Abstract
Proteins that act on DNA, or are in close proximity to it, can become inadvertently crosslinked to DNA and form highly toxic lesions, known as DNA-protein crosslinks (DPCs). DPCs are generated by different chemotherapeutics, environmental or endogenous sources of crosslinking agents, or by lesions on DNA that stall the catalytic cycle of certain DNA processing enzymes. These bulky adducts impair processes on DNA such as DNA replication or transcription, and therefore pose a serious threat to genome integrity. The large diversity of DPCs suggests that there is more than one canonical mechanism to repair them. Indeed, many different enzymes have been shown to act on DPCs by either processing the protein, the DNA or the crosslink itself. In addition, the cell cycle stage or cell type are likely to dictate pathway choice. In recent years, a detailed understanding of DPC repair during S phase has started to emerge. Here, we review the current knowledge on the mechanisms of replication-coupled DPC repair, and describe and also speculate on possible pathways that remove DPCs outside of S phase. Moreover, we highlight a recent paradigm shifting finding that indicates that DPCs are not always detrimental, but can also play a protective role, preserving the genome from more deleterious forms of DNA damage.
Keywords: DNA-protein crosslinks (DPCs), DNA replication, DNA repair
1. DNA-protein crosslinks: a diverse class of lesions
In contrast to other types of DNA lesions, DPCs are highly heterogeneous and differ from one another depending on the nature of the protein adducted to DNA, the chemistry of the crosslink and the DNA surrounding the adduct. Thus, a thorough classification of DPCs is challenging since each of the three components forming a DPC is likely to contribute to the mechanism of repair. Here, we present a simple classification based on the DNA component of the crosslink, which can greatly impact the faith of the replisome encountering the lesion (e.g. by inducing replisome stalling or replication fork collapse), and the way the DPC is sensed and repaired.
1.1. DPCs on double-stranded DNA
Proteins crosslink to uninterrupted double-stranded DNA (dsDNA) by the action of different crosslinking agents, including chemotherapeutics, reactive aldehydes, ultraviolet (UV) light, ionizing radiation (IR) and different transition metals [1,2]. DPCs induced by crosslinking agents can, in theory, link any protein in close proximity to DNA. Thus, these DPCs are highly diverse and exhibit different sizes and chemistries.
1.1.1. Endogenous crosslinking agents
An important endogenous source of crosslinking agents is aldehydes. Reactive aldehydes, such as formaldehyde, are found at high concentration in human plasma [3]. Formaldehyde is generated as a byproduct of different metabolic processes such as histone demethylation or lipid peroxidation [[4], [5], [6]]. Formaldehyde treatment is often used as a proxy of DPC sensitivity in cells. This is because formaldehyde crosslinks proteins to DNA via a methylene bridge that involves the exocyclic amine of DNA bases and different protein amino acids (mainly lysine, cysteine, histidine or tryptophan) (Fig. 1A, i) [7], although a recent study suggests a two carbon atoms linkage instead [8]. Acetaldehyde, a byproduct of ethanol metabolism [9], is also able to crosslink proteins to DNA. Acetaldehyde can form different types of DNA adducts with 1,N2-propano-2′-deoxyguanosine (PdG) being the most toxic one. The subsequent formation of DPCs involves the ring-opened isomer of PdG, containing a free aldehyde group that most likely forms a Schiff base-mediated crosslink with a basic amino acid such as arginine or lysine [10,11]. Other reactive aldehydes present in cells, such as malondialdehyde and acrolein, have also been shown to crosslink DNA and proteins together [12,13]. Importantly, reactive aldehydes are known to generate a wide spectrum of other lesions (e.g. protein-protein crosslinks, DNA adducts, and DNA interstrand crosslinks (ICLs)) [14]. In the case of formaldehyde, it likely favors protein-protein linkages over protein-DNA linkages because DPC formation by formaldehyde requires the prior disruption of Watson-Crick base pairing to expose DNA’s exocyclic amines [15]. Thus, it is often unclear how these crosslinking agents induce cellular toxicity, and caution should be taken when assuming that the cytotoxic effect of formaldehyde is mainly driven by DPCs.
Fig. 1.
DPC classification based on the DNA component of the crosslink.
(A) Illustration of DPCs on dsDNA. (B) Illustration of a specific DPC that crosslinks to AP sites on ssDNA. (C) and (D) illustrate specific DPCs flanked by a SSB or by a DSB, respectively.
1.1.2. Chemotherapeutics
Although chemotherapeutics, such as cisplatin derivatives and nitrogen mustards, are mainly known to form DNA adducts and DNA ICLs, they can also form DPCs [16]. Cisplatin can induce DPCs by attacking the N7 atom of purine bases, which can then be connected to cysteine, arginine, and lysine side chains by a subsequent nucleophilic attack on the platinum center (Fig. 1A, ii) [17]. Nitrogen mustards, which are one of the oldest classes of chemotherapeutics [18], can also generate DPCs via sequential alkylation of nucleophilic sites in DNA and proteins, mainly involving the N7 position of guanine and cysteine amino side chains [19]. Studies indicate that cells deficient in DPC removal (e.g. SPRTN-deficient cells) are sensitive to cisplatin treatment, suggesting that DPCs contribute to the cytotoxic effects of some of these chemotherapeutics [20,21]. However, it is currently unknown how large the fraction of DPCs generated by cisplatin derivatives is in cancer patients, and a big challenge is to understand whether DPC generation and/or repair contribute to the therapeutic effects and/or tumor resistance development.
Another drug, 5′-aza-2-deoxycytidine (5-aza-dC), commonly used in the treatment of myelodysplastic syndromes, generates DPCs by trapping the DNA (cytosine-5)-methyltransferase 1 (DNMT1) on its recognition site. DNMT1 maintains the methylation pattern on DNA following DNA replication. It recognizes hemi-methylated DNA and transfers methyl groups to the newly synthesized strand [22]. Incorporation of the cytosine analog 5-aza-dC during replication causes trapping of DNMT1 behind the replication fork. When DNMT1 methylates the daughter strand, the lack of a proton at the N5 position of the triazine ring disables DNMT1 release by β-elimination and results in the permanent trapping of DNMT1 (Fig. 1A, iii) [23,24]. This causes global hypomethylation in cells, which leads to re-expression of tumor suppressor genes [25,26]. The therapeutic effect of 5-aza-dC treatment likely originates from DNA hypomethylation [27], but whether DNMT1-DPCs also contribute to this effect remains unclear. In analogy with 5-aza-dC, 5-fluoro-2-deoxycytosine also traps cytosine DNA methyltransferases, and this method can be used to model DPCs on dsDNA [28,29].
1.1.3. Other exogenous sources of crosslinking agents
UV and IR cause a spectrum of different lesions on DNA, such as base damage, single-strand breaks (SSBs), double-strand breaks (DSBs) and DPCs. Interestingly, the proportional distribution of these lesions generated by UV or IR is dependent on the oxygen levels in the cellular environment [30]. Under hypoxic conditions, as can be found in solid tumors, DPCs are thought to be the main lesion caused by these agents, exceeding the number of DNA breaks [31]. Molecularly, IR induces DNA radicals that can either decompose or react with proteins. Vice versa, it can also form protein radicals that can decompose or react with DNA. The lack of oxygen promotes formation of crosslinks over decomposition [[32], [33], [34]]. Potentially, the combination of radiotherapy and inhibition of DPC repair could be a future approach for treatment of solid tumors.
1.2. DPCs on single-stranded DNA
The crosslinking agents listed above should also crosslink proteins on single-stranded nucleic acids. In fact, some crosslinkers might favor linkages on ssDNA (or RNA). This is the case for formaldehyde, which reacts with exocyclic amines of DNA that are usually protected by hydrogen bonding on duplex DNA [15]. Although the chemistry of the crosslink should be identical, ssDNA, in contrast to dsDNA, will trigger rapid DPC proteolysis (see below). Strikingly, while DPCs are considered a threat to genome stability, a recent study identified a protein that purposely crosslinks to ssDNA to protect the genome. The Cortez laboratory showed that 5-hydroxymethylcytosine (5hmC) binding, ES-cell-specific (HMCES) crosslinks to abasic sites on ssDNA via a thiazoline linkage to prevent more deleterious processing of these sites (Fig. 1B, i) [35,36]. Abasic sites are one of the most abundant lesions in the genome [37,38]. They can be formed by spontaneous depurination but also as an intermediate of the base excision repair (BER) reaction. For example, different DNA glycosylases recognize and remove damaged or oxidized bases (e.g. 8-oxoG). Abasic sites can also be generated via cytosine deamination to uracil and subsequent removal of uracil by uracil-DNA glycosylase (UDG). The apurinic/apyrimidinic (AP) site is then processed by an AP endonuclease (APE1) that cleaves the DNA backbone to initiate downstream gap filling repair to replace the lesion [39]. While APE1 mainly acts on dsDNA, it can also cleave AP sites on ssDNA, albeit with reduced efficiency [40]. Thus, if BER or another pathway acted on a ssDNA AP site, it would be highly detrimental for cells as it would generate a DSB, the most deleterious form of DNA damage. Crosslinking of HMCES shields the AP site, and thereby prevents DSB formation [35,41]. HMCES contains a single SOS response associated peptidase (SRAP) domain, which is essential for crosslinking. Interestingly, nearly all organisms encode a SRAP domain protein and the mechanism of crosslinking is conserved in bacteria, where the HMCES ortholog yedK can also bind and crosslink to AP sites [35]. Thus, HMCES-DPCs are part of a protective and highly conserved mechanism that maintains genomic integrity.
1.3. DPCs flanked by a single-strand break
DPCs can also be flanked by DNA breaks. This type of protein adduct is typically generated when proteins that act on DNA become covalently crosslinked to DNA in an intermediate step of their catalytic cycle. The most prominent example are topoisomerases.
1.3.1. TOP1 cleavage complexes (TOP1ccs)
Topoisomerase 1 (TOP1) acts on DNA to release torsional stress during replication or transcription [42]. To do so, its catalytic tyrosine attacks the sugar phosphate backbone of DNA, introducing a SSB confined by a phosphotyrosyl linkage. The free DNA strand can then rotate to release torsional stress. Finally, the DNA 5′ OH attacks the phosphotyrosine linkage and the nick is religated, releasing the covalent protein adduct. However, neighboring DNA lesions or intercalating agents (such as camptothecin or topotecan) can displace the 5′ OH, inhibiting religation of the nick and trapping the enzyme at the 3′ end of DNA (Fig. 1C, i) [43]. Because TOP1 lesions are particularly detrimental to highly proliferative cells, TOP1 poisons are widely used in the clinic to treat ovarian, cervical and lung cancers. The TOP1 covalent intermediate is called TOP1 cleavage complex (TOP1cc) and requires tyrosyl-DNA phosphodiesterase 1 (TDP1) to reverse the crosslink [44]. TDP1 attacks the phosphotyrosyl bond, replacing TOP1 at the 3′ end of DNA. A mutation in TDP1 that generates a substitution of histidine 493 with an arginine, causes a rare autosomal neurodegenerative disorder known as spinocerebellar ataxia with axonal neuropathy (SCAN1) [45]. This mutation, reduces TDP1 levels and activity leading to the accumulation of TOP1ccs in cells [46]. Interestingly, this mutation also impairs TDP1 release from DNA generating another DPC at the 3′ DNA end. However, TDP1-DPCs are expected to be very rare, restricted to this mutation, and of unclear physiological significance.
1.3.2. Flp-nick recombinase system
TOP1cc-type repair can be mimicked using the Flp-nick recombinase system. This system allows the generation of a single irreversible protein adduct flanked by a nick at a specific FRT site introduced in the genome of eukaryotic cells [47]. The Flp recombinase catalyzes site-specific recombination in Saccharomyces cerevisiae and is widely used for chromosomal gene insertion [48]. Mutation of the Flp recombinase (FlpH305L) causes it to become irreversibly linked to the 3′ end of a DNA single-strand nick via a phosphotyrosyl linkage. Thus, its similarity with a TOP1cc makes the Flp-nick system a relevant and attractive tool to study a single, site-specific TOP1-like DPC, and to monitor its impact on DNA replication [47]. However, although crosslinking of the FlpH305L to one strand of the FRT site is preferred, it can also crosslink to the opposite strand [47]. Thus, it is currently impossible to unequivocally assess the impact of Flp-DPCs on leading versus lagging strand templates.
1.3.3. Pol β-type crosslinks
Other examples of DPCs flanked by a SSB are the ones generated at oxidized abasic sites. Free radicals can generate the oxidized abasic site 2-deoxyribonolactone (dL), which can cause enzymes that exhibit AP lyase activity, such as polymerase β (Pol β), to become crosslinked upon their attempt to repair the AP site (Fig. 1C, ii) [[49], [50], [51], [52]]. In contrast to TOP1ccs, Pol β crosslinks to the 5′ end of the SSB, which might suggest a different mechanism of removal.
1.3.4. PARP1 crosslinking
The DNA repair factor poly(ADP-ribose) polymerase 1 (PARP1) can also be crosslinked to abasic sites. PARP1 also exhibits weak AP lyase activity. Following binding to the AP site, PARP1 forms a Schiff base intermediate between the C1’ atom of deoxyribose and one of its primary amine-containing amino acids. If the Schiff base is reduced (potentially by the intrinsic redox capacity of PARP1) the DNA becomes nicked with PARP1 now covalently trapped on the 3′ DNA end [[53], [54], [55]]. PARP1 can also crosslink to the 5′ end of a DNA nick following APE1 cleavage during BER [54,56]. Although most research has been done in vitro, some evidence in cells supports the existence of PARP1-DPCs, which are stimulated by the combined treatment with DNA alkylating agents and PARP1 inhibitors [[54], [55], [56]].
1.4. DPCs flanked by a double-strand break
1.4.1. TOP2 cleavage complexes (TOP2ccs)
While TOP1 induces single-strand nicks to relax DNA, topoisomerase 2 (TOP2) acts as a homodimer that generates DSBs. The TOP2 homodimer induces a break on each DNA strand and, in contrast to TOP1, forms a covalent bond with the 5′ ends of the break (Fig. 1D, i; note that TOP2ccs can also be flanked by a ssDNA break if only one of the subunits becomes trapped [57]). As observed with TOP1, intercalating agents such as etoposide and doxorubicin can prevent the religation of the nick and cause TOP2 to be covalently entrapped in a TOP2 cleavage complex (TOP2cc), which can be released by tyrosyl-DNA phosphodiesterase 2 (TDP2) [58]. Mutations in TDP2 cause intellectual disability in humans, which is thought to arise from the accumulation of DSBs linked to defective TOP2cc repair [59]. Interestingly, a self-trapping mutant of another topoisomerase, TOP3B (R338W), was also recently shown to crosslink to 5′ ends of both DNA and RNA [60]. However, whether endogenous TOP3Bccs arise in cells and the impact of these lesions on DNA or RNA processes are still unknown.
1.4.2. SPO11 cleavage complexes (SPO11ccs)
The protein SPO11, primarily expressed in germ cells, also generates DSBs to initiate meiotic recombination. It forms a topoisomerase-like phosphotyrosyl linkage on the 5′ ends of DNA that is rapidly removed by endonucleolytic processing of the DNA ends by the MRN (MRX in yeast) endo/exonuclease [[61], [62], [63], [64], [65]]. SPO11cc intermediates were recently detected in mice spermatocytes and shown to accumulate in the absence of the DSB signaling kinase ATM, which phosphorylates and activates MRN [65]. Thus, in the absence of MRN, SPO11ccs accumulate in cells and may be ultimately processed by another DPC removal pathway. The required high activity of topoisomerase-like enzymes in germ cells (TOP1/2 and SPO11) pose a threat to the genome, which is critical to maintain early in development to secure reproductive success and survival of the species. This might explain why germ cells have evolved specific DPC proteases such as the acidic repeat containing protease (ACRC), which might represent an alternative or backup pathway to remove TOP1/2- and SPO11ccs (see below).
In conclusion, DPCs are highly heterogenous DNA lesions generated by many chemotherapeutic agents, aldehydes or oxygen species present in in cells, or different DNA processing enzymes. Because of their heterogeneity, it has been difficult to quantitatively measure DPC abundance in the genome and current methodologies that monitor DPCs in cells remain crude. In the future, we might be able to adapt quantitative mass spectrometry methods to identify and accurately measure the wide spectrum of different protein adducts present in the genome.
2. Replication-coupled DPC repair
The enormous diversity of DPCs suggests the existence of multiple pathways that recognize DPCs and target their removal. Because protein adducts on DNA represent a major roadblock for DNA replication and DNA transcription, it was initially postulated that DNA replication could act as a sensing mechanism that recognizes DPCs to trigger their removal [66]. Formal proof of this model came in 2014, when replication-coupled DPC repair was recapitulated using a cell-free system derived from Xenopus eggs [67]. These original experiments described a rather safe mechanism of DPC repair that occurs without replisome disassembly nor generation of DNA DSBs, two major sources of genomic instability. Instead, they indicated the involvement of a proteolytic pathway, whose activity is stimulated by DNA replication [67].
At the same time, Stingele and colleagues identified the first DPC protease via a genetic screen in yeast [68]. Wss1 was shown to specifically degrade covalently and non-covalently bound proteins on ssDNA and to confer resistance to crosslinking agents, such as formaldehyde, via its conserved SprT protease domain [[68], [69], [70]]. Its orthologue in vertebrates, SPRTN, was soon shown to also degrade DPCs during DNA replication [21,71], validating the original observations made in yeast and frogs. Importantly, SPRTN mutations that affect its protease activity induce a human syndrome characterized by accelerated aging and early onset hepatocellular carcinoma [72]. Without SPRTN, cells accumulate DPCs, exhibit impaired replication fork progression and genome instability [21,[71], [72], [73], [74], [75], [76]]. Together, these experiments highlight the importance of DPC repair in maintaining genome integrity and human health. However, how SPRTN is coupled to DNA replication remained an important unanswered question.
Using the Xenopus in vitro system it was shown that a DPC on duplex DNA is resolved during DNA replication by the parallel actions of at least two proteases, SPRTN and/or the proteasome [67,77]. Importantly, this work provided clues on how these proteases are targeted to DPCs during replication.
2.1. Replication-coupled repair of DPCs on double-stranded DNA
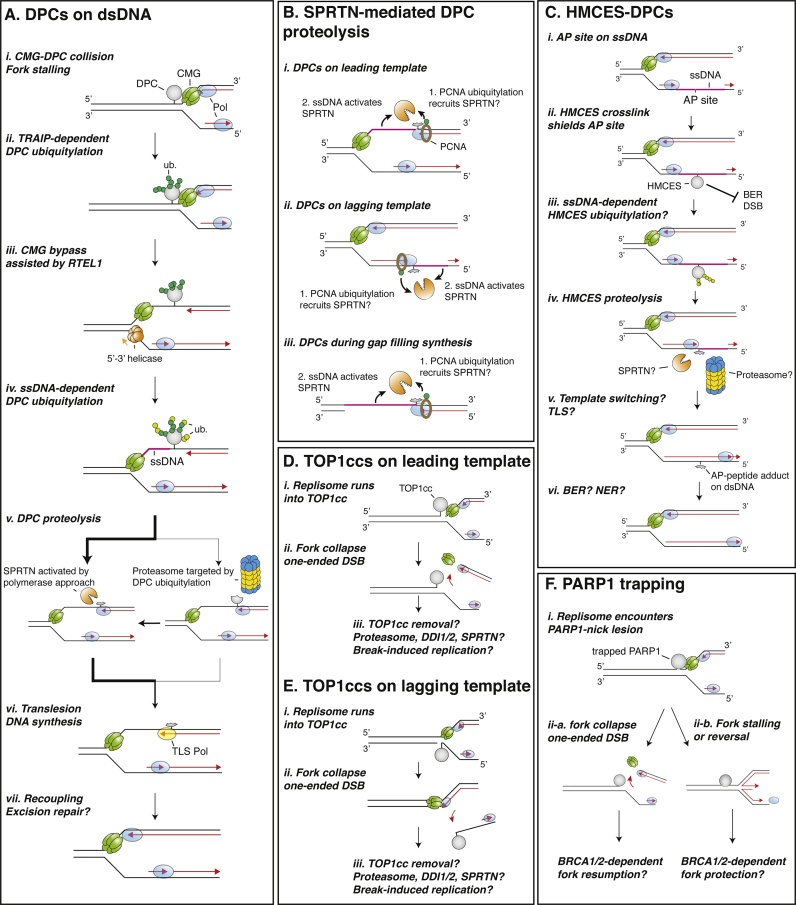
DNA methyltransferases such as HpaII (M.HpaII; 45 kDa) can be trapped at a fluorinated sequence on a plasmid, thereby modelling a DPC on dsDNA [28]. Using this approach, it was shown that DPC repair in Xenopus egg extracts is stimulated by DNA replication [67]. When a DPC is encountered by the replisome on the leading strand template, the replisome stalls. This is because the replicative helicase, CMG, which encircles and translocates on the leading strand template [78], stops at the protein adduct (Fig. 2A, i). This initial stalling event, which lasts 10−15 min in egg extracts, triggers the ubiquitylation of the protein adduct by the ubiquitin ligase TRAIP (Fig. 2A, ii) [77]. TRAIP travels with the replisome and appears to non-discriminately ubiquitylate proteins in front of CMG that inhibit its progression. The consequence of this ubiquitylation in the context of DPC repair is not fully understood, but TRAIP’s function at the replisome is not restricted to DPCs. Indeed, TRAIP was shown to ubiquitylate opposing CMGs following fork convergence during ICL repair in order to recruit the glycosylase NEIL3, which can unhook certain ICLs, or to trigger p97-mediated CMG removal, which is essential to activate the Fanconi anemia route of ICL repair [79]. If CMG stalling persists at a single fork, cyclin-mediated TRAIP activation triggers CMG ubiquitylation in cis and allows CMG removal, which is essential for downstream mitotic DNA synthesis (MiDAS) [[80], [81], [82]]. In the context of DPCs, TRAIP-mediated ubiquitylation appears to first stimulate CMG bypass of the protein adduct and then stimulate the recruitment of the proteasome. CMG can bypass protein roadblocks on its translocating strand in a process that is greatly stimulated by the generation of ssDNA beyond the protein adduct by the 5′ to 3′ DNA helicase RTEL1 (Fig. 2A, iii) [83]. Once bypassed by CMG, the protein adduct is now safely positioned behind the replication fork, where it is processed by SPRTN and/or the proteasome (Fig. 2A, iv-v) [77,83].
Fig. 2.
Replication-coupled DPC repair mechanisms.
(A) Schematic illustrating the repair of DPCs on dsDNA in Xenopus egg extracts. (B) Model for SPRTN-mediated DPC proteolysis triggered by polymerase stalling at a DPC on the leading (i) or lagging (ii) strand template, or during gap filling DNA synthesis (iii). (C) Putative repair mechanism of HMCES-DPCs. (D–E) Impact of TOP1ccs when encountered by the replisome on leading (D) or lagging (E) strand templates. Note that in neither scenario can a polymerase encounter a TOP1cc. (F) Potential outcomes of trapped PARP1-SSB lesions. (ii-a) Fork collapse results in a one-ended DSB, that requires BRCA1/2-mediated HR for downstream repair. (ii-b) Fork stalling and reversal at the roadblock requires BRCA1/2 for fork protection.
2.1.1. SPRTN-mediated DPC degradation
Surprisingly, while SPRTN contains ubiquitin interacting domains that are essential for DPC degradation, SPRTN could degrade DPCs that have not undergone ubiquitylation, albeit with slower kinetics. This was demonstrated by methylating the lysine residues of M.HpaII, which blocked its ubiquitylation but not its degradation by SPRTN [77]. Thus, how does SPRTN degrade DPCs and at the same time avoid other key replisome components? While most replisome components are replaceable if degraded (e.g. PCNA, DNA polymerases, RPA) one critical factor is the CMG helicase, which cannot be reloaded if removed from chromatin during S phase. Thus, it is critical to protect CMG and several safeguarding mechanisms operate to avoid its destruction. First, CMG’s ability to bypass DPCs on the leading strand template ensures that DPC processing by SPRTN occurs behind the replication fork, allowing CMG to be long gone before proteolysis occurs [83]. Inhibiting polymerase synthesis 16 nucleotides upstream of the DPC with a small peptide adduct, which did not inhibit CMG translocation, abolished SPRTN-mediated DPC degradation [77]. Thus, SPRTN requires a DNA polymerase to approach within a few nucleotides of the DPC, which constitutes a second protection mechanism against unwanted CMG degradation (Fig. 2A, v). Importantly, since many DPCs on lagging strand templates will not impact CMG translocation [84], targeting SPRTN by polymerase approach ensures that DPCs can be equally degraded on both leading or lagging strand templates (Figs. 2B, i-ii). It also suggests that SPRTN could act throughout the cell cycle, during any gap filling synthesis process that occurs in the absence of a replication fork (Fig. 2B, iii) [77]. Supporting this notion, SPRTN-depleted worms are sensitive to formaldehyde even when these are arrested in a non-dividing larval state [21]. Strikingly, although DPC ubiquitylation is not necessary for SPRTN activity, SPRTN’s ubiquitin-binding zinc finger (UBZ) domains are essential to degrade DPCs [75,77], suggesting that another ubiquitylated protein targets SPRTN to DPCs. Polymerase stalling at a DNA lesion induces replisome uncoupling and ubiquitylation of PCNA [85,86]. Given that SPRTN interacts in vitro with ubiquitylated PCNA via its PCNA-interacting protein (PIP) and UBZ domains [87], this is likely how SPRTN is ultimately targeted to DPCs by polymerase approach (Fig. 2B, i-iii). Supporting this idea, SPRTN-mediated DPC removal in human cells was shown to be epistatic with RAD18, which ubiquitylates PCNA [75].
Several parallel observations support a polymerase approach targeting model. Stingele and colleagues first introduced the DNA switch activation concept [68]. Wss1 proteolytic activity was shown to require DNA and to be particularly stimulated by ssDNA [68,69]. Moreover, while both dsDNA and ssDNA could activate SPRTN, dsDNA led to autocatalytic activity, while ssDNA induced full protease activation and degradation of protein substrates [21]. Thus, we envision that polymerase approach targets SPRTN to the DPC (possibly via PCNA ubiquitylation) and that the ssDNA context beyond the DPC, ultimately channels SPRTN activity to the protein adduct (Fig. 2B, i-iii). In accordance, structural analysis of SPRTN confirmed its activation by binding to ssDNA. A Zn2+-binding sub-domain (ZBD) of the SprT domain binds preferentially to ssDNA via its aromatic pocket, where it engages with unpaired DNA bases and thereby positions the active site optimally for cleavage of protein adducts [88]. In the absence of binding to ssDNA, the ZBD shields the active site of SPRTN, which likely prevents unwanted cleavage of proteins in the absence of DNA.
Additionally, SPRTN is also regulated by a ubiquitin switch. SPRTN monoubiquitylation excludes it from chromatin [21]. In the presence of DPCs, SPRTN is deubiquitylated by an unknown deubiquitylating enzyme (DUB) and relocates to chromatin [21]. However, in unperturbed cells and in Xenopus egg extracts, a predominant fraction of SPRTN is not ubiquitylated yet neither bound to chromatin. Hence, the ubiquitin switch of SPRTN is unlikely a simple on and off switch activated exclusively upon DPC formation. Identification of the DUB that regulates SPRTN’s ubiquitylation will probably clarify the full meaning of this regulation.
2.1.2. The proteasome pathway
Using Xenopus egg extracts combined with quantitative high-resolution mass spectrometry, the specific enrichment of the entire 26S proteasome on replicating DPC plasmids was detected at the time of proteolysis [77]. While SPRTN or proteasome inhibition alone had little effect on M.HpaII degradation, combined inactivation of the two proteases significantly inhibited DPC degradation, indicating that SPRTN or the proteasome can degrade DPCs independently of each other. When DPC ubiquitylation was prevented via lysine methylation of the DPC, proteasome recruitment no longer occurred, indicating that the proteasome, in contrast to SPRTN, is exclusively targeted by DPC ubiquitylation (Fig. 2A, v). However, DPC ubiquitylation and degradation by the proteasome could also occur without TRAIP or an active replisome, when the DPC was located on ssDNA [77]. This suggested the existence of a second ubiquitin ligase that targets DPCs on ssDNA. Detailed analysis of replication-coupled DPC ubiquitylation with and without TRAIP, and with or without CMG bypass, revealed that the second ligase most likely acts downstream of TRAIP during DNA replication, once CMG has bypassed the DPC, and the protein adduct is located on ssDNA (Fig. 2A, iv). Although the interplay between TRAIP and this second unknown ligase remains unclear, it is tempting to speculate that different ubiquitin ligases have evolved the capacity to target DPCs under different scenarios. For example, TRAIP could target DPCs to degradation by the proteasome for DPCs that are unpassable by CMG. In contrast, the unknown ssDNA-activated ubiquitin ligase could target DPCs that block DNA synthesis and are inaccessible to SPRTN. In both of these scenarios, proteasome and SPRTN-mediated DPC degradation might act sequentially, where the proteasome first denatures and trims the protein to allow further processing by SPRTN (Fig. 2A, v). Importantly, SPRTN depletion inhibited TLS in Xenopus egg extracts while proteasome inhibition did not. Thus, SPRTN is likely the ultimate protease that reduces polypeptide chains to very short peptide adducts amenable for bypass, and we envision that the proteasome represents a backup mechanism for DPCs that are not accessible by SPRTN (e.g. due to their position in front of the replisome or because their conformation shields the ssDNA or renders the DPC inaccessible to SPRTN’s proteolytic site) (Fig. 2A, v). In accordance with the proteasome acting as a backup mechanism, short proteasome inhibition in cells in the presence of SPRTN did not significantly stabilize DPCs, as is observed in Xenopus egg extracts [89].
2.1.3. Other replication-coupled DPC proteases?
Although SPRTN and the proteasome participate in DPC degradation in egg extracts, in their absence, the DPC is still degraded, highlighting the existence of at least one additional protease that can degrade DPCs [77]. Moreover, in the absence of DPC ubiquitylation, DPC degradation became exclusively dependent on SPRTN [77]. Thus, the unknown protease(s) is (are) likely targeted by DPC ubiquitylation. Recently, it was shown in Saccharomyces cerevisiae, that the DNA-damage inducible-1 (Ddi1) aspartic protease can remove Top1ccs during replication, independently of the proteasome and Wss1 [90]. Ddi1 requires long ubiquitin chains on its substrates to act [91,92]. Thus, polyubiquitylation of DPCs could target them for proteolytic cleavage by Ddi1, particularly in the absence of SPRTN, which leads to longer ubiquitin chains on DPCs [77]. In human cells, the orthologs DDI1 and DDI2 are essential for cell survival after replication stress by promoting restart of stalled replisomes [93]. DDI1/2 can shuttle replisome components for proteasomal degradation [93], and DDI2 was recently shown to be a ubiquitin-targeted endoprotease [92]. Whether they can also bring the proteasome to the replication fork is still unknown. Another recently identified SprT containing protein is ACRC (also known as GCNA). However, preliminary observations suggest that ACRC’s function in DPC repair is mostly independent of DNA replication [94]. Furthermore, the putative serine protease family with sequence similarity 111 member A (FAM111A) has recently been shown to protect the replisome from stalling at proteinaceous roadblocks such as trapped PARP1 or TOP1ccs [95]. FAM111A interacts with PCNA and DNA and requires its trypsin-like protease domain to ensure proper fork progression [95]. A recent study also observed HMCES accumulation in cells upon FAM111A knock down but whether HMCES is a direct target of FAM111A remains to be addressed [96]. Thus, it appears that several DPC proteases can operate during S phase and that there might be more to identify. The main challenge resides in understanding under which condition each of these proteases becomes active. It is likely that the existence of multiple proteases with different proteolytic and activation modes allows the replisome to deal with the enormous diversity of DPCs.
2.1.4. TLS across the peptide adduct
Once bypassed by CMG, DPCs represent another block to DNA polymerases and DPC bypass becomes dependent on TLS (Fig. 2A, vi). In egg extracts, immunodepletion of REV1/Pol ζ abolished bypass of M.HpaII-DPCs [67,77]. However, given their heterogeneity in protein and attachment chemistry, it is likely that the nature of the DPC dictates which TLS polymerase is employed to bypass the adducted base. In vitro polymerase bypass studies have shown different abilities of TLS polymerases to synthesize across and past peptide adducts. Moreover, large peptide adducts blocked synthesis, while smaller adducts could be bypassed by Pol κ, Pol η or Pol ν [[97], [98], [99], [100], [101], [102]], supporting the notion that DPC degradation facilitates polymerase bypass.
We note, however, that DPC degradation is not always an absolute requirement for TLS. In the absence of DPC ubiquitylation and SPRTN, M.HpaII crosslinks are stable in egg extracts but still bypassed by TLS, albeit with slower kinetics [77]. We speculate that SPRTN-mediated DPC degradation and TLS are competing mechanisms that act at stalled polymerases. If a TLS polymerase can insert and rapidly extend past a peptide adduct, it might operate before SPRTN-mediated proteolysis occurs. Alternatively, if a replicative or TLS polymerase is stuck at the lesion, it would provide more time for SPRTN to act. It is thinkable that SPRTN also degrades stalled DNA polymerases and thereby stimulates polymerase or template switching events to bypass a DPC or any other polymerase stalling lesion. This interesting idea is suggested by different studies that demonstrate that SPRTN interacts with POLD3, one of the accessory subunits of both Pol δ and Pol ζ [103,104]. Although attractive, a model of SPRTN-mediated polymerase switching still requires formal demonstration.
In summary, replication-coupled repair of DPCs on dsDNA is a complex multi-step mechanism that involves many different enzymes that act at different stages of repair. Some enzymes act in front of CMG to ensure proper replication fork progression (e.g. TRAIP, RTEL1) while others operate behind the replication fork to ensure proper gap filling synthesis (e.g. SPRTN, REV1/Pol ζ), offering many different chemotherapeutic opportunities to target and inhibit DPC bypass.
2.2. Replication-coupled DPC repair of other DPCs
The replication-coupled DPC repair pathway recapitulated in Xenopus egg extracts illustrates how a DPC on dsDNA is processed during replication. In the following section, we comment and speculate on how other types of DPCs might be degraded during replication.
2.2.1. DPCs on ssDNA
HMCES was recently shown to crosslink to abasic sites on ssDNA during replication, reporting the first potentially “beneficial” endogenous DPC (Fig. 2C, i-ii). The authors also reported that HMCES becomes ubiquitylated and degraded by the proteasome following crosslinking (Fig. 2C, iii-iv) [35]. These observations are consistent with data in egg extract that indicate that DPCs on ssDNA are ubiquitylated and targeted to the proteasome [77]. Whether the same ubiquitin ligase is responsible for both processes remains to be determined. Nevertheless, their ssDNA context suggests that HMCES-DPCs would also be an ideal substrate for SPRTN. Indeed, rapid gap filling DNA synthesis would extend the nascent strand up to the DPC and thereby target and activate SPRTN to degrade HMCES, allowing subsequent bypass by template switching or TLS (Fig. 2C, iv-v). Although cells have to now deal with DPCs, which are likely mutagenetic lesions, it is suggested that HMCES-DPCs protect the abasic site from other types of more deleterious DNA damage. For example, if the AP site was to be processed on ssDNA, it would generate a detrimental DSB. Accordingly, HMCES-deficient cells rely on HR proteins for survival and accumulate DSBs upon APOBEC3 overexpression, which stimulates the formation of abasic sites [41,105]. Thus, by crosslinking to the AP site, HMCES might ensure that the AP site is not processed until gap filling synthesis has been completed and the lesion is safely located on dsDNA (Fig. 2C, ii and v-vi). Whether BER or another excision pathway could act and remove an AP-HMCES peptide adduct on dsDNA remains to be addressed.
2.2.2. DPCs flanked by DNA breaks
Chemotherapeutics like topotecan or camptothecin trap TOP1 to the 3′ end of a single-stranded nick. TDP1, the enzyme responsible for reversing the crosslink, cannot access the crosslink without prior trimming of the protein [106,107]. Several recent reports, indicate that SPRTN protects cells from TOP1 inhibition by degrading TOP1ccs [71,76,108]. However, the model of SPRTN activation by polymerase approach is incompatible with SPRTN degrading TOP1ccs during replication since a 3′ end DPC cannot be encountered by a DNA polymerase (Fig. 2D and E, i-iii). Thus, if SPRTN-mediated TOP1cc degradation truly occurs, it must involve a yet unidentified mechanism to recruit SPRTN. Potentially, in the context of TOP1cc repair, TOP1 ubiquitylation could target SPRTN via SPRTN’s ubiquitin binding motifs. Alternatively, another protein might target SPRTN to TOP1ccs, as was recently suggested for the testis-expressed protein 264 (TEX264) [108].
It is perhaps more conceivable that TOP1ccs can be repaired during replication by other pathways. In yeast, Ddi1 is recruited to Top1ccs in the absence of Tdp1 and Wss1 and its timing coincides with the start of S phase [90]. It is unclear what triggers its recruitment, but the generation of ssDNA and subsequent TOP1cc ubiquitylation could potentially target the protease [91]. For this to be a viable mechanism, DPC degradation and repair of the DNA nick would need to occur before CMG runs off through the nick and induces fork collapse. Perhaps, additional non-covalent TOP1cc interactions with DNA are strong enough to inhibit CMG translocation ahead of the nick, which would allow time for repair. However, TOP1 poisons induce DSBs in S phase most likely because the replisome runs off through the DNA nick (Fig. 2D and E, ii). Similarly, a replication fork running into TOP2ccs is very detrimental. This suggests that TOP1/2ccs should be preferably repaired in a replication-uncoupled manner (see below).
2.2.3. Is trapped PARP1 a DPC-like lesion?
PARP inhibitors (PARPi) are widely used in the clinic to treat homologous recombination (HR) deficient breast, ovarian and prostate cancers [[109], [110], [111], [112]]. The different efficacy of PARPi appears to relate to their ability to trap PARP1 on DNA lesions [112,113]. While all PARPi target and inactivate the catalytic center of the enzyme, PARPi exert different allosteric effects on PARP1, which can either promote its release from DNA (weak trapper) or retain it on a DNA break (strong trapper) [114]. Why PARPi are particularly effective in tumors with BRCA1/2 deficiencies is still unclear. PARP1 trapping at SSBs is expected to shield and protect DNA nicks from repair (Fig. 2F, i) [113,115]. When a replication fork encounters this lesion two outcomes are possible. Either the replisome runs off, converting the SSB to a one-ended DSB, which would explain the requirement of BRCA1/2 for downstream repair (Fig. 2F, ii-a). Alternatively, entrapped PARP1 might stall the replication fork ahead of the nick, which would also require BRCA1/2 to stabilize and protect the fork (Fig. 2F, ii-b) [[116], [117], [118]]. However, the impact of trapped PARP1 on CMG and replisome progression is currently unclear although some evidence suggests that PARPi does not stall replication forks but actually accelerates them [119]. To clarify how PARPi are exquisitely lethal in a HR deficient background, it remains to be tested whether PARP1 trapping can block CMG translocation, thereby mimicking a DPC. Importantly, a recent study from the Caldecott laboratory indicates that PARP activity during DNA replication is mostly triggered by lagging strand maturation, which occurs behind the replication fork [120]. Whether BRCA1/2 requirement upon PARP1 inhibition is linked to unprocessed Okazaki fragments is an interesting idea that warrants further investigation. Last, PARP inhibition can also impair PARP1 function during BER and induce PARP1-DPCs that could also contribute to the therapeutic effects of PARPi [56].
2.2.4. Aldehyde-induced DPCs
As discussed above, a common cause of endogenous crosslinks are reactive aldehydes, which are produced via different metabolic processes. They generate different crosslinks within DNA, such as DNA adducts, DNA intra- and inter-strand crosslinks as well as DPCs. Recently, a dedicated replication-coupled repair pathway for acetaldehyde-induced ICLs has been observed and suggests a specific enzyme that could unhook the ICL, in analogy to NEIL3, which unhooks ICLs induced by abasic sites or psoralen [121,122]. Thus, it is tempting to speculate that specific yet unidentified enzymes have evolved the capacity to resolve abundant endogenous crosslinks. In the context of formaldehyde-induced DPCs, such enzymes might be specialized in reversing methylene bridges. A pre-requisite might be the trimming of DPCs by proteolysis to expose the crosslink. This could account for the formaldehyde sensitivity of SPRTN- or proteasome-deficient cells [21,123].
In summary, the mechanism of DPC repair in S phase depends on the nature of the DPC. While DPCs on dsDNA are safely degraded and bypassed during DNA replication via a process that avoids replication fork collapse, the faith and full outcomes of DPCs linked to DNA breaks (e.g. TOP1/2ccs) remain uncertain. Moreover, we now know that some DPCs are specifically formed and processed during DNA replication to protect the genome from more deleterious damage (e.g. HMCES-DPCs). Further research is likely to identify the full spectrum of DPCs that are preferentially formed and processed during DNA replication, and the different enzymes that participate in DPC removal during S phase. The challenge will reside in delineating under what conditions each enzyme becomes critical. As originally proposed, we view DNA replication as a sensing mechanism that detects and removes DPCs that would otherwise escape recognition or repair in other phases of the cell cycle.
3. Replication-independent DPC repair
In addition to the type of DPC, the cell cycle stage and cell identity also influence the route of repair. DPC repair outside of S phase might be critical for non-dividing cells or for DPCs that are formed post DNA replication, but need to be resolved before mitosis to ensure proper cell division. Other processes on DNA, like DNA transcription or chromatinization could also act as sensors to trigger repair in analogy to DNA replication. In this section, we review our knowledge about DPC repair mechanisms operating outside of S phase and comment on recent observations that indicate that DPC SUMOylation might be one of the important sensing mechanisms that triggers DPC removal in the absence of DNA replication.
3.1. The role of nucleotide excision repair
Cells deficient in NER are moderately sensitive to formaldehyde treatment suggesting that NER removes protein adducts from DNA [[124], [125], [126]]. However, in vitro, NER is unable to act on full-size DPCs but can incise DNA surrounding short peptide adducts (smaller than ∼10−15 kDa) [[127], [128], [129], [130]]. Thus, we envision that NER removes the remaining DNA-peptide adducts left behind by replication-coupled DPC proteolysis (Fig. 3A, i-iii). Alternatively, NER could also act throughout the cell cycle on small DPCs, or following another mechanism of DPC degradation on duplex DNA (e.g. following the STUbL pathway described below).
Fig. 3.
Replication-independent DPC repair mechanisms.
(A) Removal of peptide-DNA adducts via nucleotide excision repair. (B) Schematic illustrating TOP1cc repair. (C) Schematic illustrating TOP2cc repair. (D) Mechanism of nuclease-dependent SPO11cc removal. (E) Putative SUMO-driven DPC removal pathways by ACRC (iii-a) or by STUbL-mediated proteolysis (iii-b and iv).
3.2. TOP1cc repair
In cells, TOP1ccs were first shown to be both SUMOylated and ubiquitylated via lysine K48 linked ubiquitin chains [[131], [132], [133]]. Moreover, proteasome inhibition stabilized TOP1ccs [131]. Thus, it was postulated that the proteasome provides the proteolytic activity that is required for TDP1-mediated crosslink reversal. However, proteasome inhibition has many pleotropic effects (e.g. depletion of free ubiquitin pools), and whether the proteasome or another protease degrades TOP1ccs is still up for debate. In this regard, SPRTN, Ddi1 or FAM111A (reviewed above) were also shown to participate in TOP1cc removal [76,90,95]. Thus, the current challenge is to understand whether these different proteases truly act on TOP1ccs and if so, whether they are activated under specific conditions or cell cycle phases. Moreover, while TOP1ccs are thought to be generated during processes that require TOP1 activity to release torsional stress (e.g. DNA replication or transcription), whether TOP1cc repair is also coupled to these processes is unclear. Because mutation in TDP1 that inhibits its crosslink reversal activity triggers a disease that mainly affects post-mitotic non-replicating neurons (SCAN1) [45], it appears that TOP1cc repair efficiently occurs outside of S phase. Supporting this notion, TDP1 is part of the single-strand break repair (SSBR) pathway that operates throughout the cell cycle [134]. Thus, there could be a ubiquitin ligase that recognizes and targets TOP1ccs independently of DNA replication. In this regard, the ssDNA-activated ubiquitin ligase activity detected in Xenopus egg extracts could also act on TOP1ccs if enough ssDNA is generated by resecting the nick. Following TOP1cc degradation and crosslink reversal by TDP1, the resulting 3′ end phosphate is now amenable to the polynucleotide kinase 3′-phosphatase (PNKP) and processed by the canonical SSB repair machinery (Fig. 3B) [134,135]. Alternatively to TDP1, a recent report indicates that the nuclease APE2 can remove blocked 3′ DNA ends like the ones generated following TOP1cc proteolysis [136]. Moreover, some evidence in cells also suggest that TOP1ccs could be removed via DNA incision by the 5′ endonuclease XPF–ERCC1 [137].
3.3. TOP2cc repair
Trapped TOP2 is also linked to DNA via its active site tyrosine, but in contrast to TOP1, TOP2 crosslinks as a homodimer to the 5′ ends of a DSB. These 5′ phosphotyrosyl bonds can be hydrolyzed by TDP2 [58,138]. While previous reports suggested that TOP2 is also ubiquitylated and targeted to degradation by the proteasome [139], a recent report indicates that the SUMO ligase Zinc finger protein Associated with TDP2 and TOP2 (ZATT, also known as ZNF451) catalyzes the removal of TOP2ccs by TDP2 in a proteasome-independent process (Fig. 3C, ii) [140]. SUMOylation of TOP2ccs by ZATT promoted recruitment of TDP2 via a “split-SUMO interacting motif (SIM)”, formed by the association of two distinct regions of the catalytic domain of TDP2. As a result, ZATT allowed TDP2 to release intact TOP2 from DNA in vitro. ZATT-mediated SUMOylation alters the conformation of TOP2ccs, presumably to make the crosslink accessible for TDP2-dependent hydrolysis [140]. However, DPC proteases targeted by SUMOylation might also assist this process (Fig. 3C, iii). In this regard, ACRC was recently shown to participate in TOP2cc removal in ovaries and early embryos of different model organisms [141,142]. Following TDP2 crosslink reversal, DNA ends are ready for ligation and mainly repaired by the non-homologous end joining (NHEJ) machinery (Fig. 3C, iv) [143]. TOP2ccs and other DPCs at DSBs, like SPO11 adducts or artificial biotin-streptavidin linkages, can also be repaired by DSB processing nucleases such as MRN (MRX) (Fig. 3D) [62,65,[144], [145], [146]]. The MRN (MRX) complex has endonuclease activity that is stimulated by a protein adduct at the 5′ end of DNA (Fig. 3D, i-iii) [144,145]. MRN processing results in DPC-free DSB ends containing a 3′ overhang that can subsequently trigger HR (Fig. 3D, iv). Thus, the repair pathway choice of 5′ end DPCs like TOP2/SPO11ccs might depend on whether the lesion should be preferentially repaired by HR (via MRN) or NHEJ (via TDP2).
3.4. DPC SUMOylation
Both TOP1 and TOP2 have also been known to become SUMOylated in response to cellular treatment with topoisomerase poisons [132,147]. Accordingly, a recent report indicates that DPCs generated by formaldehyde or DNMT1 trapping are also SUMOylated, which is critical for their removal and for cellular fitness (Fig. 3E, i-ii) [94]. SUMOylated DPCs could be a substrate of ACRC (Fig. 3E, iii-a), which contains multiple SIMs that are essential for its localization to DPCs [94]. Curiously, ACRC is almost exclusively expressed in germ cells [148]. In the absence of ACRC, germ cells of different organisms accumulate DPCs [141,142] and although ACRC has yet to be demonstrated to function as a DPC protease in vitro, its conserved SprT catalytic residue is essential to confer resistance to crosslinking agents and to counteract the accumulation of DPCs [94,141,142]. Surprisingly, although ACRC is conserved across the animal kingdom, the SprT protease domain is lost in Mus musculus [142]. Thus, further research is needed to understand the role of ACRC as a DPC protease and its other putative functions ensured by other conserved regions of the protein (e.g. the acidic intrinsically disordered region (IDR)). Moreover, under what circumstances ACRC becomes activated and why it is specific to germ cells remain open questions. In Xenopus egg extracts, M.HpaII-DPCs also undergo SUMOylation in the absence of DNA replication [77]. Although, ACRC is present [149], SUMOylated DPCs are stable over a period of three hours in interphase extracts [77]. Thus, although DPC SUMOylation is required to recruit ACRC in cells, it might not be sufficient to trigger its activity and another stimulus that might be cell cycle regulated or specific to germline cells could cooperate with SUMOylation to activate ACRC-mediated DPC degradation.
Interestingly, DPC-SUMOylation has been reported to trigger subsequent ubiquitylation and removal of TOP1/2ccs by the proteasome in somatic cells (Fig. 3E, iii-b and iv) [150]. The data presented indicates that the SUMO E3 ligase PIAS4 attaches SUMO-2/3 chains to TOP1/2ccs, which then triggers the ubiquitylation of the DPC via the SUMO-targeted ubiquitin ligase (STUbL) RNF4. This ubiquitylation ultimately drives the degradation of the DPC by the 26S proteasome. Although this represents an alternative way to remove DPCs, it is unclear whether this pathway is the preferred route of repair or whether it only becomes relevant when other routes become limiting (e.g. upon treatment with high doses of TOP1/2 poisons).
3.5. Can DNA transcription trigger DPC repair?
DPCs are not only a roadblock to DNA replication, but also to DNA transcription [151]. In vitro transcription assays have shown that the T7 RNA polymerase cannot bypass large protein adducts, but it can if these are reduced to small peptides by proteolytic degradation [152]. Hereby, the attachment site within DNA determined the fidelity of the RNA polymerase [152]. In vivo stalling of RNA polymerases at DPCs might serve as a sensor to initiate repair. We envision that the appearance of ssDNA might trigger DPC ubiquitylation and subsequent proteolysis as seen during replication. Transcription-coupled DPC repair would on one side minimize transcriptional mutations and simultaneously remove DPCs from the genome as it has been suggested for other DNA lesions that also hinder DNA transcription [153].
In summary, several pathways have been shown to repair DPCs outside of S phase. While some canonical pathways like NER or SSBR will operate on defined substrates (e.g. small peptide adducts or DPCs flanked by a single-strand break), some pathways might be global and represent a backup mechanism to remove DPCs outside S phase (e.g. SUMO-dependent DPC removal).
4. Concluding remarks
DPCs are a highly heterogeneous class of DNA lesions, which contributes to the complexity and diversity of their repair mechanisms. Cells have to deal with different proteins being crosslinked by endogenous as well as exogenous agents at different cell cycle stages. Since the discovery of the first DPC protease, research on DPC repair has greatly intensified, revealing many novel ways to remove DPCs. The current challenge consists in understanding under what circumstances these different repair routes become relevant and how they interact with each other or compensate for one another. In the light of recent findings that show that DPCs can also be part of the physiological response of cells to DNA lesions, the field is offered with great research opportunities. It is likely that more endogenous DPCs involved in genome protection mechanisms are yet to be discovered, which will inevitably offer novel chemotherapeutic opportunities. Thus, we envision that instead of inducing DPCs and/or inhibiting their repair, future chemotherapeutics might also inhibit their formation to induce other toxic forms of DNA damage.
Declaration of Competing Interest
No conflict of interest to declare.
Acknowledgments
We thank Andres López-Contreras, Keith Caldecott, Yuichi Machida and members of the Duxin laboratory for valuable feedback on the manuscript. The Novo Nordisk Foundation Center for Protein Research is supported financially by the Novo Nordisk Foundation(grant agreement no. NNF14CC0001). This project has received funding from the European Research Council (ERC)under the European Union’s Horizon 2020 research and innovation programme (grant agreement no. 715975). U.K. is supported by the Novo Nordisk Foundation(grant agreement no. NNF17CC0026748).
References
- 1.Ide H., Nakano T., Salem A.M.H., Shoulkamy M.I. DNA–protein cross-links: formidable challenges to maintaining genome integrity. DNA Repair (Amst.) 2018;71:190–197. doi: 10.1016/j.dnarep.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Barker S., Weinfeld M., Murray D. DNA-protein crosslinks: their induction, repair, and biological consequences. Mutat. Res. - Rev. Mutat. Res. 2005;589:111–135. doi: 10.1016/j.mrrev.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Luo W., Li H., Zhang Y., Ang C.Y.W. Determination of formaldehyde in blood plasma by high-performance liquid chromatography with fluorescence detection. J. Chromatogr. B Biomed. Sci. Appl. 2001;753:253–257. doi: 10.1016/S0378-4347(00)00552-1. [DOI] [PubMed] [Google Scholar]
- 4.Esterbauer H., Cheeseman K.H., Dianzani M.U., Poli G., Slater T.F. Separation and characterization of the aldehydic products of lipid peroxidation stimulated by ADP-Fe2+ in rat liver microsomes. Biochem. J. 1982;208:129–140. doi: 10.1042/bj2080129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., Casero R.A., Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Tsukada Y.I., Fang J., Erdjument-Bromage H., Warren M.E., Borchers C.H., Tempst P., Zhang Y. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–816. doi: 10.1038/nature04433. [DOI] [PubMed] [Google Scholar]
- 7.Lu K., Ye W., Zhou L., Collins L.B., Chen X., Gold A., Ball L.M., Swenberg J.A. Structural characterization of formaldehyde-induced cross-links between amino acids and deoxynucleosides and their oligomers. J. Am. Chem. Soc. 2010;132:3388–3399. doi: 10.1021/ja908282f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tayri-Wilk T., Slavin M., Zamel J., Blass A., Cohen S., Motzik A., Sun X., Shalev D.E., Ram O., Kalisman N. Mass spectrometry reveals the chemistry of formaldehyde cross-linking in structured proteins. BioRxiv. 2019 doi: 10.1101/820779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens U.J., Hoerner M., Iasker J.M., Lieber C.S. Formation of acetaldehyde adducts with ethanol-inducible P450IIE1 in vivo. Biochem. Biophys. Res. Commun. 1988;154:584–590. doi: 10.1016/0006-291X(88)90180-5. [DOI] [PubMed] [Google Scholar]
- 10.Sako M., Inagaki S., Esaka Y., Deyashiki Y. Histones accelerate the cyclic 1,N2-propanoguanine adduct-formation of DNA by the primary metabolite of alcohol and carcinogenic crotonaldehyde. Bioorg. Med. Chem. Lett. 2003;13:3497–3498. doi: 10.1016/S0960-894X(03)00800-X. [DOI] [PubMed] [Google Scholar]
- 11.Kurtz A.J., Lloyd R.S. 1,N2-deoxyguanosine adducts of acrolein, crotonaldehyde, and trans-4-hydroxynonenal cross-link to peptides via Schiff base linkage. J. Biol. Chem. 2003;278:5970–5976. doi: 10.1074/jbc.M212012200. [DOI] [PubMed] [Google Scholar]
- 12.Tuma D.J., Thiele G.M., Xu D., Klassen L.W., Sorrell M.F. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23:872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 13.Li L., Jiang L., Geng C., Cao J., Zhong L. The role of oxidative stress in acrolein-induced DNA damage in HepG2 cells. Free Radic. Res. 2008;42:354–361. doi: 10.1080/10715760802008114. [DOI] [PubMed] [Google Scholar]
- 14.Voulgaridou G.P., Anestopoulos I., Franco R., Panayiotidis M.I., Pappa A. DNA damage induced by endogenous aldehydes: current state of knowledge. Mutat. Res. - Fundam. Mol. Mech. Mutagen. 2011;711:13–27. doi: 10.1016/j.mrfmmm.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman E.A., Frey B.L., Smith L.M., Auble D.T. Formaldehyde crosslinking: a tool for the study of chromatin complexes. J. Biol. Chem. 2015;290:26404–26411. doi: 10.1074/jbc.R115.651679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Groehler A., Degner A., Tretyakova N.Y. Mass spectrometry-based tools to characterize DNA–Protein cross-linking by bis-electrophiles. Basic Clin. Pharmacol. Toxicol. 2017;121:63–77. doi: 10.1111/bcpt.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chválová K., Brabec V., Kašpárková J. Mechanism of the formation of DNA-protein cross-links by antitumor cisplatin. Nucleic Acids Res. 2007;35:1812–1821. doi: 10.1093/nar/gkm032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawley P.D. Alkylation of DNA and its aftermath. BioEssays. 1995;17:561–568. doi: 10.1002/bies.950170615. [DOI] [PubMed] [Google Scholar]
- 19.Loeber R.L., Michaelson-Richie E.D., Codreanu S.G., Liebler D.C., Campbell C.R., Tretyakova N.Y. Proteomic analysis of DNA-protein cross-linking by antitumor nitrogen mustards. Chem. Res. Toxicol. 2009;22:1151–1162. doi: 10.1021/tx900078y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosbech A., Gibbs-Seymour I., Kagias K., Thorslund T., Beli P., Povlsen L., Nielsen S.V., Smedegaard S., Sedgwick G., Lukas C., Hartmann-Petersen R., Lukas J., Choudhary C., Pocock R., Bekker-Jensen S., Mailand N. DVC1 (C1orf124) is a DNA damage-targeting p97 adaptor that promotes ubiquitin-dependent responses to replication blocks. Nat. Struct. Mol. Biol. 2012;19:1084–1092. doi: 10.1038/nsmb.2395. [DOI] [PubMed] [Google Scholar]
- 21.Stingele J., Bellelli R., Alte F., Hewitt G., Sarek G., Maslen S.L., Tsutakawa S.E., Borg A., Kjær S., Tainer J.A., Skehel J.M., Groll M., Boulton S.J. Mechanism and regulation of DNA-Protein crosslink repair by the DNA-Dependent metalloprotease SPRTN. Mol. Cell. 2016;64:688–703. doi: 10.1016/j.molcel.2016.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gujar H., Weisenberger D.J., Liang G. The roles of human DNA methyltransferases and their isoforms in shaping the epigenome. Genes (Basel) 2019;10 doi: 10.3390/genes10020172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wu J.C., Santi D.V. Kinetic and catalytic mechanism of HhaI methyltransferase*. J. Org. Chem. 1987;262:4778–4786. http://www.ncbi.nlm.nih.gov/pubmed/3558369 [PubMed] [Google Scholar]
- 24.Schermelleh L., Spada F., Easwaran H.P., Zolghadr K., Margot J.B., Cardoso M.C., Leonhardt H. Trapped in action: direct visualization of DNA methyltransferase activity in living cells. Nat. Methods. 2005;2:751–756. doi: 10.1038/nmeth794. [DOI] [PubMed] [Google Scholar]
- 25.Wilson V.L., Jones P.A., Momparler R.L. Inhibition of DMA methylation in L1210 leukemic cells by 5-Aza-2’-deoxy- cytidine as a possible mechanism of chemotherapeutic action. Cancer Res. 1983;43:3493–3496. [PubMed] [Google Scholar]
- 26.Robert M.F., Morin S., Beaulieu N., Gauthier F., Chute I.C., Barsalou A., MacLeod A.R. DNMT1 is required to maintain CpG methylation and aberrant gene silencing in human cancer cells. Nat. Genet. 2003;33:61–65. doi: 10.1038/ng1068. [DOI] [PubMed] [Google Scholar]
- 27.Chen R.Z., Pettersson U., Beard C., Jackson-Grusby L., Jaenisch R. DNA hypomethylation leads to elevated mutation rates. Nature. 1998;395:89–93. doi: 10.1038/25779. [DOI] [PubMed] [Google Scholar]
- 28.Chen L., MacMillan A.M., Chang W., Ezaz-Nikpay K., Verdine G.L., Lane W.S. Direct identification of the active-site nucleophile in a DNA (Cytosine-5)-methyltransferase. Biochemistry. 1991;30:11018–11025. doi: 10.1021/bi00110a002. [DOI] [PubMed] [Google Scholar]
- 29.MacMillan A.M., Chen L., Verdine G.L. Synthesis of an oligonucleotide suicide substrate for DNA methyltransferases. J. Org. Chem. 1992;57:2989–2991. doi: 10.1021/jo00037a006. [DOI] [Google Scholar]
- 30.Nakano T., Xu X., Salem A.M.H., Shoulkamy M.I., Ide H. Radiation-induced DNA–protein cross-links: mechanisms and biological significance. Free Radic. Biol. Med. 2017;107:136–145. doi: 10.1016/j.freeradbiomed.2016.11.041. [DOI] [PubMed] [Google Scholar]
- 31.Murray D., Meyn R.E., Vanankeren S.C. Variations in the spectrum of lesions produced in the DNA of cells from mouse tissues after exposure to γrays in air-breathing or in artificially anoxic animals. Int. J. Radiat. Biol. 1988;53:921–933. doi: 10.1080/09553008814551291. [DOI] [PubMed] [Google Scholar]
- 32.Meyn R.E., VanAnkeren S.C., Jenkins W.T. The induction of DNA-protein crosslinks in hypoxic cells and their possible contribution to cell lethality. Radiat. Res. 1987;109:419. doi: 10.2307/3577042. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H., Wheeler K.T. Radiation-induced DNA damage in tumors and normal tissues: I. Feasibility of estimating the hypoxic fraction. Radiat. Res. 1993;136:77. doi: 10.2307/3578643. [DOI] [PubMed] [Google Scholar]
- 34.Nakano T., Mitsusada Y., Salem A.M.H., Shoulkamy M.I., Sugimoto T., Hirayama R., Uzawa A., Furusawa Y., Ide H. Induction of DNA–protein cross-links by ionizing radiation and their elimination from the genome. Mutat. Res. Mol. Mech. Mutagen. 2015;771:45–50. doi: 10.1016/j.mrfmmm.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Mohni K.N., Wessel S.R., Zhao R., Wojciechowski A.C., Luzwick J.W., Layden H., Eichman B.F., Thompson P.S., Mehta K.P.M., Cortez D. HMCES maintains genome integrity by shielding abasic sites in single-strand DNA. Cell. 2018;176:1–10. doi: 10.1016/j.cell.2018.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thompson P.S., Amidon K.M., Mohni K.N., Cortez D., Eichman B.F. Protection of abasic sites during DNA replication by a stable thiazolidine protein-DNA cross-link. Nat. Struct. Mol. Biol. 2019;26:613–618. doi: 10.1038/s41594-019-0255-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindahl T., Nyberg B. Rate of depurination of native deoxyribonucleic acid. Biochemistry. 1972;11:3610–3618. doi: 10.1021/bi00769a018. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura J., Mutlu E., Sharma V., Collins L., Bodnar W., Yu R., Lai Y., Moeller B., Lu K., Swenberg J. The endogenous exposome. DNA Repair (Amst.) 2014;19:3–13. doi: 10.1016/j.dnarep.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robson C.N., Hickson I.D. Isolation of cDNA clones encoding a human apurini/apyrimidinic endonuclease that corects DNA repair and mutagenisis defects in E. coli xth (exonuclease III) mutants. Nucleic Acids Res. 1991;19:5519–5523. doi: 10.1093/nar/19.20.5519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marenstein D.R., Wilson D.M., Teebor G.W. Human AP endonuclease (APE1) demonstrates endonucleolytic activity against AP sites in single-stranded DNA. DNA Rep. (Amst.) 2004;3:527–533. doi: 10.1016/j.dnarep.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Mehta K.P.M., Lovejoy C.A., Zhao R., Heintzman D.R., Cortez D. HMCES maintains replication fork progression and prevents double-strand breaks in response to APOBEC deamination and abasic site formation. Cell Rep. 2020;31:107705. doi: 10.1016/j.celrep.2020.107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Champoux J.J., Dulbecco R. An activity from mammalian cells that untwists superhelical DNA--a possible swivel for DNA replication (polyoma-ethidium bromide-mouse-embryo cells-dye binding assay) Proc. Natl. Acad. Sci. U. S. A. 1972;69:143–146. doi: 10.1073/pnas.69.1.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pommier Y. DNA topoisomerase I Inhibitors: chemistry, biology, and interfacial inhibition. Chem. Rev. 2009;109:2894–2902. doi: 10.1021/cr900097c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang S.W., Burgin A.B., Huizenga B.N., Robertson C.A., Yao K.C., Nash H.A. A eukaryotic enzyme that can disjoin dead-end covalent complexes between DNA and type I topoisomerases. Proc. Natl. Acad. Sci. U. S. A. 1996;93:11534–11539. doi: 10.1073/pnas.93.21.11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takashima H., Boerkoel C.F., John J., Saifi G.M., Salih M.A.M., Armstrong D., Mao Y., Quiocho F.A., Roa B.B., Nakagawa M., Stockton D.W., Lupski J.R. Mutation of TDP1, encoding a topoisomerase I-dependent DNA damage repair enzyme, in spinocerebellar ataxia with axonal neuropathy. Nat. Genet. 2002;32:267–272. doi: 10.1038/ng987. [DOI] [PubMed] [Google Scholar]
- 46.Interthal H., Chen H.J., Kehl-Fie T.E., Zotzmann J., Leppard J.B., Champoux J.J. SCAN1 mutant Tdp1 accumulates the enzyme-DNA intermediate and causes camptothecin hypersensitivity. EMBO J. 2005;24:2224–2233. doi: 10.1038/sj.emboj.7600694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nielsen I., Bentsen I.B., Lisby M., Hansen S., Mundbjerg K., Andersen A.H., Bjergbaek L. A Flp-nick system to study repair of a single protein-bound nick in vivo. Nat. Methods. 2009;6:753–757. doi: 10.1038/nmeth.1372. [DOI] [PubMed] [Google Scholar]
- 48.Gates C.A., Cox M.M. FLP recombinase is an enzyme. Proc. Natl. Acad. Sci. 1988;85:4628–4632. doi: 10.1073/pnas.85.13.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Demott M.S., Beyret E., Wong D., Bales B.C., Hwang J.T., Greenberg M.M., Demple B. Covalent trapping of human DNA polymerase β by the oxidative DNA lesion 2-deoxyribonolactone. J. Biol. Chem. 2002;277:7637–7640. doi: 10.1074/jbc.C100577200. [DOI] [PubMed] [Google Scholar]
- 50.Ide H., Kotera M. Human DNA glycosylases involved in the repair of oxidatively damaged DNA. Biol. Pharm. Bull. 2004;27:480–485. doi: 10.1248/bpb.27.480. [DOI] [PubMed] [Google Scholar]
- 51.Sung J., Park I.K. Formation of DNA‐protein cross‐links mediated by C1’‐oxidized abasic lesion in mouse embryonic fibroblast cell‐free extracts. Integr. Biol. (Camb.) 2005;9:79–85. doi: 10.1080/17386357.2005.9647255. [DOI] [Google Scholar]
- 52.Quiñones J.L., Thapar U., Yu K., Fang Q., Sobol R.W., Demple B. Enzyme mechanism-based, oxidative DNA-protein cross-links formed with DNA polymerase β in vivo. Proc. Natl. Acad. Sci. U. S. A. 2015;112:8602–8607. doi: 10.1073/pnas.1501101112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Khodyreva S.N., Prasad R., Ilina E.S., Sukhanova M.V., Kutuzov M.M., Liu Y., Hou E.W., Wilson S.H., Lavrik O.I. Apurinic/apyrimidinic (AP) site recognition by the 5’-dRP/AP lyase in poly(ADP-ribose) polymerase-1 (PARP-1) Proc. Natl. Acad. Sci. 2010;107:22090–22095. doi: 10.1073/pnas.1009182107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prasad R., Horton J.K., Chastain P.D., Gassman N.R., Freudenthal B.D., Hou E.W., Wilson S.H. Suicidal cross-linking of PARP-1 to AP site intermediates in cells undergoing base excision repair. Nucleic Acids Res. 2014;42:6337–6351. doi: 10.1093/nar/gku288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Prasad R., Horton J.K., Dai D.-P., Wilson S.H. Repair pathway for PARP-1 DNA-protein crosslinks. DNA Rep. (Amst.) 2018:0–1. doi: 10.1016/j.dnarep.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prasad R., Horton J.K., Wilson S.H. Requirements for PARP-1 covalent crosslinking to DNA (PARP-1 DPC) DNA Rep. (Amst.) 2020;89:102824. doi: 10.1016/j.dnarep.2020.102824. [DOI] [PubMed] [Google Scholar]
- 57.Bandele O.J., Osheroff N. Annu. Rev. Biochem. 2009. Cleavage of plasmid DNA by eukaryotic topoisomerase II; pp. 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ledesma F.C., El Khamisy S.F., Zuma M.C., Osborn K., Caldecott K.W. A human 5′-tyrosyl DNA phosphodiesterase that repairs topoisomerase-mediated DNA damage. Nature. 2009;461:674–678. doi: 10.1038/nature08444. [DOI] [PubMed] [Google Scholar]
- 59.Gómez-Herreros F., Schuurs-Hoeijmakers J.H.M., McCormack M., Greally M.T., Rulten S., Romero-Granados R., Counihan T.J., Chaila E., Conroy J., Ennis S., Delanty N., Cortés-Ledesma F., De Brouwer A.P.M., Cavalleri G.L., El-Khamisy S.F., De Vries B.B.A., Caldecott K.W. TDP2 protects transcription from abortive topoisomerase activity and is required for normal neural function. Nat. Genet. 2014;46:516–521. doi: 10.1038/ng.2929. [DOI] [PubMed] [Google Scholar]
- 60.Saha S., Sun Y., Huang S.-Y., Jo U., Zhang H., Tse-Dinh Y.-C., Pommier Y. Topoisomerase 3B (TOP3B) DNA and RNA cleavage complexes and pathway to repair TOP3B-linked RNA and DNA breaks. BioRxiv. 2020 doi: 10.1101/2020.03.22.002691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neale M.J., Pan J., Keeney S. Endonucleolytic processing of covalent protein-linked DNA double-strand breaks. Nature. 2005;436:1053–1057. doi: 10.1038/nature03872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hartsuiker E., Mizuno K., Molnar M., Kohli J., Ohta K., Carr A.M. Ctp1CtIP and Rad32Mre11 nuclease activity are required for Rec12Spo11 removal, but Rec12Spo11 removal is dispensable for other MRN-dependent meiotic functions. Mol. Cell. Biol. 2009;29:1671–1681. doi: 10.1128/mcb.01182-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Milman N., Higuchi E., Smith G.R. Meiotic DNA double-strand break repair requires two nucleases, MRN and Ctp1, to produce a single size class of Rec12 (Spo11)-Oligonucleotide complexes. Mol. Cell. Biol. 2009;29:5998–6005. doi: 10.1128/mcb.01127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mimitou E.P., Yamada S., Keeney S. A global view of meiotic double-strand break end resection. Science (80-.) 2017;355:40–45. doi: 10.1126/science.aak9704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paiano J., Wu W., Yamada S., Sciascia N., Callen E., Paola Cotrim A., Deshpande R.A., Maman Y., Day A., Paull T.T., Nussenzweig A. ATM and PRDM9 regulate SPO11-bound recombination intermediates during meiosis. Nat. Commun. 2020;11:1–15. doi: 10.1038/s41467-020-14654-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Reardon J.T., Cheng Y., Sancar A. Repair of DNA-protein cross-links in mammalian cells. Cell Cycle. 2006;5:1366–1370. doi: 10.4161/cc.5.13.2892. [DOI] [PubMed] [Google Scholar]
- 67.Duxin J.P., Dewar J.M., Yardimci H., Walter J.C. Repair of a DNA-Protein crosslink by replication-coupled proteolysis. Cell. 2014;159:346–357. doi: 10.1016/j.cell.2014.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Stingele J., Schwarz M.S., Bloemeke N., Wolf P.G., Jentsch S. A DNA-dependent protease involved in DNA-protein crosslink repair. Cell. 2014;158:327–338. doi: 10.1016/j.cell.2014.04.053. [DOI] [PubMed] [Google Scholar]
- 69.Balakirev M.Y., Mullally J.E., Favier A., Assard N., Sulpice E., Lindsey D.F., Rulina A.V., Gidrol X., Wilkinson K.D. Wss1 metalloprotease partners with Cdc48/Doa1 in processing genotoxic SUMO conjugates. Elife. 2015;4:1–30. doi: 10.7554/eLife.06763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Maddi K., Sam D.K., Bonn F., Prgomet S., Tulowetzke E., Akutsu M., Lopez-Mosqueda J., Dikic I. Wss1 promotes replication stress tolerance by degrading histones. Cell Rep. 2020;30:3117–3126. doi: 10.1016/j.celrep.2020.02.018. e4. [DOI] [PubMed] [Google Scholar]
- 71.Vaz B., Popovic M., Newman J.A., Fielden J., Aitkenhead H., Halder S., Singh A.N., Vendrell I., Fischer R., Torrecilla I., Drobnitzky N., Freire R., Amor D.J., Lockhart P.J., Kessler B.M., McKenna G.W., Gileadi O., Ramadan K. Metalloprotease SPRTN/DVC1 orchestrates replication-coupled DNA-Protein crosslink repair. Mol. Cell. 2016;64:704–719. doi: 10.1016/j.molcel.2016.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lessel D., Vaz B., Halder S., Lockhart P.J., Marinovic-Terzic I., Lopez-Mosqueda J., Philipp M., Sim J.C.H., Smith K.R., Oehler J., Cabrera E., Freire R., Pope K., Nahid A., Norris F., Leventer R.J., Delatycki M.B., Barbi G., Von Ameln S., Högel J., Degoricija M., Fertig R., Burkhalter M.D., Hofmann K., Thiele H., Altmüller J., Nürnberg G., Nürnberg P., Bahlo M., Martin G.M., Aalfs C.M., Oshima J., Terzic J., Amor D.J., Dikic I., Ramadan K., Kubisch C. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat. Genet. 2014;46:1239–1244. doi: 10.1038/ng.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maskey R.S., Kim M.S., Baker D.J., Childs B., Malureanu L.A., Jeganathan K.B., Machida Y., Van Deursen J.M., Machida Y.J. Spartan deficiency causes genomic instability and progeroid phenotypes. Nat. Commun. 2014;5 doi: 10.1038/ncomms6744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lopez-Mosqueda J., Maddi K., Prgomet S., Kalayil S., Marinovic-Terzic I., Terzic J., Dikic I. SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks. Elife. 2016;5:1–19. doi: 10.7554/eLife.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mórocz M., Zsigmond E., Tóth R., Zs Enyedi M., Pintér L., Haracska L. DNA-dependent protease activity of human Spartan facilitates replication of DNA-protein crosslink-containing DNA. Nucleic Acids Res. 2017;45:3172–3188. doi: 10.1093/nar/gkw1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maskey R.S., Flatten K.S., Sieben C.J., Peterson K.L., Baker D.J., Nam H.J., Kim M.S., Smyrk T.C., Kojima Y., Machida Y., Santiago A., Van Deursen J.M., Kaufmann S.H., Machida Y.J. Spartan deficiency causes accumulation of Topoisomerase 1 cleavage complexes and tumorigenesis. Nucleic Acids Res. 2017;45:4564–4576. doi: 10.1093/nar/gkx107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsen N.B., Gao A.O., Sparks J.L., Gallina I., Wu R.A., Mann M., Räschle M., Walter J.C., Duxin J.P. Replication-coupled DNA-Protein crosslink repair by SPRTN and the proteasome in Xenopus egg extracts. Mol. Cell. 2019;73:574–588. doi: 10.1016/j.molcel.2018.11.024. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fu Y.V., Yardimci H., Long D.T., Ho V., Guainazzi A., Bermudez V.P., Hurwitz J., Van Oijen A., Schärer O.D., Walter J.C. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell. 2011;146:931–941. doi: 10.1016/j.cell.2011.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wu R.A., Semlow D.R., Kamimae-Lanning A.N., Kochenova O.V., Chistol G., Hodskinson M.R., Amunugama R., Sparks J.L., Wang M., Deng L., Mimoso C.A., Low E., Patel K.J., Walter J.C. TRAIP is a master regulator of DNA interstrand crosslink repair. Nature. 2019;567:267–272. doi: 10.1038/s41586-019-1002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moreno S.P., Jones R.M., Poovathumkadavil D., Scaramuzza S., Gambus A. Mitotic replisome disassembly depends on TRAIP ubiquitin ligase activity. Life Sci. Alliance. 2019;2:1–12. doi: 10.26508/lsa.201900390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Deng L., Wu R.A., Sonneville R., Kochenova O.V., Labib K., Pellman D., Walter J.C. Mitotic CDK promotes replisome disassembly, fork breakage, and complex DNA rearrangements. Mol. Cell. 2019;73:915–929. doi: 10.1016/j.molcel.2018.12.021. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sonneville R., Bhowmick R., Hoffmann S., Mailand N., Hickson I.D., Labib K. TRAIP drives replisome disassembly and mitotic DNA repair synthesis at sites of incomplete DNA replication. Elife. 2019;8:1–19. doi: 10.7554/eLife.48686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sparks J.L., Chistol G., Gao A.O., Räschle M., Larsen N.B., Mann M., Duxin J.P., Walter J.C. The CMG helicase bypasses DNA-Protein cross-links to facilitate their repair. Cell. 2019;176:167–181. doi: 10.1016/j.cell.2018.10.053. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kose H.B., Larsen N.B., Duxin J.P., Yardimci H. Dynamics of the eukaryotic replicative helicase at lagging-strand protein barriers support the steric exclusion model. Cell Rep. 2019;26:2113–2125. doi: 10.1016/j.celrep.2019.01.086. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoege C., Pfander B., Moldovan G.L., Pyrowolakis G., Jentsch S. RAD6-dependent DNA repair is linked to modification of PCNA by ubiquitin and SUMO. Nature. 2002;419:135–141. doi: 10.1038/nature00991. [DOI] [PubMed] [Google Scholar]
- 86.Stelter P., Ulrich H.D. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 87.Centore R.C., Yazinski S.A., Tse A., Zou L. Spartan/C1orf124, a reader of PCNA ubiquitylation and a regulator of UV-induced DNA damage response. Mol. Cell. 2012;46:625–635. doi: 10.1016/j.molcel.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li F., Raczynska J.E., Chen Z., Yu H. Structural insight into DNA-Dependent activation of human metalloprotease spartan. Cell Rep. 2019;26:3336–3346. doi: 10.1016/j.celrep.2019.02.082. e4. [DOI] [PubMed] [Google Scholar]
- 89.Popovic M. SPRTN protease and SUMOylation coordinate DNA-protein crosslink repair to prevent genome instability. BioRxiv Cell Biol. 2020 doi: 10.1101/2020.02.14.949289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Serbyn N., Noireterre A., Bagdiul I., Plank M., Michel A.H., Loewith R., Kornmann B., Stutz F. The aspartic protease Ddi1 contributes to DNA-Protein crosslink repair in yeast. Mol. Cell. 2020;77:1066–1079. doi: 10.1016/j.molcel.2019.12.007. e9. [DOI] [PubMed] [Google Scholar]
- 91.Yip M.C.J., Bodnar N.O., Rapoport T.A. Ddi1 is a ubiquitin-dependent protease. Proc. Natl. Acad. Sci. 2020;117:7776–7781. doi: 10.1073/pnas.1902298117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dirac-Svejstrup A.B., Walker J., Faull P., Encheva V., Akimov V., Puglia M., Perkins D., Kümper S., Hunjan S.S., Blagoev B., Snijders A.P., Powell D.J., Svejstrup J.Q. DDI2 is a ubiquitin-directed endoprotease responsible for cleavage of transcription factor NRF1. Mol. Cell. 2020:1–10. doi: 10.1016/j.molcel.2020.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kottemann M.C., Conti B.A., Lach F.P., Smogorzewska A. Removal of RTF2 from stalled replisomes promotes maintenance of genome integrity. Mol. Cell. 2018;69:24–35. doi: 10.1016/j.molcel.2017.11.035. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Borgermann N., Ackermann L., Schwertman P., Hendriks I.A., Thijssen K., Liu J.C., Lans H., Nielsen M.L., Mailand N. SUMOylation promotes protective responses to DNA‐protein crosslinks. EMBO J. 2019;38:e101496. doi: 10.15252/embj.2019101496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kojima Y., Machida Y., Palani S., Caulfield T.R., Radisky E.S., Kaufmann S.H., Machida Y.J. FAM111A protects replication forks from protein obstacles via its trypsin-like domain. Nat. Commun. 2020;11 doi: 10.1038/s41467-020-15170-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rios-Szwed D.O., Garcia-Wilson E., Sanchez-Pulido L., Alvarez V., Jiang H., Bandau S., Lamond A., Ponting C.P., Alabert C. FAM111A regulates replication origin activation and cell fitness. BioRxiv. 2020 doi: 10.1101/2020.04.22.055574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Minko I.G., Yamanaka K., Kozekov I.D., Kozekova A., Indiani C., O’Donnell M.E., Jiang Q., Goodman M.F., Rizzo C.J., Lloyd R.S. Replication bypass of the acrolein-mediated deoxyguanine DNA-peptide cross-links by DNA polymerases of the DinB family. Chem. Res. Toxicol. 2008;21:1983–1990. doi: 10.1021/tx800174a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Yamanaka K., Minko I.G., Takata K.I., Kolbanovskiy A., Kozekov I.D., Wood R.D., Rizzo C.J., Lloyd R.S. Novel enzymatic function of DNA Polymerase v in translesion DNA synthesis past major groove DNA-peptide and DNA-DNA cross-links. Chem. Res. Toxicol. 2010;23:689–695. doi: 10.1021/tx900449u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yeo J.E., Wickramaratne S., Khatwani S., Wang Y.C., Vervacke J., Distefano M.D., Tretyakova N.Y. Synthesis of site-specific DNA-protein conjugates and their effects on DNA replication. ACS Chem. Biol. 2014;9:1860–1868. doi: 10.1021/cb5001795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wickramaratne S., Boldry E.J., Buehler C., Wang Y.C., Distefano M.D., Tretyakova N.Y. Error-prone translesion synthesis past DNA-peptide cross-links conjugated to the major groove of DNA via C5 of thymidine. J. Biol. Chem. 2015;290:775–787. doi: 10.1074/jbc.M114.613638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tretyakova N.Y., Groehler A., Ji S. DNA-protein cross-links: formation, structural identities, and biological outcomes. Acc. Chem. Res. 2015;48:1631–1644. doi: 10.1021/acs.accounts.5b00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ji S., Fu I., Naldiga S., Shao H., Basu A.K., Broyde S., Tretyakova N.Y. 5-Formylcytosine mediated DNA–protein cross-links block DNA replication and induce mutations in human cells. Nucleic Acids Res. 2018;46:6455–6469. doi: 10.1093/nar/gky444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ghosal G., Leung J.W.C., Nair B.C., Fong K.W., Chen J. Proliferating Cell Nuclear Antigen (PCNA)-binding protein C1orf124 is a regulator of translesion synthesis. J. Biol. Chem. 2012;287:34225–34233. doi: 10.1074/jbc.M112.400135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kim M.S., Machida Y., Vashisht A.A., Wohlschlegel J.A., Pang Y.P., Machida Y.J. Regulation of error-prone translesion synthesis by Spartan/C1orf124. Nucleic Acids Res. 2013;41:1661–1668. doi: 10.1093/nar/gks1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Srivastava M., Su D., Zhang H., Chen Z., Tang M., Nie L., Chen J. HMCES safeguards replication from oxidative stress and ensures error‐free repair. EMBO Rep. 2020:1–14. doi: 10.15252/embr.201949123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Debéthune L., Kohlhagen G., Grandas A., Pommier Y. Processing of nucleopeptides mimicking the topoisomerase I-DNA covalent complex by tyrosyl-DNA phosphodiesterase. Nucleic Acids Res. 2002;30:1198–1204. doi: 10.1093/nar/30.5.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Interthal H., Champoux J.J. Effects of DNA and protein size on substrate cleavage by human tyrosyl-DNA phosphodiesterase 1. Biochem. J. 2011;436:559–566. doi: 10.1042/BJ20101841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fielden J., Wiseman K., Torrecilla I., Li S., Hume S., Chiang S.C., Ruggiano A., Narayan Singh A., Freire R., Hassanieh S., Domingo E., Vendrell I., Fischer R., Kessler B.M., Maughan T.S., El-Khamisy S.F., Ramadan K. TEX264 coordinates p97- and SPRTN-mediated resolution of topoisomerase 1-DNA adducts. Nat. Commun. 2020;11:1–16. doi: 10.1038/s41467-020-15000-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bryant H.E., Schultz N., Thomas H.D., Parker K.M., Flower D., Lopez E., Kyle S., Meuth M., Curtin N.J., Helleday T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 110.Farmer H., McCabe N., Lord C.J., Tutt A.N.J.H.J., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., Martin N.M.B.B., Jackson S.P., Smith G.C.M.M., Ashworth A., McCabe H., Lord C.J., Tutt A.N.J.H.J., Johnson D.A., Richardson T.B., Santarosa M., Dillon K.J., Hickson I., Knights C., Martin N.M.B.B., Jackson S.P., Smith G.C.M.M., Ashworth A. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 111.Fong P.C., Boss D.S., Yap T.A., Tutt A., Wu P., Mergui-Roelvink M., Mortimer P., Swaisland H., Lau A., O’Connor M.J., Ashworth A., Carmichael J., Kaye S.B., Schellens J.H.M., de Bono J.S. Inhibition of poly(ADP-Ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009;361:123–134. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 112.Pommier Y., O’Connor M.J., De Bono J. Laying a trap to kill cancer cells: PARP inhibitors and their mechanisms of action. Sci. Transl. Med. 2016;8:362ps17. doi: 10.1126/scitranslmed.aaf9246. [DOI] [PubMed] [Google Scholar]
- 113.Murai J., Huang S.N., Das B.B., Renaud A., Zhang Y., Doroshow J.H., Ji J., Takeda S., Pommier Y. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–5599. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zandarashvili L., Langelier M.-F., Velagapudi U.K., Hancock M.A., Steffen J.D., Billur R., Hannan Z.M., Wicks A.J., Krastev D.B., Pettitt S.J., Lord C.J., Talele T.T., Pascal J.M., Black B.E. Structural basis for allosteric PARP-1 retention on DNA breaks. Science (80-.) 2020;368 doi: 10.1126/science.aax6367. eaax6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strom C.E., Johansson F., Uhlen M., Szigyarto C.A.-K., Erixon K., Helleday T. Poly (ADP-ribose) polymerase (PARP) is not involved in base excision repair but PARP inhibition traps a single-strand intermediate. Nucleic Acids Res. 2011;39:3166–3175. doi: 10.1093/nar/gkq1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lomonosov M., Anand S., Sangrithi M., Davies R., Venkitaraman A.R. Stabilization of stalled DNA replication forks by the BRCA2 breast cancer susceptibility protein. Genes Dev. 2003;17:3017–3022. doi: 10.1101/gad.279003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schlacher K., Christ N., Siaud N., Egashira A., Wu H., Jasin M. Double-strand break repair-independent role for BRCA2 in blocking stalled replication fork degradation by MRE11. Cell. 2011;145:529–542. doi: 10.1016/j.cell.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Schlacher K., Wu H., Jasin M. A distinct replication fork protection pathway connects fanconi Anemia tumor suppressors to RAD51-BRCA1/2. Cancer Cell. 2012;22:106–116. doi: 10.1016/j.ccr.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Maya-Mendoza A., Moudry P., Merchut-Maya J.M., Lee M., Strauss R., Bartek J. High speed of fork progression induces DNA replication stress and genomic instability. Nature. 2018:1. doi: 10.1038/s41586-018-0261-5. [DOI] [PubMed] [Google Scholar]
- 120.Hanzlikova H., Kalasova I., Demin A.A., Pennicott L.E., Cihlarova Z., Caldecott K.W. The importance of poly(ADP-Ribose) polymerase as a sensor of unligated okazaki fragments during DNA replication. Mol. Cell. 2018;71:319–331. doi: 10.1016/j.molcel.2018.06.004. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Semlow D.R., Zhang J., Budzowska M., Drohat A.C., Walter J.C. Replication-dependent unhooking of DNA interstrand cross-links by the NEIL3 glycosylase. Cell. 2016;167:498–511. doi: 10.1016/j.cell.2016.09.008. e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hodskinson M.R., Bolner A., Sato K., Kamimae-Lanning A.N., Rooijers K., Witte M., Mahesh M., Silhan J., Petek M., Williams D.M., Kind J., Chin J.W., Patel K.J., Knipscheer P. Alcohol-derived DNA crosslinks are repaired by two distinct mechanisms. Nature. 2020;579:603–608. doi: 10.1038/s41586-020-2059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ortega-Atienza S., Green S.E., Zhitkovich A. Proteasome activity is important for replication recovery, CHK1 phosphorylation and prevention of G2 arrest after low-dose formaldehyde. Toxicol. Appl. Pharmacol. 2015;286:135–141. doi: 10.1016/j.taap.2015.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Quievryn G., Zhitkovich A. Loss of DNA-protein crosslinks from formaldehyde-exposed cells occurs through spontaneous hydrolysis and an active repair process linked to proteosome function. Carcinogenesis. 2000;21:1573–1580. doi: 10.1093/carcin/21.5.573. [DOI] [PubMed] [Google Scholar]
- 125.Ridpath J.R., Nakamura A., Tano K., Luke A.M., Sonoda E., Arakawa H., Buerstedde J.M., Gillespie D.A.F., Sale J.E., Yamazoe M., Bishop D.K., Takata M., Takeda S., Watanabe M., Swenberg J.A., Nakamura J. Cells deficient in the FANC/BRCA pathway are hypersensitive to plasma levels of formaldehyde. Cancer Res. 2007;67:11117–11122. doi: 10.1158/0008-5472.CAN-07-3028. [DOI] [PubMed] [Google Scholar]
- 126.Rosado I.V., Langevin F., Crossan G.P., Takata M., Patel K.J. Formaldehyde catabolism is essential in cells deficient for the Fanconi anemia DNA-repair pathway. Nat. Struct. Mol. Biol. 2011;18:1432–1434. doi: 10.1038/nsmb.2173. [DOI] [PubMed] [Google Scholar]
- 127.Reardon J.T., Sancar A. Repair of DNA-polypeptide crosslinks by human excision nuclease. Proc. Natl. Acad. Sci. U. S. A. 2006;103:4056–4061. doi: 10.1073/pnas.0600538103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Baker D.J., Wuenschell G., Xia L., Termini J., Bates S.E., Riggs A.D., O’Connor T.R. Nucleotide excision repair eliminates unique DNA-protein cross-links from mammalian cells. J. Biol. Chem. 2007;282:22592–22604. doi: 10.1074/jbc.M702856200. [DOI] [PubMed] [Google Scholar]
- 129.Nakano T., Morishita S., Katafuchi A., Matsubara M., Horikawa Y., Terato H., Salem A.M.H., Izumi S., Pack S.P., Makino K., Ide H. Nucleotide excision repair and homologous recombination systems commit differentially to the repair of DNA-protein crosslinks. Mol. Cell. 2007;28:147–158. doi: 10.1016/j.molcel.2007.07.029. [DOI] [PubMed] [Google Scholar]
- 130.Nakano T., Katafuchi A., Matsubara M., Terato H., Tsuboi T., Masuda T., Tatsumoto T., Pil Pack S., Makino K., Croteau D.L., Van Houten B., Iijima K., Tauchi H., Ide H. Homologous recombination but not nucleotide excision repair plays a pivotal role in tolerance of DNA-protein cross-links in mammalian cells. J. Biol. Chem. 2009;284:27065–27076. doi: 10.1074/jbc.M109.019174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Desai S.D., Liu L.F., Vazquez-Abad D., D’Arpa P. Ubiquitin-dependent destruction of topoisomerase I is stimulated by the antitumor drug camptothecin. J. Biol. Chem. 1997;272:24159–24164. doi: 10.1074/jbc.272.39.24159. [DOI] [PubMed] [Google Scholar]
- 132.Mo Y.-Y., Yu Y., Shen Z., Beck W.T. Nucleolar delocalization of human topoisomerase I in response to topotecan correlates with sumoylation of the protein. J. Biol. Chem. 2002;277:2958–2964. doi: 10.1074/jbc.M108263200. [DOI] [PubMed] [Google Scholar]
- 133.Lin C.P., Ban Y., Lyu Y.L., Desai S.D., Liu L.F. A ubiquitin-proteasome pathway for the repair of topoisomerase I-DNA covalent complexes. J. Biol. Chem. 2008;283:21074–21083. doi: 10.1074/jbc.M803493200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Caldecott K.W. Single-strand break repair and genetic disease. Nat. Rev. Genet. 2008;9:619–631. doi: 10.1038/nrg2380. [DOI] [PubMed] [Google Scholar]
- 135.Pommier Y., Sun Y., Huang S.Y.N., Nitiss J.L. Roles of eukaryotic topoisomerases in transcription, replication and genomic stability. Nat. Rev. Mol. Cell Biol. 2016;17:703–721. doi: 10.1038/nrm.2016.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Álvarez-Quilón A., Wojtaszek J.L., Mathieu M., Patel T., Appel C.D., Hustedt N., Rossi S.E., Wallace B.D., Setiaputra D., Adam S., Ohashi Y., Melo H., Cho T., Gervais C., Muñoz I.M., Grazzini E., Young J.T.F., Rouse J., Zinda M., Williams R.S., Durocher D. Endogenous DNA 3′ blocks are vulnerabilities for BRCA1 and BRCA2 deficiency and are reversed by the APE2 nuclease. Mol. Cell. 2020:1–14. doi: 10.1016/j.molcel.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Zhang Y.W., Regairaz M., Seiler J.A., Agama K.K., Doroshow J.H., Pommier Y. Poly(ADP-ribose) polymerase and XPF-ERCC1 participate in distinct pathways for the repair of topoisomerase I-induced DNA damage in mammalian cells. Nucleic Acids Res. 2011;39:3607–3620. doi: 10.1093/nar/gkq1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zeng Z., Cortés-Ledesma F., El Khamisy S.F., Caldecott K.W. TDP2/TTRAP is the major 5′-tyrosyl DNA phosphodiesterase activity in vertebrate cells and is critical for cellular resistance to topoisomerase II-induced DNA damage. J. Biol. Chem. 2011;286:403–409. doi: 10.1074/jbc.M110.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mao Y., Desai S.D., Ting C.Y., Hwang J., Liu L.F. 26 S proteasome-mediated degradation of topoisomerase II cleavable complexes. J. Biol. Chem. 2001;276:40652–40658. doi: 10.1074/jbc.M104009200. [DOI] [PubMed] [Google Scholar]
- 140.Schellenberg M.J., Lieberman J.A., Herrero-Ruiz A., Butler L.R., Williams J.G., Muñoz-Cabello A.M., Mueller G.A., London R.E., Cortés-Ledesma F., Williams R.S. ZATT (ZNF451)–mediated resolution of topoisomerase 2 DNA-protein cross-links. Science (80-.) 2017;357:1412–1416. doi: 10.1126/science.aam6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bhargava V., Goldstein C.D., Russell L., Xu L., Ahmed M., Li W., Casey A., Servage K., Kollipara R., Picciarelli Z., Kittler R., Yatsenko A., Carmell M., Orth K., Amatruda J.F., Yanowitz J.L., Buszczak M. GCNA preserves genome integrity and fertility across species. Dev. Cell. 2020;52:38–52. doi: 10.1016/j.devcel.2019.11.007. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Dokshin G.A., Davis G.M., Sawle A.D., Eldridge M.D., Nicholls P.K., Gourley T.E., Romer K.A., Molesworth L.W., Tatnell H.R., Ozturk A.R., de Rooij D.G., Hannon G.J., Page D.C., Mello C.C., Carmell M.A. GCNA interacts with spartan and topoisomerase II to regulate genome stability. Dev. Cell. 2020;52:53–68. doi: 10.1016/j.devcel.2019.11.006. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gómez-Herreros F., Romero-Granados R., Zeng Z., Álvarez-Quilón A., Quintero C., Ju L., Umans L., Vermeire L., Huylebroeck D., Caldecott K.W., Cortés-Ledesma F. TDP2-dependent non-homologous end-joining protects against topoisomerase II-Induced DNA breaks and genome instability in cells and in vivo. PLoS Genet. 2013;9 doi: 10.1371/journal.pgen.1003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cannavo E., Cejka P. Sae2 promotes dsDNA endonuclease activity within Mre11-Rad50-Xrs2 to resect DNA breaks. Nature. 2014;514:122–125. doi: 10.1038/nature13771. [DOI] [PubMed] [Google Scholar]
- 145.Deshpande R.A., Lee J.H., Arora S., Paull T.T. Nbs1 converts the human Mre11/Rad50 nuclease complex into an endo/exonuclease machine specific for protein-DNA adducts. Mol. Cell. 2016;64:593–606. doi: 10.1016/j.molcel.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 146.Hoa N.N., Shimizu T., Zhou Z.W., Wang Z.Q., Deshpande R.A., Paull T.T., Akter S., Tsuda M., Furuta R., Tsusui K., Takeda S., Sasanuma H. Mre11 is essential for the removal of lethal topoisomerase 2 covalent cleavage complexes. Mol. Cell. 2016;64:580–592. doi: 10.1016/j.molcel.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 147.Mao Y., Desai S.D., Liu L.F. SUMO-1 conjugation to human DNA topoisomerase II isozymes. J. Biol. Chem. 2000;275:26066–26073. doi: 10.1074/jbc.M001831200. [DOI] [PubMed] [Google Scholar]
- 148.Carmell M.A., Dokshin G.A., Skaletsky H., Hu Y.-C., van Wolfswinkel J.C., Igarashi K.J., Bellott D.W., Nefedov M., Reddien P.W., Enders G.C., Uversky V.N., Mello C.C., Page D.C. A widely employed germ cell marker is an ancient disordered protein with reproductive functions in diverse eukaryotes. Elife. 2016;5:1–25. doi: 10.7554/eLife.19993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wühr M., Güttler T., Peshkin L., McAlister G.C., Sonnett M., Ishihara K., Groen A.C., Presler M., Erickson B.K., Mitchison T.J., Kirschner M.W., Gygi S.P. The nuclear proteome of a vertebrate. Curr. Biol. 2015;25:2663–2671. doi: 10.1016/j.cub.2015.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun Y., Jenkins L.M.M., Su Y.P., Nitiss K.C., Nitiss J.L., Pommier Y. A conserved SUMO-Ubiquitin pathway directed by RNF4/SLX5-SLX8 and PIAS4/SIZ1 drives proteasomal degradation of topoisomerase DNA-protein crosslinks. BioRxiv. 2019 doi: 10.1101/707661. [DOI] [Google Scholar]
- 151.Nakano T., Ouchi R., Kawazoe J., Pack S.P., Makino K., Ide H. T7 RNA polymerases backed up by covalently trapped proteins catalyze highly error prone transcription. J. Biol. Chem. 2012;287:6562–6572. doi: 10.1074/jbc.M111.318410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ji S., Thomforde J., Rogers C., Fu I., Broyde S., Tretyakova N.Y. Transcriptional bypass of DNA-protein and DNA-peptide conjugates by T7 RNA polymerase. ACS Chem. Biol. 2019;14:2564–2575. doi: 10.1021/acschembio.9b00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Vermeulen W., Fousteri M. Mammalian transcription-coupled excision repair. Cold Spring Harb. Perspect. Biol. Med. 2013;5:a012625. doi: 10.1101/cshperspect.a012625. [DOI] [PMC free article] [PubMed] [Google Scholar]