Abstract
Background
Not all non-small cell lung cancer (NSCLC) patients possess drug-targetable driver mutations, and response rates to immune checkpoint blockade therapies also remain unsatisfactory. Therefore, more effective treatments are still needed. Here, we report the results of a phase 2 clinical trial of adoptive cell therapy using zoledronate-expanded autologous Vγ9Vδ2 T-cells for treatment-refractory NSCLC.
Methods
NSCLC patients who had undergone at least two regimens of standard chemotherapy for unresectable disease or had had at least one treatment including chemotherapy or radiation for recurrent disease after surgery were enrolled in this open-label, single-arm, multicenter, phase 2 study. After preliminary testing of Vγ9Vδ2 T-cell proliferation, autologous peripheral blood mononuclear cells were cultured with zoledronate and IL-2 to expand the Vγ9Vδ2 T-cells. Cultured cells (>1×109) were intravenously administered every 2 weeks for six injections. The primary endpoint of this study was progression-free survival (PFS), and secondary endpoints included overall survival (OS), best objective response rate (ORR), disease control rate (DCR), safety and immunomonitoring. Clinical efficacy was defined as median PFS significantly >4 months.
Results
Twenty-five patients (20 adenocarcinoma, 4 squamous cell carcinoma and 1 large cell carcinoma) were enrolled. Autologous Vγ9Vδ2 T-cell therapy was administered to all 25 patients, of which 16 completed the foreseen course of 6 injections of cultured cells. Median PFS was 95.0 days (95% CI 73.0 to 132.0 days); median OS was 418.0 days (179.0–479.0 days), and best overall responses were 1 partial response, 16 stable disease (SD) and 8 progressive disease. ORR and DCR were 4.0% (0.1%–20.4%) and 68.0% (46.5%–85.1%), respectively. Severe adverse events developed in nine patients, mostly associated with disease progression. In one patient, pneumonitis and inflammatory responses resulted from Vγ9Vδ2 T-cell infusions, together with the disappearance of a massive tumor.
Conclusions
Although autologous Vγ9Vδ2 T-cell therapy was well tolerated and may have an acceptable DCR, this trial did not meet its primary efficacy endpoint.
Trial registration number
UMIN000006128
Keywords: immunity, cellular, immunotherapy, immunotherapy, adoptive, lung neoplasms
Introduction
Although the prevalence of lung cancer has been gradually declining over the past decade, it remains the most common tumor and the leading cause of cancer mortality worldwide, with 2.1 million new cases (11.6% of all cancers) and 1.8 million deaths (18.4% of all cancer deaths) in 2018.1 2 Approximately 85% of lung cancers are non-small cell lung cancer (NSCLC), of which lung adenocarcinoma (LUAD) and lung squamous cell carcinoma are the most common subtypes. For many years, standard first-line therapy for patients with advanced NSCLC has been platinum-based doublet therapy with the option of maintenance therapy.3 In the second-line setting, docetaxel, with or without the anti-vascularendothelial growth factor (VEGF) receptor-2 antibody ramucirumab, represented the standard of care.4 The identification of targetable gene alterations, such as epidermal growth factor receptor (EGFR) gene alterations and EML4-ALK gene rearrangements in LUAD, has led to the development of targeted drug therapy, which can achieve remarkable responses in selected patients treated with the appropriate drugs.5 The development of targeted therapies resulted in genetic alteration-guided and personalized therapy for lung cancer. Furthermore, the advent of immune checkpoint blockade has opened new avenues for lung cancer treatment and achieved robust and durable responses in a minority of patients.5 6 Nevertheless, response rates remain unsatisfactory, with clinical responses usually achieved in only a minority of patients. Therefore, the development of more effective therapies remains an unmet clinical need in treatment-refractory NSCLC.
To this end, we have been developing an adaptive Vγ9Vδ2 T-cell transfer immunotherapy protocol for the treatment of NSCLC. Vγ9Vδ2 T-cells are a unique population of lymphocytes that mediate responses to diverse immune challenges, infectious diseases and cancer.7 8 Human γδ T-cells are primarily of two types, Vδ1 and Vδ2.9 Of these, Vγ9Vδ2 T-cells are abundant in blood and contribute to first-line defense against infection and cancer. In tumor cells, the accumulation of isopentenyl pyrophosphate (IPP), an intermediate metabolite of the mevalonate pathway, is sensed by Vγ9Vδ2 T-cells.10 11 Nitrogen-containing bisphosphonates (N-BPs), such as zoledronate, inhibit farnesyl pyrophosphate synthesis in the mevalonate pathway, leading to increased levels of the upstream metabolite IPP in tumor cells, and rendering them targets of Vγ9Vδ2 T-cells.12 Vγ9Vδ2 T-cells also express natural killer (NK) cell-activating receptors such as NKG2D, which recognizes the stress-inducible ligands MICA, MICB and UL-16-binding proteins (ULBPs) on target cells.13–15 Thus, the recognition of tumor cells by Vγ9Vδ2 T-cells is not restricted by the processing and presentation of peptides by majorhistocompatibility complex (MHC) class I molecules. On recognition of their ligands, Vγ9Vδ2 T-cells secrete Th1 cytokines16 and kill tumor cells by perforin/granzyme, TNF-relatedapoptosis-inducing ligand (TRAIL) and Fas/FasL pathways.17 18 These properties encouraged us to develop Vγ9Vδ2 T-cell-based cancer immunotherapies.
Using zoledronate and IL-2, we cultured autologous Vγ9Vδ2 T-cells from patients with advanced cancer under clinical-grade conditions.19–22 Unfortunately, Vγ9Vδ2 T-cells cannot be sufficiently expanded in all patients23; but when they can be expanded, they retain cytotoxic activity and Th1 cytokine secretion very well. Therefore, we conducted phase I clinical studies to evaluate the safety and potential antitumor effects of the adoptive transfer of autologous Vγ9Vδ2 T-cells expanded from patients with NSCLC,24 colorectal cancer,22 pancreatic cancer25 and gastric cancer.26 In addition, there have been many other clinical trials performed using Vγ9Vδ2 T-cells expanded using phosphoantigens or N-BPs either in vivo or in vitro.27 28 The results of these clinical studies indicate that Vγ9Vδ2 T-cell therapy is safe and may provide some clinical benefit; however, results of the different trials were dissimilar, and objective responses were seldom achieved.
Hence, large scale clinical studies are warranted for the evaluation of Vγ9Vδ2 T-cell therapies for the treatment of cancer. Future directions for improving Vγ9Vδ2 T-cell-based immunotherapy could be the exploitation of engineering chimeric antigen receptor (CAR) into Vγ9Vδ2 T-cells or combination treatments with other immunotherapies.29 For these purposes, a more extensive database is required to understand the functional properties of Vγ9Vδ2 T-cells in vivo, including their survival, homing and cytotoxic capacity. Here, we report the results of a phase 2 clinical trial of adoptive Vγ9Vδ2 T-cell transfer therapy for treatment-refractory NSCLC patients. We evaluated progression-free survival (PFS), overall survival (OS), response rate, quality of life (QOL) and safety as well as the in vivo dynamics of Vγ9Vδ2 T-cells in patients receiving Vγ9Vδ2 T-cells every 2 weeks up to a planned total of 6 infusions.
Patients, materials and methods
Patient selection
Patients with pathologically confirmed NSCLC who had undergone at least two regimens of standard chemotherapy for unresectable disease, or who had undergone at least one treatment, including chemotherapy or radiation for recurrent disease, were enrolled in this study. Inclusion criteria were as follows: patients≥20 years of age; antitumor response evaluable by CT including non-measurable lesions; life expectancy at least 6 months; Vγ9Vδ2 T-cells ≥0.5% of peripheral blood mononuclear cells (PBMC) and expandable in vitro; Eastern Cooperative Oncology Group performance status 0 or 1; and normal kidney, liver and bone marrow function. Exclusion criteria were as follows: positive for serum HBs antigen, anti-HCV antibody, anti-HIV antibody or anti-HTLV antibody; uncontrolled infection; other malignant diseases; severe cardiovascular disease; receiving systemic steroid therapy; active enterocolitis; severe drug allergy; a history of cryoglobulinemia; active autoimmune disease; currently taking HMG-CoA reductase inhibitor; and pregnant or lactating. If immune checkpoint inhibitors (ICI) were given, a 3-month interval was required between the last dose of ICI and the first injection of Vγ9Vδ2 T-cells.
Study design
This study was an open-label, single-arm, multicenter, phase 2 study. It was undertaken in the Department of Thoracic Surgery, The University of Tokyo Hospital, Japan and the Department of Pulmonary Medicine, Keio University Hospital, Japan, based on the Act on Securement of Safety of Regenerative Medicine, Japan (Registration number: PC3150215) and conducted in accordance with the Declaration of Helsinki. The treatment was provided under the auspices of the Advanced Medical Treatment system in Japan.
The primary endpoint of this study was PFS. Secondary endpoints included OS, response rate (percentage of patients with complete response (CR) or partial response (PR)) according to the Response Evaluation Criteria in Solid Tumors guidelines (V.1.1), disease control rate (DCR; the percentage of patients with CR, PR or stable disease (SD)); QOL and safety. Duration of disease control ≥6 months was defined as long SD. Immunological profiling to support the efficacy and safety assessment was included. PFS, OS, RR, DCR, QOL and safety were analyzed in all patients who received at least one Vγ9Vδ2 T-cell infusion. Subjects deviating from the protocol were excluded from the analysis.
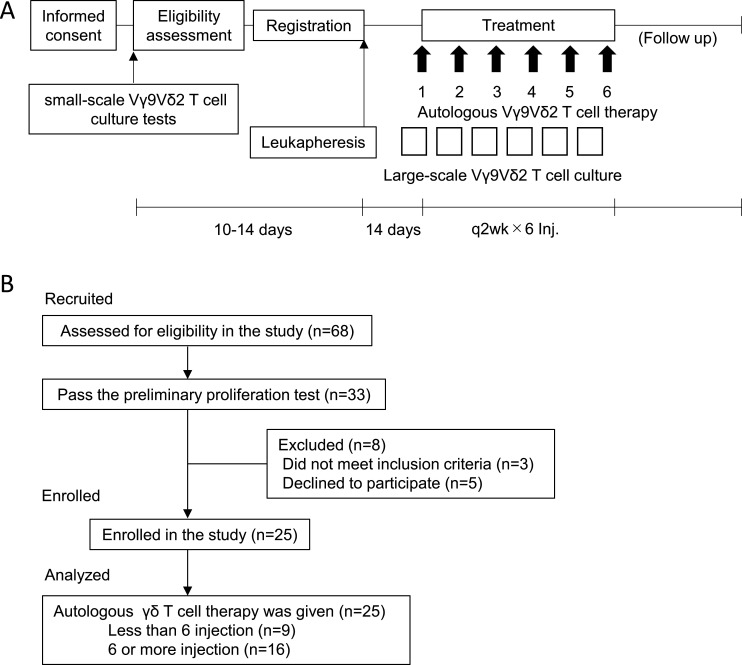
Before enrolment into the study, and after obtaining written informed consent, small-scale T-cell culture screening tests were performed to confirm the feasibility of expanding each patient’s Vγ9Vδ2 T-cells (figure 1A). Proliferation was assessed on day 10 of culture. Cutoffs for purity were defined as the percentage of Vγ9Vδ2 T-cells >70% and >90% of these positive for NKG2D, with upregulated CD69 expression. Following confirmation of feasibility, the degree of leukapheresis that would be necessary to obtain a sufficient number of Vγ9Vδ2 T-cells for treatment (ie, >1×109 cells for each of six injections) was estimated (keeping this at <10% of body weight). Cultured Vγ9Vδ2 T-cell infusions were given intravenously at intervals of 2 weeks. Vγ9Vδ2 T-cell cultures were established at the cell processing center of the Department of Immunotherapeutics, the University of Tokyo Hospital, Japan 14 days before every infusion. Autologous Vγ9Vδ2 T-cells (>1×109) were infused every 2 weeks for a total of 6 planned injections. If the clinical benefit was observed (non-PD), the patient could continue treatment until disease progression. At each patient visit, prior to beginning T-cell infusions, heparinized venous blood was collected and PBMC isolated and cryopreserved, as well as plasma samples, for further analysis.
Figure 1.
Study design and participant flow chart. (A) Study design of autologous Vγ9Vδ2 T-cell transfer therapy. After informed consent, small-scale Vγ9Vδ2 T-cell culture testing was performed to examine the feasibility of Vγ9Vδ2 T-cell expansion for therapeutic use. Eligible patients were leukapheresed and expanded Vγ9Vδ2 T-cells were infused back to the same patient every 2 weeks. (B) Participant flow chart. Twenty-five patients were enrolled in the study.
A complete physical examination and standard laboratory assessments were conducted every 2 weeks before Vγ9Vδ2 T-cell injection. Any adverse events (AEs) were assessed at each visit and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events V.4.0. QOL of the patients was assessed using the Functional Assessment of Cancer Therapy-Biologic Response Modifier (FACT-BRM) questionnaire.30 The patients answered the questionnaire at every hospital visit prior to each Vγ9Vδ2 T-cell infusion.
Generation of autologous Vγ9Vδ2 T-cell products for infusion
Vγ9Vδ2 T-cells were expanded from patients’ PBMC as previously described.19 21 24–26 In brief, PBMC was thawed and stimulated with 5 μmol/L zoledronate (Novartis Pharma KK Tokyo, Japan) in AlyS203 γδ medium (Cell Science and Technology Institute, Sendai, Japan) containing 1000 IU/mL human recombinant IL-2 (Proleukin; Chiron, Amsterdam, The Netherlands) and 10% autologous plasma. Fresh medium containing IL-2 (1000 IU/mL) was added every 2–3 days and the cultures were transferred into new flasks or culture bags as necessitated by the degree of cell growth to maintain cell density below 1×106/mL. Fourteen days after in vitro stimulation, ex vivo expanded Vγ9Vδ2 T-cells were harvested and screened for their sterility (negative for endotoxin, bacteria, fungi and mycoplasma contamination) and purity (>70%). Cell surface expression of the activation marker CD69 and NKG2D as a surrogate marker for cytotoxic activity were examined by flow cytometry. Following approval for use after these tests, the cultured cells were washed twice with RPMI-1640 (ThermoFisher Scientific, Waltham, Massachusetts USA) and resuspended in normal saline (FUSO Pharmaceutical Industries, Osaka, Japan) for administration to the patient. Additional information is provided in online supplemental materials and methods.
jitc-2020-001185supp001.pdf (97.2KB, pdf)
Phenotypic characterization of PBMC and infused Vγ9Vδ2 T-cells
Cryopreserved PBMCs collected at each patient visit were thawed and divided into different aliquots for staining with the indicated antibody panels. For the small-scale Vγ9Vδ2 T-cell culture screening and quality controls for the Vγ9Vδ2 T-cell products before infusion, each batch of cultured cells was analyzed at the time, without cryopreservation. The purity (percentages of Vγ9Vδ2 T-cells, αβ T-cells, NK cells, B cells and monocytes in cultured cell populations) and phenotypes (CD27, CD45RA, CD69 and NKG2D expression by Vγ9Vδ2 T-cells) were determined by flow cytometry using a Gallios (Beckman Coulter) and Kaluza software (Beckman Coulter). The fluorescent dye-conjugated mAbs used in this study are described in online supplemental materials and methods.
Functional characterization of the infused Vγ9Vδ2 T-cells
Cytotoxicity assay
Flow cytometry-based cytotoxicity assays were performed as described previously.20 31 Daudi cells were obtained from the RIKEN BRC Cell Bank (Ibaraki, Japan) and grown in RPMI-1640 medium (Wako, Osaka, Japan) containing 10% fetal calf serum, streptomycin (100 µg/mL) and penicillin (100 U/mL). Daudi cells resuspended in Diluent C (Sigma, St Louis, MO) were stained with freshly prepared PKH-26 (2 μmol/L, Sigma) at room temperature for 2 min. After extensive washing, target cells were coincubated with Vγ9Vδ2 T-cells at an E/T ratio of 25:1. Cells were stained with 5 µL of Annexin-V-FITC (Miltenyi Biotec Japan, Tokyo, Japan) and 20 µg/mL of 7-aminoactinomycin D (7-AAD) (Sigma). By gating on PKH-26-positive target cells, the proportion of Annexin-V-FITC- and 7-AAD-positive subpopulations was determined by flow cytometry. The percentage cytotoxicity in the PKH-26-gated cell population was calculated by subtracting the value of nonspecific Annexin-V-FITC- or 7-AAD-positive target cells, measured in appropriate controls without effector cells.
CD107 externalization assay
The CD107 externalization assay was performed to evaluate cytotoxic function.32 Cells (2×105) were cocultured with the same number of Daudi cells in the presence of 10 μmol/L Monensin (Sigma) and 5 μL of FITC-conjugated anti-CD107a and CD107b antibody (BD) or isotype control (mouse IgG1, BD). After 4 hours incubation, CD107 expression was determined by flow cytometry as an indicator of degranulation of cytotoxic molecules. At the same time, culture supernatants were harvested and analyzed for cytokine and chemokine production using Bio-Plex Pro Human Cytokine Grp I panel 27-plex (BIO-RAD, Hercules, California, USA).
Repertoire analysis by sequencing TRD CDR3 regions
Total RNA was extracted from PBMCs and cultured Vγ9Vδ2 T-cells of indicated patients. Next-generation sequencing was performed with an unbiased TCR repertoire analysis technology as described previously.33 Primers and adaptors used were listed in online supplemental table S1. Detailed information is described in online supplemental materials and methods.
jitc-2020-001185supp002.pdf (105.4KB, pdf)
Evaluation of clinical responses and statistical analysis
Clinical responses were evaluated by CT performed at baseline, after the third and fifth infusions, and 4 and 12 weeks after the sixth infusion. Clinical efficacy of the treatment or the expected PFS of 4 months was determined from the results of our previous phase I study in which the median PFS was 126 days.24 Because median PFS of patients on docetaxel, pemetrexed, gefitinib or erlotinib (all approved for NSCLC as second-line treatment at the time of trial design), was 3.4, 2.9, 2.0 or 2.2 months, respectively,34–38 the threshold PFS for the present study was defined as 3 months. The target sample size was set to be 85. Median PFS and OS with a 95% CI were estimated by the Kaplan-Meier method. The differences in PFS and OS between EGFR-positive and EGFR-negative groups were evaluated using the log-rank test. At each immune monitoring time point, the mean, SE and 95% CI of each variable is indicated. All available postbaseline data were analyzed by Wilcoxon rank-sum testing. JMP Pro15.0.0 (SAS Institute) was used for these analyzes.
Results
Patient characteristics at baseline
From June 1 2012 to December 31 2018, written informed consent was obtained from 68 patients (figure 1B). The study and patients follow-up was continued until May 24 2019. Of the 68 patients who underwent preliminary testing for Vγ9Vδ2 T-cell proliferative capacity, expansion sufficient for treatment was achieved in 33. Excluding three patients who did not meet the inclusion criteria (low Performance Status due to disease progression) and a further five who opted for a different therapy, autologous Vγ9Vδ2 T-cell therapy was finally administered to 25 patients. Sixteen patients completed the series of six Vγ9Vδ2 T-cell infusions, and the median number of infusions for the patient cohort was 6 (range 2–12).
The demographic and clinical characteristics of the patients are shown in table 1. Of the 25 patients receiving Vγ9Vδ2 T-cell therapy, 11 were female, 20 had adenocarcinoma, 4 squamous cell carcinoma and 1 large cell carcinoma. Surgery was performed in 15 patients, 11 of whom achieved complete resection. Two patients with local recurrence after surgery underwent radiotherapy, but eventually progressed and were enrolled in this study. These two patients had severe Chronic obstructive pulmonary disease (COPD)with low pulmonary function reserve, and they refused cytotoxic chemotherapy. Tyrosine kinase inhibitors were given to a total of 11 adenocarcinomas patients, including seven with EGFR gene mutations.
Table 1.
Baseline characteristics
| N=25 | |
| Gender, n (%) | |
| Female | 11 (44) |
| Male | 14 (56) |
| Age, median years (range) | 66 (33–86) |
| Body mass index (kg/m2), median (range) | 19.9 (16.1–27.0) |
| Race, n (%) | |
| Asian | 25 (100) |
| Histology, n (%) | |
| Adenocarcinoma | 20 (80) |
| EGFRm (+) | 7 (28) |
| EGFRm (-) | 9 (36) |
| EGFRm NA | 4 (16) |
| Squamous cell carcinoma | 4 (16) |
| Large cell carcinoma | 1 (4) |
| Prior treatment history* | |
| Surgery + Chemo | 7 (28) |
| Surgery + Rad | 2 (8) |
| Surgery + Chemo + Rad | 6 (24) |
| Chemo | 5 (20) |
| Chemo + Rad | 5 (20) |
| Checkpoint blockade | 3 (12) |
*Tyrosine kinase inhibitors are included in Chemo (+).
Small-scale Vγ9Vδ2 T-cell culture screening tests
One of the major hurdles for cell therapy is the interpatient heterogeneity of cell therapy products. To guarantee a minimal quality of Vγ9Vδ2 T-cell therapy, we performed a small-scale Vγ9Vδ2 T-cell culture screening test in all 68 recruited patients to determine whether it was likely that a sufficient number of cells (>1×109) of sufficient purity (>70%) would likely be possible on scale-up to large-scale culture for generating the therapeutic product. For 33 patients, it was estimated that this should be the case. Factors associated with adequate Vγ9Vδ2 T-cell proliferation were investigated in 67 patients; one 72-year-old patient with good Vγ9Vδ2 T-cell proliferation was nonetheless excluded because of a lack of information about prior therapy (online supplemental table S2). As reported previously,23 the numbers and phenotypes of Vγ9Vδ2 T-cells in PBMC are the most important factors predicting successful Vγ9Vδ2T-cell cultures (p=0.0000565). Sex, the total number of prior therapies, history of surgery or radiation, or type of earlier regimen was not associated with the outcome of Vγ9Vδ2 T-cell culturing.
jitc-2020-001185supp008.xlsx (13.5KB, xlsx)
Quality of the Vγ9Vδ2 T-cell products
For each injection, Vγ9Vδ2 T-cells were prepared from the same batch of biobanked cryopreserved PBMC. Quality was evaluated for each preparation by flow cytometry. As shown in online supplemental tables S3-7, high-quality Vγ9Vδ2 T-cell preparations were invariably obtained, the purity of which was around 90% (online supplemental table S3). The numbers of Vγ9Vδ2 T-cells administered were around 4×109 cells per injection (online supplemental table S4). Upregulation of CD69 expression and NKG2D expression of cultured Vγ9Vδ2 T-cells indicated that well-activated T-cells had been obtained (online supplemental figure S1, online supplemental tables S5 and S6). Although the interpatient variation of these markers was seen, intrapatient batch variations were minimal. Cytotoxicity of the cultured Vγ9Vδ2 T-cells was assessed at first or second injection (online supplemental table S7); the percentages of CD107+ cells were 38.9±3.9 and percent cytotoxicity against Daudi cells was 44.8±4.5. These cultured Vγ9Vδ2 T-cells promptly secreted IFNγ, TNFα, RANTES and MIP-1α on stimulation with Daudi cells (online supplemental figure S2).
jitc-2020-001185supp009.xlsx (16KB, xlsx)
jitc-2020-001185supp003.pdf (200.6KB, pdf)
jitc-2020-001185supp004.pdf (433.4KB, pdf)
Clinical responses to Vγ9Vδ2 T-cell therapy
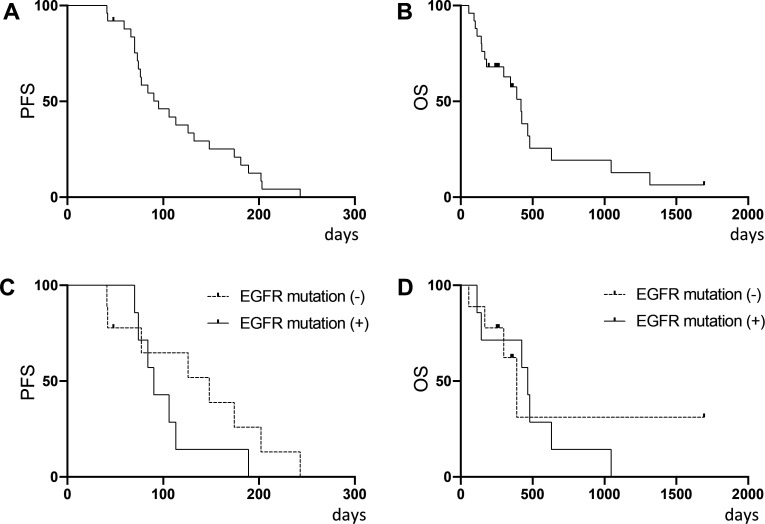
A summary of clinical endpoints is shown in online supplemental table S8. The median PFS was 95.0 days (95% CI 73.0 to 132.0 days), median OS was 418.0 days (95% CI 179.0 to 479.0 days) and best overall responses were 1 PR, 16 SD, including 4 patients whose time to progression was >180 days (long SD) and 8 PD. Objective response rate (ORR) and DCR were 4.0% (95% CI 0.1% to 20.4%) and 68.0% (46.5%–85.1%), respectively. Kaplan-Meier curves for PFS and OS are shown in figure 2A, B. There was no difference in either PFS and OS in the subgroup analysis of patients stratified by EGFR mutational status in adenocarcinoma (figure 2C, D).
Figure 2.
Kaplan-Meier analysis of 25 patients who underwent autologous Vγ9Vδ2 T-cell therapy. (A) PFS and (B) OS. Subgroup analysis by EGFR mutational status in adenocarcinoma patients for (C) PFS and (D) OS. EGFRm (+) n=7, EGFRm (-) n=9. OS, overall survival; PFS, progression-free survival.
Safety assessment
Vγ9Vδ2 T-cell therapy was well tolerated and safe even in two patients who had severe COPD with low pulmonary function reserve and could not tolerate chemotherapy. All AEs during treatment are shown in table 2. Grade ≥3 AEs developed in 12 patients during the study, including four cough, 3 anorexia, 2 pleural effusion, 2 productive cough, 2 lung infection (pneumonia), 2 tumor pain, 1 dyspnea, 1 respiratory failure, 1 pneumonitis, 1 dyspepsia, 1 ascites, 1 upper respiratory infection, 1 fever and 1 intracranial hemorrhage. Most of these AEs were associated with disease progression, and no grade ≥3 AEs were obviously related to the Vγ9Vδ2 T-cell therapy except for one patient with pneumonitis which might have been due to cytokine release syndrome following tumor cell lysis after Vγ9Vδ2 T-cell injection.
Table 2.
Adverse events (CTCAE V.4.0)
| Any grade | Grade ≧3 | |
| Adverse events, total no of patients (%) | 19 (76) | 12 (48) |
| Respiratory, thoracic and mediastinal disorders | 13 (52) | 8 (32) |
| Cough | 7 (28) | 4 (16) |
| Dyspnea | 6 (24) | 1 (4) |
| Pleural effusion | 2 (8) | 2 (8) |
| Productive cough | 2 (8) | 2 (8) |
| Pharyngeal mucositis | 1 (4) | 0 (0) |
| Respiratory failure | 1 (4) | 1 (4) |
| Pneumonitis | 1 (4) | 1 (4) |
| Hoarseness | 1 (4) | 0 (0) |
| Bronchopulmonary hemorrhage | 1 (4) | 0 (0) |
| Gastrointestinal disorders | 7 (28) | 1 (4) |
| Constipation | 4 (16) | 0 (0) |
| Nausea | 2 (8) | 0 (0) |
| Gastroesophageal reflux disease | 2 (8) | 0 (0) |
| Vomiting | 2 (8) | 0 (0) |
| Diarrhea | 1 (4) | 0 (0) |
| Dyspepsia | 1 (4) | 1 (4) |
| Stomach pain | 1 (4) | 0 (0) |
| Ascites | 1 (4) | 1 (4) |
| Metabolism and nutrition disorders | 7 (28) | 3 (12) |
| Anorexia | 7 (28) | 3 (12) |
| Infections and infestations | 6 (24) | 3 (12) |
| Upper respiratory infection | 2 (8) | 1 (4) |
| Nail infection | 1 (4) | 0 (0) |
| Lung infection | 2 (8) | 2 (8) |
| Nervous system disorders | 4 (16) | 1 (4) |
| Dizziness | 2 (8) | 0 (0) |
| Intracranial hemorrhage | 1 (4) | 1 (4) |
| Headache | 1 (4) | 0 (0) |
| Dysgeusia | 1 (4) | 0 (0) |
| Neoplasms benign, malignant and unspecified | 4 (16) | 2 (8) |
| Tumor pain | 4 (16) | 2 (8) |
| General disorders and administration site conditions | 3 (12) | 1 (4) |
| Fever | 3 (12) | 1 (4) |
| Fatigue | 1 (4) | 0 (0) |
| Skin and subcutaneous tissue disorders | 3 (12) | 0 (0) |
| Rash | 2 (8) | 0 (0) |
| Other | 1 (4) | 0 (0) |
| Psychiatric disorders | 2 (8) | 0 (0) |
| Depression | 1 (4) | 0 (0) |
| Insomnia | 1 (4) | 0 (0) |
| Cardiac disorders | 1 (4) | 0 (0) |
| Palpitations | 1 (4) | 0 (0) |
CTCAE, Common Terminology Criteria for Adverse Events.
Pneumonitis associated with Vγ9Vδ2 T-cell infusion
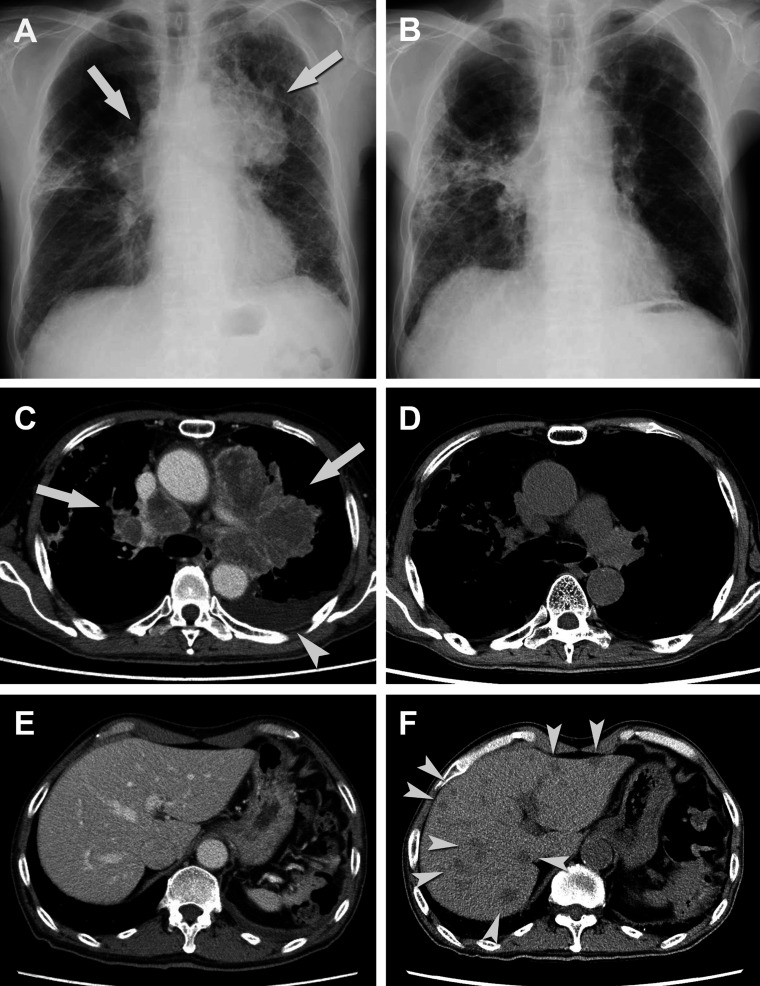
The one patient with pneumonitis was a 66-year-old male with left upper lobe primary lung cancer and brain metastasis (TU-2844). Transbronchial lung biopsy revealed adenocarcinoma; however, resection of brain metastasis was performed and the pathological diagnosis was large cell neuroendocrine carcinoma. Although whole-brain irradiation and systemic chemotherapy with CDDP+VP-16 was given, the disease progressed. Second-line chemotherapy with TS-1 was started considering the history of interstitial pneumonia but was stopped due to diarrhea, at which point the patient was referred to our hospital and was enrolled in the present study. Five days after the first injection of Vγ9Vδ2 T-cells, the patient exhibited low-grade fever, mild cough and dyspnea. CT showed signs of exacerbation of interstitial pneumonia; systemic steroid with antibiotics was, therefore, given and the patient recovered soon after. The second injection of Vγ9Vδ2 T-cells was given 5 weeks after the first; 3 days later, he again developed fever, cough and dyspnea. Systemic steroids and antibiotics were given once more, and the symptoms improved. During treatment, the lung tumor and swollen lymph nodes decreased markedly in size (figure 3). However, multiple liver metastases were found and the treatment was discontinued.
Figure 3.
Chest X-ray and CT scan of the chest and liver in a patient (TU-2844). Chest X-ray and CT scan of unresectable left upper lobe lung cancer and multiple lymph node metastases(arrows) were taken at the baseline (A, C) and 4 weeks after the second injection (B, D). While no liver metastasis was detected at the baseline (E), multiple liver metastases (arrow heads) were detected 4 weeks after the second injection (F).
QOL assessment
To evaluate QOL in patients receiving treatment, FACT-BRM scores were monitored. Total scores were essentially stable over the course of treatment, except for one patient (TU-2815) who developed grade 4 pleural effusion and ascites due to disease progression (online supplemental figure 3).
jitc-2020-001185supp005.pdf (51.7KB, pdf)
Immunological assessment
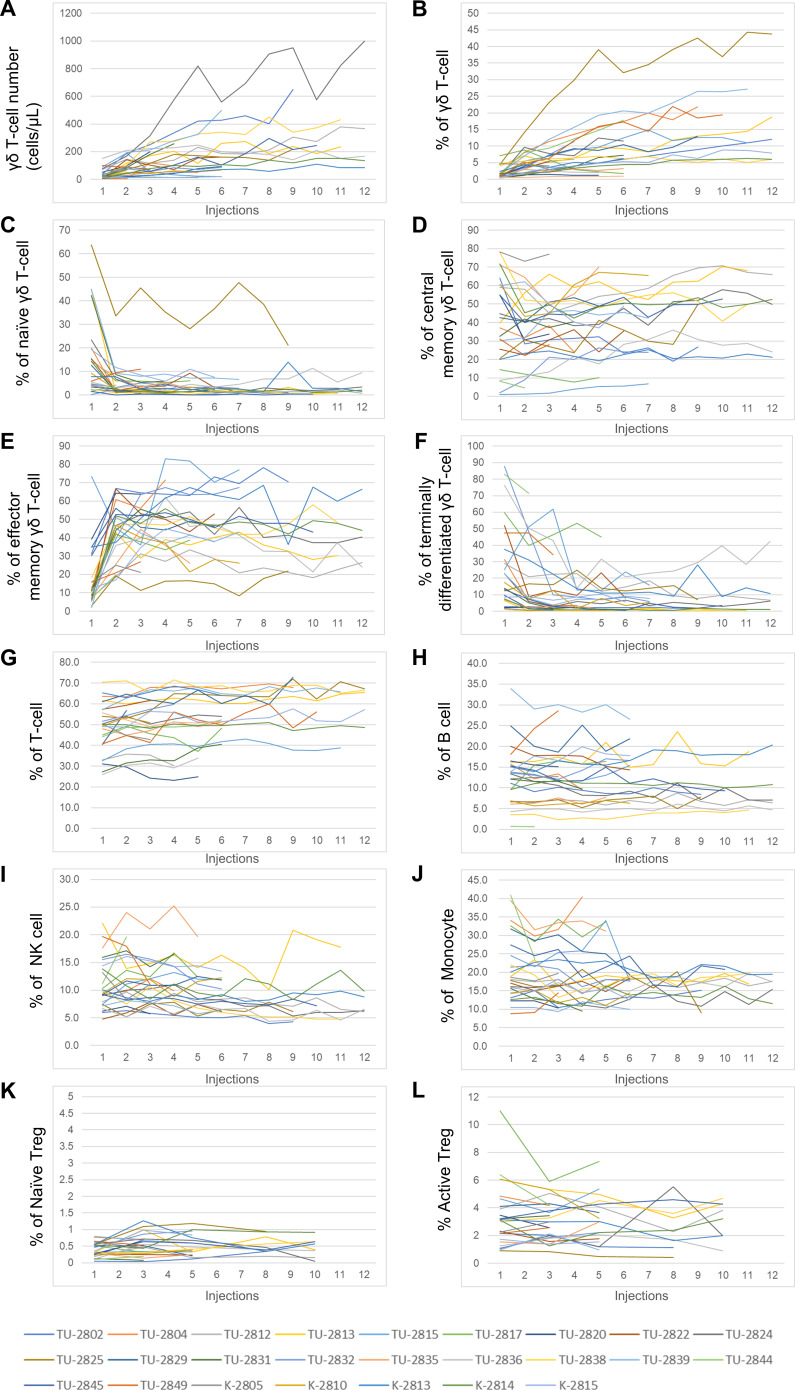
Immunological assessment was performed in 25 patients who received Vγ9Vδ2 T-cell injections. The frequency of Vγ9Vδ2 T-cells with subtypes, CD3+ T-cells, CD19+ B cells, CD3-CD56+ NK cells, CD14+ monocytes, CD45RA+FOXP3+ naïve regulatory T cells (Treg) and CD45RA-FOXP3++ activated Treg in patients’ PBMC was monitored at every visit (figure 4). Vγ9Vδ2 T-cell number and percentage in PBMC gradually increased with each injection over the course of treatment (figure 4A, B). Adoptively transferred Vγ9Vδ2 T-cells displayed CD45RA-CD27- effector memory type (online supplemental figure S1), which increased in PBMC (figure 4E). Inversely, the percentage of CD45RA+CD27+ naïve Vγ9Vδ2 T-cells decreased after one injection (figure 4C). The accumulation of Vγ9Vδ2 T-cells in PBMC was evaluated by the area under the curve of the percentage of Vγ9Vδ2 T-cells in PBMC-time curve (figure 4B and online supplemental figure S4). In contrast, percentages of T-cells, B cells, NK cells, monocytes and Tregs remained relatively stable, although there were interpatient variations in numbers (figure 4G–L). These results indicate that the adoptively transferred Vγ9Vδ2 T-cells did not perturb homeostasis of other immune cells.
Figure 4.
Immunomonitoring of the 25 patients enrolled. (A) Number of γδ T-cells per μL in blood at the time of injection. (B) The percentage of γδ T-cells in PBMC. (C) The percentage of CD45RA+CD27+ naïve γδ T-cells in PBMC. (D) The percentage of CD45RA-CD27+ central memory γδ T-cells in PBMC. (E) The percentage of CD45RA-CD27- effector memory γδ T-cells in PBMC. (F) The percentage of CD45RA+CD27- terminally differentiated γδ T-cells in PBMC. (G) The percentage of CD3+ T-cells in PBMC. (H) The percentage of CD19+ B cells in PBMC. (I) The percentage of CD3-CD56+ NK cells in PBMC. (J) The percentage of CD14+ monocytes in PBMC. (K) The percentage of CD45RA+FOXP3+ naïve regulatory T cells (Treg) in PBMC. (L) The percentage of CD45RA-FOXP3++active Treg in PBMC. Each color-coded line indicates one patient.
jitc-2020-001185supp006.pdf (468.3KB, pdf)
Blood was drawn at every patient visit before Vγ9Vδ2 T-cell injection and the levels of serum cytokines and chemokines were monitored (online supplemental figure 5). In the most patients, no characteristic changes were associated with Vγ9Vδ2 T-cell injections, although the interpatient variation was prominent. Everything measured was high in patient K-2814, irrespective of Vγ9Vδ2 T-cell injections. Interestingly, serum IL-6 and IP10 were high in PR patient TU-2844 before injection (online supplemental figure 5E, S). These factors were further increased after the Vγ9Vδ2 T-cell injections. Because cultured Vγ9Vδ2 T-cells express CXCR3, a ligand for IP10 (online supplemental figure 1), IP10 recruited Vγ9Vδ2 T-cells into the tumor that might result in the robust antitumor activity in this patient (figure 3)
jitc-2020-001185supp007.pdf (2.1MB, pdf)
Sequencing the TCR δ-chain
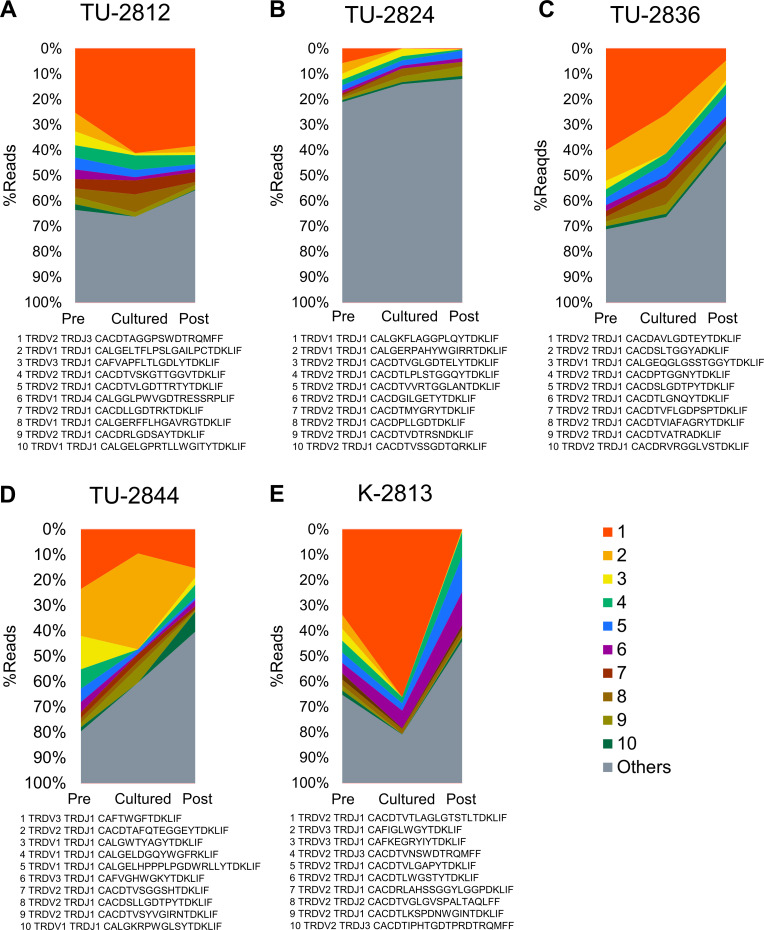
TCR δ-chain (TRD) repertoire was analyzed in five patients (TU-2812, TU-2824, TU-2836, TU-2844, K-2813) whose cryopreserved samples were available. Total RNAs were extracted from pretreatment PBMCs, cultured Vγ9Vδ2 T-cells and post-treatment PBMC harvested at last injection; the CDR3 region of the TRD was amplified and subjected to NGS. To evaluate TCR diversity or clonality, productive rearrangements with unique nucleotide sequences generated through VJ recombination were quantified (figure 5 and online supplemental tables S9-14). Top 10 clonotypes were depicted in figure 5 and the top 50 clonotypes were listed in online supplemental tables S10–S14. As previously reported,39 40 a substantial interpatient heterogeneity in Vγ9Vδ2 T-cells was observed; only one clonotype, TRDV2 TRDJ1 CACDTLGDTDKLIF, was shared between TU-2812 and TU-2836 (online supplemental table S10 and S12). While TRDV1, TRDV2 and TRDV3 clonotypes were detected in PBMC, TRDV2 clonotypes were selectively expanded by zoledronate. There was no difference in the Shannon-Weaver index between cultured Vγ9Vδ2 T-cells and pre-PBMC (4.03±1.13 vs 3.98±1.69), suggesting the polyclonal expansion of Vγ9Vδ2 T-cells by zoledronate and IL-2 (online supplemental table S9). In PR patient TU-2844 (figure 5D), the most frequent clonotype in pretreatment PBMC was TRDV3 TRDJ1 CAFTWGFTDKLIF. In cultured Vγ9Vδ2 T-cells, the clonotype TRDV2 TRDJ1 CACDTAFQTEGGEYTDKLIF was expanded and accounted for 37.7% of TRD clonotypes (online supplemental table S13). However, TRDV3 TRDJ1 CAFTWGFTDKLIF clonotype remained in cultured Vγ9Vδ2 T-cells (9.49%) and detected in PBMC after injections (15.3%).
Figure 5.
TCR δ-chain (TRD) clonotypes. TRDVJ rearrangements were analyzed by CDR3 sequencing in five patients, TU-2812 (A), TU-2824 (B), TU-2836 (C), TU-2844 (D) and K-2813 (E). PBMC before first Vγ9Vδ2 T-cell injection (Pre), zoledronate-expanded Vγ9Vδ2 T-cells (Cultured) and PBMC at the last Vγ9Vδ2 T-cell injection of each patient were compared. TRD ranking top 10 clonotypes were sorted according to the frequency of preinjection PBMC (Pre) samples and shown.
jitc-2020-001185supp010.xlsx (14.6KB, xlsx)
jitc-2020-001185supp011.xlsx (14.3KB, xlsx)
jitc-2020-001185supp012.xlsx (16KB, xlsx)
jitc-2020-001185supp013.xlsx (15.9KB, xlsx)
Discussion
The introduction into the clinic of a number of molecular targeted drugs and immune checkpoint blockade dramatically changed the therapeutic landscape for NSCLC in the last decade.5 6 However, some patients remain refractory to treatment and still require alternative or complementary treatments. Here, we present the outcomes of adoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in 25 patients with treatment-refractory NSCLC, 16 of whom completed the foreseen course of 6 injections. The eligibility criteria for patients in this study did not match the current standard of care for NSCLC that requires histological subtyping, molecular testing for EGFR, ALK, ROS1, BRAF and PD-L1 testing. The clinical benefit seen in the present study should, therefore, be evaluated separately based on these patients' heterogeneous backgrounds. Although the present study was initially intended to assess Vγ9Vδ2 T-cell infusion as third-line therapy, accrual was challenging and patients who were considered eligible had in fact already received an average of 4 prior treatments. Therefore, we terminated the study after 25 patients had been treated, although the planned sample size had been set at 85. Nonetheless, we endeavored to retrieve as much information as possible from this study to share this experience with the community and contribute to the future development of Vγ9Vδ2 T-cell-based immunotherapy.
The median PFS of the 25 patients studied was 95.0 days (95% CI, 73.0 to 132.0 days) and median OS was 418.0 days (179.0–479.0 days) (figure 2). The best overall responses were 1 PR, 16 SD, including four patients whose time to progression was >180 days, and eight PD. ORR and DCR were 4.0% (95% CI 0.1% to 20.4%) and 68.0% (95% CI 46.5% to 85.1%), respectively (online supplemental table S8). This did achieve the predefined threshold PFS of 3 months, but failed to achieve the expected PFS of 4 months. Therefore, we must conclude that we failed to demonstrate the efficacy of adoptive autologous Vγ9Vδ2 T-cell immunotherapy for treatment-refractory NSCLC, at least according to this criterion.
Clinical responses to ICI depend on the invigoration of pre-existing αβ T-cells that recognize tumor-associated antigens or neoantigens presented by MHC class I molecules and are therefore primarily restricted to tumors with high mutational burden.41 Additionally, tumor cells acquire resistance by losing the expression of neoantigens or mutating genes related to antigen processing and presentation.42 Therefore, the therapeutic use of Vγ9Vδ2 T-cells that recognize tumor cells in an MHC-independent manner would not be expected to be influenced by these tumor escape mechanisms. However, to establish the clinical benefits of Vγ9Vδ2 T-cell infusions, study design should be modified to select ICI-resistant patients or combine adoptive cell transfer with targeted drugs specific for the corresponding driver mutations.
In the present study, autologous Vγ9Vδ2 T-cells were expanded ex vivo and transferred back to the patients every 2 weeks using cultured cells expanded over the preceding 14 days from the patient’s cryopreserved PBMC. To control for quantity and quality of the cell therapy products, each preparation was assessed before infusion after screening for patients by initially conducting small-scale culture tests. In this way, we were able to select 33 treatable patients from 68 NSCLC patients and could treat them with a sufficient quantity of high-quality autologous Vγ9Vδ2 T-cells (online supplemental tables S3–S7). The necessity of prescreening the patients made it difficult to design a randomized, double-blinded study or to examine combination therapies with other drugs, such as molecular target drugs or ICI. Thus, allogeneic γδ T-cells might be used as off-the-shelf cell products because of their HLA-independent recognition of antigens.43
As reported previously,24–26 adoptive transfer of autologous Vγ9Vδ2 T-cells is safe and feasible. Most AEs observed in the present study were associated with disease progression (table 2). However, in patient TU-2844, pneumonitis or a severe inflammatory response was repeatedly observed after both of two injections of Vγ9Vδ2 T-cells, in concert with the disappearance of remarkably large tumors, lymph node metastasis and pleural effusion (figure 3). The patient experienced fever and hypoxia 2–3 days after Vγ9Vδ2 T-cell infusions, but unfortunately, was not treated in our hospital, and no samples were available for investigation during these episodes. The patient’s symptoms were similar to those caused by cytokine release syndrome, a systemic inflammatory response mediated by cytokines released by infused CAR-T-cells.44 Such a strong response was not detected in any other patients receiving Vγ9Vδ2 T-cell infusions. There are at least three possibilities to explain why robust anti-tumor activity was observed only in this single patient. One may be related to the intrinsic quality of the Vγ9Vδ2 T-cells. It has been reported that there is a substantial diversity of Vγ9Vδ2 TCR, the majority of which are of low-affinity.45 In this particular patient, cultured Vγ9Vδ2 T-cells contained TRDV3 TRDJ1 CAFTWGFTDKLIF clonotype (9.49%) in addition to TRDV2 TRDJ1 CACDTAFQTEGGEYTDKLIF (37.74%) (figure 5 and online supplemental table S13). CAFTWGFTDKLIF clonotype was detected in post-injection PBMCs (15.31%), while CACDTAFQTEGGEYTDKLIF clonotypes decreased to 3.63%. Although we could not identify which clonotype was responsible for the antitumor activity, those Vγ9Vδ2 T-cells might have high-affinity TCR, resulting in greater cytokine secretion or killing capacity in receptor ligation. Second, the tumor might produce IP10 that recruited CXCR3+ Vγ9Vδ2 T-cells into the tumor, because the serum IP10 was quite high in this patient (online supplemental figure S1 and S5S). Third, the tumor cells might have express larger amounts of the ligands recognized by the infused Vγ9Vδ2 T-cells. In any event, we conclude that the antitumor effector functions of Vγ9Vδ2 T-cells can be as robust as CAR-T-cells if they can appropriately recognize cancer cells. Biomarkers to assist in identifying those patients who might respond to Vγ9Vδ2 T-cell therapy are urgently required.
Theoretically, such biomarkers to evaluate the sensitivity to Vγ9Vδ2 T-cells would be the expression of antigens and ligands for their receptors by the tumor cells, and to select for those T-cells with high-affinity TCRs. Intensive investigations on the molecular mechanisms of antigen recognition by Vγ9Vδ2 T-cells are currently underway in the field. It is known that in the target cells, phosphoantigen binding causes a conformational change in the B30.2 domain of BTN3A1 that results in the activation of these T-cells.46 This process is mediated by the GTPase activity and relocalization of RhoB in the tumor cells; single-nucleotide polymorphisms (SNPs) near RhoB are associated with poor Vγ9Vδ2 T-cell activation.47 To date, biochemical detection of minute amounts of the phosphoantigens is not yet feasible for routine use in the clinic. Nonetheless, defined SNPs, GTPase activity of RhoB, relocalization of RhoB from the nucleus to extranuclear sites, and detection of the conformational changes of BTN3A1 are potential biomarkers to help select patients with a greater chance of benefitting from Vγ9Vδ2 T-cell therapy. It should be noted that in addition to their TCR, Vγ9Vδ2 T-cells can also recognize tumor cells via NK-like activating receptors such as NKG2D and DNAX accessory molecule 1 (DNAM1), as well as NK inhibitory receptors. The differential levels of expression of the NKG2D ligands MICA and MICB, ULBP, or the DNAM1 ligands nectin-2 and polyomavirus receptor are also potential candidates for biomarkers that would inform the likelihood of tumor recognition. However, the problem is that no single one of these markers can predict the reactivity of Vγ9Vδ2 T-cells.
Thus, the transfer of expanded autologous Vγ9Vδ2 T-cells has been found safe but ineffective for most NSCLC patients. One further reason for this failure may be because the infused T cells might not be able to infiltrate into the tumor, as well as not being able to recognize tumor cells once they did. In the most patients, the recognition of tumor cells by unmanipulated Vγ9Vδ2 T-cells might not have been sufficient to allow the detection of tumor cell killing. However, when they do reach the tumor site and recognize tumor cells in certain patients, they can mediate robust antitumor effector activity, as illustrated by the eradication of a massive tumor in one NSCLC patient in this study (figure 3). They may also effectively eliminate tumor cells and ascites in patients with gastric cancer.26 Reliably exploiting this potential robust effector function of Vγ9Vδ2 T-cells, future research directions may be to combine adoptive immunotherapy with other immune-modulating agents or engineering the Vγ9Vδ2 T-cells themselves with more highly active tumor-specific αβ TCRs or CARs.48–50 Although currently no appropriate targets for CAR-T-cells in solid tumors have been identified, our results regarding the expansion process of Vγ9Vδ2 T-cells with zoledronate and their in vivo dynamics support the feasibility of developing engineered Vγ9Vδ2 T-cells for immunotherapy. We successfully expanded Vγ9Vδ2 T-cells in 33 of 68 heavily pretreated NSCLC patients (online supplemental table S2) and showed that patients’ backgrounds, including the number of prior therapies or exposures to cytotoxic drugs, were not associated with the expansion failure (online supplemental table S2). We also documented that biweekly administration of Vγ9Vδ2 T-cells resulted in their persistence and accumulation in patients’ blood, even without coadministration of IL-2 or IL-15 (figure 4A–F) and that they did not disturb the homeostasis of other immune cells (figure 4G–L). During and after the treatment, no autoimmune responses were detected, suggesting that these Vγ9Vδ2 T-cells did not recognize or cross-react with normal tissues, that is, toxicity mediated by endogenous Vγ9Vδ2 receptors was negligible. Thus, zoledronate expanded Vγ9Vδ2 T-cells are easily generated and manipulated; they may be excellent carriers for tumor-specific TCRs or CARs.
In conclusion, we conducted a phase 2 study of the adoptive transfer of zoledronate-expanded autologous Vγ9Vδ2 T-cells in patients with treatment-refractory NSCLC. The study was terminated before the number of enrolled patients (n=25) reached the target number (n=85). We failed to demonstrate clinical efficacy of adoptive transfer of Vγ9Vδ2 T-cells in the form reported here in that a PFS of 95 days did not meet the pre-defined expected PFS of 4 months. However, we noted a very robust antitumor activity of Vγ9Vδ2 T-cells in a single patient. Selecting patients with biomarkers, improving study design by focusing on patients with unmet medical needs and gene-engineering with TCRs or CARs may be required to reveal the power of Vγ9Vδ2 T-cells for the treatment of cancer.
jitc-2020-001185supp014.pdf (2.1MB, pdf)
Acknowledgments
We thank Takamichi Izumi, Shin-nosuke Kimura, Fumiya Kobayshi and Atsushi Kondo (all employees of MEDINET) and MEDINET for supporting Vγ9Vδ2 T-cell culture, and the Clinical Research Promotion Center, the University of Tokyo Hospital, for supporting the implementation of the study.
Footnotes
Contributors: KKa, HM, KS and JN designed the clinical study. KKa, HM and YK checks of the production process and of the infusion product. KKa, HM, KM, SI, KKi, IK, TM, TT, TE, KaN, HY, MS, KS and JN treated the patients. KKa, HM and YK produced the infusion product and performed all analysis. KKa, HM, TK, YK, KoN, MS, HY, KS and JN analyzed and interpreted the patient data. YN and RS performed TCR analysis. KKa, HM and JN designed and interpreted the monitoring experiments. KKa, TK, YK and KoN wrote the manuscript and all authors read and approved the final manuscript.
Funding: This research was partially supported by AMED under Grant Number JP16lk0201041.
Competing interests: Kakimi reports grants from TAKARA BIO, grants from MSD, outside the submitted work; the Department of Immunotherapeutics, The University of Tokyo Hospital was endowed by Medinet until May 2019. Other authors have no competing interests to disclose.
Patient consent for publication: Not required.
Ethics approval: This study was registered as a trial at the University Hospital Medical Information Network Clinical Trials Registry (UMIN-CTR) as UMIN000006128. The study was approved by the Institutional Review Board at each Institution (P2011018-11Z, 2014-282-1) and the Certified Committee for Regenerative Medicine of the University of Tokyo.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information. The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- 1. Bray F, Ferlay J, Soerjomataram I, et al. . Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3. Masters GA, Temin S, Azzoli CG, et al. . Systemic therapy for stage IV non-small-cell lung cancer: American Society of clinical oncology clinical practice guideline update. J Clin Oncol 2015;33:3488–515. 10.1200/JCO.2015.62.1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garon EB, Ciuleanu T-E, Arrieta O, et al. . Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014;384:665–73. 10.1016/S0140-6736(14)60845-X [DOI] [PubMed] [Google Scholar]
- 5. Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446–54. 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 6. Planchard D, Popat S, Kerr K, et al. . Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2018;29:iv192–237. 10.1093/annonc/mdy275 [DOI] [PubMed] [Google Scholar]
- 7. Vantourout P, Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nat Rev Immunol 2013;13:88–100. 10.1038/nri3384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chien Y-hsiu, Meyer C, Bonneville M. γδ T cells: first line of defense and beyond. Annu Rev Immunol 2014;32:121–55. 10.1146/annurev-immunol-032713-120216 [DOI] [PubMed] [Google Scholar]
- 9. Silva-Santos B, Serre K, Norell H. γδ T cells in cancer. Nat Rev Immunol 2015;15:683–91. 10.1038/nri3904 [DOI] [PubMed] [Google Scholar]
- 10. Tanaka Y, Morita CT, Tanaka Y, et al. . Natural and synthetic non-peptide antigens recognized by human gamma delta T cells. Nature 1995;375:155–8. 10.1038/375155a0 [DOI] [PubMed] [Google Scholar]
- 11. Gober H-J, Kistowska M, Angman L, et al. . Human T cell receptor gammadelta cells recognize endogenous mevalonate metabolites in tumor cells. J Exp Med 2003;197:163–8. 10.1084/jem.20021500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dieli F, Gebbia N, Poccia F, et al. . Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood 2003;102:2310–1. 10.1182/blood-2003-05-1655 [DOI] [PubMed] [Google Scholar]
- 13. Das H, Groh V, Kuijl C, et al. . Mica engagement by human Vgamma2Vdelta2 T cells enhances their antigen-dependent effector function. Immunity 2001;15:83–93. 10.1016/S1074-7613(01)00168-6 [DOI] [PubMed] [Google Scholar]
- 14. Rincon-Orozco B, Kunzmann V, Wrobel P, et al. . Activation of V gamma 9V delta 2 T cells by NKG2D. J Immunol 2005;175:2144–51. 10.4049/jimmunol.175.4.2144 [DOI] [PubMed] [Google Scholar]
- 15. Lança T, Correia DV, Moita CF, et al. . The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to gammadelta T-cell cytotoxicity. Blood 2010;115:2407–11. 10.1182/blood-2009-08-237123 [DOI] [PubMed] [Google Scholar]
- 16. Wesch D, Glatzel A, Kabelitz D. Differentiation of resting human peripheral blood gamma delta T cells toward Th1- or Th2-phenotype. Cell Immunol 2001;212:110–7. 10.1006/cimm.2001.1850 [DOI] [PubMed] [Google Scholar]
- 17. Dieli F, Troye-Blomberg M, Ivanyi J, et al. . Granulysin-dependent killing of intracellular and extracellular Mycobacterium tuberculosis by Vgamma9/Vdelta2 T lymphocytes. J Infect Dis 2001;184:1082–5. 10.1086/323600 [DOI] [PubMed] [Google Scholar]
- 18. Dalton JE, Howell G, Pearson J, et al. . Fas-Fas ligand interactions are essential for the binding to and killing of activated macrophages by gamma delta T cells. J Immunol 2004;173:3660–7. 10.4049/jimmunol.173.6.3660 [DOI] [PubMed] [Google Scholar]
- 19. Kondo M, Izumi T, Fujieda N, et al. . Expansion of human peripheral blood γδ T cells using zoledronate. J Vis Exp 2011. 10.3791/3182. [Epub ahead of print: 09 Sep 2011]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sato K, Kondo M, Sakuta K, et al. . Impact of culture medium on the expansion of T cells for immunotherapy. Cytotherapy 2009;11:936–46. 10.3109/14653240903219114 [DOI] [PubMed] [Google Scholar]
- 21. Kondo M, Sakuta K, Noguchi A, et al. . Zoledronate facilitates large-scale ex vivo expansion of functional gammadelta T cells from cancer patients for use in adoptive immunotherapy. Cytotherapy 2008;10:842–56. 10.1080/14653240802419328 [DOI] [PubMed] [Google Scholar]
- 22. Izumi T, Kondo M, Takahashi T, et al. . Ex vivo characterization of γδ T-cell repertoire in patients after adoptive transfer of Vγ9Vδ2 T cells expressing the interleukin-2 receptor β-chain and the common γ-chain. Cytotherapy 2013;15:481–91. 10.1016/j.jcyt.2012.12.004 [DOI] [PubMed] [Google Scholar]
- 23. Odaira K, Kimura S-N, Fujieda N, et al. . CD27(-)CD45(+) γδ T cells can be divided into two populations, CD27(-)CD45(int) and CD27(-)CD45(hi) with little proliferation potential. Biochem Biophys Res Commun 2016;478:1298–303. 10.1016/j.bbrc.2016.08.115 [DOI] [PubMed] [Google Scholar]
- 24. Sakamoto M, Nakajima J, Murakawa T, et al. . Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded γδTcells: a phase I clinical study. J Immunother 2011;34:202–11. 10.1097/CJI.0b013e318207ecfb [DOI] [PubMed] [Google Scholar]
- 25. Aoki T, Matsushita H, Hoshikawa M, et al. . Adjuvant combination therapy with gemcitabine and autologous γδ T-cell transfer in patients with curatively resected pancreatic cancer. Cytotherapy 2017;19:473–85. 10.1016/j.jcyt.2017.01.002 [DOI] [PubMed] [Google Scholar]
- 26. Wada I, Matsushita H, Noji S, et al. . Intraperitoneal injection of in vitro expanded Vγ9Vδ2 T cells together with zoledronate for the treatment of malignant ascites due to gastric cancer. Cancer Med 2014;3:362–75. 10.1002/cam4.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Godfrey DI, Le Nours J, Andrews DM, et al. . Unconventional T cell targets for cancer immunotherapy. Immunity 2018;48:453–73. 10.1016/j.immuni.2018.03.009 [DOI] [PubMed] [Google Scholar]
- 28. Sebestyen Z, Prinz I, Déchanet-Merville J, et al. . Translating gammadelta (γδ) T cells and their receptors into cancer cell therapies. Nat Rev Drug Discov 2020;19:169–84. 10.1038/s41573-019-0038-z [DOI] [PubMed] [Google Scholar]
- 29. Garber K. γδ T cells bring unconventional cancer-targeting to the clinic - again. Nat Biotechnol 2020;38:389–91. 10.1038/s41587-020-0487-2 [DOI] [PubMed] [Google Scholar]
- 30. Bacik J, Mazumdar M, Murphy BA, et al. . The functional assessment of cancer therapy-BRM (FACT-BRM): a new tool for the assessment of quality of life in patients treated with biologic response modifiers. Qual Life Res 2004;13:137–54. 10.1023/B:QURE.0000015297.91158.01 [DOI] [PubMed] [Google Scholar]
- 31. Fischer K, Andreesen R, Mackensen A. An improved flow cytometric assay for the determination of cytotoxic T lymphocyte activity. J Immunol Methods 2002;259:159–69. 10.1016/S0022-1759(01)00507-5 [DOI] [PubMed] [Google Scholar]
- 32. Betts MR, Brenchley JM, Price DA, et al. . Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods 2003;281:65–78. 10.1016/S0022-1759(03)00265-5 [DOI] [PubMed] [Google Scholar]
- 33. Kitaura K, Shini T, Matsutani T, et al. . A new high-throughput sequencing method for determining diversity and similarity of T cell receptor (TCR) α and β repertoires and identifying potential new invariant TCR α chains. BMC Immunol 2016;17:38. 10.1186/s12865-016-0177-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mukohara T, Takeda K, Miyazaki M, et al. . Japanese experience with second-line chemotherapy with low-dose (60 mg/M2) docetaxel in patients with advanced non-small-cell lung cancer. Cancer Chemother Pharmacol 2001;48:356–60. 10.1007/s002800100362 [DOI] [PubMed] [Google Scholar]
- 35. Hanna N, Shepherd FA, Fossella FV, et al. . Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 2004;22:1589–97. 10.1200/JCO.2004.08.163 [DOI] [PubMed] [Google Scholar]
- 36. Scagliotti G, Hanna N, Fossella F, et al. . The differential efficacy of pemetrexed according to NSCLC histology: a review of two phase III studies. Oncologist 2009;14:253–63. 10.1634/theoncologist.2008-0232 [DOI] [PubMed] [Google Scholar]
- 37. Maruyama R, Nishiwaki Y, Tamura T, et al. . Phase III study, V-15-32, of gefitinib versus docetaxel in previously treated Japanese patients with non-small-cell lung cancer. J Clin Oncol 2008;26:4244–52. 10.1200/JCO.2007.15.0185 [DOI] [PubMed] [Google Scholar]
- 38. Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. . Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med 2005;353:123–32. 10.1056/NEJMoa050753 [DOI] [PubMed] [Google Scholar]
- 39. Ryan PL, Sumaria N, Holland CJ, et al. . Heterogeneous yet stable Vδ2 (+) T-cell profiles define distinct cytotoxic effector potentials in healthy human individuals. Proc Natl Acad Sci U S A 2016;113:14378–83. 10.1073/pnas.1611098113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vyborova A, Beringer DX, Fasci D, et al. . γ9δ2T cell diversity and the receptor interface with tumor cells. J Clin Invest 2020. 10.1172/JCI132489. [Epub ahead of print: 04 Aug 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Havel JJ, Chowell D, Chan TA. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat Rev Cancer 2019;19:133–50. 10.1038/s41568-019-0116-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sharma P, Hu-Lieskovan S, Wargo JA, et al. . Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–23. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Alnaggar M, Xu Y, Li J, et al. . Allogenic Vγ9Vδ2 T cell as new potential immunotherapy drug for solid tumor: a case study for cholangiocarcinoma. J Immunother Cancer 2019;7:36. 10.1186/s40425-019-0501-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: recognition and management. Blood 2016;127:3321–30. 10.1182/blood-2016-04-703751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Grunder C, van Dorp S, Hol S, et al. . gamma9 and delta2CDR3 domains regulate functional avidity of T cells harboring gamma9delta2TCRs. Blood 2012;120:5153–62. [DOI] [PubMed] [Google Scholar]
- 46. Sandstrom A, Peigné C-M, Léger A, et al. . The intracellular B30.2 domain of butyrophilin 3A1 binds phosphoantigens to mediate activation of human Vγ9Vδ2 T cells. Immunity 2014;40:490–500. 10.1016/j.immuni.2014.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sebestyen Z, Scheper W, Vyborova A, et al. . RhoB Mediates Phosphoantigen Recognition by Vγ9Vδ2 T Cell Receptor. Cell Rep 2016;15:1973–85. 10.1016/j.celrep.2016.04.081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harrer DC, Simon B, Fujii S-I, et al. . RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: a safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer 2017;17:551. 10.1186/s12885-017-3539-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Capsomidis A, Benthall G, Van Acker HH, et al. . Chimeric antigen Receptor-Engineered human gamma delta T cells: enhanced cytotoxicity with retention of cross presentation. Mol Ther 2018;26:354–65. 10.1016/j.ymthe.2017.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fisher J, Anderson J. Engineering approaches in human gamma delta T cells for cancer immunotherapy. Front Immunol 2018;9:1409. 10.3389/fimmu.2018.01409 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2020-001185supp001.pdf (97.2KB, pdf)
jitc-2020-001185supp002.pdf (105.4KB, pdf)
jitc-2020-001185supp008.xlsx (13.5KB, xlsx)
jitc-2020-001185supp009.xlsx (16KB, xlsx)
jitc-2020-001185supp003.pdf (200.6KB, pdf)
jitc-2020-001185supp004.pdf (433.4KB, pdf)
jitc-2020-001185supp005.pdf (51.7KB, pdf)
jitc-2020-001185supp006.pdf (468.3KB, pdf)
jitc-2020-001185supp007.pdf (2.1MB, pdf)
jitc-2020-001185supp010.xlsx (14.6KB, xlsx)
jitc-2020-001185supp011.xlsx (14.3KB, xlsx)
jitc-2020-001185supp012.xlsx (16KB, xlsx)
jitc-2020-001185supp013.xlsx (15.9KB, xlsx)
jitc-2020-001185supp014.pdf (2.1MB, pdf)