Abstract
Background
In response to the COVID-19 epidemic, China implemented a series of interventions that impacted tuberculosis (TB) control in the country.
Methods
Based on routine surveillance data and questionnaires, the study analyzed TB notification, follow-up examinations, and treatment outcomes. The data were split into three phases in relation to outbreak, lockdown and reopen when the nationwide COVID-19 response started in 2020: control (11 weeks prior), intensive (11 weeks during and immediately after), and regular (4 additional weeks). Data from 2017–2019 were used as baseline.
Findings
The notified number of TB patients decreased sharply in the 1st week of the intensive period but took significantly longer to rebound in 2020 compared with baseline. The percentages of TB patients undergoing sputum examination within one week after 2 months treatment and full treatment course in the intensive period were most affected and decreased by 8% in comparison with control period. 75•2% (221/294) of counties reallocated CDC and primary health care workers to fight the COVID-19 epidemic, 26•9% (725/2694) of TB patients had postponed or missed their follow-up examinations due to travel restrictions and fear of contracting COVID-19.
Interpretation
In the short term, the COVID-19 epidemic mostly affected TB notification and follow-up examinations in China, which may lead to a surge of demand for TB services in the near future. To cope with this future challenge, an emergency response mechanism for TB should be established.
Funding
National Health Commission of China–Bill & Melinda Gates Foundation TB Collaboration project (OPP1137180).
Keywords: COVID-19, Tuberculosis, Impact, China
Research in context.
Evidence before this study
We searched PubMed, and two preprint servers (bioRxiv and medRxiv) for articles describing the impact of COVID-19 on TB control with key words “COVID-19 or SARS-CoV-2 or 2019-nCoV or novel coronavirus” and “tuberculosis” up to May 29, 2020. Some studies have discussed, anticipated, or modelled the possible impact of COVID-19 on TB control, some studies only focus on one aspect of impact on TB testing, notification, or clinical course of TB patients co-infected with COVID-19. There were no studies that fully described the impact on TB notification, follow-up examinations, and treatment outcomes with detailed data, and the impact remains unclear.
Added value of this study
To our knowledge, our study is the first to address the short-term impact of COVID-19 on TB control in different periods of COVID-19 epidemic based on national TB surveillance system and specific questionnaires. The study reported the detailed impact on TB control caused by the non-pharmaceutical interventions for COVID-19 epidemic, including TB notification, follow-up examinations and treatment outcomes. However, we didn't evaluate the long-term impact on TB incidence and death which need to be further studied.
Implications of all the available evidence
The impact of COVID-19 on TB control, including notification, follow-up examinations, and treatment outcomes, can be examined from both patients and health service providers. The demand for TB services suppressed during the COVID-19 epidemic needs to be responded in future. An emergency response mechanism regarding on human resource, anti-TB drug delivery, laboratory testing capacity, etc., should be established to improve accessibility and convenience of the health service system for all TB patients.
Alt-text: Unlabelled box
1. Introduction
As of August 11, 2020, the number of people who have died from the novel coronavirus disease 2019 (COVID-19) since January reached 728,013 of 19,718,030 confirmed cases, reported from 216 countries/regions globally [1]. Noting the high morbidity and mortality rates from the disease and its characteristics of high transmission, Li et al. [2], [3], [4], [5] more and more countries began to mobilize and dedicate a large amount of public health resources to fight the epidemic, including the suspension or reduction of outpatient and inpatient services, reallocation of medical staff to COVID-19, and reinforcement of laboratory testing [1]. This has affected the resolution of other public health issues that deserve the public's concern and should be addressed [6,7]. In China, most of hospitals received COVID-19 cases used to be TB designated hospitals, responsible for tuberculosis(TB) diagnosis and treatment. We assessed the effects of COVID-19 epidemic on TB control in China, including the reduction in cases for the intensive intervention period of 76 days of lockdown and afterwards, as well as follow-up examinations and treatment outcomes.
TB, like COVID-19, is an airborne transmitted infectious disease. It is the leading infectious disease killer globally, surpassing HIV/AIDS and malaria, with 10 million new cases and 1•5 million deaths estimated in 2019 [8]. In response to the global TB epidemic, the United Nations Sustainable Development Goal proposes the target of End TB by 2030 [9]. Despite the sheer numbers globally, TB remains overshadowed by HIV and malaria and currently the COVID-19 epidemic [10], and the impact of COVID-19 on TB outcomes is of serious concern [11]. The current studies either modeled the potential impact of COVID-19 on TB control under different scenarios based on assumptions without hard data as input [12], [13], [14], or only focused on the impact of COVID-19 on TB notification or testing [15], [16], [17], and there is no study fully discussing the short-term impact on TB notification, follow-up examination and treatment outcomes with real data of country emerged from this crisis.
At the very beginning of the COVID-19 outbreak in China, the government implemented a series of multifaceted public health interventions and enforced them strictly throughout the country. These included the cancellation of all public transportation, the prohibition of all public gatherings, and the requirement for all residents to stay at home. These interventions proved successful in mitigating the spread of COVID-19 [18], [19], [20]. However, these measures could have a potential impact on TB control in China. On one hand, these measures may limit people's seeking medical behavior and the provision of health care services in hospitals. On the other hand, the measures will reduce the transmission of TB occurring outside the household. As a country with a high TB burden, China has placed great emphasis on TB control and achieved substantial progress over the past decades [21], but TB remains a major public health issue in the country. The government issued a three-year action plan to stop TB in 2019 [22]. To successfully achieve these goals and the ultimate target of End TB, it is critical for China to address the impact of the COVID-19 epidemic on TB control and develop effective solutions to be used in China and potentially use for reference by other countries.
2. Methods
2.1. Sources of data
This study used two sources of data: routine national surveillance data and questionnaires.
Routine national surveillance data: Medical records of TB patients from 2017 to 2020 were extracted from the national TB Information Management System (TBIMS) on May 29, 2020, including demographic characteristics (e.g., age, sex, and address), diagnosis information (e.g., date of first symptom onset, date of first seeking medical care, and diagnosis result), and follow-up examinations and treatment outcomes (e.g., date and result of sputum smear after 2 month treatment and full treatment course, the reason to stop treatment). All information was entered into the TBIMS via the internet by medical staff from authorized hospitals for TB diagnosis and treatment (i.e.,“TB-designated hospital”) by the local health administration [23].
Questionnaires: Chinese Tuberculosis control network is composed of the Centers for Disease Control and Prevention (CDC), TB-designated hospitals, and primary health care units (PHCs) at each county [24]. Among them, 294 counties (10% of all counties) were randomly selected by province, and questionnaire survey was conducted to all of them, which include 2 counties from Wuhan city and 8 counties from the other cities of Hubei province. Health facilities were requested to answer the questions about daily operation of the TB outpatient clinic and hospitalization, laboratory testing work, medical staff reassignment to COVID-19 units, anti-TB drug supply, traffic restrictions within and beyond the county, and suggestions for next steps. About eighteen TB patients per county were also surveyed for a sample size of 4257. These patients were interviewed by local CDC workers via telephone regarding follow-up examinations, treatment adherence, and similar matters.
2.2. Key time points and periods
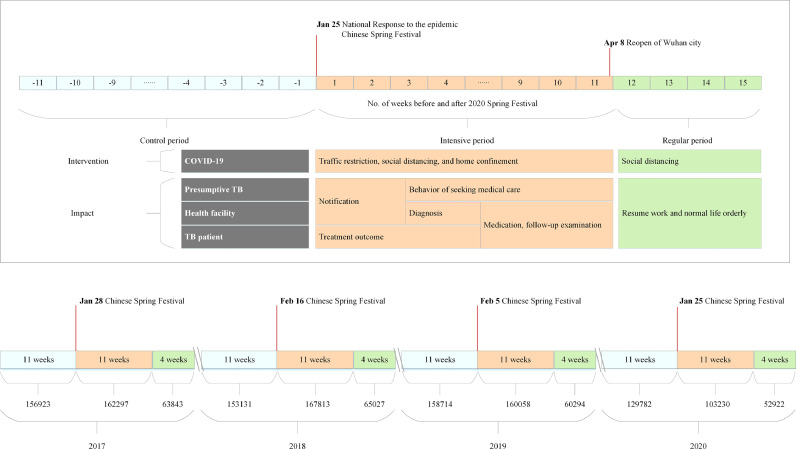
To better understand the potential impact of COVID-19 on TB control in China, this study used three periods based on two key time points: January 25, 2020 (The nationwide COVID-19 response started), and April 8, 2020 (Wuhan reopened). Eleven weeks before January 24, 2020, was considered as the control phase without any interventions for COVID-19. The second period of 11 weeks, from January 25, 2020, to April 8, 2020, was considered as the intensive phase due to the COVID-19 epidemic. The final phase of 4 weeks began on April 9, 2020, with regular interventions for the epidemic. The three years prior to the epidemic, 2017–2019, were used as the baseline and were split into 3 counterpart periods by date (Fig. 1).
Fig. 1.
TB notification breakdown by three time periods, 2017-2020. TB Notification refers to TB cases newly diagnosed by health facilities. The lower part of the figure shows TB notification 11 weeks before and 15 weeks after the Chinese Spring Festival for the years of 2017-2020. The upper part of the figure shows the two periods (intensive and regular) of 2020 with different responses to COVID-19 epidemic and potential impact on TB control in China.
In the intensive period, all people were supposed to stay at home due to containment measures in response to the COVID-19 epidemic. These measures included traffic restrictions, social distancing, and home confinement. In China most TB-designated hospitals were appointed as COVID-19 hospitals. Similarly, the medical staff, especially in the laboratories, were reassigned to fight against COVID-19, and certain areas, such as TB outpatient clinics, were directly closed.
On April 8, 2020, China lifted the 76-day lockdown on Wuhan city where the outbreak of the COVID-19 crisis started in China. The reopening of Wuhan city meant the whole country could begin to resume work and normal life in an orderly fashion.
In summary, three time periods were classified to better understand and compare the impact of the COVID-19 epidemic on TB control in China. Firstly, there was no impact on TB control during the COVID-19 epidemic in the control phase. Secondly, there was major influence from COVID-19 in intensive phase, Thirdly, there was moderate impact on TB control in the regular phase.
The Spring Festival is an important holiday in China, beginning on Chinese New Year's Eve and lasting for seven days. During this one-week national vacation, most people in China avoid seeking medical care and instead reunite with family. Yet in 2020, the Central government decided to form a Leading Group and start the nationwide COVID-19 emergence response on January 25, which was also at the beginning of one-week national holiday. Thus we selected the time point of Chinese New Year of 2017-2019 and eleven weeks before and 15 weeks after the time point as baseline.
2.3. Statistical analysis
TB notification breakdown by weeks, as well as the percentage of laboratory confirmed and migrant cases, were calculated to address the possible difference. Patient delay (from date of symptom onset to date of seeking medical care), diagnosis delay (from date of seeking medical care to date of confirmed diagnosis), and treatment delay (from date of confirmed diagnosis to date of initiating therapy) were compared by 3 periods (before, during and after Chinese Spring Festival) during 2017–2020 to reflect any changes from the impact of COVID-19 outbreak.
In China, all TB patients are requested to have sputum smear microscopy examination after 2 months treatment, as well as after the full treatment course. If the follow-up sputum smear microscopy examinations are in 7 days overdue, it will be calculated as none of show-up for following-up. The sputum conversion rate after 2 months treatment and cured rate for smear positive patients were calculated to evaluate the treatment outcomes.
Cochran-Armitage Trend Test were used for categorical data, and Kruskal-Wallis Test was used for continual data. All statistical tests were two-tailed, and the p value <0∙05 was considered significant. All the tests were done by Base R (version 3.6.3).
2.4. Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
3.1. 2020 COVID-19 periods
The TB case numbers notified in the control, intensive, and regular periods of 2020 from routine national surveillance data were 129782, 103230, and 52922 respectively (Fig. 1). The percentage of laboratory confirmed cases decreased from 53•2% in the control period to 50•8% in the regular period, and the percentage of migrant cases declined from 29•3% in the control period to 25•3% in the intensive period (Table 1).
Table 1.
TB notification, follow-up and treatment outcome during the COVID-19 periods.
| Periods | p value | |||
|---|---|---|---|---|
| Control | intensive | regular | ||
| Notification | ||||
| Laboratory confirmed cases (%) | 68996/129782 (53•2%) |
52935/103230 (51•3%) |
26898/52922 (50•8%) |
<0•0001 |
| Migrant cases (%) | 38072/129782 (29•3%) |
26136/103320 (25•3%) |
13611/52922 (25•7%) |
<0•0001 |
| Follow-up examination | ||||
| Sputum examination after 2 months treatment (%) | 94198/136963 (68•8%) |
70183/116509 (60•2%) |
20684/31917 (64•8%) |
<0•0001 |
| Sputum examination after full treatment course (%) | 97274/178314 (54•6%) |
67264/144984 (46•4%) |
26311/52892 (49•7%) |
<0•0001 |
| Treatment outcome of smear positive | ||||
| Sputum conversion rate after 2 months treatment (%) | 41716/44816 (93•1%) |
33165/35423 (93•6%) |
8771/9548 (91•9%) |
0•7475 |
| Cured rate (%) | 44252/51998 (85•1%) |
33862/39992 (84•7%) |
10997/12988 (84•7%) |
0•6159 |
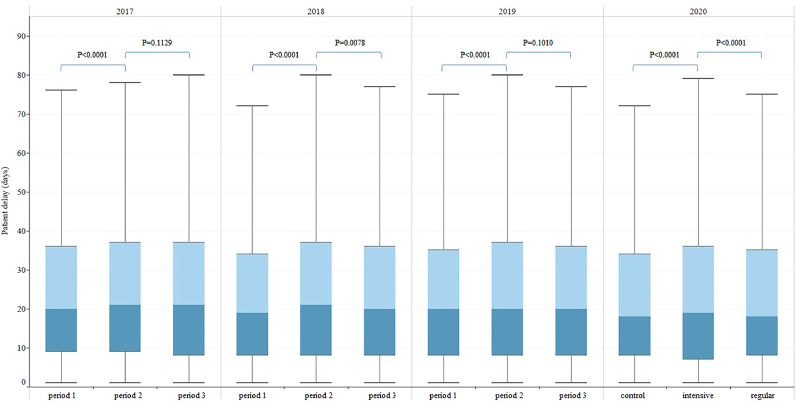
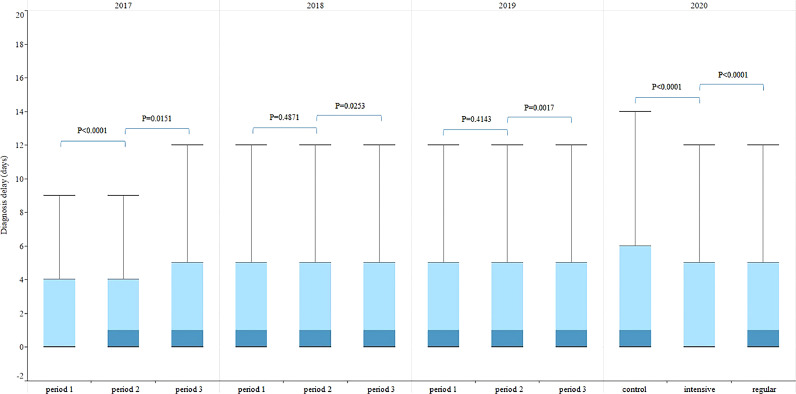
The median (interquartile range, IQR) of patient delay in the control, intensive and regular periods of 2020 were 18(8-34), 19(7-36) and 18(8-34) days respectively. The median (IQR) of diagnosis delay in the control, intensive and regular periods were 1(0-6), 0(0-5) and 1(0-5) days respectively.
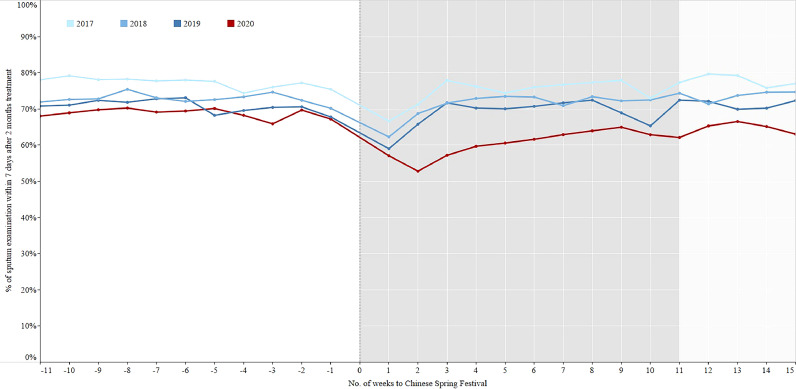
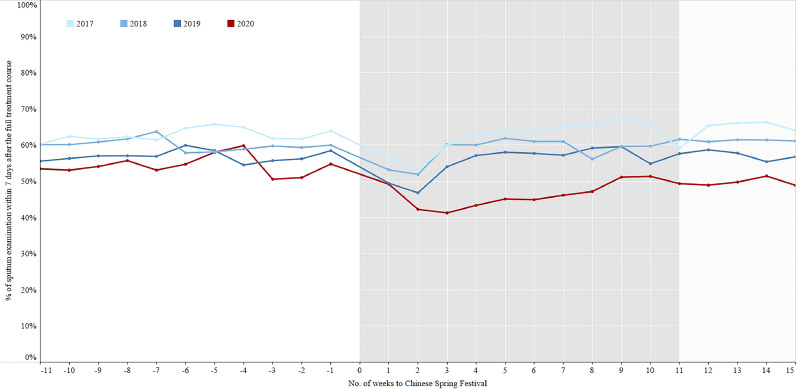
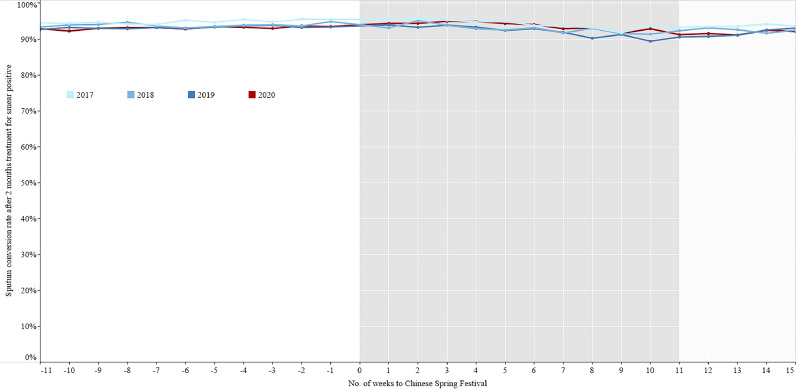
In 2020, the percentage of TB patients who had a sputum examination within one week after 2 months treatment decreased from 68•8% in the control period to 60•2% in the intensive period, the percentage of sputum examination within one week after the full treatment course declined from 54•6% in the control period to 46•4% in the intensive period. The sputum conversion rates after 2 months treatment for smear positive patients who had sputum examination in the control, intensive, and regular periods of 2020 were 93•1%, 93•6%, and 91•9% respectively. The cured rates for smear positive patients who had sputum examination after full treatment course in the control, intensive, and regular periods were 85•1%, 84•7%, and 84•7% respectively (Table 1).
3.2. Comparison between 2020 COVID-19 periods with 2017-2019 baseline.
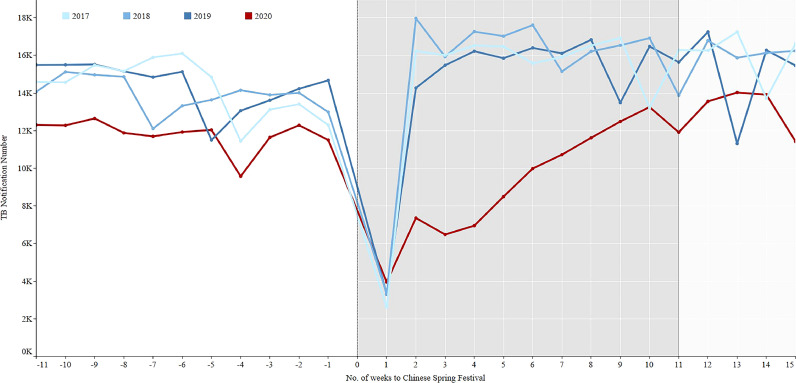
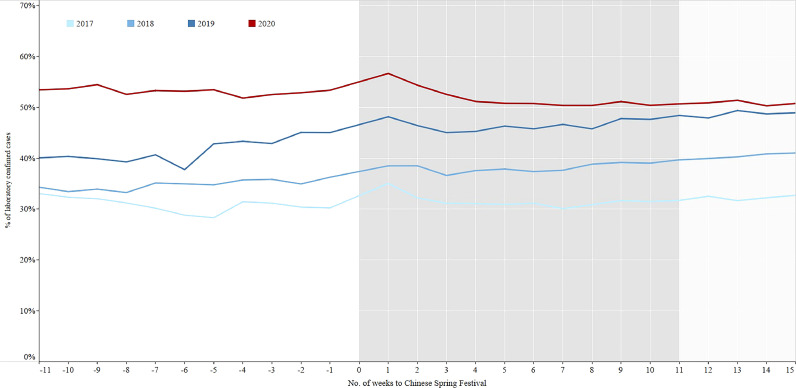
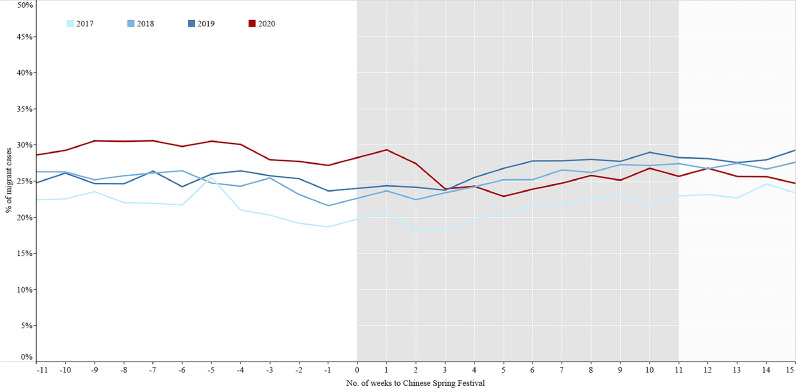
In the three years prior to COVID-19, the average notification of TB patients decreased sharply from 13,697 to 3202 in the 1st week after the Chinese Spring Festival and then quickly rebounded to the previous level from 3202 to 16,562 in the 2nd week of the three years. However, in 2020 it took about 10 weeks to return to the previous level (Fig. 2). The percentages of laboratory confirmed cases in 2017–2019 increased from period 1 to period 3 (Fig. 3, Table 2). On the contrary, the percentages continuously decreased from control period to regular period in 2020. The percentages of migrant cases increased from period 1 to period 3 in 2018–2019 (Fig. 4, Table 2), while the percentage in the intensive period of 2020 was the lowest among the three periods.
Fig. 2.
TB notification in 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020 in China.
Fig. 3.
The percentage of laboratory confirmed cases in 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020 in China. Laboratory confirmed cases: Mycobacterium tuberculosis was detected in the patient's sputum specimen by smear, culture and other diagnostics.
Table 2.
TB notification by 3 periods from year 2017 to 2020 in China.
| Year | Period | TB notification | Laboratory confirmed cases | Migrant cases | |||
|---|---|---|---|---|---|---|---|
| n (%) | p value | n (%) | p value | ||||
| 2017 | •• | •• | •• | <0•0001 | •• | <0•0001 | |
| Period 1 | 156923 | 48343(30•8%) | 34242(21•8%) | ||||
| Period 2 | 162297 | 50781(31•3%) | 34168(21•1%) | ||||
| Period 3 | 63843 | 20604(32•3%) | 14924(23•4%) | ||||
| 2018 | •• | •• | •• | <0•0001 | •• | <0•0001 | |
| Period 1 | 153131 | 53192(34•7%) | 38341(25•0%) | ||||
| Period 2 | 167813 | 64127(38•2%) | 42631(25•4%) | ||||
| Period 3 | 65027 | 26347(40•5%) | 17628(27•1%) | ||||
| 2019 | •• | •• | •• | <0•0001 | •• | <0•0001 | |
| Period 1 | 158714 | 65789(41•5%) | 40035(25•2%) | ||||
| Period 2 | 160058 | 74441(46•5%) | 42984(26•9%) | ||||
| Period 3 | 60294 | 29331(48•6%) | 17041(28•3%) | ||||
| 2020 | •• | •• | •• | <0•0001 | •• | <0•0001 | |
| Control | 129782 | 68996(53•2%) | 38072(29•3%) | ||||
| Intensive | 103230 | 52935(51•3%) | 26136(25•3%) | ||||
| Regular | 52922 | 26898(50•8%) | 13611(25•7%) | ||||
Fig. 4.
The percentage of migrant TB cases in 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020 in China. Migrant cases: The patient's current residence address and household registration do not belong to the same county (patient floats to the place rather than his/her origins).
The patient delays in the three periods of 2017-2019 were similar to that in 2020, first increased and then decreased (Fig. 5). There was no significant difference in diagnosis delay between period 1 and period 2 of 2018-2019, which was different from that of 2020 (Fig. 6). The median (IQR) of treatment delay for all three periods of 2017-2019 were all 0(0-0) days, the same as in 2020.
Fig. 5.
TB patient delay in China breakdown by three time periods, 2017–2020. Patient delay: from date of first symptom onset to date of first seeking medical care. Boxplot was used for describing the distribution of delay along y axis, the junction of different colors in the box was median. p value: Kruskal-Wallis Test, p value <0∙05 is considered significant.
Fig. 6.
TB diagnosis delay in China breakdown by three time periods, 2017–2020. Diagnosis delay: from date of first seeking medical care to date of confirmed diagnosis. p value: Kruskal-Wallis Test, p value <0∙05 is considered significant.
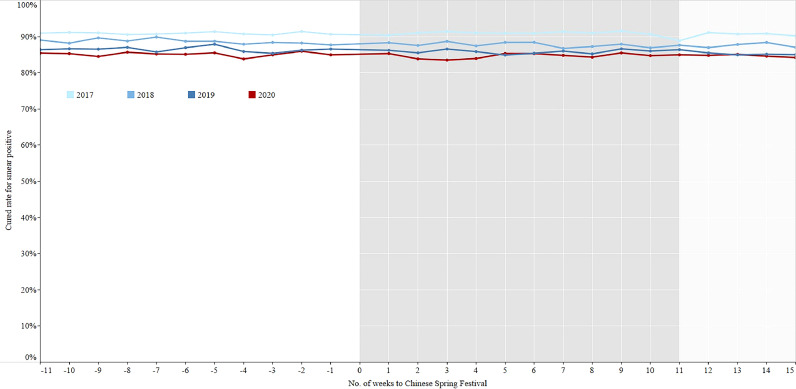
There were no significant differences for the percentage of sputum examination within 7 days after 2 months treatment and full treatment course among the three phases of 2018-2019 (Figs. 7–8, Table 3), while the percentages in the intensive period of 2020 were the lowest. The sputum conversion rate after 2 months treatment for smear positive patients who had sputum examination was no significant difference among the three phases of 2017-2019, which were similar to that of 2020. The cured rate for smear positive patients who had sputum examination at the end of prescribed treatment course had a totally same trend as the sputum conversion rate after 2 months treatment (Figs. 9–10, Table 4).
Fig. 7.
The percentage of sputum examination within 7 days after 2 months treatment in the period of 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020.
Fig. 8.
The percentage of sputum examination within 7 days after the full treatment course in the period of 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020.
Table 3.
Sputum examination within 7days after 2 months treatment and full treatment course in China, 2017–2020.
| Year | Period | Examination after 2 months treatment | Examination after full treatment course | |||
|---|---|---|---|---|---|---|
| n (%) | p value | n (%) | p value | |||
| 2017 | •• | •• | <0•0001 | •• | <0•0001 | |
| Period 1 | 119293/154437(77•2%) | 106021/168572(62•9%) | ||||
| Period 2 | 109980/146612(75•0%) | 94397/151355(62•4%) | ||||
| Period 3 | 50332/66090(76•2%) | 41235/62018(66•5%) | ||||
| 2018 | •• | •• | 0•4838 | •• | 0•2154 | |
| Period 1 | 110889/152204(72•9%) | 101432/168824(60•1%) | ||||
| Period 2 | 103589/145250(71•3%) | 92006/156417(58•8%) | ||||
| Period 3 | 49948/67826(73•6%) | 34934/57058(61•2%) | ||||
| 2019 | •• | •• | 0•1383 | •• | •0621 | |
| Period 1 | 116371/164580(70•7%) | 98750/173358(57•0%) | ||||
| Period 2 | 100345/145303(69•1%) | 89977/161568(55•7%) | ||||
| Period 3 | 45481/63943(71•1%) | 35122/61433(57•2%) | ||||
| 2020 | •• | •• | <0•0001 | •• | <0•0001 | |
| Control | 94198/136963(68•8%) | 97274/178314(54•6%) | ||||
| Intensive | 70183/116509(60•2%) | 67264/144984(46•4%) | ||||
| Regular | 20684/31917(64•8%) | 26311/52892(49•7%) | ||||
Fig. 9.
Sputum conversion rate after 2 months treatment for smear positive patients in the period of 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020. Sputum conversion rate after 2 months treatment: the result of sputum smear microscopy is converted from positive to negative after 2 months treatment.
Fig. 10.
Cured rate for smear positive patients in the period of 11 weeks before and 15 weeks after the Chinese Spring Festival, 2017–2020. Cured rate: TB patients complete the full treatment course, and the results of two consecutive smears (one is at the end of treatment course) are negative.
Table 4.
Treatment outcome among smear positive TB patients in China, 2017–2020.
| Year | Period | Sputum conversion rate after 2 months treatment | Cured rate | |||
|---|---|---|---|---|---|---|
| n (%) | p value | n (%) | p value | |||
| 2017 | •• | •• | 0•3277 | •• | 0•9080 | |
| Period 1 | 44368/46796(94•8%) | 46977/51677(90•9%) | ||||
| Period 2 | 38162/40485(94•3%) | 43836/48201(90•9%) | ||||
| Period 3 | 16685/17808(93•7%) | 17757/19571(90•7%) | ||||
| 2018 | •• | •• | 0•1602 | •• | 0•2300 | |
| Period 1 | 42605/45375(93•9%) | 46135/52048(88•6%) | ||||
| Period 2 | 37041/39886(92•9%) | 43324/49370(87•8%) | ||||
| Period 3 | 17723/19174(92•4%) | 15691/17925(87•5%) | ||||
| 2019 | •• | •• | 0•1917 | •• | 0•2022 | |
| Period 1 | 41938/44993(93•2%) | 46031/53226(86•5%) | ||||
| Period 2 | 36809/39897(92•3%) | 42570/49568(85•9%) | ||||
| Period 3 | 17293/18828(91•8%) | 15098/17734(85•1%) | ||||
| 2020 | •• | •• | 0•7475 | •• | 0•6159 | |
| Control | 41716/44816(93•1%) | 44252/51998(85•1%) | ||||
| Intensive | 33165/35423(93•6%) | 33862/39992(84•7%) | ||||
| Regular | 8771/9548(91•9%) | 10997/12988(84•7%) | ||||
3.3. Questionnaire results about the impact of COVID-19 on TB control
From the perspective of health providers, the survey results indicated that 75•2% (221/294) of counties reallocated CDC and primary health care workers to fight the COVID-19 epidemic, and 37•8% (111/294) of counties sent the TB laboratory staff to work for COVID-19 testing. 14•6% (43/294) and 13•6% (40/294) of counties were temporarily closed TB outpatient clinics and TB laboratory respectively during the intensive period. 4•4% (13/294) of counties faced a shortage of laboratory reagents for TB, and 8•2%(24/294) of counties experienced a shortage of anti-TB drug during the epidemic.
The survey indicated that TB cases could not easily access to health facilities, due to 84•0% (247/294) and 71•1% (209/294) of counties applied strict intra-county and inter-city travel restrictions. 26•9% (725/2694) of TB patients had postponed or missed going for their follow-up examinations, due to travel restrictions and fear of contracting COVID-19.
4. Discussion
Two previous studies showed that TB is a seasonal disease in China with a predominant spring peak based on monthly notification data [25,26], however, they did not reveal the reason behind the phenomena. In our study, the weekly notification data indicated a sharp drop immediately after the Chinese Spring Festival and a spike soon afterwards, which could explain the spring peak. During the weeklong Spring Festival holiday, most people staying at home with their families, the medical care–seeking behavior of presumptive TB is greatly influenced. Meanwhile, the TB outpatient clinics of many designated hospitals are closed during this time.
However, the trend in 2020 was quite different from that of 2017–2019: the speed of bouncing back to the previous level was much slower than that in previous years, which could be caused by nationwide COVID-19 interventions. The change was similar to other studies based China's provincial surveillance data [27,28]. There are two possible explanations: (1) TB service delivery system disruptions. The presumptive TB tracing and referral was stopped due to the reallocation workers of CDCs and PHCs to fight the COVID-19 epidemic among 75•2% of counties. 14•6% of counties couldn't provide clinical service as they temporarily closed TB outpatient clinics during the intensive period. (2) Lack of access. Due to the majority of counties applied strict inter-city and intra-county travel restriction, presumptive TB had difficulties in visiting health facilities and seeking medical care.
The gold standard for TB diagnosis is to find Mycobacterium tuberculosis in patient clinical samples that include sputum or other specimen [29]. The “13th Five-Year Plan" of the National TB Control Program issued by the Chinese government requires strengthening laboratory construction and ramping up use of molecular biology equipment all over the country to increase the percentage of bacteriologically confirmed TB patients to not less than 50% by 2020 [30]. From 2017 to 2019, this indicator rose gradually significant, which was either indicated the progress of TB control program in China, or likely due to the large sample sizes [31]. By the end of 2019 (the control period of 2020), the percentage had exceeded 50%, but it fell off below 50% after the holiday, which was caused by reduced laboratory capacity, e.g., TB laboratory was closed, TB laboratory staff was transferred for COVID-19 testing, and a shortage of laboratory reagents for TB occurred. In addition, the strict inter-city travel ban adopted by the majority of counties led to decline the number of migrant population, the same as migrant TB patients.
The change trend of diagnosis delay in 2020 was different from the other three years. As some symptoms of TB are similar to COVID-19, the presumptive TB will be treated in priority and diagnosed quickly during the epidemic. In China, all diagnosed TB in the designated hospital will be provided with free anti-TB drug and that's why there were no changes for treatment delay. However, due to the absence of presumptive TB from visiting hospital during the pandemic, the possibly prolonged patients delay remains unknown and needs further monitoring.
In terms of follow-up examinations, there was a clear slump in the intensive period for sputum examination after both two months treatment and full treatment course. This was due to several factors. First, as mentioned above, many counties transferred their TB laboratory to COVID-19 testing and temporarily closed their TB outpatient clinic during the intensive period, leaving TB patients with nowhere to go for sputum examination. Second, some TB patients had postponed or missed their sputum examination in fear of infection with COVID-19 [32], which was similar to the impact of Ebola on TB control [33].
Although a few counties experienced shortage of anti-TB drug during the epidemic, health care workers tried their best to solve the problem and deliver the drugs to patients. Almost all primary health care units were unable to visit TB patients as required to ensure regular medication and treatment adherence, thus TB patients used self-administered therapy or directly observed therapy (DOT) by family members. However, there was no significant difference among the sputum conversion rate of smear positive patients after 2 months treatment in the three periods, as well as the cured rate. This is consistent with previous studies that DOT improves neither TB treatment completion and cured rate nor microbiologic failure and relapse, when it was compared to self-administered therapy [34], [35], [36]. Other studies, however, show a decline in relapse rates with DOT [37,38]. As some patients didn't visit the TB clinic during the epidemic and the treatment outcomes remain unknown, our study only evaluated TB patients who visited the TB clinic. The long-term impact of COVID-19 on treatment adherence, cure rates, incidence and mortality in China still need to be further studied.
5. Conclusion
The impact of COVID-19 on TB control in China can be examined from two sides. Patients were either unable to access medical services due to closures and travel restrictions or hesitated due to concerns about infection with COVID-19. It might be a positive impact on less transmission occurring outside the household and negative impact on more transmission within the household during the lockdown phase. Meanwhile, health service providers working in TB experienced lack of both human resources and laboratory capacity. In the aftermath of the epidemic, there will likely be pent-up demand for TB services, for which it is necessary to prepare. To cope with this future challenge, an emergency response mechanism should be established, including increased human resources, scale up of laboratory testing, active case finding activities, household contact management, enhanced anti-TB drug delivery, and other measures as needed to ensure timely TB diagnosis and treatment for all those who need it.
5.1. Limitations
This study was subject to a few limitations. First, since the timing of the response to COVID-19 was not exactly the same across the country, and the control measures varied by region, the impact on TB control may also differ. This study did not attempt to quantitatively analyze this effect. Second, staying at home during the epidemic may have led to increased close contact among family members of TB patients. Health service disruption may lead to increase TB incidence and mortality. The long-term impact of COVID-19 on TB control, which needs further study, will surface beyond the time frame of this study and cannot be evaluated in this paper. Third, given that the incidence of multidrug-resistant TB (MDR-TB) is not high in China, and the number of MDR-TB patients is not large, this study did not analyze the specific impact of the COVID-19 outbreak on MDR-TB diagnosis and control.
CRediT authorship contribution statement
Huang Fei: Conceptualization, Visualization, Formal analysis, Writing - original draft. Xia Yinyin: Conceptualization, Visualization, Formal analysis, Writing - original draft. Chen Hui: Conceptualization, Data curation, Writing - original draft. Wang Ni: Conceptualization, Data curation, Writing - original draft. Du Xin: Conceptualization, Data curation. Chen Wei: Conceptualization, Data curation. Li Tao: Conceptualization, Data curation, Writing - original draft. Huan Shitong: Conceptualization, Data curation. Sun Miaomiao: Conceptualization, Data curation, Writing - original draft. Chen Mingting: Conceptualization, Data curation. Salmaan Keshavjee: Conceptualization, Visualization, Supervision. Zhao Yanlin: Conceptualization, Visualization, Writing - original draft, Supervision. Daniel P. Chin: Conceptualization, Visualization, Supervision. Liu Jianjun: Conceptualization, Supervision.
Declaration of Competing Interest
The authors have no competing interests.
Acknowledgments
Data sharing
Data sharing requests will be considered by China CDC upon written request to the corresponding author. Aggregated patient data will be available subject to a written proposal and a signed data sharing agreement.
Acknowledgments
This work was supported by the National Health Commission of China–Bill & Melinda Gates Foundation TB Collaboration project (OPP1137180).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2020.100032.
Contributor Information
Zhao Yanlin, Email: zhaoyl@chinacdc.cn.
Daniel P. Chin, Email: daniel.chin@gatesfoundation.org.
Liu Jianjun, Email: liujj@chinacdc.cn.
Appendix. Supplementary materials
References
- 1.World Health Organization. Coronavirus disease (COVID-19) pandemic. https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed August 112020).
- 2.Li Q, Guan X, Wu P, Wang X, Zhou L, Tong Y. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hunter P. The spread of the COVID-19 coronavirus: health agencies worldwide prepare for the seemingly inevitability of the COVID-19 coronavirus becoming endemic. EMBO Rep. 2020;21(4):e50334. doi: 10.15252/embr.202050334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao S, Lin Q, Ran J, Musa SS, Yang G, Wang W. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int J Infect Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wong JEL, Leo YS, Tan CC. COVID-19 in Singapore-current experience: critical global issues that require attention and action. JAMA. 2020;323(13):1243–1244. doi: 10.1001/jama.2020.2467. [DOI] [PubMed] [Google Scholar]
- 6.Burdorf A, Porru F, Rugulies R. The COVID-19 (Coronavirus) pandemic: consequences for occupational health. Scand J Work Environ Health. 2020;46(3):229–230. doi: 10.5271/sjweh.3893. [DOI] [PubMed] [Google Scholar]
- 7.Adepoju P. Tuberculosis and HIV responses threatened by COVID-19. Lancet HIV. 2020;7(5):e319–e320. doi: 10.1016/S2352-3018(20)30109-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization . World Health Organization; Geneva: 2019. Global tuberculosis report 2019. [Google Scholar]
- 9.United Nations. Sustainable development goals. https://sustainabledevelopment.un.org/topics/sustainabledevelopmentgoals (accessed May 302020).
- 10.Alagna R, Besozzi G, Codecasa LR, Gori A, Migliori GB, Raviglione M. Celebrating world tuberculosis day at the time of COVID-19. Eur Respir J. 2020;55(4) doi: 10.1183/13993003.00650-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wingfield T, Cuevas LE, MacPherson P, Millington KA, Squire SB. Tackling two pandemics: a plea on world tuberculosis day. Lancet Respir Med. 2020;8(6):536–538. doi: 10.1016/S2213-2600(20)30151-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hogan AB, Jewell BL, Sherrard-Smith E, Vesga JF, Watson OJ, Whittaker C. Potential impact of the COVID-19 pandemic on HIV, tuberculosis, and malaria in low-income and middle-income countries: a modelling study. Lancet Glob Health. 2020;8(9):e1132–e1141. doi: 10.1016/S2214-109X(20)30288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McQuaid CF, McCreesh N, Read JM, Sumner T, Houben R, White RG. The potential impact of COVID-19-related disruption on tuberculosis burden. Eur Respir J. 2020;56(2):2001718. doi: 10.1183/13993003.01718-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stop-TB-Partnership. The potential impact of the COVID-19 response on tuberculosis in high-burden countries: a modelling analysis. 2020. http://www.stoptb.org/assets/documents/news/Modeling%20Report_1%20May%202020_FINAL.pdf (accessed Jun 14 2020).
- 15.Buonsenso D, Iodice F, Sorba Biala J, Goletti D. COVID-19 effects on tuberculosis care in Sierra Leone. Pulmonology. 2020;S2531-0437(20):30130–30136. doi: 10.1016/j.pulmoe.2020.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai CC, Yu WL. The COVID-19 pandemic and tuberculosis in Taiwan. J Infect. 2020;81(2):e159–ee61. doi: 10.1016/j.jinf.2020.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Institute for Communicable Diseases, South Affria. Impact of COVID-19 intervention on TB testing in South Africa. https://www.nicd.ac.za/wp-content/uploads/2020/05/Impact-of-Covid-19-interventions-on-TB-testing-in-South-Africa-10-May-2020.pdf (Accessed August 11, 2020)
- 18.Pan A, Liu L, Wang C, Guo H, Hao X, Wang Q. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA. 2020;323(19):1915–1923. doi: 10.1001/jama.2020.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian H, Liu Y, Li Y, Wu CH, Chen B, Kraemer MUG. An investigation of transmission control measures during the first 50 days of the COVID-19 epidemic in China. Science. 2020;368(6491):638–642. doi: 10.1126/science.abb6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Litvinova M, Liang Y, Wang Y, Wang W, Zhao S. et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science. 2020;368(6498):1481–1486. doi: 10.1126/science.abb8001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L, Zhang H, Ruan Y, Chin DP, Xia Y, Cheng S. Tuberculosis prevalence in China, 1990-2010; a longitudinal analysis of national survey data. Lancet. 2014;383(9934):2057–2064. doi: 10.1016/S0140-6736(13)62639-2. [DOI] [PubMed] [Google Scholar]
- 22.National Health Commission of the People's Republic of China . Beijing: National Health Commission; 2019. Action plan to stop TB (2019-2022) [Google Scholar]
- 23.Huang F, Cheng S, Du X, Chen W, Scano F, Falzon D. Electronic recording and reporting system for tuberculosis in China: experience and opportunities. J Am Med Inform Assoc. 2014;21(5):938–941. doi: 10.1136/amiajnl-2013-002001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Jiang W, Liu Y, Zhang L, Zhu A, Tang S. Transforming tuberculosis (TB) service delivery model in China: issues and challenges for health workforce. Hum Resour Health. 2019;17(1):83. doi: 10.1186/s12960-019-0420-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Tian CW, Wang WM, Luo XM. Time-series analysis of tuberculosis from 2005 to 2017 in China. Epidemiol Infect. 2018;146(8):935–939. doi: 10.1017/S0950268818001115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang X, Duan Q, Wang J, Zhang Z, Jiang G. Seasonal variation of newly notified pulmonary tuberculosis cases from 2004 to 2013 in Wuhan, China. PLoS One. 2014;9(10) doi: 10.1371/journal.pone.0108369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu Z, Chen J, Xia Z, Pan Q, Yuan Z, Zhang W, Shen X. Impact of the COVID-19 pandemic on the detection of TB in Shanghai, China.(Accessed September 8, 2020) [DOI] [PubMed]
- 28.Liu Q, Lu P, Shen Y, Li C, Wang J, Zhu L. Collateral impact of the Covid-19 pandemic on tuberculosis control in Jiangsu Province, China. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng T, Cheng Y, Yu S, Jiang F, Su M, Chen J. A clinical TB detection method based on molecular typing technique with quality control. Comput Math Methods Med. 2019;2019 doi: 10.1155/2019/9872425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Office of the State Council, People's Republic of China . Beijing: National TB Control Program; 2017. "Thirteenth five-year plan" national TB control programme. [Google Scholar]
- 31.Thiese MS, Ronna B, Ott U. P value interpretations and considerations. J Thorac Dis. 2016;8(9):E928–EE31. doi: 10.21037/jtd.2016.08.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen H, Zhang K. Insight into impact of COVID-19 epidemic on tuberculosis burden in China. Eur Respir J. 2020;2002710 doi: 10.1183/13993003.02710-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Desta KT, Kessely DB, Daboi JG. Evaluation of the performance of the national tuberculosis program of Liberia during the 2014-2015 Ebola outbreak. BMC Public Health. 2019;19(1):1221. doi: 10.1186/s12889-019-7574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karumbi J, Garner P. Directly observed therapy for treating tuberculosis. Cochrane Database Syst Rev. 2015;5 doi: 10.1002/14651858.CD003343.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pasipanodya JG, Gumbo T. A meta-analysis of self-administered vs directly observed therapy effect on microbiologic failure, relapse, and acquired drug resistance in tuberculosis patients. Clin Infect Dis. 2013;57(1):21–31. doi: 10.1093/cid/cit167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chien JY, Lai CC, Tan CK, Chien ST, Yu CJ, Hsueh PR. Decline in rates of acquired multidrug-resistant tuberculosis after implementation of the directly observed therapy, short course (DOTS) and DOTS-Plus programmes in Taiwan. J Antimicrob Chemother. 2013;68(8):1910–1916. doi: 10.1093/jac/dkt103. [DOI] [PubMed] [Google Scholar]
- 37.Weis SE, Slocum PC, Blais FX, King B, Nunn M, Matney GB. The effect of directly observed therapy on the rates of drug resistance and relapse in tuberculosis. N Engl J Med. 1994;330(17):1179–1184. doi: 10.1056/NEJM199404283301702. [DOI] [PubMed] [Google Scholar]
- 38.Balasubramanian VN, Oommen K, Samuel R. DOT or not? Direct observation of anti-tuberculosis treatment and patient outcomes, Kerala State, India. Int J Tuberc Lung Dis. 2000;4(5):409–413. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.