Abstract
Objective
We used resting-state functional connectivity (rsFC) to evaluate the integrity of the neural circuits associated with primary and secondary rewards in bipolar disorder (BD) with different mood phases.
Methods
Sixty patients with BD [21 patients with depressive episode of BD (BDD) and 41 patients with maniac episode of BD (BDM)] and 42 healthy controls (HCs) underwent resting-state functional magnetic resonance imaging. rsFC was assessed using region of interest-wise analyses.
Results
Attenuation of rsFC at the orbitofrontal cortex (OFC) and the left ventral striatum (LVS) was observed in the secondary reward circuit of patients with BD compared to that of HCs. Among BDD, BDM and HCs, the rsFC between OFC and LVS in BDM was intermediate, while the rsFC between OFC and right ventral striatum/right amygdala in BDM was the highest; the corresponding rsFC values in BDD were the lowest. Furthermore, a positive correlation was found between rsFC and Young Mania Rating Scale scores in BDM.
Conclusions
This study suggests that there may be an abnormal rsFC between OFC and LVS in the second reward of patients with BD and the discrepant patterns of rsFC may exist between different mood states in patients with BD.
Keywords: bipolar disorder, primary reward circuit, secondary reward circuit, resting state, functional connectivity
Introduction
Bipolar disorder (BD) is considered as a relapsing–remitting condition with episodes of melancholic lows and inertia (depressive episodes) that are explicitly separated from dizzying and capricious highs (manic episodes) and interspersed with remission. The aggregate lifetime prevalence of bipolar spectrum is up to 2.4% (Merikangas et al., 2011). There are many controversies about the pathophysiology of BD. A particular important question that has not been well addressed yet is the neurobiology mechanisms that drive patients with BD to present extreme and opposite emotional shifts over time. A prominent theory proposes that BD may be caused by the dysregulation of a ‘behavioral activation system’, in which temporary increases result in mania and temporary decreases result in depression (Urosevic et al., 2008). Some studies suggest that this system is particularly relevant to the reward circuit, also known as the mesocorticolimbic circuit (Dutra et al., 2017; Jimenez et al., 2018).
The prospect of rewards drives much of our daily life. Rewards are traditionally categorized as primary and secondary. Primary rewards have an innate value and directly relate to survival and reproduction such as food and sex. In contrast, secondary rewards are not essential for maintenance of homeostasis and only gain value through learned association with lower level rewards, including money or power. Both animal and human neuroimaging studies suggest that primary and secondary rewards may be represented phylogenetically in distinct brain regions (Schultz, 2006; Hikosaka et al., 2008). A meta-analysis showed that the right anterior orbitofrontal cortex (OFC) and the bilateral ventral striatum were more likely to be activated by monetary rewards rather than food and erotic rewards (Sescousse et al., 2013). In contrast, it appeared that the somatosensory cortex and the dorsal anterior insula were more likely to be activated by food compared to monetary and erotic rewards. Finally, the brain areas more robustly activated by erotic rather than by monetary and food rewards were located in the ventral anterior insula and the bilateral amygdala.
Neuroimaging is an ideal tool that can help us visualize cerebral activity throughout the whole brain. The number of neuroimaging studies focusing on regional brain activation during primary reward tasks in BD is considerably smaller compared to those related to secondary reward tasks. Linke et al. (2012) reported increased activation in the left medial OFC and amygdala among remitted individuals with BD compared to HCs during a task, in which participants could risk monetary gain or loss by playing in a trial or pass to the next trial without gain or loss. Using a similar task, Caseras et al. (2013) proved a greater bilateral ventral striatal activity in euthymic BD than in healthy subjects. In a socially rewarding stimulus (happy or sad face), elevated ventral striatal activity and left amygdala activity have also been observed in patients with BD (Lawrence et al., 2004; Almeida et al., 2010). Therefore, in addition to some reports (Schreiter et al., 2016), it is likely that in patients with BD abnormalities exist within the prefrontal cortex and subcortical structures such as the striatum and the amygdala.
While these data are fundamental, the discrepancy among findings regarding changes in local activation also suggests that the abnormalities may lie at a circuit level rather than in localized brain regions, since these regions do not operate independently but communicate through multiple pathways between different parts of the circuit (Sporns, 2013). Therefore, a vital next step is to begin to explore the resting-state functional connectivity (rsFC) between regions or structure simplicated in BD that may be coupled to neuronal activity and are not caused by physiological effects originating from the task paradigms (Biswal et al., 1995; Salvador et al., 2005). Brady et al. (2016) conducted a whole-brain analysis of rsFC to test the hypothesis that bipolar mania is associated with altered connectivity between cortical regions and subcortical structures, such as the amygdala and striatum, and demonstrated that patients in the manic state showed a disrupted functional connectivity between brain regions involved in the regulation of emotion and the amygdala.
Taken together, we hypothesized that the rsFC of the neural circuit associated with reward presented abnormalities in patients with BD. In addition, distinct brain regions are implicated in primary and secondary rewards, and previous studies found that both neural circuits associated with primary and secondary rewards appeared abnormalities in patients with schizophrenia, while only neural circuits associated with secondary reward were shown to be abnormal in patients with BD and few studies reported about neural circuits associated with primary reward in patients with BD (Grimm et al., 2012; de Leeuw et al., 2015; Dutra et al., 2015, 2017). So, we hypothesized that the abnormality of rsFC in patients with BD was different in neural circuits associated with primary and secondary rewards. It is also important to note that the functional connectivity patterns across mood symptomatology within different diagnostic categories (i.e. BDD vs BDM) may be completely different. Man et al. (Man et al., 2018) delineated that a distinguished amygdala–striatum connectivity profile was driven by the current mood phase of the participant, where BDD was differentiated from BDM with a well-validated passive picture-viewing task. Therefore, in this study, we examined the hypothesis that mood stages arise from the rsFC in neural circuits associated with primary and secondary rewards.
Methods
Participants
Sixty-two inpatients with BD (36 men and 26 women) were recruited from the Psychiatric Hospital of Zhumadian (a Zhumadian city-owned psychiatric hospital, Henan Province, China). All patients were Han Chinese and right-handed. Patients met the Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition (DSM-IV) diagnosis of BD based on the Structured Clinical Interview (American Psychiatric Association, 1996), which was confirmed by two psychiatrists. The Young Mania Rating Scale (YMRS), 17-items Hamilton Depression Scale (HAMD-17), Hamilton Anxiety Scale (HAS) and the Positive and Negative Syndrome Scale were used to assess the severity of patients’ symptoms. The consistency of assessment on these scales between psychiatrists was greater than 0.89. Forty-one BD patients met the DSM-IV criteria for hypomania (≥4 days) or mania (≥7 days) and were included in a manic group (manic episode of BD, BDM). According to Wood et al. (1995)) and Rich et al. (2008), the manic state is defined by YMRS > 26; the hypomanic state by YMRS > 12 but <18. Twenty-one BD patients were in a depressed mood state (evaluated as a HAMD-17 ≥ 18), and gave a history of clear-cut episodes of mania or hypomania. These patients were recruited in a BD depression group (bipolar disorder depression episode, BDD). Details about patients’ demographic characteristics and clinical information are presented in Table 1. Exclusion criteria included diagnoses of schizophrenia, mental retardation, dementia and other cognitive disorders, history of head injury that resulted in loss of consciousness, cardiovascular or neurological disease, magnetic resonance imaging (MRI) contraindications, such as claustrophobia and mental implants, or meeting the DSM-IV criteria for substance dependence.
Table 1.
Demographic and clinical characteristics of patients with BD and controls
| BDD (n = 21) | BDM (n = 41) | Controls (n = 42) | t/F/χ2 | P | |
|---|---|---|---|---|---|
| Sex (male/female) | 12/9 | 24/1 | 22/20 | 2.37 | 0.306 |
| age | 28.29 ± 6.69 | 28.06 ± 6.65 | 31.7 ± 6.65 | 3.68 | 0.028 |
| Years of education (years) | 11.14 ± 3.06 | 10.14 ± 2.96 | 14.19 ± 2.97 | 19.72 | 0.000 |
| Age at onset (years) | 25.05 ± 8.17 | 22.89 ± 6.81 | - | 0.99 | 0.326 |
| Duration of illness (years) | 5.37 ± 4.24 | 5.10 ± 5.00 | - | -0.95 | 0.345 |
| Number of manic episodes | 1.21 ± 0.70 | 2.95 ± 1.57 | - | -5.77 | 0.000 |
| Number of depression episodes | 2.50 ± 1.83 | 1.29 ± 0.93 | - | 2.36 | 0.031 |
| Mood stabilizers, n (%) | 20 (95.2%) | 41 (100%) | - | 0.13 | 0.83 |
| Antidepressants, n (%) | 21 (100%) | 0 | - | 24.41 | 0.000 |
| Antipsychotics, n (%) | 8 (38.1%) | 22 (53.7%) | - | 1.35 | 0.246 |
| YMRS | 1.90 ± 1.04 | 27.80 ± 7.41 | - | -17.09 | 0.000 |
| HAS | 20.71 ± 8.65 | 2.91 ± 2.83 | - | 19.86 | 0.000 |
| HAMD-17 | 24.43 ± 6.03 | 5.04 ± 3.93 | - | 11.10 | 0.000 |
| Medication | |||||
| Antipsychotics, n (%) | 8 (38.1) | 31 (75.6) | |||
| Lithium, n (%) | 9 (42.9) | 23 (56.1) | |||
| Valproate, n (%) | 11 (52.4) | 18 (43.9) | |||
| Lamotrigine, n (%) | 1 (4.7) | - |
Forty-two HCs (22 men and 20 women) were also recruited from the local community. All were Han Chinese and right-handed from the Zhumadian area. All participants were in good physical health, and none of them had any personal or family history of (or demonstrated any symptoms of) a clinical psychiatric disorder.
All participants gave written informed consent approved by the Institutional Review Board of the Psychiatric Hospital of Zhumadian that authorized this research project (No.160401033). A detailed questionnaire including sociodemographic characteristics, general information and medical and psychological conditions was administered to each participant by a member of the research staff. Additional information was collected from available medical records.
Image acquisition
All MRI examinations were performed using a GE Signa HDxT 3.0T MRI scanner (GE Medical Systems, LLC, USA). The participants were placed in a birdcage head coil and individually fitted to a bite bar partially composed of dental impression compound attached to the coil to reduce head motion. Conventional T1W1 and T2W1 were performed to rule out structural abnormalities. For the resting-state scan, participants were asked to keep their eyes closed, stay awake and not think of anything in particular. Resting-state functional MRI (fMRI) data were acquired using the echo planar imaging (EPI) sequence as follows: repetition time (TR)/echo time (TE) = 2000/30 ms, slice number = 33, thickness = 3 mm, matrix size = 64 × 64, field of view (FOV) = 210 × 210 mm2, flip angle = 90° and 210 volumes (7 min). For spatial normalization and registration purposes, corresponding high-resolution anatomical T1-weighted images were obtained covering the whole brain with sagittal 3D-MPRAGE (magnetization prepared rapid acquisition gradient echo) sequence: TR/TE = 1600/2.5 ms, thickness = 1 mm, matrix size = 512 × 448, FOV = 224 × 256 mm2, flip angle = 7° and 176 volumes. An experienced neuroradiologist screened all MRI scans for pathological radiological indications and artifacts.
Preprocessing of resting-state fMRI data
Resting blood oxygen level-dependent data preprocessing was performed using the Data Processing Assistant for Resting-State fMRI (http://rfmri.org/DPARSF) (Yan et al., 2009). All software programs were run based on Statistical Parametric Mapping 8 (SPM8, http://www.fil.ion.ucl.ac.uk/spm/) and REST software (http://www.restfmri.net) on the MATLAB platform (The MathWorks, Natick, MA, USA).
For each participant, the first 10 volumes were discarded for reducing magnetization disequilibrium and participants’ adaptation to the scanning noise, followed by slice timing with the 17th slice as the reference; spatial realignment for head motion correction (mean head motion, FD Jenk) exceeding 0.2 mm/degree was excluded. One patient in BDM group was excluded from statistical analysis. The number of outlier volumes did not differ significantly between the two groups (t1,102 = 0.019, P = 0.83). Both groups did not show any difference in the composite average (t1,102 = 1.33, P = 0.57) and maximum measures of head motion (t1,102 = 0.59, P = 0.69). fMRI images of each patient were then registered to their segmented high-resolution T1-weighted anatomical images, regressing nuisance variables, including white matter and cerebral spinal fluid signals; normalizing fMRI images to the standard of the Montreal Neurological Institute (MNI) EPI template with a resampled resolution of 3 × 3 × 3 mm3 and spatial smoothing with an isotropic 6 mm full-width at half-maximum of Gaussian kernel. Finally, detrending and temporal band-pass filtering (0.01–0.08 Hz) were performed to remove respiratory and cardiovascular noise.
Functional connectivity analysis
Although the basic anatomy of structures of the reward circuit that we call as the ‘common reward circuit’ are well established (Everitt and Robbins, 2005; Haber and Knutson, 2010), neuroimaging studies suggest that primary and secondary rewards may be represented in phylogenetically distinct brain regions (Schultz, 2000; Knutson et al., 2007). A previous meta-analysis study (Sescousse et al., 2013) identified ‘reward type-specific’ regions that were defined as those more reliably and robustly activated by one reward compared to the other. Based on its results, we investigated the following regions as spherical regions of interest (ROIs) for primary (erotic) reward: left amygdala (MNI peak coordinates x, y and z: −22, −4 and −18), right amygdala (22, −6 and −16), right nucleus accumbens (4, 10 and −8), left anterior insula (−38, 14 and −12), right anterior insula (34, 10 and −6) and ventromedial prefrontal cortex (vmPFC) (0, 38 and 8). Additionally, the following regions were chosen as ROIs for secondary (monetary) reward: left ventral striatum (LVS, −14, 10 and −12), right ventral striatum (14, 10 and −8), right amygdale (24, −2 and −18), right posterior ventrolateral thalamus (22, −24 and −8) and anterior OFC (12, 48 and −22). A spherical ROI centered on the coordinates with a radius of 6 mm was defined. The size of the ROIs was defined on the basis of previous studies (Lin et al., 2015; Duff et al., 2018) (see Figure 1). In primary or secondary reward, each ROI was visually inspected to avoid overlap and to assure localization within anatomical boundaries. Subsequently, we extracted the time courses of each ROI by averaging the time courses of all voxels within the ROI. The correlation coefficients (CCs) between every two ROIs were calculated and then transformed in Z-score using Fisher’s z transformation.
Fig. 1.
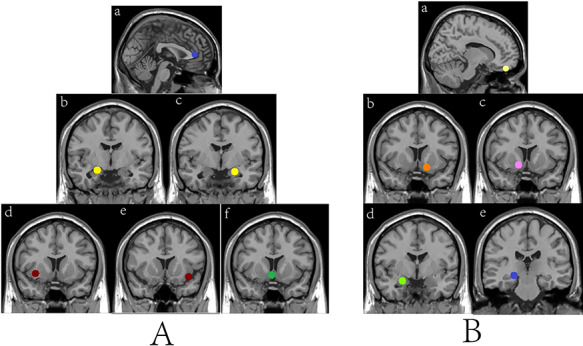
ROIs for the primary reward circuit (A) and secondary reward circuit (B). In (A), (a) left ventromedial prefrontal cortex, (b) right amygdala, (c) left amygdala, (d) right anterior insula, (e) left anterior insula and (f) right nucleus accumbens are shown. In (B), (a) anterior orbitofrontal cortex, (b) LVS, (c) right ventral striatum, (d) right amygdala and (e) right posterior ventrolateral thalamus are shown. ROIs were defined as 6-mm radius spheres.
Statistical analysis
Statistical analyses were conducted using the SPSS software (Version 20.0). The BrainNet Viewer was used for the visualization of the neuroimaging results (https://www.nitrc.org/projects/bnv).
Group differences in demographic and clinical characteristics
Sixty-two patients with BD and 42 HCs were enrolled in the present study. Patients with BD were sub-grouped into BDD and BDM based on their clinical features. Demographic features were compared among BDD, BDM and normal controls using a one-way analysis of variance followed by a least significant difference test for continuous variables and a chi-square test for categorical variables. Clinical characteristics were compared between BDD and BDM using a Student’s two-sample t-test.
Group differences in ROI-wise rsFC
An analysis of covariance was used to compare the group differences in ROI-wise rsFC data between HCs and BD or among HCs, BDD and BDM, where the rsFC between every two ROIs was dependent variable and the group (between HCs and BD or among HCs, BDD and BDM) was fixed factor with age, sex, years of education and head motion as covariates. Post hoc group contrasts comparing BDD, BDM and HCs were adjusted using the Bonferroni post hoc correction. Tests were two-tailed and the difference was considered significant when an adjusted P-value was less than 0.05 after Bonferroni correction for multiple comparisons. The adjusted P-value was calculated as uncorrected P-value × the test number for every two ROIs rsFC. For example, the test number was 15 for the primary reward, since six ROIs were investigated for the primary reward. The test number was 10 for the second reward as five ROIs were investigated for the second reward.
Correlation analysis
Only those variables that showed statistically significant group differences in ROI-wise rsFC were included in the following analysis. Pearson product–moment CCs were used to examine the correlation between rsFC and clinical symptoms. Bonferroni correction was used to correct for multiple comparisons. Results were considered significant when the adjusted P-value <0.05.
Results
Demographic characteristics
Demographic and clinical characteristics are summarized in Table 1. HCs were significantly older (F2,103 = 3.68, P = 0.028) and had significantly more years of education than both BDD and BDM (F2,103 = 9.72, P = 0.000). There were no significant differences between BDD and BDM on age and years of education (P > 0.05). However, the number of manic episodes (t1,61 = −5.77, P = 0.000), number of depression episodes (t1,61 = 2.36, P = 0.031) and all symptoms scales, including YMRS (t1,61 = −17.09, P = 0.000), HAS (t1,61 = 19.86, P = 0.000) and HAMD-17 (t1,61 = 11.10, P = 0.000), were significantly different between BDD and BDM. All patients in both BDD and BDM groups were under medication. The two bipolar groups did not differ in frequency of mood stabilizers (χ21,61 = 0.13, P = 0.83) and antipsychotics (χ21,61 = 1.35, P = 0.246).
Differences in ROI-wise rsFC between HCs and BD
In the primary reward circuit, a univariate analysis showed the strength of rsFC at vmPFC and left amygdala was significantly lower in BD than HCs (F1,100 = 4.38, P = 0.026), while no significant difference was found in rsFC after Bonferroni correction (adjusted P > 0.05). No other difference in rsFC was observed between BD and HCs for ROIs in the primary reward circuit.
In the secondary reward circuit, univariate analysis indicated that there was a significant group difference in the strength of rsFC between BD and HCs at the OFC and the LVS after Bonferroni correction (F1,100 = 9.46, adjusted P = 0.018), which was significantly lower in the BD group compared to HCs (Figure 2). No other difference in rsFC was observed between BD and HCs for ROIs in the secondary reward circuit.
Fig. 2.
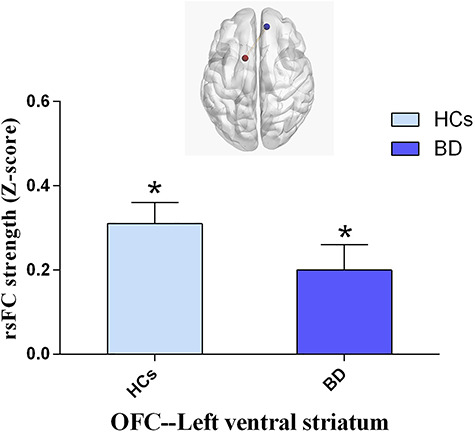
Group comparisons of functional connectivity between BD (n = 62) and HCs (n = 42). *P < 0.05, compared to HCs.
Differences in ROI-wise rsFC among HCs, BDD and BDM
In the primary reward circuit, there was no significant group difference in the strength of rsFC between ROIs after Bonferroni correction (all adjusted P > 0.05).
In the secondary reward circuit, the univariate analysis for rsFC revealed significant group differences (among HCs, BDD and BDM) in the rsFC between the OFC and the LVS (F2,99 = 9.48, adjusted P = 0.017), OFC and right ventral striatum (F2,99 = 7.45, adjusted P = 0.031), OFC and right amygdale (F2,99 = 5.11, adjusted P = 0.039) and LVS and right amygdala (F2,99 = 4.27, adjusted P = 0.038) after Bonferroni correction. Bonferroni post hoc tests displayed that the strength of rsFC between the OFC and the LVS in HCs was significantly higher than in BDD (P = 0.025); the strength of rsFC in BDM was intermediate between that in HCs and BDD, while the difference was not statistically significant (P > 0.05). Between the OFC and the right ventral striatum, the strength of rsFC in BDM was significantly higher than that in HCs (P = 0.021) and BDD (P = 0.014); the strength of rsFC in BDD, although lower than that in HCs, was not significantly different, showing a similar pattern to the strength of rsFC between the OFC (P = 0.006) and the right amygdala (P = 0.034). Between the LVS and the right amygdala, the strength of rsFC in BDM was significantly higher than that in BDD (P = 0.008), while there was no significant difference compared with that in HCs. Post hoc group contrasts among HCs, BDD and BDM are summarized in Figure 3.
Fig. 3.
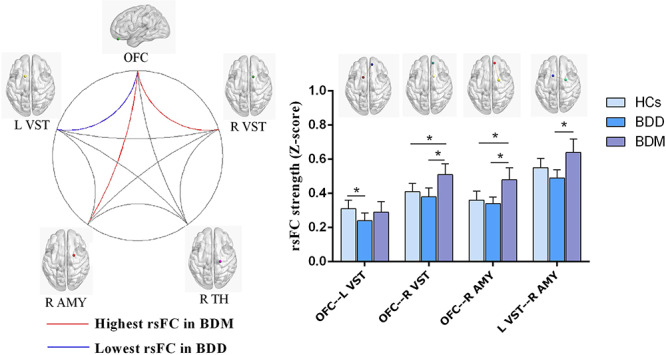
Group comparisons of functional connectivity among HCs (n = 42), BDD (n = 21) and BDM (n = 41). L, left; VST, ventral striatum; R, right; AMY, amygdala; TH, thalamus. *P < 0.05.
Correlation analysis between rsFC and clinical symptoms
We then studied the relationships We then studied the relationships between rsFCs and the scores of YMRS, HAS and HAMD-17. Results revealed significant differences among HCs, BDD and BDM. rsFC and the scores of YMRS showed a significant positive correlation between the OFC and the right ventral striatum (r = 0.44, P = 0.001, adjusted P = 0.012) in the BDM group (Figure 4). After controlling for age, sex, years of education and head motion, the correlation remained significant (r = 0.46, P = 0.001, adjusted P = 0.012). Additional correlation results are presented in Supplementary Table S1.
Fig. 4.
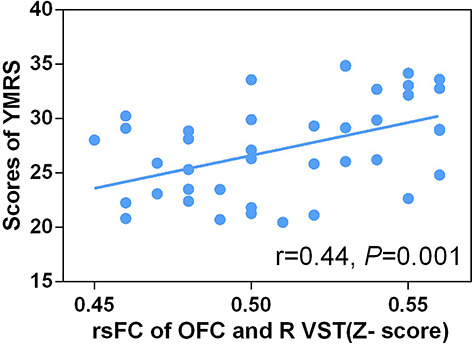
The relationship between the scores of YMRS and functional connectivity of OFC and R VST in BDM (n = 41).
Discussion
This study presents three major findings: (i) the strength of rsFC at the OFC and the LVS in BD was decreased only in the secondary reward circuit; (ii) in the secondary reward circuit, rsFC between the OFC and the LVS in BDM was intermediate between that of HCs and BDD; the rsFC between OFC and right ventral striatum/right amygdala in BDM was the highest among the three groups; these rsFCs in BDD were the lowest among the three groups; (iii) in BDM, the rsFC showed a positive correlation with the scores of YMRS between the OFC and the right ventral striatum. To the best of our knowledge, this study is the first to examine the neural circuits associated with primary and secondary rewards using resting-state correlates of BD by differing mood stages. Provided this observation is replicated in the future studies, rsFC of neural circuits associated with primary and secondary rewards in patients with BD could be a potential marker that can be investigated in conjunction with relapse signatures such as minor mood fluctuations and sleep disturbance to predict changes in ongoing mood states.
Our finding that the rsFC within the neural circuit associated with secondary reward, which primarily involves the ventral striatum, amygdala, thalamus and OFC, was lower in BD than HCs was consistent with our hypothesis: the rsFC of reward circuit presents an abnormality in BD not detected in HCs and this abnormality is different between neural circuits associated with primary and secondary rewards. The reward circuit is a complex neural network forming the basis of evaluating the possible results of different choices effectively. Although the rsFC is not a direct reflex of brain activation during task performance, it provides complementary insights into brain function. Therefore, a lower rsFC may compensate a greater cerebral flow induced by a reward-related task (Dutra et al., 2015) and the increased amplitude of low frequency fluctuations observed within the cortical and subcortical limbic system in BD (Xu et al., 2014). Another possibility is that the relationship between task performance and resting state in the human brain is dissociated or unrelated. Theoretically, resting state may reflect a spontaneous brain activity non-specific to task, whereas task performance may reflect more reactivity to task stimulus. The results of some studies support this view. For example, the research by Damme et al. (2019) examining both a task-based and rsFC in individuals at clinical high risk for psychosis demonstrated that there were distinct and opposite profiles of functional connectivity during self-reference task and resting state. Another study of Whitfield-Gabrieli et al. (2011) enrolled HCs indicated a similar conclusion.
Similar rsFC changes in BD have been found in previous studies. Altinay et al. (2018) (Anand et al., 2009) reported decreased rsFC between OFC and striatum in unmedicated BD patients compared to HCs, which can be elevated after 8 weeks of lithium monotherapy. Using the same ROIs, Shi et al. (2018) found nearly universally lower rsFC strength within the reward circuit among manifested BD compared to matched HCs and unipolar depression, suggesting that BD may differ from unipolar depression in the reward circuit at the resting state. Another study, employing a within- and between-subjects design utilizing a monetary and social incentive delay task among BD and HCs during fMRI, observed that decreased FC between OFC and VST was associated with consideration of behavioral alternatives after omission of expected rewards (Dutra et al., 2017). Consistent with these studies, the lower rsFC in the reward circuit observed in the current study suggests that BD may be driven by the dysfunction of the reward circuit.
It is also noteworthy that some studies have reported enhanced FC between the cortical area and limbic system or indistinguishable FC in patients with BD compared to HCs (Wessa et al., 2014; Damme et al., 2017). Previous discrepancies may be caused by many factors: demographic characteristics of BD samples, such as age and sex, may be a source of heterogeneity across studies; differences in MRI acquisition protocols and processing; differences in selection of ROIs and statistical analyses performed. More importantly, the clinical characteristics of patients with BD further complicate the interpretation of previous studies. For example, a systematic review on rsFC in individuals with BD depicted that patients in mania showed higher amygdala hyperconnectivity than those in bipolar depression (Syan et al., 2018), while Syan et al. (2017) found no differences in the rsFC of the amygdala between euthymic patients with BD and HCs.
As mentioned above, we expected that functional connectivity patterns were different between mood stages; in other words, functional connectivity would depend on mood symptomatology. Our subgroup analysis based on the clinical phases of the BD group revealed that in BDM, the rsFC between OFC and LVS was lower than that in HCs, while rsFC between OFC and right ventral striatum was stronger than that of HCs. Previous studies have reported similar contrasting patterns of rsFC in mania and in euthymia or in depression (Brady et al., 2016). This result suggested that mood states in BD may be related to frontal cortex abnormality in hemispheric lateralization. Previous studies found that depressive state in BD was associated with increased left orbital frontal cortical activation (Liu et al., 2012) and increased left amygdala–OFC functional connectivity (Versace et al., 2010), suggesting that a depressive episode may present abnormalities on the left side. Meanwhile, another study supported that abnormality on the right side was characteristic feature of manic state in BD (Hulvershorn et al., 2012). These are consistent with our result. Although there were some divergent reports (Li et al., 2015; Wei et al., 2017), our finding can be considered to further confirm that the mood asymmetry may exist to a certain extent. Anatomically, the ventral striatum is located at the junction of multiple corticobasal ganglion loops involving limbic, sensorimotor and associative functions (Haber and Knutson, 2010). The ventral striatum is part of the limbic loop and receives many projections from the OFC, midbrain and ACC. Hence, it is an ideal place to integrate cognitive, motor and emotional information and to influence goal-oriented behavior independently of reward (Delgado, 2007; Haber and Knutson, 2010). As early as 1992, there was experimental evidence (Tomarken et al., 1992) supporting that greater left hemisphere activity was associated with positive emotion, whereas more right hemisphere activity was associated with negative emotion, suggesting that the left and right hemispheres were responsible for processing positive and negative emotions, respectively. This hypothesis, known as the ‘affective valence hypothesis’, is also supported by recent studies based on electroencephalogram, MRI or positron emission tomography scans (Kop et al., 2011; Poole and Gable, 2014; Quaedflieg et al., 2016). We, therefore, speculate that greater rsFC between the OFC and the right ventral striatum in BDM may hint excessive control from the frontal cortex to the ventral striatum. This is based on the hypothesis that cortical regions provide a ‘top-down’ regulation of emotions generated in the limbic regions they target, leading to over control of negative emotions. This results in patients in BDM to be overactive and overexcited, even when in mania. Similarly, reduced rsFC in the left hemisphere in BDM may indicate a loosen control on positive emotion, resulting in manifestation of more positive emotions. This view is also supported by our correlation analysis showing a positive correlation between the scores of YMRS and the rsFC between the OFC and the right ventral striatum for BDM; the stronger the control the cortex exerts on the right striatum, the more severe the manic symptoms are.
Our other notable result was that no significant group difference was found for rsFC among ROIs in the neural circuit associated with primary reward, and it was in the neural circuit associated with second reward that rsFC among ROIs revealed significant group differences. This was consent with our hypothesis that the abnormality of rsFC in patients with BD was different in neural circuits associated with primary and secondary rewards. Primary rewards are closely related to situation-specific biological needs; how individuals use primary rewards to satisfy these needs may depend on mechanisms evolved in the evolutionary life processes (Lea and Webley, 2006). Secondary rewards, especially those represented by money, have evolved relatively recently in human evolutionary history and are unique to the human species. The performance and perception of individuals in social interaction involving secondary rewards may, thus, depend more on their social and cultural contexts (Henrich et al., 2005). A study (Dutra et al., 2017) employing a monetary and social incentive delay task between patients with BD and HCs revealed a disrupted corticolimbic connectivity during reward processing and the other study (Dutra et al., 2015) from their team supported this findings, suggesting that patients with BD present abnormal in the secondary reward. However, there were few reports on the primary reward for patients with BD despite the fact that there were a few reports for patients with schizophrenia (Grimm et al., 2012; de Leeuw et al., 2015). We infer that patients with BD may have a malfunction in some advanced functional brain circuit rather than in a primary circuit. Another possibility is that patients with BD have a more pronounced dysfunction in some advanced functional brain circuit in addition to a dysfunction of a primary functional circuit.
Our study has the following limitations. First, it was a cross-sectional study; hence, the relationship between the change of rsFC and the change of mood states could not be determined. Second, our sample size was comparatively small, especially in the BDD group, which could lead to false-positive or negative results due to the weak statistical power. Third, although this study suggested a decreased strength of rsFC only in the secondary reward circuit, it was insufficient to directly examine the specificity of this effect to the secondary reward circuit over and above the primary reward. Fourth, the collection of imaging data based on reward-related tasks could have helped us verify the results. Finally, in this study, there was a significant difference between the BDD and BDM subgroups in the usage of antidepressants due to different disease states, and we were not able to control such confounding factors. Cullen et al. (2016) revealed that antidepressant treatment increased the rsFC between the frontal cortex and right amygdala and treatment response is positively associated with increased the rsFC between the frontal cortex and right amygdala in the study focusing on the effect of a selective serotonin reuptake inhibitor on rsFC for 8 weeks. Further, we did not investigate the potential impact of other psychotropic medications on our outcome measures.
Conclusions
To summarize, this study demonstrated that patients with BD had a reduction of rsFC between the OFC and the LVS and the pattern of rsFC was different between BDD and BDM, suggesting that the rsFC of the neural circuit associated with second reward may be abnormal in BD. In addition, the finding that contrasting and convergent patterns of rsFC may exist between different mood states in patients with BD reminds that different treatment strategies need to be considered for BDD and BDM and rsFC of neural circuits associated with primary and secondary rewards in patients with BD may be a state marker rather than a trait one.
Availability of data and materials
The materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality under the circumstances without conflicting to our further research.
Authors’ contributions
J.S. and H.G. appraised all potential studies and wrote and revised the draft manuscript. Z.W., S.T. and Y.T. designed the study and revised the draft manuscript and subsequent manuscripts. S.L., W.X. and F.F. assisted with the presentation of findings and assisted with drafting and revising the manuscript. H.A. and F.Y. conceived and designed the study, assisted with searches, appraised relevant studies and assisted with drafting and revising the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by Beijing Excellent Talent Training Program (2016000021469G175), Beijing Hospital Authority Training Plan (PX2017024), Beijing Municipal Science and Technology Project (Z181100001518005, Z171100001017021), the Beijing Municipal Special Foundation for High-level Health Technology Personnel Construction, Beijing Municipal Hospital Development-Program (PX2016010), the National Natural Science Foundation of China (31671145), Beijing Municipal Science & Technology Commission grant (Z141107002514016), Beijing Natural Science Foundation (7162087) and Beijing Municipal Administration of Hospitals Clinical medicine Development of special funding (XMLX201609).
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Psychiatric Hospital of Zhumadian, and all participants volunteered to participate in the study and signed informed consent.
Consent to publish
Informed consents to publish were obtained from the patients in this research.
Conflict of interest
There was no conflict of interest in our study.
Supplementary Material
Contributor Information
Jing Shi, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Hua Guo, Present Office, The Psychiatric Hospital of Zhumadian, Zhumadian, Henan 463000, China.
Sijia Liu, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Wei Xue, Department of Clinical Pharmacology, Beijing Hospital of the Ministry of Health, Beijing 100730, P.R. China.
Fengmei Fan, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Hongzhen Fan, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Huimei An, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Zhiren Wang, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Shuping Tan, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Fude Yang, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
Yunlong Tan, Psychiatry Research Center, Beijing HuiLongGuan Hospital, Peking University Huilongguan Clinical Medical School, Beijing 100096, China.
References
- Almeida J.R., Versace A., Hassel S., Kupfer D.J., Phillips M.L. (2010). Elevated amygdala activity to sad facial expressions: a state marker of bipolar but not unipolar depression. Biological Psychiatry, 67(5), 414–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altinay M., Karne H., Anand A. (2018). Lithium monotherapy associated clinical improvement effects on amygdala-ventromedial prefrontal cortex resting state connectivity in bipolar disorder. Journal of Affective Disorders, 225, 4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A., Li Y., Wang Y., Lowe M.J., Dzemidzic M. (2009). Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Research, 171(3), 189–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association (1996). The Diagnostic and Statistical Manual for Mental Disorders-Fourth Edition, Taiwan: Heji Publishing House. [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. (1995). Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine, 34(4), 537–41. [DOI] [PubMed] [Google Scholar]
- Brady R.O. Jr., Masters G.A., Mathew I.T., et al. (2016). State dependent cortico-amygdala circuit dysfunction in bipolar disorder. Journal of Affective Disorders, 201, 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caseras X., Lawrence N.S., Murphy K., Wise R.G., Phillips M.L. (2013). Ventral striatum activity in response to reward: differences between bipolar I and II disorders. The American Journal of Psychiatry, 170(5), 533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen K.R., Klimes-Dougan B., Vu D.P., et al. (2016). Neural correlates of antidepressant treatment response in adolescents with major depressive disorder. Journal of Child and Adolescent Psychopharmacology, 26(8), 705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damme K.S., Young C.B., Nusslock R. (2017). Elevated nucleus accumbens structural connectivity associated with proneness to hypomania: a reward hypersensitivity perspective. Social Cognitive and Affective Neuroscience, 12(6), 928–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damme K.S.F., Pelletier-Baldelli A., Cowan H.R., Orr J.M., Mittal V.A. (2019). Distinct and opposite profiles of connectivity during self-reference task and rest in youth at clinical high risk for psychosis. Human Brain Mapping, 40(11), 3254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R. (2007). Reward-related responses in the human striatum. Annals of the New York Academy of Sciences, 1104, 70–88. [DOI] [PubMed] [Google Scholar]
- Duff E.P., Makin T., Cottaar M., Smith S.M., Woolrich M.W. (2018). Disambiguating brain functional connectivity. NeuroImage, 173, 540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra S.J., Cunningham W.A., Kober H., Gruber J. (2015). Elevated striatal reactivity across monetary and social rewards in bipolar I disorder. Journal of Abnormal Psychology, 124(4), 890–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutra S.J., Man V., Kober H., Cunningham W.A., Gruber J. (2017). Disrupted cortico-limbic connectivity during reward processing in remitted bipolar I disorder. Bipolar Disorders, 19(8), 661–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. (2005). Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience, 8(11), 1481–9. [DOI] [PubMed] [Google Scholar]
- Grimm O., Vollstadt-Klein S., Krebs L., Zink M., Smolka M.N. (2012). Reduced striatal activation during reward anticipation due to appetite-provoking cues in chronic schizophrenia: a fMRI study. Schizophrenia Research, 134(2–3), 151–7. [DOI] [PubMed] [Google Scholar]
- Haber S.N., Knutson B. (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J., Boyd R., Bowles S., et al. (2005). Economic man in cross-cultural perspective: behavioral experiments in 15 small-scale societies. The Behavioral and Brain Sciences, 28(6), 795–815. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Bromberg-Martin E., Hong S., Matsumoto M. (2008). New insights on the subcortical representation of reward. Current Opinion in Neurobiology, 18(2), 203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulvershorn L.A., Karne H., Gunn A.D., et al. (2012). Neural activation during facial emotion processing in unmedicated bipolar depression, euthymia, and mania. Biological Psychiatry, 71(7), 603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez E., Sole B., Arias B., et al. (2018). Characterizing decision-making and reward processing in bipolar disorder: a cluster analysis. European Neuropsychopharmacology, 28(7), 863–74. [DOI] [PubMed] [Google Scholar]
- Knutson B., Rick S., Wimmer G.E., Prelec D., Loewenstein G. (2007). Neural predictors of purchases. Neuron, 53(1), 147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kop W.J., Synowski S.J., Newell M.E., Schmidt L.A., Waldstein S.R., Fox N.A. (2011). Autonomic nervous system reactivity to positive and negative mood induction: the role of acute psychological responses and frontal electrocortical activity. Biological Psychology, 86(3), 230–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence N.S., Williams A.M., Surguladze S., et al. (2004). Subcortical and ventral prefrontal cortical neural responses to facial expressions distinguish patients with bipolar disorder and major depression. Biological Psychiatry, 55(6), 578–87. [DOI] [PubMed] [Google Scholar]
- Lea S.E., Webley P. (2006). Money as tool, money as drug: the biological psychology of a strong incentive. The Behavioral and Brain Sciences, 29(2), 161–76discussion 176-209. [DOI] [PubMed] [Google Scholar]
- Leeuw M., Kahn R.S., Vink M. (2015). Fronto-striatal dysfunction during reward processing in unaffected siblings of schizophrenia patients. Schizophrenia Bulletin, 41(1), 94–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Huang C., Deng W., et al. (2015). Contrasting and convergent patterns of amygdala connectivity in mania and depression: a resting-state study. Journal of Affective Disorders, 173, 53–8. [DOI] [PubMed] [Google Scholar]
- Lin F., Zhou Y., Du Y., Zhao Z., Qin L., Xu J., Lei H. (2015). Aberrant corticostriatal functional circuits in adolescents with internet addiction disorder. Frontiers in Human Neuroscience, 9, 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linke J., King A.V., Rietschel M., et al. (2012). Increased medial orbitofrontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. The American Journal of Psychiatry, 169(3), 316–25. [DOI] [PubMed] [Google Scholar]
- Liu J., Blond B.N., Dyck L.I., Spencer L., Wang F., Blumberg H.P. (2012). Trait and state corticostriatal dysfunction in bipolar disorder during emotional face processing. Bipolar Disorders, 14(4), 432–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man V., Gruber J., Glahn D.C., Cunningham W.A. (2018). Altered amygdala circuits underlying valence processing among manic and depressed phases in bipolar adults. Journal of Affective Disorders, 245, 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas K.R., Jin R., He J.P., et al. (2011). Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Archives of General Psychiatry, 68(3), 241–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole B.D., Gable P.A. (2014). Affective motivational direction drives asymmetric frontal hemisphere activation. Experimental Brain Research, 232(7), 2121–30. [DOI] [PubMed] [Google Scholar]
- Quaedflieg C.W., Smulders F.T., Meyer T., Peeters F., Merckelbach H., Smeets T. (2016). The validity of individual frontal alpha asymmetry EEG neurofeedback. Social Cognitive and Affective Neuroscience, 11(1), 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich B.A., Fromm S.J., Berghorst L.H., et al. (2008). Neural connectivity in children with bipolar disorder: impairment in the face emotion processing circuit. Journal of Child Psychology and Psychiatry, 49(1), 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvador R., Suckling J., Coleman M.R., Pickard J.D., Menon D., Bullmore E. (2005). Neurophysiological architecture of functional magnetic resonance images of human brain. Cerebral Cortex, 15(9), 1332–42. [DOI] [PubMed] [Google Scholar]
- Schreiter S., Spengler S., Willert A., et al. (2016). Neural alterations of fronto-striatal circuitry during reward anticipation in euthymic bipolar disorder. Psychological Medicine, 46(15), 3187–98. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2000). Multiple reward signals in the brain. Nature Reviews. Neuroscience, 1(3), 199–207. [DOI] [PubMed] [Google Scholar]
- Schultz W. (2006). Behavioral theories and the neurophysiology of reward. Annual Review of Psychology, 57, 87–115. [DOI] [PubMed] [Google Scholar]
- Sescousse G., Caldu X., Segura B., Dreher J.C. (2013). Processing of primary and secondary rewards: a quantitative meta-analysis and review of human functional neuroimaging studies. Neuroscience and Biobehavioral Reviews, 37(4), 681–96. [DOI] [PubMed] [Google Scholar]
- Shi J., Geng J., Yan R., et al. (2018). Differentiation of transformed bipolar disorder from unipolar depression by resting-state functional connectivity within reward circuit. Frontiers in Psychology, 9, 2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporns O. (2013). Structure and function of complex brain networks. Dialogues in Clinical Neuroscience, 15(3), 247–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syan S.K., Minuzzi L., Smith M., Allega O.R., Hall G.B., Frey B.N. (2017). Resting state functional connectivity in women with bipolar disorder during clinical remission. Bipolar Disorders, 19(2), 97–106. [DOI] [PubMed] [Google Scholar]
- Syan S.K., Smith M., Frey B.N., et al. (2018). Resting-state functional connectivity in individuals with bipolar disorder during clinical remission: a systematic review. Journal of Psychiatry & Neuroscience, 43(5), 298–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken A.J., Davidson R.J., Wheeler R.E., Doss R.C. (1992). Individual differences in anterior brain asymmetry and fundamental dimensions of emotion. Journal of Personality and Social Psychology, 62(4), 676–87. [DOI] [PubMed] [Google Scholar]
- Urosevic S., Abramson L.Y., Harmon-Jones E., Alloy L.B. (2008). Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: review of theory and evidence. Clinical Psychology Review, 28(7), 1188–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A., Thompson W.K., Zhou D., et al. (2010). Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biological Psychiatry, 67(5), 422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei S., Geng H., Jiang X., et al. (2017). Amygdala-prefrontal cortex resting-state functional connectivity varies with first depressive or manic episode in bipolar disorder. Neuroscience Letters, 641, 51–5. [DOI] [PubMed] [Google Scholar]
- Wessa M., Kanske P., Linke J. (2014). Bipolar disorder: a neural network perspective on a disorder of emotion and motivation. Restorative Neurology and Neuroscience, 32(1), 51–62. [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S., Moran J.M., Nieto-Castanon A., Triantafyllou C., Saxe R., Gabrieli J.D. (2011). Associations and dissociations between default and self-reference networks in the human brain. NeuroImage, 55(1), 225–32. [DOI] [PubMed] [Google Scholar]
- Wood A., Kroll L., Moore A., Harrington R. (1995). Properties of the mood and feelings questionnaire in adolescent psychiatric outpatients: a research note. Journal of Child Psychology and Psychiatry, 36(2), 327–34. [DOI] [PubMed] [Google Scholar]
- Xu K., Liu H., Li H., et al. (2014). Amplitude of low-frequency fluctuations in bipolar disorder: a resting state fMRI study. Journal of Affective Disorders, 152-154, 237–42. [DOI] [PubMed] [Google Scholar]
- Yan C., Liu D., He Y., et al. (2009). Spontaneous brain activity in the default mode network is sensitive to different resting-state conditions with limited cognitive load. PLoS One, 4(5), 5743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching participant confidentiality under the circumstances without conflicting to our further research.


