We report a case of COVID‐19‐induced extracorporeal membrane oxygenation treated by late i.v. steroid administration. Our case suggests the value of prospective clinical trials for the evaluation of steroid use in severe COVID‐19‐induced extracorporeal membrane oxygenation.
Keywords: ARDS, COVID‐19, ECMO, SARS‐CoV‐2, steroid
Abstract
Background
The efficacy of steroid treatment for coronavirus disease (COVID‐19) is unknown.
Case presentation
A 67‐year‐old man was transported to our hospital due to impaired consciousness and respiratory failure. After admission, tracheal aspirate of the patient was harvested, and it tested positive for severe acute respiratory syndrome coronavirus 2 nucleic acid. He required veno‐venous extracorporeal membrane oxygenation to sustain his oxygenation. However, his respiratory failure did not improve for 20 days. On day 20 of admission, we started to use i.v. steroid therapy. On day 23, lung opacity on the chest X‐ray cleared and the patient’s oxygen saturation improved significantly. We successfully removed extracorporeal membrane oxygenation on day 27.
Conclusion
Our case report encourages more future trials to evaluate the therapeutic use of i.v. steroid in severe COVID‐19‐induced acute respiratory distress syndrome.
Introduction
Coronavirus disease (COVID‐19) is caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) and was first reported in Wuhan, China, in December 2019. As of 20 April, 2020, a total of 2,319,066 infected cases and 157,970 fatalities were confirmed worldwide (https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019). Considering the current situation, identifying an effective treatment is urgent and indispensable. Corticosteroid use was not routinely recommended during previous pandemics such as SARS and Middle East respiratory syndrome (MERS). 1 However, Mehta et al. reported that hyperinflammation including elevated interleukin (IL)‐6 could be a driver of severity in COVID‐19. 2 Based on this hypothesis, immunosuppressive therapy involving corticosteroids could be beneficial. We report here a case of severe acute respiratory distress syndrome (ARDS) caused by COVID‐19, successfully treated with late corticosteroids use.
Case Report
A 67‐year‐old man complained of fatigue and loss of appetite. His symptoms gradually worsened for 6 days until he was brought to our emergency room for impaired consciousness. He had a history of smoking, hypertension, and diabetes. Upon arrival at the hospital, his Glasgow Coma Scale score was 11 (E3V3M5) and he was hemodynamically unstable (heart rate, 130 b.p.m.; blood pressure, 80/52 mmHg). The stabilization of hemodynamics was achieved with bolus fluid resuscitation and norepinephrine. He also had respiratory failure with a respiratory rate of 35 breaths/min and oxygen saturation (SpO2) of 87% under reservoir oxygen mask at 10 L/min; hence, he was immediately intubated and mechanically ventilated. Arterial blood gas analysis showed severe metabolic acidosis with pH 6.90 and base excess −22.5, and severe respiratory failure, with partial pressure of arterial oxygen (PaO2) of 85 mmHg and partial pressure of arterial carbon dioxide (PaCO2) of 52 mmHg under fraction of inspired oxygen (FiO2) of 1.0. Chest X‐ray and computed tomography showed extensive consolidation and ground‐glass opacities in the lung fields bilaterally. Laboratory tests indicated acute systemic inflammation and hepatic/renal injury, characterized by elevated white blood cell count, and levels of C‐reaction protein, liver enzymes, lactate dehydrogenase, and creatine.
The patient’s clinical course during hospitalization is shown in Figure 1. After admission, tracheal aspirate of the patient was harvested, and it tested positive for SARS‐CoV‐2 nucleic acid by the fluorescence quantitative reverse transcription–polymerase chain reaction method. He was treated with meropenem (2 g/day), levofloxacin (0.5 g/day), and nafamostat (300 mg/day). Nafamostat was used as a potential inhibitor of SARS‐CoV‐2 infection. 3 Nevertheless, the patient’s respiratory failure continued to be severe, with a PaO2 of 60 mmHg under ventilator setting of FiO2 1.0 and positive end‐expiratory pressure of 15 cmH2O. We initiated veno‐venous extracorporeal membrane oxygenation (VV‐ECMO) on day 2. The procedure was safely carried out with no complications. The devices used for our VV‐ECMO system were CAPIOX Oxygenators (Terumo Instrument, Tokyo, Japan) and HLS Cannulae (draining cannula, 25 Fr 38 cm; reinfusion cannula, 17 Fr 15 cm) (Getinge, Gothenburg, Sweden). The draining cannula was placed in the right internal jugular vein and the reinfusion cannula was placed in the right femoral vein. Initial blood flow was 4.5 L/min with 2000 pump rotations. Continuous renal replacement therapy (blood flow, 100 mL/min; polyethylenimine‐coated polyacrylonitrile (AN69ST) membrane hemofilter) was also initiated on the same day. During ECMO support, we reduced both the tidal volume and the plateau pressure of the ventilator following the lung rest strategy. The patient received deep sedation and occasionally neuromuscular blockade to reduce patient–ventilator desynchrony. We continued ECMO support for 2 weeks; however, his respiratory failure did not improve. From day 16, we initiated prone‐positioning care for 20 h per day, three times a week. The combination of VV‐ECMO and prone positioning is known to improve oxygenation and respiratory compliance without absence of serious adverse events. 4 At least five medical staff members, including physicians and mechanical engineers, were required for the procedure. A physician was in charge for the safety of the location of the tracheal tube and others managed the catheter of the ECMO or the positioning. We followed the suggested protocol for prone positioning under VV‐ECMO. 4 Despite this intensive treatment, oxygen saturation and consolidation on chest X‐ray showed no improvement. On day 20, we decided to use adjunctive steroid therapy to suppress the hyperinflammation of the lung injury; 500 mg methylprednisolone was given for 3 days, followed by 80 mg prednisolone. On day 23, lung opacity on chest X‐ray and oxygen saturation improved significantly (Fig. 2). We could successfully remove ECMO on day 27. We are successfully tapering steroids without serious adverse events, including recurrent respiratory failure, nosocomial infection, or gastrointestinal bleeding. Mild controllable hyperglycemia was observed during the clinical course. The patient was removed from the ventilator on day 51. At the time of publication, he was able to stand up unassisted without any difficulty and continues rehabilitation to discharge to his home.
Fig. 1.
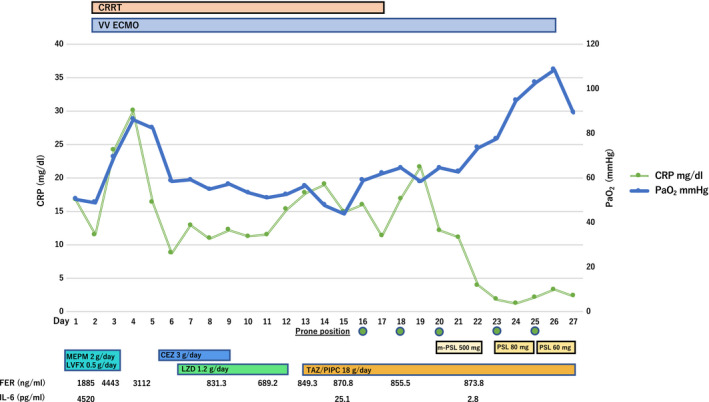
Clinical course of a 67‐year‐old man during hospitalization for severe COVID‐19‐induced acute respiratory distress syndrome. After initiation of corticosteroid treatment on day 20 of hospitalization, partial pressure of arterial oxygen (PaO2) gradually improved and inflammatory marker C‐reaction protein (CRP) decreased. Fraction of inspired oxygen (FiO2) of the ventilator was set at 1.0 before extracorporeal membrane oxygenation (ECMO) deployment. During and after ECMO support, FiO2 was set at 0.4. CEZ, cefazolin; CRP, C‐reaction protein; CRRT, continuous renal replacement therapy; FER, ferritin; IL‐6, interleukin‐6; LVFX, levofloxacin; LZD, linezolid; MEPM, meropenem; m‐PSL, methylprednisolone; PIPC, piperacillin; PSL, prednisolone; TAZ, tazobactam; VV‐ECMO, veno‐venous ECMO.
Fig. 2.
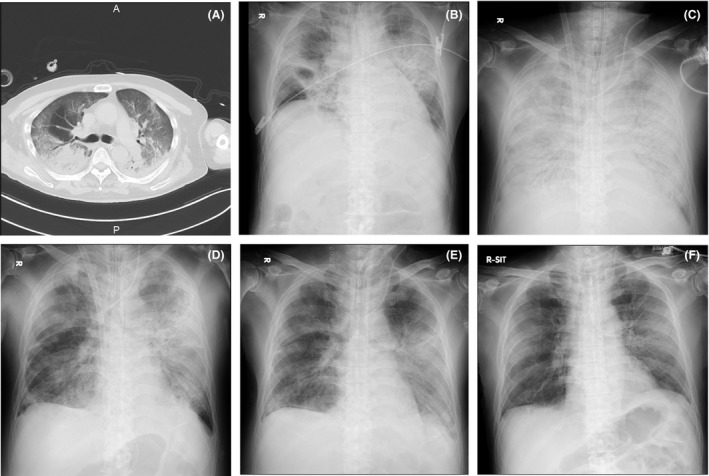
Chest computed tomography (CT) and chest X‐ray images in time series of a 67‐year‐old man during hospitalization for severe COVID‐19‐induced acute respiratory distress syndrome. A, Chest CT on day 1 shows bilateral basal consolidation with peripheral ground‐glass opacity. B, Chest X‐ray on day 1 shows bilateral pulmonary infiltrate mainly in hilar region. C, Chest X‐ray on day 20 shows bilateral diffuse pulmonary infiltrates with air bronchogram. D, Chest X‐ray on day 23 shows improved bilateral lung opacity (mainly in the right lobe of lungs). E, Chest X‐ray on day 31 (extracorporeal membrane oxygenation removed). F, Chest X‐ray on day 52 (ventilator removed).
Discussion
The efficacy of corticosteroids in the treatment of COVID‐19‐induced ARDS is unclear. Some reports discourage corticosteroid treatment in COVID‐19 pneumonia because no clinical benefit was seen in respiratory viral infections including respiratory syncytial virus, influenza, SARS‐CoV, or MERS‐CoV. 1 , 5 , 6 , 7 The available observational data also show the harmful effect of steroids in respiratory viral infections resulting in increased mortality, impaired viral clearance, and secondary infections. 6 , 8
However, corticosteroid treatment might improve the outcome of ARDS. 9 Acute respiratory distress syndrome is partly caused by the host immune response‐induced hyperinflammation, which persists after clearance of the pathogens. 10 In confirmed severe cases of COVID‐19 in the intensive care unit, plasma concentrations of inflammatory cytokines such as IL‐2, IL‐7, IL‐10, and tumor necrosis factor‐α are higher than in non‐intensive care unit patients. 11 Moreover, elevated ferritin and IL‐6 levels were detected in non‐survivors than in survivors among 150 confirmed COVID‐19 cases in Wuhan, China. 12 This evidence supports the assumption that the cytokine storm syndrome resembling hemophagocytic lymphohistiocytosis might be one of the factors leading to poor patient outcomes of COVID‐19. 2 Theoretically, immunosuppressive therapy such as corticosteroids could be beneficial in severe ARDS induced by COVID‐19 with hyperinflammation, although COVID‐19 might not lead to typical ARDS. 13 It should be noted that a very recent randomized controlled trial showed the beneficial effect of dexamethasone treatment on survival outcome in patients with COVID‐19 who were receiving either invasive mechanical ventilation or oxygen alone. 14 However, the proper timing of corticosteroid therapy is still unclear.
We did not decide to use steroids in the earlier phase due to the lack of evidence for the use of steroids in specific COVID‐19‐induced ARDS at that time, even though some guidelines suggested the use of corticosteroids in moderate to severe ARDS patients within 14 days of onset. 15 However, the lung‐rest strategy with ECMO and prone positioning for a certain period did not improve the oxygen saturation of the patient. Although systemic inflammatory markers such as IL‐6 decreased over the time course, chest X‐rays for the patient still showed an exudative appearance. Without clinical signs and laboratory evidence of bacterial infection, we assumed that sustained exudative appearance on chest X‐rays might reflect persisting local lung tissue inflammation. In addition, semiquantitative polymerase chain reaction of tracheal aspirates revealed decreased levels of SARS‐CoV‐2 gene expression over time. Therefore, we hypothesized that adjunctive steroids as potent anti‐inflammatory, antifibrotic, and immunomodulator drugs to suppress the hyperinflammation of lung injury would be beneficial when used during minimized viral load, although this decision is against the evidence reported by Steinberg et al., which showed that corticosteroid use at least 14 days after the onset of ARDS was associated with significantly increased 60‐ and 180‐day mortality rates. 16 The dose of methylprednisolone was determined after discussion among clinicians. Referring to published guidelines by the Society of Critical Care Medicine and European Society of Intensive Care Medicine that suggested the use of methylprednisolone in late ARDS at a higher dose than in early ARDS, 15 we decided to use initial 500 mg methylprednisolone therapy with consideration of the severity of the patients. Although we cannot exclude the possibility that the recovery was due to the natural course of the disease, lung opacity on chest X‐ray and oxygen saturation significantly improved immediately after adjunctive steroid therapy.
In conclusion, this was a case of severe COVID‐19‐induced ARDS that was successfully treated by late administration of i.v. steroids. The current case report boosts more future trials to evaluate the best timing or dose of corticosteroids, as well as selection of the best population for COVID‐19‐induced ARDS with different severity.
Disclosure
Approval of the research protocol: The ethics committee at our institution approved the publication of this case report.
Informed consent: Consent for publication was obtained from the patient’s family.
Registry and registration no. of the study: N/A.
Animal studies: N/A.
Conflict of interest: None.
Acknowledgements
This research was supported by JSPS KAKENHI Grant Number 20H03784. The authors would like to thank physicians, nursing teams, mechanical engineering teams, and all other medical staff at Juntendo University Urayasu Hospital who are fighting against COVID‐19. We would also like to thank Editage for English language editing.
Funding Information
Japan Society for the Promotion of Science : 20H03784
References
- 1. Russell CD, Millar JE, Baillie JK. Clinical evidence does not support corticosteroid treatment for 2019‐nCoV lung injury. Lancet 2020; 395: 473–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020; 395: 1033–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Asakura H, Ogawa H. Potential of heparin and nafamostat combination therapy for COVID‐19. J. Thromb. Haemost. 2020; 18: 1521–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kimmoun A, Roche S, Bridey C, et al Prolonged prone positioning under VV‐ECMO is safe and improves oxygenation and respiratory compliance. Ann. Int. Care 2015; 5: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arabi YM, Mandourah Y, Al‐Hameed F, et al Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018; 197: 757–67. [DOI] [PubMed] [Google Scholar]
- 6. Ni YN, Chen G, Sun J, Liang BM, Liang ZA. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta‐analysis. Crit. Care 2019; 23: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Medicine 2006; 3: e343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Arabi YM, Balkhy HH, Hayden FG, et al Middle East Respiratory Syndrome. N. Engl. J. Med. 2017; 376: 584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meduri GU, Bridges L, Shih MC, Marik PE, Siemieniuk RAC, Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients' data from four randomized trials and trial‐level meta‐analysis of the updated literature. Intensive Care Med. 2016; 42: 829–40. [DOI] [PubMed] [Google Scholar]
- 10. Peiris JS, Chu CM, Cheng VC, et al Clinical progression and viral load in a community outbreak of coronavirus‐associated SARS pneumonia: a prospective study. Lancet 2003; 361: 1767–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang C, Wang Y, Li X, et al Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020; 46: 846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID‐19 does not lead to a "Typical" acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020; 201: 1299–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Horby P, Lim WS, Emberson JR, et al Dexamethasone in Hospitalized Patients with Covid‐19 ‐ Preliminary Report. N. Engl. J. Med. 2020. 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Annane D, Pastores SM, Rochwerg B, et al Guidelines for the diagnosis and management of critical illness‐related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017; 43: 1751–63. [DOI] [PubMed] [Google Scholar]
- 16. Steinberg KP, Hudson LD, Goodman RB, et al Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. N. Engl. J. Med. 2006; 354: 1671–84. [DOI] [PubMed] [Google Scholar]


