Abstract
Objectives
To approximate the rate of familial myasthenia gravis and the coexistence of other autoimmune disorders in the patients and their families.
Design
Retrospective cohort study.
Setting
Clinics across North America.
Participants
The study included 1032 patients diagnosed with acetylcholine receptor antibody (AChR)-positive myasthenia gravis.
Methods
Phenotype information of 1032 patients diagnosed with AChR-positive myasthenia gravis was obtained from clinics at 14 centres across North America between January 2010 and January 2011. A critical review of the epidemiological literature on the familial rate of myasthenia gravis was also performed.
Results
Among 1032 patients, 58 (5.6%) reported a family history of myasthenia gravis. A history of autoimmune diseases was present in 26.6% of patients and in 28.4% of their family members.
Discussion
The familial rate of myasthenia gravis was higher than would be expected for a sporadic disease. Furthermore, a high proportion of patients had a personal or family history of autoimmune disease. Taken together, these findings suggest a genetic contribution to the pathogenesis of myasthenia gravis.
Keywords: epidemiology, neuromuscular disease, genetics, neurology, neurogenetics
Strengths and limitations of this study.
A strength of this study is that it analyses a large cohort of patients with myasthenia gravis with complete data on each patient, allowing multiple clinical correlations to be made.
A strength of this study is that standardised criteria were used to diagnose patients with myasthenia gravis, including establishing the specific subtype of the disease for each patient.
A strength of this study is that the cohort of patients with myasthenia gravis was sufficiently large to allow the generation of evidence confirming a genetic contribution to the disease.
A limitation of this study is the reliance on self-reported family history status for both myasthenia gravis and other autoimmune diseases by the patients.
A limitation of this study is its retrospective design, which precludes ascertaining additional information from individual patients.
Introduction
Myasthenia gravis is a rare autoimmune disease that is characterised by antibody-mediated interference with neuromuscular transmission at the neuromuscular junction. Originally, the role for an autoimmune attack against the acetylcholine receptor (AChR) on the postsynaptic side of the neuromuscular junction was recognised.1 However, over the past decade, additional autoimmune targets have been identified: muscle-specific tyrosine kinase (MuSK), agrin and lipoprotein receptor-related protein 4 (LRP4).2–4 All of these proteins play essential roles in maintaining the structure and function of the neuromuscular junction.
Myasthenia gravis manifests clinically with muscle weakness and fatigability. Symptoms of ocular muscle weakness are observed early in 85% of patients with myasthenia gravis.5 These symptoms include diplopia and/or ptosis. Weakness of the bulbar musculature is observed in 60% of patients with myasthenia gravis, with symptoms including dysarthria and/or dysphagia.5
A recent literature review of 31 epidemiological studies suggests that the annual incidence of myasthenia gravis may range between 3 and 30 cases per million people.6 A common theme among myasthenia gravis epidemiological studies is that there is a significant degree of variability between cohorts. For example, a study of a Hong Kong Chinese cohort reported an incidence of 4 per million people per year, whereas a study of an English cohort reported an incidence of 30 per million people per year.7 8 This substantial degree of variability may be the result of inconsistent diagnostic criteria, varying case ascertainment, lack of physician awareness concerning myasthenia gravis or it may reflect different occurrence across populations.
Historically, adult-onset myasthenia gravis has been regarded as a sporadic disease with only a minimal genetic component.9 However, genome-wide association studies, fine-mapping studies and epidemiological studies of myasthenia gravis suggest a genetic contribution to the disease.10 11 In fact, studies have described patients with myasthenia gravis that have a family history of myasthenia gravis and/or a family history of autoimmune diseases.12–16 In this study, we performed a literature search of the familial rate reported by myasthenia gravis epidemiological studies and, using our cohort of 1032 North American patients with myasthenia gravis, approximated the prevalence of familial myasthenia gravis, compared the characteristics of familial disease with sporadic disease and assessed the comorbidity of other autoimmune diseases among patients and among their families.
Methods
Patient ascertainment
Phenotype information of 1032 patients diagnosed with myasthenia gravis was obtained from myasthenia gravis clinics at 14 centres across North America between January 2010 and January 2011.10 The numbers of patients with myasthenia gravis attending each of these clinics were not available for this study. Patients were diagnosed by neurologists specialising in myasthenia gravis. Each myasthenia gravis diagnosis was based on standard clinical criteria that included, but was not limited to, weakness, fatigability and electrophysiological, pharmacological (edrophonium test) and/or serological abnormalities. Inclusion criteria for this study were as follows: confirmed diagnosis of myasthenia gravis, non-Hispanic white ethnicity and the presence of anti-AChR antibodies. Patients with anti-MuSK antibodies were excluded from the study. The LRP4 antibody was discovered after the collection of the cohort was complete. Thus, the LRP4 antibody status of the patients was not known. Family histories of myasthenia gravis and other autoimmune diseases were systematically obtained for each subject using a simple structured questionnaire (see online supplementary table S1). A positive family history was defined as having a first-degree (~50% of DNA in common), second-degree (~25% of DNA in common) or third-degree (~12.5% of DNA in common) relative with the disease. DNA samples were collected from each subject and used for genetic analyses as previously reported.10 Patients with genetic forms of myasthenia gravis were not explicitly excluded, though none in the cohort was known to have such a mutation.
bmjopen-2020-037909supp001.pdf (40.2KB, pdf)
Literature review methodology
To find studies about the epidemiology of familial myasthenia gravis, the PubMed and Medline databases were searched using permutations of search terms: ‘epidemiology of familial myasthenia gravis’, ‘epidemiology of myasthenia gravis’ and ‘heritability of myasthenia gravis’. After reviewing 31 epidemiological studies between the years of 1950 through 2018, only 10 studies explicitly referenced a family history of myasthenia gravis. Five of those papers provided metrics about family members with myasthenia gravis.12–16
Patient and public involvement statement
No patients or members of the public were actively involved with co-producing the research presented in this article.
Results
North American cohort of patients with myasthenia gravis
Clinical data were collected from a total of 1032 patients across 14 centres in North America and were analysed in this study. All of the patients had positive anti-AChR antibodies. The median age of symptom onset in this clinic-based cohort was 58 years of age (range=4–91; median onset age for females=46; median onset age for males=62). The female-to-male ratio was 1:1.27. Early-onset disease (<40 years of age) was observed among 248 (24.0%) of the cohort. Consistent with other reports, nearly one-third of the patients in our North American study cohort (305, 29.6%) had undergone thymectomy.
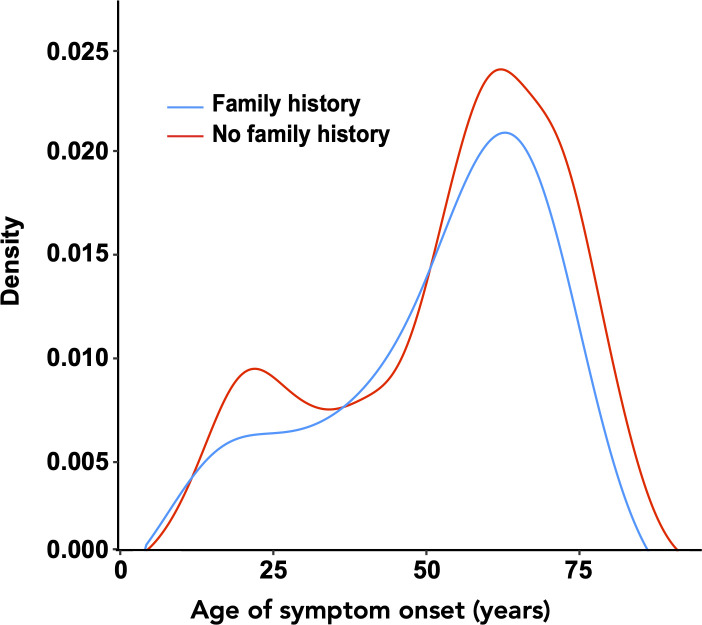
Fifty-eight (5.6%) patients had a family history of myasthenia gravis. Sibling–sibling (31.0%) and parent–child (32.8%) were the most common types of familial relationship, followed by uncle/aunt–nephew/niece (13.8%), cousin–cousin (12.1%) and grandparent–grandchild (5.2%) relationships. Of note, the indicated percentage of parent–child cases might be inflated because neonatal myasthenia gravis cases (which are not believed to be genetic) could not be discerned from non-neonatal cases. Age at symptom onset was similar among patients with a family history compared with patients without a family history (57.5 years of age, range=8–80 years vs 58.5 years, range=4–91 years, p value=0.183, Wilcoxon rank-sum test, figure 1, table 1).
Figure 1.
Symptom-onset age distribution of sporadic and familial cases. The density represents the relative probability of myasthenia gravis at each age point.
Table 1.
Comparison of familial and sporadic cases among a cohort of patients diagnosed with myasthenia gravis (n=1032)
| Familial | Sporadic | P value | |
| Number of myasthenia gravis cases (%) | 58 (5.6) | 974 (94.4) | – |
| Median age of disease onset (years) (range) | 57.5 (8–80) | 58.5 (4–91) | 0.183 |
| Number of patients with early-onset disease (<40 years) (%) | 15 (25.9) | 233 (23.9) | 0.86 |
| Number of females (%) | 25 (43.1) | 429 (44.0) | 0.997 |
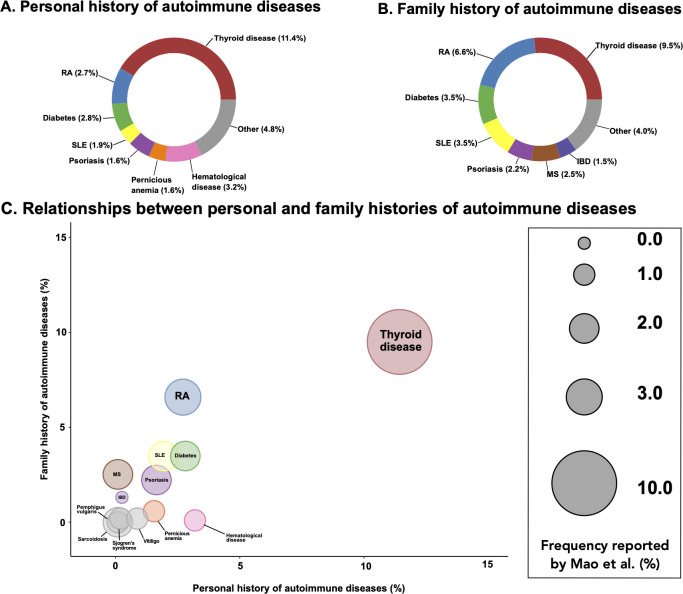
A personal history of an autoimmune disease other than myasthenia gravis was reported in 275 (26.6%) of study participants. A total of 293 (28.4%) subjects had a family history of an autoimmune disease. The prevalence of autoimmune disease in the general population is ~3%–9%, indicating that the rates observed among our myasthenia gravis subjects are significantly increased.17 A breakdown of the specific autoimmune diseases for both personal history and family history of disease is shown in figure 2A, B.
Figure 2.
Autoimmune diseases in a cohort of 1032 patients with myasthenia gravis. (A) Occurrence of autoimmune diseases among patients with myasthenia gravis (n=275). (B) Occurrence of autoimmune diseases among familial relatives of patients with myasthenia gravis (n=293). (C) Comparisons of autoimmune diseases among patients with myasthenia gravis and among relatives. Size of each circle represents the percentage previously reported by Mao et al.18
The most common autoimmune diseases present concomitantly in patients with myasthenia gravis were thyroid disease (n=118, 11.4%), haematological disease (n=33, 3.2% consisting of autoimmune haemolytic anaemia and autoimmune thrombocytopenic purpura) and rheumatoid arthritis (n=28, 2.7%). This was similar to the rates previously reported by Mao et al (figure 2C).18
The three most common autoimmune diseases present in the families of patients with myasthenia gravis were thyroid disease (n=98, 9.5%), rheumatoid arthritis (n=68, 6.6%) and type 1 diabetes (n=36, 3.5%).
Literature review
Literature review concerning the epidemiology of familial myasthenia gravis identified five studies that discussed the patient’s family history of myasthenia gravis with sample sizes that ranged from 264 to 6638 (table 2).12–16 The frequency of familial myasthenia gravis ranged from 0.2% to 7.2% across studies, the upper limit having been reported for a Finnish cohort.15 Among these, three studies reported that familial myasthenia gravis cases had an earlier symptom onset age than the sporadic cases.12 14 15 Only three studies reported on the patient history of other autoimmune diseases and/or on the family history of other autoimmune diseases.12 14 15 Among these, thyroid disease, rheumatoid arthritis, systemic lupus erythematosus and Sjogren’s syndrome were the most common other autoimmune diseases reported in patients’ personal and/or family histories.12 14 15
Table 2.
Studies reporting the rate of familial disease in myasthenia gravis
| Source | Origin | Sample size | Familial rate (%) | Study type |
| 12 | Taiwan | 6638 | 0.2 | Population |
| 13 | Japan | 3141 | 0.7 | Population |
| 14 | Spain | 462 | 3.5 | Clinic |
| 15 | Finland | 264 | 7.2 | Population |
| 16 | United States | 702 | 3.8 | Clinic |
Discussion
We found the prevalence of familial myasthenia gravis in our North American cohort to be 5.6%. Our previous genetic analysis of this cohort showed the heritability of myasthenia gravis to be 25.5%.10 This is comparable to the prevalence reported in other studies, where the frequency ranged from 0.2% to 7.2% of patients with myasthenia gravis having a family history of myasthenia gravis depending on the cohort studied.12–16 Although the vast majority of myasthenia gravis cases are still non-familial, a prevalence of 5.6% represents a several hundred-fold increase for a disease with an overall prevalence of 1 in 5–10 000.5 The two studies of myasthenia gravis based on Asian cohorts reported substantially lower rates of familial disease compared with our North American cohort and other European cohorts. For example, two studies of Taiwanese (n=6638) and Japanese (n=3141) cohorts reported rates of familial myasthenia gravis at 0.2% and 0.7%, respectively.12 13 Three studies of Spanish, American, and Finnish cohorts reported rates of 3.5%, 3.8% and 7.2% (table 2).14–16 The familial rate reported in our cohort of over 1000 patients were, as expected, closer in value to the rates reported among European and American cohorts. Overall, these data suggest that there is population variation in the inheritance of myasthenia gravis that warrants further study to identify the genetic contribution to disease risk.
A notable feature of our cohort is that males had a slightly higher prevalence of familial myasthenia gravis compared with females (1:1.32), suggesting that there might be sex-specific differences in the occurrence of familial versus sporadic disease. This observation may reflect the different age distribution of myasthenia gravis cases in males versus females observed across all myasthenia gravis cases. The reason for this is unclear, though we note that our previous genome-wide association study of myasthenia gravis indicated that the genetic architecture was different among younger and older age groups.10
Myasthenia gravis is an immunological disorder and, generally speaking, autoimmune diseases are known to have heritable components.19 Likewise, approximately one-third of our cohort had a personal history and/or a family history of another autoimmune disease, which is much higher than the prevalence of 3%–9%, which has been historically reported in the general population.17 We found that 19% (11/58) of the familial myasthenia gravis cases also had a personal history of autoimmune diseases; this was more than the sporadic myasthenia gravis cases in which only 10% (98/974) had a personal history of autoimmune diseases. Similar to previous reports, we found thyroid disease, rheumatoid arthritis, systemic lupus erythematosus and type 1 diabetes to be the most commonly identified comorbidities (figure 2C).18 19 Interestingly, the frequency of thyroid disease in this cohort (11.4%) is similar to the frequency range of thyroid disease reported in a study by Kiessling et al.20 The increased prevalence of familial myasthenia gravis and increased prevalence of other autoimmune disorders both suggest that these autoimmune diseases may share a common predisposition that may be genetic in origin. We speculate that the link between thyroid disease and myasthenia gravis may be due to a common genetic background or an immunological cross-reactivity against epitopes or auto-antigens shared by the thyroid and other tissues relevant to myasthenia gravis.21 22 Future studies focusing on specific genes and genomic variants encountered in patients with familial myasthenia gravis offer the promise to more precisely identify any genetic contributions to the disease.
While our study has some notable strengths, it also has an inherent limitation related to its reliance on self-reported family histories, which could have overestimated or underestimated the prevalence of the diseases studied. For example, it is plausible that a patient could self-report as not having a family history of myasthenia gravis because a family member was never clinically diagnosed with the disease or did not live long enough for the disease to manifest. Similarly, other studies have found an overestimation of some autoimmune diseases, especially thyroid disease and rheumatoid arthritis, related to self-reporting.23
Our analyses provide evidence of a genetic contribution to myasthenia gravis based on the higher than expected rate of familial disease observed among our North American patient cohort as well as the co-occurrence of autoimmune diseases known to have a genetic basis among this population. More work needs to be done to further elucidate the genetic aetiology of this archetypal autoimmune disease.
Supplementary Material
Acknowledgments
We thank the patients and research participants who contributed samples for this study.
Footnotes
DBD and BJT contributed equally.
Contributors: The study concept and design were proposed by DBD and BJT. The acquisition, analysis and/or interpretation of data were performed by all authors. The manuscript was drafted by JDG and BT. Critical revisions, intellectual contributions and final approval were provided by DD, RJB, EB, MB, DB, VC, MC, AC, MMD, AE, JF, MF, JFH, TJ, HJK, JK, WJK, BL, MiM, MaM, JMM, AM, MMe, SM, MWN, JO, RMP, MP, AP, CP, RR, DPR, JR, DBS, ZS, AS, GIW and CW. The statistical analysis for this study was provided by JDG.
Funding: Study funding by the National Institutes of Health Intramural Research Program. The work was also supported by the Myasthenia Gravis Foundation (Drs Drachman and Traynor), a generous bequest by Geraldine Weinrib, and a gift from Philip Swift. Support was provided by Mr and Mrs Don Brandon and the Department of Neurology, University of Kansas Medical Center.
Disclaimer: The funders had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript and decision to submit the manuscript for publication.
Competing interests: RJB served as a consultant for NuFactor and Momenta Pharmaceutical and receives research support from PTC Therapeutics, Ra Pharma, Orphazyme, Sanofi Genzyme, FDA OOPD, NIH and PCORI. MB reports grant support from Muscular Dystrophy Association, ALS Association, ALS Recovery Fund, Kimmelman Estate, Target ALS, Eli Lilly & Company and the National Institutes of Health (NIH) during the conduct of the study. He also reports grant support from FDA, CDC and DOD; research support from Alexion Pharmaceuticals, UCB, Cytokinetics, Neuraltus, Biogen and Orphazyme A/S; and personal fees from NMD Pharma, Ra Pharmaceuticals, Mitsubishi-Tanabe, Avexis, UCB and Denali outside the submitted work. VC served as a consultant for review and expert testimony for the Department of Health and Human Services and the Department of Justice under the Vaccine Injury and Compensation Program. Dr Chaudhry has received royalty for total neuropathy score (TNS) patented (through Johns Hopkins University) for license of TNS use from AstraZeneca, Genentech, Seattle Genetics, Calithera Biosciences, Merrimack Pharmaceuticals, Levicept, and Acetylon Pharmaceuticals. MMD serves or recently served as a consultant for ArgenX, Catalyst, CSL-Behring, Kezar, Momenta, NuFactor, RMS Medical, Sanofi Genzyme, Shire Takeda, and Spark Therapeutics. Dr Dimachkie received grants from Alexion, Alnylam Pharmaceuticals, Amicus, Biomarin, Bristol-Myers Squibb, Catalyst, CSL-Behring, FDA/OOPD, GlaxoSmithKline, Genentech, Grifols, Mitsubishi Tanabe Pharma, MDA, NIH, Novartis, Sanofi Genzyme, Octapharma, Orphazyme, Sarepta Therapeutics, Shire Takeda, Spark, UCB Biopharma, Viromed & TMA. AE was a member of the advisory board for Alexion, a scientific award jury member for Grifols and safety data monitor for UCB. MF has received honoraria for serving on advisory boards for ARGNX pharma, Alexion. MF also has research support from Catalyst, Ra pharma, Amicus, Orphazyme, Alexion, Momenta and Alnylam. JFH reports research support and grants from Alexion Pharmaceuticals, argenx BVBA, Centers for Disease Control and Prevention, Muscular Dystrophy Association, NIH (including the National Institute of Neurologic Disorders and Stroke and the National Institute of Arthritis and Musculoskeletal and Skin Disease), PCORI (Patient-Centered Outcomes Research Institute) and Ra Pharmaceuticals; and nonfinancial support from Alexion Pharmaceuticals, argenx BVBA, Ra Pharmaceuticals and Toleranzia. HJK is funded by the Muscular Dystrophy Association (508240) and by NIH grant U54NS115054; is a consultant for Alnylam Pharmaceuticals, Ra Pharmaceuticals, and UCB Pharmaceuticals; and is CEO of ARC Biotechnology, LLC, which receives support from the NIH (R41NS110331). He serves on the Editorial Board of Experimental Neurology. MMe has received honoraria as a speaker and/or moderator from Alnylam, Akcea, Pfizer and CSL Behring. She has served on Advisory Boards for Pfizer, Alnylam and Akcea. She serves as an investigator for clinical trials with Alnylam and Biogen. SM has served on advisory board meetings for Alexion and argenx. MP served on advisory board for CSL Behring, Alexion pharmaceuticals, Argenx Pharmaceuticals and has been consultant for Momenta Pharmaceuticals. DPR receives research funding from a Sponsored Research Agreement from Cabaletta Bioscience. BJT holds an American and European Union patent on the clinical testing and therapeutic intervention for the hexanucleotide repeat expansion of C9orf72, and has received research grants from The Myasthenia Gravis Foundation, the Robert Packard Center for ALS Research, the ALS Association (ALSA), the Italian Football Federation (FIGC), the Center for Disease Control and Prevention (CDC), the Muscular Dystrophy Association (MDA), Merck and Microsoft Research. BJT receives funding through the Intramural Research Program at the National Institutes of Health.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Patient consent for publication: Obtained.
Ethics approval: Written informed consent was obtained from all patients who participated in this study. Institution review board (IRB) approval was obtained at all participating institutions. Lead institutes for IRB=National Institutes of Health, protocol 03-AG-N329 (https://clinicaltrials.gov/ct2/show/NCT02014246).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. The data, consisting of patient family history of myasthenia gravis and other autoimmune diseases in addition to patient personal history of other autoimmune diseases, are not publicly available because of patient privacy concerns, but de-identified participant data are available upon request by contacting the corresponding author. In the interests of scientific rigour, the code used for analysis of the dataset is available on GitHub (https://github.com/neurogenetics/Familial-Myasthenia-Gravis).
Author note: Statistical analysis: conducted by Joshua Green, BS, and Dr Bryan Traynor, MD, PhD, National Institutes of Health.
References
- 1.Fambrough DM, Drachman DB, Satyamurti S. Neuromuscular junction in myasthenia gravis: decreased acetylcholine receptors. Science 1973;182:293–5. 10.1126/science.182.4109.293 [DOI] [PubMed] [Google Scholar]
- 2.Hoch W, McConville J, Helms S, et al. Auto-antibodies to the receptor tyrosine kinase MuSK in patients with myasthenia gravis without acetylcholine receptor antibodies. Nat Med 2001;7:365–8. 10.1038/85520 [DOI] [PubMed] [Google Scholar]
- 3.Gasperi C, Melms A, Schoser B, et al. Anti-agrin autoantibodies in myasthenia gravis. Neurology 2014;82:1976–83. 10.1212/WNL.0000000000000478 [DOI] [PubMed] [Google Scholar]
- 4.Higuchi O, Hamuro J, Motomura M, et al. Autoantibodies to low-density lipoprotein receptor-related protein 4 in myasthenia gravis. Ann Neurol 2011;69:418–22. 10.1002/ana.22312 [DOI] [PubMed] [Google Scholar]
- 5.Jayam Trouth A, Dabi A, Solieman N, et al. Myasthenia gravis: a review. Autoimmune Dis 2012;2012:874680 10.1155/2012/874680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrogan A, Sneddon S, de Vries CS. The incidence of myasthenia gravis: a systematic literature review. Neuroepidemiology 2010;34:171–83. 10.1159/000279334 [DOI] [PubMed] [Google Scholar]
- 7.Yu YL, Hawkins BR, Ip MS, et al. Myasthenia gravis in Hong Kong Chinese. 1. epidemiology and adult disease. Acta Neurol Scand 1992;86:113–9. 10.1111/j.1600-0404.1992.tb05050.x [DOI] [PubMed] [Google Scholar]
- 8.MacDonald BK, Cockerell OC, Sander JW, et al. The incidence and lifetime prevalence of neurological disorders in a prospective community-based study in the UK. Brain 2000;123:665–76. 10.1093/brain/123.4.665 [DOI] [PubMed] [Google Scholar]
- 9.Spillane J, Higham E, Kullmann DM. Myasthenia gravis. BMJ 2012;345:e8497. 10.1136/bmj.e8497 [DOI] [PubMed] [Google Scholar]
- 10.Renton AE, Pliner HA, Provenzano C, et al. A genome-wide association study of myasthenia gravis. JAMA Neurol 2015;72:396–404. 10.1001/jamaneurol.2014.4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giraud M, Vandiedonck C, Garchon H-J. Genetic factors in autoimmune myasthenia gravis. Ann N Y Acad Sci 2008;1132:180–92. 10.1196/annals.1405.027 [DOI] [PubMed] [Google Scholar]
- 12.Liu F-C, Kuo C-F, See L-C, et al. Familial aggregation of myasthenia gravis in affected families: a population-based study. Clin Epidemiol 2017;9:527–35. 10.2147/CLEP.S146617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Murai H, Yamashita N, Watanabe M, et al. Characteristics of myasthenia gravis according to onset-age: Japanese nationwide survey. J Neurol Sci 2011;305:97–102. 10.1016/j.jns.2011.03.004 [DOI] [PubMed] [Google Scholar]
- 14.Salvado M, Canela M, Ponseti JM, et al. Study of the prevalence of familial autoimmune myasthenia gravis in a Spanish cohort. J Neurol Sci 2016;360:110–4. 10.1016/j.jns.2015.11.049 [DOI] [PubMed] [Google Scholar]
- 15.Pirskanen R. Genetic aspects in myasthenia gravis. A family study of 264 Finnish patients. Acta Neurol Scand 1977;56:365–88. [PubMed] [Google Scholar]
- 16.Namba T, Brunner NG, Brown SB, et al. Familial myasthenia gravis. Report of 27 patients in 12 families and review of 164 patients in 73 families. Arch Neurol 1971;25:49–60. 10.1001/archneur.1971.00490010059009 [DOI] [PubMed] [Google Scholar]
- 17.Cooper GS, Bynum MLK, Somers EC. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J Autoimmun 2009;33:197–207. 10.1016/j.jaut.2009.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mao Z-F, Yang L-X, Mo X-A, et al. Frequency of autoimmune diseases in myasthenia gravis: a systematic review. Int J Neurosci 2011;121:121–9. 10.3109/00207454.2010.539307 [DOI] [PubMed] [Google Scholar]
- 19.Ceccarelli F, Agmon-Levin N, Perricone C. Genetic factors of autoimmune diseases. J Immunol Res 2016;2016:1–2. 10.1155/2016/3476023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiessling WR, Finke R, Kotulla P, et al. Circulating TSH-binding inhibiting immunoglobulins in myasthenia gravis. Acta Endocrinol 1982;101:41–6. 10.1530/acta.0.1010041 [DOI] [PubMed] [Google Scholar]
- 21.Marinó M, Ricciardi R, Pinchera A, et al. Mild clinical expression of myasthenia gravis associated with autoimmune thyroid diseases. J Clin Endocrinol Metab 1997;82:438–43. 10.1210/jc.82.2.438 [DOI] [PubMed] [Google Scholar]
- 22.Masood I, Yasir M, Kudyar RP. Autoimmune thyroid disease with myasthenia gravis in a 28-year-old male: a case report. Cases J 2007;2:8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Rourke JA, Ravichandran C, Howe YJ, et al. Accuracy of self-reported history of autoimmune disease: a pilot study. PLoS One 2019;14:e0216526. 10.1371/journal.pone.0216526 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-037909supp001.pdf (40.2KB, pdf)