Abstract
Foodborne diseases represent a global health threat besides the great economic losses encountered by the food industry. These hazards necessitate the implementation of food preservation methods to control foodborne pathogens, the causal agents of human illnesses. Until now, most control methods rely on inhibiting the microbial growth or eliminating the pathogens by applying lethal treatments. Natural antimicrobials, which inhibit microbial growth, include traditional chemicals, naturally occurring antimicrobials, or biological preservation (e.g. beneficial microbes, bacteriocins, or bacteriophages). Although having great antimicrobial effectiveness, challenges due to the adaptation of foodborne pathogens to such control methods are becoming apparent. Such adaptation enables the survival of the pathogens in foods or food-contact environments. This imperative concern inspires contemporary research and food industry sector to develop technologies which do not target microbial growth but disarming microbial virulence factors. These technologies, referred to as "antivirulence", render the microbe non-capable of causing the disease with very limited or no opportunities for the pathogenic microorganisms to develop resistance. For the sake of safer and fresh-like foods, with no effect on the sensory properties of foods, a combination of two or more natural antimicrobials or with other stressors, is now widespread, to preserve foods. This review introduces and critically describes the traditional versus the emerging uses of natural antimicrobials for controlling foodborne pathogens in foods.
Development of biological control strategies using natural antimicrobials proved to be effective in inhibiting microbial growth in foods and allowing improved food safety. In the meanwhile, discovery of new antivirulence agents could be a transformative strategy in food preservation in the far future.
Keywords: Food science, Food safety, Microbiology, Foodborne pathogens, Food preservation, Antimicrobial technologies, Antivirulence strategy, Natural antimicrobials
Food science; Food safety; Microbiology; Foodborne pathogens; Food preservation; Antimicrobial technologies; Antivirulence strategy; Natural antimicrobials.
1. Introduction
Food is a persistent need, nutritionally, for human life. However, undesirable microorganisms may contaminate food products, food processing facilities or manufacturing environments leading to risk of human diseases. Foodborne pathogens are those microorganisms which cause human diseases via virulence machinery, even sometimes at their low infectious dose (Yousef and Abdelhamid, 2019). Control of such microorganisms has evolved through the human history to allow for extension of the food shelf-life, and production of safer and nutritious foods. Microbial control methods aim mainly to inhibit the growth of undesirable microorganisms via the application of physical, or natural antimicrobials-based strategies. In this review, natural antimicrobials which comprise chemical and biological-based technologies will be discussed.
Combination of two or more natural antimicrobials or with physical methods, also known as “hurdle approach”, gained the interest of food industry because of their minimal impact on the nutritive value and sensory properties of foods (Singh and Shalini, 2016). Physical methods of food preservation aim to inhibit or kill undesirable microorganisms from foods or food processing environments. Research continues regarding developing thermal processing which is still used for preservation of many foods until today. While thermal processing, in which foods are heated up to a certain temperature between 50 to 150 °C, is effective to kill pathogenic microorganisms, it causes changes in sensory and organoleptic characteristics of foods (Kong et al., 2007). On the other hand, non-thermal physical technologies such as high pressure processing, irradiation, and pulsed electric field (Amit et al., 2017) have been developed, and gained high consumer acceptance because it is less disruptive to food quality as compared to traditional thermal processing. Combination of natural antimicrobials with, particularly, non-thermal technologies will be such an asset because of the high lethality, they could achieve, with maintaining the sensory properties of foods.
Emerging technologies targeting factors by which microorganisms cause disease, also known as antivirulence technologies, have received attention in the last decade as a new line of food safety research.
In this review, we aim to critically describe the significance of natural antimicrobials, and their underlying mechanisms, in controlling foodborne pathogens. Here, natural antimicrobials are categorized into those which target; i) Microbial growth, and ii) Microbial virulence factors.
2. Control of microbial growth
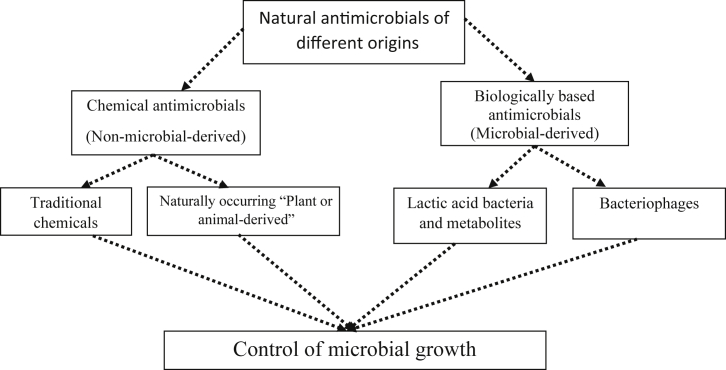
The optimal intrinsic and extrinsic factors essential for microbial growth in foods are targeted, so they cannot support microbial growth, or their survival. Intrinsic factors are those related to the food itself, e.g. pH value, water activity, the nutritional composition, existing antimicrobials or redox potential while extrinsic factors include those related to the environment surrounding the food such as storage temperature, and relative humidity surrounding the food (Hamad, 2012). Natural antimicrobials control microbial growth via disruption of cell structure and function. In the current review, such antimicrobials were classified based on their origin as shown in Figure 1, and were divided into 1) Chemical antimicrobials or "preservatives"; and 2) Biologically-based preservatives.
Figure 1.
Schematic representation of classifying natural antimicrobials which control microbial growth as presented in the current study.
2.1. Chemical antimicrobials
Chemical antimicrobials sometimes are known as preservatives. However, "preservative" is a broader term than "antimicrobial" when used alone. Antimicrobials inhibit microbial growth, but do not eliminate the microbe. They act on specific microbial metabolic targets such as cell wall, cell membrane, genetic determinants or enzymes (Pisoschi et al., 2018). Different antimicrobials have different mechanisms to stop microbial growth and could be combined to provide a synergistic effect rather than the effect of individual agents.
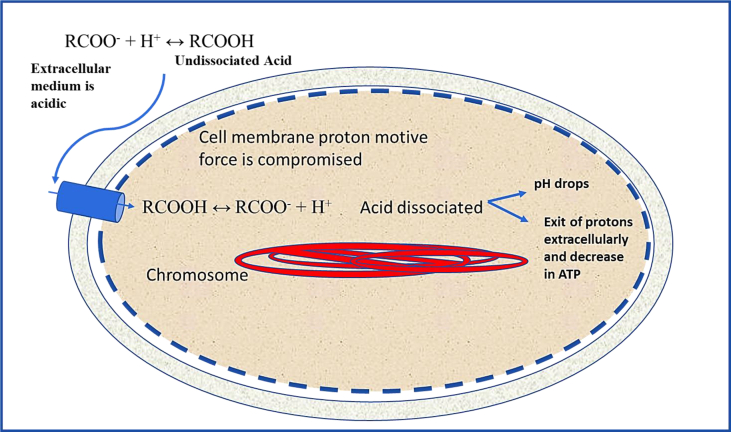
Chemical antimicrobials could be classified into two classes, “traditional" and “naturally occurring" (Raybaudi-Massilia et al., 2009; Davidson et al., 2013). Traditional chemical antimicrobials have been used since many decades and approved by many countries. Traditional chemicals used in food preservation include but not limited to sulfites, and nitrites. Organic acids such as benzoic, sorbic, acetic, lactic and propionic acids are commonly used as traditional food preservatives. Organic acids, in their undissociated form, pass through the cytoplasmic membrane lipid bilayer into the cytoplasm where they dissociate into anions and protons (Stratford and Eklund, 2003). Protons decrease the intracellular pH, and consequently inhibit glycolysis and active transport. Bacteria tend to exclude protons outside the cell on the expense of cellular energy (Raybaudi-Massilia et al., 2009). Thus, the antimicrobial effect of organic acids is likely attributed to such cascade of cellular events (Figure 2). Traditional antimicrobials face some considerations because some could have risk to human health (e.g. nitrites link to cancer in young children), impact important nutrients for the consumer or affect food flavor. For example, sulphite causes degradation of thiamine, an essential vitamin (Garcia-Fuentes et al., 2015).
Figure 2.
Mechanisms of the antimicrobial effect of “weak” organic acids against foodborne pathogenic bacteria.
On the other hand, naturally occurring food preservatives are mostly organic, and extracted from natural sources. Naturally-occurring antimicrobials include lysozymes, spices, essential oils, isothiocyanate, avidin, lactoferrin, phenolic compounds, and garlic oil (Raybaudi-Massilia et al., 2009; Davidson et al., 2013). These chemical preservatives could extend shelf-life of food products, but a few have some shortcomings (Davidson et al., 2013). Of these, certain naturally occurring antimicrobials are present in very low amounts and if higher levels are used, they could affect the taste and smell of foods (e.g. spices).
Chemical antimicrobials may play a vital role in controlling microbial toxins produced in foods. Microbial toxins in foods mostly include bacterial or mold toxins. Bacterial toxins, produced by pathogens growing in food, could be heat labile or stable (AL-Mamun et al., 2018). Studies illustrated different methods, e.g. the use of plant-derived compounds, to reduce bacterial toxins production via down-regulation of the toxin production genes in microorganisms (De Souza et al., 2010; Upadhyay et al., 2015). Many species of molds produce secondary metabolites, grouped as mycotoxins (e.g. aflatoxins produced by Aspergillus flavus), which are toxic for humans, animals and birds (Upadhyay et al., 2015). Mycotoxins such as patulin, ochratoxin A, zearalenone, aflatoxins, and fumonisins, commonly contaminate human food products (e.g. cereals) leading to several human diseases (Upadhyay et al., 2015). Several approaches to prevent mycotoxicosis, a disease caused by mycotoxins, include reducing the load of molds or their spores. Furthermore, some chemicals such as hydrogen peroxide, have been found effective to inactivate mycotoxins such as aflatoxins (Gardner et al., 1971).
The use of antimicrobials in foods still lacks the presence of approved methods for determining their antimicrobial efficacy in foods. This means when a company has an interest in an ingredient, it should develop its own industrial validation method (Davidson et al., 2013). More importantly, the consumer perception and need for fresh-like foods may lead to increasing scientific efforts and industrial shifts to natural antimicrobials.
2.2. Biologically-based antimicrobials
2.2.1. Lactic acid bacteria
Antimicrobials of biological origin (mainly microbial) mostly use lactic acid bacteria (LAB), their metabolites or both to prevent the growth of undesirable microorganisms and improve the safety and quality of foods. LAB are considered a form of biopreservation through food fermentation. They are accepted by the consumers as natural biopreservatives and health promoting microbes. In this aspect, LAB are added to the food to produce lactic acid which results in biopreservation by controlled acidification. The efficacy of lactic acid production, by LAB, depends on several factors such as food's fermentable carbohydrates, initial pH, and growth rate of LAB strains (Gobbetti and Di Cagno, 2017). Other LAB metabolites such as diacetyl, hydrogen peroxide and most importantly, bacteriocins could inhibit growth of foodborne pathogens (O'Bryan et al., 2015). In the past wo decades, small molecules such as bioactive peptides, produced by LAB, were prepared and exploited in food applications which target suppression of microbial virulence. Details about these emerging applications against microbial virulence are discussed later in this review.
Biologically-based antimicrobials, of microbial origin, showed potential to mitigate mycotoxins in foods. For example, the biological control of aflatoxins in corn grains based on the fact that competitive exclusion of toxigenic strains could be achieved by the use of non-toxigenic strains, showed some success (Abbas et al., 2006). Several strains of Lactobacillus spp. were effective in the management and control of aflatoxins (Sangsila et al., 2016; Palumbo et al., 2006). Contemporarily, high-throughput approaches such as the application of genetic engineering tools are exploited to mitigate aflatoxins (Kumar et al., 2017).
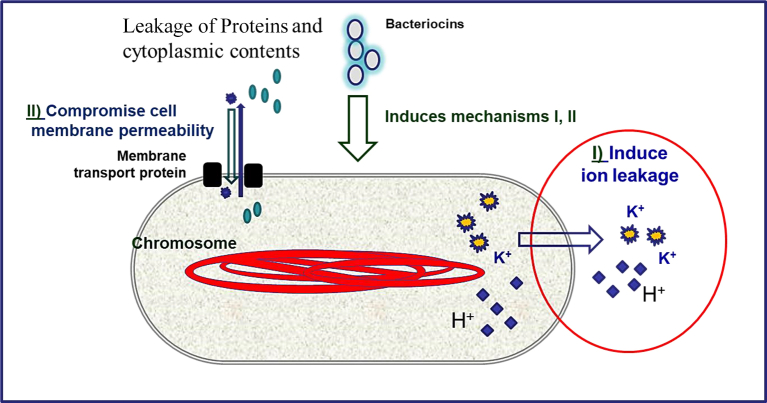
Bacteriocins are antimicrobial proteins that are produced by different members of LAB (Jack et al., 1995). They do not kill producer bacteria and are ribosomally synthesized. Their mode of action is thought to affect Gram-positive bacteria only, but some may antagonize Gram-negatives. Bacteriocins, in most of the cases, cause cell membrane damage in target bacteria and their use is expanding in food safety. Bacteriocins cause conformational changes in the cytoplasmic membrane with pore creation, resulting in increased permeability and consequently leakage of ions and molecules from within the cell (Raybaudi-Massilia et al., 2009) as demonstrated in Figure 3. Nisin is the well-known bacteriocin, approved in approximately 50 countries, and used as a preservative of processed vegetables, canned foods, and fresh cheese (De Vuyst and Vandamme, 1994). Nisin has broad spectrum inhibitory activity against Gram-positive bacteria such as staphylococci and prevents spore germination in Clostridium and Bacillus (Biswaro et al., 2018; Gut et al., 2011). For example, addition of L. lactis to a cheese model resulted in prevention of spore outgrowth in C. beijerinckii INIA 63 (Garde et al., 2011). Furthermore, nisin delayed growth of Listeria monocytogenes, inoculated onto meat, for more than 2 weeks when stored at 5 °C and did not allow Staphylococcus aureus to grow at the same storage conditions (Chung et al., 1989). Pediocin, a bacteriocin produced by Pediococcus spp., is also another example of bacteriocins with antagonistic potential against Gram-positive and some Gram-negative foodborne pathogens (Ghosh et al., 2019). Discovery of novel bacteriocins, similar to or more effective than nisin or pediocin, is a growing field in food safety research (Yang et al., 2016; Wu et al., 2019).
Figure 3.
Mode of action of bacteriocins against foodborne pathogenic bacteria.
2.2.2. Bacteriophages
Bacteriophages are viruses that infect bacteria, replicate within the host and those which are lytic, cause cell lysis (Labrie et al., 2010). The application of bacteriophages in the biocontrol of foodborne pathogens has gained increasing interest throughout recent years while their use as preservatives is relatively new. Their specificity, effective mode of action (Spricigo et al., 2013), reduced effect on organoleptic properties of foods (Sharma et al., 2005) and ubiquity (Hughes et al., 1998) are the main advantages of using lytic bacteriophages to eradicate foodborne pathogens. A growing body of evidence about the efficacy of bacteriophages to control spoilage, pathogenic and biofilm forming microorganisms is promising. Recent food applications of bacteriophages against some well-known foodborne pathogens are presented in Table 1. Large numbers of bacteriophages were isolated and have been used in different food matrices to control Gram-positive (e.g. S. aureus and L. monocytogenes) and Gram-negative (e.g. Salmonella spp., E. coli O157:H7, and Pseudomonas syringae) bacterial pathogens and their effect was food-type and phage-concentration dependent. In terms of food type, bacteriophage DT1 and DT6 caused complete reduction of E. coli O157:H7 in milk (Tomat et al., 2013) whereas bacteriophage cocktail sprayed on spinach blades resulted in 4.5 log colony forming unit (CFU)/blade reduction (Patel et al., 2011). This suggests effectiveness of bacteriophages is higher in liquid foods than solid foods. Although not a constant rule of thumb, the higher titers (usually referred to as plaque forming unit (PFU)) of bacteriophages used in food application, the larger inactivation rates of foodborne pathogens. Commercial bacteriophage preparations have been approved for use in food such as ListShield™, SalmoFresh™, and EcoShield™ (Intralytics, Columbia, MD, USA) that target L. monocytogenes, Salmonella spp. and E. coli, respectively, in food and food processing environments. With all these remarkable findings, future research is needed to maximize antimicrobial action of bacteriophages in food matrices and reduce their host-contact time needed for attachment to, and lysis of the foodborne pathogen (Sillankorva et al., 2012).
Table 1.
Applications of bacteriophages in controlling foodborne pathogens.
| Foodborne pathogen | Bacteriophage (phage) | Food/process application | Application outcomes | Reference |
|---|---|---|---|---|
| E. coli O157:H7 | Phage DT1 and DT6 | In milk during milk fermentation | 100% reduction in CFU∗/ml within an hour | (Tomat et al., 2013) |
| Phage cocktail | Spinach blades | Spraying of phage cocktail resulted in a 4.5 log CFU reduction after 2 h | (Patel et al., 2011) | |
| Campylobacter | Phage CP220 | Administration into Campylobacter-colonized chicken | 2 log reduction in CFU/g after 48 h in cecal Campylobacter | (El-Shibiny et al., 2009) |
| Phage Cj6 | On top of raw and cooked beef | largest reductions were recorded at high host cell densities | (Bigwood et al., 2009) | |
| P. syringae | Phage φ6 | Canker of kiwifruit | Reduction of 3.9 log CFU/mL using MOI∗∗ 1 | (Pinheiro et al., 2019) |
| S. aureus | Phage cocktail | During cheese manufacturing | Undetectable levels of S. aureus after 6 h in fresh cheese | (Bueno et al., 2012) |
| Cronobacter sakazakii | Phage CR5 | Infant formula milk | Complete inhibition of Cronobacter using MOI 105. | (Lee et al., 2016) |
| L. monocytogenes | Listeria phage A511 | Soft ripened white mold and red-smear cheeses | 6 logs reduction of bacterial growth with MOI 106 | (Guenther and Loessner, 2011) |
| Phage P100 | Catfish and salmon fillet | Reduction by 1.6–2.3 CFU/g at 22 °C on upon surface application | (Soni et al., 2010) | |
| Phage P100 | Cooked ham surface | 100-fold reduction after 14–28 days of storage on cooked ham surface | (Holck and Berg, 2009) | |
| Phage P100 | Red smear soft cheese | 3.5 logs to complete eradication following phage application during rind washing | (Carlton et al., 2005) | |
| Phage cocktail | Melon pieces | Listeria population reduction by 6.8 log units after 7 days of storage | (Leverentz et al., 2004) | |
| Salmonella Newport | Phage cocktail | Biocontrol of S. Newport on cherry tomato | Using MOI 5 leads to about 4.4 log reductions | (El-Dougdoug et al., 2019) |
| Salmonella Typhimurium | Phage F01-E2 | Turkey deli meats, chocolate milk and hot dogs | 5-log reduction of CFU on turkey deli meats, chocolate milk and a 3-log reduction when applied to hot dogs | (Guenther et al., 2012) |
| Salmonella Typhimurium | Phage cocktail PC1 | Meat skin | More than 99 % reduction in CFU at MOI 10 or above | (Hooton et al., 2011) |
| Salmonella Enteritidis | LPSE1 | Milk | Reduced recoverable Salmonella by approximately 1.44 log CFU/mL at MOI 1 and 2.37 log CFU/mL at MOI 100 | (Huang et al., 2018) |
| Sausage | At MOI 1 in sausage, Salmonella count decreased 0.52 log CFU/mL of sausage homogenate at 28 °C. At MOI 100, the count decreased 0.49 log CFU/ml at 4 °C. |
|||
| Lettuce | Reduction by 2.02, 1.71, and 1.45 log CFU/mL of lettuce homogenate, MOI 1, 10, and 100, respectively |
CFU denotes for colony forming unit.
A multiplicity of infection (MOI) which denotes for the ration of phage titre to the bacterial concentration.
2.3. Developments for enhancing biological control using natural antimicrobials
Since efficacy of natural antimicrobials can be challenged by several physical or chemical factors in the food environment, strategies pertaining to improve their activity gained a mounting interest. These strategies can be grouped into different categories as described below.
2.3.1. Increasing efficacy of antimicrobials through synergies
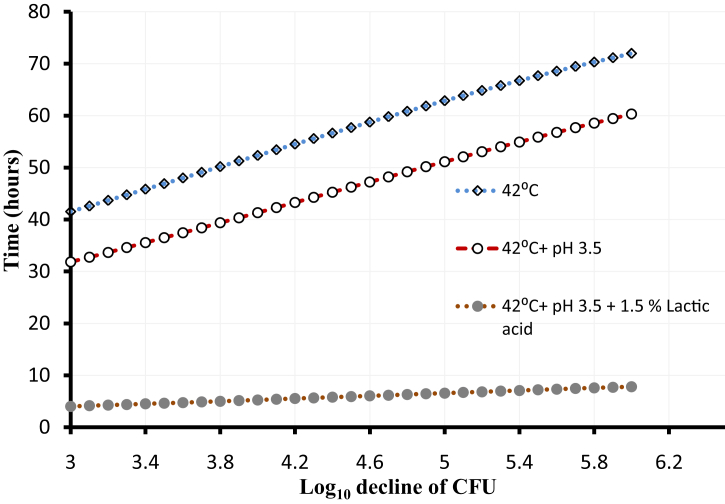
To improve the antimicrobial activity of natural antimicrobials, synergism between two or more antimicrobials or between antimicrobials and food-related stressors was feasible. Such synergistic interaction, also known as hurdle approach, aims to get maximum lethality against foodborne pathogens (Leistner, 2000). For example, sequential application of the lipopeptide paenibacterin and desiccation stress resulted in significant population reduction (1.5–1.9 log CFU/ml) of S. enterica serovars compared to desiccation stress alone (<0.1 log CFU/ml) (Abdelhamid and Yousef, 2019). Another example was that three antimicrobials, nisin A, lactoperoxidase, and reuterin, synergized to control L. monocytogenes and S. aureus in a dairy product with 4-log reduction after incubation at 10 °C for 12 days, compared to that reduction by any of the two antimicrobials (Arqués et al., 2008). Successful synergism between antimicrobials, against foodborne pathogens, included the combined use of nisin A and cinnamaldehyde (Shi et al., 2017), Pediocin PA-1 and sodium diacetate (Schlyter et al., 1993), or nisin A and lactoferrin (Murdock et al., 2007). A modeled example of combining the antimicrobial lactic acid with other stressors to inactivate L. monocytogenes is illustrated in Figure 4. Figure 4 shows that the time (7.8 h) required for 6-log reduction of L. monocytogenes in broth culture using combination of heat, acidic pH, and lactic acid is shorter, than that time (60.3 h) required by heat and acidic pH combination or heat treatment alone (71.9 h).
Figure 4.
Inactivation of L. monocytogenes in broth using hurdle technology. Data represent a plot of time (h) required for 3–6 log CFU reduction of L. monocytogenes exposed to heat treatment (42 °C) combined with pH 3.5 and 1.5 % lactic acid (solid spheres) compared to combination of heat treatment (42 °C) and pH 3.5 (open spheres) or heat treatment only (open diamond). Inactivation of L. monocytogenes profiles was predicted by USDA Pathogen Modeling program [version 8] [https://www.ars.usda.gov/northeast-area/wyndmoor-pa/eastern-regional-research-center/residue-chemistry-and-predictive-microbiology-research/docs/pathogen-modeling-program/].
2.3.2. Improving efficacy of antimicrobials by controlled delivery
The inhibition of target foodborne pathogens can be enhanced by gradual release of antimicrobials into the food environment. For example, a bimodal approach of nisin delivery from its carrier was designed where initial quick rate of release occurred, and as time progressed, nisin release becomes slower (Balasubramanian et al., 2011). Such approach of controlled delivery was more effective in inhibiting Micrococcus luteus, a model microorganism, than instant addition of nisin. On the other hand, combination of instant addition of nisin and slowly released nisin showed effectiveness to reduce counts of L. monocytogenes in broth (Chi-Zhang et al., 2004). Thus, the controlled delivery of antimicrobials aims to sustain the inhibition of target microorganisms over long time of storage. However, more research is needed to prove the efficacy of delivery approaches in real foods to streamline their applications.
2.3.3. Antimicrobial packaging systems
The use of natural antimicrobials as coatings or their incorporation into packaging materials tends to reduce counts of foodborne pathogens and extending the self-life of food products. For example, chitosan films containing 60% lysozyme were efficient to reduce E. coli by 2.7 log units (Park et al., 2004). Polypropylene films with ethylene-vinyl alcohol copolymer containing citral and oregano showed profound antimicrobial activity against L. monocytogenes, E. coli and S. enterica in packaged salad (Muriel-Galet et al., 2013, 2012). Chitosan films containing nisin and peptide P34 showed enhanced antimicrobial activity against a group of foodborne pathogens (Cé et al., 2012). Soy protein films incorporated with grape seed extract, Ethylenediaminetetraacetic acid, and nisin resulted in significant reduction by 3, 2, and 1 log CFU/ml of L. monocytogenes, E. coli O157:H7, and S. Typhimurium populations, respectively (Sivarooban et al., 2008). Hence, packaging materials could be efficient delivery system for antimicrobials in foods to control foodborne pathogens; however, it is advantageous that further research can optimize the best combination of antimicrobials/their concentration and type of the packaging material used.
3. Control of microbial virulence
Antivirulence strategies have now become an emerging area of controlling pathogenic bacteria in foods. Virulence factors are bacterial products by which they, adhere, colonize, invade, circumvent host immune system or damage the host itself (Defoirdt, 2018). The production of virulence factors is controlled by various regulatory mechanisms. Of these, quorum sensing (QS), is known to regulate the production of several virulence factors when the pathogen reaches high cell density, and thus has been a pivotal target for anti-virulence agents (Schütz and Empting, 2018). Moreover, biofilm forming ability is a strategy used by pathogens to evade the host immune responses (Watters et al., 2016). In addition, proper folding of virulence factors through the bacterial machinery is important for their biological functions. Therefore, interference of virulence factor's functions (e.g. toxin neutralization) is used for anti-virulence strategies (Rudkin et al., 2017).
Antivirulence approaches intend to disrupt any of the virulence mechanisms/their regulatory machinery or interfere with the virulence factors with the goal to prevent or treat infections. Research emphasized the success of using beneficial bacterial cells (e.g. Lactobacillus and Bifidobacterium), chemical (e.g. chalcone), or biological molecules (e.g. mucin) to inhibit the virulence of Gram-positive and Gram-negative pathogenic bacteria. Examples of the most effective antivirulence agents, their mode of action and the target pathogen such as Salmonella Typhimurium, E. coli O157:H7, C. difficile or S. aureus are summarized in Table 2. In this regard, probiotics which are defined as “live microorganisms when administered in adequate amounts confer a health benefit on the host” (Abdelhamid et al., 2019; Hill et al., 2014), are showing great promise. Small molecules, peptide in nature, secreted by probiotics were able to reduce virulence of E. coli O157:H7, Salmonella Typhimurium and C. jejuni (Bayoumi and Griffiths, 2012; Ding et al., 2005; Medellin-Peña and Griffiths, 2009). Virulent C. difficile is known to cause severe C. difficile infections (CDIs) in which disease progression is driven by two exotoxins, namely, TcdA and TcdB (Di Bella et al., 2016). Downregulation of expression of virulence genes encoding TcdA and TcdB in C. difficile has been successful by using cell free preparations of probiotics (Yun et al., 2014).
Table 2.
Examples of antivirulence activities against foodborne pathogens.
| Antivirulent Agent∗ | Foodborne pathogen | Activity | Reference |
|---|---|---|---|
| Probiotics | |||
| Bifidobacterium lactis Bb12/Lactobacillus rhamnosus LGG |
Salmonella Typhimurium Enteropathogenic E. coli |
Anti-adhesive | (Bernet et al., 1994) |
| L. acidophilus A4 | E. coli O157:H7 | Anti-adhesive | (Kim et al., 2008) |
| E. coli Nissle | S. Typhimurium | Anti-invasive | (Altenhoefer et al., 2004) |
| Bifidobacteria | Shiga toxin-producing E. coli | Antitoxin effect | (Asahara et al., 2004) |
| Bifidobacteria and lactobacilli | Clostridium difficile | Antitoxin effect | (Valdés-Varela et al., 2016) |
| L. acidophilus A4 | E. coli O157:H7 | Antibiofilm | (Kim et al., 2009) |
| L. acidophilus La-5 | E. coli O157:H7 | Anti-quorum sensing | (Medellin-Pena et al., 2007) |
| Probiotic Bacillus subtilis | Staphylococcus aureus | Decolonization | (Piewngam et al., 2018) |
| Chemical or biological molecules | |||
| T315 compound | S. Typhimurium | Antibiofilm | (Moshiri et al., 2018) |
| Chalcone | S. aureus | Anti-toxin Anti-QS Antibiofilm |
(Zhang et al., 2017) |
| Carvacrol, thymol, trans-cinnamaldehyde | E. coli O157:H7 | Antibiofilm Reduced expression of virulence genes |
(Yuan and Yuk, 2019) |
| Mucin glycans | Pseudomonas aeruginosa | Downregulation of virulence gene expression Anti-QS |
(Wheeler et al., 2019) |
| Surface-layer protein extract | E. coli O157:H7 | Anti-adhesive | (Johnson-Henry et al., 2007) |
| Cell-free preparations fromLactobacillusandBifidobacteriumsp. | Campylobacter jejuni | Anti-QS Reduced expression of virulence genes |
(Mundi et al., 2013) |
| Small molecules F12 and F19 | S. aureus | Anti-toxin Block of the transcription factor AgrA |
(Greenberg et al., 2018) |
| Methylthioadenosine | S. Typhimurium | Reduced motility Anti-invasive |
(Bourgeois et al., 2018) |
| Resveratrol |
S. aureus L. monocytogenes E. coli O157:H7 Campylobacter jejuni |
Antibiofilm | (Ma et al., 2018) |
| Green pepper essential oil | Pseudomonas aeruginosa KM01 | Anti-enzymatic | (Myszka et al., 2019) |
| Sodium citrate and cinnamic aldehyde |
E. coli O157:H7 S. aureus |
Antibiofilm | (Liu et al., 2019) |
Antivirulence agents were divided into circumstances where whole cells of probiotics were used or those circumstances where pure chemicals/biological-derived molecules were subject to the cited study.
The rise of antibiotic resistance and the evolving understanding of virulence factors from a wide array of pathogens spurred the growing interest in antivirulence approaches. The encouraging promises of antivirulence agents are attributed to their rapid target inactivation, little impact on commensal microbiota, and less pressure imposed on the target pathogen which decreases opportunities for developing resistance (Fleitas Martínez et al., 2019; Langdon et al., 2016; Vale et al., 2016). Of the highly emerging antivirulence strategies is QS inhibitors. QS is a communication system that coordinates the behavior of bacteria (e.g. orchestrate gene expression of virulence genes) in a cell-density dependent manner, and is achieved by signal molecules known as “autoinducers” (Papenfort and Bassler, 2016). Anti-QS agents aim to destroy the autoinducers or interfere with their binding to the host leading to decreased expression of virulence genes (Mundi et al., 2013). QS autoinducers are also involved in the ability of microbial pathogens to form biofilms. Biofilm is a multicellular phenotype of bacterial cells when they aggregate, attach to surfaces and surrounded by a polymeric matrix. Biofilm is critical for pathogenesis in some microbial infections (Wu et al., 2015). Examples of anti-QS and anti-biofilm activities exerted by chemical or natural agents are listed in Table 2.
Encouraging applications of antivirulence approach could extend to prevention of polymicrobial infections (Puga et al., 2018). This can be accomplished by antivirulence compounds which target commonly shared virulence factors among different pathogens (Maura et al., 2016). Additional biotechnological paths of such compounds include protection of unvaccinated individuals or immune-compromised people. However, there are some challenges that face antivirulence agents such as requirement of their use in a combined form to act on various virulence factors produced by the same pathogenic strain to ensure effectiveness of virulence disruption (Fleitas Martínez et al., 2019). Additionally, rapid pathogen identification is necessary to determine its underlying virulence factors, which will act as targets for antivirulence agents. Assessment of bacterial persistence after antivirulence agent's withdrawal, and the need for developing narrow spectrum antivirulence agents against certain forms of microbial diseases are further considerations to be met (Dickey et al., 2017). Yet, antivirulence agents are mostly needed for targeting antibiotic-resistant bacterial pathogens, microbial diseases without available vaccination or toxin-related diseases. For example, FDA has approved immunoglobulins purified from donor plasma for the treatment of botulism, a disease caused by a foodborne pathogen C. botulinum, through neutralizing botulinum neurotoxins (Dickey et al., 2017). Further research is still needed to allow identification of antivirulence agents, expanding knowledge about their mode of action, and developing methods for their appropriate use in foods. This paves the pathway to broaden the use of antivirulence approaches in real-world applications to improve safety of foods and promote human health.
4. Conclusion
Natural antimicrobials have a great a potential to suppress growth of foodborne pathogens in foods by targeting microbial cellular structures, or microbial cell homeostasis. The antimicrobial activity of these natural agents in food decreases due to the physical and chemical components of the food environment. Therefore, strategies are continuously developed to maximize the antimicrobial efficacy via synergism, controlling the delivery of antimicrobials in foods, or their incorporation into packaging materials. The proper selection of the antimicrobial agent, and the method of use ultimately boost the pathogen control in foods. On the other hand, antivirulence strategies, which is target-specific, present a promising strategy to design either narrow spectrum or broad spectrum agents to interfere with/halt the function of pathogen's virulence factors. Appropriate choice of antivirulence agents and their target virulence mechanism increases the competitiveness of such agents for use in microbial decontamination of foods.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Abbas H., Zablotowicz R., Bruns H.A., Abel C. Biocontrol of aflatoxin in corn by inoculation with non-aflatoxigenic Aspergillus flavus isolates. Biocontrol Sci. Technol. 2006;16:437–449. [Google Scholar]
- Abdelhamid A.G., El-Masry S.S., El-Dougdoug N.K. Probiotic Lactobacillus and Bifidobacterium strains possess safety characteristics, antiviral activities and host adherence factors revealed by genome mining. EPMA J. 2019;10:337–350. doi: 10.1007/s13167-019-00184-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamid A.G., Yousef A.E. The microbial lipopeptide paenibacterin disrupts desiccation resistance in Salmonella enterica serovars Tennessee and Eimsbuettel. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00739-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- AL-Mamun M., Chowdhury T., Biswas B., Absar N. Food Safety and Preservation. Elsevier; 2018. Food poisoning and intoxication: a global leading concern for human health; pp. 307–352. [Google Scholar]
- Altenhoefer A., Oswald S., Sonnenborn U., Enders C., Schulze J., Hacker J., Oelschlaeger T.A. The probiotic Escherichia coli strain Nissle 1917 interferes with invasion of human intestinal epithelial cells by different enteroinvasive bacterial pathogens. FEMS Immunol. Med. Microbiol. 2004;40:223–229. doi: 10.1016/S0928-8244(03)00368-7. [DOI] [PubMed] [Google Scholar]
- Amit S.K., Uddin M.M., Rahman R., Islam S.M.R., Khan M.S. A review on mechanisms and commercial aspects of food preservation and processing. Agric. Food Secur. 2017;6:51. [Google Scholar]
- Arqués J.L., Rodríguez E., Nuñez M., Medina M. Antimicrobial activity of nisin, reuterin, and the lactoperoxidase system on Listeria monocytogenes and Staphylococcus aureus in Cuajada, a semisolid dairy product manufactured in Spain. J. Dairy Sci. 2008;91:70–75. doi: 10.3168/jds.2007-0133. [DOI] [PubMed] [Google Scholar]
- Asahara T., Shimizu K., Nomoto K., Hamabata T., Ozawa A., Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga Toxin-Producing Escherichia coli O157:H7. Infect. Immun. 2004;72:2240–2247. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian A., Lee D.S., Chikindas M.L., Yam K.L. Effect of nisin’s controlled release on microbial growth as modeled for Micrococcus luteus. Probiotics Antimicrob. Proteins. 2011;3:113–118. doi: 10.1007/s12602-011-9073-8. [DOI] [PubMed] [Google Scholar]
- Bayoumi M.A., Griffiths M.W. In vitro inhibition of expression of virulence genes responsible for colonization and systemic spread of enteric pathogens using Bifidobacterium bifidum secreted molecules. Int. J. Food Microbiol. 2012;156:255–263. doi: 10.1016/j.ijfoodmicro.2012.03.034. [DOI] [PubMed] [Google Scholar]
- Bernet M.F., Brassart D., Neeser J.R., Servin A.L. Lactobacillus acidophilus LA 1 binds to cultured human intestinal cell lines and inhibits cell attachment and cell invasion by enterovirulent bacteria. Gut. 1994;35:483–489. doi: 10.1136/gut.35.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigwood T., Hudson J.A., Billington C. Influence of host and bacteriophage concentrations on the inactivation of food-borne pathogenic bacteria by two phages. FEMS Microbiol. Lett. 2009;291:59–64. doi: 10.1111/j.1574-6968.2008.01435.x. [DOI] [PubMed] [Google Scholar]
- Biswaro L.S., da Costa Sousa M.G., Rezende T.M.B., Dias S.C., Franco O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018;9 doi: 10.3389/fmicb.2018.00855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeois J.S., Zhou D., Thurston T.L.M., Gilchrist J.J., Ko D.C. Methylthioadenosine suppresses Salmonella virulence. Infect. Immun. 2018;86 doi: 10.1128/IAI.00429-18. e00429-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueno E., García P., Martínez B., Rodríguez A. Phage inactivation of Staphylococcus aureus in fresh and hard-type cheeses. Int. J. Food Microbiol. 2012;158:23–27. doi: 10.1016/j.ijfoodmicro.2012.06.012. [DOI] [PubMed] [Google Scholar]
- Carlton R.M., Noordman W.H., Biswas B., De Meester E.D., Loessner M.J. Bacteriophage P100 for control of Listeria monocytogenes in foods: genome sequence, bioinformatic analyses, oral toxicity study, and application. Regul. Toxicol. Pharmacol. 2005;43:301–312. doi: 10.1016/j.yrtph.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Cé N., Noreña C.P.Z., Brandelli A. Antimicrobial activity of chitosan films containing nisin, peptide P34, and natamycin. CyTA - J. Food. 2012;10:21–26. [Google Scholar]
- Chi-Zhang Y., Yam K.L., Chikindas M.L. Effective control of Listeria monocytogenes by combination of nisin formulated and slowly released into a broth system. Int. J. Food Microbiol. 2004;90:15–22. doi: 10.1016/s0168-1605(03)00168-5. [DOI] [PubMed] [Google Scholar]
- Chung K.T., Dickson J.S., Crouse J.D. Effects of nisin on growth of bacteria attached to meat. Appl. Environ. Microbiol. 1989;55:1329–1333. doi: 10.1128/aem.55.6.1329-1333.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson P.M., Critzer F.J., Taylor T.M. Naturally occurring antimicrobials for minimally processed foods. Annu. Rev. Food Sci. Technol. 2013;4:163–190. doi: 10.1146/annurev-food-030212-182535. [DOI] [PubMed] [Google Scholar]
- De Souza E.L., de Barros J.C., de Oliveira C.E.V., da Conceição M.L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 2010;137:308–311. doi: 10.1016/j.ijfoodmicro.2009.11.025. [DOI] [PubMed] [Google Scholar]
- De Vuyst L., Vandamme E.J. Bacteriocins of Lactic Acid Bacteria. Springer US; Boston, MA: 1994. Lactic acid bacteria and bacteriocins: their practical importance; pp. 1–11. [Google Scholar]
- Defoirdt T. Quorum-sensing systems as targets for antivirulence therapy. Trends Microbiol. 2018;26:313–328. doi: 10.1016/j.tim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- Di Bella S., Ascenzi P., Siarakas S., Petrosillo N., di Masi A. Clostridium difficile toxins A and B: insights into pathogenic properties and extraintestinal effects. Toxins. 2016;8:134. doi: 10.3390/toxins8050134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey S.W., Cheung G.Y.C., Otto M. Different drugs for bad bugs: antivirulence strategies in the age of antibiotic resistance. Nat. Rev. Drug Discov. 2017;16:457–471. doi: 10.1038/nrd.2017.23. [DOI] [PubMed] [Google Scholar]
- Ding W., Wang H., Griffiths M.W. Probiotics down-regulate flaA sigma28 promoter in Campylobacter jejuni. J. Food Protect. 2005;68:2295–2300. doi: 10.4315/0362-028x-68.11.2295. [DOI] [PubMed] [Google Scholar]
- El-Dougdoug N., Cucic S., Abdelhamid A., Brovko L., Kropinski A., Griffiths M., Anany H. Control of Salmonella Newport on cherry tomato using a cocktail of lytic bacteriophages. Int. J. Food Microbiol. 2019:60–71. doi: 10.1016/j.ijfoodmicro.2019.01.003. [DOI] [PubMed] [Google Scholar]
- El-Shibiny A., Scott A., Timms A., Metawea Y., Connerton P., Connerton I. Application of a Group II Campylobacter bacteriophage to reduce strains of Campylobacter jejuni and Campylobacter coli colonizing broiler chickens. J. Food Protect. 2009;72:733–740. doi: 10.4315/0362-028x-72.4.733. [DOI] [PubMed] [Google Scholar]
- Fleitas Martínez O., Cardoso M.H., Ribeiro S.M., Franco O.L. Recent advances in anti-virulence therapeutic strategies with a focus on dismantling bacterial membrane microdomains, toxin Neutralization, Quorum-sensing interference and biofilm inhibition. Front. Cell. Infect. Microbiol. 2019;9 doi: 10.3389/fcimb.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Fuentes A., Wirtz S., Vos E., Verhagen H. Short review of sulphites as food additives. Eur. J. Nutr. Food Saf. 2015;5:113–120. [Google Scholar]
- Garde S., Ávila M., Arias R., Gaya P., Nuñez M. Outgrowth inhibition of Clostridium beijerinckii spores by a bacteriocin-producing lactic culture in ovine milk cheese. Int. J. Food Microbiol. 2011;150:59–65. doi: 10.1016/j.ijfoodmicro.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Gardner H.K., Koltun S.P., Dollear F.G., Rayner E.T. Inactivation of aflatoxins in peanut and cottonseed meals by ammoniation. J. Am. Oil Chem. Soc. 1971;48:70–73. doi: 10.1007/BF02635688. [DOI] [PubMed] [Google Scholar]
- Ghosh B., Sukumar G., Ghosh A.R. Purification and characterization of pediocin from probiotic Pediococcus pentosaceus GS4, MTCC 12683. Folia Microbiol. 2019;64:765–778. doi: 10.1007/s12223-019-00689-0. [DOI] [PubMed] [Google Scholar]
- Gobbetti M., Di Cagno R. Cheese. Elsevier; 2017. Extra-Hard varieties; pp. 809–828. [Google Scholar]
- Greenberg M., Kuo D., Jankowsky E., Long L., Hager C., Bandi K., Ma D., Manoharan D., Shoham Y., Harte W., Ghannoum M.A., Shoham M. Small-molecule AgrA inhibitors F12 and F19 act as antivirulence agents against Gram-positive pathogens. Sci. Rep. 2018;8:14578. doi: 10.1038/s41598-018-32829-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guenther S., Herzig O., Fieseler L., Klumpp J., Loessner M.J. Biocontrol of Salmonella Typhimurium in RTE foods with the virulent bacteriophage FO1-E2. Int. J. Food Microbiol. 2012;154:66–72. doi: 10.1016/j.ijfoodmicro.2011.12.023. [DOI] [PubMed] [Google Scholar]
- Guenther S., Loessner M.J. Bacteriophage biocontrol of Listeria monocytogenes on soft ripened white mold and red-smear cheeses. Bacteriophage. 2011;1:94–100. doi: 10.4161/bact.1.2.15662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gut I.M., Blanke S.R., van der Donk W.A. Mechanism of inhibition of Bacillus anthracis spore outgrowth by the lantibiotic nisin. ACS Chem. Biol. 2011;6:744–752. doi: 10.1021/cb1004178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamad S.H. Progress in Food Preservation. Wiley-Blackwell; Oxford, UK: 2012. Factors affecting the growth of microorganisms in food; pp. 405–427. [Google Scholar]
- Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Pot B., Morelli L., Canani R.B., Flint H.J., Salminen S., Calder P.C., Sanders M.E. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- Holck A., Berg J. Inhibition of Listeria monocytogenes in cooked ham by virulent bacteriophages and protective cultures. Appl. Environ. Microbiol. 2009;75:6944–6946. doi: 10.1128/AEM.00926-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooton S.P.T., Atterbury R.J., Connerton I.F. Application of a bacteriophage cocktail to reduce Salmonella Typhimurium U288 contamination on pig skin. Int. J. Food Microbiol. 2011;151:157–163. doi: 10.1016/j.ijfoodmicro.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Huang C., Virk S.M., Shi J., Zhou Y., Willias S.P., Morsy M.K., Abdelnabby H.E., Liu J., Wang X., Li J. Isolation, characterization, and application of bacteriophage LPSE1 against Salmonella enterica in Ready to Eat (RTE) foods. Front. Microbiol. 2018;9:1046. doi: 10.3389/fmicb.2018.01046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K.A., Sutherland I.W., Clark J., Jones M.V. Bacteriophage and associated polysaccharide depolymerases - novel tools for study of bacterial biofilms. J. Appl. Microbiol. 1998;85:583–590. doi: 10.1046/j.1365-2672.1998.853541.x. [DOI] [PubMed] [Google Scholar]
- Jack R.W., Tagg J.R., Ray B. Bacteriocins of gram-positive bacteria. Microbiol. Mol. Biol. Rev. 1995;59:171–200. doi: 10.1128/mr.59.2.171-200.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson-Henry K.C., Hagen K.E., Gordonpour M., Tompkins T.A., Sherman P.M. Surface-layer protein extracts from Lactobacillus helveticus inhibit enterohaemorrhagic Escherichia coli O157:H7 adhesion to epithelial cells. Cell Microbiol. 2007;9:356–367. doi: 10.1111/j.1462-5822.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- Kim Y., Kim S.-H., Whang K.-Y., Kim Y.-J., Oh S. Inhibition of Escherichia coli O157:H7 attachment by interactions between lactic acid bacteria and intestinal epithelial cells. J. Microbiol. Biotechnol. 2008;18:1278–1285. [PubMed] [Google Scholar]
- Kim Y., oh S., Kim S.H. Released exopolysaccharide (r-EPS) produced from probiotic bacteria reduce biofilm formation of enterohemorrhagic Escherichia coli O157:H7. Biochem. Biophys. Res. Commun. 2009;379:324–329. doi: 10.1016/j.bbrc.2008.12.053. [DOI] [PubMed] [Google Scholar]
- Kong F., Tang J., Rasco B., Crapo C. Kinetics of salmon quality changes during thermal processing. J. Food Eng. 2007;83:510–520. doi: 10.1111/j.1750-3841.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Kumar P., Mahato D.K., Kamle M., Mohanta T.K., Kang S.G. Aflatoxins: a global concern for food safety, human health and their management. Front. Microbiol. 2017 doi: 10.3389/fmicb.2016.02170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labrie S.J., Samson J.E., Moineau S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010;8:317–327. doi: 10.1038/nrmicro2315. [DOI] [PubMed] [Google Scholar]
- Langdon A., Crook N., Dantas G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016;8:39. doi: 10.1186/s13073-016-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J.-H., Bai J., Shin H., Kim Y., Park B., Heu S., Ryu S. A novel bacteriophage targeting Cronobacter sakazakii is a potential biocontrol agent in foods. Appl. Environ. Microbiol. 2016;82:192–201. doi: 10.1128/AEM.01827-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leistner L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000;55:181–186. doi: 10.1016/s0168-1605(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Leverentz B., Conway W.S., Janisiewicz W., Camp M.J. Optimizing concentration and timing of a phage spray application to reduce Listeria monocytogenes on honeydew melon tissue. J. Food Protect. 2004;67:1682–1686. doi: 10.4315/0362-028x-67.8.1682. [DOI] [PubMed] [Google Scholar]
- Liu L., Ye C., Soteyome T., Zhao X., Xia J., Xu W., Mao Y., Peng R., Chen J., Xu Z., Shirtliff M.E., Harro J.M. Inhibitory effects of two types of food additives on biofilm formation by foodborne pathogens. Microbiol. 2019;8 doi: 10.1002/mbo3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D.S.L., Tan L.T.-H., Chan K.-G., Yap W.H., Pusparajah P., Chuah L.-H., Ming L.C., Khan T.M., Lee L.-H., Goh B.-H. Resveratrol—potential antibacterial agent against foodborne pathogens. Front. Pharmacol. 2018;9:102. doi: 10.3389/fphar.2018.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maura D., Ballok A.E., Rahme L.G. Considerations and caveats in anti-virulence drug development. Curr. Opin. Microbiol. 2016;33:41–46. doi: 10.1016/j.mib.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medellin-Pena M., Wang H., Johnson R., Anand S., Griffiths M.W., Medellin-Pena M.J., Wang H., Johnson R., Anand S., Griffiths M.W. Probiotics affect virulence-related gene expression in Escherichia coli O157:H7. Appl. Environ. Microbiol. 2007;73:4259–4267. doi: 10.1128/AEM.00159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medellin-Peña M.J., Griffiths M.W. Effect of molecules secreted by Lactobacillus acidophilus strain La-5 on Escherichia coli O157:H7 colonization. Appl. Environ. Microbiol. 2009;75:1165–1172. doi: 10.1128/AEM.01651-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshiri J., Kaur D., Hambira C.M., Sandala J.L., Koopman J.A., Fuchs J.R., Gunn J.S. Identification of a small molecule anti-biofilm agent against Salmonella enterica. Front. Microbiol. 2018;9:2804. doi: 10.3389/fmicb.2018.02804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundi A., Delcenserie V., Amiri - Jami M., Moorhead S., Griffiths M.W. Cell-free preparations of Lactobacillus acidophilus strain La-5 and Bifidobacterium longum Strain NCC2705 affect virulence gene expression in Campylobacter jejuni. J. Food Protect. 2013;76:1740–1746. doi: 10.4315/0362-028X.JFP-13-084. [DOI] [PubMed] [Google Scholar]
- Murdock C.A., Cleveland J., Matthews K.R., Chikindas M.L. The synergistic effect of nisin and lactoferrin on the inhibition of Listeria monocytogenes and Escherichia coli O157:H7. Lett. Appl. Microbiol. 2007;44:255–261. doi: 10.1111/j.1472-765X.2006.02076.x. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V., Cerisuelo J.P., López-Carballo G., Lara M., Gavara R., Hernández-Muñoz P. Development of antimicrobial films for microbiological control of packaged salad. Int. J. Food Microbiol. 2012;157:195–201. doi: 10.1016/j.ijfoodmicro.2012.05.002. [DOI] [PubMed] [Google Scholar]
- Muriel-Galet V., Cerisuelo J.P., López-Carballo G., Aucejo S., Gavara R., Hernández-Muñoz P. Evaluation of EVOH-coated PP films with oregano essential oil and citral to improve the shelf-life of packaged salad. Food Contr. 2013;30:137–143. [Google Scholar]
- Myszka K., Olejnik A., Majcher M., Sobieszczańska N., Grygier A., Powierska-Czarny J., Rudzińska M. Green pepper essential oil as a biopreservative agent for fish-based products: antimicrobial and antivirulence activities against Pseudomonas aeruginosa KM01. LWT. 2019;108:6–13. [Google Scholar]
- O’Bryan C.A., Crandall P.G., Ricke S.C., Ndahetuye J.B. Handbook of Natural Antimicrobials for Food Safety and Quality. Elsevier; 2015. Lactic acid bacteria (LAB) as antimicrobials in food products; pp. 117–136. [Google Scholar]
- Palumbo J.D., Baker J.L., Mahoney N.E. Isolation of bacterial antagonists of Aspergillus flavus from almonds. Microb. Ecol. 2006;52:45–52. doi: 10.1007/s00248-006-9096-y. [DOI] [PubMed] [Google Scholar]
- Papenfort K., Bassler B.L. Quorum sensing signal–response systems in Gram-negative bacteria. Nat. Rev. Microbiol. 2016;14:576–588. doi: 10.1038/nrmicro.2016.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.-I., Daeschel M.A., Zhao Y. Functional properties of antimicrobial lysozyme-chitosan composite films. J. Food Sci. 2004;69:M215–M221. [Google Scholar]
- Patel J., Sharma M., Millner P., Calaway T., Singh M. Inactivation of Escherichia coli O157:H7 attached to spinach harvester blade using bacteriophage. Foodb. Pathog. Dis. 2011;8:541–546. doi: 10.1089/fpd.2010.0734. [DOI] [PubMed] [Google Scholar]
- Piewngam P., Zheng Y., Nguyen T.H., Dickey S.W., Joo H.-S., Villaruz A.E., Glose K.A., Fisher E.L., Hunt R.L., Li B., Chiou J., Pharkjaksu S., Khongthong S., Cheung G.Y.C., Kiratisin P., Otto M. Pathogen elimination by probiotic Bacillus via signalling interference. Nature. 2018;562:532–537. doi: 10.1038/s41586-018-0616-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro L.A.M., Pereira C., Frazão C., Balcão V.M., Almeida A. Efficiency of phage ϕ6 for biocontrol of Pseudomonas syringae pv. syringae: an in vitro preliminary study. Microorganisms. 2019;7:286. doi: 10.3390/microorganisms7090286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisoschi A.M., Pop A., Georgescu C., Turcuş V., Olah N.K., Mathe E. An overview of natural antimicrobials role in food. Eur. J. Med. Chem. 2018;143:922–935. doi: 10.1016/j.ejmech.2017.11.095. [DOI] [PubMed] [Google Scholar]
- Puga C.H., Rodríguez-López P., Cabo M.L., SanJose C., Orgaz B. Enzymatic dispersal of dual-species biofilms carrying Listeria monocytogenes and other associated food industry bacteria. Food Contr. 2018;94:222–228. [Google Scholar]
- Raybaudi-Massilia R.M., Mosqueda-Melgar J., Soliva-Fortuny R., Martín-Belloso O. Control of pathogenic and spoilage microorganisms in fresh-cut fruits and fruit juices by traditional and alternative natural antimicrobials. Compr. Rev. Food Sci. Food Saf. 2009;8:157–180. doi: 10.1111/j.1541-4337.2009.00076.x. [DOI] [PubMed] [Google Scholar]
- Rudkin J.K., McLoughlin R.M., Preston A., Massey R.C. Bacterial toxins: offensive, defensive, or something else altogether? PLoS Pathog. 2017;13 doi: 10.1371/journal.ppat.1006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sangsila A., Faucet-Marquis V., Pfohl-Leszkowicz A., Itsaranuwat P. Detoxification of zearalenone by Lactobacillus pentosus strains. Food Contr. 2016;62:187–192. [Google Scholar]
- Schlyter J.H., Glass K.A., Loeffelholz J., Degnan A.J., Luchansky J.B. The effects of diacetate with nitrite, lactate, or pediocin on the viability of Listeria monocytegenes in Turkey slurries. Int. J. Food Microbiol. 1993;19:271–281. doi: 10.1016/0168-1605(93)90019-d. [DOI] [PubMed] [Google Scholar]
- Schütz C., Empting M. Targeting the Pseudomonas quinolone signal quorum sensing system for the discovery of novel anti-infective pathoblockers. Beilstein J. Org. Chem. 2018;14:2627–2645. doi: 10.3762/bjoc.14.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma M., Ryu J.H., Beuchat L.R. Inactivation of Escherichia coli O157:H7 in biofilm on stainless steel by treatment with an alkaline cleaner and a bacteriophage. J. Appl. Microbiol. 2005;99:449–459. doi: 10.1111/j.1365-2672.2005.02659.x. [DOI] [PubMed] [Google Scholar]
- Shi C., Zhang X., Zhao X., Meng R., Liu Z., Chen X., Guo N. Synergistic interactions of nisin in combination with cinnamaldehyde against Staphylococcus aureus in pasteurized milk. Food Contr. 2017;71:10–16. [Google Scholar]
- Sillankorva S.M., Oliveira H., Azeredo J. Bacteriophages and their role in food safety. Internet J. Microbiol. 2012;863945:1–13. doi: 10.1155/2012/863945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S., Shalini R. Effect of hurdle technology in food preservation: a Review. Crit. Rev. Food Sci. Nutr. 2016;56:641–649. doi: 10.1080/10408398.2012.761594. [DOI] [PubMed] [Google Scholar]
- Sivarooban T., Hettiarachchy N.S., Johnson M.G. Physical and antimicrobial properties of grape seed extract, nisin, and EDTA incorporated soy protein edible films. Food Res. Int. 2008;41:781–785. [Google Scholar]
- Soni K.A., Nannapaneni R., Hagens S. Reduction of Listeria monocytogenes on the surface of fresh channel catfish fillets by bacteriophage Listex P100. Foodb. Pathog. Dis. 2010;7:427–434. doi: 10.1089/fpd.2009.0432. [DOI] [PubMed] [Google Scholar]
- Spricigo D.A., Bardina C., Cortés P., Llagostera M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013;165:169–174. doi: 10.1016/j.ijfoodmicro.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Stratford M., Eklund T. Food Preservatives. Springer; Boston, MA: 2003. Organic acids and esters; pp. 48–84. [Google Scholar]
- Tomat D., Mercanti D., Balagué C., Quiberoni A. Phage biocontrol of enteropathogenic and shiga toxin-producing Escherichia coli during milk fermentation. Lett. Appl. Microbiol. 2013;57 doi: 10.1111/lam.12074. [DOI] [PubMed] [Google Scholar]
- Upadhyay A., Mooyottu S., Yin H., Nair M., Bhattaram V., Venkitanarayanan K. Inhibiting microbial toxins using plant-derived compounds and plant extracts. Medicines. 2015;2:186–211. doi: 10.3390/medicines2030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdés-Varela L., Alonso-Guervos M., García-Suárez O., Gueimonde M., Ruas-Madiedo P. Screening of bifidobacteria and lactobacilli able to antagonize the cytotoxic effect of Clostridium difficile upon intestinal epithelial HT29 monolayer. Front. Microbiol. 2016;7:577. doi: 10.3389/fmicb.2016.00577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale P.F., McNally L., Doeschl-Wilson A., King K.C., Popat R., Domingo-Sananes M.R., Allen J.E., Soares M.P., Kümmerli R. Beyond killing. Evol. Med. Public Heal. 2016:148–157. doi: 10.1093/emph/eow012. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watters C., Fleming D., Bishop D., Rumbaugh K.P. Progress in Molecular Biology and Translational Science. 2016. Host responses to biofilm; pp. 193–239. [DOI] [PubMed] [Google Scholar]
- Wheeler K.M., Cárcamo-Oyarce G., Turner B.S., Dellos-Nolan S., Co J.Y., Lehoux S., Cummings R.D., Wozniak D.J., Ribbeck K. Mucin glycans attenuate the virulence of Pseudomonas aeruginosa in infection. Nat. Microbiol. 2019;4:2146–2154. doi: 10.1038/s41564-019-0581-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H., Moser C., Wang H.Z., Høiby N., Song Z.J. Strategies for combating bacterial biofilm infections. Int. J. Oral Sci. 2015;7:1–7. doi: 10.1038/ijos.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou L., Lu F., Bie X., Zhao H., Zhang C., Lu Z., Lu Y. Discovery of a novel antimicrobial lipopeptide, brevibacillin V, from Brevibacillus laterosporus fmb70 and its application on the preservation of skim milk. J. Agric. Food Chem. 2019;67:12452–12460. doi: 10.1021/acs.jafc.9b04113. [DOI] [PubMed] [Google Scholar]
- Yang X., Huang E., Yuan C., Zhang L., Yousef A.E. Isolation and structural elucidation of brevibacillin, an antimicrobial lipopeptide from Brevibacillus laterosporus that combats drug-resistant Gram-positive bacteria. Appl. Environ. Microbiol. 2016;82:2763–2772. doi: 10.1128/AEM.00315-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousef A.E., Abdelhamid A.G. Behavior of microorganisms in food: growth, survival, and death. In: Doyle M.P., Diez-Gonzalez F., Hill C., Doyle M., Diez-Gonzalez F., Hill C., editors. Food Microbiology: Fundamentals and Frontiers. fifth ed. ASM Press; Washington, DC: 2019. pp. 3–21. ASM Press, Washington, DC, USA. [Google Scholar]
- Yuan W., Yuk H.-G. Effects of sublethal thymol, carvacrol, and trans -cinnamaldehyde adaptation on virulence properties of Escherichia coli O157:H7. Appl. Environ. Microbiol. 2019;85 doi: 10.1128/AEM.00271-19. AEM-00271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun B., Oh S., Griffiths M.W. Lactobacillus acidophilus modulates the virulence of Clostridium difficile. J. Dairy Sci. 2014;97:4745–4758. doi: 10.3168/jds.2014-7921. [DOI] [PubMed] [Google Scholar]
- Zhang B., Teng Z., Li X., Lu G., Deng X., Niu X., Wang J. Chalcone attenuates Staphylococcus aureus virulence by targeting sortase A and alpha-Hemolysin. Front. Microbiol. 2017;8:1715. doi: 10.3389/fmicb.2017.01715. [DOI] [PMC free article] [PubMed] [Google Scholar]