Abstract
We reviewed the current status and future perspectives regarding the role of surgery in multidisciplinary treatment strategies for locally advanced esophageal squamous cell carcinoma (ESCC). The treatment and management of ESCC have been improved by dramatic advances in diagnostic techniques and the development of surgery, chemotherapy, radiotherapy, and immunotherapy. The current standard treatment for locally advanced ESCC is preoperative chemotherapy followed by surgery in Japan, whereas preoperative chemoradiotherapy is a globally recommended approach. Differences of recognition regarding the role for surgery between Japan and many Western countries may have created peculiar preferences for preoperative therapy. The clinical significance of conversion strategy and salvage surgery for patients with ESCC should be further evaluated in terms of curability and safety. Although strategies to identify patients who would benefit from preoperative therapy are strongly required to avoid performing unnecessary treatment, it remains difficult to predict the efficacy of preoperative therapy prior to treatment. Prospective clinical trials and basic research to identify predictive biomarkers for response to chemotherapy, radiotherapy, and immunotherapy are needed to promote the development of multidisciplinary treatment strategies for patients with ESCC.
Keywords: chemotherapy, esophageal cancer, immunotherapy, radiotherapy, surgery
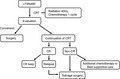
In this review, we summarized the recent development and future perspectives of multidisciplinary therapy for locally advanced esophageal squamous cell carcinoma with a focus on the role of surgery. The treatment strategies were categorized into perioperative treatment (chemotherapy and chemoradiotherapy), conversion surgery, salvage surgery, and immunotherapy as a novel treatment strategy. In particular, we focused on the difference in recognition regarding the role of surgery in multidisciplinary treatment strategy between Japan and many Western countries.
1. INTRODUCTION
Esophageal cancer is the sixth leading cause of death from cancer and eighth most common cancer in the world. 1 Esophageal squamous cell carcinoma (ESCC) is the predominant histological type of esophageal cancer worldwide, and it accounts for approximately 90% of all cases in Japan. 2 Conversely, adenocarcinoma of the esophagus is the most common type in many Western countries. 1 The remarkable variation in the prevalence of these histological types in different regions means each area has particular environmental risk factors for esophageal carcinogenesis. 3 This has therefore encouraged us to attempt to understand the cancer biology and clinical features of each histological type.
The treatment and management of ESCC have evolved in recent years, with dramatic advances in diagnostic techniques and the development of surgery, chemotherapy, radiotherapy, and immunotherapy. 4 With a curative intent, esophagectomy with radical mediastinal lymph node dissection remains the standard therapy. 5 , 6 However, the systemic nature of this disease is often responsible for the failure of surgery alone. Thus, a better understanding of the current status for multimodal therapy should provide us with valuable clues to improve the treatment of ESCC.
In this review, we summarized the recent development and future perspectives of multidisciplinary therapy for locally advanced ESCC with a focus on the role of surgery. This review was categorized into perioperative treatment (chemotherapy and chemoradiotherapy [CRT]), conversion surgery, salvage surgery, and immunotherapy as a novel treatment strategy.
2. PERIOPERATIVE THERAPY
2.1. Chemotherapy
There remains much controversy regarding the current standard of care for patients with potentially resectable locally advanced ESCC. Many trials on postoperative or preoperative therapy have been conducted to investigate the survival benefit of various strategies (Table 1).
Table 1.
Prospective randomized trials of perioperative therapy for locally advanced esophageal squamous cell carcinoma
| Study | Author | Year | Histology | Treatment | n | Five‐year survival rate | P‐value |
|---|---|---|---|---|---|---|---|
| JCOG9204 7 | Ando et al | 2003 | SCC | Post‐CF | 120 | 55% (DFS) | .037 |
| Surgery alone | 122 | 45% (DFS) | |||||
| OEO2 9 | Allum et al | 2009 | AC/SCC | Pre‐CF | 320 | 23.0% (OS) | .03 |
| 66.5%/30.8% | Surgery alone | 335 | 17.1% (OS) | ||||
| JCOG9907 10 | Ando et al | 2012 | SCC | Pre‐CF | 164 | 55% (OS) | .04 |
| Post‐CF | 166 | 43% (OS) | |||||
| NCT012255231 3 | Zhao et al | 2015 | SCC | Peri‐PCF | 175 | 31% (RFS) | <.001 |
| Pre‐PCF | 171 | 17% (RFS) | |||||
| TROG 14 | Burmeister et al | 2005 | AC/SCC | Pre‐CRT | 128 | 22.2 mo (median OS) | NS |
| 62%/37% | Surgery alone | 128 | 19.3 mo (median OS) | ||||
| CROSS 15 | van Hagen et al | 2012 | AC/SCC | Pre‐CRT | 178 | 47% (OS) | .003 |
| 75%/23% | Surgery alone | 188 | 34% (OS) | ||||
| JCOG1109 16 (ongoing) | ‐ | ‐ | SCC | Pre‐CF | ‐ | ‐ | |
| Pre‐DCF | ‐ | ‐ | |||||
| Pre‐CRT | ‐ | ‐ |
Abbreviations: AC, adenocarcinoma; DFS, disease‐free survival; NS, not significant; OS, overall survival; Peri‐PCF, perioperative paclitaxel + CDDP +5‐FU; Post‐CF, postoperative CDDP + 5‐FU; Pre‐CF, preoperative CDDP + 5‐FU; Pre‐CRT, preoperative chemoradiotherapy; Pre‐DCF, preoperative docetaxel + CDDP +5‐FU; Pre‐PCF, preoperative paclitaxel + CDDP +5‐FU; RFS, relapse‐free survival; SCC, squamous cell carcinoma.
Concerning postoperative chemotherapy, the JCOG9204 trial, which compared surgery alone vs surgery followed by postoperative chemotherapy using the cisplatin plus 5‐fluorouracil (CF) regimen, was performed in Japan. This trial revealed that postoperative CF therapy extended the disease‐free survival of patients with node‐positive cStage II/III ESCC. 7 A meta‐analysis including three randomized controlled trials and six retrospective studies supported that surgery followed by postoperative chemotherapy (compared with surgery alone) was as an independent favorable prognostic factor for ESCC. 8
Preoperative approaches have been considered for patients with locally advanced disease for the purpose of down‐staging disease to allow definite resection and improving long‐term prognosis. Additionally, treatment efficacy can be evaluated preoperatively by monitoring clinical responses and postoperatively via pathologic evaluation of the resected tumor tissue. This allows more tailored treatment because therapy can be modified or changed in patients who did not respond to the preoperative therapy. Another merit of preoperative chemotherapy is the higher dose intensity of chemotherapeutic agents relative to that permissible during postoperative chemotherapy because esophagectomy for ESCC usually is highly invasive. The OEO2 trial conducted by the Medical Research Council in the UK suggested that preoperative CF therapy in comparison with surgery alone had a significant survival benefit with a hazard ratio (HR) of 0.84. The treatment effect was also demonstrated to be consistent in both adenocarcinoma and ESCC. 9 JCOG9907, a randomized trial of patients with cStageII/III ESCC (excluding T4), demonstrated that preoperative CF therapy followed by surgery improved overall survival (OS) compared with the effects of postoperative CF therapy. 10 In this study, the planned interim analysis was conducted after the completion of patient accrual. Although progression‐free survival (PFS), the primary endpoint of this study, did not reach the stopping boundary, OS in the preoperative group was superior to that of postoperative group (P = .01). Updated analyses demonstrated that the 5‐year OS rate was 43% in the postoperative group vs 55% in the preoperative group (HR = 0.73, P = .04). Based on the results, preoperative CF therapy is currently the standard treatment for cStage II/III ESCC in Japan. Of note, for patients who undergo upfront surgery, postoperative CF is recommended if the pathologic examination reveals lymph node metastasis. 11
Although JCOG 9907 demonstrated that preoperative CF therapy improved OS, down‐staging and R0 resection were reported to be less beneficial in Stage III patients than in Stage II patients in a subgroup analysis. This suggests that a more powerful preoperative treatment may be necessary for patients with locally advanced ESCC. Thus, the JCOG1109 (NExT study) trial, a three‐arm phase III trial, was started to confirm the superiority of docetaxel and cisplatin plus 5‐fluorouracil (DCF) over CF and of CRT over CF as preoperative therapy for ESCC. 12 This trial is currently ongoing to identify the optimal preoperative treatment regimen for ESCC.
Whether preoperative chemotherapy alone is sufficient for locally advanced ESCC is the next clinical question. Zhao et al assessed a perioperative (pre‐ and postoperative) regimen of paclitaxel, cisplatin, and 5‐fluorouracil for patients with curable ESCC in comparison with preoperative chemotherapy alone. 13 Among 346 patients, the perioperative chemotherapy group had higher 5‐year relapse‐free survival (RFS) and OS rates without an increased risk of severe toxic events. Thus, assessments of perioperative therapeutic strategy combined with other treatment regimens, as well as studies in other countries, are necessary.
3. CRT
In many Western countries, CRT has been preferably administered to patients with ESCC to achieve preoperative down‐staging because surgeons assume that CRT is more powerful than chemotherapy alone concerning local tumor control.
Burmeister et al reported that preoperative CRT consisting of 35 Gy of radiation and CF therapy did not significantly improve OS in comparison with surgery alone. 14 However, subgroup analysis indicated that patients with ESCC had better histological complete response and PFS rates than those with adenocarcinoma. In the CROSS study, preoperative CRT (carboplatin, paclitaxel, and radiation plus 41.4 Gy of radiation) improved survival among patients with potentially curable esophageal or esophagogastric junction cancer. The median OS was 49 months in the preoperative CRT group, vs 24 months in the surgery alone group, and moreover, the benefit of preoperative CRT was confirmed in an ESCC subgroup analysis. 15 Some meta‐analyses have emphasized the superiority of preoperative CRT followed by surgery over surgery alone, whereas other reports did not identify differences in outcomes between the approaches. A meta‐analysis conducted by Gebski et al indicated that the HR for all‐cause mortality for preoperative CRT vs surgery alone was 0.81, and the results were similar among different histological types, namely 0.84 for ESCC and 0.75 for adenocarcinoma. 16 Two meta‐analyses demonstrated that preoperative CRT improves the pathological response rate, local and regional control rate, and OS compared with the outcomes for surgery alone. 17 , 18 Another meta‐analysis reported that preoperative CRT is associated with a small, statistically insignificant improvement in OS. 19 In a meta‐analysis of nine randomized trials, preoperative CRT provided a clearly significant survival benefit to patients with ESCC. 20 The results appear reasonable because some studies have emphasized that histological complete response was more common in patients with ESCC than in those with adenocarcinoma. 14 These meta‐analyses support the survival benefits of preoperative CRT for ESCC. Differences in the odds ratios are probably attributable to differences in the collection of randomized trials.
In terms of long‐term survival after preoperative CRT for ESCC, the response to preoperative CRT has been reported to be the most important factor. 21 , 22 , 23 Swisher et al emphasized that the pathological response is an independent factor for survival and proposed a revision of the esophageal cancer staging system to accommodate pathological responses following preoperative CRT. 21 It was also reported that non‐responders to preoperative CRT experienced no benefit and even worse outcomes than those undergoing primary resection for locally advanced ESCC. 24 Active surveillance after the completion of preoperative CRT for esophageal cancer is currently being assessed in a phase III randomized controlled trial (SANO trial; NTR6803). 25 In this trial, surgical resection is being offered only to patients in whom locoregional regrowth without distant dissemination is highly suspected or proven. The ESOSTRATE trial is also comparing active surveillance with standard surgery in patients with clinical complete responses after preoperative CRT (NCT02551458). Such an organ‐preserving strategy can have great advantages, but it is only justified if its long‐term survival is non‐inferior to that of preoperative CRT followed by surgery.
The European Society for Medical Oncology guidelines 26 describe that patients with locally advanced ESCC benefit from preoperative chemotherapy or they are most likely to benefit to a greater extent from preoperative CRT, with higher rates of complete tumor resection and better local tumor control and survival. The CROSS regimen can be recommended as a contemporary standard of care. Preoperative CRT is also a recommended approach for advanced ESCC according to the National Comprehensive Cancer Network guidelines (version 2. 2018), and both the CROSS and FOLFOX 27 regimens are preferred. The PROTECT trial is an ongoing randomized phase II trial comparing two preoperative CRT regimens (CROSS vs FOLFOX) in patients with resectable ESCC or adenocarcinoma. 28
4. CONVERSION SURGERY
The term “conversion surgery” is generally used to describe surgical treatment with a curative intent for tumors that responded to preceding therapy after initially being deemed technically or oncologically unresectable or only marginally resectable. Although conversion therapy is commonly considered for patients with colorectal cancer, the clinical significance of this strategy for patients with ESCC remains unclear.
Esophageal cancers have an increased tendency of invasion to adjacent organs, such as the trachea, bronchus, aorta, and lungs, because of its anatomical location and the lack of serosa in the esophageal wall. A phase II trial investigating the safety and efficacy of induction chemotherapy using DCF and subsequent conversion surgery for initially unresectable locally advanced ESCC was performed in Japan. 29 In the protocol, DCF therapy was followed by conversion surgery if the lesion was resectable or by CRT if the lesion was unresectable. Conversion surgery was performed in 41.7% of patients, and R0 resection was achieved in 39.6% of patients. The 1‐year OS rate of the enrolled patients was 67.9%. These results imply that induction DCF therapy followed by conversion surgery is a promising strategy for patients with locally advanced, unresectable ESCC. A phase III trial comparing this strategy vs definitive CRT plus salvage surgery (JCOG1510) is currently underway. 30 CRT for clinical stage T4b (cT4b) esophageal cancer ideally aims to achieve complete response, but it is mostly performed to control local invasion before the subsequent treatment. The prognosis of unresectable cT4b esophageal cancer with surrounding invasion is markedly poor, and usually CRT is the initial treatment for these patients. The tumors are usually considered unresectable if peripheral invasion is not resolved by CRT. The treatment strategy for cT4b esophageal cancer without distant organ metastasis remains under debate.
Our principle for treating cT4b patients is as follows. CRT is performed for patients with unresectable tumors invading other organs without distant organ metastasis as an initial treatment. Following 40 Gy of radiation, patients are evaluated to determine whether peripheral invasion has been resolved and whether R0 surgical resection is feasible. If curative resection is deemed possible and the patient is assumed to tolerate surgery, then conversion surgery is considered. We perform conversion surgery after an interval of approximately 4 weeks and after obtaining adequate informed consent. Conversely, if curative resection is impossible because of insufficient tumor reduction or because the patient declines surgery, we continue CRT to 60 Gy.
We previously evaluated 147 patients with cT4b ESCC without distant organ metastasis at our institute between 1997 and 2016. Among them, 43 patients underwent curative resection of the tumor at the midterm evaluation, 104 patients continued with definitive CRT (dCRT), and salvage surgery was performed for 21 patients. Multivariate analysis for disease‐specific survival illustrated that response at the midterm evaluation and surgical intervention (conversion or salvage surgery) were significantly good prognostic factors. We therefore concluded that surgery could be a useful option for eligible patients after carefully considering the risks and proper timing. 31
5. SALVAGE SURGERY
dCRT is generally administered for obvious T4b (non‐resectable) esophageal cancer as a standard treatment. 32 , 33 Following the results of RTOG85‐01, 34 dCRT including radiotherapy with a total dose of 50 Gy or more plus CF therapy was established as a treatment strategy for advanced esophageal cancer. In addition, dCRT is sometimes preferred for potentially resectable, locally advanced esophageal cancer to avoid the risk of esophagectomy. 35 In the JCOG9906 study, the median survival time after dCRT for Stage II/III ESCC was 29 months with manageable acute toxicities. 36 Although the survival data are inferior compared with those of standard preoperative CF therapy following surgery, this option is valuable from the perspective of preserving the esophagus. However, CRT alone achieves complete response in only 40% of patients, and residual disease and posttreatment regrowth are major problems. 34 Many of these patients are at increased risk during salvage esophagectomy because they completed prior radiation treatment at high doses. 37 , 38
Salvage endoscopic submucosal dissection is recommended if target lesions are diagnosed as intramucosal or submucosal tumors without metastases. 39 Although salvage surgery is considered for patients with more advanced tumors, the indication should be decided on the basis of both resectability and the patient's general condition. Many previous reports concerning salvage surgery reported higher postoperative morbidity and mortality rates. 40 , 41 Our previous study indicated that recurrence rather than residual tumor after dCRT was a favorable indicator for salvage esophagectomy. 42 Tachimori et al recommended a technical artifice for the prevention of tracheal necrosis, preservation of right bronchial artery, and omission of cervical lymphadenectomy to preserve blood supply from the inferior thyroid artery. 43
Contrarily, some studies reported low mortality rates after salvage surgery, 44 , 45 suggesting that advances in surgical techniques and perioperative management are improving postoperative outcomes. Kumagai et al found that salvage surgery offers significant benefits regarding long‐term survival compared with the effects of second‐line CRT, although salvage surgery carries the potential risk of high treatment‐related mortality. 46 At present, a non‐randomized validation study for dCRT with or without salvage surgery is being conducted for Stage II/III esophageal cancer, excluding T4 (JCOG0909). The results of this study will influence the future treatment strategy for recurrent or residual esophageal cancer.
6. IMMUNOTHERAPY
Immune checkpoint inhibitors targeting the PD‐1/PD‐L1 pathway have revolutionarily changed the treatment landscape for many different cancers. Concerning ESCC, a single‐arm, multicenter phase II trial (ATTRACTION‐1) was first undertaken to assess the activity of nivolumab, a human IgG4 monoclonal antibody against PD‐1, after the failure of fluoropyrimidine‐, platinum‐, and taxane‐based chemotherapies. 47 Nivolumab exhibited promising anti‐tumor efficacy with an objective response rate of 17%, and median OS was remarkable (10.8 months) with some patients achieving durable responses. Next, in the randomized, open‐label phase III ATTRACTION‐3 trial, nivolumab and chemotherapy were compared in patients with advanced ESCC refractory or who were intolerant to previous fluoropyrimidine‐based and platinum‐based chemotherapy. 48 The results revealed that treatment with nivolumab was associated with significant improvement in median OS (10.9 months vs 8.4 months) and a favorable safety profile vs chemotherapy in previously treated patients with advanced ESCC. The survival benefit of nivolumab was not affected by patients’ tumor PD‐L1 expression levels. Based on these results, nivolumab was recommended as a new standard second‐line treatment option for patients with advanced ESCC. The phase 3 KEYNOTE‐181 trial was conducted to compare pembrolizumab (a humanized IgG4 monoclonal antibody against PD‐1) with chemotherapy as a second‐line treatment in patients with esophageal and esophagogastric junction cancer, including ESCC. 49 The superiority of pembrolizumab over chemotherapy in terms of OS was demonstrated in patients with PD‐L1–positive tumors. These results suggest that pembrolizumab is a new therapeutic option for second‐line treatment in patients with PD‐L1–positive esophageal cancer.
Irradiation can upregulate PD‐L1 expression in human ESCC cells, implying that radiotherapy may positively enhance the therapeutic efficacy of immunotherapy. 50 Therefore, a number of clinical trials are underway to study the effect of radiotherapy combined with immune checkpoint blockade in patients with ESCC. A phase II trial is scheduled to evaluate the benefit of preoperative CRT plus pembrolizumab followed by surgery in patients with ESCC, and the study is expected to be completed in 2022 (NCT02844075). A multicenter phase I/II trial of CRT combined with nivolumab in the treatment of locally advanced ESCC is ongoing (NCT03278626). A study evaluating concurrent treatment with pembrolizumab and CRT as a preoperative therapeutic strategy for locally advanced esophageal cancer is also ongoing (NCT03064490).
Immune checkpoint blockade is also being studied in the adjuvant setting. A double‐blind phase III trial (EORTC18071) was the first trial to test immune checkpoint inhibitors in the adjuvant setting. In the study, 951 patients with stage III cutaneous melanoma following adequate lymph node resection were assigned to receive ipilimumab or placebo. 51 Patients who received adjuvant ipilimumab experienced significantly improved RFS. 51 In ESCC, a phase III investigational study of nivolumab or placebo in participants with resected esophageal or esophagogastric junction cancer (CheckMate 577) is underway (NCT02743494). With the rapid progress made in cancer immunotherapy in the metastatic and adjuvant setting, there has also been increasing interest in applying immune checkpoint blockade in the preoperative setting. A feasibility phase I trial of nivolumab with preoperative CF or DCF therapy for locally advanced esophageal carcinoma is ongoing (NCT03914443). Representative prospective trials of immunotherapy for ESCC are summarized in Table 2.
Table 2.
Prospective trials of immunotherapy for esophageal squamous cell carcinoma
| Study | Author | Year | Histology | Therapy line/Phase | Treatment | n | Median OS (months) | P‐value |
|---|---|---|---|---|---|---|---|---|
| ATTRACTION‐3 48 | Kato et al | 2019 | SCC | 2nd line/Phase 3 | Nivolumab vs chemotherapy | 419 | 10.9 vs. 8.4 | .019 |
| KEYNOTE‐181 49 | Metges et al | 2019 | AC/SCC | 2nd line/Phase 3 | Pembrolizumab vs chemotherapy | 628 | (All patients) | |
| 8.2 vs. 7.1 | 0.0095 | |||||||
| NCT02844075 (ongoing) | ‐ | ‐ | SCC | Preoperative/Phase 2 | Pembrolizumab with CRT | ‐ | ‐ | ‐ |
| NCT03278626 (ongoing) | ‐ | ‐ | SCC | 1st line/Phase 1, 2 | Nivolumab with CRT | ‐ | ‐ | ‐ |
| NCT03064490 (ongoing) | ‐ | ‐ | AC/SCC | Preoperative/Phase 2 | Pembrolizumab with CRT | ‐ | ‐ | ‐ |
| plus postoperative pembrolizumab | ||||||||
| NCT02743494 (ongoing) | ‐ | ‐ | AC/SCC | Postoperative/Phase 3 | Nivolumab vs placebo | ‐ | ‐ | ‐ |
| NCT03914443 (ongoing) | ‐ | ‐ | SCC | Preoperative/Phase 1 | Nivolumab with chemotherapy | ‐ | ‐ |
Abbreviations: AC, adenocarcinoma; CRT, chemoradiotherapy; OS, overall survival; SCC, squamous cell carcinoma.
7. COMMENTS
Esophagectomy with three‐field lymph node dissection has been proposed for decades, and it is currently the main surgical procedure for esophageal cancer in Japan. Although many studies have demonstrated its survival benefit, 52 , 53 this aggressive operation is not widely used globally because of its complicated surgical techniques and high invasiveness. Japanese surgeons generally believe that surgery is adequate for local control; thus, they are skeptical about the necessity of preoperative (chemo) radiotherapy. Conversely, preoperative chemotherapy is usually understood to play a role in the control of micrometastasis but not local tumor control. Differences in recognition regarding the role of surgery between Japan and most Western countries may have created peculiar preferences for preoperative therapy, i.e. chemotherapy or chemoradiotherapy.
The possible risk of increased postoperative complications after preoperative therapy should be also discussed because patients are in a potentially immunosuppressive condition after preoperative therapy. 54 In particular, preoperative CRT for esophageal cancer has been reported to increase the incidence of postoperative complications. 55 , 56 In terms of postoperative mortality, individual studies revealed no increases in mortality rates after preoperative CRT followed by surgery compared with those after surgery alone. However, a subgroup analysis of preoperative CRT for ESCC in a meta‐analysis based on 23 relevant studies suggested increased risks of total postoperative mortality and treatment‐related mortality. 57 The significance of preoperative CRT for potentially resectable ESCC differs from that of conversion surgery or salvage surgery. It is reported that the development of postoperative complications is an independent disease‐specific poor prognostic factor after curative resection for patients with ESCC. 58 We should pay especially close attention to safety in patients who receive preoperative CRT.
The worldwide discrepancy observed in the results of preoperative therapy for ESCC may be partly explained by heterogeneous tumor biology in the patient population. Therefore, it is essential to improve the accuracy of preoperative molecular diagnostics to identify specific patients who will benefit from this treatment. One approach for identifying biomarkers for predicting the efficacy of multidisciplinary therapy is to use molecular biology assessments for particular tumor characteristics. 59 We recently identified positive RAD51 expression as a useful biomarker for predicting resistance to preoperative therapy and poor prognosis in patients who received preoperative CRT for ESCC based on a large‐cohort, retrospective, observational study. 60 Both clinical trials and basic research are ongoing to develop treatment strategies for locally advanced ESCC. We hope that significant evidence will be produced from well‐designed clinical and basic research studies in the near future.
DISCLOSURE
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
ACKNOWLEDGEMENTS
We thank Joe Barber Jr, PhD, from Edanz Group (https://en‐author‐services.edanzgroup.com/) for editing a draft of this manuscript.
Saeki H, Sohda M, Sakai M, Sano A, Shirabe K. Role of surgery in multidisciplinary treatment strategies for locally advanced esophageal squamous cell carcinoma. Ann Gastroenterol Surg. 2020;4:490–497. 10.1002/ags3.12364
REFERENCES
- 1. Pennathur A, Gibson MK, Jobe BA, et al. Oesophageal carcinoma. Lancet. 2013;381:400–12. [DOI] [PubMed] [Google Scholar]
- 2. Tachimori Y, Ozawa S, Numasaki H, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus. 2019;16:221–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hongo M, Nagasaki Y, Shoji T. Epidemiology of esophageal cancer: Orient to Occident. Effects of chronology, geography and ethnicity. J Gastroenterol Hepatol. 2009;24:729–35. [DOI] [PubMed] [Google Scholar]
- 4. Watanabe M, Otake R, Kozuki R, et al. Recent progress in multidisciplinary treatment for patients with esophageal cancer. Surg Today. 2020;50:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fujita H, Sueyoshi S, Tanaka T, et al. Three‐field dissection for squamous cell carcinoma in the thoracic esophagus. Ann Thorac Cardiovasc Surg. 2002;8:328–35. [PubMed] [Google Scholar]
- 6. Tachimori Y, Nagai Y, Kanamori N, et al. Pattern of lymph node metastases of esophageal squamous cell carcinoma based on the anatomical lymphatic drainage system. Dis Esophagus. 2011;24:33–8. [DOI] [PubMed] [Google Scholar]
- 7. Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study–JCOG9204. J Clin Oncol. 2003;21:4592–6. [DOI] [PubMed] [Google Scholar]
- 8. Zhao P, Yan W, Fu H, et al. Efficacy of postoperative adjuvant chemotherapy for esophageal squamous cell carcinoma: A meta‐analysis. Thorac Cancer. 2018;9:1048–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Allum WH, Stenning SP, Bancewicz J, et al. Long‐term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7. [DOI] [PubMed] [Google Scholar]
- 10. Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5‐fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74. [DOI] [PubMed] [Google Scholar]
- 11. Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nakamura K, Kato K, Igaki H, et al. Three‐arm phase III trial comparing cisplatin plus 5‐FU (CF) versus docetaxel, cisplatin plus 5‐FU (DCF) versus radiotherapy with CF (CF‐RT) as preoperative therapy for locally advanced esophageal cancer (JCOG1109, NExT study). Jpn J Clin Oncol. 2013;43:752–5. [DOI] [PubMed] [Google Scholar]
- 13. Zhao Y, Dai Z, Min W, et al. Perioperative versus Preoperative Chemotherapy with Surgery in Patients with Resectable Squamous Cell Carcinoma of Esophagus: A Phase III Randomized Trial. J Thorac Oncol. 2015;10:1349–56. [DOI] [PubMed] [Google Scholar]
- 14. Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–68. [DOI] [PubMed] [Google Scholar]
- 15. van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84. [DOI] [PubMed] [Google Scholar]
- 16. Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta‐analysis. Lancet Oncol. 2007;8:226–34. [DOI] [PubMed] [Google Scholar]
- 17. Urschel JD, Vasan H. A meta‐analysis of randomized controlled trials that compared neoadjuvant chemoradiation and surgery to surgery alone for resectable esophageal cancer. Am J Surg. 2003;185:538–43. [DOI] [PubMed] [Google Scholar]
- 18. Fiorica F, Di Bona D, Schepis F, et al. Preoperative chemoradiotherapy for oesophageal cancer: a systematic review and meta‐analysis. Gut. 2004;53:925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Greer SE, Goodney PP, Sutton JE, et al. Neoadjuvant chemoradiotherapy for esophageal carcinoma: a meta‐analysis. Surgery. 2005;137:172–7. [DOI] [PubMed] [Google Scholar]
- 20. Kranzfelder M, Schuster T, Geinitz H, et al. Meta‐analysis of neoadjuvant treatment modalities and definitive non‐surgical therapy for oesophageal squamous cell cancer. Br J Surg. 2011;98:768–83. [DOI] [PubMed] [Google Scholar]
- 21. Swisher SG, Hofstetter W, Wu TT, et al. Proposed revision of the esophageal cancer staging system to accommodate pathologic response (pP) following preoperative chemoradiation (CRT). Ann Surg. 2005;241: 810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berger AC, Farma J, Scott WJ, et al. Complete response to neoadjuvant chemoradiotherapy in esophageal carcinoma is associated with significantly improved survival. J Clin Oncol. 2005;23:4330–7. [DOI] [PubMed] [Google Scholar]
- 23. Saeki H, Morita M, Tsuda Y, et al. Multimodal treatment strategy for clinical T3 thoracic esophageal cancer. Ann Surg Oncol. 2013;20:4267–73. [DOI] [PubMed] [Google Scholar]
- 24. Hsu PK, Chien LI, Huang CS, et al. Comparison of survival among neoadjuvant chemoradiation responders, non‐responders and patients receiving primary resection for locally advanced oesophageal squamous cell carcinoma: does neoadjuvant chemoradiation benefit all? Interact Cardiovasc Thorac Surg. 2013;17:460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Noordman BJ, Wijnhoven BPL, Lagarde SM, et al. Neoadjuvant chemoradiotherapy plus surgery versus active surveillance for oesophageal cancer: a stepped‐wedge cluster randomised trial. BMC Cancer. 2018;18:142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2016;27:v50–v57. [DOI] [PubMed] [Google Scholar]
- 27. Conroy T, Galais MP, Raoul JL, et al. Definitive chemoradiotherapy with FOLFOX versus fluorouracil and cisplatin in patients with oesophageal cancer (PRODIGE5/ACCORD17): final results of a randomised, phase 2/3 trial. Lancet Oncol. 2014;15:305–14. [DOI] [PubMed] [Google Scholar]
- 28. Messager M, Mirabel X, Tresch E, et al. Preoperative chemoradiation with paclitaxel‐carboplatin or with fluorouracil‐oxaliplatin‐folinic acid (FOLFOX) for resectable esophageal and junctional cancer: the PROTECT‐1402, randomized phase 2 trial. BMC Cancer. 2016;16:318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5‐fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Terada M, Hara H, Daiko H, et al. Phase III study of tri‐modality combination therapy with induction docetaxel plus cisplatin and 5‐fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous‐cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol. 2019;49:1055–60. [DOI] [PubMed] [Google Scholar]
- 31. Sohda M, Miyazaki T, Sakai M, et al. Multidisciplinary therapy for locally advanced oesophageal cancer with special reference to surgical conversion and salvage. Anticancer Res. 2019;39:3167–75. [DOI] [PubMed] [Google Scholar]
- 32. Kaneko K, Ito H, Konishi K, et al. Definitive chemoradiotherapy for patients with malignant stricture due to T3 or T4 squamous cell carcinoma of the oesophagus. Br J Cancer. 2003;88:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Crosby TD, Brewster AE, Borley A, et al. Definitive chemoradiation in patients with inoperable oesophageal carcinoma. Br J Cancer. 2004;90:70–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cooper JS, Guo MD, Herskovic A, et al. Chemoradiotherapy of locally advanced esophageal cancer: long‐term follow‐up of a prospective randomized trial (RTOG 85–01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- 35. Teoh AY, Chiu PW, Yeung WK, et al. Long‐term survival outcomes after definitive chemoradiation versus surgery in patients with resectable squamous carcinoma of the esophagus: results from a randomized controlled trial. Ann Oncol. 2013;24:165–71. [DOI] [PubMed] [Google Scholar]
- 36. Kato K, Muro K, Minashi K, et al. Phase II study of chemoradiotherapy with 5‐fluorouracil and cisplatin for Stage II‐III esophageal squamous cell carcinoma: JCOG trial (JCOG 9906). Int J Radiat Oncol Biol Phys. 2011;81:684–90. [DOI] [PubMed] [Google Scholar]
- 37. Sakuraba M, Kimata Y, Hishinuma S, et al. Importance of additional microvascular anastomosis in esophageal reconstruction after salvage esophagectomy. Plast Reconstr Surg. 2004;113:1934–9. [DOI] [PubMed] [Google Scholar]
- 38. Urschel JD, Sellke FW. Complications of salvage esophagectomy. Med Sci Monit. 2003;9:RA173‐80. [PubMed] [Google Scholar]
- 39. Takeuchi M, Kobayashi M, Hashimoto S, et al. Salvage endoscopic submucosal dissection in patients with local failure after chemoradiotherapy for esophageal squamous cell carcinoma. Scand J Gastroenterol. 2013;48:1095–101. [DOI] [PubMed] [Google Scholar]
- 40. Nishimura M, Daiko H, Yoshida J, et al. Salvage esophagectomy following definitive chemoradiotherapy. Gen Thorac Cardiovasc Surg. 2007;55: 461–65. [DOI] [PubMed] [Google Scholar]
- 41. Miyata H, Yamasaki M, Takiguchi S, et al. Salvage esophagectomy after definitive chemoradiotherapy for thoracic esophageal cancer. J Surg Oncol. 2009;100:442–6. [DOI] [PubMed] [Google Scholar]
- 42. Sohda M, Kumakura Y, Saito H, et al. Clinical Significance of Salvage Esophagectomy for Patients with Esophageal Cancer and Factors of Influencing Long‐term Survival. Anticancer Res. 2017;37:5045–51. [DOI] [PubMed] [Google Scholar]
- 43. Tachimori Y, Kanamori N, Uemura N, et al. Salvage esophagectomy after high‐dose chemoradiotherapy for esophageal squamous cell carcinoma. J Thorac Cardiovasc Surg. 2009;137:49–54. [DOI] [PubMed] [Google Scholar]
- 44. Yoo C, Park JH, Yoon DH, et al. Salvage esophagectomy for locoregional failure after chemoradiotherapy in patients with advanced esophageal cancer. Ann Thorac Surg. 2012;94:1862–8. [DOI] [PubMed] [Google Scholar]
- 45. Chao YK, Chan SC, Chang HK, et al. Salvage surgery after failed chemoradiotherapy in squamous cell carcinoma of the esophagus. Eur J Surg Oncol. 2009;35:289–94. [DOI] [PubMed] [Google Scholar]
- 46. Kumagai K, Mariosa D, Tsai JA, et al. Systematic review and meta‐analysis on the significance of salvage esophagectomy for persistent or recurrent esophageal squamous cell carcinoma after definitive chemoradiotherapy. Dis Esophagus. 2016;29:734–9. [DOI] [PubMed] [Google Scholar]
- 47. Kudo T, Hamamoto Y, Kato K, et al. Nivolumab treatment for oesophageal squamous‐cell carcinoma: an open‐label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–9. [DOI] [PubMed] [Google Scholar]
- 48. Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION‐3): a multicentre, randomised, open‐label, phase 3 trial. Lancet Oncol. 2019;20:1506–17. [DOI] [PubMed] [Google Scholar]
- 49. Metges J, Francois E, Shah M, et al. The phase 3 KEYNOTE‐181 study: pembrolizumab versus chemotherapy as second‐line therapy for advanced esophageal cancer. Ann Oncol. 2019;30:iv130. [Google Scholar]
- 50. Chen MF, Chen PT, Chen WC, et al. The role of PD‐L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL‐6 and T‐cell immunosuppression. Oncotarget. 2016;7:7913–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Eggermont AM, Chiarion‐Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high‐risk stage III melanoma (EORTC 18071): a randomised, double‐blind, phase 3 trial. Lancet Oncol. 2015;16:522–30. [DOI] [PubMed] [Google Scholar]
- 52. Akiyama H, Tsurumaru M, Udagawa H, et al. Radical lymph node dissection for cancer of the thoracic esophagus. Ann Surg. 1994;220: 364–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Udagawa H, Ueno M, Shinohara H, et al. The importance of grouping of lymph node stations and rationale of three‐field lymphoadenectomy for thoracic esophageal cancer. J Surg Oncol. 2012;106:742–7. [DOI] [PubMed] [Google Scholar]
- 54. Saeki H, Masuda T, Okada S, et al. Impact of perioperative peripheral blood values on postoperative complications after esophageal surgery. Surg Today. 2010;40:626–31. [DOI] [PubMed] [Google Scholar]
- 55. Avendano CE, Flume PA, Silvestri GA, et al. Pulmonary complications after esophagectomy. Ann Thorac Surg. 2002;73:922–6. [DOI] [PubMed] [Google Scholar]
- 56. Fink U, Stein HJ, Wilke H, et al. Multimodal treatment for squamous cell esophageal cancer. World J Surg. 1995;19:198–204. [DOI] [PubMed] [Google Scholar]
- 57. Kumagai K, Rouvelas I, Tsai JA, et al. Meta‐analysis of postoperative morbidity and perioperative mortality in patients receiving neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal and gastro‐oesophageal junctional cancers. Br J Surg. 2014;101:321–38. [DOI] [PubMed] [Google Scholar]
- 58. Saeki H, Tsutsumi S, Tajiri H, et al. Prognostic significance of postoperative complications after curative resection for patients with esophageal squamous cell carcinoma. Ann Surg. 2017;265:527–33. [DOI] [PubMed] [Google Scholar]
- 59. Okumura H, Uchikado Y, Setoyama T, et al. Biomarkers for predicting the response of esophageal squamous cell carcinoma to neoadjuvant chemoradiation therapy. Surg Today. 2014;44:421–8. [DOI] [PubMed] [Google Scholar]
- 60. Saeki H, Jogo T, Kawazoe T, et al. RAD51 expression as a biomarker to predict efficacy of preoperative therapy and survival for esophageal squamous cell carcinoma: a large‐cohort observational study (KSCC1307). Ann Surg. 2014;21(2):597–604. [DOI] [PubMed] [Google Scholar]


