Abstract
We report the draft metagenome-assembled genome of a member of the Chloroflexi family Herpetosiphonaceae from microbial biofilms developed in a circumneutral, iron-rich hot spring in Japan. This taxon represents a novel genus and species—here proposed as Candidatus Anthektikosiphon siderophilum—that expands the known taxonomic and genetic diversity of the Herpetosiphonaceae and helps orient the evolutionary history of key traits like photosynthesis and aerobic respiration in the Chloroflexi.
Keywords: chloroflexota, Herpetosiphon, predatory bacteria, aerobic respiration, metagenomics
The Chloroflexi family Herpetosiphonaceae is made up of aerobic, nonphototrophic filamentous bacteria, and is the sister group to the clade of well known, photosynthetic Chloroflexia that includes the genera Roseiflexus and Chloroflexus (Kiss et al., 2011; Ward et al., 2015; Ward et al., 2018a). The Herpetosiphonaceae family is best known for the predatory bacteria of the genus Herpetosiphon (e.g. Castenholz, 2012), but the ecological and physiological diversity of this family is not yet well constrained. Deeper sampling of the metabolic and genetic diversity of the Herpetosiphonaceae is critical for understanding the evolution of aerobic respiration, phototrophy, and carbon fixation (variably via the Calvin cycle and 3-hydroxyproprionate bicycle) within the Chloroflexi—by orienting the relative order and timing of acquisition of these traits within this group (e.g. Shih et al., 2017; Ward et al., 2018a). We discovered a draft genome of a novel genus in the Herpetosiphonaceae recovered from genome-resolved metagenomic sequencing of samples of mineralized biofilms from Okuoku-hachikurou Onsen—a carbonate-forming, iron-rich hot spring in Akita Prefecture, Japan, that shares some mineralogical features with iron formation deposits observed in the early parts of the sedimentary record (Takashima et al., 2011; Ward et al., 2017a). This taxon provided valuable insight into the aerobic history of the Chloroflexia and expanded the known diversity of Herpetosiphonaceae.
OHK22 was recovered from genome-resolved metagenomic sequencing of samples from Okuoku-hachikurou Onsen (OHK) in Akita Prefecture, Japan. The geochemistry and microbial diversity and ecology of OHK has been characterized previously (Takashima et al., 2011; Ward et al., 2017a). In brief, OHK is an iron-carbonate hot spring in Akita Prefecture, Japan, with source waters supersaturated in CO2, no detectable dissolved oxygen, pH 6.8, ~45°C, and containing 114 μM dissolved Fe2+ (Ward et al., 2017a). As the spring water flows downstream, the water cools, exchanges gases with the atmosphere, and the ferrous iron is oxidized and deposited forming mixed aragonite-iron oxide laaminated rocks. Samples for metagenomics were collected in September 2016 from the “Shallow Source” and “Canal” sites described in Ward et al., 2017a (Fig. 1). Thin biofilms (<1 mm-thick) were scraped from mineral precipitates using sterile forceps and spatulas (~0.25 cm3 of material). Cells were lysed and DNA preserved in the field using a Zymo Terralyzer BashingBead Matrix and Xpedition Lysis Buffer (Zymo Research). Cells were disrupted immediately by attaching the polyethylene sample tubes to the blade of a cordless reciprocating saw (Makita JR101DZ), and operating for 1 min. Following return to the lab, microbial DNA was extracted and purified with a Zymo Soil/Fecal DNA extraction kit (Zymo Research). DNA was quantified with a Qubit 3.0 fluorimeter (Life Technologies) according to manufacturer’s instructions following DNA extraction. Purified DNA was submitted to SeqMatic LLC (Fremont) for library preparation sequencing. Library preparation was performed using an Illumina Nextera XT DNA Library Preparation Kit per the manufacturer’s protocols, producing constructs with an average size of ~519 bp prior to 2×100 bp paired-end sequencing via Illumina HiSeq 4000 technology. Raw sequence reads were preprocessed and quality controlled (adapter trimming, contaminant filtering, and merging of paired reads) with BBTools (Bushnell, 2014) prior to coassembling the two samples with MegaHit v. 1.02 (Li et al., 2016) and genome bins constructed based on differential coverage using MetaBAT (Kang et al., 2015), MaxBin (Wu et al., 2014), and CONCOCT (Alneberg et al. 2013. CONCOCT: clustering contigs on coverage and composition. arXiv preprint arXiv:1312.4038.) followed by replication and refinement by DAS Tool (Sieber et al., 2018). Completeness and contamination/redundancy were determined with CheckM v1.1.2 using the bacterial marker set UID1452 (Parks et al., 2015). The genome was uploaded to RAST v2.0 for annotation and characterization (Aziz et al., 2008). Presence or absence of metabolic pathways of interest was predicted using MetaPOAP v1.0 (Ward et al., 2018b). Taxonomic assignment was determined with GTDB-Tk v0.3.2 (Parks et al., 2018). Genomes were compared with AAI (Rodriguez-R and Konstantinidis, 2014) to verify species and genus-level divisions. Organismal phylogenies were built using concatenated ribosomal proteins following methods from Hug et al. (2016). Protein sequences were extracted from genomes using the tblastn function of BLAST+ (Camacho et al., 2009) and aligned using MUSCLE (Edgar, 2004). Trees were calculated using RAxML v8.2.12 (Stamatakis, 2014) on the Cipres science gateway (Miller et al., 2010). Transfer bootstrap support values were calculated by BOOSTER (Lemoine et al., 2018), and trees were visualized with the Interactive Tree of Life viewer (Letunic and Bork, 2016). Presence of genes associated with iron cycling was determined with FeGenie (Garber et al., 2020). Comparison of genome contents was made based on annotations in RAST (Aziz et al., 2008) together with analyses by OrthoVenn2 (Xu et al., 2019) and KEGG-decoder (Graham et al., 2018). All software was run with default parameters; further testing and optimization of parameters for each computational analysis may allow future improvement of assembly and MAG quality.
Fig. 1.
Oku-okuhachikurou Onsen (OHK), Japan, and the source of Ca. Anthektikosiphon siderophilum. A: Overview map of Japan showing the location of OHK in Akita Prefecture. Modified from Ward et al. (2017a). B: Panorama of OHK pools and channels at the time of sampling. Source pool occurs at the left, with flow downstream to the right. Scale bar ~1 m. Samples were collected from the “Shallow Source” (C, scale bar ~ 30 cm) and “Canal” (D, scale bar ~10 cm) sites as scrapings of thin green-colored biofilms overlying iron-oxide and aragonite travertine along the water line.
Metagenomic sequencing was performed on biofilm and mineralized samples from the “Shallow Source” and “Canal” sites at OHK Onsen (Fig. 1) (Ward et al., 2017a). Both samples consisted of layered iron oxide and calcium carbonate travertine with very thin (<–1 mm) biofilms submerged in flowing hot spring water ~44°C with relatively low dissolved O2 (<62 μM) and high dissolved ferrous iron (>83 μM). Previous work has suggested that the microbial community in this environment is capable of only limited rates of primary productivity and organic carbon accumulation and is fueled primarily by iron oxidation and aerobic respiration with only small contributions from photosynthesis (Ward et al., 2017a).
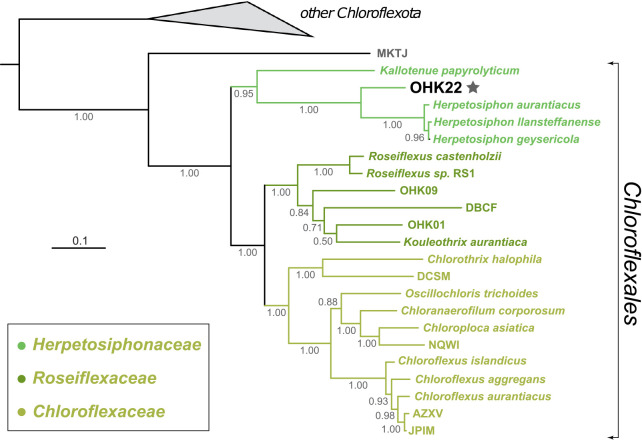
Metagenomic sequencing of these two samples from OHK Onsen resulted in 52 GB of raw sequence data which produced a 579 Mb coassembly with an N50 of 27,219 nt. Binning of this metagenome assembly to produce metagenome-assembled genomes (MAGs) produced a number of high quality genomes following current standards (Bowers et al., 2017) including OHK22 described here and OHK40 which has been described elsewhere (Ward et al. 2019. Genomic evidence for phototrophic oxidation of small alkanes in a member of the chloroflexi phylum. bioRxiv 531582.). Reads mapped to the OHK22 MAG were recovered with an average of 5-fold coverage from the “Canal” metagenome and 48-fold coverage from the “Shallow Source” metagenome. The OHK22 genome is 6,335,032 nt and 62.48% GC, and was recovered as 285 contigs with an N50 of 33,814 and a minimum contig length of 2,609 nt. The genome was estimated to be 96.97% complete with 0.91% contamination/redundancy and 0% strain heterogeneity. The genome contained 45 tRNAs and 5,771 coding sequences. We determined that OHK22 belonged to the Chloroflexi family Herpetosiphonaceae via analysis with concatenated ribosomal protein phylogenies and with GTDB-Tk, but results indicated that this taxon could not be assigned to any previously described genus in this family (Fig. 2).
Fig. 2.
Phylogeny of the Chloroflexia built with concatenated ribosomal protein sequences and labeled with family- and order-level taxonomy as determined by GTDB-tk. OHK22 (marked with star) branches between Herpetosiphon and Kallotenue within the Herpetosiphonaceae family. For metagenome-assembled genomes without species or strain names, leaves are labeled with NCBI WGS database IDs.
A 16S rRNA sequence was not recovered in the OHK22 MAG; however, our initial 16S amplicon sequencing survey from the same sites (Ward et al., 2017a) revealed the presence of three closely related Herpetosiphonaceae OTUs at OHK with total relative abundance at the “Canal” and “Shallow Source” sites of ~0.3%. Estimates from the later acquired samples which are the focus of this study are consistent with this: the relative abundance of OHK22 in the microbial community based on the proportion of raw metagenomic reads and the coassembly that map onto the OHK22 genome suggest that this organism makes up no more than ~1% of the sequenced microbial community.
The previously characterized genomic diversity of the Herpetosiphonaceae consisted of Kallotenue papyrolyticum (Hedlund et al., 2015) and three species within the genus Herpetosiphon (H. aurantiacus, Kiss et al., 2011; H. geysericola, Ward et al., 2015; and H. llansteffanense, Livingstone et al., 2018). Other Herpetosiphon strains have been isolated including members of the species H. gulosus and H. giganteus (Pan et al., 2017), but to our knowledge genome sequences are not yet available for these organisms. Phylogenetic analyses (Fig. 2) and classification via GTDB-Tk placed OHK22 on a branch between Herpetosiphon and Kallotenue. AAI analyses (Rodriguez-R and Konstantinidis, 2014) displayed <60% similarity between OHK22 and other Herpetosiphonaceae—a level consistent with genus-level divergence. A 16S rRNA sequence was not recovered in the OHK22 MAG; however, 16S amplicon sequencing of the same samples (Ward et al., 2017a) revealed the presence of a three closely related OTUs of Herpetosiphonaceae that likely represent OHK22 and/or closely related strains. These OTUs displayed ~86% similarity to Herpetosiphon aurantiacus, again consistent with genus-level divergence. Sequences >90% similar to these OHK Herpetosiphonaceae OTUs have been recovered from other harsh environments, particularly cold desert soils (e.g. Sattin et al., 2009; Borin et al., 2010; Mapelli et al. 2012. Succession of a bacterial rhizospheric community along a chronosequence of a cold desert. In BIODESERT International Workshop-Microbial Diversity in Desert Extreme Environment “Microarrays from theory to application”). This pattern implies that there is a broad environmental distribution for this genus-level clade in regions with low nutrient availability and high osmotic and/or temperature stress.
While members of the Chloroflexi are best known from thermophilic environments like hot springs (e.g. Klatt et al., 2013; Thiel et al., 2018), much of the known diversity of this phylum was derived from mesophilic or even psychrophilic environments (e.g. Inagaki et al., 2003; Hug et al., 2013; Mehrshad et al., 2018). Within the Herpetosiphonaceae, only the basal lineage Kallotenue papyrolyticum has previously been shown to thrive under high temperatures >40°C (Table 1). OHK22 was recovered from a hot spring that maintains a temperature of ~44°C, and therefore expanded the known diversity of thermophilic Herpetosiphonaceae.
Table 1.
Herpetosiphonaceae genomes compared in this study.
| MAG Id | GTDB-Tk taxonomy | Source environment | Source and/or optimal growth T | Reference | NCBI WGS accession number | Genbank assembly accession | Completeness | Contamination | Strain heterogeneity | Contigs | Size (Mb) | GC% | Coding sequences |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OHK22 | d__Bacteria; p__Chloroflexota; c__Chloroflexia; o__Chloroflexales; f__Herpetosiphonaceae; g__;s__ |
Iron-rich hot spring | Source at 44°C | This study | JABXJS | 96.97 | 0.91 | 0 | 285 | 6.35 | 62.48 | 5131 | |
| Kallotenue papyrolyticum | d__Bacteria; p__Chloroflexota; c__Chloroflexia; o__Chloroflexales; f__Herpetosiphonaceae; g__JKG1; s__JKG1 sp000526415 |
cellulolytic enrichment in Great Boiling Spring, Nevada | Optimum 55°C | Cole et al., 2013; Hedlund et al., 2015 | JAGA | GCA_000526415.1 | 97.58 | 0 | 0 | 4 | 4.475263 | 65.77 | 3948 |
| Herpetosiphon aurantiacus | d__Bacteria; p__Chloroflexota; c__Chloroflexia; o__Chloroflexales; f__Herpetosiphonaceae; g__Herpetosiphon; s__Herpetosiphon aurantiacus |
Slimy coating of a freshwater alga | Holt and Lewin, 1968; Kiss et al., 2011 | N/A | GCA_000018565.1 | 98.18 | 1.82 | 0 | 3 | 6.346587 | 50.9 | 5617 | |
| Herpetosiphon geysericola | d__Bacteria; p__Chloroflexota; c__Chloroflexia; o__Chloroflexales; f__Herpetosiphonaceae; g__Herpetosiphon; s__Herpetosiphon geysericola |
Biofilm adjacent to a hot springin Baja California, Mexico | Maximum T of 38–40°C | Lewin, 1970; Ward et al., 2015 | LGKP | GCA_001306135.1 | 99.09 | 0.91 | 0 | 46 | 6.140412 | 50.75 | 5445 |
| Herpetosiphon llanstefanensis | d__Bacteria; p__Chloroflexota; c__Chloroflexia; o__Chloroflexales; f__Herpetosiphonaceae; g__Herpetosiphon; s__Herpetosiphon sp003205875 |
Stream-edge soil | Better growth at 30°C than 37°C or 42°C | Livingstone et al., 2018 | PUBZ | GCA_003205875.1 | 99.09 | 1.82 | 0 | 169 | 6.140145 | 50.77 | 5401 |
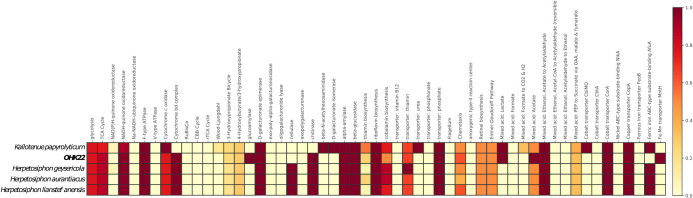
While prediction of function from protein sequences alone are tentative in the absence of isolate-based experimental verification, we found that the metabolic pathways encoded in the OHK22 genome were broadly similar to that of other members of the Herpetosiphonaceae (Kiss et al., 2011; Hedlund et al., 2015; Ward et al., 2015; Livingstone et al., 2018); these are consistent with a lifestyle as an aerobic heterotroph (Fig. 3). OHK22 included genes for a high-potential electron transport chain feeding aerobic respiration (i.e. a bc-type Complex III and an A-family heme copper O2 reductase). The OHK22 MAG did not recover genes associated with denitrification (e.g. nirS, nirK, qNor, cNor), sulfate reduction (e.g. aprA, aprB, dsrA, dsrB), carbon fixation (e.g. rubisco, ATP-citrate lyase, or malyl-CoA lyase), nitrogen fixation (e.g. nifHDK), or phototrophy (e.g. pufL, pufM, bchX, bchY, bchZ). Given the high completeness of the OHK22 MAG, MetaPOAP estimated the likelihood of False Negatives for the presence of these pathways to be very low (e.g. ~7×10–10 for phototrophy based on the absence of the key marker genes pufL, pufM, bchX, bchY, and bchZ). This provides confidence that these pathways are actually absent in OHK22. Analysis of the genome with CXXCH_finder (McGlynn et al., 2015) revealed that no proteins encoded by OHK22 have more than 2 heme-binding domains, suggesting that despite the iron oxide-rich environment this organism is not capable of dissimilatory iron reduction (or oxidation) via extracellular electron transfer mechanisms. FeGenie found fewer genes associated with siderophore synthesis, iron transport, iron regulation, and iron storage in OHK22 as compared to other Herpetosiphonaceae (e.g. 4 genes for iron transport in OHK22 versus 17 in Herpetosiphon aurantiacus and 11 in Kallotenue papyrolyticum, Supplemental Table S1). This may reflect adaptation of OHK22 to an iron-rich environment in which less energetic investment in iron acquisition is necessary as compared to the relatively iron-poor environments from which other Herpetosiphonaceae have been recovered. FeGenie identified one protein encoded by OHK22 similar to sulfocyanin (SoxE) which has been implicated in iron oxidation in some acidophilic iron oxiders (e.g. Castelle et al., 2015) but no other proteins related to iron oxidation or reduction.
Fig. 3.
KEGG-decoder heatmap of relative abundance of functional genes between OHK22 and previously described genomes of Herpetosiphonaceae.
OHK22 lacks a B-family heme copper O2 reductase complex that is found in a number of thermophilic Chloroflexi (including phototrophs and nonphototrophs from the classes Anaerolineae, Ardenticatenia, and Chloroflexia, e.g. Hemp et al., 2015; Ward et al., 2019a). The distribution of B-family heme copper O2 reductases in Chloroflexi is unlike that of A-family heme-copper O2 reductases, the latter of which appears to be ancestral in many class-level Chloroflexi clades; instead, the B-family heme-copper O2 reductases appear to be derived traits that have been acquired independently through horizontal gene transfer in certain Chloroflexi lineages (Ward et al., 2018a). The absence of B-family heme copper O2 reductases, Alternative Complex III, phototrophy, and carbon fixation in OHK22 indicate that the ancestral electron transport chains of Chloroflexia most likely contained high-potential metabolism built around A-family heme-copper O2 reductases and bc-type Complex III. This further highlights the rich and delayed history of phototrophy relative to aerobic respiration within the Chloroflexia (Ward et al., 2015; Shih et al., 2017; Ward et al., 2018a).
Genomic comparisons using OrthoVenn2 (Xu et al., 2019) revealed 331 protein sequences in 117 clusters encoded in OHK22 genome that were not present in other Herpetosiphonaceae. These primarily consisted of short, incomplete sequences and/or hypothetical proteins of unknown function. Further examination of the suite of genes present in OHK22 but not Herpetosiphonaceae via analyses from RAST and KEGG-decoder revealed that OHK22 possessed additional genes associated with metal tolerance and transport of metals other than iron. These included protein-coding genes annotated as a P-type Mg2+ transport ATPase, the Fe-Mn transporter MntH, and magnesium and cobalt efflux protein CorC. The relative enrichment of metal tolerance and transport genes in this taxon may reflect adaptation to the metal-rich environment in which OHK22 lives.
While it is not currently possible to determine whether or not OHK22 is a predatory bacterium like members of Herpetosiphon in the absence of an enrichment or isolate, we found that OHK22 lacks many genes found in members of Herpetosiphon that have been associated with predatory activity. These include hydroxymethyl glutaryl CoA lyase, mevalonate kinase, NADPH-dependent flavin mononucleotide (FMN) reductase, tryptophan 2,3-dioxygenase, serine protease, and von Willebrand factor (Livingstone et al., 2018). However, OHK22 did encode proteins for degrading extracellular polymers including chitinase, glucoamylase, and multiple peptidoglycan hydrolases. It may be that OHK22 fills an ecological niche more similar to Kallotenue (Cole et al., 2013) than to Herpetosiphon (Lewin, 1970)—as a nonpredatory aerobic heterotroph targeting extracellular organic carbon sources such as polysaccharides produced by other microbes in the environment.
While a variety of carotenoid pigments are known to be synthesized by many Chloroflexia, including members of the Herpetosiphonaceae (e.g. H. aurantiacus, Kiss et al., 2011), the OHK22 MAG did not contain downstream components of carotenoid synthesis pathways found in closely related organisms. The OHK22 genome encoded proteins such as phytoene synthase (CrtB) and phytoene desaturase (CrtI), but lacked lycopene cyclase (CruA), γ-carotene 1′,2′-hydratase (CruF), and other downstream carotenoid modification proteins seen in other Herpetosiphonaceae genomes (Kiss et al., 2011; Hedlund et al., 2015; Ward et al., 2015; Livingstone et al., 2018). It therefore seems likely OHK22 is capable of synthesizing lycopene but lacks the ability to produce more complex carotenoids.
The OHK22 genome did not encode a flagellum and its mode of motility is uncertain. Because genetic markers for the gliding motility of other Herpetosiphonaceae have not yet been identified (e.g. Kiss et al., 2011), it cannot be determined at this time whether or not OHK22 is a motile organism. Like other Chloroflexi (e.g. Sutcliffe, 2011; Pace et al., 2015; Ward et al., 2018a)—but unlike the closely related phyla Armatimonadetes and Ca. Eremiobacterota (WPS-2) (Ward et al., 2017b, 2019b)—OHK22 does not encode LPS biosynthesis or outer membrane proteins. This supports the hypothesis of a monoderm nature for the Chloroflexi membrane (Sutcliffe, 2010, 2011); current relationships support a derived, secondary loss of the outer membrane from a diderm last common ancestor of Chloroflexi, Armatimonadetes, and Ca. Eremiobacterota (Ward et al., 2019b).
Conclusions
The environment and apparent ecological niche, genomic content (e.g. number of coding sequences, GC content), and putative metabolic capabilities of OHK22 suggest that this organism may reflect a transitional form of the Herpetosiphonaceae between ancestral high temperature polysaccharide degraders like Kallotenue and more derived low temperature predatory bacteria like Herpetosiphon. Together with this evidence for the evolutionary and ecological divergence of OHK22 from the described genera of Herpetosiphonaceae, accepted metrics for genome-based taxonomy indicate that OHK22 represents a novel genus-level lineage. We therefore proposed the assignment of OHK22 to a novel candidate genus and species within the Herpetosiphonaceae, with the name Candidatus Anthektikosiphon (from the Greek anthektikós, durable or tough, and siphon, tube or pipe, in reference to the apparently typical extreme habitats of these organisms and the filamentous morphology typical of Chloroflexia) siderophilum (from the Greek sideros, iron, and philos, loving, in reference to the high iron adaptation of this species). We propose Ca. Anthektikosiphon siderophilum OHK22 as the type genome for the genus and species, with official classification pending isolation and characterization of at least one strain of these taxa.
Molecular clock studies estimated that Kallotenue and Herpetosiphon lineages diverged from each other—and from other members of Chloroflexia (i.e. the phototrophic families Chloroflexaceae and Roseiflexaceae)— approximately 1.1–1.3 billion years ago, followed by divergence of other genus-level lineages of Chloroflexia >400 million years ago (Shih et al., 2017). If those estimates are even broadly correct, it is reasonable to predict that Ca. Anthektikosiphon diverged from other Herpetosiphonaceae lineages roughly several hundred million years ago. Given recent estimates for the rate of diversification of bacterial lineages over geological time (Louca et al., 2018) it is expected that many more lineages of Ca. Anthektikosiphon will have arisen in this time. While some of these lineages may have subsequently gone extinct, it seems likely that thorough sampling of appropriate environments will yield discovery of more extant diversity of Ca. Anthektikosiphon and other related lineages.
Data availability
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JABXJS000000000. The version described in this paper is version JABXJS010000000.
Citation
Ward, L. M., Fischer, W. W., and McGlynn, S. E. (2020) Candidatus Anthektikosiphon siderophilum OHK22, a New Member of the Chloroflexi Family Herpetosiphonaceae from Oku-okuhachikurou Onsen. Microbes Environ 35: ME20030.
https://doi.org/10.1264/jsme2.ME20030
Supplementary Material
Acknowledgements
Sequencing was performed at SeqMatic LLC (Fremont, CA). LMW acknowledges support from NASA NESSF (#NNX16AP39H), NSF (#OISE 1639454), NSF GROW (#DGE 1144469), the Agouron Institute, and the Simons Foundation. WWF acknowledges support from the Caltech Center for Environment-Microbe Interactions and the Simons Foundation Collaboration on the Origins of Life (SCOL). SEM acknowledges support from the Astrobiology Center Program of the National Institutes of Natural Sciences (grant no. AB311013)
References
- Aziz R.K., Bartels D., Best A.A., DeJongh M., Disz T., Edwards R.A., et al. (2008) The RAST Server: rapid annotations using subsystems technology. BMC Genomics 9: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borin S., Ventura S., Tambone F., Mapelli F., Schubotz F., Brusetti L., et al. (2010) Rock weathering creates oases of life in a high Arctic desert. Environ Microbiol 12: 293–303. [DOI] [PubMed] [Google Scholar]
- Bowers R.M., Kyrpides N.C., Stepanauskas R., Harmon-Smith M., Doud D., Reddy T.B.K., et al. (2017) Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat Biotechnol 35: 725–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell, B. (2014) BBTools Software Package. URL http://sourceforge.net/projects/bbmap
- Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., and Madden T.L. (2009) BLAST+: architecture and applications. BMC Bioinf 10: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelle C.J., Roger M., Bauzan M., Brugna M., Lignon S., Nimtz M., et al. (2015) The aerobic respiratory chain of the acidophilic archaeon Ferroplasma acidiphilum: A membrane-bound complex oxidizing ferrous iron. Biochim Biophys Acta, Bioenerg 1847: 717–728. [DOI] [PubMed] [Google Scholar]
- Castenholz, R.W. (2012) Order II. Herpetosiphonales. In Bergey’s Manual of Systematic Bacteriology, vol. 1. Boone, D.R., and Castenholz, R.W. (eds). Hoboken, NJ: John Wiley & Sons, pp. 444–466. [Google Scholar]
- Cole J.K., Gieler B.A., Heisler D.L., Palisoc M.M., Williams A.J., Dohnalkova A.C., et al. (2013) Kallotenue papyrolyticum gen. nov., sp. nov., a cellulolytic and filamentous thermophile that represents a novel lineage (Kallotenuales ord. nov., Kallotenuaceae fam. nov.) within the class Chloroflexia. Int J Syst Evol Microbiol 63: 4675–4682. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32: 1792–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber A.I., Nealson K.H., Okamoto A., McAllister S.M., Chan C.S., Barco R.A., and Merino N. (2020) FeGenie: A Comprehensive Tool for the Identification of Iron Genes and Iron Gene Neighborhoods in Genome and Metagenome Assemblies. Front Microbiol 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham E.D., Heidelberg J.F., and Tully B.J. (2018) Potential for primary productivity in a globally-distributed bacterial phototroph. ISME J 12: 1861–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund B.P., Murugapiran S.K., Huntemann M., Clum A., Pillay M., Palaniappan K., et al. (2015) High-quality draft genome sequence of Kallotenue papyrolyticum JKG1T reveals broad heterotrophic capacity focused on carbohydrate and amino acid metabolism. Genome Announc 3: e01410-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemp J., Ward L.M., Pace L.A., and Fischer W.W. (2015) Draft genome sequence of Ardenticatena maritima 110S, a thermophilic nitrate-and iron-reducing member of the Chloroflexi class Ardenticatenia. Genome Announc 3: e01347-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J.G., and Lewin R.A. (1968) Herpetosiphon aurantiacus gen. et sp. n., a new filamentous gliding organism. J Bacteriol 95: 2407-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug L.A., Castelle C.J., Wrighton K.C., Thomas B.C., Sharon I., Frischkorn K.R., et al. (2013) Community genomic analyses constrain the distribution of metabolic traits across the Chloroflexi phylum and indicate roles in sediment carbon cycling. Microbiome 1: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug L.A., Baker B.J., Anantharaman K., Brown C.T., Probst A.J., Castelle C.J., et al. (2016) A new view of the tree of life. Nat Microbiol 1: 16048. [DOI] [PubMed] [Google Scholar]
- Inagaki F., Suzuki M., Takai K., Oida H., Sakamoto T., Aoki K., et al. (2003) Microbial communities associated with geological horizons in coastal subseafloor sediments from the Sea of Okhotsk. Appl Environ Microbiol 69: 7224–7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D.D., Froula J., Egan R., and Wang Z. (2015) MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3: e1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss H., Nett M., Domin N., Martin K., Maresca J.A., Copeland A., et al. (2011) Complete genome sequence of the filamentous gliding predatory bacterium Herpetosiphon aurantiacus type strain (114-95T). Stand Genomic Sci 5: 356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatt C.G., Liu Z., Ludwig M., Kühl M., Jensen S.I., Bryant D.A., and Ward D.M. (2013) Temporal metatranscriptomic patterning in phototrophic Chloroflexi inhabiting a microbial mat in a geothermal spring. ISME J 7: 1775–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemoine F., Entfellner J.B.D., Wilkinson E., Correia D., Felipe M.D., Oliveira T., and Gascuel O. (2018) Renewing Felsenstein’s phylogenetic bootstrap in the era of big data. Nature 556: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I., and Bork P. (2016) Interactive tree of life (iTOL) v3: an online tool for the display and annotation of phylogenetic and other trees. Nucleic Acids Res 44: W242–W245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewin R.A. (1970) New Herpetosiphon species (Flexibacterales). Can J Microbiol 16: 517–520. [DOI] [PubMed] [Google Scholar]
- Li D., Luo R., Liu C.M., Leung C.M., Ting H.F., Sadakane K., et al. (2016) MEGAHIT v1. 0: a fast and scalable metagenome assembler driven by advanced methodologies and community practices. Methods (Amsterdam, Neth) 102: 3–11. [DOI] [PubMed] [Google Scholar]
- Livingstone P.G., Morphew R.M., Cookson A.R., and Whitworth D.E. (2018) Genome analysis, metabolic potential, and predatory capabilities of herpetosiphon llansteffanense sp. nov. Appl Environ Microbiol 84: e01040-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louca S., Shih P.M., Pennell M.W., Fischer W.W., Parfrey L.W., and Doebeli M. (2018) Bacterial diversification through geological time. Nat Ecol Evol 2: 1458–1467. [DOI] [PubMed] [Google Scholar]
- McGlynn S.E., Chadwick G.L., Kempes C.P., and Orphan V.J. (2015) Single cell activity reveals direct electron transfer in methanotrophic consortia. Nature 526: 531–535. [DOI] [PubMed] [Google Scholar]
- Mehrshad M., Salcher M.M., Okazaki Y., Nakano S.I., Šimek K., Andrei A.S., and Ghai R. (2018) Hidden in plain sight—highly abundant and diverse planktonic freshwater Chloroflexi. Microbiome 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, M.A., Pfeiffer, W., and Schwartz, T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway Computing Environments Workshop (GCE). New Orleans, LA, Institute of Electrical and Electronics Engineers (IEEE), pp. 1–8. [Google Scholar]
- Pace L.A., Hemp J., Ward L.M., and Fischer W.W. (2015) Draft genome of Thermanaerothrix daxensis GNS-1, a thermophilic facultative anaerobe from the Chloroflexi class Anaerolineae. Genome Announc 3: e01354-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X., Kage H., Martin K., and Nett M. (2017) Herpetosiphon gulosus sp. nov., a filamentous predatory bacterium isolated from sandy soil and Herpetosiphon giganteus sp. nov., nom. rev. Int J Syst Evol Microbiol 67: 2476–2481. [DOI] [PubMed] [Google Scholar]
- Parks D.H., Imelfort M., Skennerton C.T., Hugenholtz P., and Tyson G.W. (2015) CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res 25: 1043–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks D.H., Chuvochina M., Waite D.W., Rinke C., Skarshewski A., Chaumeil P.A., and Hugenholtz P. (2018) A standardized bacterial taxonomy based on genome phylogeny substantially revises the tree of life. Nat Biotechnol 36: 996–1004. [DOI] [PubMed] [Google Scholar]
- Rodriguez-R L.M., and Konstantinidis K.T. (2014) Bypassing cultivation to identify bacterial species. Microbe 9: 111–118. [Google Scholar]
- Sattin S.R., Cleveland C.C., Hood E., Reed S.C., King A.J., Schmidt S.K., et al. (2009) Functional shifts in unvegetated, perhumid, recently-deglaciated soils do not correlate with shifts in soil bacterial community composition. J Microbiol 47: 673–681. [DOI] [PubMed] [Google Scholar]
- Shih P.M., Ward L.M., and Fischer W.W. (2017) Evolution of the 3-hydroxypropionate bicycle and recent transfer of anoxygenic photosynthesis into the Chloroflexi. Proc Natl Acad Sci U S A 114: 10749–10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieber C.M., Probst A.J., Sharrar A., Thomas B.C., Hess M., Tringe S.G., and Banfield J.F. (2018) Recovery of genomes from metagenomes via a dereplication, aggregation and scoring strategy. Nat Microbiol 3: 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30: 1312–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe I.C. (2010) A phylum level perspective on bacterial cell envelope architecture. Trends Microbiol 18: 464–470. [DOI] [PubMed] [Google Scholar]
- Sutcliffe I.C. (2011) Cell envelope architecture in the Chloroflexi: a shifting frontline in a phylogenetic turf war. Environ Microbiol 13: 279–282. [DOI] [PubMed] [Google Scholar]
- Takashima C., Okumura T., Nishida S., Koike H., and Kano A. (2011) Bacterial symbiosis forming laminated iron‐rich deposits in Okuoku‐hachikurou hot spring, Akita Prefecture, Japan. Isl Arc 20: 294–304. [Google Scholar]
- Thiel V., Tank M., and Bryant D.A. (2018) Diversity of chlorophototrophic bacteria revealed in the omics era. Annu Rev Plant Biol 69: 21–49. [DOI] [PubMed] [Google Scholar]
- Ward L.M., Hemp J., Pace L.A., and Fischer W.W. (2015) Draft genome sequence of Herpetosiphon geysericola GC-42, a nonphototrophic member of the Chloroflexi class Chloroflexia. Genome Announc 3: e01352-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L.M., Idei A., Terajima S., Kakegawa T., Fischer W.W., and McGlynn S.E. (2017a) Microbial diversity and iron oxidation at Okuoku-hachikurou Onsen, a Japanese hot spring analog of Precambrian iron formations. Geobiology 15: 817–835. [DOI] [PubMed] [Google Scholar]
- Ward L.M., McGlynn S.E., and Fischer W.W. (2017b) Draft genome sequences of a novel lineage of Armatimonadetes recovered from Japanese hot springs. Genome Announc 5: e00820-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L.M., Hemp J., Shih P.M., McGlynn S.E., and Fischer W.W. (2018a) Evolution of phototrophy in the Chloroflexi phylum driven by horizontal gene transfer. Front Microbiol 9: 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L.M., Shih P.M., and Fischer W.W. (2018b) MetaPOAP: presence or absence of metabolic pathways in metagenome-assembled genomes. Bioinformatics 34: 4284–4286. [DOI] [PubMed] [Google Scholar]
- Ward L.M., Idei A., Nakagawa M., Ueno Y., Fischer W.W., McGlynn, S.E. (2019a) Geochemical and metagenomic characterization of Jinata Onsen, a Proterozoic-analog hot spring, reveals novel microbial diversity including iron-tolerant phototrophs and thermophilic lithotrophs. Microbes Environ 34: 278–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward L.M., Cardona T., and Holland-Moritz H. (2019b) Evolutionary implications of anoxygenic phototrophy in the bacterial phylum Candidatus Eremiobacterota (WPS-2). Front Microbiol 10: 1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.W., Tang Y.H., Tringe S.G., Simmons B.A., and Singer S.W. (2014) MaxBin: an automated binning method to recover individual genomes from metagenomes using an expectation-maximization algorithm. Microbiome 2: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Dong Z., Fang L., Luo Y., Wei Z., Guo H., et al. (2019) OrthoVenn2: a web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res 47: W52–W58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This Whole Genome Shotgun project has been deposited at DDBJ/ENA/GenBank under the accession JABXJS000000000. The version described in this paper is version JABXJS010000000.