Abstract
This study offers a suitable and easy proliposome-liposome method that enhances the encapsulation ability of liposome structures on poor water-soluble extracts. Pollen phenolic extract (PPE) was studied to show applicability in the proposed method. The poor water-soluble PPE (0.2%, w/v) was encapsulated by liposomes generated from proliposomes (P-liposomes) that were prepared via high-pressure homogenization technique without using any organic solvents and high temperature. Only a few drops of ethanol were used to dissolve poor water-soluble compounds in PPE during the preparation of P-liposomes. The trace amount of ethanol maintained the improvement of PPE solubility in P-liposome dispersion, hence the in vitro bioaccessibility and bioactivity of PPE incorporated in P-liposomes increased. Thus, higher encapsulation efficiency was found in P-liposomes compared to conventional liposomes (C-liposomes) in which the EE was 75 and 73%, respectively. To increase the physical stability of liposome structures, the surface of both P-liposomes and C-liposomes was covered with chitosan. There were found small changes between P-liposomes and C-liposomes in terms of mean diameter size and zeta potential. On the other hand, the bioactivity of encapsulated PPE showed differences in P-liposomes and C-liposomes. The antioxidant capacity of PPE in P-liposomes enhanced approximately two times in CUPRAC and three times in DPPH assays. Also, in vitro bioaccessibility of PPE in P-liposomes increased approximately 4 and 2 folds, respectively, regarding total phenolics and flavonoids. To our knowledge, this is the first report about the increment of encapsulation behavior of liposome structures on low water-soluble extract within an aqueous media.
Keywords: Food science, Food technology, Natural product chemistry, Pollen extract, Phenolics, Proliposome, Liposome, In vitro bioaccessibility
Food science, Food technology, Natural product chemistry, Pollen extract, Phenolics, Proliposome, Liposome, In vitro bioaccessibility.
1. Introduction
Pollens are microspores of seed plants and are sources of protein for young adult honey bees. While a worker bee is passing through anthers of flowers, pollen sticks to the body. The flowers transmit the special movements of the legs alone and the right pollen to the tibiae cavity in the hind legs. During this process, a pollen load occurs on each of these legs. It is collected by humans from the entrance of the hive, so it is produced without any processing (Crane, 2009).
After bee pollen is defined as a nutrient in food legislation, the nutritional values of pollen have gained importance (Campos et al., 2010). Bee pollen contains about 50% polysaccharides, 1–20% fats and lipids, 6–28% protein, 6% amino acids, 4–10% simple sugars and accompanied by minerals (Cu, Fe, Zn, K, Na), several vitamins, (provitamin A, vitamin E, biotin, folic acid, thiamine, niacin) and a variety of secondary plant products such as terpenes, carotenoids, flavonoids, and phenolic acids (Campos et al., 2008). In particular, phenolic compounds composed of flavonoids and phenolic acids exhibit strong antioxidant activity. These natural bioactive compounds have a variety of beneficial effects including anti-carcinogen, anti-inflammatory, anti-tumor, and anti-oxidant (Duan et al., 2019). However, phenolic compounds are sensitive and strongly influenced by adverse environmental and process conditions. They also have poor bioaccessibility. In this sense, encapsulation techniques could improve their stability and bioaccessibility (Motilva et al., 2016). Liposomes consisting of phospholipid vesicles are versatile carriers for both hydrophilic and hydrophobic bioactive molecules such as antimicrobials, flavors, antioxidants, and bioactive compounds (Moraes et al., 2013). They are considered as promising delivery systems for phenolic compounds. Depending on the solubility of the bioactive molecules, there are several conventional and novel liposome preparation methods divided by mean size, polydispersity, and lamellarity of liposomes provided (Patil and Jadhav, 2014). In all these methods, there are three main steps for the preparation of the liposomes including removing lipids from an organic solvent, spreading the lipid in an aqueous phase, and purifying the liposomes. While sonication, high-pressure homogenization and supercritical fluid treatment are suitable for liposomal encapsulation of hydrophilic compounds; ethanol/ether injection and reverse phase evaporation methods are suitable for liposomal entrapment of lipophilic compounds (Lin et al., 2019; Jaafar-Maalej et al., 2010). Since pollen extract contains lipophilic and poorly water-soluble compounds, liposome preparation methods for lipophilic compounds could be applied to encapsulate pollen phenolics. To increase the solubility of lipophilic compounds and poorly water-soluble compounds, organic solvents such as ethanol, isopropyl ether at high temperature are required in these methods (Lin et al., 2019). In the literature, several authors reported liposome preparation techniques for lipophilic compounds using organic solvents (Charcosset et al., 2015; Sebaaly et al., 2016; Toniazzo et al., 2017). Among these organic solvents, ethanol is harmless and accepted by the authorities, but even ethanol is not preferred to be used by consumers. Therefore, the aim of this study was to encapsulate poorly water-soluble pollen extract into liposome using an aqueous phase without any organic solvent. In this concept, the proliposome-liposome method based on the conversion of the initial proliposome preparation into a liposome dispersion by dilution with an aqueous phase was employed with some modifications. In the proliposome-liposome method, organic solvents are replaced by an aqueous solution. This replacement was performed by stepwise addition of the aqueous phase to the ethanolic phase. Then, primary liposomes were produced by high-pressure homogenization technique. To our knowledge, there is limited study regarding the proliposome-liposome method. Even in these reports, the researchers used Tris-HCl and heating treatment, which were not included in this study. In addition, the surface of liposomes that were generated from poliposomes was covered with a natural polymer, chitosan layer in this study. Since liposomes are fragile particles, surface covering improves their kinetic stability (Altin et al., 2018a). In the current study, the encapsulation ability of conventional liposomes and liposomes generated from proliposomes were compared and the impact of surface covering on them was evaluated. In this perspective, surface charge (the zeta (ζ) potential), particle size, and encapsulation efficiency of each liposome structure were determined. To understand the effect of extract solubility on encapsulation behavior, the content and location of pollen phenolic extract within each liposome structure and in vitro bioaccessibility of encapsulated pollen phenolic extract were analyzed.
2. Material and methods
2.1. Materials
Pollen samples were kindly provided by a local beekeeper in Istanbul. Lecithin (soybean phospholipids 97% Ultralec P) was a gift from Rotel, Turkey. Chitosan with 80% DDA (degree of deacylation) was granted by Primex (Sighufjordur, Iceland). Sephadex G50 was obtained from GE Healthcare Life Sciences (Uppsala, Sweden). Other chemicals, standards, and reagents were purchased from Sigma-Aldrich Co. (St. Louis, USA).
2.2. Preparation of pollen phenolic extract (PPE)
The extraction of pollen phenolics was performed according to the method of de Florio Almeida et al. (2017) with some modifications. Pollen samples were ground using liquid nitrogen. To remove moisture, pollen samples were freeze-dried almost for 16 h (Christ Alphna 1–2 LDplus, Osterode am Harz, Germany). Then, phenolic compounds were extracted by ethanol/MQ water (80%, v/v) with a ratio of 1:10 (w/v) in an ultrasonic water bath at 30 °C for 90 min. After centrifugation at 11.000 rpm for 20 min at 30 °C, the top phase was collected. The extraction process was repeated at three times and collected top phases were pooled. Then, ethanol was removed by a rotary evaporator (Bibby Sterilin RE-100, Bibby Scientific Limited, Staffordshire, UK) at 40 °C. The remained extract was freze-dried and stored at -20 °C.
2.3. Preparation of primary and secondary liposomes
Liposomes were prepared described by Gültekin-Özgüven et al. (2016) with some modifications. To prepare primary liposomes, lecithin powder 2% (w/v) was dissolved in acetate buffer (pH = 3.5 ± 0.1; 0.1 M) overnight at room temperature. Conventionally, freeze-dried PPEs (0.1 and 0.2 w/v) were dissolved in this lecithin solution. In the modified proliposome-liposome method, only a few drops of ethanol were used to dissolve freeze-dried PPEs (0.1 and 0.2%, w/v) before the addition of the water phase containing lecithin. Conventional liposomes and liposomes generated from proliposomes with and without PPE were prepared by homogenizing dispersions with a high shear disperser (DI-25 Yellowline, IKA) at 9.500 rpm for 10 min before the dispersions were passed five times via high-pressure homogenizer (Microfluidizer Processor M-110L, Microfluidics, Newton, USA) at a homogenization pressure of 25.000 psi. To obtain secondary liposomes, all liposome dispersions with and without PPE were added to chitosan solution (0.4%, w/w) dissolved in an acetate buffer solution with a ratio of 1:1 (w/w) and stirred overnight at room temperature.
2.4. Measurement of zeta potential and particle size distribution
The average size of liposomes was measured by a static light-scattering instrument (Mastersizer, 2000; Malvern Instruments, Malvern, United Kingdom). The zeta potential of the liposomes was measured with a zeta sizer (Mastersizer 2000; Malvern Instruments, Malvern, United Kingdom). Prior to measurement of the particle size and zeta potential, an aliquot of liposomal suspensions was diluted with an acetate buffer solution at pH 3.5. All measurements were repeated at least five times.
2.5. Removal of unencapsulated extract by gel filtration
According to the study described by Gibis et al. (2016), sephadex gel filtration process was used to remove both chitosan not bound to liposomal surfaces and the unencapsulated extract in liposomes. Sephadex G50 gel solution (5%, w/w in deionized water) was prepared and loaded into the injectors (6 mL) until a gel layer of approximately 3 cm was formed. Then, injectors were placed into falcon tubes and 1.5 mL of sample was loaded onto the column located in these falcon tubes. After centrifugation of falcon tubes at 3000 rpm for 20 min, unbound chitosan and the unencapsulated extract was removed by the gel solution.
2.6. Spectrophometric analysis
2.6.1. Determination of total phenolic content (TPC)
Total phenolic content was determined according to the Folin-Ciocalteu method (Chen et al., 2015). 1.5 mL of 10 times diluted Folin-Ciocalteu reagent and 1.2 ml of 7.5 % (w/v) sodium carbonate solution were added on 200 μL of the diluted sample. After standing at 25 °C for 48 min in the dark, the extinction of the mixture was measured on a UV-Vis spectrophotometer (BioTek Instruments, Winooski, USA) at 765 nm. The results were expressed as mg gallic acid per L sample. All samples were analyzed in triplicate.
2.6.2. Determination of total flavonoid content (TFC)
To determine the total flavonoid content, 250 μL sample was mixed with 1.25 mL of MQ water. At times of zero, 75 μL of NaNO2 (5%, w/v) solution; at the 6th min, 150 μL of aluminum chloride solution (10% w/v); at the 11th min., 500 μL of NaOH (1M) were mixed. After the addition of 2.5 mL of MQ water, the extinction of the mixture was measured at 510 nm. Results were expressed as mg of rutin equivalents per L sample (Dewanto et al., 2002; Valcarcel et al., 2015). All samples were analyzed in triplicate.
2.6.3. Determination of total antioxidant capacity (TAC)
To determine total antioxidant capacity, CUPRAC (cupric ion reducing antioxidant capacity) and DPPH (2,2-diphenyl-1-picrylhydrazyl) assays were employed.
2.6.3.1. Determination of CUPRAC antioxidant capacity
In CUPRAC assay, 100 μL sample was mixed with 1 mL of ammonium acetate (pH:7), 1mL of neocuproine solution (7.5 × 10−3 M), 1 mL of copper (II) chloride solution (10−2 mM), and 1mL of MQ water, respectively. The mixture was left to stand for 30 min in dark and its extinction was determined at 450 nm (Apak et al., 2004).
2.6.3.2. Determination of DPPH antioxidant capacity
In DPPH assay, 100 μL sample and 2 mL of DPPH solution in methanol (10−1 mM) were mixed. After standing 30 min in the dark, extinction of the mixture was measured at 517 nm. The results were expressed as mg Trolox equivalents (TE) per L sample for both CUPRAC and DPPH assays. All samples were analyzed in triplicate (Kumaran and Karunakaran, 2006).
2.7. Determination of encapsulation efficiency
To decide the optimum PPE concentration for encapsulation, two different concentrations of PPE (0.1–0.2%, w/v) were selected based on our previous experiences and their encapsulation efficiency. For this purpose; after gel filtration, the phenolic content of the samples was measured to detect the content of extract in the liposomal bilayer. Then, gel filtered liposomes were destabilized by the addition of 3 mL of % 0.15 (w/v). Triton X-100 to detect the phenolic content inside the liposomes by the subtraction of phenolic content in intact liposomes and phenolic content in destabilized liposomes (Gibis et al., 2016). To measure phenolics, the Folin-Ciocalteu method described in 2.6.1 was performed. Encapsulation efficiency (%) was calculated according to the following Eq. (1)
| (1) |
2.8. In vitro gastrointestinal digestion
In vitro bioaccessibility studies were performed according to the method described by Altin et al., 2018a, Altin et al., 2018b. In brief, 13.5 ml of basal saline (140 mM NaCl and 5mM KCl) and 1.5 mL of sample was mixed in a shaking water bath (New Brunswick Scientific Co., Inc., New Jersey, USA) at 37 °C for 10 min. To start the gastric digestion, 4.5 mL of simulated gastric fluid containing 3.2 g/L pepsin in 1M HCl was added to the mixture followed by adjusting pH to 2.0. After samples were incubated for 1 h at 37 °C, the pH of the mixture was set to 7.5 using 1.0 M NaOH. Finally, 4.5 mL simulated intestinal fluid (4.76 mg/ml pancreatin and 5.16 mg/ml porcine bile extract in PBS, pH 7.5) was added to the mixture. During 2 h incubation stimulating intestinal environment, the pH of the mixture was maintained at 7.5. To terminate enzymatic reactions, digested samples were centrifuged at 6000 rpm for 10 min at 4 °C, and collected top layers were acidified. Then, they were kept at -20 °C for further analysis. The bioaccessibility (%) was calculated according to the following Eq. (2).
| (2) |
2.9. Quantification and identification of phenolic compounds in PPE by high pressure liquid chromotography (HPLC)
The HPLC analysis was performed using a Shimadzu 20A series ultra-fast liquid chromatography, coupled with degasser, autosampler, column oven, and SPD M20A model PDA detector (UFLC, Shimadzu Corporation, Kyoto, Japan). The chromatographic separations were performed on an Inertsil C18 column (150 mm x 4,6 mm, 3 μm). A gradient of mobile phase A (water with 0.75 % formic acid) and mobile phase B (methanol with 0.75 % formic acid) was used. The flow rate was 1 mL/ml, the injection volume was 10 μL and the column temperature was set to 40 °C. A 55 min gradient program was used with the gradient profile as follows: 0–3 min: 5% B, 3–18 min: 40% B, 18–45 min: 80% B, 48–50 min: 100% B, 52–55 min: 5% B.
2.10. Statistical analysis
IBM SPSS Statistics 24.0 (Chicago, IL, USA) software was employed for statistical analysis. All analyses were performed at least in triplicate. The results were expressed as mean ± standard deviation. Differences were analyzed by Turkey's post-test comparisons and significant differences were determined using p-value of <0.05.
3. Results and discussion
3.1. Characterization of primary and secondary C-Liposomes and P-Liposomes
Primary and secondary C-liposomes and P-liposomes with and without PPE were characterized by according to their particle size and surface charges, where results were represented in Table 1. The incorporation of PPE into the liposomal dispersions caused a reduction of particle size. This decrement could be related to the formation of covalent links between lipids and phenolics. The lipophilic character of PPE had triggered the placement of the PPE on the surface of the liposomes that caused the formation of lipophenol structures on the surface of liposome particles (Van Dael and Ceuterickx, 1984). In the proliposome stage, the solubility of lipophilic PPE was increased. Thus, more PPE could interact with the lipid surface of liposomes, which might be caused to the formation of smaller particles in proliposome-liposome samples (Table 1). On the other hand, the addition of cationic polymer, chitosan, to the structures caused at least 4 folds increment in particle size both in P-liposomes and C-liposomes with and without PPE (Table 1). Moreover, the surface charge of the structures change from negative to positive, which means that positively charged chitosan deposited on the surface of the encapsulates in each case (Table 1). The increment of particle size and alteration of the surface charge of the liposomes are two important parameters for successful surface coating. In our previous studies, we have reported that the introduction of the negatively charged phenolic extract to the anionic liposomal dispersion increase the negativity of the total zeta potential of liposomal dispersions (Gültekin-Özgüven et al., 2016; Altin et al., 2018b; Akgün et al., 2020). However, the surface charge of phenolic pollen extract in pH between 3.0-3.5 has been found as neutral (Sebii et al., 2019). For this reason, the addition of zero charged pollen phenolic extract to the dispersions of P-liposomes and C-liposomes did not affect the zeta potential of particles (Table 1).
Table 1.
Particle size and z epotential of primary and secondary liposome dispersions with and without pollen extract.
| Particle Size (nm) |
ζ -potential (mV) |
|||
|---|---|---|---|---|
| C-Liposome | P-Liposome | C-Liposome | P-Liposome | |
| Primary liposomes without pollen extract | 95.43 ± 1.1 | 96.28 ± 1.3 | -31.2 ± 2.1 | -30.6 ± 1.2 |
| Primary liposomes with pollen extract | 86.89 ± 1.3 | 83.42 ± 1.5 | -31.7 ± 1.5 | -30.3 ± 2.2 |
| Secondary liposomes without pollen extract | 355 ± 4.2 | 394 ± 3.8 | 52 ± 1.8 | 54.13 ± 1.2 |
| Secondary liposomes with pollen extract | 395 ± 4.3 | 402 ± 4.1 | 51 ± 2.2 | 42.03 ± 1.5 |
∗Values are presented as mean values ±standard deviation (n = 3).
3.2. Encapsulation efficiency (EE), content and location of PPE in primary and secondary C-Liposomes and P-Liposomes
In several studies (Akgün et al., 2020; Altin et al., 2018b; Gibis et al., 2014; Gültekin-Özgüven et al., 2016), it has been indicated that the optimum encapsulation efficiency with liposomes can be reached using 0.1–0.2% (w/v) of phenolic concentration. To compare the encapsulation ability of P-liposomes respect to the C-liposomes in terms of optimum encapsulation efficiency, PPEs with a concentration of 0.1 and 0.2% (w/v) were studied. The EE was found to be 85% and 81% at a concentration of 0.1% (w/v) of pollen phenolic extract in C-liposomes and P-liposomes, respectively. However, there was a reduction of EE when the concentration of pollen phenolic extract was increased. Thus, the formulation with 0.2% (w/v) concentration of PPE had 73 and 75% of EE in C-liposomes and P-liposomes, respectively. According to the EE results, it can be said that C-liposomes and P-liposomes had a similar encapsulation capacity. In other words, the conversion of proliposomes to liposomes did not affect the encapsulation process. In fact, the EE results are comparable with our previous studies (Altin et al., 2018b; Akgün et al., 2020). While the formulations with 0.1% (w/v) of PPE resulted in the highest encapsulation efficiency, 0.2% (w/v) concentration of PPE was selected to make the spectrophotometric measurements easier due to multiple dilutions in the further steps. The content and location of the PPE in terms of TAC, TPC, and TFC were shown in Table 2. TAC and TFC results showed that more PPE was loaded on P-liposomes compared to C-liposomes in both primary and secondary liposomes. The lipid structure of the liposome surface led to an accumulation of the low water-soluble PPE on it (Table 2). According to the findings, the surface of primary C-liposomes contained more PPE compared to the primary P-liposomes in terms of TAC-CUPRAC, TAC-DPPH, and TFC. Increment of the PPE solubility by a few drops of ethanol led to encapsulation of more PPE in P-liposomes (interior).
Table 2.
Content and location of TPC (mg GAE/L), TFC (mg CE/L), and TAC (mg TE/L) and in primary and secondary liposome dispersions with pollen extact.
| TAC-CUPRAC (mg/L) |
TAC-DPPH (mg/L) |
TFC (mg/L) |
TPC (mg/L) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surface of liposome | Interior of liposome | Total | Surface of liposome | Interior of liposome | Total | Surface of liposome | Interior of liposome | Total | Surface of liposome | Interior of liposome | Total | |
| Primary C-Liposomes |
60.21 ± 3.2a | 458.5 ± 3.5a | 518,7a | 85.71 ± 6.9a | 419.9 ± 5.4a | 505.61a | 109.± 4.5a | 519.4.± 4.5a | 628,4a | 48.08.±3.1a | 476.7 ± 3.6a | 524.78a |
| Primary P-liposomes |
21.04 ± 5.8b | 504.7 ± 19b | 525,74b | 27.4 ± 4.85b | 506.5.± 24b | 533.9b | 135.6 ± 3.6b | 514.8.± 1.3b | 650.4b | 17.13.±1.3b | 446.2 ± 0.87b | 463.33b |
| Secondary C-liposomes |
42.02 ± 3.5c | 504.5 ± 17bc | 546,52c | 71.83 ± 2.3c | 531 ± 7.8c | 602.83c | 162.5.±2.7c | 548.6.± 1.6c | 711.1c | 33 ± 1.14c | 551.1 ± 3.4c | 584.1c |
| Secondary P-liposomes | 45.4 ± 5.76cd | 512.5 ± 24d | 557,9d | 39 ± 6.76d | 503.1 ± 8.3bd | 542.1d | 188.± 8.5d | 557 ± 16.8d | 745d | 41.4.± 2.1d | 551.1.± 27cd | 592,5d |
∗∗Values are presentes as mean values ±standard deviation (n = 3). Different small letters in the columns represent statistically significant differences (p < 0.05) in each.
TPC: Total phenolic content, TFC: total flavonoid content, TAC: total antioxidant capacity, GAE: gallic acid equivalents, TE: trolox equivalents.
The more antioxidant compound was detected in the DPPH method compared to the CUPRAC which was related to the working mechanism of each method. While DPPH allows determining the lipophilic antioxidative compounds, in CUPRAC method both lipophilic and hydrophilic antioxidative compounds can be analyzed (Apak et al., 2007).
The TPC was determined in higher amounts in C-liposomes both in primary and secondary structures compared to the P-liposomes (Table 2) which might be related to the low accessibility of TPC reagents to the phenolic groups in P-liposomes. As it explained on particle size results, the formation of covalently linked lipohenol structures was maintained by the increment of the solubility of PPE in proliposome step within the liposomal dispersion. Therefore, the accessibility of phenolic groups was reduced against the Folin-Ciocalteu reagent. These findings were compatible with particle size results (Table 1).
3.3. Effect of in vitro digestion on PPE in C-liposomes and P-liposomes
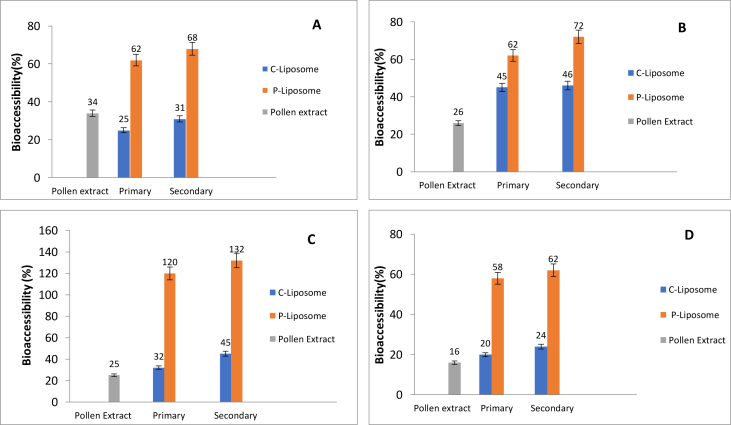
The bioaccessibility of bioactive compounds of PPE (0.2 %, w/v) was investigated in C-liposomes and P-liposomes (primary and secondary) under in vitro gastro intestinal digestion model (Figure 1). Freeze-dried PPE (0.2 % (w/v) which was dissolved in acetate buffer (pH 3.5), PPE (0.2 % (w/v) which was dissolved in a few drops of ethanol) were studied as control groups. The amount of TPC, TAC, and TFC of the in vitro digested samples and bioaccessibility (%) of PPE were calculated. The results showed that the pure PPE had the lowest bioaccessibility compared to both C-liposomes and P-liposomes with PPE. Moreover, the encapsulation of PPE via P-liposomes resulted in a dramatic increment of the bioaccessibility of the PPE. In the proliposome step, the addition of a few drops of ethanol caused to the improvement of the solubility of PPE in liposome dispersion. Thus, PPE became more bioaccessible within the encapsulation formulation. Indeed, compared to the pure PPE, C-liposome encapsulation did not improve the in vitro bioaccessibility of PPE in terms of TAC (Figure 1 A, B). The surface coating of liposomes with cationic chitosan resulted in an increment of the in vitro bioaccessibility. Because, the presence of a polymer layer on the surface of the liposomes helped the elimination of both enzymatic and acidic degradation of PPE, which was located on the surface of the liposomes, in the gastric phase. Hence, PPE which was located in the surface as well as inside of the liposomes accessed to the intestine phase (Altin et al., 2018a). Moreover, regarding TPC and TFC, the bioaccessibility of PPE phenolics was increased approximately 4 and 2 folds, respectively, when the PPE encapsulated by P-liposomes. In addition, TAC (CUPRAC and DPPH assays) of PPE enhanced approximately two and three times in P-liposomes, respectively.
Figure 1.
The bioaccessibility of (A) antioxidant (DPPH), (B) flavonoid, (C) phenolic, (D) antioxidant (CUPRAC) compound in pollen extracts where in freeze dried form (0.2 %), in primary liposome (0.2 %) and secondary liposome forms.
3.4. Identification of the changes in the polyphenol profile of PPE during encapsulation before and after in-vitro digestion
Six different polyphenol compounds namely gallic acid, 4 (p-)hydroxybenzoic acid, pectolinaringenin, rutin, luteolin, and apigenin have been determined in PPE (Table 3). These compounds were detected in higher amounts in primary P-liposome compared to PPE, which corresponded to the protection of liposomal encapsulation of phenolic compounds against adverse degradation factors. However, in secondary P-liposome, all phenolic compounds were found in a lower amount compared to PPE and P-liposomes. This situation can be explained by the interaction between polyphenol compounds and chitosan (Popa et al., 2000). While the formation of polyphenol-chitosan complex restricted the detection of individual phenolic compounds in HPLC analysis, phenolics can still show the antioxidant activity within this complex. Thus, the amount of TAC, TPC, and TFC of secondary P-liposome was higher than the primary P-liposome and PPE (Table 2).
Table 3.
Changes in the polyphenol profile of PPE during encapsulation before and after in-vitro digestion.
| Phenolic compound |
Before in vitro digestion |
After in vitro digestion |
||||
|---|---|---|---|---|---|---|
| PPE (0.2%) |
Primary P-liposome with PPE (0.2%) |
Secondary P-liposome with PPE (0.1%) |
PPE (0.2%) |
Primary P-liposome with PPE (0.2%) |
Secondary P-liposome with PPE (0.1%) |
|
| Concentration (mg/100ml) | ||||||
| Gallic acid | 0.37 | 2.80 | <0.1 | 0.97 | <0.1 | ND |
| 4 (p-)Hydroxybenzoic Acid | 5.40 | 6.20 | ND | 2.08 | 0.33 | ND |
| Pectolinaringenin | 16.63 | 17.50 | 14.93 | 5.97 | 16.68 | ND |
| Rutin | 1.82 | 1.84 | <0.1 | 2.81 | 2.07 | 0.03 |
| Luteolin | 5.35 | 6.80 | 0.56 | 4.97 | 4.10 | 2.38 |
| Apigenin | 0.55 | 1.10 | <0.1 | 0.40 | 0.51 | 0.96 |
PPE: Pollen phenolic extract.
The polyphenol profile of in vitro digested samples is also shown in Table 3. It has been found that the amount of polyphenol compounds namely 4 (p-)hydroxybenzoic acid, pectolinaringenin, luteolin, and apigenin were reduced after in vitro digestion. On the other hand, there was an increase in gallic acid amount in the digested sample of PPE. Gallic acid can be found as a free form or as a component of hydrolyzable tannins (Amarowicz and Janiak, 2019). The increment of the gallic acid amount in the digested sample of PPE can be related to the degradation of hydrolyzable tannins to gallic acid and other derivatives during in vitro digestion. In the digested sample of primary P-liposome, gallic acid was found <0.1 mg/100 mL. Gallic acid undergoes structural changes during the gastric and intestine phase where 4-O-methyl gallic acid, 3-O-methyl gallic acid, and 3,4-O-dimethylgallic acid are the major metabolites of gallic acid (Yucetepe et al., 2020). Because of these structural changes, the detected amount of gallic acid in digested samples was found low amount in HPLC. The increment of the detected amount of rutin, luteolin, and apigenin in the digested samples of secondary P-liposome might be related to the breaking of the chemical bonds of polyphenol-chitosan complexes in the gastric and intestine environment. Compared to the digested PPE sample, pectolinaringenin content was approximately 2.8 times higher in the primary digested sample of P-liposomes. This situation may be explained by the fact that poor water solubility of pectolinaringenin increases by increasing pH up 8.5 (Lucas-Abellán et al., 2019). Naringenin, a derivative of pectolinaringenin, was reported to be the most efficiently encapsulated with β-cyclodextrin at pH 8.5. Since pH increased up to only 7.5 during in-vitro digestion and enzymatic reaction was terminated by acidification at the end of the incubation period, pectolinaringenin was probably not detected in the digested sample of secondary P-liposome. Regarding C-liposomes, individual phenolic compounds of PPE could not be detected (under the limit of quantification) by HPLC due to poor solubility of pollen extract in water.
4. Conclusions
The low solubility of PPE in an aqueous liposomal dispersion was increased in the proliposome step. Increasing the solubility of PPE in this step led to increment of encapsulation efficiency (75%) and in vitro bioaccessibility of PPE in P-liposome structure. Compared to the primary C-liposome, the in vitro bioaccessibility of PPE was increased at least 1.5, 3, and 4 folds in terms of TAC (CUPRAC), TAC (DPPH), and TPC in primary P-liposome, respectively. In fact, primary C-liposomes did not increase the in vitro bioaccessibility of PPE except TFC. The in vitro bioaccessibility of TPC in PPE was increased approximately 2 folds in both primary C-liposome and P-liposomes. Gallic acid, 4 (p-)hydroxybenzoic acid, pectolinaringenin, rutin, luteolin, and apigenin have been determined in PPE. The uncharged PPE extract in pH 3–3.5 did not affect the surface charge of either C-liposomes or P-liposomes. However, covering the surface of liposomes with cationic chitosan altered the surface charge and increased the particle size of both C-liposome and P-liposomes with and without PPE. Beyond that, the presence of chitosan on the liposome surface resulted in a dramatic increment on the in vitro bioaccessibility of PPE in both C-liposome and P-liposomes.
In conclusion, this study represented a novel, suitable, and easy approach on the proliposome-liposome method to encapsulate the poor water-soluble extract such as PPE by liposome structures. We showed that PPE dissolved by a few drops of ethanol in the proliposome step. P-liposomes with a trace amount of ethanol offers a suitable platform to encapsulate more extract and provide the increment of in vitro bioaccessibility of encapsulated extract. Moreover, P-liposomes allow a surface covering with chitosan, which enhanced the stability of P-liposomes as well as improved the in vitro bioaccessibility of encapsulated PPE. The results of this study have the potential to be an innovation in the technology of this type of encapsulation.
Declarations
Author contribution statement
İlayda Hızır-Kadı, Mine Gültekin-Özgüven, Gokce Altin, Evren Demircan, Beraat Özçelik: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Akgün D., Gültekin-Özgüven M., Yücetepe A., Altin G., Gibis M., Weiss J., Özçelik B. Stirred-type yoghurt incorporated with sour cherry extract in chitosan-coated liposomes. Food Hydrocolloids. 2020;101:105532. [Google Scholar]
- Altin G., Gültekin-Ozgüven M., Ozcelik B. Chitosan coated liposome dispersions loaded with cacao hull waste extract: effect of spray drying on physico-chemical stability and in vitro bioaccessibility. J. Food Eng. 2018;223:91–98. [Google Scholar]
- Altin G., Gültekin-Özgüven M., Ozcelik B. Liposomal dispersion and powder systems for delivery of cocoa hull waste phenolics via Ayran (drinking yoghurt): comparative studies on in-vitro bioaccessibility and antioxidant capacity. Food Hydrocolloids. 2018;81:364–370. [Google Scholar]
- Amarowicz R., Janiak M. Hydrolysable tannins. Encyclopedia of food Chemistry. Ref. Module Food Sci. 2019:337–343. [Google Scholar]
- Apak R., Güçlü K., Özyürek M., Karademir S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004;52(26):7970–7981. doi: 10.1021/jf048741x. [DOI] [PubMed] [Google Scholar]
- Apak R., Güçlü K., Demirata B., Özyürek M., Çelik S.E., Bektaşoğlu B., Berker K.I., Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules. 2007;12(7):1496–1547. doi: 10.3390/12071496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos M.G., Bogdanov S., de Almeida-Muradian L.B., Szczesna T., Mancebo Y., Frigerio C., Ferreira F. Pollen composition and standardisation of analytical methods. J. Apicult. Res. 2008;47(2):154–161. [Google Scholar]
- Campos M.G.R., Frigerio C., Lopes J., Bogdanov S. What is the future of Bee-Pollen. J. ApiProduct ApiMed. Sci. 2010;2(4):131–144. [Google Scholar]
- Charcosset C., Juban A., Valour J.P., Urbaniak S., Fessi H. Preparation of liposomes at large scale using the ethanol injection method: effect of scale-up and injection devices. Chem. Eng. Res. Des. 2015;94:508–515. [Google Scholar]
- Chen L.Y., Cheng C.W., Liang J.Y. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015;170:10–15. doi: 10.1016/j.foodchem.2014.08.038. [DOI] [PubMed] [Google Scholar]
- Crane E. Encyclopedia of Insects. Academic Press; 2009. Bee products; pp. 71–75. [Google Scholar]
- de Florio Almeida J., dos Reis A.S., Heldt L.F.S., Pereira D., Bianchin M., de Moura C.…Carpes S.T. Lyophilized bee pollen extract: a natural antioxidant source to prevent lipid oxidation in refrigerated sausages. LWT-Food Sci. Technol. 2017;76:299–305. [Google Scholar]
- Dewanto V., Wu X., Liu R.H. Processed sweet corn has higher antioxidant activity. J. Agric. Food Chem. 2002;50(17):4959–4964. doi: 10.1021/jf0255937. [DOI] [PubMed] [Google Scholar]
- Duan H., Dong Z., Li H., Li W.R., Shi S.X., Wang Q.…Zhai K.F. Quality evaluation of bee pollens by chromatographic fingerprint and simultaneous determination of its major bioactive components. Food Chem. Toxicol. 2019;134:110831. doi: 10.1016/j.fct.2019.110831. [DOI] [PubMed] [Google Scholar]
- Gibis M., Zeeb B., Weiss J. Formation, characterization, and stability of encapsulated hibiscus extract in multilayered liposomes. Food Hydrocolloids. 2014;38:28–39. [Google Scholar]
- Gibis M., Ruedt C., Weiss J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016;88:105–113. doi: 10.1016/j.foodres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Gültekin-Özgüven M., Karadağ A., Duman Ş., Özkal B., Özçelik B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016;201:205–212. doi: 10.1016/j.foodchem.2016.01.091. [DOI] [PubMed] [Google Scholar]
- Jaafar-Maalej C., Diab R., Andrieu V., Elaissari A., Fessi H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010;20(3):228–243. doi: 10.3109/08982100903347923. [DOI] [PubMed] [Google Scholar]
- Kumaran A., Karunakaran R.J. Antioxidant and free radical scavenging activity of an aqueous extract of Coleus aromaticus. Food Chem. 2006;97:109e114. [Google Scholar]
- Lin L., Gu Y., Sun Y., Cui H. Characterization of chrysanthemum essential oil triple-layer liposomes and its application against Campylobacter jejuni on chicken. LWT- Food Sci. Technol. 2019;107:16–24. [Google Scholar]
- Lucas-Abellán C., Pérez-Abril M., Castillo J., Serrano A., Mercader M.T., Fortea M.I.…Núñez-Delicado E. Effect of temperature, pH, β-and HP-β-cds on the solubility and stability of flavanones: Naringenin and hesperetin. LWT- Food Sci. Technol. 2019;108:233–239. [Google Scholar]
- Moraes M., Carvalho J.M.P., Silva C.R., Cho S., Sola M.R., Pinho S.C. Liposomes encapsulating beta-carotene produced by the proliposomes method: characterisation and shelf life of powders and phospholipid vesicles. Int. J. Food Sci. Technol. 2013;48(2):274–282. [Google Scholar]
- Motilva M.J., Macià A., Romero M.P., Rubió L., Mercader M., González-Ferrero C. Human bioavailability and metabolism of phenolic compounds from red wine enriched with free or nano-encapsulated phenolic extract. J. Funct. Foods. 2016;25:80–93. [Google Scholar]
- Patil Y.P., Jadhav S. Novel methods for liposome preparation. Chem. Phys. Lipids. 2014;177:8–18. doi: 10.1016/j.chemphyslip.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Popa M.I., Aelenei N., Popa V.I., Andrei D. Study of the interactions between polyphenolic compounds and chitosan. React. Funct. Polym. 2000;45(1):35–43. [Google Scholar]
- Sebaaly C., Greige-Gerges H., Stainmesse S., Fessi H., Charcosset C. Effect of composition, hydrogenation of phospholipids and lyophilization on the characteristics of eugenol-loaded liposomes prepared by ethanol injection method. Food Biosci. 2016;15:1–10. [Google Scholar]
- Sebii H., Karra S., Bchir B. 2019. Physico-chemical, Surface and Thermal Properties of Date Palm Pollen as a Novel Nutritive Ingredient. [Google Scholar]
- Toniazzo T., Peres M.S., Ramos A.P., Pinho S.C. Encapsulation of quercetin in liposomes by ethanol injection and physicochemical characterization of dispersions and lyophilized vesicles. Food Biosci. 2017;19:17–25. [Google Scholar]
- Valcarcel J., Reilly K., Gaffney M., O’Brien N.M. Antioxidant activity, total phenolic and total flavonoid content in sixty varieties of potato (Solanum tuberosum L.) grown in Ireland. Potato Res. 2015;58(3):221–244. [Google Scholar]
- Van Dael H., Ceuterickx P. The interaction of phenol with lipid bilayers. Chem. Phys. Lipids. 1984;35(2):171–181. doi: 10.1016/0009-3084(84)90023-9. [DOI] [PubMed] [Google Scholar]
- Yücetepe A., Altin G., Özçelik B. A novel antioxidant source: evaluation of in vitro bioaccessibility, antioxidant activity and polyphenol profile of phenolic extract from black radish peel wastes (Raphanus sativus L. var. Niger) during simulated gastrointestinal digestion. Int. J. Food Sci. Technol. 2020 [Google Scholar]