Abstract
Objective
Previous studies have reported that propofol has antitumor, anti-inflammatory, and antioxidant effects in addition to its anesthetic properties. To confirm this, a retrospective investigation was conducted to determine whether different anesthetic agents, particularly propofol and inhalation anesthetics, have an effect on the recurrence of hepatocellular carcinoma (HCC) in patients who were diagnosed with primary HCC and underwent laparoscopic hepatectomy.
Subjects and Methods
Patients with Barcelona Clinic Liver Cancer stages 0, A, and B HCC, who underwent laparoscopic hepatic resection, were enrolled in this study. Postoperative HCC recurrence, which was determined from postoperative liver CT, was evaluated 24 months postoperatively with respect to the main anesthetic agents. The characteristics of HCC and other patient-related or surgery-related variables were evaluated together.
Results and Conclusion
During the 24-month period after hepatic resection, less HCC patients in the propofol group than in the inhalation group recurred (p = 0.046). The mean time to recurrence was 20.8 months (95% CI, 19.7–22.0) and 19.1 months (95% CI, 17.8–20.4) in the propofol group and the inhalation group, respectively. In addition, multivariable Cox proportional regression analysis revealed that the propofol group showed significantly decreased recurrence versus the inhalation group (hazard ratio, 0.57; 95% CI, 0.47–0.69; p = 0.029). When propofol was used as the main general anesthetic agent for laparoscopic hepatic resection, the postoperative 2-year recurrence rate decreased in early- and intermediate-stage HCC.
Keywords: Anesthesia, Hepatic resection, Hepatocellular carcinoma, Inhalation anesthetic, Propofol, Tumor progression
Significance of the Study
Propofol and inhalation anesthetics can differently influence postoperative recurrence of hepatocellular carcinoma (HCC) in a clinical setting.
Postoperative 2-year HCC recurrence was decreased when propofol was used as the main anesthetic.
Propofol should be considered as optimal anesthetic for patients with early-stage HCC undergoing hepatic resection.
Introduction
Hepatocellular carcinoma (HCC) is a solid type of cancer that has high recurrence and mortality rates [1]. Although locoregional treatments such as radiofrequency tumor ablation (RFA) and transarterial chemoembolization (TACE) can be applied, surgical resection or liver transplantation is the ultimate curative option for patients with early-stage HCC [1, 2]. Nevertheless, postoperative HCC recurrence has previously been determined to be about 50% in 2 years [3, 4], and several characteristics of HCC, such as tumor differentiation, tumor size and number, and vascular invasion, are risk factors for recurrence [3, 5]. In addition, nuclear factor (NF)-κB, natural killer (NK) cells, and several interleukins (IL) have been reported to be linked with the progression or recurrence of HCC [6, 7].
Propofol and inhalation anesthetics, which are widely used anesthetics, function quite differently. Inhalation anesthetics decrease the activity of NK cells, while propofol does not suppress NK cell activity [8]. Moreover, propofol reduces the level of NF-κB, tumor necrosis factor(TNF)-α, IL-1β, and IL-6, thus providing antioxidant and anti-inflammatory effects [9, 10]. Additionally, propofol has been shown to inhibit the migration and invasion of human cancer cells [11, 12]. Previous studies reported better outcome in HCC patients when propofol was used for sedation or general anesthesia [13, 14].
In this study, patients with early- or intermediate-stage HCC were enrolled, and a retrospective investigation was performed to evaluate the difference in the recurrence rate after hepatectomy for HCC according to the main anesthetic agents used, propofol or inhalation anesthetic.
Subjects and Methods
Study Population and Data Collection
This study was approved by the local Institutional Review Board of the Seoul National University Bundang Hospital. The requirement for written informed consents was waived. The cohorts included comprised patients who were diagnosed with primary HCC and underwent elective laparoscopic hepatic resection between April 2003 and June 2013. The Barcelona Clinic Liver Cancer (BCLC) staging classification was limited to 0, A, and B.
General Anesthesia
General anesthesia was usually performed in the following manner. Midazolam was given to the patients commonly at the preoperative holding area. General anesthesia was induced under routine monitoring, which included electrocardiogram, invasive arterial pressure measurement, and pulse oximetry. Three anesthesiologists had chosen an inhalation anesthetic, and the other 2 anesthesiologists had performed total intravenous anesthesia during anesthesia maintenance for laparoscopic hepatic resection. When the inhalation anesthetic was chosen as the main anesthetic, propofol, rocuronium, and remifentanil were given only for initial sedation, neuromuscular block, and pain control, respectively. When total intravenous anesthesia was planned, propofol and remifentanil were administered via the target-controlled infusion method, and rocuronium was given intermittently.
Assessment of Postoperative HCC Recurrence
Postoperative HCC recurrence was evaluated for 24 months postoperatively. Postoperative serial liver computed tomography (CT) was evaluated to judge the recurrence of HCC at intervals of 3–6 months. All liver CT scans were finally evaluated by certified radiologists. When HCC recurrence was visualized on liver CT, the time to recurrence after hepatic resection was recorded together.
Other Outcome Variables
Various factors that could influence the recurrence of HCC were evaluated as follows: (1) Child-Pugh score and BCLC staging classification; (2) preoperative laboratory results; (3) preoperative RFA or TACE; (4) surgical procedure, operator, and operation time; (5) pathologic findings of HCC; (6) main anesthetic agent and anesthesia time; and (7) perioperative transfusion.
Statistical Analysis
Continuous or categorical variables were expressed as the mean ± SD or number (proportion). Normality test was performed for continuous data using a Shapiro-Wilk test. Student's t test or χ2 test was used where appropriate. The progression-free survival (PFS) was calculated and analyzed with the Kaplan-Meier survival analysis with log-rank test. Univariate Cox proportional regression analysis was performed to determine individual relationships among all covariates. Then the covariates with p < 0.2 were selected to form the univariate Cox proportional regression model and analyzed via multivariate Cox proportional regression model. The results of the Cox proportional regression analyses were presented as hazard ratios with 95% confidence interval (CI). IBM® SPSS® statistics version 22.0 (IBM Corporation, NY, USA) was used for all analyses and p ≤ 0.05 was considered statistically significant.
Results
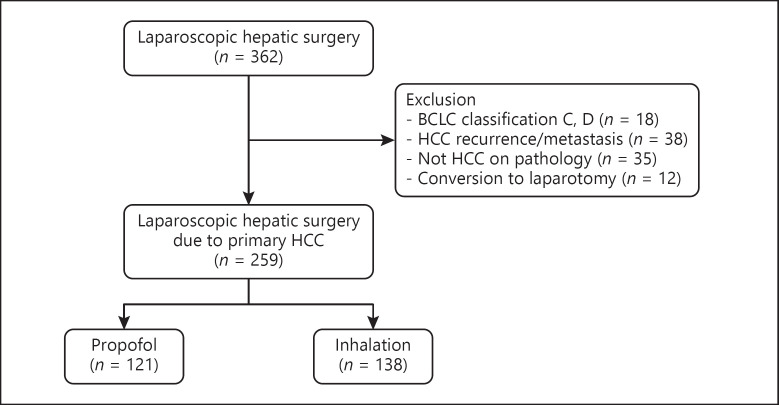
The following cases were excluded: patients who had HCC recurrence or metastasis to other organs when undergoing the operation, diagnosis other than HCC on the postoperative pathology, and intraoperative conversion from laparoscopic procedure to laparotomy. Finally, 259 patients were enrolled in this study for the analysis; 121 patients received propofol (propofol group) and 138 received inhalation anesthetic (inhalation group) (Fig. 1). The characteristics of the patients, surgery, and HCC are shown in Table 1.
Fig. 1.
Flow diagram of patient enrollment. BCLC, Barcelona Clinic Liver Cancer; HCC, hepatocellular carcinoma.
Table 1.
Characteristics of the patients, surgery, and hepatocellular carcinoma
| Propofol (n = 121) | Inhalation anesthetic (n = 138) | p value | |
|---|---|---|---|
| Patient characteristics | |||
| Age, years | 61.4±10.3 | 64.5±11.4 | 0.155 |
| Gender, males/females | 94/27 (77.7/22.3) | 106/32 (76.8/23.2) | 0.985 |
| Height, cm | 166.4±7.9 | 163.4±7.8 | 0.048 |
| Weight, kg | 66.5±9.6 | 63.3±10.2 | 0.108 |
| Child-Pugh A/B | 111/10 (91.7/8.3) | 130/8 (94.2/5.8) | 0.593 |
| BCLC 0/A/B | 29/69/23 (24.0/57.0/19.0) | 30/88/20 (21.7/63.8/14.5) | 0.737 |
| Preoperative laboratory findings | |||
| HBsAg+ | 81 (66.9) | 99 (71.7) | 0.483 |
| Anti-HCV+ | 13 (10.7) | 10 (7.2) | 0.442 |
| α-Fetoprotein, ng mL−1 | 201.6±599.7 | 210.7±615.1 | 0.939 |
| PIVKA-II, mAU mL−1 | 33.0±34.8 | 50.2±50.9 | 0.246 |
| ICG test | 9.3±8.1 | 11.1±10.0 | 0.394 |
| Hb, g dL−1 | 14.2±1.5 | 13.9±1.7 | 0.264 |
| Platelets, ×103 μL−1 | 152±47 | 144±59 | 0.468 |
| PT INR | 1.06±0.09 | 1.10±0.12 | 0.060 |
| aPTT, s | 36.8±3.9 | 37.3±3.5 | 0.489 |
| AST, IU L−1 | 32.9±19.5 | 39.5±25.9 | 0.166 |
| ALT, IU L−1 | 34.1±27.2 | 41.7±49.5 | 0.381 |
| ALP, IU L−1 | 81.4±24.8 | 84.8±34.1 | 0.590 |
| γ-GT, IU L−1 | 63.9±85.5 | 61.1±54.1 | 0.830 |
| Total bilirubin, mg dL−1 | 0.9±0.3 | 0.8±0.5 | 0.852 |
| Albumin, g dL−1 | 4.2±0.4 | 4.1±0.4 | 0.109 |
| Creatinine, mg dL−1 | 0.9±0.3 | 1.0±0.9 | 0.447 |
| Glucose, mg dL−1 | 109.0±29.3 | 114.1±48.4 | 0.551 |
| Surgical findings | |||
| Operation time, min | 315.3±167.2 | 275.4±151.9 | 0.190 |
| Operator A/B/C/D | 92/23/6/0 | 105/16/14/3 | 0.085 |
| (76.0/19.0/5.0/0.0) | (76.1/11.6/10.1/2.2) | ||
| Surgical procedure | 0.496 | ||
| Tumorectomy/wedge resection | 37 (30.6) | 39 (28.3) | |
| Subsegmentectomy | 7 (5.8) | 8 (5.8) | |
| Segmentectomy | 20 (16.5) | 31 (22.5) | |
| Sectionectomy | 47 (38.8) | 43 (31.2) | |
| Hemihepatectomy | 10 (8.3) | 17 (12.3) | |
| Perioperative transfusion | 23 (19.0) | 30 (21.7) | 0.697 |
| Pathologic findings and tumor staging | |||
| Solitary/multifocal HCC | 111/10 (91.7/8.3) | 130/8 (94.2/5.8) | 0.593 |
| HCC size (≥5 cm) | 7 (5.8) | 12 (8.7) | 0.511 |
| Grade of differentiation I/II/III | 3/30/88 (2.5/24.8/72.7) | 3/46/78 (2.4/36.2/61.4) | 0.147 |
| Capsular infiltration | 37 (30.6) | 40 (31.5) | 0.985 |
| BD invasion | 3 (2.5) | 0 (0.0) | 0.201 |
| Microvascular invasion | 34 (28.1) | 43 (33.9) | 0.400 |
| HCC TNM stage 1/2/3A | 81/33/7 (66.9/27.3/5.8) | 86/40/12 (62.3/29.0/8.7) | 0.519 |
| Cirrhosis of nontumorous liver | 61 (50.4) | 70 (51.7) | 0.941 |
| Minimal resection margin, mm | 12.3±9.6 | 16.3±15.2 | 0.075 |
Data are presented as means ± SD or numbers (%). BCLC, Barcelona Clinic Liver Cancer; HBsAg, surface antigen of hepatitis B virus; anti-HCV, anti-hepatitis C virus antibody; PIVKA-II, protein induced by vitamin K absence/antagonist II; ICG, indocyanine green; PT INR, international normalized ratio of prothrombin time; aPTT, activated partial thromboplastin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; γ-GT, γ-glutamyl transpeptidase; HCC, hepatocellular carcinoma; BD, bile duct; TNM stage, tumor-node-metastasis stage. Data are missing for the following variables: γ-GT (9 cases), PIVKA-II (80 cases), α-fetoprotein (4 cases), ICG test (30 cases), grade of differentiation, capsular infiltration, and microvascular invasion (11 cases, respectively).
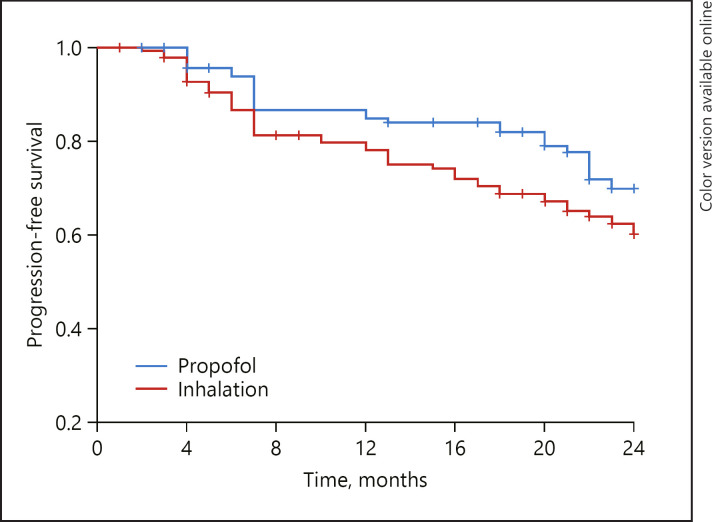
The Kaplan-Meier curve of 24-month PFS according to the main anesthetic is shown in Figure 2. HCC recurred less in the propofol group than in the inhalation group (p = 0.046), and the mean time to recurrence was 20.8 months (95% CI, 19.7–22.0) and 19.1 months (95% CI, 17.8–20.4) in the propofol and the inhalation group, respectively.
Fig. 2.
Recurrence-free survival after hepatic resection for HCC. +, censored data in each group.
The overall 24-month PFS rates after surgery were 77.7 and 65.2% in the propofol and the inhalation group, respectively (p = 0.038). The HCC recurrence according to the main anesthetic agent and other variables was compared separately in a univariate and subsequently in a multivariate Cox proportional regression model. The propofol group showed significantly lower recurrence rates than the inhalation group (hazard ratio, 0.66; 95% CI, 0.42–1.05; p = 0.016). The multivariate Cox proportional regression model also presented results similar to those exhibited by the propofol group, which significantly decreased recurrence rates (hazard ratio, 0.57; 95% CI, 0.47–0.69; p = 0.029) compared with the inhalation group (Table 2). In addition, other variables that significantly increased the 24-month recurrence rate were microvascular invasion and higher TNM stage in the multivariate Cox proportional regression model.
Table 2.
Cox proportional regression analysis for progression-free survival
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |
| Anesthesia, inhalation (ref.: inhalation) | 0.66 (0.42–1.05) | 0.016 | 0.57 (0.47–0.69) | 0.029 |
| Age | 1.01 (0.98–1.03) | 0.486 | ||
| Female (ref.: male) | 0.46 (0.46–0.20) | 0.070 | 0.21 (0.03–1.60) | 0.133 |
| BCLC stage (ref.: 0) | ||||
| A | 1.19 (0.59–2.40) | 0.633 | ||
| B | 1.25 (0.51–3.09) | 0.622 | ||
| HbsAg (ref.: negative) | 1.03 (0.56–1.89) | 0.938 | ||
| Anti-HCV (ref.: negative) | 1.19 (0.43–3.29) | 0.742 | ||
| α-Fetoprotein | 1.26 (0.38–4.17) | 0.004 | 1.00 (0.99–1.01) | 0.129 |
| PIVKA-II | 1.58 (0.46–5.44) | 0.002 | 1.05 (0.40–2.72) | 0.330 |
| ICG test | 1.00 (1.00–1.01) | 0.323 | ||
| Operation time | 1.00 (1.00–1.00) | 0.291 | ||
| Transfusion (ref.: no) | 1.22 (0.66–2.23) | 0.524 | ||
| Tumor number (ref.: single) | 1.75 (0.75–4.08) | 0.197 | 1.76 (0.30–3.12) | 0.945 |
| HCC size | 1.99 (1.00–3.95) | 0.049 | 1.96 (0.57–5.39) | 0.323 |
| Grade of differentiation (ref.: I) | ||||
| II | 0.65 (0.09–4.77) | 0.670 | ||
| III | 0.70 (0.08–6.25) | 0.748 | ||
| Capsular infiltration (ref.: no) | 0.82 (0.45–1.49) | 0.507 | ||
| BD invasion | 2.38 (0.33–17.26) | 0.391 | ||
| Microvascular invasion (ref.: no) | 1.21 (1.06–1.74) | 0.028 | 1.53 (0.89–2.64) | 0.015 |
| TNM stage (ref.: 1) | ||||
| 2 | 1.85 (1.02–3.38) | 0.044 | 1.58 (0.46–5.44) | 0.001 |
| 3A | 6.44 (2.98–13.89) | <0.001 | 7.34 (1.99–27.13) | 0.006 |
| Cirrhosis of nontumorous liver | 1.24 (0.72–2.13) | 0.434 | ||
| Minimal resection margin | 1.76 (0.57–5.39) | 0.323 | ||
Discussion
This retrospective study showed that HCC patients with BCLC stage 0, A, or B had less postoperative recurrence when propofol was used as the main anesthetic agent compared to the use of inhalation anesthetic.
HCC is an inflammation-related cancer, and several risk factors for recurrence after surgical resection have been established: larger tumor size, multinodularity, worse differentiation, microvascular invasion, liver disease stage, age, and sex [3, 5]. In addition to these well-known pathological risk factors, it has recently been revealed that several molecular pathways with mediators such as IL-6, NF-κB, NK cells, TNF-α, and signal transducer and activator of transcription 3 are related to hepatocyte regeneration and development of HCC [6, 7].
Previous studies have reported results similar to those of the present study. When propofol was used for sedation or general anesthesia, better survival was observed in HCC patients [13, 14]. In the present study, only patients with primary HCC of BCLC stage 0, A, or B were enrolled, and the surgical procedure was limited to laparoscopic hepatic resection in order to recruit early- or intermediate-stage HCC, limit the basal characteristics to specific conditions, and reduce confounding factors as much as possible. As such, the characteristics and risk factors of HCC as described above seem to be comparable between both groups. Accordingly, other important risk factors for HCC recurrence such as macrovascular invasion to the hepatic artery, hepatic vein, or portal vein [3, 5] were not observed in any patient. Moreover, all laparoscopic hepatic resections resulted in R0 resection, and no patient underwent postoperative adjuvant therapy in this study population. Our results may provide further reinforcing evidence regarding the nonanesthetic advantage of propofol in early- or intermediate-stage HCC patients.
Previous studies have reported that propofol has antitumor, anti-inflammatory, and antioxidant effects in addition to its anesthetic properties [15]. On the contrary, inhalation anesthetics were reported to have immunosuppressive effects, although this remains a controversial issue [16].
Propofol has been reported to decrease the viability, proliferation, and invasion of HCC cells in vitro by controlling some microribonucleic acids [17, 18]. The number and proportion of NK cells have been shown to decrease and its function impaired in HCC patients [19]; NK cell suppression increases the susceptibility of tumor progression [20]. While inhalation anesthetics decreased NK cell function, propofol did not inhibit NK cell activity, contributing to upregulation of interferon-γ and reduce tumor cell retention or metastasis [8, 21]. NF-κB is involved intricately in the progression of HCC as well as other kinds of cancers [22]. NF-κB activation is observed in HBV- or HCV-infected livers and advanced liver diseases [23]. Promotion of HCC development was also observed by NF-κB activation in Mdr2-knockout mice [24]. Propofol is known to downregulate NF-κB [9, 10], but inhalation anesthetics have been reported to increase the nuclear level and transcription activity of NF-κB [25]. The TNF inflammatory pathway is also involved in liver carcinogenesis, judging from the reduced tumorigenesis in TNF receptor type 1-knockout mice [26]. IL-6-knockout mice presented less hepatocarcinogenesis [27], which corresponded with the elevated IL-6 levels in HCC patients [28]. Therapeutic propofol levels have been shown to reduce the biosynthesis of several proinflammatory cytokines, such as TNF-α, IL-6, and IL-1β in activated macrophages [10]. However, each inhalation anesthetic agent seems to have varied complex effects on pro- or anti-inflammatory cytokines [16]. Besides, hypoxia-inducible factorα (HIF)-1α has been known to be correlated with tumor development and expressed in various kinds of human tumor cells [29], and propofol, unlike the inhalation anesthetic, inhibited HIF-1α activation [30]. These antitumor, anti-inflammatory, and antioxidant effects of propofol on the HCC-related molecular pathway and several mediators may explain the low postoperative recurrence rate of HCC in this study.
A major limitation of this study is that the duration of observation for HCC recurrence was limited to 2 years postoperatively; hence, we were unable to verify the long-term prognosis. However, it has previously been established that early recurrence of HCC has mostly occurred within 1–2 years of resection [4]. Another study with a larger group of patients, including cases with higher BCLC stages, showed similar results at long-term follow-up [14]. Second, the recurrence of HCC was evaluated only by CT in the present study. However, other examinations, such as positron emission tomography-CT or magnetic resonance imaging, could be used to detect postoperative HCC recurrence [5].
Conclusions
Postoperative 2-year recurrence of early- and intermediate-stage HCC decreased when propofol was used as the main anesthetic during general anesthesia for laparoscopic hepatic resection. Propofol needs to be reconsidered as an optimal anesthetic for early- and intermediate-stage HCC patients undergoing hepatic resection surgery.
References
- 1.Dhanasekaran R, Limaye A, Cabrera R. Hepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis, and therapeutics. Hepat Med. 2012 May;4:19–37. doi: 10.2147/HMER.S16316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhardwaj N, Perera MT, Silva MA. Current Treatment Approaches to HCC with a Special Consideration to Transplantation. J Transplant. 2016;2016:7926264. doi: 10.1155/2016/7926264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shah SA, Cleary SP, Wei AC, Yang I, Taylor BR, Hemming AW, et al. Recurrence after liver resection for hepatocellular carcinoma: risk factors, treatment, and outcomes. Surgery. 2007 Mar;141((3)):330–9. doi: 10.1016/j.surg.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 4.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, et al. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006 Feb;243((2)):229–35. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colecchia A, Schiumerini R, Cucchetti A, Cescon M, Taddia M, Marasco G, et al. Prognostic factors for hepatocellular carcinoma recurrence. World J Gastroenterol. 2014 May;20((20)):5935–50. doi: 10.3748/wjg.v20.i20.5935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berasain C, Castillo J, Perugorria MJ, Latasa MU, Prieto J, Avila MA. Inflammation and liver cancer: new molecular links. Ann N Y Acad Sci. 2009 Feb;1155((1)):206–21. doi: 10.1111/j.1749-6632.2009.03704.x. [DOI] [PubMed] [Google Scholar]
- 7.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006 Feb;43((2 Suppl 1)):S45–53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 8.Welden B, Gates G, Mallari R, Garrett N. Effects of anesthetics and analgesics on natural killer cell activity. AANA J. 2009 Aug;77((4)):287–92. [PubMed] [Google Scholar]
- 9.Sánchez-Conde P, Rodríguez-López JM, Nicolás JL, Lozano FS, García-Criado FJ, Cascajo C, et al. The comparative abilities of propofol and sevoflurane to modulate inflammation and oxidative stress in the kidney after aortic cross-clamping [table of contents.] Anesth Analg. 2008 Feb;106((2)):371–8. doi: 10.1213/ane.0b013e318160580b. [DOI] [PubMed] [Google Scholar]
- 10.Chen RM, Chen TG, Chen TL, Lin LL, Chang CC, Chang HC, et al. Anti-inflammatory and antioxidative effects of propofol on lipopolysaccharide-activated macrophages. Ann N Y Acad Sci. 2005 May;1042((1)):262–71. doi: 10.1196/annals.1338.030. [DOI] [PubMed] [Google Scholar]
- 11.Xu YB, Du QH, Zhang MY, Yun P, He CY. Propofol suppresses proliferation, invasion and angiogenesis by down-regulating ERK-VEGF/MMP-9 signaling in Eca-109 esophageal squamous cell carcinoma cells. Eur Rev Med Pharmacol Sci. 2013 Sep;17((18)):2486–94. [PubMed] [Google Scholar]
- 12.Wu KC, Yang ST, Hsia TC, Yang JS, Chiou SM, Lu CC, et al. Suppression of cell invasion and migration by propofol are involved in down-regulating matrix metalloproteinase-2 and p38 MAPK signaling in A549 human lung adenocarcinoma epithelial cells. Anticancer Res. 2012 Nov;32((11)):4833–42. [PubMed] [Google Scholar]
- 13.Puijk RS, Ziedses des Plantes V, Nieuwenhuizen S, Ruarus AH, Vroomen LG, de Jong MC, et al. Propofol Compared to Midazolam Sedation and to General Anesthesia for Percutaneous Microwave Ablation in Patients with Hepatic Malignancies: A Single-Center Comparative Analysis of Three Historical Cohorts. Cardiovasc Intervent Radiol. 2019 Nov;42((11)):1597–608. doi: 10.1007/s00270-019-02273-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai HC, Lee MS, Lin C, Lin KT, Huang YH, Wong CS, et al. Propofol-based total intravenous anaesthesia is associated with better survival than desflurane anaesthesia in hepatectomy for hepatocellular carcinoma: a retrospective cohort study. Br J Anaesth. 2019 Aug;123((2)):151–60. doi: 10.1016/j.bja.2019.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasileiou I, Xanthos T, Koudouna E, Perrea D, Klonaris C, Katsargyris A, et al. Propofol: a review of its non-anaesthetic effects. Eur J Pharmacol. 2009 Mar;605((1-3)):1–8. doi: 10.1016/j.ejphar.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 16.Stollings LM, Jia LJ, Tang P, Dou H, Lu B, Xu Y. Immune Modulation by Volatile Anesthetics. Anesthesiology. 2016 Aug;125((2)):399–411. doi: 10.1097/ALN.0000000000001195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang J, Zhang D, Wu GQ, Feng ZY, Zhu SM. Propofol inhibits the adhesion of hepatocellular carcinoma cells by upregulating microRNA-199a and downregulating MMP-9 expression. Hepatobiliary Pancreat Dis Int. 2013 Jun;12((3)):305–9. doi: 10.1016/s1499-3872(13)60048-x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Shan WF, Jin TT, Wu GQ, Xiong XX, Jin HY, et al. Propofol exerts anti-hepatocellular carcinoma by microvesicle-mediated transfer of miR-142-3p from macrophage to cancer cells. J Transl Med. 2014 Oct;12((1)):279. doi: 10.1186/s12967-014-0279-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai L, Zhang Z, Zhou L, Wang H, Fu J, Zhang S, et al. Functional impairment in circulating and intrahepatic NK cells and relative mechanism in hepatocellular carcinoma patients. Clin Immunol. 2008 Dec;129((3)):428–37. doi: 10.1016/j.clim.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 20.Berrien-Elliott MM, Romee R, Fehniger TA. Improving natural killer cell cancer immunotherapy. Curr Opin Organ Transplant. 2015 Dec;20((6)):671–80. doi: 10.1097/MOT.0000000000000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inada T, Kubo K, Shingu K. Promotion of interferon-gamma production by natural killer cells via suppression of murine peritoneal macrophage prostaglandin E₂ production using intravenous anesthetic propofol. Int Immunopharmacol. 2010 Oct;10((10)):1200–8. doi: 10.1016/j.intimp.2010.06.027. [DOI] [PubMed] [Google Scholar]
- 22.Vlahopoulos SA, Cen O, Hengen N, Agan J, Moschovi M, Critselis E, et al. Dynamic aberrant NF-κB spurs tumorigenesis: a new model encompassing the microenvironment. Cytokine Growth Factor Rev. 2015 Aug;26((4)):389–403. doi: 10.1016/j.cytogfr.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luedde T, Schwabe RF. NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2011 Feb;8((2)):108–18. doi: 10.1038/nrgastro.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004 Sep;431((7007)):461–6. doi: 10.1038/nature02924. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Zhang J, Yang L, Dong Y, Zhang Y, Xie Z. Isoflurane and sevoflurane increase interleukin-6 levels through the nuclear factor-kappa B pathway in neuroglioma cells. Br J Anaesth. 2013 Jun;110(Suppl 1):i82–91. doi: 10.1093/bja/aet115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knight B, Yeoh GC, Husk KL, Ly T, Abraham LJ, Yu C, et al. Impaired preneoplastic changes and liver tumor formation in tumor necrosis factor receptor type 1 knockout mice. J Exp Med. 2000 Dec;192((12)):1809–18. doi: 10.1084/jem.192.12.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007 Jul;317((5834)):121–4. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 28.Soresi M, Giannitrapani L, D'Antona F, Florena AM, La Spada E, Terranova A, et al. Interleukin-6 and its soluble receptor in patients with liver cirrhosis and hepatocellular carcinoma. World J Gastroenterol. 2006 Apr;12((16)):2563–8. doi: 10.3748/wjg.v12.i16.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, et al. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000 Aug;157((2)):411–21. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang H, Benzonana LL, Zhao H, Watts HR, Perry NJ, Bevan C, et al. Prostate cancer cell malignancy via modulation of HIF-1α pathway with isoflurane and propofol alone and in combination. Br J Cancer. 2014 Sep;111((7)):1338–49. doi: 10.1038/bjc.2014.426. [DOI] [PMC free article] [PubMed] [Google Scholar]