Abstract
Objective
Recent scientific literature has widely described a possible major role of smell dysfunction as a specific symptom of coronavirus disease 2019. This systematic review may provide a more holistic approach to current knowledge of the disease.
Methods
A systematic review was completed using Embase, PubMed and Web of Science databases that considered original articles focused on olfactory evaluation in coronavirus disease 2019 patients, published between March and May 2020, in English language.
Results
From the 483 research papers initially identified, 32 original studies were selected, comprising a total of 17 306 subjects with a laboratory confirmed diagnosis of coronavirus disease 2019. Individual study sample sizes ranged from 6 to 6452 patients. This comprehensive analysis confirmed that olfactory disorders represent an important clinical feature in coronavirus disease 2019, with a prevalence of 11–100 per cent in included patients, although there was heterogeneity in terms of assessment tools and population selection criteria.
Conclusion
The results indicate that an accurate clinical evaluation should be carried out using structured questionnaires and tests with olfactory substances.
Key words: COVID-19, SARS-CoV-2, Smell, Olfactory Nerve Diseases, Olfaction Disorders
Introduction
Infection by the new pathogen severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has highlighted a possible major role of chemosensory dysfunction, with a particular reference to smell disorders, often in association with taste disorders.1,2
Focusing on smell impairment, it is known that post-viral anosmia could be a fairly common sequela of upper airway disease.3 However, the clinical presentation of smell disorders during coronavirus disease 2019 (Covid-19) does not seem to be ‘univocal’, ranging from patient reports of normal smell, to reports of partial loss of smell (hyposmia) or total loss of smell (anosmia), or even altered perception of smell (dysosmia).
Many research teams have evaluated olfactory dysfunction in patients affected by Covid-19, highlighting a possible role of the viral invasion of the olfactory bulb by SARS-CoV-2 as the main aetiopathogenic mechanism of olfactory dysfunction.4 Bulfamante et al. recently described the autoptic presence of numerous particles, likely referable to virions of SARS-CoV-2, at the level of the olfactory nerve.5
However, it remains difficult to establish the exact prevalence of smell disorders, the expected timing of onset, the smell outcome, the associated risk factors, the relationship with taste disorders and, above all, the aetiopathogenetic mechanisms of damage.
A small number of systematic reviews6–12 have been published already, during the early stages of the pandemic in Europe and USA. However, in light of continuous scientific updating, we believe that our study can provide a more holistic approach to current knowledge of the disease. Furthermore, we believe that accurate identification of an olfactory disorder and its characteristics could facilitate our understanding of pathogenetic mechanisms, with particular reference to possible involvement of the central nervous system, thus ultimately enhancing our wider understanding of the role of smell dysfunction in Covid-19.
Materials and methods
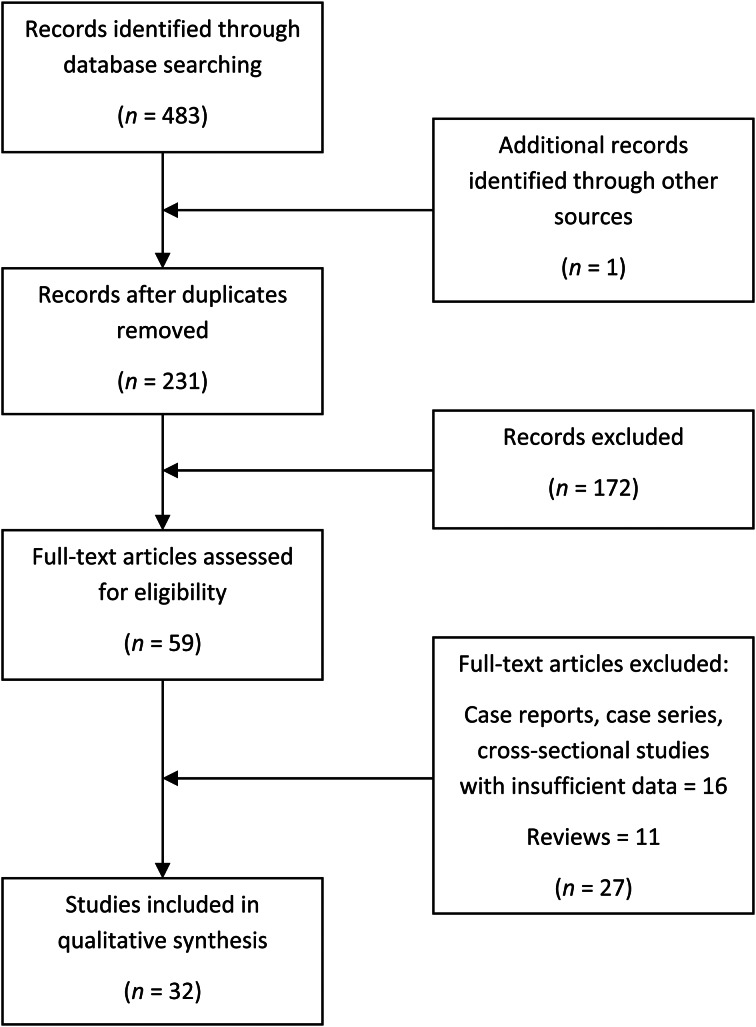
This research was conducted using PubMed, Embase and Web of Science databases (Table 1), focusing on papers published up to 31th May 2020. The search was carried out according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRISMA’) reporting guidelines,13 as shown in Figure 1. Specifically, we performed a systematic electronic search of original articles published between March and May 2020, in English language, considering studies focused on olfactory evaluation in Covid-19 patients. Although there are many studies that consider smell dysfunction in patients affected by Covid-19, we chose to specifically consider only those which carried out an in-depth assessment focused on chemosensory disorders, particularly smell impairment, in Covid-19 patients.
Table 1.
Summary of search strategies
| Database | Search strategy | Date of search | Unique papers found (n) |
|---|---|---|---|
| PubMed | ((“COVID” OR “COVID-19” OR “SARS-COV-2” OR “coronavirus”)) AND (“smell” or “anosmia” or “dysosmia” or “hyposmia” or “parosmia” or “olfaction” or “olfactory”) | 31 May 2020 | 199 |
| Embase | (‘coronavirus’ OR ‘covid’ OR ‘covid 19’ OR ‘sars cov 2’) AND (‘smell’ OR ‘anosmia’ OR ‘dysosmia’ OR ‘hyposmia’ OR ‘parosmia’ OR ‘olfaction’ OR ‘olfactory’) | 31 May 2020 | 216 |
| Web of Science | (TS= (Covid 19 OR Covid OR Coronavirus OR SARS-COV-2)) AND (TS= (Smell OR anosmia OR dysosmia OR hyposmia OR parosmia OR olfaction OR olfactory)) | 31 May 2020 | 68 |
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (‘PRISMA’) flowchart.
Inclusion criteria were: a laboratory confirmed diagnosis of Covid-19 infection; and the presence of a smell evaluation assessed through anamnestic and/or database data collection, a simple survey, a validated questionnaire focused on olfactory ability, and/or chemosensitive tests with odorants. We excluded from our investigation all systematic and narrative reviews, case reports, and all studies without specific data on patients affected by Covid-19. For a more precise analysis, we also excluded studies in which the patient's setting and/or smell evaluation method was not clearly explained.
The references of review articles were checked for cross-referencing purposes. The research process was conducted by two different authors (EF and AMS). Disagreements regarding the final selection of studies were discussed by the two authors and a final consensus was reached.
For each included article, we recorded: the number of Covid-19 patients, the number of patients with olfactory dysfunction, the country (and city if available) in which the study was performed, the type of study, patients’ data, the adopted method for smell evaluation (anamnestic data collection, simple survey, elaborated questionnaire focused on olfactory ability and/or chemosensitive tests with odorant), the time of evaluation, the time of disease onset, the concomitant evaluation of taste disorders, the patient setting (in-patient and/or out-patient) and evaluation results.
The selected studies were assessed for quality and methodological bias using the National Heart, Lung, and Blood Institute Study Quality Assessment Tools.14 The level of evidence was assessed according to the Oxford Centre for Evidence-Based Medicine level of evidence guide.15
Patients, intervention, comparison and outcomes criteria
The patients, intervention, comparison and outcomes (‘PICO’) criteria for the review were considered as follows: (1) patients – patients with SARS-CoV-2 infection certified on laboratory tests who underwent a clinical evaluation of smell impairment using anamnestic data, a smell questionnaire and/or olfactory tests; (2) intervention – clinical evaluation of olfactory disorders; (3) comparison – different methods of evaluating olfactory function (subjective and objective); and (4) outcome – prevalence and characteristics of olfactory dysfunction in Covid-19 patients.
Results
Of the 483 research papers initially identified, 32 original studies were finally selected, comprising a total of 17 306 subjects with a laboratory confirmed diagnosis of Covid-19. Individual study sample sizes ranged from 6 to 6452 patients. The studies’ characteristics are described in Table 2.2,16–46 Over half of the selected studies were carried out in European countries.
Table 2.
Summary of included studies
| Study authors | Covid-19 patient population size (n) | Patients with olfactory dysfunction | Study location | Study type | Oxford level of evidence | NHI-SQAT score |
|---|---|---|---|---|---|---|
| Aggarwal et al.16 | 16 | 3 (19%) subjective olfactory &/or taste dysfunction | Des Moines, USA | Retrospective cohort study | 4 | Fair |
| Beltrán-Corbellini et al.17 | 79 | 25 (31.65%) subjective olfactory dysfunction | Madrid, Spain | Case series | 4 | Fair |
| Carignan et al.18 | 134 | 87 (64.9%) subjective olfactory &/or taste dysfunction | Quebec Eastern Townships, Canada | Retrospective cohort study | 4 | Fair |
| Giacomelli et al.19 | 59 | 20 (33.9%) subjective olfactory &/or taste dysfunction | Milan, Italy | Cross-sectional study | 4 | Fair |
| Hornuss et al.20 | 45 | 38 (84%) objective olfactory dysfunction | Freiburg, Germany | Cross-sectional study | 4 | Good |
| Kai Chua et al.21 | 31 | 7 (22.6%) subjective olfactory dysfunction | Singapore | Cross-sectional study | 4 | Fair |
| Kim et al.22 | 213 | 68 (31.9%) subjective olfactory dysfunction | Seoul, South Korea | Cross-sectional study | 4 | Good |
| Klopfenstein et al.23 | 114 | 54 (47.4%) subjective olfactory dysfunction | Trévenans, France | Retrospective cohort study | 4 | Fair |
| Lechien et al.24 | 417 | 357 (85.6%) subjective olfactory dysfunction, with validated tool | 12 European hospitals | Cross-sectional study | 4 | Good |
| Lechien et al.25 | 2013; subset of 93 patients were eligible for objective olfactory evaluation | 1754 (87%) subjective olfactory dysfunction | 18 European hospitals | Cross-sectional study | 4 | Good |
| Lechien et al.26 | 86 | 53 (62%) objective olfactory dysfunction | Mons, Belgium | Cross-sectional study | 4 | Good |
| Lechien et al.27 | 1420 | 70.2% subjective olfactory dysfunction | 18 European hospitals | Cross-sectional study | 4 | Fair |
| Lee et al.28 | 3191 | 488 (15.3%) subjective mixed olfactory &/or taste dysfunction in patients at early stage of Covid-19 | Daegu, South Korea | Cross-sectional study | 4 | Good |
| Li et al.29 | 145 | 16 (11%) objective olfactory dysfunction 25 days from symptom onset | Wuhan, China | Cross-sectional study | 4 | |
| Luers et al.30 | 72 | 53 (73.61%) subjective dysfunction | Cologne, Germany | Retrospective cohort study | 4 | Fair |
| Menni et al.31 | 6452 in UK, 726 in USA | 64.8% in UK & 67.5% in USA had subjective olfactory &/or taste dysfunction | UK & USA | Cross-sectional study | 4 | Good |
| Moein et al.32 | 60 | 59 (98.33%) objective olfactory dysfunction, 21 (35%) subjective olfactory &/or taste dysfunction | Teheran, Iran | Case series | 4 | Fair |
| Noh et al.33 | 199 | 52 (26.1%) subjective olfactory dysfunction | Gyeongju, Republic of Korea | Cross-sectional study | 4 | Good |
| Ottaviano et al.34 | 6 | 6 (100%) objective olfactory dysfunction | Padova, Italy | Case series | 4 | Fair |
| Paderno et al.35 | 508 (295 hospitalised + 213 home-quarantined) | 44% in hospitalised group & 72% in home-quarantined group had subjective olfactory dysfunction | Brescia, Italy | Cross-sectional study | 4 | Good |
| Speth et al.36 | 103 | 61.2% subjective olfactory dysfunction | Aarau, Switzerland | Cross-sectional study | 4 | Good |
| Spinato et al.37 | 202 | 130 (64.36%) subjective mixed olfactory &/or taste dysfunction | Treviso, Italy | Cross-sectional study | 4 | Fair |
| Tostmann et al.38 | 79 | 37 (46.8%) subjective olfactory dysfunction | Nijmegen, Netherlands | Cross-sectional study | 4 | Fair |
| Trubiano et al.39 | 28 | 11 (39.3%) subjective mixed olfactory &/or taste dysfunction | Melbourne, Australia | Retrospective cohort study | 4 | Fair |
| Tsivgoulis et al.40 | 22 | 16 (72.7%) objective olfactory dysfunction | Athens, Greece | Cross-sectional study | 4 | Good |
| Vaira et al.41 | 345 | 256 (74.2%) subjective chemosensitive disorders, but 30.1% of 89 patients who did not report dysfunction proved objectively hyposmic | Sassari, Salerno, Milan & Bologna, Italy | Cross-sectional study | 4 | Good |
| Vaira et al.42 | 72 | 60 (83.33%) objective dysfunction; 44 (61.1%) subjective dysfunction | Sassari, Italy | Case series | 4 | Good |
| Vaira et al.43 | 33 | 25 (75.76%) had dysfunction on objective & self-administered test; 17 (51.52%) had subjective dysfunction | Sassari, Bologna & Salerno, Italy | Cross-sectional study | 4 | Fair |
| Wee et al.44 | 154 | 22.7% subjective mixed olfactory &/or taste dysfunction | Singapore | Cross-sectional study | 4 | Poor |
| Yan et al.2 | 59 | 40 (67.8%) subjective dysfunction | La Jolla, USA | Cross-sectional study | 4 | Good |
| Yan et al.45 | 128 | 75 (58.59%) subjective dysfunction | La Jolla, USA | Cross-sectional study | 4 | Good |
| Zayet et al.46 | 95 | 60 (63.2%) dysfunction | Trévenans, France | Retrospective cohort study | 4 | Good |
Covid-19 = coronavirus disease 2019; NHI-SQAT = National Heart, Lung, and Blood Institute Study Quality Assessment Tools
Olfactory ability was assessed by: using validated questionnaires focused on smell dysfunction, in three studies; obtaining objective information on smell impairment through standardised chemosensitive tests with odorants, in five studies; and considering both methods, in five studies (Table 3).18,20,24–26,29,32,34,37,40–43,47–59 The remaining studies assessed olfactory ability through anamnestic data collection, simple surveys and/or structured, non-validated questionnaires (Table 4).2,16,17,19,21–23,27,28,30,31,33,35,36,38,39,44–46
Table 3.
Summary of smell-related outcomes assessed via validated questionnaires and/or objective tests
| Study authors | Patient age (years) | Setting | Olfactory evaluation(s) | Evaluation time point | Evaluation results | Olfactory dysfunction onset |
|---|---|---|---|---|---|---|
| Carignan et al.18 | Median 57.1; IQR 41.2–64.5 | Out-patients (except 3 Covid-19 patients admitted to hospital) | Adapted questions from Self-reported Mini Olfactory Questionnaire47 | Within 72 hours’ (before or after) SARS-CoV-2 testing | Anosmia 69 (51.5%), dysgeusia 85 (63.4%) | 3 (2.2%) reported anosmia & dysgeusia as presenting manifestations |
| Hornuss et al.20 | Median 56 ± 16.9 | In-patients | Self-report questionnaire, Burghart Sniffin’ Sticks smell test48,49 | N/A | 44% of anosmic & 50% of hyposmic patients on objective tests did not report smelling problems | N/A |
| Lechien et al.24 | Average 36.9 ± 11.4; IQR 19–77 | Non-ICU in-patients & infected healthcare workers across Europe | sQOD-NS50 | Average of 9.2 ± 6.2 days after first symptoms onset | Anosmia 284, 73 hyposmia | Olfactory dysfunction appeared before (11.8%), after (65.4%) or at same time as appearance of general or ENT symptoms (22.8%) |
| Lechien et al.25 | Average 39.50; IQR 12.10 | 161 (8%) in-patients & 1852 (92%) out-patients | Standardised online validated questionnaire NAHNES;51 a subset of patients had Burghart Sniffin’ Sticks smell test48,49 | Mean (SD) time from end of disease to evaluation was 7.8 (6.8) days | Mean duration of olfactory dysfunction was 8.4 days (SD, 5.1) | Before other symptoms (15%), concomitant with other symptoms (25%) or after other symptoms (57%) (considering patients with smell dysfunction) |
| Lechien et al.26 | Mean 41.7 ± 11.8 | Out-patients | NAHNES,51 sQOD-NS,50 SNOT-22 (French version), Burghart Sniffin' Sticks smell test48,49 | Mean duration of olfactory dysfunction at evaluation time was 17 ± 11 days for anosmic & 18 ± 11 days for hyposmic patients | Objective olfactory testing: 41 (47.7%) anosmic, 12 (14.0%) hyposmic | 61.4% of patients described total loss of smell at disease onset |
| Li et al.29 | Average 49 (range, 13–80) | Multicentre prospective cohort study | Smell identification testing using a T&T olfactometer based scoring system52 with odours generally familiar to Chinese population | N/A | Dysosmia of: garlic in 7 (5%), pineapple in 13 (9%), mint in 11 (8%) & ginger in 38 (26%) | Average from symptom onset of 62 days (range, 25–95) |
| Moein et al.32 | Average 46.55 ± 12.17 (overall population) | In-patients in single hospital | UPSIT smell test,53 single question | Patients dismissible within 4 days | Anosmia in 15; microsmia was severe in 20, moderate in 16 & mild in 8 | N/A |
| Ottaviano et al.34 | N/A | N/A | Objective olfactory test ‘Le Nez Du Vin’ quick olfaction test,54 PROMs,34 SNOT-22,55 smell & taste VAS55 | N/A | Alterations in smell & taste; nasal symptoms other than olfaction or taste were found to be irrelevant | N/A |
| Spinato et al.37 | Median 56; IQR 45–67 | Out-patients in single hospital | ARTIQ,56 SNOT-2255 | Patients were asked if had experienced sudden onset of altered smell or taste in 2 weeks before swab | SNOT-22 grades: 5 very mild, 23 mild, 27 moderate, 27 severe, 48 as bad as it can be | Timing of altered sense of smell or taste onset in relation to other symptoms occurred before other symptoms in 24 (11.9%), at same time in 46 (22.8%) & after other symptoms in 54 (26.7%) |
| Tsivgoulis et al.40 | Mean 55 ± 10 | In-patients | SNOT-22,55 Q-SIT (Sensonics, Haddon Heights, NJ, USA)57 | N/A | Microsomia in 15, anosmia in 1 | N/A |
| Vaira A et al.41 | Average 48.5 ± 12.8 (range, 23–88) | 184 in-patients & 161 out-patients | CCCRC orthonasal olfaction test58,59 administered for hospitalised patients; test with 7 groups of odorants for home-quarantined patients | 9.9 ± 5.8 (range, 1–28) days from positive swab; 14.8 ± 7.4 (range, 2–35) days from Covid-19 symptoms onset | Normal findings in 104 (30.1%); hyposmia was mild in 76 (22%), moderate in 59 (17.1%), severe in 45 (13%); anosmia in 61 (17.7%) | High frequencies of olfactory disorders throughout observation period, ranging between 77.4% (days 1–4) & 69.2% (days 25–35) |
| Vaira et al.42 | Average 49.2 ± 13.7 (range, 26–90) | In-patients in single teaching hospital & infected healthcare workers | CCCRC orthonasal olfaction test,58,59 single questions | Average 19.3 ± 4.5 days from onset; 15.6 ± 4.3 days from positive swab. Prevalence over whole disease course | Hypogeusia was mild in 22, moderate in 33, severe in 3; ageusia in 2; olfactory dysfunction in 44 | N/A |
| Vaira et al.43 | Average 47.2 ± 10 (range, 26–64) | Out-patients in 3 hospitals | CCCRC orthonasal olfaction test,58,59 self-administered home test, single questions | Average 20.1 ± 3.9 days from onset; 17.5 ± 3.1 days from positive swab. Prevalence over whole disease course | Of 21 with chemosensory dysfunctions, 4 had hyposmia only, 4 had anosmia hyposmia only, & 13 reported olfactory & taste disorders | N/A |
IQR = interquartile range; Covid-19 = coronavirus disease 2019; SARS-CoV-2 = severe acute respiratory syndrome coronavirus-2; N/A = not applicable; ICU = intensive care unit; sQOD-NS = short version of the Questionnaire of Olfactory Disorders – Negative Statements (a seven-item patient-reported outcome questionnaire including social, eating, annoyance and anxiety questions; NAHNES = National Health and Nutrition Examination Survey; SD = standard deviation; UPSIT = University of Pennsylvania Smell Identification Test; PROM = patient-reported outcome measures; SNOT-22 = Sino-Nasal Outcome Test-22; VAS = visual analogue scale; ARTIQ = Acute Respiratory Tract Infection Questionnaire; Q-SIT = Quick Smell Identification Test; CCCRC = Connecticut Chemosensory Clinical Research Center
Table 4.
Summary of smell-related outcomes assessed via anamnestic data collection, simple surveys and/or non-validated questionnaires
| Study authors | Patient age (years) | Setting | Olfactory evaluation(s) | Evaluation time point | Evaluation results | Olfactory dysfunction onset |
|---|---|---|---|---|---|---|
| Aggarwal et al.16 | Mean 65.5 | In-patients in single hospital | Electronic medical record database | N/A | Anosmia 3 (19%), dysgeusia 3 (19%) | N/A |
| Beltrán-Corbellini et al.17 | Average 61.6 ± 17.4 | In-patients in multiple (n = 2) tertiary care hospitals | Non-validated questionnaire | N/A | Anosmia 14 (17.7%), ageusia 14 (17.7%) | 22 (27.8%) had acute onset of olfactory &/or taste dysfunction; first symptom in 11 (13.9%) |
| Giacomelli et al.19 | Median 60; IQR 50–74 | In-patients in single tertiary care hospitals | Non-validated questionnaire (single question) | Median of 15 days after first symptoms onset | Anosmia 7 (11.9%), hyposmia 7 (11.9%) | N/A |
| Kai Chua et al.21 | N/A | Patients referred to single tertiary care hospital with acute respiratory symptoms | Non-validated questionnaire | N/A | Hyposmia 3 (9.6%), anosmia 4 (12.9%) | N/A |
| Kim et al.22 | Median 26; IQR 22–47 | Community designated for isolation of Covid-19 patients | Non-validated questionnaire survey | N/A | Of 68 individuals with hyposmia, 61 had accompanying symptoms such as hypogeusia, nasal congestion or rhinorrhoea | N/A |
| Klopfenstein et al.23 | Average 47 ± 16 (for patients with olfactory disorders only) | In-patients & out-patients in single hospital | Non-validated questionnaire (single question) | Prevalence over whole disease course | Anosmia 54 (47.4%) | Olfactory dysfunction was never first symptom; onset 4.4 days after |
| Lechien et al.27 | Mean 39.17 ± 12.09 | In-patients & out-patients | Non-validated standardised questionnaire | N/A | Loss of smell 70.2%, nasal obstruction 67.8%, rhinorrhoea 60.1%, gustatory dysfunction 54.2% | Loss of smell persisted at least 7 days after disease in 37.5% of cured patients. Mean duration of olfaction dysfunction was 8.41 ± 5.05 days |
| Lee et al.28 | Average 36.5 (range, 24.5–54.0) | Out-patients awaiting hospitalisation or facility isolation | Single question | Early stage of disease | Anosmia & ageusia in 254 of 488 (52.0%), ageusia only in 99 (20.3%), anosmia only in 135 (27.7%) | Early stage of Covid-19 |
| Luers et al.30 | Average 38 ± 13 (range, 21–87) for overall population | Out-patients in single teaching hospital | Single question | Average of 13 ± 3 days after first symptoms; 7 ± 1 after positive swab | Olfactory dysfunction 53 (73.61%) | N/A |
| Menni et al.31 | Average of 41.25 ± 12.18 in UK cohort & 44.65 ± 14.31 in US cohort | Out-patients | Self-reported symptoms – ‘COVID RADAR Symptom Tracker app’ (question on symptoms) | N/A | 64.8% in UK & 67.5% in USA had subjective olfactory &/or taste dysfunction | N/A |
| Noh et al.33 | Mean 38.0 | Patients in residential treatment centre | Single questions | N/A | 52 (26.1%) anosmia, 45 (22.6%) ageusia | Duration of anosmia ranged 2–28 days (median, 7 days) |
| Paderno et al.35 | Mean 55 ± 15 | In-patients & out-patients | Non-validated, survey-based questionnaire focusing on olfactory & gustatory dysfunctions | Mean lag time between swab & survey was 11 ± 8 days | Subjective olfactory dysfunction in 44% in hospitalised group & in 72% in home-quarantined group | Mean lag time between symptom onset & survey was 18 ± 7 days |
| Speth et al.36 | Mean 46.8 ± 15.9 | In-patients & out-patients | Non-validated standardised questionnaire | N/A | 14.6% hyposmia, 46.6% anosmia | Olfactory dysfunction was experienced on 1st day of disease by 8.7% |
| Tostmann et al.38 | N/A | Healthcare workers in single teaching hospital | Non-validated questionnaire | N/A | 37 (46.8%) subjective olfactory dysfunction | N/A |
| Trubiano et al.39 | Median 55 (IQR 46, 63.5) | Patients previously assessed in single hospital | Hospital dataset | N/A | 7 (25%) anosmia (with or without ageusia); 7 (25%) ageusia (with or without anosmia); 3 (10.7%) anosmia & ageusia | N/A |
| Wee et al.44 | N/A | In-patients & out-patients in single hospital | Non-validated questionnaire including self-reported olfactory & gustatory dysfunctions | N/A | N/A | |
| Yan et al.2 | N/A | Out-patients & in-patients in single hospital | Single question | Prevalence over whole disease course | Olfactory dysfunction in 40 | 22% reported anosmia as first symptom |
| Yan et al.45 | Median 53.5 & IQR 40–65 for in-patients; median 43 & IQR 34–54 for out-patients | Out-patients & in-patients in single hospital | Single question | Prevalence over whole disease course | olfactory dysfunction in 75 | N/A |
| Zayet et al.46 | Mean 39.8 ± 12.2 (range, 18–73) | Out-patient in single hospital | Non-validated standardised questionnaire | N/A | Dysgeusia & anosmia in 52 (54.7%), dysgeusia &/or anosmia in 70 (73.7%) | N/A |
N/A = not applicable; IQR = interquartile range; Covid-19 = coronavirus disease 2019
Discussion
Our review confirmed that olfactory disorders represent an important clinical feature in individuals affected by Covid-19, with a prevalence ranging from 11 per cent to 100 per cent of included patients, although there was heterogeneity in terms of assessment tools and population selection criteria.
The reported data show that smell dysfunction was, overall, more prevalent in patients investigated with validated questionnaires and/or tests with odorants (Table 3), compared to individuals evaluated using anamnestic data, simple surveys and/or non-validated questionnaires. This is in agreement with the findings of Moein et al.32 and further studies,60 which indicate that self-reported evaluations of olfactory loss are not in line with the more reliable outcomes of standardised tests. There are exceptions to this general trend, however, as highlighted by the papers of Lechien et al.26 and Li et al.,29 but in these latter manuscripts there are some possible biases that may affect the data.
The variations in reported outcomes may be a result of the different methods of evaluation; however, the variations might also be because of other important factors. Primarily, non-validated tests are only focused on smell disorders of new onset and do not investigate the presence of olfactory dysfunction prior to Covid-19. In contrast, a validated questionnaire and/or objective olfactory test allows greater accuracy regarding the real prevalence of olfactory disorders, the exact timing of onset and their characteristics.
Furthermore, our analysis does not suggest any significant differences in terms of the age or gender of the enrolled subjects, although younger patients often seem to show a greater prevalence of smell disorders than older ones. These data seem difficult to understand until we consider that the elderly population has a higher prevalence of smell disorders overall. In the context of Covid-19, younger patients are more likely to have a new onset of olfactory dysfunction (more evident with a non-validated questionnaire analysis), and frequently have less severe respiratory symptoms, resulting in more susceptibility to olfactory problems. Therefore, we believe that age should be considered as a possible bias, at least regarding the elderly population, given that the estimated prevalence of smell impairment in the general population aged above 80 years ranges between 43.1 per cent and 84.9 per cent.61
Regarding the hospital setting, our review highlighted a lower prevalence of smell disorders in hospitalised patients compared with home-quarantined patients. Two studies focused specifically on this comparison,2,35 emphasising a greater prevalence of the disorder in individuals with low-to-mild disease compared to those who needed hospital treatment. Once again, this difference could be related to greater attention devoted to olfactory impairment in patients in an overall better health condition.
Another relevant source of heterogeneity is linked to the different timings of smell evaluation with respect to the onset of symptoms. According to our data, smell dysfunction seems to occur mostly in early stages of the disease, and tends to decrease or resolve within the two weeks following virologic healing in the majority of the patients; therefore, all evaluations that take place during an advanced or unspecified disease stage could underestimate olfactory dysfunction.
Finally, we should consider that the large prevalence of smell disorders apparently became evident only when the SARS-CoV-2 infection hit Europe. In the first studies performed in China and Singapore, patients were frequently unaware of olfactory dysfunction.62–64 It is striking that more than half of the reviewed studies were carried out in European countries. This could be related to a higher prevalence of Covid-19 associated smell disorders in Caucasian people, although other factors should be taken into account. A possible bias could be presented by the fact that some scientific reports are written in original Chinese language and are difficult to access. In addition, we should bear in mind that – with the exception of China – the scholarly production on Covid-19 and olfactory dysfunction follows the outbreak spread, which is already peaking in Europe and Western Asia, has flourished in North America and is in an earlier stage in South America.
While these data confirm what has already been included in earlier reviews, our paper is able to present a somewhat later analysis of the issue of smell impairment in Covid-19. It discusses more complete and well-defined data than other previously published papers, and includes a significantly greater number of patients.
Nevertheless, many problems need to be addressed to allow a holistic evaluation of smell impairments in Covid-19 patients. In order to allow further and stronger meta-analytic papers, smell assessment tools should converge into validated questionnaires and odorant tests. In addition, important reported biases (e.g. age, hospital setting and patients’ overall condition) should be appropriately addressed in the context of well-designed future prospective studies.
Conclusion
In the wake of the relevance of olfactory dysfunction in individuals with Covid-19, we believe that olfactory assessment is essential in every patient with a new diagnosis of SARS-CoV-2 infection in the early stage. Furthermore, we think that smell disorders of new onset should be considered a possible symptom for suspected SARS-CoV-2 infection. Our study suggests the need for a clinical standardised evaluation carried out using structured questionnaires and, if possible, tests with olfactory substances. Finally, ENT assessment in Covid-19 patients should be routinely proposed to ensure the correct evaluation of chemosensitive disorders and the possible need for therapeutic strategies.
Acknowledgments
Competing interests
None declared
References
- 1.Hopkins C, Kumar N. Loss of Sense of Smell as Marker of COVID-19 Infection. London: ENT UK, Royal College of Surgeons of England, 2020 [Google Scholar]
- 2.Yan CH, Faraji F, Prajapati DP, Boone CE, DeConde AS. Association of chemosensory dysfunction and Covid-19 in patients presenting with influenza-like symptoms. Int Forum Allergy Rhinol 2020;10:806–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seiden AM. Postviral olfactory loss. Otolaryngol Clin North Am 2004;37:1159–66 [DOI] [PubMed] [Google Scholar]
- 4.Butowt R, Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci 2020;11:1200–3 [DOI] [PubMed] [Google Scholar]
- 5.Bulfamante G, Chiumello D, Canevini MP, Priori A, Mazzanti M, Centanni S et al. First ultrastructural autoptic findings of SARS-Cov-2 in olfactory pathways and brainstem. Minerva Anestesiol 2020;86:678–9 [DOI] [PubMed] [Google Scholar]
- 6.Tong JY, Wong A, Zhu D, Fastenberg JH, Tham T. The prevalence of olfactory and gustatory dysfunction in COVID-19 patients: a systematic review and meta-analysis. Otolaryngol Head Neck Surg 2020;163:3–11 [DOI] [PubMed] [Google Scholar]
- 7.Krajewska J, Krajewski W, Zub K, Zatoński T. COVID-19 in otolaryngologist practice: a review of current knowledge. Eur Arch Otorhinolaryngol 2020;277:1885–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lovato A, De Filippis C. Clinical presentation of COVID-19: a systematic review focusing on upper airway symptoms. Ear Nose Throat J 2020;13:145561320920762. Epub 2020 Apr 13 [DOI] [PubMed] [Google Scholar]
- 9.Pellegrino R, Cooper KW, Di Pizio A, Joseph PV, Bhutani S, Parma V. Corona viruses and the chemical senses: past, present, and future. Chem Senses 2020;14:bjaa031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Printza A, Constantinidis J. The role of self-reported smell and taste disorders in suspected COVID-19. Eur Arch Otorhinolaryngol 2020;277:2625–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sedaghat AR, Gengler I, Speth MM. Olfactory dysfunction: a highly prevalent symptom of COVID-19 with public health significance. Otolaryngol Head Neck Surg 2020;163:12–15 [DOI] [PubMed] [Google Scholar]
- 12.Soler ZM, Patel ZM, Turner JH, Holbrook EH. A primer on viral-associated olfactory loss in the era of COVID-19. Int Forum Allergy Rhinol 2020;10:814–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Heart, Lung, and Blood Institute (NHLBI). Study Quality Assessment Tools. In: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [8 September 2020]
- 15.Centre for Evidence-Based Medicine. OCEBM Levels of Evidence. In: https://www.cebm.net/index.aspx?o=5653Website [8 September 2020]
- 16.Aggarwal S, Garcia-Telles N, Aggarwal G, Lavie C, Lippi G, Henry BM. Clinical features, laboratory characteristics, and outcomes of patients hospitalized with coronavirus disease 2019 (COVID-19): early report from the United States. Diagnosis (Berl) 2020;7:91–6 [DOI] [PubMed] [Google Scholar]
- 17.Beltrán-Corbellini Á, Chico-García JL, Martínez-Poles J, Rodríguez-Jorge F, Alonso-Cánovas A. Acute-onset smell and taste disorders in the context of COVID-19: a pilot multicentre polymerase chain reaction based case-control study. Eur J Neurol 2020. Epub 2020 May 16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carignan A, Valiquette L, Grenier C, Musonera JB, Nkengurutse D, Marcil-Héguy A et al. Anosmia and dysgeusia associated with SARS-CoV-2 infection: an age-matched case-control study. CMAJ 2020;192:E702–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Giacomelli A, Pezzati L, Conti F, Bernacchia D, Siano M, Oreni L et al. Self-reported olfactory and taste disorders in SARS-CoV-2 patients: a cross-sectional study. Clin Infect Dis 2020;71:889–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornuss D, Lange B, Schröter N, Rieg S, Kern WN, Wagner D. Anosmia in COVID-19 patients. Clin Microbiol Infect 2020. Epub 2020 May 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua AJ Kai, Chan EC Yun, Loh JP, Charn TC. Acute olfactory loss is specific for Covid-19 at the emergency department. Ann Emerg Med 2020. Epub 2020 May 14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim GU, Kim MJ, Ra SH, Lee J, Bae S, Jung J et al. Clinical characteristics of asymptomatic and symptomatic patients with mild COVID-19. Clin Microbiol Infect 2020;26:948.e1–948.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klopfenstein T, Kadiane-Oussou NJ, Toko L, Royer PY, Lepiller Q, Gendrin V et al. Features of anosmia in COVID-19. Med Mal Infect 2020;50:436–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;277:2251–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lechien JR, Chiesa-Estomba CM, Hans S, Barillari MR, Jouffe L, Saussez S. Loss of smell and taste in 2013 European patients with mild to moderate COVID-19. Ann Intern Med 2020. Epub 2020 May 26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lechien JR, Cabaraux P, Chiesa-Estomba CM, Khalife M, Hans S, Calvo-Henriquez C et al. Objective olfactory evaluation of self-reported loss of smell in a case series of 86 COVID-19 patients. Head Neck 2020;42:1583–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lechien JR, Chiesa-Estomba CM, Place S, Laethem YV, Cabaraux P, Mat Q et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med 2020;288:335–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee Y, Min P, Lee S, Kim SW. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J Korean Med Sci 2020;35:e174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Long X, Zhu C, Wang H, Wang T, Lin Z et al. Olfactory dysfunction in recovered coronavirus disease 2019 (COVID-19) patients. Mov Disord 2020;35:1100–1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luers JC, Rokohl AC, Loreck N, Wawer Matos PA, Augustin M, Dewald F et al. Olfactory and gustatory dysfunction in coronavirus disease 19 (COVID-19). Clin Infect Dis 2020. Epub 2020 May 1 [DOI] [PMC free article] [PubMed]
- 31.Menni C, Valdes AM, Freidin MB, Sudre CH, Nguyen LH, Drew DA et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat Med 2020;26:1037–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moein ST, Hashemian SMR, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol 2020;10:944–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noh JY, Yoon JG, Seong H, Choi WS, Sohn JW, Cheong HJ et al. Asymptomatic infection and atypical manifestations of COVID-19: comparison of viral shedding duration. J Infect 2020. Epub 2020 May 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ottaviano G, Carecchio M, Scarpa B, Marchese-Ragona R. Olfactory and rhinological evaluations in SARS-CoV-2 patients complaining of olfactory loss. Rhinology 2020;58:400–1 [DOI] [PubMed] [Google Scholar]
- 35.Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T et al. Smell and taste alterations in Covid-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol 2020;10:955–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speth MM, Singer-Cornelius T, Obere M, Gengler I, Brockmeier SJ, Sedaghat AR. Olfactory dysfunction and sinonasal symptomatology in COVID-19: prevalence, severity, timing, and associated characteristics. Otolaryngol Head Neck Surg 2020;163:114–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA 2020;323:2089–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tostmann A, Bradley J, Bousema T, Yiek WK, Holwerda M, Bleeker-Rovers C et al. Strong associations and moderate predictive value of early symptoms for SARS-CoV-2 test positivity among healthcare workers, the Netherlands, March 2020. Euro Surveill 2020;25:2000508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trubiano JA, Vogrin S, Kwong JC, Homes N. Alterations in smell or taste - classic COVID-19? Clin Infect Dis 2020. Epub 2020 May 28 [DOI] [PMC free article] [PubMed]
- 40.Tsivgoulis G, Fragkou PC, Delides A, Karofylakis E, Dimopoulou D, Sfikakis PP et al. Quantitative evaluation of olfactory dysfunction in hospitalized patients with coronavirus [2] (COVID-19). J Neurol 2020;267:2193–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaira LA, Hopkins C, Salzano G, Petrocelli M, Melis A, Cucurullo M et al. Olfactory and gustatory function impairment in COVID-19 patients: Italian objective multicenter-study. Head Neck 2020;42:1560–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vaira LA, Deiana G, Fois AG, Pirina P, Madeddu G, De Vito A et al. Objective evaluation of anosmia and ageusia in COVID-19 patients: single-center experience on 72 cases. Head Neck 2020;42:1252–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vaira LA, Salzano G, Petrocelli M, Deiana G, Salzano FA, De Riu G. Validation of a self-administered olfactory and gustatory test for the remotely evaluation of COVID-19 patients in home quarantine. Head Neck 2020;42:1570–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wee LE, Chan YFZ, Teo NWY, Cherng BPZ, Thien SY, Wong HM et al. The role of self-reported olfactory and gustatory dysfunction as a screening criterion for suspected COVID-19. Eur Arch Otorhinolaryngol 2020;277:2389–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yan CH, Faraji F, Prajapati DP, Ostrander BT, DeConde AS. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol 2020;10:821–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zayet S, Klopfenstein T, Mercier J, Kadiane-Oussou NJ, Lan Cheong Wah L, Royer PY, et al. Contribution of anosmia and dysgeusia for diagnostic of COVID-19 in outpatients. Infection 2020. Epub 2020 May 14 [DOI] [PMC free article] [PubMed]
- 47.Zou LQ, Linden L, Cuevas M, Metasch ML, Welge-Lüssen A, Hähner A et al. Self-reported Mini Olfactory Questionnaire (Self-MOQ): a simple and useful measurement for the screening of olfactory dysfunction. Laryngoscope 2019. Epub 2019 Nov 20 [DOI] [PubMed]
- 48.Hummel T, Konnerth C-G, Rosenheim K, Kobal G. Screening of olfactory function with a four-minute odor identification test: reliability, normative data, and investigations in patients with olfactory loss. Ann Otol Rhinol Laryngol 2001;110:976–81 [DOI] [PubMed] [Google Scholar]
- 49.Hinz A, Luck T, Riedel-Heller SG, Herzberg PY, Rolffs C, Wirkner K et al. Olfactory dysfunction: properties of the Sniffin’ Sticks Screening 12 test and associations with quality of life. Eur Arch Otorhinolaryngol 2019;276:389–95 [DOI] [PubMed] [Google Scholar]
- 50.Mattos JL, Edwards C, Schlosser RJ, Hyer M, Mace JC, Smith TL et al. A brief version of the questionnaire of olfactory disorders in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol 2019;9:1144–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rawal S, Hoffman HJ, Bainbridge KE, Huedo-Medina TB, Duffy VB. Prevalence and risk factors of self-reported smell and taste alterations: results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses 2016;41:69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Okutani F, Hirose K, Kobayashi T, Kaba H, Hyodo M. Evaluation of “Open Essence” odor-identification test card by application to healthy volunteers. Auris Nasus Larynx 2013;40:76–80 [DOI] [PubMed] [Google Scholar]
- 53.Doty RL, Agrawal U. The shelf life of the University of Pennsylvania Smell Identification Test (UPSIT). Laryngoscope 1989;99:402–4 [DOI] [PubMed] [Google Scholar]
- 54.McMahon C, Scadding GK. Le Nez du Vin--a quick test of olfaction. Clin Otolaryngol Allied Sci 1996;2:278–80 [DOI] [PubMed] [Google Scholar]
- 55.Rimmer J, Hellings P, Lund VJ, Alobid I, Beale T, Dassi C et al. European position paper on diagnostic tools in rhinology. Rhinology 2019;57(suppl S28):1–41 [DOI] [PubMed] [Google Scholar]
- 56.Aabenhus R, Thorsen H, Siersma V, Brodersen J. The development and validation of a multidimensional sum-scaling questionnaire to measure patient-reported outcomes in acute respiratory tract infections in primary care: the Acute Respiratory Tract Infection Questionnaire. Value Health 2013;16:987–92 [DOI] [PubMed] [Google Scholar]
- 57.Jackman AH, Doty RL. Utility of a three-item smell identification test in detecting olfactory dysfunction. Laryngoscope 2005;115:2209–12 [DOI] [PubMed] [Google Scholar]
- 58.Cain WS, Gent JF, Goodspeed RB, Leonard G. Evaluation of olfactory dysfunction in the Connecticut chemosensory clinical research center. Laryngoscope 1988;98:83–8 [DOI] [PubMed] [Google Scholar]
- 59.Veyseller B, Ozucer B, Karaaltin AB, Yildirim Y, Degirmenci N, Aksoy F et al. Connecticut (CCCRC) olfactory test: normative values in 426 healthy volunteers. Indian J Otolaryngol Head Neck Surg 2014;66:31–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Soter A, Kim J, Jackman A, Tourbier I, Kaul A, Doty RL. Accuracy of self-report in detecting taste dysfunction. Laryngoscope 2008;118:611–17 [DOI] [PubMed] [Google Scholar]
- 61.Doty RL. Epidemiology of smell and taste dysfunction. Handb Clin Neurol 2019;164:3–13 [DOI] [PubMed] [Google Scholar]
- 62.Guan WJ, Ni ZY, Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang X, Yu Y, Xu J, Liang WH, Ou CQ, He JX et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pung R, Chiew CJ, Young BE, Chin S, Chen MIC, Clapham HE et al. Investigation of three clusters of COVID-19 in Singapore: implications for surveillance and response measures. Lancet 2020;395:1039–46 [DOI] [PMC free article] [PubMed] [Google Scholar]