Abstract
Background
The isolation of symptomatic cases and tracing of contacts has been used as an early COVID-19 containment measure in many countries, with additional physical distancing measures also introduced as outbreaks have grown. To maintain control of infection while also reducing disruption to populations, there is a need to understand what combination of measures—including novel digital tracing approaches and less intensive physical distancing—might be required to reduce transmission. We aimed to estimate the reduction in transmission under different control measures across settings and how many contacts would be quarantined per day in different strategies for a given level of symptomatic case incidence.
Methods
For this mathematical modelling study, we used a model of individual-level transmission stratified by setting (household, work, school, or other) based on BBC Pandemic data from 40 162 UK participants. We simulated the effect of a range of different testing, isolation, tracing, and physical distancing scenarios. Under optimistic but plausible assumptions, we estimated reduction in the effective reproduction number and the number of contacts that would be newly quarantined each day under different strategies.
Results
We estimated that combined isolation and tracing strategies would reduce transmission more than mass testing or self-isolation alone: mean transmission reduction of 2% for mass random testing of 5% of the population each week, 29% for self-isolation alone of symptomatic cases within the household, 35% for self-isolation alone outside the household, 37% for self-isolation plus household quarantine, 64% for self-isolation and household quarantine with the addition of manual contact tracing of all contacts, 57% with the addition of manual tracing of acquaintances only, and 47% with the addition of app-based tracing only. If limits were placed on gatherings outside of home, school, or work, then manual contact tracing of acquaintances alone could have an effect on transmission reduction similar to that of detailed contact tracing. In a scenario where 1000 new symptomatic cases that met the definition to trigger contact tracing occurred per day, we estimated that, in most contact tracing strategies, 15 000–41 000 contacts would be newly quarantined each day.
Interpretation
Consistent with previous modelling studies and country-specific COVID-19 responses to date, our analysis estimated that a high proportion of cases would need to self-isolate and a high proportion of their contacts to be successfully traced to ensure an effective reproduction number lower than 1 in the absence of other measures. If combined with moderate physical distancing measures, self-isolation and contact tracing would be more likely to achieve control of severe acute respiratory syndrome coronavirus 2 transmission.
Funding
Wellcome Trust, UK Engineering and Physical Sciences Research Council, European Commission, Royal Society, Medical Research Council.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spread rapidly across multiple countries in early 2020.1, 2, 3 A staple public health control measure for outbreaks of emerging, directly transmitted infections involves the isolation of symptomatic cases as well as the tracing, testing, and quarantine of their contacts.2 The effectiveness of this measure in containing new outbreaks depends on both the transmission dynamics of the infection and the proportion of transmission that occurs from infections without symptoms.4 Evidence exists that SARS-CoV-2 has a reproduction number (R) of about 2–3 in the early stages of an outbreak,1, 5 and many infections can occur without symptoms,6 which means isolation of symptomatic cases and contact tracing alone are unlikely to contain an outbreak unless a high proportion of cases are isolated and contacts successfully traced and quarantined.7
Several countries have used combinations of non-pharmaceutical interventions to reduce SARS-CoV-2 transmission.3, 8 As well as isolating symptomatic individuals and tracing and quarantining their contacts, measures have included general physical distancing, school closures, remote working, community testing, and cancellation of events and mass gatherings. It has also been suggested that the effectiveness of contact tracing could be enhanced through app-based digital tracing.9 The effectiveness of contact tracing and the extent of resources required to implement it successfully will depend on the social interactions within a population.10 Targeted interventions such as contact tracing also need to consider individual-level variations in transmission: high variation can lead to superspreading events, which could result in larger numbers of contacts needing to be traced.11 Several examples exist of such events occurring for COVID-19, including meals, parties, and other gatherings involving close contacts.12
Research in context.
Evidence before this study
We searched PubMed, BioRxiv, and MedRxiv for articles published in English from inception to April 15, 2020, with the following keywords: “2019-nCoV”, “novel coronavirus”, “COVID-19”, “SARS-CoV-2” AND “contact tracing” AND “model*”. Early modelling studies of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) suggested that isolation and tracing alone might not be sufficient to control outbreaks and additional measures might be required; these measures have since been explored in population-level models. However, an analysis with setting-specific social contact data to quantify the potential effect of combined contact tracing and physical distancing measures on reducing individual-level transmission of SARS-CoV-2 has not been done.
Added value of this study
We use data from more than 40 000 individuals to assess contact patterns and potential SARS-CoV-2 transmission in different settings and compare how combinations of self-isolation, contact tracing, and physical distancing could reduce secondary cases. We assessed a range of combined physical distancing and testing and tracing measures, including app-based tracing, remote working, limits on different sized gatherings, and mass population-based testing. We also estimated the number of contacts that would be quarantined under different strategies.
Implications of all the available evidence
Several characteristics of SARS-CoV-2 make effective isolation and contact tracing challenging, including high transmissibility, a relatively short serial interval, and transmission that can occur without symptoms. Combining isolation and contact tracing with physical distancing measures—particularly measures that reduce contacts in settings that would otherwise be difficult to trace—could therefore increase the likelihood of achieving sustained control of SARS-CoV-2 transmission.
We used social-contact data from a large-scale UK study of over 40 000 participants13 to explore a range of different control measures for SARS-CoV-2, including self-isolation of symptomatic cases; household quarantine; manual tracing of acquaintances (ie, contacts that have been met before); manual tracing of all contacts; app-based tracing; mass testing regardless of symptoms; limits on daily contacts made outside home, school, and work; and having a proportion of the adult population work from home. We estimated the reduction in transmission under different scenarios, and we estimated how many primary cases and contacts would be quarantined per day in different strategies for a given level of symptomatic case incidence.
Methods
Transmission model
For this mathematical modelling study, our analysis was based on data of 40 162 UK participants with recorded social contacts from the BBC Pandemic dataset.13 In the BBC Pandemic dataset, collected in 2017–18, a contact was defined as an interaction that either involved a face-to-face conversation or physical contact, which broadly reflect the types of close contact that have been linked to SARS-CoV-2 transmission clusters to date.12 Using these data, we simulated 25 000 individual-level transmission events by repeatedly generating contact distributions for a primary case and randomly generating infections among these contacts. In each simulation, we randomly specified a primary case as either younger than 18 years or aged 18 years and older, on the basis of UK demography, in which 21% of the population are younger than 18 years.14 We then generated contacts by randomly sampling values from the marginal distributions of daily contacts made in three different settings for their age group (ie, younger than 18 years or adults): in household (defined as household size minus one), at work or school, and in other settings (figure 1A, B ). We used the marginal distributions rather than raw participant data to ensure non-identifiability and reproducibility in our model code. Information was provided and consent obtained from all participants in the BBC Pandemic study before the app recorded any data. Our study was approved by the London School of Hygiene & Tropical Medicine Observational Research Ethics Committee (ref 14400).
Figure 1.
Model of social interactions and SARS-CoV-2 transmission and control
(A) Distribution of daily contacts made at home, work, school, and other settings in the BBC Pandemic dataset. (B) Examples of daily social contact patterns for four randomly selected individuals in the model. (C) Factors that influence whether an individual is isolated and whether contacts are successfully traced in the model (parameters presented in table 1). (D) Implementation of contact tracing in the model. The timeline shows a primary case with four daily contacts self-isolating either 1 or 3 days after onset of symptoms. We assumed the household contact to be the same person throughout, whereas other contacts are made independently. Had the primary case not been isolated, seven secondary cases would have occurred in this illustration (shown with circulations). For isolation 1 day after onset, four secondary infections were prevented immediately. Then seven contacts were potentially traceable, three of whom were infected. In this example, two infected contacts pre-isolation were successfully traced and quarantined (ie, one was missed), so overall the isolation-and-tracing control measure resulted in a 4 + 2=6 reduction in the effective reproduction number. A similar illustration is shown for isolation 3 days after onset. SARS-CoV-2=severe acute respiratory syndrome coronavirus 2.
In our model, we assumed infected individuals had a certain probability of being symptomatic and of being tested if symptomatic, as well as an infectious period that depended on when or if they self-isolated after the onset of symptoms (table 1 ). We assumed a mean delay of 2·6 days from onset to isolation in our baseline scenario (appendix, p 2). We assumed individuals became infectious 1 day before onset of symptoms. During each day of the effective infectious period, individuals made a given number of contacts equal to their simulated daily contacts. To avoid double-counting household members, household contacts were not tallied over the entire infectious period, but instead were fixed. Once individual-level contacts had been defined, we generated secondary infections at random on the basis of assumed secondary attack rates among contacts made in different settings, and we estimated how many contacts would be successfully traced in each of these settings under different scenarios (full description in the appendix, p 1). First, we generated the number of secondary cases without any control measures in place. Second, we randomly sampled the proportion of these secondary cases that were either successfully traced and quarantined, and hence removed from the potentially infectious pool, or averted through isolation of the primary case. The difference between these two values gave the overall number of secondary cases that would contribute to further transmission, the effective R (Reff; figure 1C, D).
Table 1.
Parameter definitions and assumptions for the baseline model
| Assumed value | Details and references | |
|---|---|---|
| Individual-level dynamics | ||
| R in absence of control measures | 2·6 | SARs were chosen to be consistent with empirical estimates (table 2) and produce an R consistent with a meta-analysis of early studies;15 sensitivity analysis shown in the appendix (p 4) |
| Duration of infectiousness | 5 days (for cases that will become symptomatic, first day is pre-symptomatic) | Given incubation period of about 5 days, this assumption implies serial interval of about 6·5 days;16 |
| Relative infectiousness of asymptomatic cases | 50% | One published point estimate was 65%,17 but secondary cases from asymptomatic individuals were more likely to, in turn, be asymptomatic, suggesting lower contribution to transmission; sensitivity analysis shown in the appendix (p 3) |
| Proportion of cases who are eventually symptomatic | 30% of children, 70% of adults | Based on evidence synthesis of age-stratified COVID-19 data;18 sensitivity analysis shown in the appendix (p 3) |
| Probability that symptomatic individual will eventually self-isolate and be tested | 90% | We assumed that the virus is only detectable by PCR during the infectious period; 90% of UK survey respondents said they would likely comply with app request to self-isolate if rapid test available19 |
| Effective duration of infectiousness if an individual self-isolates when symptomatic | Mean delay from onset to isolation of 2·6 days; distribution shown in the appendix (p 2) | We assumed that individuals are most likely to self-isolate 0–4 days after onset (ie, 1–5 days after becoming infectious); for 269 cases with known date of onset and confirmation in Singapore, of those who were confirmed within 5 days, 2% were confirmed on date of onset, 26% on day 2, 27% on day 3, 14% on day 4, and 31% on day 5;20 we assumed that isolation could occur 1 day before confirmation; sensitivity analysis shown in the appendix (p 5) |
| SAR among contacts in home | 20% | Details in the SAR section of the Methods |
| SAR among other contacts | 6% | Details in the SAR section of the Methods |
| Contact tracing | ||
| Proportion of contacts who are acquaintances (ie, have been met before) | 100% in household, 90% at school, 79% at work, 52% in other settings | Data from BBC Pandemic dataset;13 for each contact reported, participants were asked “have you met this person before?” |
| Proportion of potentially traceable household contacts who are successfully traced | 100% | Assumed |
| Proportion of potentially traceable workplace, school, or other scenario contacts who are successfully traced | 95% | Assumed, with sensitivity analysis shown in figure 2 |
| Probability that traced contacts adhere to quarantine | 90% | Proportion of traced contacts who are successfully removed from the potentially infectious group; we assumed virus is only detectable by PCR during the infectious period; 90% of UK survey respondents said they would likely comply with app request to self-isolate if rapid test available;19 we assumed contacts traced by app would be quarantined immediately and manually traced contacts would take 2 days to quarantine after isolation of the index case9, 16 |
| App-based tracing | ||
| Proportion of population that would have the app installed | 53% | We assumed that 71% of the population were smartphone users (details in appendix, p 1); 75% of UK survey respondents said they would probably or definitely download the app;19 therefore, we assume that 71% × 75% = 53% of the population would have the app installed. |
| Mass testing | ||
| Proportion of the population that are tested per week | 5% (ie, 460 000 tests per day for UK) | 0·7% of the population tested per day, which is equal to the highest number of daily per person tests done anywhere in world as of mid-April, 2020 (details in appendix, p 1) |
R=reproduction number. SAR=secondary attack rate.
Secondary attack rate data sources
To estimate the risk of transmission per contact in different community settings, we collated contact tracing studies for COVID-19 from multiple settings that stratified contacts within and outside households (table 2 ). Across studies, the estimated secondary attack rate within households was 10–20%, with a much smaller secondary attack rate (ranging from 0% to 5%) estimated among close contacts made outside households. However, all these studies were done in a so-called under control scenario (ie, effective R<1) and some reported relatively few contacts (ie, fewer than ten per case), which might omit superspreading events, and isolation outside of household. These findings suggest that SARS-CoV-2 infection might be driven by community transmission events as well as household contacts. In our main analysis, we assumed a secondary attack rate of 20% for households and 6% for all contacts, which led to an overall R of 2·6 in our model when no control measures were in place. This value is consistent with the estimated R values in the early stages of the epidemic.1, 5
Table 2.
Secondary attack rates estimated from COVID-19 contact tracing studies by location
| Secondary attack rate among household contacts (%) | Secondary attack rate among close contacts outside household (%) | Contacts traced per case | Observed reproduction number | |
|---|---|---|---|---|
| Shenzhen16 | 12·9% | 0·9% | 3·0 | 0·4 |
| USA21 | 10·5% | 0·0% | 44·5 | 0·20 |
| Guangzhou22 | 10·1% | 0·5% | 14·3 | 0·34 |
| Taiwan23 | 6·6% | 0·4% | 27·6 | 0·21 |
| Ningbo17 | 13·3% | 5·1% | 11·2 | 0·69 |
| Guangzhou24 | 19·3% | 5·3% | 9·8 | 0·62 |
Table includes two separate analyses of contact tracing data from Guangzhou and differing estimates are likely to be influenced by control measures in place at the time.
Scenarios
We considered several scenarios, both individually and in combination (appendix, p 2). These scenarios included no control, self-isolation of symptomatic cases within and away from household, household quarantine, quarantine of work or school contacts, manual tracing of acquaintances (ie, contacts that have been met before), manual tracing of all contacts, app-based tracing, mass testing of cases regardless of symptoms, a limit on daily contacts made in other settings (with the baseline limit being four contacts, equal to the mean number reported by adults in the BBC Pandemic data), and a proportion of the population with no school or work contacts. In the self-isolation only scenario, we assumed that individuals who were successfully isolated either had no risk of onward transmission (even to household members) or had no risk to contacts outside the household, but household members could still be infected. Otherwise, we assumed household quarantine was in place alongside other measures. For app-based tracing to be successfully implemented in a given simulation, both the infectious individual and their contacts needed to have and use the app. We assumed that individuals younger than 10 years or older than 80 years would not use a smartphone app (table 1). In the scenario with mass testing of cases regardless of symptoms, we assumed that infected individuals would be identified and immediately self-isolate at a random point during or after their 5-day infectious period. We assumed that infected individuals would not test positive if they were tested during the latent period. No other measures (eg, self-isolation when symptomatic) were in place for this scenario. In the baseline scenario for reduced work contacts, we assumed that 50% of the population had no work contacts because 54% of respondents in a UK social contact survey reported not visiting work in the days after lockdown was introduced on March 23, 2020.25 For each intervention scenario, we simulated 25 000 primary cases, generating individual-level contact distributions and secondary cases with and without the control measure in place, as previously described. The model code is available online.
Role of the funding source
The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Under the control measures considered, we found that combined testing and tracing strategies reduced the Reff more than mass testing or self-isolation alone (table 3 ). If self-isolation of symptomatic cases alone was included, our optimistic scenario resulted in a mean transmission reduction of 29% if self-isolation was within the household and 35% if self-isolation was outside the household. The addition of household quarantine resulted in an overall mean reduction of 37%. In simulations, self-isolation and household quarantine with the addition of manual contact tracing of all contacts reduced transmission by 64%; the addition of manual tracing of acquaintances only led to a 57% reduction in transmission. We estimated that the addition of app-based tracing only, with our baseline assumption of 53% coverage, reduced transmission by 47%. Contact tracing measures also substantially reduced the probability that a primary symptomatic case would generate more than one secondary case (table 3).
Table 3.
Mean reduction in Reff under different control measures
| Self-Isolation | Contact tracing | Non-HH contacts who are potentially traceable (%) | Cases who have R>1 (%) | Reff | Mean reduction in Reff | |
|---|---|---|---|---|---|---|
| No control | No | No | NA | 50% | 2·6 | 0% |
| Self-isolation within home | Yes | No | NA | 40% | 1·8 | 29% |
| Self-isolation outside home | Yes | NA | NA | 37% | 1·7 | 35% |
| Self-isolation plus HHQ | Yes | HH | NA | 35% | 1·6 | 37% |
| Self-isolation plus HHQ plus work or school contact tracing | Yes | HH and work or school | 100% | 27% | 1·2 | 53% |
| Self-isolation plus HHQ plus manual contact tracing of acquaintances | Yes | All | 90% school, 79% work, and 52% other | 26% | 1·1 | 57% |
| Self-isolation plus HHQ plus manual contact tracing of all contacts | Yes | All | 100% | 21% | 0·94 | 64% |
| Self-isolation plus HHQ plus app-based tracing | Yes | All | 53% | 30% | 1·4 | 47% |
| Self-isolation plus HHQ plus manual contact tracing of acquaintances plus app-based tracing | Yes | All | 90% school, 79% work, and 52% other with manual tracing; 53% with app tracing | 23% | 1 | 61% |
| Self-isolation plus HHQ plus manual contact tracing of acquaintances plus limit to four daily contacts with other individuals | Yes | All | 90% school, 79% work, and 52% other | 21% | 0·93 | 64% |
| Self-isolation plus HHQ plus manual contact tracing of acquaintances plus app-based tracing plus limit to four daily contacts with other individuals | Yes | All | 90% school, 79% work, and 52% other with manual tracing; 53% with app tracing | 20% | 0·87 | 66% |
| Mass testing of 5% of population per week | No | NA | NA | 49% | 2·5 | 2% |
Results from 20 000 simulated setting-specific secondary transmissions, assuming a secondary attack rate of 20% among household contacts and 6% among other contacts. Results under the assumption of some workplace restrictions remaining in place are shown in table 4. Estimates are shown to two significant figures. HH=household. HHQ=household quarantine. NA=not applicable. Reff=effective reproduction number.
We estimated that if some level of physical distancing were maintained, it could supplement reductions in transmission from contact tracing. For example, if daily contacts in other settings (ie, outside the home, work, and school) were limited to four people (the mean number in our dataset), manual tracing of acquaintances only led to a mean 64% reduction in transmission, and the addition of app-based tracing alongside this gave a mean 66% reduction overall. We estimated that mass random testing of 5% of the population each week would reduce transmission by only 2%, because substantially fewer infections would be detected than in other scenarios and many of those that were would have already transmitted infection.
We also considered the number of contacts that would be traced under different strategies. In a scenario where 20 000 new symptomatic cases occurred per day, most contact tracing strategies would require over 500 000 contacts to be newly quarantined each day on average (table 4 ). We should note that if contact tracing is triggered on the basis of suspected COVID-19-like symptoms rather than confirmation of COVID-19, the number of symptomatic cases in these scenarios would reflect the total incidence of illness and not just of confirmed COVID-19 cases. Although we estimated a similar reduction in transmission from manual tracing of all contacts and from manual tracing of only acquaintances with a limit to four daily contacts in other settings (table 3), manual tracing of acquaintances with a four-person limit required fewer people to be quarantined each day (table 4). We obtained similar results for the relative reductions in transmission and number of contacts traced when we assumed a higher secondary attack rate within household or among other contacts, which corresponded to baseline R values of 2·6–2·9 (appendix, p 4).
Table 4.
Number of additional people quarantined per symptomatic case under different scenarios for the absolute number of new symptomatic cases per day
| Median number of people quarantined per detected case (90% prediction interval) | Mean newly quarantined people per day assuming 20 000 new symptomatic cases per day | Mean newly quarantined people per day assuming 5000 new symptomatic cases per day | Mean newly quarantined people per day assuming 1000 new symptomatic cases per day | |
|---|---|---|---|---|
| SI and HHQ | 2 (0–4) | 38 000 | 9400 | 1900 |
| SI plus HHQ plus work or school CT | 13 (1–110) | 540 000 | 140 000 | 27 000 |
| SI plus HHQ plus manual CT of acquaintances | 22 (1–120) | 650 000 | 160 000 | 32 000 |
| SI plus HHQ plus manual CT of all contacts | 29 (1–140) | 830 000 | 210 000 | 41 000 |
| SI plus HHQ plus app-based CT | 4 (1–69) | 310 000 | 76 000 | 15 000 |
| SI plus HHQ plus manual CT of acquaintances plus app-based CT | 25 (1–130) | 740 000 | 180 000 | 37 000 |
| SI plus HHQ plus manual CT of acquaintances plus limit to four daily contacts with other individuals | 17 (1–110) | 560 000 | 140 000 | 28 000 |
| SI plus HHQ plus manual CT of acquaintances plus app-based CT plus limit to four daily contacts with other individuals | 21 (1–110) | 630 000 | 160 000 | 32 000 |
We assumed that quarantined contacts are independent. Estimates shown to two significant figures. If contact tracing is initiated on the basis of suspected rather than confirmed COVID-19 cases, the symptomatic case numbers here would reflect total incidence of COVID-19-like illness, which might be considerably higher than the number of confirmed cases. CT=contact tracing. HHQ=household quarantine. SI=self isolation.
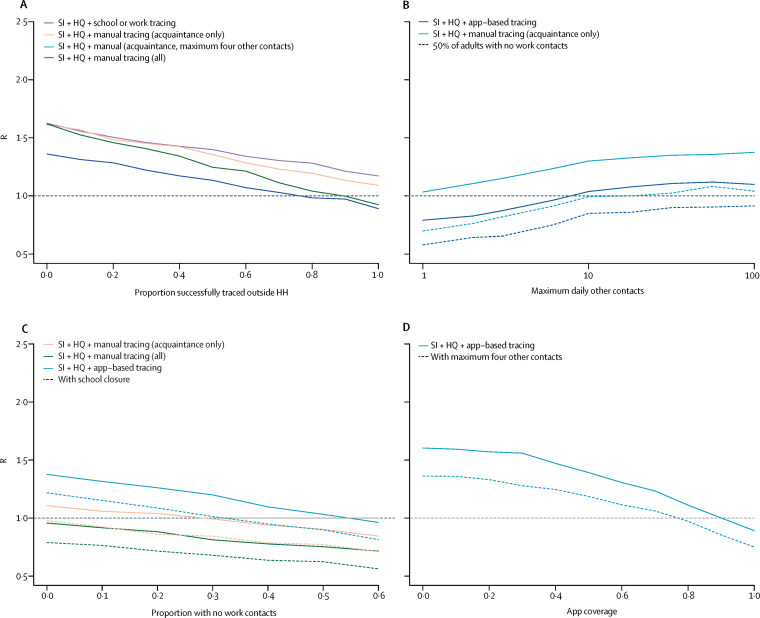
We found that the effectiveness of manual contact-tracing strategies was highly dependent on how many contacts were successfully traced, with a high level of tracing required to ensure Reff lower than 1 in our baseline scenario (figure 2A ). If contact tracing was combined with a maximum limit to daily contacts made in other settings (eg, by restricting gatherings), we found that this limit would have to be small (ie, fewer than ten or 20 contacts) before a discernible effect could be seen on Reff. The limit would have to be small (ie, fewer than about ten contacts) to ensure Reff lower than 1 for app-based tracing, even if half of adults also had no work contacts (figure 2B). When app-based tracing was in place, we estimated that if work contacts alone were restricted, a substantial proportion of the adult population would need to have zero work contacts to ensure Reff lower than 1 (figure 2C). Under our baseline assumptions, we estimated that app-based tracing would require a high level of coverage to ensure Reff lower than 1 (figure 2D) because both primary case and contacts would need to install and use the app.
Figure 2.
Impact of contact tracing effectiveness and physical distancing on reduction in R (baseline R 2·6)
Reduction in R under different strategies for different proportions of work, school, and other contacts that are successfully traced (A); effect of the maximum limit on the number of daily contacts in other settings and control tracing strategies on R, either when adults are working as normal, or when 50% have no work contacts (B); effect of proportion of population with no work contacts (C); and effect of app-based tracing under different assumptions about app coverage (D). In all panels, other parameters are as presented in table 1. HQ=household quarantine. R=reproduction number. SI=self isolation.
We also considered the effect of the proportion of infections assumed to be symptomatic and the relative contribution of asymptomatic individuals to transmission. We estimated that if a high proportion of cases were symptomatic, self-isolation and contact tracing measures would lead to a greater relative reduction in transmission (appendix, p 3); this is mostly because more primary cases would be detected. Control measures were slightly less effective if the relative transmissibility of asymptomatic infections was higher (appendix, p 3) because it would mean more undetectable transmission occurring. However, because our baseline scenario assumed that 70% of adults were symptomatic, the overall effect of asymptomatic individuals on transmission was less than it would be if most cases were asymptomatic. We estimated that if individuals self-isolated rapidly (ie, with 1·2 days on average rather than 2·6 days), self-isolation and household quarantine would lead to a larger reduction in transmission (appendix, p 5); correspondingly, if we assumed cases took longer to self-isolate after becoming symptomatic (ie, 3·6 days on average), these measures were less effective. However, the estimated overall reduction from self-isolation and manual contact tracing was similar across the three scenarios because although more secondary infections occurred before isolation, a large proportion of them would be traced under our baseline model assumptions.
Discussion
Using a model of setting-specific interactions, we estimated that strategies that combined isolation of symptomatic cases with tracing and quarantine of their contacts reduced the Reff more than mass testing or self-isolation alone. The effectiveness of these isolation and tracing strategies was further enhanced when combined with physical distancing measures, such as a reduction in work contacts, or a limit to the number of contacts made outside of home, school, or work settings. Not only does physical distancing reduce transmission, but it is also likely to reduce the number of unknown contacts who can be harder to trace. Several countries have achieved a prolonged suppression of SARS-CoV-2 transmission using a combination of case isolation, contact tracing, and physical distancing. In Hong Kong, isolation of cases and tracing of contacts was combined with other physical distancing measures, which resulted in an estimated Reff near 1 throughout February and March, 2020.26 In South Korea, testing and tracing has been combined with school closures and remote working.27
In our analysis, we estimated that many contacts would need to be traced and tested if the incidence of symptomatic cases was high. This logistical constraint might influence how and when it is possible to transition from ensuring an Reff lower than 1 through extensive physical distancing measures to reducing transmission predominantly through targeted isolation and tracing measures. Our estimate of many contacts potentially being traced per case in the manual tracing strategies we considered (table 4) suggests that any planning for ongoing control based on isolation and tracing should consider the probable need to do at least 30–50 additional tests for each symptomatic case reported. If contact tracing is initiated on the basis of suspected rather than confirmed SARS-CoV-2 infections, then the number of symptomatic cases that require follow-up tracing and testing might be considerably higher than the level of confirmed COVID-19 incidence. Given the role of pre-symptomatic transmission for SARS-CoV-2, the quarantine of these contacts rather than symptom monitoring alone is likely to be more effective at reducing onward transmission.28
Our analysis has several limitations. We focused on individual-level transmission between a primary case and their contacts rather than considering higher degree network effects. Therefore, our results focused on possible reductions in transmission rather than temporal ranges of outbreak size or dynamics. Network structure might also influence specific interventions. If contacts were clustered (ie, know each other), the number of contacts that would need to be traced over multiple generations of transmission could be reduced. Additionally, if an inverse relationship exists between the probability of detectable symptoms and app coverage, as might be the case for young children, it could reduce the effectiveness of symptom-based tracing for such index cases. We also assumed that contacts made within the home are the same people daily, but contacts outside home are made independently each day. Repeated contacts would also reduce the number of people that need to be traced. However, our estimates are consistent with the upper bound of numbers traced in empirical studies (table 2), as well as the analysis of UK social interactions that accounted for contacts of contacts.10 Because our data were not stratified beyond the four contact settings we considered (home, work, school, and other), we could not consider additional specific settings, such as mass gatherings. However, our finding that gatherings in other settings needed to be restricted to small sizes before a noticeable effect on transmission occurred is consistent with findings that groups between ten and 50 people have a larger effect on SARS-CoV-2 dynamics than groups of more than 50.29 In our main analysis, we used a limit of four daily contacts as an illustrative example. In reality, any control strategies would also need to consider the probable behaviour of a population in complying with social restrictions.
Our baseline assumptions were plausible but optimistic. Particularly, we assumed a delay of symptom onset to isolation of 2·6 days in the baseline scenario, and quarantine within 2 days for successfully manually traced contacts and immediately for app-based tracing, with 90% of contacts assumed to adhere to quarantine. For context, on the basis of viral shedding dynamics, onset of infectiousness typically occurs 2–3 days after exposure.6 In our model, we considered self-isolation both within and outside the household, finding that isolation outside household led to slightly higher reduction in onward transmission than within; this reduction was not larger because some pre-symptomatic transmission had often already occurred. However, our conclusions about onwards transmission in the different control tracing scenarios were not dependent on assumptions about household transmission, because in these scenarios, we assumed that household quarantine would be in place too. We also simulated contact patterns at random for each individual in our population, whereas in an outbreak, a correlation between number of contacts and infection risk is likely to occur; individuals with multiple contacts might be more likely to acquire infection and transmit it to others. If this were the case, and we assume the same secondary attack rates, the overall reduction might be lower than we have estimated; however, to keep the baseline R consistent, this correlation would have to be offset by a lower secondary attack rate among contacts. We also did not include the potential for imported infections; when local infection prevalence is low, additional screening or restrictions might need to be considered to reduce the risk of new importations of cases.
Our results highlight the challenges involved in controlling SARS-CoV-2. Consistent with previous modelling studies7, 10 and observed early global outbreak dynamics, our analysis suggests that—depending on the overall effectiveness of testing, tracing, isolation, and quarantine—a combination of self-isolation, contact tracing, and physical distancing might be required to maintain Reff lower than 1. Additionally, in a scenario where incidence is high, a considerable number of individuals might need to be quarantined to achieve control with use of strategies that involve contact tracing.
Acknowledgments
Acknowledgments
AJK was supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant 206250/Z/17/Z). MT was supported by the UK Engineering and Physical Sciences Research Council (grant EP/N509620/1). PK acknowledges support from the Royal Society (0RP\EA\180004). WJE was supported by the Medical Research Council (grant MC_PC_19065). We would like to thank Stephen Eglen for doing an independent CODECHECK on our model code.
Contributors
AJK, PK, and WJE designed the analysis. AJK developed the model. AJK, PK, AJKC, SMK, MT, HF, and JRG contributed to collection, processing, and interpretation of the original BBC Pandemic dataset, as well as interpretation of the study findings. The CMMID COVID-19 working group members contributed to interpretation of the study results. All authors contributed to writing the manuscript and approved the final version.
Declaration of interests
We declare no competing interests.
Contributor Information
CMMID COVID-19 working group:
Jon C Emery, Graham Medley, James D Munday, Timothy W Russell, Quentin J Leclerc, Charlie Diamond, Simon R Procter, Amy Gimma, Fiona Yueqian Sun, Hamish P Gibbs, Alicia Rosello, Kevin van Zandvoort, Stéphane Hué, Sophie R Meakin, Arminder K Deol, Gwen Knight, Thibaut Jombart, Anna M Foss, Nikos I Bosse, Katherine E Atkins, Billy J Quilty, Rachel Lowe, Kiesha Prem, Stefan Flasche, Carl A B Pearson, Rein M G J Houben, Emily S Nightingale, Akira Endo, Damien C Tully, Yang Liu, Julian Villabona-Arenas, Kathleen O'Reilly, Sebastian Funk, Rosalind M Eggo, Mark Jit, Eleanor M Rees, Joel Hellewell, Samuel Clifford, Christopher I Jarvis, Sam Abbott, Megan Auzenbergs, Nicholas G Davies, and David Simons
Supplementary Material
References
- 1.Cereda D, Tirani M, Rovida F. The early phase of the COVID-19 outbreak in Lombardy, Italy. ArXiv. 2020 DOI: 2003.09320 published online March 20. (preprint). [Google Scholar]
- 2.Ng Y, Li Z, Chua YX. Evaluation of the effectiveness of surveillance and containment measures for the first 100 patients with COVID-19 in Singapore—January 2–February 29, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:307–311. doi: 10.15585/mmwr.mm6911e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gudbjartsson DF, Helgason A, Jonsson H. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020 doi: 10.1056/NEJMoa2006100. published online April 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fraser C, Riley S, Anderson RM, Ferguson NM. Factors that make an infectious disease outbreak controllable. Proc Natl Acad Sci USA. 2004;101:6146–6151. doi: 10.1073/pnas.0307506101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abbott S, Hellewell J, Thompson RN. Estimating the time-varying reproduction number of SARS-CoV-2 using national and subnational case counts. Wellcome Open Research. 2020. https://wellcomeopenresearch.org/articles/5-112
- 6.He X, Lau EHY, Wu P. Temporal dynamics in viral shedding and transmissibility of COVID-19. Nat Med. 2020;26:672–675. doi: 10.1038/s41591-020-0869-5. [DOI] [PubMed] [Google Scholar]
- 7.Hellewell J, Abbott S, Gimma A. Feasibility of controlling COVID-19 outbreaks by isolation of cases and contacts. Lancet Glob Health. 2020;8:e488–e496. doi: 10.1016/S2214-109X(20)30074-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiang S, Allen D, Annan-Phan S. The effect of large-scale anti-contagion policies on the coronavirus (COVID-19) pandemic. MedRxiv. 2020 doi: 10.1101/2020.03.22.20040642. published online May 21. (preprint). [DOI] [PubMed] [Google Scholar]
- 9.Ferretti L, Wymant C, Kendall M. Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science. 2020;368 doi: 10.1126/science.abb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keeling MJ, Hollingsworth TD, Read JM. The efficacy of contact tracing for the containment of the 2019 novel coronavirus (COVID-19) MedRxiv. 2020 doi: 10.1101/2020.02.14.20023036. published online Feb 17. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Endo A, CMMID COVID-19 Working Group. Abbott S, Kucharski AJ, Funk S. Estimating the overdispersion in COVID-19 transmission using outbreak sizes outside China. Wellcome Open Res. 2020;5:67. doi: 10.12688/wellcomeopenres.15842.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leclerc QJ, Fuller NM, Knight LE, CMMID COVID-19 Working Group. Funk S, Knight GM. What settings have been linked to SARS-CoV-2 transmission clusters? Wellcome Open Res. 2020;5:83. doi: 10.12688/wellcomeopenres.15889.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klepac P, Kucharski AJ, Conlan AJ. Contacts in context: large-scale setting-specific social mixing matrices from the BBC Pandemic project. MedRxiv. 2020 doi: 10.1101/2020.02.16.20023754. published online March 5. (preprint). [DOI] [Google Scholar]
- 14.Office for National Statistics . UK Data Service; Colchester: 2011. 2011 UK census. [Google Scholar]
- 15.Davies NG, Kucharski AJ, Eggo RM, Gimma A, CMMID COVID-19 Working Group. Edmunds WJ. The effect of non-pharmaceutical interventions on COVID-19 cases, deaths and demand for hospital services in the UK: a modelling study. MedRxiv. 2020 doi: 10.1101/2020.04.01.20049908. published online April 6. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bi Q, Wu Y, Mei S. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30287-5. published online April 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Wang A, Yi B. The epidemiological characteristics of infection in close contacts of COVID-19 in Ningbo city. Chin J Epidemiol. 2020 doi: 10.3760/cma.j.cn112338-20200304-00251. published online March 4. (in Chinese). [DOI] [PubMed] [Google Scholar]
- 18.Davies NG, Klepac P, Liu Y. Age-dependent effects in the transmission and control of COVID-19 epidemics. MedRxiv. 2020 doi: 10.1101/2020.03.24.20043018. published online May 3. (preprint). [DOI] [PubMed] [Google Scholar]
- 19.Abeler J, Altmann S, Milsom L, Toussaert S, Zillessen H. Support for app-based contact tracing of COVID-19. OSFPreprint. 2020 doi: 10.2196/19857. (preprint). published online April 14. DOI: osf.io/3k57r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.nCoV-2019 Data Working Group Epidemiological data from the nCoV-2019 outbreak: early descriptions from publicly available data. 2020. https://github.com/beoutbreakprepared/nCoV2019
- 21.Burke RM, Midgley CM, Dratch A. Active monitoring of persons exposed to patients with confirmed COVID-19—United States, January–February 2020. MMWR Morb Mortal Wkly Rep. 2020;69:245–246. doi: 10.15585/mmwr.mm6909e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luo L, Liu D, Liao X. Modes of contact and risk of transmission in COVID-19 among close contacts. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042606. published online March 26. (preprint). [DOI] [Google Scholar]
- 23.Cheng H-Y, Jian S-W, Liu D-P. Contact tracing assessment of COVID-19 transmission dynamics in Taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.2020. published online May 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing Q-L, Liu M-J, Yuan J. Household secondary attack rate of COVID-19 and associated determinants. MedRxiv. 2020 doi: 10.1101/2020.04.11.20056010. published online April 15. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis CI, Van Zandvoort K, Gimma A. Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18:124. doi: 10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowling BJ, Ali ST, Ng TWY. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. 2020;5:e279–e288. doi: 10.1016/S2468-2667(20)30090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Government of the Republic of Korea . Government of Korea; Seoul: 2020. Flattening the curve on COVID-19: how Korea responded to a pandemic using ICT. [Google Scholar]
- 28.Peak CM, Kahn R, Grad YH. Individual quarantine versus active monitoring of contacts for the mitigation of COVID-19: a modelling study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30361-3. published online May 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brooks-Pollock E, Read JM, House T, Medley G, Keeling MJ, Danon L. The population attributable fraction (PAF) of cases due to gatherings and groups with relevance to COVID-19 mitigation strategies. MedRxiv. 2020 doi: 10.1101/2020.03.20.20039537. published online March 23. (preprint). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.