Graphical abstract

Keywords: Respiration, Cyanide, Mitochondria, Antidote
Highlights
-
•
Cyanide is a cytochrome c oxidase inhibitor that results in bioenergetic failure.
-
•
There is a cell-permeable succinate prodrug, NV118, that releases succinate.
-
•
Our mitochondrial-directed therapy (NV118) but not B12a improved cellular function.
-
•
There was attenuation of ROS with NV118 but not with B12a treatment.
Abstract
The objective of this study was to compare the use of hydroxocobalamin (B12a) and a succinate prodrug to evaluate for improvement in mitochondrial function in an in vitro model of cyanide poisoning. Peripheral blood mononuclear cells (PBMC) and human aortic smooth muscle cells (HASMC) incubated with 50 mM of sodium cyanide (CN) for five minutes serving as the CN group compared to controls. We investigated the following: (1) Mitochondrial respiration; (2) Superoxide and mitochondrial membrane potential with microscopy; (3) Citrate synthase protein expression. All experiments were performed with a cell concentration of 2−3 × 106 cells/ml for both PBMC and HASMC. There were four conditions: (1) Control (no exposure); (2) Cyanide (exposure only); (3) B12a (cyanide exposure followed by B12a treatment); (4) NV118 (cyanide followed by NV118 treatment). In this study the key findings include: (1) Improvement in key mitochondrial respiratory states with the succinate prodrug (NV118) but not B12a; (2) Attenuation of superoxide production with treatment of NV118 but not with B12a treatment; (3) The changes in respiration were not secondary to increased mitochondrial content as measured by citrate synthase; (4) The use of easily accessible human blood cells showed similar mitochondrial response to both cyanide and treatment to HASMC. The use of a succinate prodrug to circumvent partial CIV inhibition by cyanide with clear reversal of cellular respiration and superoxide production that was not attributed to changes in mitochondrial content not seen by the use of B12a.
1. Introduction
Cyanide is a potent Complex IV or CIV inhibitor that results in cellular bioenergetic failure from decreased ATP production [[1], [2], [3], [4]]. Cyanide exposure can occur from smoke inhalation, especially following the combustion of materials that include wool, silk, synthetic rubber, and polyurethane. Exposure can also occur as an occupational hazard and also as an agent of terriorism [[5], [6], [7], [8], [9]]. Treatment of cyanide toxicity consists primarily of supportive care and select use of antidotal therapy. One class of antidotes are the nitrites to induce methemoglobin formation, however, its primary effect may be attributed to the formation of nitric oxide (NO) that displace cyanide from CIV potentially leading to recovery in the electron transport system (ETS) [10]. Another commonly used antidote is hydroxocobalamin (B12a) that binds with cyanide forming a common vitamin cyanocobalamin (B12) that is non-toxic [[11], [12], [13]]. A significant limitation that exists is that very few antidotes address the underlying problem with cellular bioenergetic failure. There is a gap in mitochondrial therapy that directly delivers substrate to increase oxidative phosphorylation (OXPHOS) capacity. There are a class of cell-permeable succinate prodrugs that readily bypass the cell membrane independent of active transport to release succinate. While primarily developed for patients with genetically inherited Complex I or CI deficiency, its use in partial CIV inhibition is not known [14,15].
Another gap that exists is the limited ability to gauge cellular function in vivo with cyanide poisoning. There are many barriers to this approach that include cost, time and invasiveness. Whole blood cells such as peripheral blood mononuclear cells (PBMC) and platelets have been explored for their potential to act as sensitive biomarkers for mitochondrial dysfunction in lieu of invasive and time consuming tissue biopsies [[16], [17], [18]]. The measurement of mitochondrial respiration in cells isolated from human blood offers a more sensitive assay for mitochondrial poisons such as cyanide by directly measuring mitochondrial respiration as demonstrated in our previous study [19].
The goals of this in vitro investigation were to utilize a cellular model of partial complex IV inhibition to study a new approach in therapy using a succinate prodrug. We evaluated alterations in respiration, reactive oxygen species production and mitochondrial content compared to standard treatment as well as the use of blood cells as a surrogate biomarker of tissue function.
2. Materials and methods
2.1. Material
We used a cell-permeable first-generation succinate prodrug, NV101-118 or diacetoxy-methyl succinate obtained from NeuroVive Pharmaceutical AB (Lund, Sweden) as seen in Fig. 1.
Fig. 1.
Structure of a First Generation Succinate Prodrug: NV118.
2.2. Study design and cell preparation
The Institutional Review Board approved this study (Protocol number 822694) and whole blood was obtained from twenty consented participants. All eligible subjects underwent phlebotomy and samples were taken from the collection tubing and PBMC were isolated using Leucosep Centrifuge tubes from Greiner Bio-One (Kremsmünster, Austria) [19]. Adult primary human aortic smooth muscle cells (HASMC)- SKU: ATCC® PCS-100-012 were purchased from Lifeline Cell Technology (Frederick, CA, USA). The HASMC between passages one and eight were cultured in Vascular Cell Basal media from American Type Culture Collection (Manassas, VA, USA) without antibiotic use. HASMC have been used to study vascular pathophysiology and is often used as a model for vascular response to stress response. For experiments to measure mitochondrial function with fluorescence microscopy, cells were plated at a density of 10,000 cells per dish on fibronectin-coated MatTek 35-mm glass-bottom dishes purchased from MatTek Life Sciences (Ashland, MA, USA) approximately 48 h before experiments. MatTek dishes were coated with 10 μg/ml fibronectin-PBS solution for 30 min prior to plating.
2.3. High-resolution respirometry
Respiration was measured at a constant temperature of 37 °C in a high-resolution respirometry (Oxygraph-2k) with DatLab software version 7 for data recording and analysis (Oroboros Instruments, Innsbruck, Austria). All experiments were performed at an oxygen concentration range of 50−210 μM O2. For respiration measurements, both PBMC and HASMC were suspended in a mitochondrial respiration medium (MiR05) containing sucrose 110 mM, HEPES 20 mM, taurine 20 mM, K-lactobionate 60 mM, MgCl2 3 mM, KH2PO4 10 mM, EGTA 0.5 mM, BSA 1 g/l, pH 7.1. All experiments were performed with a cell concentration of 2−3 × 106 cells/ml with normalization performed for each experiment based on cell number.
There were four conditions: (1) Control (no exposure); (2) Cyanide (exposure only); (3) B12a (cyanide exposure followed by B12a treatment); (4) NV118 (cyanide followed by NV118 treatment). Initially, cells were left to stabilize at a routine respiration state, revealing resting cellular energy demands on OXPHOS. Once routine respiration was obtained, with the exception of the Control group, sodium cyanide, a 1 mM stock for a final concentration of 50 μM was used in the other three groups. A fresh batch was made prior to each experiment and immediately injected into airtight O2k chambers for consistent dosing due to off-gassing that may occur with cyanide. This concentration was determined in a dose dependent fashion to cause a 50 % reduction in respiration representing partial inhibition of CIV as a 100 % inhibition is incompatible with cell viability. 15 min. following cyanide exposure, respiration was obtained and either B12a (1 mM) or NV118 (100 μM) was used as treatment based on previous publications [14]. Dimethylsulphoxide (DMSO) was the compound vehicle for NV118 and therefore it was also used in a separate series of experiments as well as succinate and treatment alone to examine for any treatment effect in the absence of cyanide exposure. After the titration of B12a or NV118, the maximal capacity of the electron transport system (ETS) also known as maximal respiration or MAX was measured after careful titration of the protonophore, carbonyl cyanide p-(trifluoromethoxy) phenylhydrazone (CCCP (0.5 u M steps)) until no further increase in respiration was detected. Rotenone, a CI inhibitor, (2 μM) and antimycin-A, a CIII inhibitor, (2.5 u M) were then sequentially added to inhibit the ETS providing the non-mitochondrial residual oxygen consumption (ROX) that was subsequently subtracted from the different respiratory parameters in further analyses [20]. We also investigated specific CIV activity with the addition of ascorbate (2 mM) and N,N,N′,N′-Tetramethyl-p-phenylenediamine or TMPD (0.5 mM) followed by sodium azide (100 mM) which will inhibit any remaining CIV respiration based on multiple publications [[21], [22], [23]]. All chemicals used for respiration were obtained from Sigma-Aldrich (St. Louis, MO, USA) based on previous publications which include exact specifications [20,24]
2.4. Determination of superoxide production and mitochondrial inner membrane potential
The same conditions were repeated to determine superoxide production and mitochondrial inner membrane potential. Cells were imaged using a Zeiss LSM 710 Confocal microscope (Oberkochen, Germany). We used the following fluorophores: MitoSOX Red Mitochondrial Superoxide Indicator (catalog M36008); Tetramethylrhodamine, methyl ester (TMRM) mitochondrial membrane potential indicator (catalog I34361); and 4′,6-diamidino-2-phenylindole (DAPI) DNA stain (catalog D1306) purchased from Invitrogen (Carlsbad, CA, USA). The production of superoxide by mitochondria can be visualized in fluorescence microscopy using MitoSOX Red. MitoSOX Red experiments were performed with a 5 u M working solution. Fluorescence intensity was measured with ImageJ software and Corrected Total Cell Fluorescence (CTCF) was calculated through the formula (CTCF = Integrated Density – Area of Cell × Background Fluorescence). Mitochondrial membrane potential was obtained with the use of tetramethylrhodamine (TMRM). TMRM data was acquired by incubating cells in 20 nM working solution for 30 min in the dark at 37 °C. Fluorescence Intensity Ratio for each region of interest (ROI) was calculated as follows: (ROI Mean Fluorescence – Background Fluorescence) / Background Fluorescence [25].
2.5. Determination of citrate synthase (CS) relative density values
Protein quantification was performed with iWestern and gels were imaged with the iBright CL1500 by Thermo Fisher Scientific (Waltham, MA, USA). Western blot was performed to measure CS protein expression used as a marker of mitochondrial content. Western blot was performed on cells with the same conditions and concentrations used in this study with all reagents and antibodies purchased from Invitrogen (Carlsbad, CA, USA) unless otherwise noted. Cells were incubated on ice in RIPA lysis and extraction buffer (catalog 89,900) containing Pierce Protease inhibitor (catalog A32963) for 5 min. All cells types were briefly sonicated and then subjected to centrifugation at 14,000 g at 4ᵒC for 10 min. Protein concentrations of the supernatant were quantified using a Pierce BCA Protein Assay Kit from Thermo Fisher Scientific (Waltham, MA, USA). Equal protein content was loaded into each well of a 4–12 % Bis-Tris gel and separated by sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Gel proteins were transferred onto a nitrocellulose membrane and then incubated with a CS Recombinant Rabbit Monoclonal Antibody (catalog 3H8L26). Primary rabbit monoclonal anti-glyceradehyde 3-phosphate dehydrogenase (GAPDH) antibody conjugated to horseradish peroxidase (HRP) (#3683 Cell Signaling Technology; 1:2000) was used as the internal control. CS levels were detected using goat anti-rabbit IgG secondary antibody conjugated to HRP (catalog A16096; 1:400) and a chemiluminescent substrate reagent kit. Immunoblotting steps were done in an iBind Western Device (Invitrogen). iBright Analysis Software (Thermo Scientific) was used in the quantification and densitometric analysis of the blots. All experiments were performed in duplicates and the local background corrected density values were normalized against respective GAPDH values used as a housekeeping protein.
2.6. Statistical analysis
Statistics were calculated by using Prism v.8 from GraphPad Software (San Diego, CA, USA). Data are presented as mean ± standard deviation (SD) of the mean if not indicated otherwise. Analysis between groups was performed by using repeated measures ANOVA. The results were adjusted with Bonferroni correction for multiple tests. A P value of <0.05 was statistically significant.
3. Results
Whole blood for PBMC isolation were obtained from participants that included 20 healthy subjects: 10 males and 10 females, median age of 31 years of age (interquartile range = 25–36). Table 1 contains all the values presented as the mean and the SD obtained for respiration, fluorescence microscopy and Western blot in HASMC and PBMC for all 4 conditions. Fig. 2, Fig. 3 displays the oxygen consumption for key respiratory states for all conditions in both HASMC and PBMC, respectively. Key findings in the HASMC cell line include a similar baseline routine respiration prior to exposure to cyanide with a significant 50 % decrease in mitochondrial respiration after cyanide exposure with the exception of the Control group which was not exposed to cyanide. The use of NV118 showed a significant treatment effect and CII-linked respiration along with a significant increase in both maximal and CIV respiration when compared to the Cyanide and B12a group. This relationship in respiration with the treatment of B12a and NV118 was also consistent in the PBMC groups. In addition, we also performed additional experiments examining for an effect with treatment alone (B12a and NV118) with no observed differences with the exception that NV118 increased respiration in healthy PBMCs which has been noted in other works.
Table 1.
Mitochondrial respiration, MitoSOX, TMRM, and citrate synthase obtained in PBMC and HASMC. Values presented as mean ± SD.
| HASMC (pmol O2 • s−1 • 10-6 Cells) | Control Group | Cyanide group | B12a group | NV118 group | ||||
|---|---|---|---|---|---|---|---|---|
| Respiratory State | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Routine | 16.30 | 0.46 | 17.50 | 0.67 | 17.42 | 0.73 | 17.16 | 0.71 |
| Cyanide exposure | 16.30 | 0.57 | 9.46 | 2.00 | 10.14 | 0.77 | 10.20 | 0.78 |
| Treatment | 16.30 | 0.57 | 9.56 | 1.89 | 10.78 | 0.53 | 12.76 | 0.55 |
| Complex II-linked respiration | 0.34 | 0.43 | 0.12 | 0.08 | 0.16 | 0.17 | 6.38 | 0.87 |
| Maximal respiration | 25.86 | 0.83 | 21.52 | 1.51 | 23.10 | 1.00 | 27.28 | 4.18 |
| Complex IV respiration | 65.20 | 0.66 | 29.70 | 0.85 | 30.14 | 0.81 | 55.44 | 1.67 |
| PBMC (pmol O2 • s−1 • 10-6 Cells) | Control Group | Cyanide group | B12a group | NV118 group | ||||
| Respiratory State | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Routine | 20.25 | 1.13 | 22.87 | 1.26 | 23.46 | 1.80 | 20.96 | 0.90 |
| Cyanide exposure | 20.19 | 1.26 | 8.54 | 0.46 | 10.55 | 1.47 | 8.61 | 0.69 |
| Treatment | 20.19 | 1.26 | 8.89 | 0.49 | 7.17 | 1.01 | 14.44 | 0.99 |
| Complex II-linked respiration | 1.23 | 0.33 | 2.57 | 0.33 | 2.95 | 0.56 | 9.08 | 1.00 |
| Maximal respiration | 50.30 | 2.46 | 23.70 | 1.43 | 15.76 | 2.21 | 34.68 | 2.03 |
| Complex IV respiration | 72.49 | 5.61 | 25.07 | 2.60 | 33.42 | 6.12 | 56.08 | 6.61 |
| HASMC (Average CTCF) | Control Group | Cyanide group | B12a group | NV118 group | ||||
| Microscopy | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| MitoSOX | 197005.24 | 38437.69 | 483858.24 | 102987.75 | 283262.05 | 33065.41 | 239075.81 | 22207.17 |
| TMRM | 271.75 | 26.46 | 327.01 | 23.51 | 293.35 | 24.98 | 320.63 | 23.31 |
| PBMC (Average CTCF) | Control Group | Cyanide group | B12a group | NV118 group | ||||
| Microscopy | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| MitoSOX | 62148.00 | 4257.32 | 116576.70 | 10957.88 | 144530.00 | 10348.72 | 61427.45 | 3130.45 |
| TMRM | 207.14 | 17.41 | 175.52 | 11.29 | 169.62 | 14.58 | 183.55 | 10.75 |
| HASMC (Relative Density Value) | Control Group | Cyanide group | B12a group | NV118 group | ||||
| Western Blot | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Citrate synthase | 10118.05 | 398.70 | 12441.68 | 810.08 | 11635.22 | 948.47 | 12385.28 | 884.94 |
| PBMC (Relative Density Value) | Control Group | Cyanide group | B12a group | NV118 group | ||||
| Western Blot | Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM |
| Citrate synthase | 3113.41 | 590.70 | 4466.01 | 916.34 | 4420.17 | 906.23 | 4761.85 | 1161.17 |
Fig. 2.
Key Respiration States for HASMC.
Respiration obtained in HASMC in 4 groups: (1) Control (no exposure to treatment); (2) Cyanide (exposed to 50 μM); (3) B12a (exposed to cyanide followed by 1 mM of B12a); (4) NV118 (exposed to cyanide followed by 100 μM). Routine respiration was the same across all groups (not-displayed) and the y-axis ranges have been adjusted to clearly show the resolution of the reported respiratory states. Values presented as mean ± SD.
*The Control group significantly different after all other groups after cyanide exposure but before administration of treatment (P < 0.0001).
**The NV118 group is significantly higher than all the other groups (P < 0.001).
#The NV118 group is significantly higher than both the Cyanide and B12a group (P < 0.05).
##The NV118 group is significantly higher than both the Cyanide and B12a group and significantly lower than the Control group (P < 0.0001).
Fig. 3.
Key Respiration States for PBMC.
Respiration obtained in PBMC in 4 groups: (1) Control (no exposure to treatment); (2) Cyanide (exposed to 50 μM); (3) B12a (exposed to cyanide followed by 1 mM of B12a); (4) NV118 (exposed to cyanide followed by 100 μM). Routine respiration was the same across all groups (not-displayed) and the y-axis ranges have been adjusted to clearly show the resolution of the reported respiratory states. Values presented as mean ± SD.
*The control group significantly different after all other groups after cyanide exposure but before administration of treatment (P < 0.0001).
**The NV118 group is significantly lower than the Control lower (P < 0.01) but the NV118 group is significantly higher than all the other groups (P < 0.001).
#The NV118 group is significantly higher than both the Cyanide and B12a group (P < 0.05).
##The NV118 group is significantly higher than both the Cyanide and B12a group (P < 0.0001).
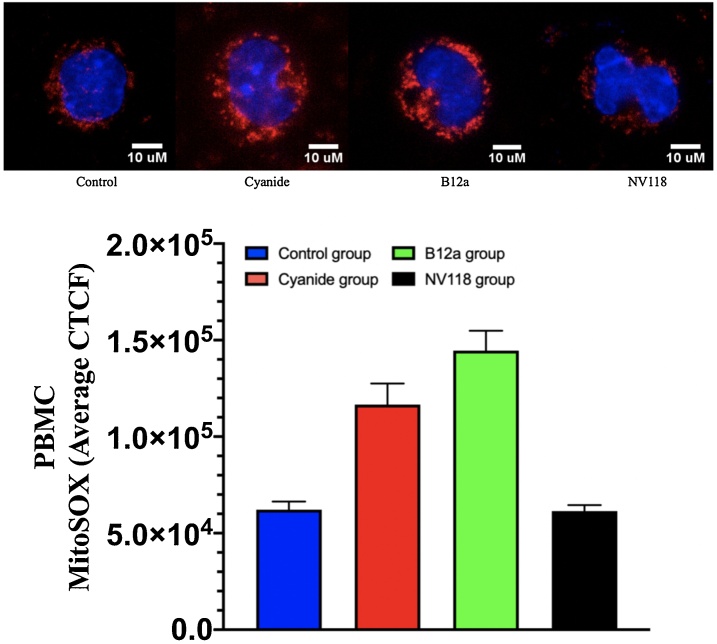
Figs. 4, 5 display the superoxide production as measured with MitoSOX fluorescence shown as the average corrected total cell fluorescence (CTCF) for each of the four groups. For HASMCs, there was a significant increase in superoxide in the Cyanide group when compared to the Control group. There was normalization of superoxide in the NV118 group when compared to the Cyanide group. There were no other significant differences between groups with respect to HASMCs. In the PBMC groups, there was a significant increase in superoxide when compared to the Control group. There was also a significant decrease of superoxide upon administration of NV118 similar to what is seen with HASMC. A different finding with PBMCs when compared to HASMCs is that in the B12a group there was also a significant increase in superoxide when compared to the Control group similar to the Cyanide group.
Fig. 4.
MitoSOX for HASMC.
Superoxide measured as MitoSOX obtained in HASMC in 4 groups: (1) Control (no exposure to treatment); (2) Cyanide (exposed to 50 μM); (3) B12a (exposed to cyanide followed by 1 mM of B12a); (4) NV118 (exposed to cyanide followed by 100 μM). Values presented as mean ± SD. Each cell shown is a representation for each condition above the bar graph.
* The Control group is significantly lower than the Cyanide group with the NV118 group significantly lower than Cyanide group (P < 0.005 for both)
Fig. 5.
MitoSOX for PBMC.
Superoxide measured as MitoSOX obtained in PBMC in 4 groups: (1) Control (no exposure to treatment); (2) Cyanide (exposed to 50 μM); (3) B12a (exposed to cyanide followed by 1 mM of B12a); (4) NV118 (exposed to cyanide followed by 100 μM). Values presented as mean ± SD. Each cell shown is a representation for each condition above the bar graph.
* The Control group is significantly lower than the Cyanide group with the NV118 group significantly lower than Cyanide group (P < 0.005 for both) and the B12a group is significantly higher than the Control group.
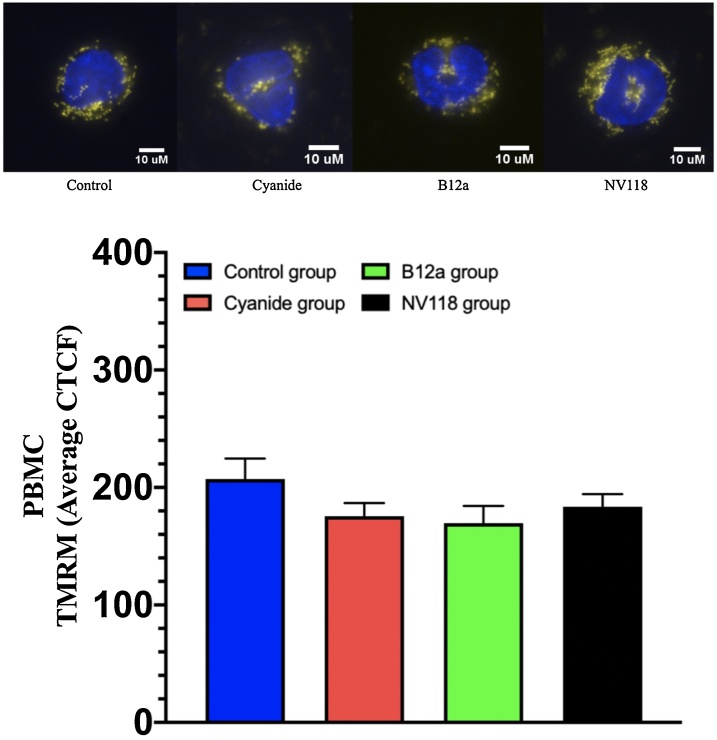
Fig. 6, Fig. 7 display the mitochondrial membrane potential obtained with TMRM displayed as the average corrected total cell fluorescence (CTCF) for each of the four groups. There were no significant differences in the TMRM signal in all 4 groups for both the HASMC and PBMC cell line. Also shown are fluorescence images of a cell from each group in both HASMC and PBMC cell lines. In addition, we also performed additional experiments for all conditions with treatment alone (B12a and NV118) and succinate with no observed differences
Fig. 6.
TMRM for HASMC.
Mitochondrial membrane potential measured as TMRM obtained HASMC in 4 groups: (1) Control (no exposure to treatment); (2) Cyanide (exposed to 50 μM); (3) B12a (exposed to cyanide followed by 1 mM of B12a); (4) NV118 (exposed to cyanide followed by 100 μM). Values presented as mean ± SD. There was no significant difference in HASMC in all conditions. Each cell shown is a representation for each condition above the bar graph.
Fig. 7.
TMRM for PBMC.
Mitochondrial membrane potential measured as TMRM obtained in PBMC in 4 groups: (1) Control (no exposure to treatment); (2) Cyanide (exposed to 50 μM); (3) B12a (exposed to cyanide followed by 1 mM of B12a); (4) NV118 (exposed to cyanide followed by 100 μM). Values presented as mean ± SD. There was no significant difference in PBMC in all conditions. Each cell shown is a representation for each condition above the bar graph.
Supplemental Fig. 1 illustrate the relative density values for CS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) used as a housekeeping protein obtained in PBMC and HASMC in the 4 groups. There was no significant difference in the relative density value of CS across all conditions with both the HASMC and PBMC cell line. Supplement Fig. 2 show the Western blot image with the −45 kDa band corresponding to the molecular weight of CS protein was detected using a polyclonal secondary antibody against a rabbit monoclonal anti-CS primary that is known to bind human CS. A second band was detected at −37 kDa using a monoclonal rabbit anti-GAPDH antibody known to detect human GAPDH.
4. Discussion
This in vitro study investigates the changes in mitochondrial function in response to cyanide. Cyanide caused impaired mitochondrial function alleviated with the administration of a cell-permeable mitochondrial substrate when compared to cells treated with B12a. The key findings include: (1) Improvement in key mitochondrial respiratory states with the application of a succinate prodrug (NV118) but not B12a in mitochondrial dysfunction imposed by cyanide; (2) Attenuation of superoxide production observed in the Cyanide group treated with NV118 but not with B12a treatment; (3) The changes in respiration were not secondary to increased mitochondrial content as measured by CS; (4) The use of easily accessible human blood cells showed similar mitochondrial response to both cyanide and treatment, demonstrating the significant potential as a peripheral serum biomarker of mitochondrial toxicity and treatment response.
We utilized a first-generation succinate prodrug (NV118) which is one of a series of in vitro test compounds [14]. There is currently a large unmet need for evidence-based treatment related to cyanide poisoning [26]. We utilized an in vitro cellular model of partial complex IV inhibition that was largely reversed with the application of the succinate prodrug but not with B12a. Importantly, these findings are consistent with the mechanism of action as a therapy for cyanide poisoning [27]. The cobalt ion in B12a combines with cyanide to form the nontoxic cyanocobalamin making the timely administration of this therapy crucial [8]. In this study, the treatment of B12a after exposure to cyanide did not result in any significant changes in key mitochondrial respiratory states such as maximal and CIV respiration when compared to NV118. As proof of target engagement and therapeutic mechanism, we also demonstrated the increased CII-linked respiration in the NV118 group which is the most likely reason for the increased maximal and CIV respiration observed in our study. There was another study that demonstrated partial inhibition of CIV respiration in an ex vivo study of carbon monoxide poisoning that showed improvement upon affected cells being given NV118. While there is reproducibility with the effect of succinate on CIV inhibition, further studies are required to fully understand the rescue effect of CIV respiration and may include measurements of ATP [28].
In both cell lines. cyanide resulted in an increase in superoxide as measured with MitoSOX fluorescence that was attenuated with administration of NV118. Cyanide has been showed to inhibit superoxide reductases which reduces superoxide to the more stable hydrogen peroxide [29]. Superoxide is a short-lived but highly reactive oxygen species that can damage cellular membranes, mitochondrial, and proteins. Over production of superoxide has been implicated in a variety of ailments such as cancer, aging, and Parkinson's disease [30,31]. The main pathway used for the cellular degradation of superoxide is through superoxide dismutase (SOD). There are both cytosolic and mitochondrial SOD where it has been shown that cyanide may inhibit cytosolic SOD more so than mitochondrial SOD depending on dose [32]. The increased MitoSOX signal from cyanide exposure may be due to overall increase in production of superoxide that overwhelms the ability of SOD to convert superoxide to hydrogen peroxide. One noted difference is in the B12a group between both cell lines where there was a clear increase in superoxide when compared to the Control group in PBMC not seen with HASMC. The difference between PBMC and HASMC may be due to the type of cells exposed to cyanide and response to treatment with B12a. In our study, the use of NV118 not only increased mitochondrial respiration but also attenuated the production of superoxide as measured by MitoSOX. By supplying the mitochondria with a substrate for CII-linked, this better supports aerobic metabolism for ATP generation.
In addition to measuring superoxide production we also evaluated mitochondrial membrane potential with the use of TMRM. TMRM is a cell-permeant, cationic, red-orange fluorescent dye that is readily sequestered by active mitochondria. Healthy cells with functioning mitochondria will demonstrate a bright signal as opposed to injured mitochondria that will demonstrate a dimmer or loss of signal indicating a decrease in membrane potential. In our study, there were no significant changes in the TMRM signal in either cell lines across the 4 conditions. The effect of cyanide on mitochondrial membrane potential is not well established whereas well known uncouplers such as carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) and 2,4-dinitrophenol (DNP) reduce mitochondrial membrane potential. Interestingly, there was one study that found the use of cyanide actually attenuated the uncoupling response of both FCCP and DNP [33]. It is important to note that the response of mitochondrial membrane potential may differ based on various factors such as the cyanide concentration, duration of exposure, cell type, and technique used to measure membrane potential.
We also measured the CS relative density value in both cell lines in all four conditions with Western blotting. CS is a pace-maker enzyme in the Krebs cycle with a molecular weight of 51,709 Da and is localized in the mitochondrial matrix. CS, therefore, is commonly used as a quantitative marker enzyme for the content of intact mitochondria. The proliferation of mitochondria in pathological states is sometimes associated with an increase in CS activity per cell, but CS activity can vary depending on the tissue type [34]. In this study we evaluated alterations in cellular function with exposure to cyanide with response to treatment. One of the possible factors in changes in both respiration and ROS generation maybe related to a change in the number of mitochondria, or content. Thus, we measured the corrected relative density of CS in both cell lines used in our study across all conditions. We demonstrate that the mitochondrial content was the same across all conditions and cell lines which makes changes in mitochondrial content as a cause of the changes seen in this study unlikely. While chronic exposure to cyanide may result in changes in mitochondrial content measured as CS, we were interested in studying cyanide poisoning in the acute setting which is a more realistic scenario to which exposure to cyanide may occur.
Finally, we used two cell types and demonstrated similar results in mitochondrial dysfunction imposed by cyanide with rescue seen with the use of NV118. The use of circulating blood cells such as white blood cells and platelets potentially serve as pathophysiologic biomarkers, or as an early warning or “canary in the coal mine” for mitochondrial dysfunction that may occur in conditions of metabolic stress in affected organ systems, such as brain and heart [16]. Once isolation of PBMC have occurred, the time to obtain key mitochondrial respiration parameters is typically less than an hour which may have relevance for time-sensitive clinically problems such as acute poisoning or sepsis. The advantage of the use of blood cells include minimally invasive method (phlebotomy), ability to perform multiple measurements, monitor response to treatment, and prognosis. Our prior work has explored the use of blood cells in a variety of acute care illnesses to gauge severity of disease [[21], [22], [23]]. One of the limitations of this method is how much of a biomarker blood cells may serve as a surrogate function for tissue. We utilized HASMC as a surrogate for the vascular function which was similar in response to both cyanide and treatment to PBMC.
In this study, we have shown that mitochondrial-directed therapy mitigates mitochondrial dysfunction imposed by cyanide when compared to B12a. Our results also suggest that the potential application of human blood cells as a surrogate marker of tissue function as the findings in PBMC mirrored that of a representative cell line affected by cyanide.
5. Limitations
One of the limitations of our study is that the first-generation succinate prodrugs are not suitable for in vivo use. Another limitation is whether blood cells serve as a precise biomarker of tissue cellular function, in our study demonstrates similar mitochondrial response to cyanide and restoration of cellular function with the use of NV118 in both PBMC and HASMC. This can be further explored in an in vivo model of cyanide poisoning as well. A third limitation is if the concentration of B12a was sufficient. We used a wide range of concentrations of B12a with no appreciable effect on respiration imposed by cyanide which is likely due to the fact B12a is not a mitochondrial substrate. Finally, a final consideration is the concentration of cyanide used. While the use of the concentration of cyanide in our study is smaller than what is used in animal studies, we choose a concentration that reliable induced a 50 % reduction in respiration to represent partial CIV inhibition. It is not known what efficacy NV118 would have with complete CIV inhibition although it is unlikely there would be survivability in a clinical scenario if there was complete CIV inhibition.
Author statement
We confirm that this manuscript has not been published elsewhere and is not under consideration by another journal and all authors have approved the manuscript and agree with its submission to Toxicology Reports.
Funding
1. NIH grant K08HL136858 (Jang)
2. NIH grant R03HL154232 (Jang)
3. NIH grant R21ES031243 (Jang)
4. NIH grant R21NS103826 (Kilbaugh)
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.toxrep.2020.09.002.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Truong D.H., Eghbal M.A., Hindmarsh W., Roth S.H., O’Brien P.J. Molecular mechanisms of hydrogen sulfide toxicity. Drug Metab. Rev. 2006;38:733–744. doi: 10.1080/03602530600959607. [DOI] [PubMed] [Google Scholar]
- 2.Chance B., Williams G.R. Respiratory enzymes in oxidative phosphorylation. IV. The respiratory chain. J. Biol. Chem. 1955;217:429–438. [PubMed] [Google Scholar]
- 3.Banea J.P., Bradbury J.H., Mandombi C. Konzo prevention in six villages in the DRC and the dependence of konzo prevalence on cyanide intake and malnutrition. Toxicol. Rep. 2015;2:609–616. doi: 10.1016/j.toxrep.2015.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shivanoor S.M., David M. Fourier transform infrared (FT-IR) study on cyanide induced biochemical and structural changes in rat sperm. Toxicol. Rep. 2015;2:1347–1356. doi: 10.1016/j.toxrep.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee S.K., Rhee J.S., Yum H.S. Cyanide poisoning deaths detected at the national forensic service headquarters in seoul of Korea: a six year survey (2005∼2010) Toxicol. Res. 2012;28:195–199. doi: 10.5487/TR.2012.28.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anseeuw K., Delvau N., Burillo-Putze G. Cyanide poisoning by fire smoke inhalation: a European expert consensus. European J. emergency med. Off. J. European Soc. Emergency Med. 2013;20:2–9. doi: 10.1097/MEJ.0b013e328357170b. [DOI] [PubMed] [Google Scholar]
- 7.Birngruber C.G., Veit F., Lang J., Verhoff M.A. Inhaled cyanide poisoning as a vital sign in a room fire victim. Forensic Sci. Int. 2017;281:e16. doi: 10.1016/j.forsciint.2017.10.037. e8. [DOI] [PubMed] [Google Scholar]
- 8.Thompson J.P., Marrs T.C. Hydroxocobalamin in cyanide poisoning. Clin. Toxicol. (Phila) 2012;50:875–885. doi: 10.3109/15563650.2012.742197. [DOI] [PubMed] [Google Scholar]
- 9.Bebarta V.S., Tanen D.A., Boudreau S. Intravenous cobinamide versus hydroxocobalamin for acute treatment of severe cyanide poisoning in a swine (Sus scrofa) model. Ann. Emerg. Med. 2014;64:612–619. doi: 10.1016/j.annemergmed.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leavesley H.B., Li L., Prabhakaran K., Borowitz J.L., Isom G.E. Interaction of cyanide and nitric oxide with cytochrome c oxidase: implications for acute cyanide toxicity. Toxicol. Sci. 2008;101:101–111. doi: 10.1093/toxsci/kfm254. [DOI] [PubMed] [Google Scholar]
- 11.Bebarta V.S. Antidotes for cyanide poisoning. European J. emergency med. Off. J. European Soc. Emergency Med. 2013;20:65–66. doi: 10.1097/MEJ.0b013e3283591730. [DOI] [PubMed] [Google Scholar]
- 12.Bebarta V.S., Brittain M., Chan A. Sodium nitrite and sodium thiosulfate are effective against acute cyanide poisoning when administered by intramuscular injection. Ann. Emerg. Med. 2017;69:718–725. doi: 10.1016/j.annemergmed.2016.09.034. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee J., Mahon S.B., Mukai D. The vitamin B12 analog cobinamide is an effective antidote for oral cyanide poisoning. J. Med. Toxicol. 2016;12:370–379. doi: 10.1007/s13181-016-0566-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehinger J.K., Piel S., Ford R. Cell-permeable succinate prodrugs bypass mitochondrial complex I deficiency. Nat. Commun. 2016;7:12317. doi: 10.1038/ncomms12317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piel S., Ehinger J.K., Elmer E., Hansson M.J. Metformin induces lactate production in peripheral blood mononuclear cells and platelets through specific mitochondrial complex I inhibition. Acta Physiol. (Oxf) 2015;213:171–180. doi: 10.1111/apha.12311. [DOI] [PubMed] [Google Scholar]
- 16.Chacko B.K., Zhi D., Darley-Usmar V.M., Mitchell T. The Bioenergetic Health Index is a sensitive measure of oxidative stress in human monocytes. Redox Biol. 2016;8:43–50. doi: 10.1016/j.redox.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer P.A., Chacko B.K., George D.J. Decreased Bioenergetic Health Index in monocytes isolated from the pericardial fluid and blood of post-operative cardiac surgery patients. Biosci. Rep. 2015:35. doi: 10.1042/BSR20150161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chacko B.K., Kramer P.A., Ravi S. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin. Sci. 2014;127:367–373. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jang D.H., Shofer F.S., Weiss S.L., Becker L.B. Impairment of mitochondrial respiration following ex vivo cyanide exposure in peripheral blood mononuclear cells. Clin. Toxicol. (Phila) 2016;54:303–307. doi: 10.3109/15563650.2016.1139712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jang D.H., Greenwood J.C., Spyres M.B., Eckmann D.M. Measurement of mitochondrial respiration and motility in acute care: sepsis, trauma, and poisoning. J. Intensive Care Med. 2017;32:86–94. doi: 10.1177/0885066616658449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang D.H., Khatri U.G.B.P.S. Alterations in mitochondrial respiration and reactive oxygen species in patients poisoned with carbon monoxide treated with hyperbaric oxygen. Intensive Care Med. Exp. 2018 doi: 10.1186/s40635-018-0169-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang D.H., Orloski C.J., Owiredu S., Shofer F.S., Greenwood J.C., Eckmann D.M. Alterations in mitochondrial function in blood cells obtained from patients with Sepsis Presenting to an emergency department. Shock. 2018 doi: 10.1097/SHK.0000000000001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang D.H., Kelly M., Hardy K., Lambert D.S., Shofer F.S., Eckmann D.M. A preliminary study in the alterations of mitochondrial respiration in patients with carbon monoxide poisoning measured in blood cells. Clin. Toxicol. (Phila) 2017;55:579–584. doi: 10.1080/15563650.2017.1288912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pesta D., Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol. Biol. 2012;810:25–58. doi: 10.1007/978-1-61779-382-0_3. [DOI] [PubMed] [Google Scholar]
- 25.Jang D.H., Greenwood J.C., Owiredu S., Ranganathan A., Eckmann D.M. Mitochondrial networking in human blood cells with application in acute care illnesses. Mitochondrion. 2017 doi: 10.1016/j.mito.2017.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Distelmaier F., Haack T.B., Wortmann S.B., Mayr J.A., Prokisch H. Treatable mitochondrial diseases: cofactor metabolism and beyond. Brain. 2017;140:e11. doi: 10.1093/brain/aww303. [DOI] [PubMed] [Google Scholar]
- 27.Bebarta V.S. Cyanide poisoning and antidotes. Emerg. Med. Australas. 2012;24(680) doi: 10.1111/1742-6723.12004. author reply 1-2. [DOI] [PubMed] [Google Scholar]
- 28.Owiredu S., Ranganathan A., Eckmann D.M. Ex vivo use of cell-permeable succinate prodrug attenuates mitochondrial dysfunction in blood cells obtained from carbon monoxide poisoned individuals. Am. J. Physiol., Cell Physiol. 2020 doi: 10.1152/ajpcell.00539.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turrens J.F. Superoxide production by the mitochondrial respiratory chain. Biosci. Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- 30.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Paradies G., Petrosillo G., Pistolese M., Di Venosa N., Federici A., Ruggiero F.M. Decrease in mitochondrial complex I activity in ischemic/reperfused rat heart: involvement of reactive oxygen species and cardiolipin. Circ. Res. 2004;94:53–59. doi: 10.1161/01.RES.0000109416.56608.64. [DOI] [PubMed] [Google Scholar]
- 32.Akyol S., Erdogan S., Idiz N. The role of reactive oxygen species and oxidative stress in carbon monoxide toxicity: an in-depth analysis. Redox Rep. 2014;19:180–189. doi: 10.1179/1351000214Y.0000000094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khailova L.S., Rokitskaya T.I., Kotova E.A., Antonenko Y.N. Effect of cyanide on mitochondrial membrane depolarization induced by uncouplers. Biochemistry (Mosc) 2017;82:1140–1146. doi: 10.1134/S0006297917100066. [DOI] [PubMed] [Google Scholar]
- 34.Sjovall F., Morota S., Hansson M.J., Friberg H., Gnaiger E., Elmer E. Temporal increase of platelet mitochondrial respiration is negatively associated with clinical outcome in patients with sepsis. Crit Care. 2010;14:R214. doi: 10.1186/cc9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.