Abstract
The phosphatidylinositol 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signalling pathway is involved in multiple cellular processes, including cell survival, proliferation, differentiation, metabolism and cytoskeletal reorganisation. The downstream effectors of this PI3K pathway are also essential for maintaining physiologic homeostasis, commonly dysregulated in most solid tumours. AKT is the key regulator in PI3K/AKT/mTOR signalling, interacting with multiple intracellular molecules. AKT activation subsequently leads to a number of potential downstream effects, and its aberrant activation results in the pathogenesis of cancer. Accordingly, as an attractive therapeutic target for cancer treatment, several AKT inhibitors are currently under development and in multiple stages of clinical trials for various types of malignancy, including gastric cancer (GC). Therefore, the authors review the significance of AKT and recent studies on AKT inhibitors in GC, focusing on the scientific background with the potential to improve treatment outcomes.
Keywords: gastric cancer, AKT
Introduction
The prognosis for metastatic or recurrent gastric cancer (GC) remains very poor, making it the third leading cause of cancer-related death worldwide.1 Although effective combination cytotoxic chemotherapies have been introduced and two targeted agents, trastuzumab and ramucirumab, have approved for GC treatment in first-line and second-line settings, the outcomes are still unsatisfactory.2–5 More recently, immune checkpoint inhibitiors (ICIs) with antiprogrammed cell death protein-1 monoclonal antibodies, such as pembrolizumab and nivolumab, have led to durable and impressive responses in a minority of GC.6 7 However, a significant proportion of patients does not respond to these therapies, plus the efficacies seem to vary depending on the tumour biology.8 GC is well known as a complex and heterogeneous disease with various treatment outcomes.9 Interestingly, two recent molecular classifications have provided insights on the heterogeneous nature of GC. The Cancer Genome Atlas Research Network suggested a comprehensive molecular characterisation of 295 GCs using various platforms, and proposed four distinct subtypes: Epstein-Barr virus-positive, microsatellite instability (MSI), genomically stable and tumours with chromosomal instability.10 Another group also described four subgroups: MSI, microsatellite stable (MSS)/epithelial-to-mesenchymal transition, MSS/TP53 positive and MSS/TP53 negative.11 Each subtype is characterised by specific gene mutations and alterations in multiple signalling pathways. Consequently, these biologic investigations have improved clinical awareness of linking tumour profiling to the selection of molecularly targeted therapies. Plus, targeting the appropriate molecules may be a promising approach for precision medicine in GC treatment.
Among these molecules, AKT, also known as protein kinase B, is a serine–threonine kinase downstream of the phosphatidylinositol 3-kinase (PI3K) signalling pathway, which controls multiple cellular processes, such as cell survival, proliferation, differentiation, metabolism and cytoskeletal reorganisation.12 13 Functioning as a major effector protein in the PI3K pathway, AKT modulates normal cellular physiology like cell growth, motility, proliferation, metabolism and survival.14 AKT is also one of the most hyperactivated kinases in human cancers, influencing various biological phenomena that are directly involved in tumourigenesis.15 Frequent activation of AKT has also been reported in approximately 78% of GC.16 Kobayashi et al demonstrated that increased AKT kinase activity was associated with a higher grade and poor prognosis in GC.17 Moreover, in another molecular classification proposed by the Singapore-Duke group, mesenchymal subtype GC cell lines were found to be particularly sensitive to the PI3K/AKT/mammalian target of rapamycin (mTOR) pathway inhibitors.18 Therefore, cumulative evidence indicates a promising potential for targeting AKT for the effective treatment of GC. This review focuses on the role of AKT and highlights the results of recent clinical trials on AKT inhibition in GC.
Overview of PI3K signalling pathway and AKT activation
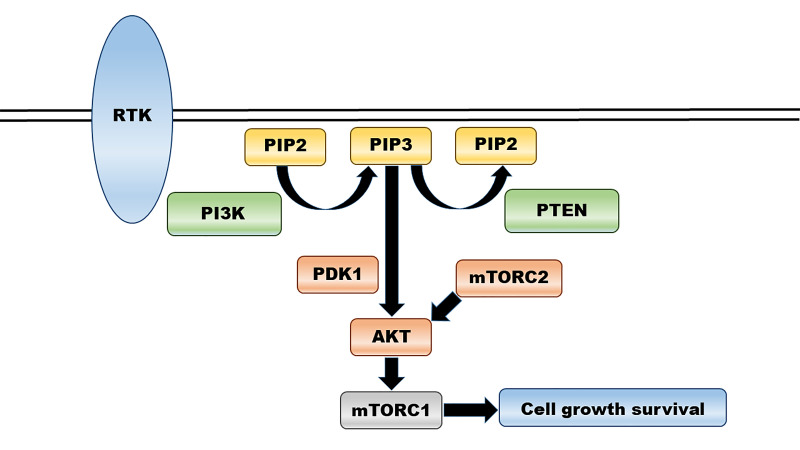
The upstream target proteins of AKT are stimuli-induced receptor tyrosine kinases (RTKs) and include PI3K, mTOR complex 2 (mTORC2) and phosphoinositide-dependent protein kinase 1 (PDK1, figure 1).14 19 The growth factor-mediated stimulation of RTKs interacts with src-homology 2 domains in PI3K, subsequently activating PI3K. This leads to the conversion of phosphatidylinositol 4,5-diphosphate (PIP2) into phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 acts as a second messenger to recruit AKT to the plasma membrane, and the binding of AKT to PIP3 induces a conformational change that results in the phosphorylation of AKT at Ser473 by mTORC2 and Thr308 by PDK1.20 Phosphorylated AKT is an active form and promotes cell growth via the phosphorylation of mTOR complex 1. Important negative regulators of the PI3K/AKT/mTOR pathway also include the tumour suppressor gene, phosphatase and tensin homologue (PTEN), which dephosphorylate PIP3 back to PIP2. As mentioned above, AKT is an important signalling hub with numerous downstream substrates, affecting cell growth, proliferation, survival, cellular metabolism, glucose uptake and even angiogenesis.20 21 AKT has been identified in three isoforms (AKT1, AKT2 and AKT3) transcribed from different genes and sharing an 80% amino acid homology.22 Each member contains three conserved domains including a plekstrin homology (PH) domain in the N-terminal, central kinase domain and hydrophobic C-terminal tail (figure 2).23 In general, AKT1 is widely expressed, the expression of AKT2 is elevated in insulin-responsive metabolic tissues and AKT3 is more highly expressed in endocrine and brain tissues.19 20 Although still unclear how each isoform carries out a specific functional role, AKT1 promotes growth and survival, while AKT2 controls cellular invasiveness and mesenchymal characteristics.22 In particular, AKT3 is known to play an essential role in physiologic brain development.24
Figure 1.
Overview of PI3K/AKT/mTOR signalling pathway. AKT, protein kinase B; mTORC1, mammalian target of rapamycin complex 1; mTORC2, mammalian target of rapamycin complex 2; PI3K, phosphatidylinositol 3-kinase; PDK1, phosphoinositide-dependent protein kinase 1; PIP2, phosphatidylinositol 4,5-diphosphate; PIP3, phosphatidylinositol 3,4,5-triphosphate; PTEN, phosphatase and tensin homologue; RTK, receptor tyrosine kinase.
Figure 2.
Schematic representation of domain structure of AKT. AKT, protein kinase B; PH, plekstrin homology.
AKT in GC and its importance
The PI3K/AKT/mTOR pathway is the most commonly dysregulated signalling pathway in human cancer.12 Common abnormalities and alterations in cancer include activating mutations or amplification of growth factors or RTKs, activating mutations in the p110α catalytic subunit of PI3K, loss of function and deletions in PTEN and activating mutations or amplification of AKT.14 19 21 For instance, aberrations in the PI3K signalling pathway have been discovered in 38% of all tumour types, with PTEN loss by immunohistochemistry (IHC) occurring most frequently (30%), followed by mutations in PIK3CA (13%), PTEN (6%) and AKT1 (1%).20 25 In GC, the PI3K/AKT/mTOR pathway has also been heavily implicated in both tumourigenesis and disease progression.26 One early study by Staal et al reported a 20-fold amplification of AKT1 in one of every five GC cases in a survey of 225 human tumours.27 It is well known that AKT2 is a frequently amplified isoform, while AKT1 is the most commonly mutated isoform in most solid tumours.20 An AKT1 point mutation in the PH domain that substitutes an amino acid (E17K) is the most predominantly reported mutation, ranging from 0% to 4%, and confers increased activity due to pathological localisation of AKT1 in the plasma membrane.25 28 However, AKT1 E17K mutation is very rare in GC.29 Notably, a study of 294 cancer tissues detected AKT2 mutations in 1 of every 51 GC cases (2.0%), yet no mutations of AKT1 or AKT3.30 In addition to E17K, E49K, L52R, C77F and Q19K mutants are known to activate AKT1, and G171R has been identified in the kinase domain of AKT3 in various types of cancer.21 23 Meanwhile, AKT2 gene amplification has been reported in 5%–15% of pancreatic, ovarian and breast cancers.15 31–34
Unlike other cancers, the amplification of AKT2 has not been fully explored in GC.28 Plus, a recent study showed a low amplification frequency of AKT1 of 0.3% in GC.35 A study of 311 GC cases found increased AKT activity in 78%, as determined by IHC.16 Cinti et al examined 50 resected GC cases and phosphorylated AKT was detected in 68% of the tumours, which correlated with the tumour aggressiveness.36 Similarly, a link has been shown between AKT overexpression and advanced disease, suggesting a prognostic role in GC.17 37 Therefore it is possible that AKT is activated through other mechanisms other than mutations. The importance of AKT in the PI3K pathway extends to its role in tumours with other known alterations or in the regulation of AKT signalling by microRNAs (miRNAs). A recent study reported that human epidermal growth factor receptor 2 (HER2) overexpression was significantly associated with phosphorylated AKT expression in GC tissues.38 In this study, phosphorylated AKT expression was also correlated with poor prognosis, indicating that the PI3K/AKT pathway plays a critical role in HER2-positive GC. Another study showed that cytoplasmic AKT expression was markedly increased in PIK3CA-mutant tumours, providing a strong rationale for the clinical exploration of PI3K/AKT inhibitor combinations. Interestingly, geridonin and paclitaxel could synergistically inhibit the proliferation of GC cells via the suppression of AKT signalling.39 Moreover, the mesenchymal subtype classified by the Singapore-Duke group is particularly sensitive to the PI3K/AKT/mTOR pathway inhibitors.18 In addition, Lu et al observed that metastasis-associated lung adenocarcinoma transcript 1 competitively binds to miRNA-181a-5p, thereby upregulating AKT3 protein levels and promoting tumour growth in GC.40 Thus, overall, these findings demonstrate the significance of AKT as a mediator of cellular proliferation and effective target for drug development in GC.
Pan-AKT inhibitors in GC
Several promising AKT inhibitors have already been developed and are currently in various stages of clinical trials. There are two categories of AKT inhibitors.20 21 The first type is a competitive or allosteric inhibitor of the ATP-biding site in the kinase domain, while the second type interacts with the PH domain of AKT, thereby preventing localisation of AKT in the plasma membrane. Most AKT inhibitors in clinical development inhibit all AKT isoforms referred as pan-AKT inhibitors.21 An overview of clinical evidence of AKT inhibitors in GC is briefly summarised in table 1.
Table 1.
Clinical evidence of AKT inhibitors in GC
| Drug | Phase | Setting | Geographic region | Primary endpoints | Treatment arms | Patient number | RR (%) | PFS (months) | OS (months) | Common AEs | Reference |
| Ipatasertib | Randomised phase II | First-line | Europe, USA, Asia | PFS* | Ipatasertib/mFOLFOX6† mFOLFOX6 | 71 82 |
52 56 |
6.57 7.52 |
12.12 15.67 |
Diarrhoea, nausea, anorecia nausea, anorexia, neuropathy |
Bang et al54 |
| Capivasertib | Phase I/II biomarker-driven umbrella trial | Second-line | South Korea | RR | Capivasertib/paclitaxel | 24 | 33.3 | – | – | Hyperglycaemic, neutropenia, anaemia, diarrhoea | Lee et al35 |
| Afuresertib | Phase Ib | Second-line | Asia | MTD/RP2D | Afuresertib/paclitaxel | 29 | Pending results | NCT02240212 | |||
| Miransertib | Preclinical study | AKT3 was upregulated in the majority of E-cadherin-deficient GCs, and mouse-derived gastric organoids lacking tumour suppressor gene CDH1 were sensitive to the apoptotic effects of miransertib. | Bougen-Zhukov et al82 | ||||||||
*The coprimary endpoints were PFS in the intention-to-treat and biomarker-defined subgroup with PTEN low patients (<10%) as determined by IHC.
†The mFOLFOX6 regimen consisted of oxaliplatin (85 mg/m2 intravenous infusion on day 1 every 14 days) coadministered with leucovorin 400 mg/m2, then 5-fluorouracil 400 mg/m2 administered as bolus infusion, followed by 5-fluorouracil 2400 mg/m2 continuous infusion over 46 to 48 hours.
AEs, adverse events; GCs, gastric cancers; IHC, immunohistochemistry; MTD, maximum tolerated dose; OS, overall survival; PFS, progression-free survival; PTEN, phosphatase and tensin homologue; RP2D, recommended phase II dose; RR, response rate.
Ipatasertib (GDC-0068)
Ipatasertib is a highly selective oral ATP-competitive pan-AKT inhibitor (IC50=5.0–18.0 nM) which preferentially targets the phosphorylated conformation of AKT.41 42 Ipatasertib exhibits antitumour effects against several cancer cell lines and xenograft models by inhibiting the PI3K/AKT pathway.43 44 A first phase I study of ipatasertib demonstrated that the maximum tolerated dose (MTD) was 600 mg once daily with the two dose-limiting toxicity (DLT) at a dose level of 800 mg.45 In terms of safety, the predominant ipatasertib-related adverse events (AEs) are diarrhoea, nausea, asthenia/fatigue and rashes. The incidence of clinically significant hyperglycaemic or rashes is relatively low with ipatasertib compared with other agents against PI3K signalling. Plus, at the MTD of 600 mg for ipatasertib, 11 (44%) of 25 patients showed the best overall response. An additional biomarker study of the same clinical samples revealed a compensatory feedback activation of extracellular-signal-regulated protein kinase and human epidermal growth factor receptor 3.46 Accordingly, several phase II studies have evaluated the efficacy and safety for patients with triple-negative breast cancer (TNBC) and castration-resistant prostate cancer (CRPC).47–49 LOng-Term follow-Up Study (LOTUS) is a randomised, double-blind, placebo-controlled, phase II study designed to investigate the efficacy of ipatasertib plus paclitaxel in treatment-naive locally advanced or metastatic TNBC.50 As a result, the combination of paclitaxel/ipatasertib demonstrated an improved PFS, one of two coprimary endpoints (6.2 vs 4.9 months, HR 0.6, p=0.037). Of note, in the subset of patients with PIK3CA/AKT1/PTEN-altered tumours (n = 42), the treatment benefit derived from ipatasertib was greater in patients with PIK3CA/AKT1/PTEN-altered tumours identified through next-generation sequencing (9.0 vs 4.9 months, HR 0.44, p=0.04). The ipatasertib–paclitaxel doublet in LOTUS was generally tolerated. AKT inhibition has also been studied in a neoadjuvant setting via the randomised phase II FAIRLANE trial.51 Although the addition of ipatasertib to neoadjuvant paclitaxel did not significantly increase the pathologic complete response (CR) rate, the overall response rate (ORR) by MRI was numerically higher with ipatasertib. Furthermore, the antitumour effect of ipatasertib was higher in the biomarker-selected patients and all the patients who showed CR had PIK3CA/AKT1/PTEN-altered tumours. Similar to these findings, a combined blockade with abiraterone and ipatasertib exhibited superior antitumour activity to abiraterone alone in patients with metastatic CRPC, especially tumours with PTEN loss.52 53 Therefore, these evidence indicates that the identification of reliable biomarkers related with the PI3K/AKT pathway is important to facilitate precise patient selection and increase the clinical benefit to this agent. Consequently, ipatasertib continues to be actively investigated in several phase I and II clinical trials and separate phase III trials for TNBC and CRPC (table 2). In addition to these combination studies, several AKT inhibitors including ipatasertib are also being studied as a main drug in biomarker-driven trials.41
Table 2.
Pan-AKT inhibitors in clinical development
| Drug | Mechanism | Tumour types | Phase | Study design/combination Partner |
Cliniclatrials.gov Number |
| Ipatasertib (GDC-0068) | ATP-competitive pan-AKT inhibitor | Breast | Ib | Trastuzumab/pertuzumab | NCT04253561 |
| Breast/ovary/prostate | Ib | Rucaparib | NCT03840200 | ||
| Breast | I/Ib | Carboplatin or carboplatin/paclitaxel | NCT03853707 | ||
| Solid tumours | I | Atezolizumab | NCT03673787 | ||
| Brain tumour | IIb | Pembrolizumab | NCT02430363 | ||
| Breast | Ib | Atezolizumb/paclitaxel | NCT03800836 | ||
| Atezolizumab/Nab-paclitaxel | |||||
| Breast | Ib | Aromatase inhibitors or fulvestrant or fulvestrant/palbociclib | NCT03959891 | ||
| Prostate | Ib/II | Abiraterone or abiraterone/apitolisib | NCT01485861 | ||
| Breast | II | Atezolizumab or atezolizumab/bevacizumab | NCT03395899 | ||
| Breast | III | Paclitaxel | NCT03337724 | ||
| Breast | III | Atezolizumab/paclitaxel | NCT04177108 | ||
| Prostate | III | Abiraterone/prednisolone | NCT03072238 | ||
| Capivasertib (AZD5363) | ATP-competitive pan-AKT inhibitor | Solid tumours | I | Olaparib | NCT02338622 |
| Solid tumours | I | Olaparib/durvalumab | NCT03772561 | ||
| Breast/ovary/endometrium | Ib | Olaparib | NCT02208375 | ||
| Prostate | I | Enzalutamide or abiraterone | NCT04087174 | ||
| Prostate | II | Enazalutamide | NCT02525068 | ||
| Breast | III | Paclitaxel | NCT03997123 | ||
| Afuresertib (GSK2110183) | ATP-competitive pan-AKT inhibitor | Prostate | I/II | LAE001/prednisolone | NCT04060394 |
| Stomach | Ib* | Paclitaxel | NCT02240212 | ||
| Uprosertib (GSK2141795) | ATP-competitive pan-AKT inhibitor | Multiple myeloma | II* | Trametinib | NCT01989598 |
| Miransertib (ARQ 092) | Allosteric pan-AKT and AKT1 E17K inhibitor | Ovary | Ib* | Paclitaxel or paclitaxel/carboplatin or anastrozole | NCT02476955 |
Biomarker-driven studies have been excluded from this list.
*Clinicaltrials.gov has noted no active patient enrolment in this study.
A recent randomised phase II trial assessed ipatasertib plus standard chemotherapy (oxaliplatin, leucovorin and 5-fluorouracil) versus chemotherapy alone in 153 patients with GC/gastro-oesophageal junction cancer as the first-line therapy.54 Two coprimary endpoints were PFS in the intention-to-treat and biomarker-defined subgroup of patients with PTEN low (<10%) as determined by IHC. However, this trial did not meet its two endpoints for PFS and no significant difference with respect to overall survival was observed between the two groups. Plus, patients with PIK3CA mutations or amplification demonstrated no benefit with the combination study treatment. The most common AEs were nausea, diarrhoea and decreased appetite. Higher rates of severe AEs were also reported in the ipatasertib arm with common toxicity being diarrhoea, vomiting and nausea. Although the safety results were consistent with known AE profiles and there was no newly reported toxicity, the lower dose intensity of ipatasertib plus chemotherapy and higher rate of withdrawal in the experimental arm may have contributed to the lower-than-expected efficacy. There is an uncertainty regarding the best combination for ipatasertib in GC, raising the question of the interplay between ipatasertib and the chemotherapy backbone. Another consideration is that the treatment arm included more patients with poor prognostic factors, including diffuse histology, peritoneal metastasis and a higher number of metastatic sites. Ipatasertib activity may also be masked by heterogeneity of the study population, highlighting the potential need to identify specific biomarkers in order to maximise the efficacy of ipatasertib. Thus, due to low efficacy and toxicity concerns, it is unclear whether ipatasertib with conventional cytotoxic chemotherapy will be developed for GC. Notwithstanding, more recent studies are investigating the combination of ipatasertib with ICIs, such as pembrolizumab and atezolizumab, in breast and brain cancer (table 2). The outcomes of these studies will hopefully provide insights on AKT inhibition combined with chemoimmunotherapy and may become an important step for future studies in GC.
Capivasertib (AZD5363)
Capivasertib is another orally bioavailable, potent ATP-competitive pan-AKT inhibitor (IC50=3.0–7.0 nM) that inhibits the phosphorylation of AKT substrates.20 Several preclinical studies have demonstrated that capivasertib inhibits the growth of xenografts derived from a range of solid tumour types.55 56 Capivasertib also significantly enhances the anticancer activity of docetaxel in GC xenograft models harbouring either PI3KCA mutation or PTEN loss.57 In a phase I study using one continuous and two intermittent dosing schedules, the recommended phase II dose was 480 mg twice daily for 4 days followed by 3 days off, where the common AEs were diarrhoea (78%), nausea (49%) and hyperglycaemic (20%).58 In particular, preliminary expansion data for patients with AKT1 E17K-mutant tumours showed very encouraging antitumour activity.59 Another phase I study conducted in Japan also confirmed an intermittent dose of 480 mg to be the recommended dose, with partial response (PR) reported in two patients with AKT1 E17K mutations.60 This data indicate that the presence of the AKT1 E17K mutation could be a potential response biomarker for this agent.20 However, as noted before, AKT1 E17K mutations are very uncommon in GC.
This has led to several phase II studies of capivasertib in combination with paclitaxel or fulvestrant in breast cancer.49 The phase II PAKT study investigated the addition of capivasertib to paclitaxel as the first-line therapy in 140 patients with metastatic TNBC.61 The addition of capivasertib to first-line paclitaxel therapy for TNBC resulted in significantly longer PFS and OS. Plus, benefits were more pronounced in patients with PIK3CA/AKT1/PTEN-altered tumours. Capivasertib was also tested in a phase I/II FAKTION study of 140 postmenopausal women with metastatic oestrogen receptor (ER)-positive/HER2-negative breast cancer who had not received more than three previous lines of endocrine treatment and up to one line of chemotherapy for metastatic disease.62 63 In this study, the primary endpoint of PFS was longer in the experimental arm (10.3 vs 4.8 months, HR 0.58, p=0.004). A further subgroup analysis also showed that the PI3K pathway alteration status did not seem to change the effect of capivasertib, In contrast to these results, a recent phase Ib/II BEECH trial investigated the efficacy of capivasertib in combination with first-line weekly paclitaxel for ER-positive/HER2-negative breast cancer show no significant PFS benefits in the overall population or PIK3CA-positive subpopulation.64 Therefore, these results require further confirmation via ongoing phase III trial. Subsequent clinical studies and biomarker-driven trials have since validated the combination of capivasertib with other agents (table 2). For example, preliminary data demonstrated a synergistic effect of capivasertib in combination with olaparib, indicating that inhibitors of the P13K/AKT pathway potentiate a cytostatic effect of poly ADP-ribose polymerase (PARP) inhibitors in a combination therapy.65 Recently, Lee et al performed a biomarker-based umbrella trial (VIKTORY; targeted agent eValuation In gastric cancer basket KORea) in GC.35 This study classified patients with metastatic GC based on eight different biomarker groups and, among 10 associated clinical trials, included a treatment arm with capivasertib plus paclitaxel for PIK3CA mutation. It is noteworthy that the PIK3CA mutation capivasertib arm demonstrated moderate antitumour activity with an ORR of 33.3% in second-line GC, especially when compared with the low response rate (<15%) for the arm with PIK3CA wild-type capivasertib. As a result, these findings provide a strong rationale that such combinations with capivasertib and paclitaxel require further evaluation in additional phase II/III trials.
Afuresertib (GSK2110183)
Afuresertib is an oral ATP-competitive pan-AKT inhibitor (IC50=0.08–2.6 nM) that has been evaluated in a phase I study involving patients with haematologic malignancies.66 On the basis of two DLTs in a 150 mg cohort, the recommended monotherapy phase II dose from this study was established at an MTD of 125 mg/day. This study showed a favourable safety profile and afuresertib appeared to be clinically active in multiple myeloma (MM). The drug-related AEs included nausea (23.3%), diarrhoea (20.5%), dyspepsia (19.2%) and fatigue (16.4%). Three patients with MM attained PR and an additional three attained minimal responses. Plus, in preclinical setting, enhanced antitumour activity was observed in MM cells when afuresertib was combined with pomalidomide plus dexamethasone. In a different phase I trial, the effect of afuresertib was examined in combination with trametinib, a mitogen-activated protein kinase (MEK) inhibitor, in 20 patients with advanced solid tumours and MM.67 For these patient groups, an intermittent dosing schedule of a trametinib/afuresertib combination was shown to be more tolerable than continuous dosing. Thereafter, afuresertib was evaluated as a monotherapy or in combination with other anticancer agents in one phase I or two phase II studies. A phase IIa trial by Arceci et al demonstrated clinical activity (ORR=31%) associated with afuresertib monotherapy in patients with langerhans cell histiocytosis.68 In a phase Ib study, a combination of afuresertib with carboplatin and paclitaxel in recurrent platinum-resistant ovarian cancer showed a favourable pharmacokinetic and toxicity profile with an MTD of afuresertib defined as 125 mg a day.69 Chen et al also reported that combination therapy with ofatumumab and afuresertib was active and well-tolerated in previously treated patients with chronic lymphocytic leukaemia.70 Based on its documented activity in other solid tumours, afuresertib in combination with paclitaxel in a second-line setting is currently testing in a phase Ib study in an unselected population (NCT02240212). Recently, many studies have revealed that the MEK/extracellular signal-regulated kinase (ERK) pathway is involved in regulating cell survival and proliferation in GC.71 Moreover, some tumours can harbour genomic alterations in both the PI3K/AKT and MEK/ERK signalling network.20 It is worth noting that afuresertib in combination with MEK inhibitor was shown to be beneficial for advanced solid tumours. Thus, this has significant implications for the clinical development of such combinations in GC. Plus, afuresertib is undergoing continued testing in combination with targeted therapies and chemotherapies in several phase I and II studies (table 2).
Uprosertib (GSK2141795)
Uprosertib is an oral ATP-competitive pan-AKT kinase inhibitor (IC50=38.0–328.0 nM) that has shown enhanced antitumour effects in combination with an MEK inhibitor in a pancreatic cancer tumour model.72 In a phase I trial of uprosertib in patients with solid tumours, the recommended phase II dose of uprosertib for once-daily dosing was 75 mg, where the most common treatment-related AEs included diarrhoea, fatigue, vomiting and a decreased appetite.73 In this study, PR was seen in two patients with a PIK3CA mutation and PTEN loss, respectively. Plus, a continuous dosing schedule of uprosertib in combination with trametinib was implemented in a separate phase I study.74 Based on these results, uprosertib was evaluated in a number of clinical studies measuring the impact of a combination with trametinib. For example, a phase II clinical trial investigated the combination of uprosertib with trametinib in patients with acute myeloid leukaemia with Kirsten ras oncogene homologue (KRAS) and Neuroblastoma ras viral oncogene homologue mutations.75 Despite the preliminary biological efficacy of this combination, no clinical benefit was observed. Exploration of the same combination in recurrent cervical cancer also revealed minimal clinical benefit.76 When exploring a dosing of uprosertib at 50 mg and trametinib at 1.5 mg, the study closed early as the drug combination caused an unacceptable toxicity profile. This dosing combination also yielded limited clinical efficacy in recurrent endometrial cancer, with no responses at the previously recommended phase II dose.77 Therefore, at this point in time, there are no ongoing studies, except for the treatment of MM (table 2).
Miransertib (ARQ 092)
Miransertib is an allosteric pan-AKT and AKT1 E17K mutant inhibitor (IC50=2.7–14.0 nM) that exhibits antitumour effects in vitro and in vivo as a monotherapy or in combination with paclitaxel.78 In preliminary data from a phase Ib trial, the combination of miransertib and anastrozole demonstrated a manageable safety profile and preliminary efficacy in patients with PIK3CA or AKT1-mutant endometrial or ovarian cancer.79 The most frequent AEs included a rash, hyperglycaemic and elevated liver enzyme. Durable PR was achieved in two patients with PIK3CA mutations. Meanwhile, miransertib is also being studied for the treatment of Proteus syndrome, which is a very rare mosaic overgrowth disorder caused by a somatic E17K activating mutation in the oncogene AKT1.80 Recently, Leoni et al reported a case of successful treatment with miransertib of a patient with Proteus syndrome that resulted in relapsed AKT1 E17K mutant ovarian cancer, and clinical and serological remission after 22 months of treatment.81 Despite insufficient data to support clinical cancer therapy, promising reports of antitumour activity in patients harbouring AKT1 E17K mutations support the use of biomarker-driven strategies in further clinical development of this drug. Interestingly, Bougen-Zhukov et al observed that AKT3 was upregulated in the majority of E-cadherin-deficient GCs, and mouse-derived gastric organoids lacking tumour suppressor gene CDH1 were sensitive to the apoptotic effects of miransertib.82 This identification may be useful for providing new therapeutic strategies for hereditary and sporadic GC with mutations in the CDH1 gene, since germline truncation mutations in the CDH1 gene are found in 30%–50% families with hereditary diffuse GC.83
Clinical perspective: resistance, toxicity and biomarkers
The PI3K/AKT/mTOR pathway comprises a complex network of crosstalk with many parallel cascades, so its inhibition induces negative feedback resulting in activation of other compensatory signalling pathways.84 Theoretically, AKT inhibitors relieve feedback inhibition of upstream molecules and reactivate the PI3K signalling pathway by increasing the expression and phosphorylation of multiple RTKs and downstream effectors.85 Indeed, emerging evidence supports the role of RTKs in restoring PI3K signalling following AKT inhibition.86 Recent studies have demonstrated that other kinases can interact with AKT and stimulate cellular transformation without requiring the PI3K signalling pathway.22 For example, in ER-positive breast cancer, a complex cycle with cross-regulatory interactions is already established between ER and the growth factor receptor network, leading to enhanced cell growth and proliferation in an AKT-independent manner.87 Alteration of PDK1 can also activate other downstream molecules of the PI3K pathway via substrates other than AKT, such as serum-inducible and glucocorticoid-inducible protein kinase, mitogen-activated protein kinase or protein kinase C alpha.22 In addition to PDK1, several studies have revealed AKT-independent protumouric features of mTORC2.88 89 Moreover, the loss of PTEN and numerous miRNAs may closely contribute to the resistance to these pan-AKT inhibitors in GC.90 91 To overcome this resistance, combination therapy regimens have been or are being tested in both preclinical and clinical settings as described in table 2. However, potential adverse effects have largely restricted the application and clinical significance of these inhibitors. While the underlying mechanisms of the PI3K/AKT/mTOR pathway inhibitor-associated toxicity are not well understood, they are likely related to a widespread role in intracellular signal transduction.92 Similar to mTOR inhibitors, AKT inhibitors often have common toxicities, such as metabolic effects, cutaneous and mucosal effects, non-infectious pneumonitis, immunosuppression and constitutional symptoms.84 Of note, pan-AKT inhibitors inevitably cause hyperglycaemic due to the influence of these inhibitors on glucose homeostasis.13 In addition, the incidence and severity of AKT inhibitor-related AEs seem to be modest and better tolerated than the AEs caused by PI3K inhibitors. To decrease these toxicities, AKT isoform-specific inhibitors like MK-2206, an allosteric inhibitor with activity against AKT1 and AKT2, could be used in conjunction with a biomarker-driven approach.13 However, concerns remain regarding the use of isoform-specific inhibitors due to a potential compensatory response that may lead to the hyperactivation of other AKT isoforms.93
Identification of more robust predictive biomarkers is critical to optimise treatment with these agents and avoid severe AEs and a subtherapeutic dose that often results in inadequate pathway inhibition and resistance to these agents.13 To date, genomic alterations related with the PI3K/AKT/mTOR signalling pathway have been widely studied as potential biomarkers of response or resistance to AKT inhibitors.94 The PIK3CA mutation status has been implemented as a predictive marker for AKT inhibitors. Plus, accumulating evidence on novel biomarkers, such as AKT mutations including AKT1 E17K, PIK3CA amplification, PTEN expression loss, KRAS mutations or the insulin level, has shown encouraging results in several studies.94 However, the relationship between these biomarkers and the therapeutic effect of AKT inhibitors still remains unclear. Tumour heterogeneity can influence such conflicting results, and the genetic alterations vary according to various types of cancer. Thus, researchers are needed to develop more precise and standardised methods for analysing these mutations to obtain convincing data. Consequently, the challenge remains to apply the data generated through further validated clinical trials into clinical practice in order to provide precision medicine for GC in the near future.
Conclusion
Multiple studies have already explored the promising potential of AKT inhibitors as monotherapies or in combination with cytotoxic and other targeted therapies for many cancers, including several validation studies for patients with GC, although recent data from a phase II study showed limited clinical activity with toxicity concerns, even in molecularly selected populations. Therefore, future research needs to focus on the detection of appropriate high-precision biomarkers with a better understanding of the complexities of the PI3K/AKT/mTOR signalling pathway, while a biomarker-driven approach, like the VIKTORY trial, can optimise the therapeutic efficacy of AKT inhibitors in GC. Plus, the potential role of the AKT1 E17K mutation as a predictive marker needs to be explored in well-designed large-scale studies. Additional efforts are also being made to identify the best combination partners for synergistic effects and lower toxicity. This may lead to a better therapeutic index and broaden the usefulness of AKT inhibitors to overcome chemoresistance, and thereby improve treatment outcomes. In particular, it is important to assess the impact of combining ICIs with AKT inhibitors, as ICIs have become an important part of treating GC, plus the PI3K/AKT/mTOR pathway plays an essential role in the immune system. In conclusion, additional trials are needed to refine the benefits of AKT inhibitors, including specific evidence for patients with GC.
Acknowledgments
IC would like to acknowledge National Health Service funding to the National Institute for Health Research Biomedical Research Centre at the Royal Marsden NHS Foundation Trust and The Institute of Cancer Research.
Footnotes
Contributors: BWK and IC contributed to this manuscript and gave final approval.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: IC—Advisory Board: Eli-Lilly, Bristol Meyers Squibb, MSD, Bayer, Roche, Merck-Serono, Five Prime Therapeutics, Astra-Zeneca, Oncologie International; Pierre FabreResearch funding: Eli-Lilly, Janssen-Cilag, Sanofi Oncology; Merck-SeronoHonorarium: Eli-Lilly.
Patient consent for publication: Not required.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Pellino A, Riello E, Nappo F, et al. Targeted therapies in metastatic gastric cancer: current knowledge and future perspectives. World J Gastroenterol 2019;25:5773–88. 10.3748/wjg.v25.i38.5773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bang Y-J, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687–97. 10.1016/S0140-6736(10)61121-X [DOI] [PubMed] [Google Scholar]
- 4.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (regard): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014;383:31–9. 10.1016/S0140-6736(13)61719-5 [DOI] [PubMed] [Google Scholar]
- 5.Wilke H, Muro K, Van Cutsem E, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (rainbow): a double-blind, randomised phase 3 trial. Lancet Oncol 2014;15:1224–35. 10.1016/S1470-2045(14)70420-6 [DOI] [PubMed] [Google Scholar]
- 6.Catenacci Daniel V, Wainberg Z, Fuchs Charles S, et al. KEYNOTE-059 cohort 3: safety and efficacy of pembrolizumab monotherapy for first-line treatment of patients (PTS) with PD-L1-positive advanced gastric/gastroesophageal (G/GEJ) cancer. Ann Oncol 2017;28:iii153 10.1093/annonc/mdx302.008 [DOI] [Google Scholar]
- 7.Kang Y-K, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;390:2461–71. 10.1016/S0140-6736(17)31827-5 [DOI] [PubMed] [Google Scholar]
- 8.Chau I. Clinical development of PD-1/PD-L1 immunotherapy for gastrointestinal cancers: facts and hopes. Clin Cancer Res 2017;23:6002–11. 10.1158/1078-0432.CCR-17-0020 [DOI] [PubMed] [Google Scholar]
- 9.Jeon J, Cheong J-H. Clinical implementation of precision medicine in gastric cancer. J Gastric Cancer 2019;19:235–53. 10.5230/jgc.2019.19.e25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014;513:202–9. 10.1038/nature13480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. Nat Med 2015;21:449–56. 10.1038/nm.3850 [DOI] [PubMed] [Google Scholar]
- 12.Fruman DA, Chiu H, Hopkins BD, et al. The PI3K pathway in human disease. Cell 2017;170:605–35. 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Janku F, Yap TA, Meric-Bernstam F. Targeting the PI3K pathway in cancer: are we making headway? Nat Rev Clin Oncol 2018;15:273–91. 10.1038/nrclinonc.2018.28 [DOI] [PubMed] [Google Scholar]
- 14.Song M, Bode AM, Dong Z, et al. AKT as a therapeutic target for cancer. Cancer Res 2019;79:1019–31. 10.1158/0008-5472.CAN-18-2738 [DOI] [PubMed] [Google Scholar]
- 15.Noorolyai S, Shajari N, Baghbani E, et al. The relation between PI3K/Akt signalling pathway and cancer. Gene 2019;698:120–8. 10.1016/j.gene.2019.02.076 [DOI] [PubMed] [Google Scholar]
- 16.Nam SY, Lee HS, Jung G-A, et al. Akt/Pkb activation in gastric carcinomas correlates with clinicopathologic variables and prognosis. APMIS 2003;111:1105–13. 10.1111/j.1600-0463.2003.apm1111205.x [DOI] [PubMed] [Google Scholar]
- 17.Kobayashi I, Semba S, Matsuda Y, et al. Significance of Akt phosphorylation on tumor growth and vascular endothelial growth factor expression in human gastric carcinoma. Pathobiology 2006;73:8–17. 10.1159/000093087 [DOI] [PubMed] [Google Scholar]
- 18.Lei Z, Tan IB, Das K, et al. Identification of molecular subtypes of gastric cancer with different responses to PI3-kinase inhibitors and 5-fluorouracil. Gastroenterology 2013;145:554–65. 10.1053/j.gastro.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 19.Hoxhaj G, Manning BD. The PI3K–AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer 2020;20:74–88. 10.1038/s41568-019-0216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown JS, Banerji U. Maximising the potential of AKT inhibitors as anti-cancer treatments. Pharmacol Ther 2017;172:101–15. 10.1016/j.pharmthera.2016.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mundi PS, Sachdev J, McCourt C, et al. AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol 2016;82:943–56. 10.1111/bcp.13021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Faes S, Dormond O. PI3K and AKT: Unfaithful partners in cancer. Int J Mol Sci 2015;16:21138–52. 10.3390/ijms160921138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Revathidevi S, Munirajan AK. Akt in cancer: mediator and more. Semin Cancer Biol 2019;59:80–91. 10.1016/j.semcancer.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez E, McGraw TE. The Akt kinases: isoform specificity in metabolism and cancer. Cell Cycle 2009;8:2502–8. 10.4161/cc.8.16.9335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millis SZ, Ikeda S, Reddy S, et al. Landscape of phosphatidylinositol-3-kinase pathway alterations across 19 784 diverse solid tumors. JAMA Oncol 2016;2:1565–73. 10.1001/jamaoncol.2016.0891 [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka T, Yashiro M. The role of PI3K/Akt/mTOR signaling in gastric carcinoma. Cancers 2014;6:1441–63. 10.3390/cancers6031441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Staal SP. Molecular cloning of the akt oncogene and its human homologues Akt1 and Akt2: amplification of Akt1 in a primary human gastric adenocarcinoma. Proc Natl Acad Sci U S A 1987;84:5034–7. 10.1073/pnas.84.14.5034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bellacosa A, Kumar CC, Di Cristofano A, et al. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res 2005;94:29–86. 10.1016/S0065-230X(05)94002-5 [DOI] [PubMed] [Google Scholar]
- 29.Kim MS, Jeong EG, Yoo NJ, et al. Mutational analysis of oncogenic AKT E17K mutation in common solid cancers and acute leukaemias. Br J Cancer 2008;98:1533–5. 10.1038/sj.bjc.6604212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soung YH, Lee JW, Nam SW, et al. Mutational analysis of AKT1, AKT2 and AKT3 genes in common human carcinomas. Oncology 2006;70:285–9. 10.1159/000096289 [DOI] [PubMed] [Google Scholar]
- 31.Altomare DA, Testa JR. Perturbations of the AKT signaling pathway in human cancer. Oncogene 2005;24:7455–64. 10.1038/sj.onc.1209085 [DOI] [PubMed] [Google Scholar]
- 32.Cheng JQ, Godwin AK, Bellacosa A, et al. AKT2, a putative oncogene encoding a member of a subfamily of protein-serine/threonine kinases, is amplified in human ovarian carcinomas. Proc Natl Acad Sci U S A 1992;89:9267–71. 10.1073/pnas.89.19.9267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cheng JQ, Ruggeri B, Klein WM, et al. Amplification of AKT2 in human pancreatic cells and inhibition of AKT2 expression and tumorigenicity by antisense RNA. Proc Natl Acad Sci U S A 1996;93:3636–41. 10.1073/pnas.93.8.3636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clark AR, Toker A. Signalling specificity in the Akt pathway in breast cancer. Biochem Soc Trans 2014;42:1349–55. 10.1042/BST20140160 [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Kim ST, Kim K, et al. Tumor genomic profiling guides patients with metastatic gastric cancer to targeted treatment: the VIKTORY umbrella trial. Cancer Discov 2019;9:1388–405. 10.1158/2159-8290.CD-19-0442 [DOI] [PubMed] [Google Scholar]
- 36.Cinti C, Vindigni C, Zamparelli A, et al. Activated Akt as an indicator of prognosis in gastric cancer. Virchows Arch 2008;453:449–55. 10.1007/s00428-008-0676-8 [DOI] [PubMed] [Google Scholar]
- 37.Murakami D, Tsujitani S, Osaki T, et al. Expression of phosphorylated Akt (pAkt) in gastric carcinoma predicts prognosis and efficacy of chemotherapy. Gastric Cancer 2007;10:45–51. 10.1007/s10120-006-0410-7 [DOI] [PubMed] [Google Scholar]
- 38.Sukawa Y, Yamamoto H, Nosho K, et al. HER2 expression and PI3K-Akt pathway alterations in gastric cancer. Digestion 2014;89:12–17. 10.1159/000356201 [DOI] [PubMed] [Google Scholar]
- 39.Wang S-Q, Wang C, Chang L-M, et al. Geridonin and paclitaxel act synergistically to inhibit the proliferation of gastric cancer cells through ROS-mediated regulation of the PTEN/PI3K/Akt pathway. Oncotarget 2016;7:72990–3002. 10.18632/oncotarget.12166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu Z, Luo T, Pang T, et al. MALAT1 promotes gastric adenocarcinoma through the MALAT1/miR-181a-5p/AKT3 axis. Open Biol 2019;9:190095–95. 10.1098/rsob.190095 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Huck BR, Mochalkin I. Recent progress towards clinically relevant ATP-competitive Akt inhibitors. Bioorg Med Chem Lett 2017;27:2838–48. 10.1016/j.bmcl.2017.04.090 [DOI] [PubMed] [Google Scholar]
- 42.Prêtre V, Wicki A. Inhibition of Akt and other AGC kinases: a target for clinical cancer therapy? Semin Cancer Biol 2018;48:70–7. 10.1016/j.semcancer.2017.04.011 [DOI] [PubMed] [Google Scholar]
- 43.Blake JF, Xu R, Bencsik JR, et al. Discovery and preclinical pharmacology of a selective ATP-competitive Akt inhibitor (GDC-0068) for the treatment of human tumors. J Med Chem 2012;55:8110–27. 10.1021/jm301024w [DOI] [PubMed] [Google Scholar]
- 44.Lin J, Sampath D, Nannini MA, et al. Targeting activated Akt with GDC-0068, a novel selective Akt inhibitor that is efficacious in multiple tumor models. Clin Cancer Res 2013;19:1760–72. 10.1158/1078-0432.CCR-12-3072 [DOI] [PubMed] [Google Scholar]
- 45.Saura C, Roda D, Roselló S, et al. A first-in-human phase I study of the ATP-competitive AKT inhibitor Ipatasertib demonstrates robust and safe targeting of Akt in patients with solid tumors. Cancer Discov 2017;7:102–13. 10.1158/2159-8290.CD-16-0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yan Y, Serra V, Prudkin L, et al. Evaluation and clinical analyses of downstream targets of the Akt inhibitor GDC-0068. Clin Cancer Res 2013;19:6976–86. 10.1158/1078-0432.CCR-13-0978 [DOI] [PubMed] [Google Scholar]
- 47.Chan JJ, Tan TJY, Dent RA. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Ther Adv Med Oncol 2019;11:1758835919880429–29. 10.1177/1758835919880429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat 2018;169:397–406. 10.1007/s10549-018-4697-y [DOI] [PubMed] [Google Scholar]
- 49.Lyons TG. Targeted therapies for triple-negative breast cancer. Curr Treat Options Oncol 2019;20:82. 10.1007/s11864-019-0682-x [DOI] [PubMed] [Google Scholar]
- 50.Kim S-B, Dent R, Im S-A, et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (Lotus): a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2017;18:1360–72. 10.1016/S1470-2045(17)30450-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oliveira M, Saura C, Nuciforo P, et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann Oncol 2019;30:1289–97. 10.1093/annonc/mdz177 [DOI] [PubMed] [Google Scholar]
- 52.de Bono JS, De Giorgi U, Rodrigues DN, et al. Randomized phase II study evaluating Akt blockade with Ipatasertib, in combination with abiraterone, in patients with metastatic prostate cancer with and without PTEN loss. Clin Cancer Res 2019;25:928–36. 10.1158/1078-0432.CCR-18-0981 [DOI] [PubMed] [Google Scholar]
- 53.Doi T, Fujiwara Y, Matsubara N, et al. Phase I study of ipatasertib as a single agent and in combination with abiraterone plus prednisolone in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2019;84:393–404. 10.1007/s00280-019-03882-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bang Y-J, Kang Y-K, Ng M, et al. A phase II, randomised study of mFOLFOX6 with or without the Akt inhibitor ipatasertib in patients with locally advanced or metastatic gastric or gastroesophageal junction cancer. Eur J Cancer 2019;108:17–24. 10.1016/j.ejca.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 55.Davies BR, Greenwood H, Dudley P, et al. Preclinical pharmacology of AZD5363, an inhibitor of Akt: pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background. Mol Cancer Ther 2012;11:873–87. 10.1158/1535-7163.MCT-11-0824-T [DOI] [PubMed] [Google Scholar]
- 56.Toren P, Kim S, Cordonnier T, et al. Combination AZD5363 with enzalutamide significantly delays Enzalutamide-resistant prostate cancer in preclinical models. Eur Urol 2015;67:986–90. 10.1016/j.eururo.2014.08.006 [DOI] [PubMed] [Google Scholar]
- 57.Li J, Davies BR, Han S, et al. The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to Taxotere. J Transl Med 2013;11:241–41. 10.1186/1479-5876-11-241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Banerji U, Dean EJ, Pérez-Fidalgo JA, et al. A Phase I open-label study to identify a dosing regimen of the Pan-AKT inhibitor AZD5363 for evaluation in solid tumors and in PIK3CA-mutated breast and gynecologic cancers. Clin Cancer Res 2018;24:2050–9. 10.1158/1078-0432.CCR-17-2260 [DOI] [PubMed] [Google Scholar]
- 59.Hyman DM, Smyth LM, Donoghue MTA, et al. AKT inhibition in solid tumors with AKT1 mutations. J Clin Oncol 2017;35:2251–9. 10.1200/JCO.2017.73.0143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamura K, Hashimoto J, Tanabe Y, et al. Safety and tolerability of AZD5363 in Japanese patients with advanced solid tumors. Cancer Chemother Pharmacol 2016;77:787–95. 10.1007/s00280-016-2987-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schmid P, Abraham J, Chan S, et al. Capivasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer: the pAkt trial. J Clin Oncol 2020;38:423–33. 10.1200/JCO.19.00368 [DOI] [PubMed] [Google Scholar]
- 62.Jones RH, Casbard A, Carucci M, et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive breast cancer (FAKTION): a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2020;21:345–57. 10.1016/S1470-2045(19)30817-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Presti D, Quaquarini E. The PI3K/AKT/mTOR and CDK4/6 pathways in endocrine resistant HR+/HER2- metastatic breast cancer: biological mechanisms and new treatments. Cancers 2019;11:1242. 10.3390/cancers11091242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Turner NC, Alarcón E, Armstrong AC, et al. BEECH: a dose-finding run-in followed by a randomised phase II study assessing the efficacy of AKT inhibitor capivasertib (AZD5363) combined with paclitaxel in patients with estrogen receptor-positive advanced or metastatic breast cancer, and in a PIK3CA mutant sub-population. Ann Oncol 2019;30:774–80. 10.1093/annonc/mdz086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gallyas F, Sumegi B, Szabo C. Role of Akt activation in PARP inhibitor resistance in cancer. Cancers 2020;12:532. 10.3390/cancers12030532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spencer A, Yoon S-S, Harrison SJ, et al. The novel AKT inhibitor afuresertib shows favorable safety, pharmacokinetics, and clinical activity in multiple myeloma. Blood 2014;124:2190–5. 10.1182/blood-2014-03-559963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tolcher AW, Patnaik A, Papadopoulos KP, et al. Phase I study of the MEK inhibitor trametinib in combination with the AKT inhibitor afuresertib in patients with solid tumors and multiple myeloma. Cancer Chemother Pharmacol 2015;75:183–9. 10.1007/s00280-014-2615-5 [DOI] [PubMed] [Google Scholar]
- 68.Arceci RJ, Allen CE, Dunkel IJ, et al. A phase IIA study of afuresertib, an oral pan-AKT inhibitor, in patients with Langerhans cell histiocytosis. Pediatr Blood Cancer 2017;64:e26325. 10.1002/pbc.26325 [DOI] [PubMed] [Google Scholar]
- 69.Blagden SP, Hamilton AL, Mileshkin L, et al. Phase Ib dose escalation and expansion study of Akt inhibitor Afuresertib with carboplatin and paclitaxel in recurrent platinum-resistant ovarian cancer. Clin Cancer Res 2019;25:1472–8. 10.1158/1078-0432.CCR-18-2277 [DOI] [PubMed] [Google Scholar]
- 70.Chen CI, Paul H, Le LW, et al. A phase 2 study of ofatumumab (Arzerra®) in combination with a pan-AKT inhibitor (afuresertib) in previously treated patients with chronic lymphocytic leukemia (CLL). Leuk Lymphoma 2019;60:92–100. 10.1080/10428194.2018.1468892 [DOI] [PubMed] [Google Scholar]
- 71.Yang M, Huang C-Z. Mitogen-activated protein kinase signaling pathway and invasion and metastasis of gastric cancer. World J Gastroenterol 2015;21:11673–9. 10.3748/wjg.v21.i41.11673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nandan D, Zhang N, Yu Y, et al. Miransertib (ARQ 092), an orally-available, selective Akt inhibitor is effective against Leishmania. PLoS One 2018;13:e0206920–e20. 10.1371/journal.pone.0206920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aghajanian C, Bell-McGuinn KM, Burris HA, et al. A phase I, open-label, two-stage study to investigate the safety, tolerability, pharmacokinetics, and pharmacodynamics of the oral AKT inhibitor GSK2141795 in patients with solid tumors. Invest New Drugs 2018;36:1016–25. 10.1007/s10637-018-0591-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tolcher AW, Kurzrock R, Valero V, et al. Phase I dose-escalation trial of the oral AKT inhibitor uprosertib in combination with the oral MEK1/MEK2 inhibitor trametinib in patients with solid tumors. Cancer Chemother Pharmacol 2020;85:673–83. 10.1007/s00280-020-04038-8 [DOI] [PubMed] [Google Scholar]
- 75.Ragon BK, Odenike O, Baer MR, et al. Oral MEK 1/2 inhibitor trametinib in combination with Akt inhibitor GSK2141795 in patients with acute myeloid leukemia with Ras mutations: a phase II study. Clin Lymphoma Myeloma Leuk 2019;19:431–40. 10.1016/j.clml.2019.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu JF, Gray KP, Wright AA, et al. Results from a single arm, single stage phase II trial of trametinib and GSK2141795 in persistent or recurrent cervical cancer. Gynecol Oncol 2019;154:95–101. 10.1016/j.ygyno.2019.05.003 [DOI] [PubMed] [Google Scholar]
- 77.Westin SN, Sill MW, Coleman RL, et al. Safety lead-in of the MEK inhibitor trametinib in combination with GSK2141795, an AKT inhibitor, in patients with recurrent endometrial cancer: an NRG Oncology/GOG study. Gynecol Oncol 2019;155:420–8. 10.1016/j.ygyno.2019.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu Y, Savage RE, Eathiraj S, et al. Targeting AKT1-E17K and the PI3K/AKT pathway with an allosteric AKT inhibitor, ARQ 092. PLoS One 2015;10:e0140479–e79. 10.1371/journal.pone.0140479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hyman D, Bonafede M, O'Cearbhaill R, et al. Abstract CT035: a phase Ib study of miransertib (ARQ 092) in combination with anastrozole in patients with PIK3CA or AKT1-mutant ER+ endometrial or ovarian cancer. Cancer Res 2018;78. [Google Scholar]
- 80.Keppler-Noreuil KM, Sapp JC, Lindhurst MJ, et al. Pharmacodynamic study of Miransertib in individuals with Proteus syndrome. Am J Hum Genet 2019;104:484–91. 10.1016/j.ajhg.2019.01.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Leoni C, Gullo G, Resta N, et al. First evidence of a therapeutic effect of miransertib in a teenager with Proteus syndrome and ovarian carcinoma. Am J Med Genet A 2019;179:1319–24. 10.1002/ajmg.a.61160 [DOI] [PubMed] [Google Scholar]
- 82.Bougen-Zhukov N, Nouri Y, Godwin T, et al. Allosteric AKT inhibitors target synthetic lethal vulnerabilities in E-Cadherin-Deficient cells. Cancers 2019;11:1359. 10.3390/cancers11091359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pharoah PD, Guilford P, Caldas C, et al. Incidence of gastric cancer and breast cancer in CDH1 (E-cadherin) mutation carriers from hereditary diffuse gastric cancer families. Gastroenterology 2001;121:1348–53. 10.1053/gast.2001.29611 [DOI] [PubMed] [Google Scholar]
- 84.Lee JJ, Loh K, Yap Y-S. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med 2015;12:342–54. 10.7497/j.issn.2095-3941.2015.0089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Costa C, Bosch A. The strategy of PIKing a target: what is AKTually most effective? Clin Cancer Res 2018;24:2029–31. 10.1158/1078-0432.CCR-17-3440 [DOI] [PubMed] [Google Scholar]
- 86.Chandarlapaty S, Sawai A, Scaltriti M, et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 2011;19:58–71. 10.1016/j.ccr.2010.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Vasan N, Toska E, Scaltriti M. Overview of the relevance of PI3K pathway in HR-positive breast cancer. Ann Oncol 2019;30:x3–11. 10.1093/annonc/mdz281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Guertin DA, Stevens DM, Saitoh M, et al. mTOR complex 2 is required for the development of prostate cancer induced by PTEN loss in mice. Cancer Cell 2009;15:148–59. 10.1016/j.ccr.2008.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Roulin D, Cerantola Y, Dormond-Meuwly A, et al. Targeting mTORC2 inhibits colon cancer cell proliferation in vitro and tumor formation in vivo. Mol Cancer 2010;9:57. 10.1186/1476-4598-9-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Papa A. The PTEN–PI3K axis in cancer. Biomolecules 2019;9:153 10.3390/biom9040153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang S-M, Huang C, Li X-F, S-m Y, Huang C LX-f, et al. miR-21 confers cisplatin resistance in gastric cancer cells by regulating PTEN. Toxicology 2013;306:162–8. 10.1016/j.tox.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 92.Sanchez V, Nichols C, Kim H, et al. Targeting PI3K signaling in acute lymphoblastic leukemia. Int J Mol Sci 2019;20:412 10.3390/ijms20020412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang Q, Chen X, Hay N. Akt as a target for cancer therapy: more is not always better (lessons from studies in mice). Br J Cancer 2017;117:159–63. 10.1038/bjc.2017.153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brandão M, Caparica R, Eiger D, et al. Biomarkers of response and resistance to PI3K inhibitors in estrogen receptor-positive breast cancer patients and combination therapies involving PI3K inhibitors. Ann Oncol 2019;30:x27–42. 10.1093/annonc/mdz280 [DOI] [PMC free article] [PubMed] [Google Scholar]