2018) introduced pH 9 and pH 9.5 as the optimal pH for immobilized trypsine (on mDE@PANI support) and free trypsine, respectively. However, some studies reported no changes in pH and temperature profiles following enzyme immobilization (Fernandez-Lafuente 2010). For instance, de Lima et al. (2018) found that the activity of both immobilized tannase (on mDE-PANI support) and free tannase maximally occurred at pH 6.0. The absence of variation in optimal parameters could be attributed to strong covalent bonds, which keep the flexibility of the enzyme unchanged after alteration of pH and temperature (Cabrera et al. 2018; Khoobi et al. 2014). Some authors argued that protein structure changed due to the exposure to excessive acidic or alkaline conditions so that the variation in optimum pH might be a function of the ionic environment around the active site of the immobilized enzyme (Fernandez-Lafuente 2010). Figure 4 revealed that the enzyme activity generally increased with an increase in pH from 4.5 to 7.5 for the free and immobilized lipases. For pH values above 7.5, a slight reduction was observed in the activity (Fig. 4). As illustrated in Fig. 4b, the immobilized PPL on mDE-APTES had more stability to alteration of the pH at the range of 4.5–10.5 in comparison with free lipase. In addition, a notable difference was found between the free and immobilized enzyme's activity at pH range of 6.5–9.5 through elevated temperature from 25 °C to 45 °C.
Fig. 4.
Residual activity of a the free and b immobilized lipase after incubation (for 15 min at various temperatures) in the presence of substrate (p-NPP in ethanol (3 mM)) and 0.9 mL of 100 mM citrate–phosphate buffer (pH 4–5) or 100 mM phosphate buffer (pH 6–8) or 100 mM glycine–NaOH buffer (pH 9–11). Each value is mean ± SD of triplicate experiments. Significance at *p < 0.05 was compared with free lipase
Kinetic studies of immobilized lipase
The Michaelis constant (Km) values of free and immobilized lipase were 1.1 ± 0.0 μM and 2.4 ± 0.1 μM, respectively (Table 3). Michaelis constant often changes following immobilization because of partitioning, diffusion, conformational, microenvironmental, and mass-transfer effects (Cabrera et al. 2018; Fernandez-Lafuente 2010). The results obtained here could be probably explained by the difficulty in the formation of enzyme–substrate complexes in the presence of PPL@mDE-APTES due to less substrate accessibility to the active site (Fernandez-Lafuente 2010). In addition, it seems that the applied support was not appropriately assisted to maintain the PPL's flexibility that led to an increase in the Km value (Rodrigues et al. 2019). According to Altinok et al. (2008), the enzymatic Km might be affected by the type of support and immobilization method. For instance, Cabrera et al. (2018) observed higher Km after covalent immobilization of invertase on polyaniline modified magnetic DE. However, de Lima et al. (2018) determined a significantly lower (p = 0.03) Km value for immobilized tannase on mDE functionalized by polyaniline (10 ± 2 μM) compared to that of the free enzyme (18 ± 7 μM). Some studies reported that the change in molecular orientation of enzymes was responsible for the alteration of Km parameter after immobilization (Altinok et al. 2008; Cabrera et al. 2018). As represented in Table 3, the value of Vmax for immobilized lipase was also altered in the present study compared to the free one, which could be attributed to the similar factors influenced Michaelis constant as mentioned above. The decrease in maximum velocity (Table 3) could be attributed to the restriction of substrate diffusion and improper conformation of the enzyme's active site that corresponding to less substrate catalysis and lower reaction rate (Rodrigues et al., 2019). In the study performed by Cabrera et al. (2018) it was found that the related Km of the immobilized enzyme (trypsin immobilized on polyaniline functionalized mDE) was lower (0.7 mM) than free enzyme (2 mM) while the Vmax of the immobilized trypsin was higher (144 U/mg protein) compared to the free one (84 U/mg protein). In the research of Zhang et al. (2018), an increase in Vmax and Km also occurred after immobilization of lipase on zwitterionic polymer-grafted silica nanoparticles. They suggested that increase in Km value might be ascribed to structural changes in the enzyme induced by immobilization that led to a lower accessibility of substrate to the active site (Zhang et al. 2018). The immobilized lipase on gigaporous polystyrene microspheres also showed similar results (increase of the Km and decrease of the Vmax values) (Li et al. 2010). Comparable with the present study, Vmax of the immobilized lipase on dopmine functionalized mesoporous onion-like silica (DPMS) was lower than the free enzyme due to protein structure changes, active site hindrance effects, and diffusive mass transfer limitations by the support employed for immobilization (Gao et al. 2017). Pimentel et al. (2007) attributed lower Km value of the immobilized lipase on ferromagnetic azide-dacron compared with the free lipase to the new conformational structure of lipase on the support after immobilization that was more suitable for its combination with the substrate.
Table 3.
Kinetic parameters of free and immobilized lipase on mDE-APTES particles in phosphate buffer (100 mM, pH 7.5)
| Biocatalyst | Vmax (µmol min–1) | Km (mM) | Kcat (min–1) |
|---|---|---|---|
| The free lipase | 16.2 ± 0.3 | 1.1 ± 0.0 | 5.2 ± 0.2 |
| PPL@mDE | 13.0 ± 0.6 | 2.4 ± 0.1 | 4.2 ± 0.1 |
Storage stability of the immobilized enzyme
Storage stability is commonly considered as a major parameter in enzyme immobilization. The effect of storage conditions on the activity of the immobilized enzyme is known as an important aspect to ensure long shelf life (Meng et al. 2013). In general, most studies have proved a decline in enzyme activity for all tested storage conditions (Fernandez-Lafuente 2010). Figure 5a represents the stability of free and immobilized PPL for all tested storage conditions. The free and the immobilized enzymes were stored in phosphate buffer (100 mM, pH 7.5) at 4 °C, 25 °C, and 37 °C and the activity measurements were carried out for a period of 18 days. The free enzyme lost its total activity within 4 days and 14 days at 37 °C and 25 °C, respectively, while the immobilized lipase was stable up to 16 days and lost only 50% of its activity after 10 days incubation at 25 °C (Fig. 5a). It was found that the immobilization process improved the stability of the enzyme and assisted to maintain the activity. The results showed suitable storage stability of the immobilized derivative, which might be attributed to the enhancement of enzyme stability through reducing its denaturation after immobilization (Fig. 5a). Imanparast et al. (2018) also observed an increase in the storage stability of the immobilized lipase onto Celite surface. Cabrera et al. (2018) reported that invertase immobilized onto mDE nanoparticles lost its activity by a rate of 5% every 30 days of storage. The results of the present study showed that PPL@mDE-APTES retained almost 80% of its activity on the 18th day of storage at 4 °C (Fig. 5a).
Fig. 5.
a Storage stability of free and immobilized lipase in phosphate buffer (100 mM, pH 7.5) after incubation at 4 °C, 25 °C, and 37 °C for different time intervals under shaking 120 rpm. *is the level of significance (p < 0.05) versus free lipase. b Reusability profile of the lipase immobilized on mDE-APTES (3 mg/mL) in phosphate buffer (100 mM, pH 7.5). After every cycle, the relative enzymatic activity of PPL@mDE was compared with the first run
Reusability of the immobilized lipase
The recovery and reusability of the immobilized enzymes are important features which assist to minimize the total cost of enzyme application (Fernandez-Lafuente 2010). Figure 5b shows that the activity of the immobilized enzyme slightly decreased with a larger number of reuses. It was also observed that 90% of the immobilized lipase activity was retained after 4 cycles of use (Fig. 5b). The present study indicated that immobilized lipase retained 53% of initial activity after the 11th cycle (Fig. 5b). Cabrera et al. (2018) reported the appreciable reusability of invertase@mDE up to nine cycles, although the relative activity fell by 50% following the 10th cycle. In addition, immobilized trypsin was reused ten times and retained approximately 100% of the initial activity through the third cycle. Goradia et al. (2005) indicated a 40% retention of free trypsin activity at the 6th cycle, whereas trypsin@mDE maintained 75% of its initial activity in the same cycle. Eventually, immobilized β-galactosidase kept 100% of the early activity through four cycles and 62% at the 10th cycle (Cabrera et al. 2018).
Esterification studies
The activity of PPL@mDE was investigated based on the esterification of isoamyl alcohol and acetic acid to produce isoamyl acetate. Over the last decades, immobilized lipases received great considerations for the synthesis of aroma esters as fragrance and flavor (Fernandez-Lafuente 2010). In this study, it was predicted that the yield of the esterification process mainly depended on the parameters including the reaction time, amount of mDE, and the molar ratio of alcohol to acid. The time course of the fragrant ester formation is shown in Fig. 6a. The highest esterification yield was observed after 3 h (Fig. 6a). In addition, a positive effect was obtained on the reaction yield by increasing alcohol to the acid ratio from 0 to 3 (Fig. 6b). This may be attributed to the effect of acid inhibition on the enzyme activity (Fernandez-Lafuente 2010). However, the esterification efficiency declined with an increase in the alcohol concentration (ratio > 4:4) (Fig. 6b), which could be explained by the inhibitory effect of alcohol on the activity of lipase (Fernandez-Lafuente 2010). The production of isoamyl acetate enhanced in parallel with PPL@mDE amount up to 6 mg, and then the esterification yield was dropped at higher PPL@mDE amount (Fig. 6c). Concurrently, a blank reaction was designed at the same conditions without adding lipase and no esterification was observed in such reaction. Oleic acid to ethanol molar ratio of 1:1 (v/v) was found as the optimum ratio for maximum production of ethyl oleate (63% esterification) by Kumar et al. (2013) using the lipase of Bacillus sp.
Fig. 6.
a Time course of isoamyl acetate synthesis using the free and immobilized lipase in n-hexane under shaking at 100 rpm and 37 °C (Significance at *p < 0.05). The influences of b isoamyl alcohol to acetic acid ratio and c amount of PPL@mDE on esterification yield (%) for 3 h at 100 rpm and 37 °C. d Reusability of PPL@mDE (6 mg) for the esterification reaction (isoamyl alcohol to an acetic acid molar ratio of 4:4 and incubation time of 3 h at 37 °C) to produce isoamyl acetate in n-hexane. Each value represents mean ± SD of three independent experiments
The reusability of PPL@mDE-APTES demonstrated a gradual decline in esterification efficiency in the n-hexane medium (Fig. 6d). Nevertheless, immobilized lipase retained about 56.3% of its initial activity during the synthesis of isoamyl acetate after 11 successive catalytic cycles (Fig. 6d). The same declining pattern was observed by Kumar et al. (2013) in the esterification curve of the immobilized lipase from Bacillus sp. following the 7th cycle of esterification. Meng et al. (2013) reported that 80% of esterification potential of lipase (from Mucor javanicus) immobilized on poly (methylmethacrylate-co-divinylbenzene) porous magnetic microsphere persisted following the 5th cycle. Furthermore, recycling of PPL@mDE-APTES up to 12 cycles would promise to notably decrease the operating costs.
GC–MS analysis
As shown in Fig. 7, the peaks of acetic acid (I in Fig. 7) and isoamyl alcohol (II in Fig. 7) with retention time (Rt) of 4.2 and 9.2 min, respectively, were dramatically dropped compared with that of the major peak of the product (isoamyl acetate, III in Fig. 7, Rt of 12.06) after 3 h treatment by PPL@mDE. The calculated esterification yield using the area under the curve (AUC) of the product in the GC pattern (Fig. 7) was found to be 96%. This value is somehow similar to that of the measured esterification yield by back titration method due to the higher precision of the GC method. Ćorović et al. (2017) reported that at 75 °C, 1 M of isoamyl alcohol, and 6 mg/mL of Candida antarctica lipase B onto Purolite® MN102, an esterification yield of 100% was achieved after a 6-h treatment. The immobilized CRL on multiwalled carbon nanotube was able to synthesize isoamyl acetate and ethyl butyrate by 75% and 78% esterification yield, respectively (Asmat et al. 2020).
Fig. 7.
Gas chromatogram profile of the lipase-treated sample. The reaction mixture contained acetic acid (I) and isoamyl alcohol (II) (molar ratio of 4:4), and PPL@mDE (6 mg) in n-hexane was incubated for 6 h to produce isoamyl acetate (III)
Conclusion
This study suggested a simple and economic method to synthesize a magnetic composite of DE as a matrix for PPL immobilization. The produced PPL@mDE biocatalyst exhibited appropriate stability (80% relative activity after 18 days’ storage at 4 °C) and remarkable reusability (53% relative activity after 11 cycles of application). Therefore, the concept of using PPL@mDE in the production of isoamyl acetate (as banana flavour) was promising, since it can be reused in several cycles without considerable and dramatic loss of its activity (about 56.3% relative activity after 11 successive cycles of isoamyl acetate synthesis). However, it merits additional investigations and optimization evaluation for probable application of the produced immobilized biocatalyst in large scales.
Acknowledgement
Research reported in this publication was supported by Elite Researcher Grant Committee under award number 976958 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran to M.A.F.
Footnotes
Fatemeh Vakili and Somayeh Mojtabavi contributed equally as first author.
Contributor Information
Hamid Forootanfar, Email: h_forootanfar@kmu.ac.ir.
Mohammad Ali Faramarzi, Email: faramarz@tums.ac.ir.
References
- Altinok H, Aksoy S, Tümtürk H, Hasirci N. Covalent immobilization of invertase on chemically activated poly (styrene-2-hydroxyethyl methacrylate) microbeads. J Food Biochem. 2008;32(3):299–315. doi: 10.1007/s11172-006-0499-1. [DOI] [Google Scholar]
- Asmat S, Anwer AH, Husain Q. Immobilization of lipase onto novel constructed polydopamine grafted multiwalled carbon nanotube impregnated with magnetic cobalt and its application in synthesis of fruit flavours. Int J Biol Macromol. 2019;140:484–495. doi: 10.1016/j.ijbiomac.2019.08.086. [DOI] [PubMed] [Google Scholar]
- Barbosa O, Ortiz C, Berenguer-Murcia Á, Torres R, Rodrigues RC, Fernandez-Lafuente R. Glutaraldehyde in bio-catalysts design: a useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014;4(4):1583–1600. doi: 10.1002/chin.201421290. [DOI] [Google Scholar]
- Bindu V, Mohanan P (2017) Enhanced stability of α-amylase via immobilization onto chitosan-TiO2 nanocomposite. Nanosci Technol 4(2):1–9. 10.15226/2374-8141/4/2/00146
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cabrera MP, Assis CR, Neri DF, Pereira CF, Soria F, Carvalho LB., Jr High sucrolytic activity by invertase immobilized onto magnetic diatomaceous earth nanoparticles. Biotechnol Rep. 2017;14:38–46. doi: 10.1016/j.btre.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrera MP, da Fonseca TF, de Souza RVB, de Assis CRD, Marcatoma JQ, da Costa MJ, Neri DFM, Soria F, de Carvalho Jr LB. Polyaniline-coated magnetic diatomite nanoparticles as a matrix for immobilizing enzymes. Appl Surf Sci. 2018;457:21–29. doi: 10.1016/j.apsusc.2018.06.238. [DOI] [Google Scholar]
- Ćorović M, Mihailović M, Banjanac K, Carević M, Milivojević A, Milosavić N, Bezbradica D. Immobilization of Candida antarctica lipase B onto Purolite® MN102 and its application in solvent-free and organic media esterification. Bioprocess Biosyst Eng. 2017;40(1):23–34. doi: 10.1007/s00449-016-1671-0. [DOI] [PubMed] [Google Scholar]
- de Lima JS, Cabrera MP, de Souza Motta CM, Converti A, Carvalho LB., Jr Hydrolysis of tannins by tannase immobilized onto magnetic diatomaceous earth nanoparticles coated with polyaniline. Food Res Int. 2018;107:470–476. doi: 10.1016/j.foodres.2018.02.066. [DOI] [PubMed] [Google Scholar]
- de los Ángeles Calixto-Romo M, Santiago-Hernández JA, Vallejo-Becerra V, Amaya-Delgado L, del Carmen Montes-Horcasitas M, and Hidalgo-Lara ME (2008) Expression, purification and immobilization of the intracellular invertase INVA, from Zymomonas mobilis on crystalline cellulose and Nylon-6. J. Ind. Microbiol. Biotechnol. 35(11):1455. 10.1007/s10295-008-0447-1 [DOI] [PubMed]
- Fernandez-Lafuente R. Lipase from Thermomyces lanuginosus: uses and prospects as an industrial biocatalyst. J Mol Catal B Enzym. 2010;62(3–4):197–212. doi: 10.1016/j.molcatb.2009.11.010. [DOI] [Google Scholar]
- Gao J, Jiang Y, Lu J, Han Z, Deng J, Chen Y. Dopamine-functionalized mesoporous onion-like silica as a new matrix for immobilization of lipase Candida sp.99–125. Sci Rep. 2017;7(1):1–9. doi: 10.1038/srep40395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goradia D, Cooney J, Hodnett B, Magner E. The adsorption characteristics, activity and stability of trypsin onto mesoporous silicates. J Mol Catal B Enzym. 2005;32(5–6):231–239. doi: 10.1016/j.molcatb.2004.12.007. [DOI] [Google Scholar]
- Imanparast S, Hamedi J, Faramarzi MA. Enzymatic esterification of acylglycerols rich in omega-3 from flaxseed oil by an immobilized solvent-tolerant lipase from Actinomadura sediminis UTMC 2870 isolated from oil-contaminated soil. Food Chem. 2018;245:934–942. doi: 10.1016/j.foodchem.2017.11.080. [DOI] [PubMed] [Google Scholar]
- Janićijević J, Krajišnik D, Čalija B, Vasiljević BN, Dobričić V, Daković A, Antonijević MD, Milić J. Modified local diatomite as potential functional drug carrier—A model study for diclofenac sodium. Int J Pharm. 2015;496(2):466–474. doi: 10.1016/j.ijpharm.2015.10.047. [DOI] [PubMed] [Google Scholar]
- Jegannathan KR, Chan ES, Ravindra P. Physical and stability characteristics of Burkholderia cepacia lipase encapsulated in κ-carrageenan. J Mol Catal B: Enzym. 2009;58(1–4):78–83. doi: 10.1016/j.molcatb.2008.11.009. [DOI] [Google Scholar]
- Kahraman MV, Bayramoğlu G, Kayaman-Apohan N, Güngör A. α-Amylase immobilization on functionalized glass beads by covalent attachment. Food Chem. 2007;104(4):1385–1392. doi: 10.1016/j.foodchem.2007.01.054. [DOI] [Google Scholar]
- Kapoor M, Gupta MN. Lipase promiscuity and its biochemical applications. Process Biochem. 2012;47(4):555–569. doi: 10.1016/j.procbio.2012.01.011. [DOI] [Google Scholar]
- Khoobi M, Motevalizadeh SF, Asadgol Z, Forootanfar H, Shafiee A, Faramarzi MA. Synthesis of functionalized polyethylenimine-grafted mesoporous silica spheres and the effect of side arms on lipase immobilization and application. Biochem Eng J. 2014;88:131–141. doi: 10.1016/j.bej.2014.04.009. [DOI] [Google Scholar]
- Kiss M, Sefanovits-Bányai É, Tóth Á, Boross L. Extractive synthesis of ethyl-oleate using alginate gel co-entrapped yeast cells and lipase enzyme. Eng Life Sci. 2004;4(5):460–464. doi: 10.1002/elsc.200420047. [DOI] [Google Scholar]
- Kumar D, Nagar S, Bhushan I, Kumar L, Parshad R, Gupta VK. Covalent immobilization of organic solvent tolerant lipase on aluminum oxide pellets and its potential application in esterification reaction. J Mol Catal B Enzym. 2013;87:51–61. doi: 10.1016/j.molcatb.2012.10.002. [DOI] [Google Scholar]
- Li Y, Gao F, Wei W, Qu JB, Ma GH, Zhou WQ. Pore size of macroporous polystyrene microspheres affects lipase immobilization. J Mol Catal B: Enzym. 2010;66(1–2):182–189. doi: 10.1016/j.molcatb.2010.05.007. [DOI] [Google Scholar]
- Mendes AA, Freitas L, de Carvalho AK, de Oliveira PC, de Castro HF. Immobilization of a commercial lipase from Penicillium camembertii (Lipase G) by different strategies. Enzyme Res. 2011;2011:2011. doi: 10.4061/2011/967239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Xu G, Zhou Q-L, Wu J-P, Yang L-R. Improvements of lipase performance in high-viscosity system by immobilization onto a novel kind of poly (methylmethacrylate-co-divinylbenzene) encapsulated porous magnetic microsphere carrier. J Mol Catal B Enzym. 2013;89:86–92. doi: 10.1016/j.molcatb.2013.01.006. [DOI] [Google Scholar]
- Mohamad NR, Marzuki NHC, Buang NA, Huyop F, Wahab RA. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip. 2015;29(2):205–220. doi: 10.1080/13102818.2015.1008192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozyilmaz G, Gezer E. Production of aroma esters by immobilized Candida rugosa and porcine pancreatic lipase into calcium alginate gel. J Mol Catal B: Enzym. 2010;64(3–4):140–145. doi: 10.1016/j.molcatb.2009.04.013. [DOI] [Google Scholar]
- Palomo JM, Fernández-Lorente G, Guisán JM, Fernández-Lafuente R. Modulation of immobilized lipase enantioselectivity via chemical amination. Adv Synth Catal. 2007;349(7):1119–1127. doi: 10.1002/adsc.200600555. [DOI] [Google Scholar]
- Pimentel MC, Leao AB, Melo EH, Ledingham WM, Filho JL, Sivewright M, Kennedy JF. Immobilization of Candida rugosa lipase on magnetized Dacron: kinetic study. Artif CellsBlood Substitutes Biotechnol. 2007;35(2):221–235. doi: 10.1080/10731190601188380. [DOI] [PubMed] [Google Scholar]
- Poornima K, Preetha R (2017) Biosynthesis of food flavours and fragrances A review. Asian J Chem 29(11):2345–2352. 10.14233/ajchem.2017.20748
- Prlainović NŽ, Bezbradica DI, Rogan JR, Uskoković PS, Mijin DŽ, Marinković AD. Surface functionalization of oxidized multi-walled carbon nanotubes: Candida rugosa lipase immobilization. C R Chim. 2016;19(3):363–370. doi: 10.1016/j.crci.2015.10.008. [DOI] [Google Scholar]
- Taghizadeh T, Talebian-Kiakalaieh A, Jahandar H, Amin M, Tarighi S, Faramarzi MA. Biodegradation of bisphenol A by the immobilized laccase on some synthesized and modified forms of zeolite Y. J Hazard Mater. 2020;386:121950. doi: 10.1016/j.jhazmat.2019.121950. [DOI] [PubMed] [Google Scholar]
- Tsai W-T, Lai C-W, Hsien K-J. Characterization and adsorption properties of diatomaceous earth modified by hydrofluoric acid etching. J Colloid Interface Sci. 2006;297(2):749–754. doi: 10.1016/j.jcis.2005.10.058. [DOI] [PubMed] [Google Scholar]
- Xie W, Huang M. Fabrication of immobilized Candida rugosa lipase on magnetic Fe3O4-poly (glycidyl methacrylate-co-methacrylic acid) composite as an efficient and recyclable biocatalyst for enzymatic production of biodiesel. Renew: Energy; 2020. [Google Scholar]
- Zhang C, Dong X, Guo Z, Sun Y. Remarkably enhanced activity and substrate affinity of lipase covalently bonded on zwitterionic polymer-grafted silica nanoparticles. J Colloid Interface Sci. 2018;519:145–153. doi: 10.1016/j.jcis.2018.02.039. [DOI] [PubMed] [Google Scholar]
- Zhang L, Tang W, Ma T, Zhou L, Hui C, Wang X, Wang P, Zhang C, Chen C. Laccase-immobilized tannic acid-mediated surface modification of halloysite nanotubes for efficient bisphenol-A degradation. RSC Adv. 2019;9(67):38935–38942. doi: 10.1039/C9RA06171A. [DOI] [PMC free article] [PubMed] [Google Scholar]







