Abstract
Emotion regulation plays a central role in empathy. Only by successfully regulating our own emotions can we reliably use them in order to interpret the content and valence of others’ emotions correctly. In an functional magnetic resonance imaging (fMRI)-based experiment, we show that regulating one’s emotion via reappraisal modulated biased emotional intensity ratings following an empathy for pain manipulation. Task-based analysis revealed increased activity in the right inferior frontal gyrus (IFG) when painful emotions were regulated using reappraisal, whereas empathic feelings that were not regulated resulted in increased activity bilaterally in the precuneus, supramarginal gyrus and middle frontal gyrus (MFG), as well as the right parahippocampal gyrus. Functional connectivity analysis indicated that the right IFG plays a role in the regulation of empathy for pain, through its connections with regions in the empathy for pain network. Furthermore, these connections were further modulated as a function of the type of regulation used: in sum, our results suggest that accurate empathic judgment (i.e. empathy that is unbiased) relies on a complex interaction between neural regions involved in emotion regulation and regions associated with empathy for pain. Thus, demonstrating the importance of emotion regulation in the formulation of complex social systems and sheds light on the intricate network implicated in this complex process.
Keywords: empathy, emotion regulation, reappraisal, gPPI, IFG
Our emotions can help us respond effectively and adaptively to the complex world that surrounds us. They can also, however, become destructive and unhelpful, making us more confused rather than providing us more clarity (Gross, 2013). For this reason, being able to regulate our emotions by amplifying those that encourage adaptive responses and diminishing those that do not is central to our wellbeing. A common strategy that individuals use to regulate their emotions is cognitive reappraisal—a process through which individuals reconstruct an emotional situation in a way that alters its emotional impact, for example by reconstructing a horror film as a parody (McRae et al., 2012).
Traditionally, the study of emotion regulation focused on intrinsic and basic emotions (e.g. fear, anger or disgust; Gross, 2013). Recently, however, growing research interest is being directed toward more complex emotional situations provoked during interpersonal interactions. One such complex emotional situation is the experience of empathy, which is the focus of this paper. Empathy is generally defined as an individual’s ability to vicariously experience the thoughts and feelings of another person, thus generating connections between individuals. As part of the empathic process, individuals use their own emotions and experiences as a reference point for understanding the mental states of others. Thus, it follows that empathy is influenced by the control individuals exert over their own emotional experiences (Decety, 2010, Naor et al. 2018).
The tendency to use one’s own emotions while at the same time regulating them is even more relevant in the context of empathy for pain, i.e. the ability to partake of the pain felt by others (Fitzgibbon et al., 2010). Empathy for pain has been the major focus of empathy research in social neuroscience and other related fields (Singer and Lamm, 2009), highlighting the importance of empathy for pain in daily life. For example, we recently demonstrated that the use of reappraisal to regulate emotions can influence the empathic process and eliminate biases in judging emotional facial intensity (Naor et al. 2018). The ability to accurately judge the intensity of emotional facial expressions can be considered to be one type of empathic accuracy (Ickes et al., 1990). The ability to identify others’ emotions based on the observation of facial expressions has been linked to the ability to share such feelings (Enticott et al., 2008), a key concept in empathy (Blais et al., 2012; Singer, 2006). Judgment of morphed faces has been used as a measure of empathic accuracy in previous works, for example in studies that showed participants dynamic facial expressions and asked them to continuously judge the intensity of the emotional expressions (e.g. Hall and Schmid Mast, 2007; Zaki et al., 2008, 2009). Furthermore, reduction in the ability to make accurate emotional intensity inferences from morphed static face images has been associated with conditions marked by impairments in empathy, such as cocaine users (Kuypers et al., 2015), patients with ventromedical prefrontal cortex lesions (Jenkins et al., 2014) and individuals with autistic spectrum disorder (Smith et al., 2010). A recent study demonstrated a cognitive bias for judgments of pain only when these judgments were made after the participant experienced empathy for pain, yielding exaggerated assessment of emotional intensity compared to the presented intensity. Nevertheless, that bias disappeared when participants used reappraisal to regulate their empathy (Naor et al. 2018).
The neural networks underlying the process of modulating empathy for pain in the context of emotion regulation have yet to be explored. Empathy relies heavily on areas of the salience network, namely the anterior insula (AI) and the anterior cingulate cortex (ACC) (Menon and Uddin 2010; Seeley et al., 2007). Conversely, emotion regulation, and mainly reappraisal-based downward regulation, is associated with executive control and limbic networks, namely the prefrontal cortex and the amygdala (Seeley et al., 2007, Menon and Uddin 2010). An accumulating body of research highlights the utility of examining functional connectivity when assessing the relationships between cognitive and affective processes, as well as their corresponding brain processes.
Hence, the current study aimed at exploring the functional connectivity among the neural networks involved both in upregulating and in downregulating empathy for pain. To this end, we employed the task developed by Naor et al. (2018) in an functional magnetic resonance imaging (fMRI) setting. In short, participants observed scenarios of painful or non-painful situations. They were then asked to rate the degree of affect in faces that depicted either a painful or a happy expression. In half of the trials, participants were asked to empathize with the scenario, while in the other half they were asked to reappraise their empathy. Empathic engagement with the painful scenario is hypothesized to lead to empathy, which will affect the participants’ emotional state and lead them to judge other people’s levels of pain inaccurately but will not affect the accuracy of their valence judgment of other emotions. Conversely, the use of reappraisal will downregulate the participant’s own emotional state, resulting in more accurate empathic judgment.
In addition, we hypothesized that (i) the experience of empathy for pain would result in increased activity in the salience network, mainly the AI and the ACC; (ii) downregulation of empathy for pain via reappraisal would result in increased activity in regions associated with executive control and decreased activity in limbic networks; and (iii) the degree of activity in the prefrontal-limbic network would affect the degree of cognitive bias, such that the greater the functional connectivity between regions related to emotion regulation and those related to empathy, the lesser the bias would be. To this end, in addition to a GLM-based fMRI data analysis, we also conducted a generalized psychophysiological interaction (gPPI) analysis to explore the functional networks underlying the differences between bias scores after observation of painful scenarios under reappraise and watch conditions. This analysis enabled us to pinpoint the brain regions that exhibit higher functional coupling during the process of downward regulation of empathy.
Methods
Participants
Thirty-three healthy participants were recruited from the student population at Ben-Gurion University of the Negev (14 male; age = 24.65; SD = 1.76) in return for payment. The Ethics Committee at Soroka Medical Centre approved the experiment (Approval Number 0114-15-SOR). All participants had normal or corrected-to-normal vision. Participants were screened for neurological or psychiatric history, as well as for any metal implants that might interfere with the scanning. All participants signed an informed consent form prior to participating. Two participants were excluded from the final analysis due to technical failures in the scanning session that resulted in the loss of behavioral data.
Materials
We used a set of 23 matched colored pictures showing hands and feet in painful and non-painful scenarios. Each painful scenario was matched with a non-painful scenario that involved all the same components except the painful element. In addition, the experiment employed a well-validated set of faces (Blais et al., 2012; http://mapageweb.umontreal.ca/gosselif/STOIC.rar). Emotional expressions were morphed with neutral ones to create a sequential morph of 100 pictures. Eight models were used (four female), each depicting two emotions (happy and painful) at six levels of intensity (40, 50, 60, 70, 80 and 90%). For more detailed information, see Naor et al. (2018).
Experimental Procedure
The behavioral procedure was similar to the procedure used in previous research at our lab (Naor et al. 2018). Each experimental trial began with a 4000 ms presentation of either a painful or a non-painful scenario picture taken from the painful/non-painful scenario pictures set. Two thousand ms into the presentation, a colorful frame appeared instructing participants which regulation strategy to employ—REAPPRAISE or EMPATHIC WATCH. The painful/non-painful scenario picture remained on the screen with the colorful frame for additional 2000 ms. After the painful/non-painful scenario picture disappeared, participants were shown a picture from the facial expression set. Participants were then given 6000 ms to judge the intensity of the emotion shown in the facial expression on a scale ranging from 1 to 100. After 6000 ms, the scale disappeared and a fixation cross appeared from 3000 to 5000 ms before the next trial. A total of four models (either a male or a female, matching the participant’s sex) were used in the experiment. For each model, six morphed painful faces and six morphed happy faces were selected. For statistical power, we ran each stimuli combination twice, yielding a total trial count of 96 (4 models × 6 morphed faces × 2 emotions × 2 repetitions), divided into two experimental runs of 48 trials each in the scanner. The complete instructions are reported in Appendix 1 (Figure 1).
Fig. 1.
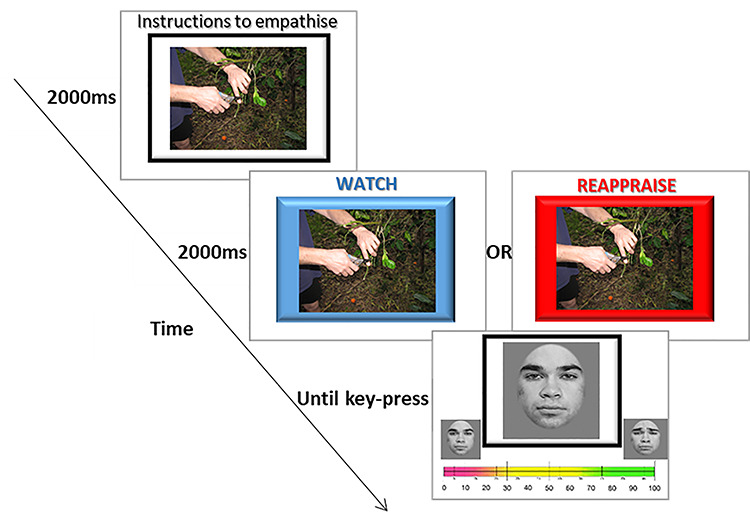
Example of a painful scenario with a neutral face. A picture depicting either a painful or a non-painful scenario appeared for 2000 ms. Then, an instruction to REAPPRAISE (red frame) or to employ EMPATHIC WATCH (blue frame) appeared for an additional 2000 ms. After participants viewed the scenario, they were shown a picture depicting an emotional version morphed between 100% neutral and 100% emotion (pain or happy) and were given 6000 ms to assess the emotional intensity of the presented face.
Behavioral analysis
A three-way repeated measures ANOVA was calculated to examine the effect of the presented scenario (painful/non-painful), the presented emotional expression (happy/painful) and the regulation strategy deployed (REAPPRAISAL/EMPATHIC WATCH) on participants’ judgment of emotional intensity. The dependent variable in the ANOVA analysis was the calculated bias score, which is the averaged difference between the actual observed intensity and the judged intensity.
To further examine the effect of scenario and expression on bias, a 2 × 2 repeated measures ANOVA was independently calculated for each regulation strategy, with the presented scenario (painful/non-painful) and the presented emotional expression (happy/painful) as dependent variables and the bias score as the independent variable.
fMRI data preprocessing
FMRI data were processed using FEAT (FMRI Expert Analysis Tool) Version 6.00, a toolbox of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl). Functional images were registered to high-resolution structural images using Boundary-Based Registration (Greve and Fischl, 2009). The high-resolution structural image was registered to the standard space using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002) and then further refined using FNIRT non-linear registration (Andersson et al., 2013). The following pre-statistical processing was applied: motion correction was carried out using MCFLIRT with options for extended motion parameters (i.e. standard motion parameters plus their derivatives and the squares of their derivatives) (Jenkinson et al., 2002); non-brain removal using the Brain Extraction Tool (BET, Smith, 2002). We further scrubbed the volumes’ framewise displacement >0.9 mm. Participants for whom >10% of the volumes passed this threshold (i.e. >38 volumes) were excluded from the analysis. Spatial smoothing using a Gaussian kernel of FWHM 5 mm; grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor; and high pass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Denoising was carried out with independent component analysis (ICA)-AROMA in FSL by conducting single-subject ICA to remove motion components from each participant’s functional data (Pruim et al., 2015a, b). ICA-AROMA was selected as it was shown to be highly effective in accounting for motion related variance (Ciric et al., 2017; Pruim et al., 2015a).
fMRI task within-participant analysis
Statistical analysis was conducted using FILM with local autocorrelation correction (Woolrich et al., 2001). The time-series model included eight EVs to account for the eight contrasts in the experimental design. Each trial lasted ~10 s (with a 500 milliseconds jitter) and included 2 s of initial scenario viewing, a 2 s regulation period and a 6 s judgment phase. A double gamma hemodynamic response function (HRF) was used, and the extended motion parameters served as an indicator function to model out single TRs identified to have excessive motion according to a framewise displacement >0.9 mm. The second-level analysis, in which contrast estimates were averaged over within-subject runs, was conducted using a fixed-effects model by forcing the random effects variance to zero in FLAME (FMRIB’s Local Analysis of Mixed Effects; Beckmann et al., 2003; Woolrich et al., 2004, Woolrich, 2008). Each 10 s trial was modeled in its entirety to ensure an optimal fit across the data. Specifically, the first 2-s period was modeled as the observation period, the next 2-s period was modeled as the regulation period and finally the subsequent 6 s were modeled as the judgment time.
fMRI task group activity analysis
Group analysis was conducted using FLAME (FMRIB’s Local Analysis of Mixed Effects) stage 1 (Beckmann et al., 2003; Woolrich et al., 2004; Woolrich, 2008). Based on Gaussian random field theory, Z (Gaussianised T/F) statistic images underwent parametric thresholding using clusters determined by z > 3.1 and a corrected cluster significance threshold of P > 0.05 (Worsley, 2001). We separately examined differences between regulation following painful scenarios and regulation following non-painful scenarios for each regulation strategy (i.e. reappraisal and empathic watch). The results of those contrasts were then contrasted themselves to account for the unique effect of each regulation type.
After the whole-brain group analysis, a gPPI analysis (Friston et al., 1997; McLaren et al., 2012; O’Reilly et al., 2012) was conducted in FSL FEAT to examine functional connectivity in networks involved in the use of reappraisal of empathy for pain. This analysis examined the interaction between activity in the seed region—which was selected based on the task analysis [i.e. the inferior frontal gyrus (IFG) and the supramarginal gyrus (SMG)]—and activity in all other voxels in the brain as a function of task condition, i.e. REAPPRAISAL (painful/non-painful) and EMPATHIC WATCH (painful/non-painful). The gPPI analysis matrix included all EVs of the original task from the group analysis to control for the main effect of task. Judgments of painful facial expression following painful scenario under the REAPPRAISE and EMPATHIC WATCH conditions were used as the psychological parameter. The physiological parameter was the time course of the seed region in the right IFG (based on the results of the task analysis, as reported in the Results section). Finally, the mathematical product of the psychological variable and the physiological variable constituted the interaction term. A mixed-effects group-level regression was employed using FLAME 1 and the results were thresholded at z > 3.1 and P < 0.05 corrected for multiple comparisons.
Results
Behavioral findings
A three-way repeated-measures ANOVA with Bonferroni correction for multiple comparisons revealed a greater bias for judgments of painful facial expressions than for those of happy facial expressions [F(1,30) = 8.759, P = 0.006, 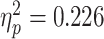 ]. In addition, main effects emerged for regulation instructions [F(1,30) = 5.210, P = 0.006
]. In addition, main effects emerged for regulation instructions [F(1,30) = 5.210, P = 0.006 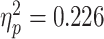 ], scenario [F(1,23) = 5.210, P = 0.03
], scenario [F(1,23) = 5.210, P = 0.03 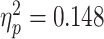 ], and the interaction between them [F(1,30) = 10.160, P = 0.003
], and the interaction between them [F(1,30) = 10.160, P = 0.003 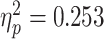 ]. As predicted, the bias for painful facial expressions was higher when participants watched the scenario empathically compared to the condition in which they reappraised their feelings [F(1,30) = 2.112, P = 0.157,
]. As predicted, the bias for painful facial expressions was higher when participants watched the scenario empathically compared to the condition in which they reappraised their feelings [F(1,30) = 2.112, P = 0.157, 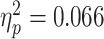 ]. The bias for painful facial expression was also higher for conditions that followed painful scenarios than for non-painful ones [F(1,30) = 2.667, P = 0.14,
]. The bias for painful facial expression was also higher for conditions that followed painful scenarios than for non-painful ones [F(1,30) = 2.667, P = 0.14, 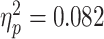 ]. Moreover, all three conditions interacted, so that the greatest bias was found during trials in which judgments of painful facial expressions were made following empathic watch of a painful scenario [F(1,30) = 4.928, P = 0.034,
]. Moreover, all three conditions interacted, so that the greatest bias was found during trials in which judgments of painful facial expressions were made following empathic watch of a painful scenario [F(1,30) = 4.928, P = 0.034, 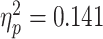 ].
].
Figure 2 portrays the results of further testing the source of the three-way interaction using a 2 × 2 repeated-measures ANOVA with Bonferroni correction for multiple comparisons. Under EMPATHIC WATCH, we found a greater bias in judgments of painful facial expressions compared to happy facial expressions [F(1,30) = 12.007, P = 0.002,  = 0.286], as well as a greater bias in judgments made after exposure to painful scenarios compared to non-painful ones [F(1,30) = 11.430, P = 0.002,
= 0.286], as well as a greater bias in judgments made after exposure to painful scenarios compared to non-painful ones [F(1,30) = 11.430, P = 0.002, 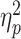 = 0.276]. In addition, an interaction emerged between facial expression and scenario [F(1,30) = 7.289, P = 0.011,
= 0.276]. In addition, an interaction emerged between facial expression and scenario [F(1,30) = 7.289, P = 0.011,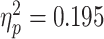 ]. Table 1 depicts the follow-up t-tests conducted to examine the source of this interaction. A paired sample t-test revealed a greater bias for painful facial expressions in judgments following painful scenarios than in judgments following non-painful scenarios (Table 1). A similar Bonferroni corrected ANOVA for REAPPRAISE trials did not yield significant results for scenario [F(1,30) = 2.667, N.S.], emotion [F(1,30) = 1.205, N.S.], or the interactions between them [F(1,30) = 1.386, N.S.]. Moreover, the bias score for painful expressions following painful scenarios in EMPATHIC WATCH trials was significantly higher than in REAPPRAISE trials (t = 3.677, df = 30, P = 0.001)1.
]. Table 1 depicts the follow-up t-tests conducted to examine the source of this interaction. A paired sample t-test revealed a greater bias for painful facial expressions in judgments following painful scenarios than in judgments following non-painful scenarios (Table 1). A similar Bonferroni corrected ANOVA for REAPPRAISE trials did not yield significant results for scenario [F(1,30) = 2.667, N.S.], emotion [F(1,30) = 1.205, N.S.], or the interactions between them [F(1,30) = 1.386, N.S.]. Moreover, the bias score for painful expressions following painful scenarios in EMPATHIC WATCH trials was significantly higher than in REAPPRAISE trials (t = 3.677, df = 30, P = 0.001)1.
Fig. 2.
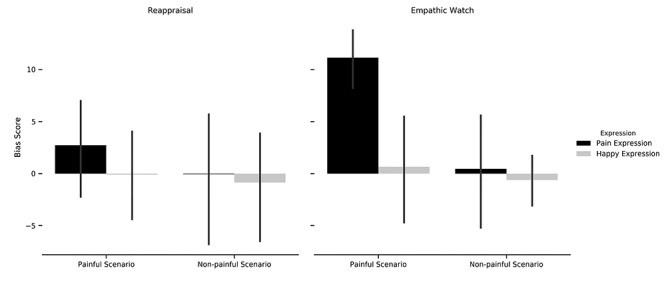
Three-way repeated-measures ANOVA of regulation strategy (reappraise/empathic watch), with scenario (painful/non-painful) and emotion (painful/happy) as within-subject factors and bias score as a dependent variable, *P < 0.005; **P < 0.001.
Table 1.
Bias scores, mean differences and t values for WATCH and REAPPRAISE conditions
| Condition | Scenario | Emotion | Bias score (SD) | Mean difference | t-test value |
|---|---|---|---|---|---|
| Watch | Non-painful | Pain | 0.461 (16.04) | 10.671 | 4.019** |
| Painful | 11.132 (7.834) | ||||
| Non-painful | Happy | −0.617 (6.946) | 1.274 | 0.556 | |
| Painful | 0.657 (15.256) | ||||
| Reappraise | Non-painful | Pain | −0.059 (17.907) | 2.781 | 2.165* |
| Painful | 2.722 (13.58) | ||||
| Non-painful | Happy | −0.852 (14.38) | 0.748 | 0.507 | |
| Painful | 0.103 (12.475) |
While the interaction between emotion and scenario in EMPATHIC WATCH trials was significant [F(1,30) = 7.289, P = 0.011,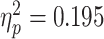 ], there was no similar interaction in REAPPRAISE trials [F(1,30) = 1.386, N.S.]. *P < 0.05; **P < 0.001.
], there was no similar interaction in REAPPRAISE trials [F(1,30) = 1.386, N.S.]. *P < 0.05; **P < 0.001.
Neuroimaging findings
Functional activity results
To examine the effect of emotion regulation following empathy for pain, we first compared (A) EMPATHIC WATCH trials during exposure to painful scenarios to (B) EMPATHIC WATCH trials during exposure to non-painful scenarios. Increased activity related to empathy for pain was found in regions of the salience network, including the bilateral Insula and the IFG, as well as in the left MFG and the right ACC (Table 2). Then, we compared (C) REAPPRAISE trials following exposure to painful scenarios to (D) REAPPRAISE trials following exposure to non-painful scenarios. Increased activity was found in regions associated with executive control, including the left parietal lobule, as well as regions from the salience network including the bilateral AI. Results of the whole-brain analysis are reported in Table 2 and Figure 3. To examine the effect of reappraisal, we subtracted the unique activity of both regulation strategies from each other [i.e. (A-B)–(C-D)]. This analysis revealed increased activity during REAPPRAISE trials in the right IFG, whereas for EMPATHIC WATCH, increased activity was found in left SMG, the right precuneus and the right MFG. The results of the whole-brain analysis are reported in Table 3 and Figure 4.
Table 2.
A whole-brain analysis of the effects of EMPATHIC WATCH for painful emotion larger than non-painful emotion and the effects of REAPPRAISE for painful emotion larger than non-painful emotion. Regions were classified using the Harvard–Oxford Atlas, z > 3.1 and (corrected) cluster significance P < 0.05. Z-MAX values represent peak activity for the cluster. MNI coordinates
| EMPATHIC WATCH painful > non-painful (A-B) | ||||||
|---|---|---|---|---|---|---|
| Voxels | Z-MAX | MAX X | MAX Y | MAX Z | R/L | |
| 949 | 4.43 | 50 | 14 | -2 | R | Insular Cortex, inferior frontal gyrus, precentral gyrus, superior temporal gyrus, post-central gyrus, frontal orbital cortex |
| 923 | 4.66 | -44 | 2 | 2 | L | Insular cortex, inferior frontal gyrus, middle frontal gyrus, precentral gyrus, superior temporal gyrus, post-central gyrus, frontal orbital cortex |
| 646 | 4.07 | -62 | -22 | 28 | L | Superior temporal gyrus, post-central gyrus, superior parietal lobule, supramarginal gyrus, anterior and posterior division, angular gyrus |
| 332 | 4.13 | 0 | 16 | 32 | Superior frontal gyrus, juxtapositional lobule cortex, paracingulate gyrus, anterior cingulate gyrus | |
| REAPPRAISE painful > non-painful (C-D) | ||||||
| 546 | 4.32 | -62 | -42 | 46 | L | Superior temporal gyrus, posterior division, superior parietal lobule post-central gyrus, supramarginal gyrus, anterior and posterior divisions, angular gyrus |
| 466 | 3.84 | 60 | -46 | 44 | R | Superior temporal gyrus, posterior division, post-central gyrus supramarginal gyrus, anterior and posterior division, angular gyrus |
| 364 | 4.21 | 46 | 24 | -4 | R | Insular cortex, inferior frontal gyrus, frontal orbital cortex |
| 272 | 3.93 | -38 | 10 | -6 | L | Insular cortex, inferior frontal gyrus |
Fig. 3.
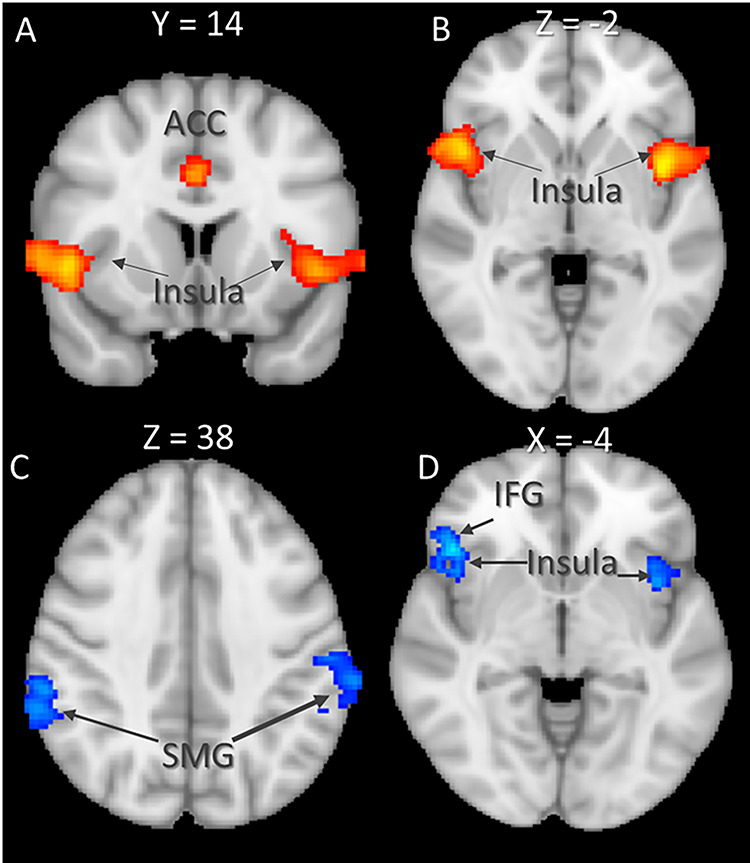
(A and B) Results of empathic watch for painful vs non-painful scenarios. (C and D) Results of reappraisal of painful vs non-painful scenarios. Clusters were derived at z > 3.1 and (corrected) cluster significance P < 0.05. IFG = inferior frontal gyrus; SMG = supramarginal gyrus.
Table 3.
Results of whole-brain analysis for EMPATHIC WATCH effect larger than REAPPRAISE effect, and REAPPRAISE effect larger than EMPATHIC WATCH effect. Regions were classified using the Harvard–Oxford Atlas, z > 3.1 and (corrected) cluster significance P < 0.05. Z-MAX values represent peak activity for the cluster. MNI coordinates
| EMPATHIC WATCH effect > REAPPRAISE effect [(A-B)—(C-D)] | ||||||
|---|---|---|---|---|---|---|
| Voxels | Z-MAX | X | Y | Z | R/L | |
| 109 | 3.72 | -30 | -56 | 50 | L | Superior parietal lobule, lateral occipital cortex—superior division, angular gyrus, supramarginal gyrus—posterior division |
| 69 | 3.58 | -6 | -98 | 0 | L | Occipital pole |
| 61 | 3.98 | 22 | -46 | -16 | R | Parahippocampal gyrus, posterior division |
| 46 | 3.53 | 4 | -66 | 22 | Precuneous cortex, cuneal cortex, supracalcarine cortex, intracalcarine cortex | |
| 25 | 3.39 | 54 | -4 | 52 | R | Precentral gyrus, post-central gyrus, middle frontal gyrus |
| REAPPRAISE effect > EMPATHIC WATCH effect [(C-D)—(A-B)] | ||||||
| 45 | 4.12 | 56 | 42 | -10 | R | Inferior frontal gyrus |
Fig. 4.
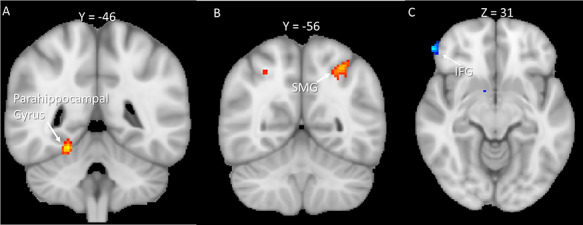
(A and B) Results of empathic watch larger than reappraisal. (C) Results of reappraisal larger than empathic watch. Clusters were derived at z > 3.1 and (corrected) cluster significance P < 0.05. SMG = supramarginal gyrus.
Functional connectivity results
Two independent gPPI analyses were carried out, employing a seed region in the right IFG based on the effect of reappraisal vs empathic watch. These analyses showed divergent patterns for empathizing with painful vs non-painful scenarios as well as for reappraising painful vs non-painful scenarios. Specifically, empathizing with painful scenarios was associated with increased connectivity with the mid-cingulate and ACC, as well as with the bilateral post-central cortex. Conversely, during reappraisal of painful vs non-painful scenarios, increased connectivity was found between the IFG and the bilateral lateral occipital cortex, as well as with the left IFG, left posterior insula and left parahippocampal gyrus. Detailed functional connectivity results are reported in Table 4, with key findings illustrated in Figure 5.
Table 4.
Results of gPPI analysis for the effects of EMPATHIC WATCH and REAPPRAISAL, with the time series of a seed in the IFG. Regions were classified using the Harvard–Oxford Atlas, z > 3.1 and (corrected) cluster significance P < 0.05. Z-MAX values represent peak activity for the cluster. MNI coordinates
| Volume | Z-MAX | X | Y | Z | R/L | |
|---|---|---|---|---|---|---|
| Functional connectivity of the IFG during EMPATHIC WATCH condition | ||||||
| Painful scenario > non-painful scenario | ||||||
| 215 | 3.041 | 20 | −50 | 14 | R | Precuneus, supracalcarine cortex |
| 110 | 3.1602 | −28 | −50 | 16 | L | Precuneus, supracalcarine cortex |
| 105 | 2.7229 | −38 | −38 | 58 | L | Post-central gyrus |
| 57 | 2.9896 | −32 | −24 | 40 | L | Post-central gyrus |
| 44 | 2.8075 | −16 | 20 | 34 | L | Middle cingulate gyrus |
| 38 | 2.8298 | 2 | −18 | 48 | R | Mid-cingulate, supplementary motor area |
| 37 | 2.6564 | −64 | 4 | −4 | L | Superior temporal gyrus |
| 35 | 2.7188 | −50 | −20 | 28 | L | Supramarginal gyrus |
| 33 | 2.473 | 4 | −2 | 36 | R | Anterior cingulate gyrus |
| 31 | 2.7528 | −4 | −28 | −4 | L | Thalamus |
| 27 | 2.6531 | 22 | −46 | 34 | R | Precuneus |
| 22 | 2.8328 | −54 | −4 | −28 | L | Middle temporal gyrus |
| 21 | 2.7157 | 54 | −40 | 54 | R | Supramarginal gyrus |
| Non-painful scenario > painful scenario | ||||||
| 110 | −2.9173 | 56 | −34 | −6 | R | Middle temporal gyrus |
| 67 | −2.9378 | −2 | −42 | −8 | L | Cerebellum left I–IV |
| 35 | −2.7737 | −44 | 22 | 12 | L | Inferior frontal gyrus |
| 34 | −2.8068 | −10 | 48 | 42 | L | Superior frontal gyrus |
| Volume | Max Int | X | Y | Z | R/L | |
| Functional connectivity of the IFG during REAPPRAISAL condition | ||||||
| Painful scenario > non-painful scenario | ||||||
| 166 | 3.0999 | −48 | −74 | 20 | L | Lateral occipital cortex |
| 80 | 3.1135 | 32 | −88 | 24 | R | Lateral occipital cortex |
| 48 | 2.6958 | −60 | 8 | 6 | L | Inferior frontal gyrus |
| 41 | 2.5736 | −40 | −38 | 18 | L | Insula |
| 40 | 2.8461 | −32 | −8 | −30 | L | Parahippocampal gyrus, temporal fusiform cortex |
| 30 | 2.591 | −58 | −34 | −6 | L | Middle temporal gyrus |
| 26 | 2.5268 | −60 | −16 | 8 | L | Planum temporale |
| 24 | 2.8386 | −22 | −64 | 70 | L | Lateral occipital cortex |
| 21 | 2.5674 | −52 | −8 | −28 | L | Inferior temporal gyrus |
| 166 | 3.0999 | −48 | −74 | 20 | L | Lateral occipital cortex |
| 80 | 3.1135 | 32 | −88 | 24 | R | Lateral occipital cortex |
| Non-painful scenario > painful scenario | ||||||
| 24 | −2.7775 | 70 | −40 | 20 | R | Supramarginal gyrus |
Fig. 5.
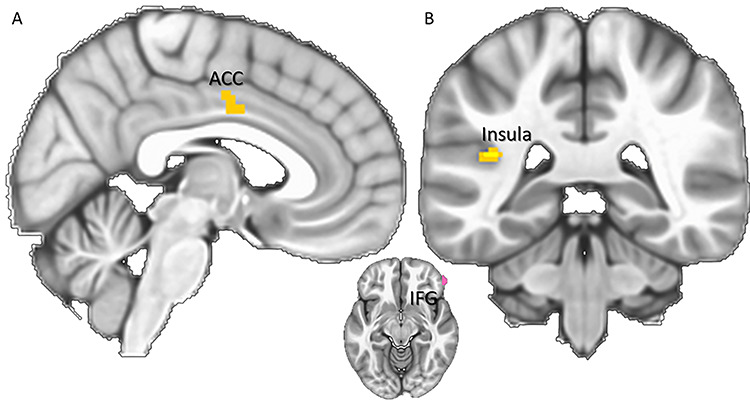
Results of the gPPI functional connectivity analyses, with the time series of a seed in the right IFG (in pink) and the activity during judgments of painful facial expression when empathically watching painful scenarios compared to neutral scenarios (A) and during judgments of painful facial expression when reappraising painful scenarios compared to neutral scenarios (B). Clusters were derived at z > 3.1 and (corrected) cluster significance P < 0.05.
Discussion
The aim of the present study was to examine the neural networks that facilitate the influence of emotion regulation on judgment biases following empathic feelings for the pain of others. To this end, we employed a paradigm that measures biases while participants judge painful facial expressions as a result of the experience of empathy for pain (Naor et al. 2018). We compared judgment biases following empathic feelings for the pain of others to conditions in which participants regulated their empathy using reappraisal.
Replicating our previous work (Naor et al. 2018), the current findings show that the experience of empathy for pain yields a cognitive bias while judging painful facial expressions after experiencing empathy for pain. These biases are eliminated following down-regulation of empathic feelings via reappraisal. Furthermore, these biases are limited to judgments of painful expressions following empathy for pain and do not occur when participants judge happy facial expressions or when they are exposed to non-painful scenarios. These findings support our previous results and show that when individuals naturally feel empathy for the pain of another, they develop a strong bias in their ability to accurately judge the intensity of the pain felt by that other, leading to their perception of the pain as stronger than it really is. We show that reappraisal can eliminate this bias in judgment, resulting in accurate emotion judgments.
The current study adds to our previous behavioral research by showing that regulation of empathy for pain via reappraisal is associated with increased activity in the right IFG. The up-regulation of empathy for pain via empathic watch led to increased activity in a diffused network of regions known to be involved in empathy for pain, including the left SMG, the right precuneus and the right MFG. In addition, neural regions previously associated both with empathy for pain and with reappraisal (e.g. anterior and posterior regions of the left SMG within the IPL as well as the parietal operculum—empathy for pain: Costantini et al., 2008; Li et al., 2019; Morawetz et al., 2015) were involved when participants employed reappraisal following exposure to painful scenarios. A gPPI analysis revealed increased functional connectivity between the right IFG and regions of the network involved in empathy for pain. Interestingly, different regulation strategies resulted in increased connectivity with different parts of the network. Empathic watch resulted in increased connectivity with regions involved in processing of self-pain, while reappraisal resulted in increased connectivity with regions involved in simulation of others pain, as well as self-pain processing (Shamay-Tsoory, 2011).
Activation in the left SMG and the right MFG was found during empathic watch only, suggesting that these two regions play a critical role and are associated with the process of feeling empathy for the pain of others. The following discussion considers the potential role of each of these regions in the regulation of empathy for pain and its influence on judgment biases.
The involvement of the right IFG in regulation of empathy for pain coincides with previous findings outlining a role for the IFG in down-regulation of emotion via reappraisal (Ochsner et al., 2012), and specifically in regulation of social emotions (Grecucci et al., 2013). Moreover, the right IFG alongside the putamen and the SMA have been implicated in emotion regulation via motor inhibition, such as in expressive suppression where individuals physically suppress emotional facial expressions in order to alter their emotions (Vanderhasselt et al., 2012). These findings suggest that the involvement of the right IFG in the current task following regulation of empathy for pain is related to the motor qualities of empathy for pain, such as simulating what happens to others or the activation of different muscles in reaction to the pain of others.
Empathic watch of painful scenarios was associated with increased activity in the left SMG, the right precuneus and the right MFG. The MFG and the adjacent precentral gyrus have been implicated in up-regulation of emotions (Grecucci et al., 2013; Frank et al., 2014). Whereas empathic watch, unlike reappraisal, is not a classical regulation strategy, it does possess all the requirements of such a strategy as it is meant to amplify and elongate a previously exciting emotion (Gross, 2013). Furthermore, among other subregions, the MFG encompasses the dorsolateral prefrontal cortex (DLPFC), which is a central region in cognitive empathy (Kalbe et al., 2010). Researchers have suggested that empathy can be viewed as a complex system that includes two distinct subsystems: emotional empathy and cognitive empathy. Whereas emotional empathy represents the ability to share others’ emotion and includes empathy for pain, cognitive empathy allows for the involvement of more cognitively complex processes, such as perspective-taking and mentalizing (Shamay-Tsoory et al., 2009). Engagement of the DLPFC in emotional empathy is an uncommon finding and may point to the involvement of cognitive empathy in the up-regulation of empathy. An additional indication of this potential role of the DLPFC in empathy for pain emerges from the functional connectivity analysis (see below).
We further examined changes in functional connectivity with the right IFG, which showed enhanced activity during the task, using gPPI analysis. One interesting result is that the IFG, a region involved in the simulation of pain, showed higher connectivity with the insula as well as with the contralateral IFG during reappraisal trials. Whereas during empathic watch trials higher connectivity was found with the ACC. While the IFG is related to the simulation of pain, both the insula and the ACC are part of the empathy for pain network, reportedly responding to observed and felt pain (Shamay-Tsoory, 2011). Yet it seems that each regulation strategy triggers different parts of that network.
Our findings have implications that go beyond a scientific examination of empathy and its functions. Indeed, our approach could add to the framework of Research Domain Criteria (RDoC) project and may serve as a basis for future therapeutic protocols. By validating experimental tasks and protocols the RDoC is aiming to change the way mental disorders are being classified (Morris and Cuthbert, 2012). Gur and Gur (2016) used a simple emotional faces recognition test to show that individuals with schizophrenia exhibit dysfunctional patterns of facial emotion identification. They concluded that an emotion identification performance index of the RDoC’s social cognition domain should be developed and could be used to improve the diagnostics, research focus and eventually treatment of schizophrenia. In line with this conclusion, the task developed in our work can serve as an implicit tool to examine emotion identification in social contexts. Furthermore, it can shed light on the impact of emotion regulation on biases in social contexts and the neural networks mediating them, among healthy individuals and clinical populations.
In this study, we sought to describe the neural network involved in regulation of empathy for pain. The study does, however, have some potential limitations. First, the stimuli used were artificially morphed images of actors portraying emotional facial expressions rather than real representations of individuals experiencing pain. Second, it is possible that the bias in judgment of painful facial expression resulted from greater sensitivity to the visual features portrayed in these expressions, which may be linked to empathic accuracy, rather than from their emotional value per se. Additionally, happy faces were used as control stimuli because they have been shown to be easy to differentiate from painful faces (Naor et al. 2018) and are therefore less likely to skew our results due to mislabeling the perceived emotion, even at low intensities. Nevertheless, we cannot rule out the possibility that the observed differences between happy and painful expressions stem from other factors, such as processing difficulty. Finally, the empathic watch cue was employed as a control condition for the reappraisal condition. However, we cannot rule out the possibility that these instructions enhanced empathy.
This paper is the first to measure and describe the functional networks underlying biased empathic accuracy following empathy for pain. Additional work is required to uncover the depth and complexity of the interaction between emotion regulation and prosocial emotions, as well as the neural networks that govern these interactions. Recent views on emotional processing maintain that such complex behavior is mediated by large cortical and subcortical dynamic brain networks (Pessoa, 2017, 2018). This research represents an initial attempt to map these networks in the context of regulation of empathy for pain and empathic accuracy. The results also raise questions about the differences and similarities between the experience of pain and that of empathy for pain. Rütgen et al. (2015) found that the experience of empathy for pain relies on the same neural responses as well as the same neurotransmitter activity associated with the first-hand experience of pain (for more on this view, see MacDonald and Leary, 2005). Conversely, Singer et al. (2004) claimed that the neural networks involved both in self-pain and in empathy for pain go only as far as regions associated with the affective qualities of pain and not those concerned with its sensory qualities (for more on this view, see Lamm et al., 2011). Whether empathy for pain and first-hand experience of pain share the same neural underpinnings and networks in full, in part or not at all, it would be interesting to compare how such experiences affect empathic accuracy, as well as to examine the effect of emotion regulation on the way such experiences bias empathic accuracy.
In this paper, we attempted to map the neural network that facilitates the regulation of empathy for pain in order to make accurate empathic judgments. Our results demonstrate the importance of emotion regulation in the formulation of complex social systems. Although the literature on empathy is based largely on the premise that adaptive empathic reactions require emotion regulation (Jackson and Decety, 2004), little research has directly explored the contribution of emotion regulation to accurate empathic responses, especially with respect to reappraisal. Indeed, even though empathy is inherently emotional in nature, research on empathy seems to remain primarily focused on shared emotions and not on the way these shared emotions are regulated. Understanding the mechanisms underlying empathy regulation is important given that the purpose of empathy is to alleviate the distress of a suffering target.
Funding
There are no funders to report for this submission.
Conflict of interest
None declared.
Appendix 1 – Full practice and experimental protocol
Slide 1 Intro
I am going to read you the instructions from paper. We are doing that to keep uniformity across all our participants, and as a result, you might feel that there is some repetition. We repeat the instructions to make sure that they are as clear and understood. In this task you are going to view emotional images. Some of these images might induce extremely negative emotions in you, while others very little negative emotions, or they might not induce any emotional reactions.
For every image you would see you would be asked to implement one of two emotion regulation strategy, reappraisal and watch, in a moment I will explain them to you, and we will practice using them. It is important that you try and remember what to do in each of this strategies, as we will ask you about it.
Slide 2 - Reappraisal instructions
We will start by going over reappraisal, the first emotion regulation strategy you will use to change the way are feeling. In reappraisal you are going to try and change the meaning of the image. That is, try and think of something to tell yourself about the image which would help you to reduce any negative feelings you might be experiencing towards it. For example, you could say something about the outcome that the situation is going to be resolved soon, or that help is on the way. You could focus a detail, or an aspect of the situation that is not as negative. However, we want you to stay focused on the image itself, and not just randomly think of other things that might make you feel better. Change something in the meaning of the image that will help you feel less negative emotions towards it.
When you are practicing reappraisal it is important that you do not think that the image is fake, or that it is taken out of a movie. Rather you should think that you are witnessing a real scenario, which you are trying to reappraise. The reason for that is that we are trying to simulate events that happened in real life, where we can not say about them that they didn’t happen.
Slide 3 - Reappraisal que
A blue frame around the image is an indication you should exercise reappraisal. Do you remember what you should do? Could you please shortly explain to me how are you implementing reappraisal?
[After participant’s replay] – Good, remember you should focus on the image, but think of it in a way that would help you reduce any negative emotions you might have.
Slide 4 - Watch instructions
The second strategy you will be asked to practice is watch. During watch you will be asked to observe the image in front of you and allow yourself to experience the emotions in an interrupted way. That is, try not to block your emotions, and not to alter how you are feeling, but allow thoughts and feeling to develop freely.
Slide 5 - Watch que
A red frame around the image is an indication you should exercise watch. Do you remember what you should do? Could you please shortly explain to me how are you implementing watch?
[After participant’s replay] – Good, for as long as the image is presented look at it and allow for your emotions and thoughts to develop freely.
Slide 6 – general explanation
Now you know both strategies – reappraisal and watch, each trial will begin with the appearance of an image on the screen. A couple of second after it’s appearance the image will be surrounded with a frame, either in blue or red. The frame will indicate to you which strategy to implement. Do you remember which color represents which strategy?
Shortly after the appearance of the que frame both frame and image would disappear, and am image of a face expressing an emotion. Using the response box you will be asked to indicate on a scale of 1 to 100 what is the intensity of the expressed emotion.
Footnotes
This comparison is not orthogonal and is shown only to demonstrate the full scope of the effect.
Contributor Information
Navot Naor, University of Maryland, Department of Psychology, College Park, MD 20742-5031, USA.
Christiane Rohr, Max Planck Institute for Human Cognitive and Brain Sciences, Department of Neurology, Leipzig 04103, Germany.
Lina H Schaare, Max Planck Institute for Human Cognitive and Brain Sciences, Department of Neurology, Leipzig 04103, Germany.
Chirag Limbachia, University of Maryland, Department of Psychology, College Park, MD 20742-5031, USA.
Simone Shamay-Tsoory, University of Haifa, Department of Psychology, Haifa 3498838, Israel.
Hadas Okon-Singer, University of Haifa, Department of Psychology, Haifa 3498838, Israel.
References
- Andersson J., Jenkinson M., Smith S. M. (2013). Non-linear registration, aka spatial normalisation FMRIB Analysis Group Technical Reports. TR07JA2. Available: www. fmrib. ox. ac. uk analysis/techrep [October 17, 2017].
- Beckmann C.F., Jenkinson M., Smith S.M. (2003). General multilevel linear modeling for group analysis in FMRI. NeuroImage, 20(2), 1052–63. [DOI] [PubMed] [Google Scholar]
- Blais C., Roy C., Fiset D., Arguin M., Gosselin F. (2012). The eyes are not the window to basic emotions. Neuropsychologia, 50(12), 2830–8. [DOI] [PubMed] [Google Scholar]
- Ciric R., Wolf D.H., Power J.D., et al. (2017). Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage, 154, 174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini M., Galati G., Romani G.L., Aglioti S.M. (2008). Empathic neural reactivity to noxious stimuli delivered to body parts and non-corporeal objects. European Journal of Neuroscience, 28(6), 1222–30. [DOI] [PubMed] [Google Scholar]
- Decety J. (2010). The neurodevelopment of empathy in humans. Developmental Neuroscience, 32(4), 257–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enticott P.G., Johnston P.J., Herring S.E., Hoy K.E., Fitzgerald P.B. (2008). Mirror neuron activation is associated with facial emotion processing. Neuropsychologia, 46(11), 2851–4. [DOI] [PubMed] [Google Scholar]
- Fitzgibbon B.M., Giummarra M.J., Georgiou-Karistianis N., Enticott P.G., Bradshaw J.L. (2010). Shared pain: from empathy to synaesthesia. Neuroscience & Biobehavioral Reviews, 34(4), 500–12. [DOI] [PubMed] [Google Scholar]
- Frank D.W., Dewitt M., Hudgens-Haney M., et al. (2014). Emotion regulation: quantitative meta-analysis of functional activity and deactivity. Neuroscience & Biobehavioral Reviews, 45, 202–11. [DOI] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. (1997). Psychophysiological and modulatory interactions in neuroimaging. NeuroImage, 6(3), 218–29. [DOI] [PubMed] [Google Scholar]
- Grecucci A., Giorgetta C., Bonini N., Sanfey A.G. (2013). Reappraising social emotions: the role of inferior frontal gyrus, temporo-parietal junction and insula in interpersonal emotion regulation. Frontiers in Human Neuroscience, 7, 523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve D.N., Fischl B. (2009). Accurate and robust brain image alignment using boundary-based registration. NeuroImage, 48(1), 63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J. (Ed.). (2013). Handbook of emotion regulation, Guilford publications. [Google Scholar]
- Gur R.C., Gur R.E. (2016). Social cognition as an RDoC domain. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 171(1), 132–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J.A., Schmid Mast M. (2007). Sources of accuracy in the empathic accuracy paradigm. Emotion, 7(2), 438. [DOI] [PubMed] [Google Scholar]
- Ickes W., Stinson L., Bissonnette V., Garcia S. (1990). Naturalistic social cognition: Empathic accuracy in mixed-sex dyads. Journal of Personality and Social Psychology, 59(4), 730. [Google Scholar]
- Jackson P.L., Decety J. (2004). Motor cognition: A new paradigm to study self–other interactions. Current opinion in Neurobiology, 14(2), 259–63. [DOI] [PubMed] [Google Scholar]
- Jenkins L.M., Andrewes D.G., Nicholas C.L., et al. (2014). Social cognition in patients following surgery to the prefrontal cortex. Psychiatry Research: Neuroimaging, 224(3), 192–203. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5(2), 143–56. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage, 17(2), 825–41. [DOI] [PubMed] [Google Scholar]
- Kalbe E., Schlegel M., Sack A.T., et al. (2010). Dissociating cognitive from affective theory of mind: a TMS study. Cortex, 46(6), 769–80. [DOI] [PubMed] [Google Scholar]
- Kuypers K.P.C., Steenbergen L., Theunissen E.L., Toennes S.W., Ramaekers J.G. (2015). Emotion recognition during cocaine intoxication. European Neuropsychopharmacology, 25(11), 1914–21. [DOI] [PubMed] [Google Scholar]
- Lamm C., Decety J., Singer T. (2011). Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage, 54(3), 2492–502. [DOI] [PubMed] [Google Scholar]
- Li Y., Zhang T., Li W., Zhang J., Jin Z., Li L. (2019). Linking brain structure and activation in anterior insula cortex to explain the trait empathy for pain. Human Brain Mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald G., Leary M.R. (2005). Why does social exclusion hurt? The relationship between social and physical pain. Psychological Bulletin, 131(2), 202. [DOI] [PubMed] [Google Scholar]
- McLaren D.G., Ries M.L., Xu G., Johnson S.C. (2012). A generalized form of context-dependent psychophysiological interactions (gPPI): a comparison to standard approaches. NeuroImage, 61(4), 1277–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K., Ciesielski B., Gross J.J. (2012). Unpacking cognitive reappraisal: goals, tactics, and outcomes. Emotion, 12(2), 250. [DOI] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. (2010). Saliency, switching, attention and control: a network model of insula function. Brain Structure and Function, 214(5-6), 655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawetz C., Bode S., Baudewig J., Kirilina E., Heekeren H.R. (2015). Changes in effective connectivity between dorsal and ventral prefrontal regions moderate emotion regulation. Cerebral Cortex, 26(5), 1923–37. [DOI] [PubMed] [Google Scholar]
- Morris S.E., Cuthbert B.N. (2012). Research domain criteria: cognitive systems, neural circuits, and dimensions of behavior. Dialogues in Clinical Neuroscience, 14(1), 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naor N., Shamay-Tsoory S.G., Sheppes G., Okon-Singer H. (2018). The impact of empathy and reappraisal on emotional intensity recognition. Cognition and Emotion, 32(5), 972–987. [DOI] [PubMed] [Google Scholar]
- O’Reilly J.X., Woolrich M.W., Behrens T.E., Smith S.M., Johansen-Berg H. (2012). Tools of the trade: psychophysiological interactions and functional connectivity. Social Cognitive and Affective Neuroscience, 7(5), 604–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. (2012). Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences, 1251(1), E1–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2017). A network model of the emotional brain. Trends in Cognitive Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L. (2018). Understanding emotion with brain networks. Current Opinion in Behavioral Sciences, 19, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruim R.H., Mennes M., Buitelaar J.K., Beckmann C.F. (2015a). Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. Neuroimage, 112, 278–87. [DOI] [PubMed] [Google Scholar]
- Pruim R.H., Mennes M., Rooij D., Llera A., Buitelaar J.K., Beckmann C.F. (2015b). ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. Neuroimage, 112, 267–77. [DOI] [PubMed] [Google Scholar]
- Rütgen M., Seidel E.M., Silani G., et al. (2015). Placebo analgesia and its opioidergic regulation suggest that empathy for pain is grounded in self pain. Proceedings of the National Academy of Sciences, 112(41), E5638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley W.W., Allman J.M., Carlin D.A., et al. (2007). Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Disease & Associated Disorders, 21(4), S50–7. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G. (2011). The neural bases for empathy. The Neuroscientist, 17(1), 18–24. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory S.G., Aharon-Peretz J., Perry D. (2009). Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain, 132(3), 617–27. [DOI] [PubMed] [Google Scholar]
- Singer T. (2006). The neuronal basis and ontogeny of empathy and mind reading: review of literature and implications for future research. Neuroscience and biobehavioral reviews, 30(6), 855–63. [DOI] [PubMed] [Google Scholar]
- Singer T., Seymour B., O'doherty J., Kaube H., Dolan R.J., Frith C.D. (2004). Empathy for pain involves the affective but not sensory components of pain. Science, 303(5661), 1157–62. [DOI] [PubMed] [Google Scholar]
- Singer T., Lamm C. (2009). The social neuroscience of empathy. Annals of the New York Academy of Sciences, 1156(1), 81–96. [DOI] [PubMed] [Google Scholar]
- Smith S.M. (2002). Fast robust automated brain extraction. Human Brain Mapping, 17(3), 143–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.J.L., Montagne B., Perrett D.I., Gill M., Gallagher L. (2010). Detecting subtle facial emotion recognition deficits in high-functioning autism using dynamic stimuli of varying intensities. Neuropsychologia, 48(9), 2777–81. [DOI] [PubMed] [Google Scholar]
- Vanderhasselt M.A., Kühn S., De Raedt R. (2012). ‘Put on your poker face’: neural systems supporting the anticipation for expressive suppression and cognitive reappraisal. Social Cognitive and Affective Neuroscience, 8(8), 903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. (2008). Robust group analysis using outlier inference. NeuroImage, 41(2), 286–301. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. (2001). Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage, 14(6), 1370–86. [DOI] [PubMed] [Google Scholar]
- Woolrich M.W., Behrens T.E., Beckmann C.F., Jenkinson M., Smith S.M. (2004). Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage, 21(4), 1732–47. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. (2001). Statistical analysis of activity images. Functional MRI: An introduction to methods, 14(1), 251–70. [Google Scholar]
- Zaki J., Bolger N., Ochsner K. (2008). It takes two: The interpersonal nature of empathic accuracy. Psychological science, 19(4), 399–404. [DOI] [PubMed] [Google Scholar]
- Zaki J., Weber J., Bolger N., Ochsner K. (2009). The neural bases of empathic accuracy. Proceedings of the National Academy of Sciences, 106(27), 11382–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


