Abstract
Central nervous system adverse effects are commonly reported with pregabalin (PGB). On the other hand, movement disorders (MDs) associated with this drug were rarely described. However, their occurrence could significantly affect the quality of life of PGB users. This literature review aims to evaluate the clinical epidemiological profile, pathological mechanisms, and management of PGB-associated MDs. Relevant reports in six databases were identified and assessed by two reviewers without language restriction. A total of 46 reports containing 305 cases from 17 countries were assessed. The MDs encountered were as follows: 184 individuals with ataxia, 61 with tremors, 39 with myoclonus, 8 with parkinsonism, 1 with restless legs syndrome, 1 with dystonia, 1 with dyskinesia, and 1 with akathisia. The mean age was 62 years (range: 23–94). The male sex was slightly predominant with 54.34%. The mean PGB dose when the MD occurred was 238 mg, and neuropathic pain was the most common indication of PGB. The time from PGB start to MD was < 1 month at 75%. The time from PGB withdrawal to recovery was < 1 week at 77%. All the individuals where the follow-up was reported had a full recovery. The most common management was PGB withdrawal. In the literature, the majority of the cases did not report information about timeline events, neurological examination details, or electrodiagnostic studies. The best management for all MDs is probably PGB withdrawal. If the patient is on dialysis program, perhaps an increased number of sessions will decrease recovery time. Furthermore, the addition of a benzodiazepine could accelerate recovery.
Keywords: CI-1008, drug induced, literature review, movement disorder, pregabalin
Introduction
Pregabalin (PGB) is also known as CI-1008, 3-isobutyl-γ-aminobutyric acid (GABA), and (S)-3-isobutyl-GABA. In 1997, the first results in animal models were published and PGB showed significant outcomes in the management of induced epilepsy and mechanical allodynia.[1] In 1999, the first studies with a small group of humans showed that the drug was effective to treat seizures, but the safety profile was still in doubt due to the reports about PGB being associated with high-frequency myoclonus (MCL).[2] Later, from 2000 to 2004, multicenter studies with large numbers of participants and with improved methodology showed that the PGB was safe, and no new reports of MCL were described.[3] In this way, LYRICA® (PGB) was approved on July 6, 2004, by the European Medicines Agency and on December 30 that year by the Food and Drug Administration (FDA).[4] It is noteworthy that PGB was originally approved by the FDA for focal-onset seizures. Furthermore, this drug is currently among the 100 most commonly prescribed medications in the United States.[5]
PGB is approved for the adjunctive therapy of partial-onset seizures (patients 4 years of age or older), neuropathic pain associated with diabetic peripheral neuropathy, postherpetic neuralgia, fibromyalgia, and neuropathic pain with spinal cord injury. This drug is also being prescribed off-label for the treatment of chronic pain, bipolar disorder, generalized anxiety disorder, insomnia, and social anxiety disorder.[6] Its structure is similar to the GABA, which is a compound that highly diffuses across the blood–brain barrier due to a modification of a lipid analog. However, PGB does not directly affect or bind to GABA receptors. The pharmacologic studies in rat models suggested that PGB mainly binds to the α2 δ auxiliary subunit of presynaptic voltage-gated calcium channels, which are expressed in the brain and spinal cord. More specifically, the PGB benefits are probably only due to their effect on subtype 1 calcium channels. Besides, it is believed that the subtype 2 is not associated with any pharmacological benefits [Figure 1].[7,8]
Figure 1.
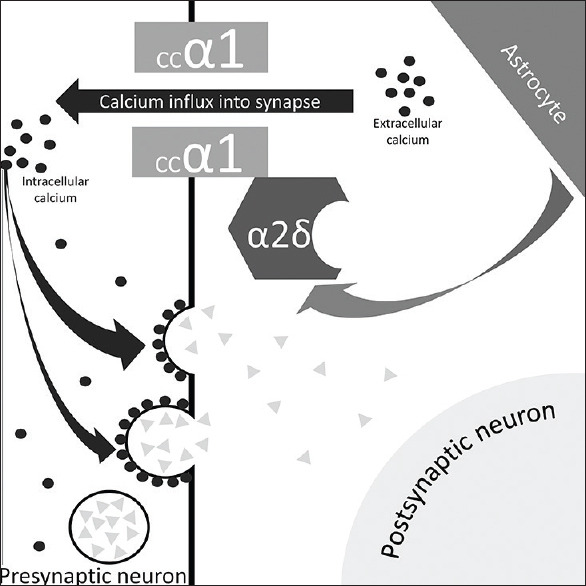
Schematic diagram of the mechanism of action of pregabalin. Pregabalin binds to the α2 δ auxiliary subunit of presynaptic voltage-gated calcium channels decreasing the calcium influx. This decreases the intracellular calcium that reduces the release of excitatory neurotransmitters. Furthermore, it is represented the most acceptable hypothesis for chronic pain, which is the activation of the astrocyte by an inciting event leading to indirect interaction with the α2 δand promoting the calcium influx causing to neurokinin release. In this way, the pregabalin binding in α2 δ decreases/interrupts this misleading pathway
The action of PGB on these types of voltage-gated calcium channels and their location could explain the adverse events already reported in the literature secondary to PGB. Central nervous system side effects are common complaints in clinical practice. Mild to moderate in degree, they generally occur within the first 2 weeks after the start of medication and can lead to almost 15% of treatment discontinuation. The most commonly reported side effects in premarketing controlled trials were blurred vision, dizziness, dry mouth, edema, somnolence, and weight gain.[9] In this context, movement disorders (MDs) associated with PGB were rarely reported. The most commonly already described MDs in multicentric trials were ataxia and tremor.[10] However, the first report was on MCL in a small study by Asconape et al. that drew attention to the safety of PGB, in which two individuals out of six using PGB developed MCL.[2] This literature review aims to evaluate the clinical epidemiological profile, pathological mechanisms, and management of PGB-associated MDs.
Methods
Search strategy
We searched six databases in an attempt to locate all existing reports on MDs associated with PGB published between 1999 and 2019 in electronic form. Excerpta Medica (Embase), Google Scholar, Latin American and Caribbean Health Sciences Literature, Medline, Scientific Electronic Library Online, and ScienceDirect were searched. Search terms were ''tremor, ataxia, dystonia, restless legs syndrome, periodic limb movement disorder, akathisia, dyskinesia, parkinsonism, tic, chorea, restlessness, hyperkinetic, hypokinetic, bradykinesia, movement disorder, movement, side effect, MCL, ballism, adverse effect, and idiosyncratic.'' These terms were combined with ''pregabalin, CI-1008.''
Inclusion and exclusion criteria
Case reports, case series, original articles, letters to the editor, bulletins, and poster presentations published from 1999 to 2019 were included in this review with no language restriction. The two authors independently screened the titles and abstracts of all papers found from the initial search. Disagreements between authors were resolved through discussion.
We exclude cases in which the MD could have a better explanation or was in the authors' differential diagnosis as a minor hypothesis. Other exclusion criteria were patients diagnosed with myoclonic epilepsy or other types of seizures where, due to clinical history, PGB could have led to the exacerbation of previous myoclonic findings. Moreover, we did not include cases that did not contain minimal information or were not accessible by electronic methods, including after a formal request to the study authors by E-mail. Cases with more than one factor contributing to the MD were evaluated based on the probability of the event occurrence based on the Naranjo algorithm.
Data extraction
A total of 8,737 papers were found; 8,064 were not eligible [Figure 2]. When provided, we extracted author, department, year of publication, country of occurrence, number of patients affected, PGB indication, time from first PGB dose till MD onset, time from PGB withdrawal to symptom improvement, patient's status at follow-up, and important findings of clinical history and management.[11,12,13] Most of the cases did not report information about timeline events, neurological examination details, or electrodiagnostic studies. Data were extracted by two independent authors, double-checked to ensure matching, and organized by whether the MD was a side effect of the PGB use.
Figure 2.
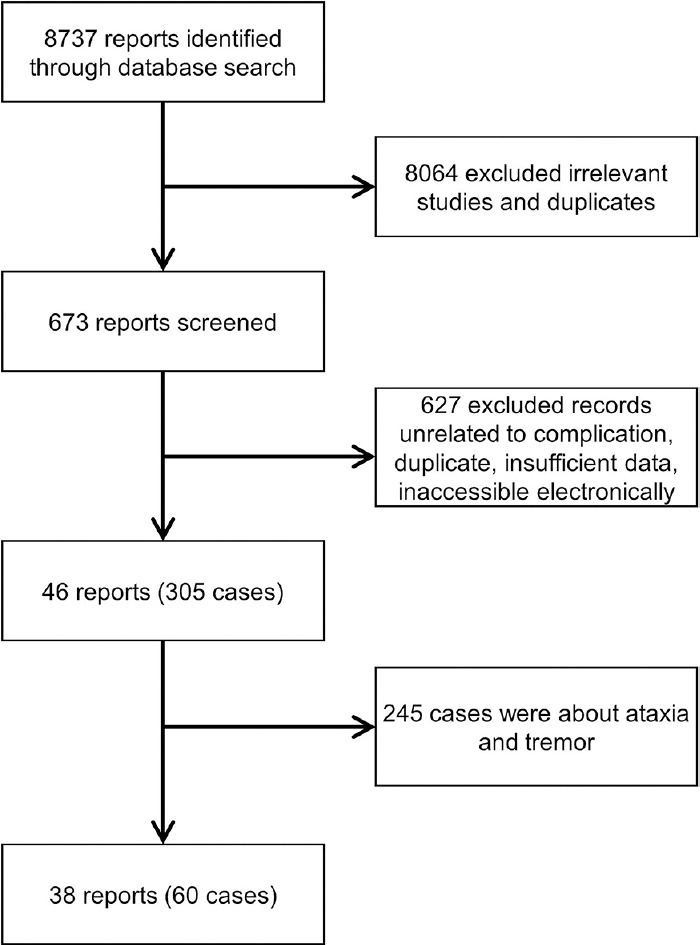
Flowchart of the screening process
Statistical analysis
Categorical variables were represented as proportions; continuous variables were represented as mean, standard deviations (SD), median, and range.
Definitions
The clinical characteristics and definitions of the MDs such as tremor, ataxia, dystonia, restless legs syndrome, periodic limb MD, akathisia, dyskinesia, parkinsonism, tic, chorea, ballism, and MCL were obtained from the reference Jankovic and Tolosa.[14] The Naranjo algorithm was used to determine the likelihood of whether an adverse drug reaction was actually due to the drug rather than the result of other factors.[15] In the cases where the non-English literature was beyond the authors' proficiency (English, Portuguese, Spanish, Italian, French, and German) and the English abstract did not provide enough data such as Japanese, Korean, Chinese, Russian, and Dutch, Google Translate service was used.[16]
Results
For the years 1999 and 2019, a total of 46 reports containing 305 cases who developed MDs after the use of PGB from 17 different countries were reported [Table 1].[2,3,10,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60] The origin was North American in 196, European in 68, Asian in 40, and South American in 1. The MDs associated with PGB found were as follows: 184 patients with ataxia, 61 with tremors, 39 with MCL, 8 with parkinsonism, 1 with restless legs syndrome, 1 with dystonia, 1 with dyskinesia, and 1 with akathisia. In the subgroup of cases not clearly defined, there were 7 individuals with MCL, 1 with akathisia, and 1 with stuttering. Three articles did not report the number of patients, but they observed MCL[39,54] and parkinsonism.[49]
Table 1.
Clinical reports of pregabalin-associated movement disorder
| Reference | country/year | No cases | Age/sex | PGB |
Important CH and CM | |
|---|---|---|---|---|---|---|
| Indication | Dose (mg) | |||||
| MCL | ||||||
| Asconape et al. | USA/1999 | 2 | NR | RFE | 450 | CH: high-frequency multifocal MCL. CM: PGB maintained for more 13 weeks with the persistence of the MCL but without serious consequences |
| A clinical trial with PGB, where 2 of 6 patients developed MCL | NR | RFE | 600 | CH: sporadic right-sided focal MCL. CM: PGB maintained for more 28 weeks with the persistence of the MCL but without serious consequences | ||
| Huppertz et al. | Germany/2001 | 4 | 44/male | RFE | 600 | CH: high-frequency multifocal MCL. CM: decreased PGB dose causes decrease MCL-frequency |
| Patients were participating in a clinical trial with PGB, where 4 of 19 patients developed MCL | 42/female | RFE | 350 | CH: sporadic multifocal MCL. CM: PGB dose increased with the persistence of MCL | ||
| 24/female | RFE | 250 | CH: sporadic left-sided focal MCL. CM: PGB dose increased with the persistence of MCL | |||
| 23/male | RFE | 50 | CH: sporadic multifocal MCL. CM: PGB dose increased with the persistence of MCL | |||
| Heckmann et al. | Germany/2005 | 2 | 89/female | PHN | 300 | CH: asterixis |
| In the discussion, Heckmann et al. added the report of a case not clearly defined and without reference of a patient that developed generalized MCL and hand DTN during PGB therapy. Furthermore, this article situation is retracted, but we did not find the editorial decision | ||||||
| Knake | Germany/2007 | 2 | 80/female | PHN | 150 | CH: MCL status epilepticus. CM: PGB replaced by flupirtine, lorazepam single dose with symptom recovery |
| 79/female | NP | 300 | CH: multifocal MCL that developed to generalized tonic–clonic seizures. CM: PGB was withdrawal, lorazepam started with symptom recovery | |||
| Han et al. | Korea/2008 | 2 | 69/female | PHN | 150 | CH: asterixis. CM: PGB stopped, and symptoms recovered |
| 75/male | NP | 150 | CH: asterixis. CM: PGB withdrawal with symptom recovery | |||
| Hellwig and Amtage | Germany/2008 | 1 | 71/male | NP | 100 | CH: chronic renal failure; asterixis (cortical negative MCL). CM: PGB withdrawal and clonazepam started with symptom recovery |
| Modur and Milteer | USA/2008 | 2 | 25/male | Refractory epilepsy | 300 | CH: multifocal MCL that increased frequency with PGB dose increase. CM: PGB dose decrease with the persistence of symptoms after PGB was discontinued symptom recovery |
| 49/male | Refractory epilepsy | 350 | CH: multifocal MCL that increased frequency with PGB dose increase. CM: PGB dose decrease the symptoms recovered | |||
| Murphy and Mosher | Canada/2008 | 1 | 67/female | NP | 600 | CH: multifocal MCL. CM: PGB withdrawal with symptom resolution |
| Healy et al. | UK/2009 | 1 | 47/male | NP | 150 | CH: chronic renal failure; multifocal MCL; previous gabapentin-induced MCL. CM: PGB withdrawal with symptom recovery |
| Yoo et al. | USA/2009 | 1 | 30/female | NP | 225 | CH: chronic renal failure; multifocal MCL. CM: PGB withdrawal and dialysis done with residual tremor; after another dialysis the following day, her symptoms resolved |
| Gosavi et al. | Singapore/2011 | 1 | 73/male | NP | NR | CH: multifocal MCL. CM: PGB replaced by gabapentin |
| Lee et al. | Korea/2011 | 2 | 67/male | NP | 450 | CH: multifocal MCL previous chronic renal failure. CM: PGB withdrawal and three hemodialysis sessions with symptom resolution. |
| 43/male | Fibromyalgia | 75 | CH: multifocal MCL previous chronic renal failure. CM: PGB withdrawal with symptom resolution. | |||
| Shimizu et al. | Japan/2012 | 1 | 91/male | NP | 150 | CH: multifocal MCL. CM: Diazepam was administered with symptom recovery after PGB was withdrawal |
| Kirac et al. | Turkey/2013 | 2 | 56/female | NP | 300 | CH: MCL status epilepticus; possible association with melphalan. CM: PGB withdrawal and levetiracetam started with symptom recovery |
| 73/female | NP | 150 | CH: MCL status epilepticus. CM: PGB was withdrawal | |||
| Courtois et al. | Belgium/2014 | 1 | 64/female | NP | 150 | CH: MCL status epilepticus. CM: PGB withdrawal, she needed intensive care management with benzodiazepines for the control of seizures |
| Olszewska et al. | Ireland/2015 | 1 | 66/male | NP | 75 | CH: CKD; multifocal MCL including speech. CM: PGB was withdrawal with symptom resolution |
| Kim et al. | Korea/2017 | 9 | 64/female | NP | 150 | CM: PGB withdrawal |
| 50/male | 150 | |||||
| 91/female | 150 | |||||
| 70/male | 300 | |||||
| 59/female | 300 | |||||
| 72/male | 150 | |||||
| 73/male | 300 | |||||
| 67/male | 150 | |||||
| 84/male | 300 | |||||
| Park et al. | Korea/2018 | 1 | 80/male | NP | 150 | CH: multifocal negative MCL. CM: PGB withdrawal with lorazepam start resolved the symptoms |
| Desai et al. | USA/2019 | 2 | 51/male | NP | 150 | CH: multifocal MCL. CM: PGB withdrawal |
| 54/female | Arthralgia | 150 | CH: multifocal MCL. CM: PGB withdrawal | |||
| Hernandez et al. | Spain/2019 | 1 | 94/female | PHN | 225 | CM: PGB dose was decreased with the resolution of symptoms |
| PKN | ||||||
| Lloret et al. | Argentina/2009 | 1 | 64/female | Diabetic neuropathy | 150 | CH: she was in previous use of gabapentin. Then, PGB was added. CM: PGB was withdrawal |
| Matsuki et al. | Japan/2012 | 2 | 69/male | NP | 300 | CM: PGB was replaced to gabapentin with a resolution of symptoms |
| 75/male | PHN | 75 | CM: PGB was replaced by clonazepam with symptom resolution. | |||
| Matsuki et al. described the cases as muscle rigidity. We believed that description is a possible diagnosis of PKN with predominant rigidity | ||||||
| Masmoudi et al. | France/2016 | 1 | 58/female | Fibromyalgia | 300 | CM: PGB withdrawal |
| Prado-Mel et al. | Spain/2018 | 1 | 58/female | NP | 150 | CH: Possible interaction because symptoms developed four days after an iodinated contrast abdominal computed tomography. CM: PGB withdrawal |
| Ari et al. | Turkey/2019 | 1 | 53/female | Fibromyalgia | 150 | CM: PGB was withdrawal |
| RLS | ||||||
| Park et al. | Korea/2009 | 1 | 64/female | NP | 300 | CH: Previous history of depression was in the use of mirtazapine after PGB was added and the RLS symptoms started. CM: PGB was maintained, MTZ was replaced by bupropion with symptom resolution |
| DTN | ||||||
| Kwong et al. | China/2011 | 1 | 24/male | NP | 450 | CH: oromandibular DTN. Differential diagnosis of tetanus. CM: diphenhydramine started with minimal improvement. He was admitted to the intensive care unit. |
| DKN | ||||||
| Aksoy et al. | Turkey/2013 | 1 | 70/male | RLS | 150 | CH: He had DKN with pramipexole, PGB, and gabapentin. Only after clonazepam, he had RLS-symptom recovery. CM: PGB withdrawal |
| AKT | ||||||
| Dag et al. | Turkey/2013 | 1 | 58/male | Diabetic neuropathy | 150 | CH: He was in use of metoprolol for coronary artery disease. CM: PGB was withdrawal. After 6 weeks, gabapentin was started with no new symptoms |
| Clinical trials, literature reviews, and not clearly defined cases | ||||||
| French et al. | 2003/USA | 34 | Ataxia | A total of 453 patients in a double-blind, randomized, placebo-controlled, parallel-group study to assess the efficacy, safety, and tolerability of PGB administered twice daily | ||
| 19 | Tremor | |||||
| Arroyo et al. | USA/2004 | 13 | Tremor | Randomized, double-blind, placebo-controlled study of PGB in adults with focal epilepsy. Tremor individuals 3 (150 mg) + 10 (600 mg). The MCL patients had mild symptoms and were in use of 600 mg daily, other characteristics are not specified. Ataxia group 2 (150 mg) + 16 (600 mg) | ||
| 4 | MCL | |||||
| 18 | Ataxia | |||||
| Beydoun et al. | USA/2005 | 57 | Ataxia | A total of 378 participants in a multicenter, double-blind, randomized, parallel-group, placebo-controlled trial to evaluate the efficacy and safety of PGB | ||
| Elger et al. | USA/2005 | 41 | Ataxia | A double-blind, placebo-controlled study with 204 patients to assess PGB for partial seizures administered as fixed dose or as flexible dose | ||
| Sluyts et al. | Belgium/2007 | 1 | MCL | Poster presentation about an individual with an impaired renal function that developed MCL secondary to PGB | ||
| Huber et al. | Germany/2008 | 4 | Tremor | A retrospective study with 32 intellectual disabled participants who used PGB for resistant epilepsy. The specific characteristics are not provided | ||
| 5 | Ataxia | |||||
| Lee et al. | Korea/2009 | 5 | Ataxia | Double-blind, randomized, placebo-controlled, multicenter trial with 178 participants assessed the efficacy of PGB for refractory partial epilepsies | ||
| 6 | Tremor | |||||
| Baulac et al. | France/2010 | 20 | Ataxia | A total of 434 individuals with partial seizures were randomized to PGB, lamotrigine, or placebo as adjunctive therapy for 17 weeks of double-blind treatment | ||
| 16 | Tremor | |||||
| Saif et al. | Greece/2010 | 3 | Tremor | Efficacy of PGB for the management of oxaliplatin-induced sensory neuropathy in 23 individuals | ||
| 4 | Ataxia | |||||
| Zaccara et al. | Italy/2011 | NA | Ataxia | A systematic review with meta-analyses of the randomized controlled trials found that ataxia has a relative of 4.77 of occurring when PGB is used | ||
| Giray et al. | Turkey/2016 | 1 | Stuttering | A 31-year-old female was diagnosed with chronic regional pain syndrome and PGB 75 mg was started. During the second dose, she developed stuttering. PGB was withdrawal. After 1 week, she had a full recovery | ||
| Hamlyn et al. | USA/2017 | 1 | AKT | A 53-year-old male was taking PGB 150 mg for his NP. The individual stopped the medication without medical advice. After 7 days, he developed AKT. CM: benzodiazepines trials did not improve the symptoms, PGB was restarted with symptom recovery | ||
| Slocum et al. | USA/2018 | 1 | MCL | A 54-year-old female ingested PGB 3825 mg. On examination, rotary nystagmus and MCL were observed. A single dose of lorazepam was administered | ||
| Ozturka et al. | Turkey/2017 | 1 | MCL | A 23-year-old female took PGB >3000 mg intranasal. The patient developed multifocal MCL. The management was PGB withdrawal and valproic acid starts with symptom resolution within 7 days | ||
| Kwon and Park | Korea/2019 | NR | MCL | Poster presentation that assessed retrospectively cases of a determinate hospital. They conclude that PGB-induced MCL can develop even in patients with normal renal function with only 14.3% having a renal injury. Furthermore, NP was the main indication | ||
| Reyad et al. | Egypt/2019 | NR | MCL | Role of PGB in the postmastectomy pain syndrome. At least one case, but no specific information is provided | ||
| Pacheco-Paez et al. | Canada/2019 | NA | PKN | Assessed the reports in the World Health Organization and VigiBase. They found that the use of PGB is associated with an odds ratio of 2.43 (2.36–2.50) | ||
CH: Clinical history, CM: Clinical management, AKT: Akathisia, MCL: Myoclonus, NA: Not available/ not applicable, NR: Not reported, NP: Neuropathic pain, PGB: Pregabalin, PKN: Parkinsonism, RFE: Refractory focal epilepsy, RLS: Restless legs syndrome, DTN: Dystonia, DKN: Dyskinesia
The following results describe the subgroup without ataxia or tremor, which involved 60 patients. The mean and median reported age was about 62.89 (SD: 18.12) and 66.5 years (age range: 23–94), respectively. The male was the predominant sex, with a percentage of 54.34% (25/46). The most common clinical indication of PGB was neuropathic pain 56.25% (27/48), followed by refractory epilepsy (8), postherpetic neuralgia (5), fibromyalgia (3), diabetic neuropathy (2), arthralgia (1), chronic back pain (1), and restless legs syndrome (1).
The mean and median PGB dose when the MD occurred was 238 (SD: 136.95) and 150 mg (dose range: 50–600 mg), respectively. The number of individuals using each dosage was, respectively: 1 patient using 50 mg, 3 with 75 mg, 1 with 100 mg, 20 with 150 mg, 2 with 225 mg, 1 with 250 mg, 11 with 300 mg, 2 with 350 mg, 3 with 450 mg, and 3 with 600 mg. The time since the onset of PGB and abnormal movement occurrence was specified in 37 reports. From these, in 28 individuals, the interval of time from the beginning of PGB until the MD onset was <1 month (onset range: 1 day–9 months). The time from PGB withdrawal to abnormal movement recovery was specifically reported by 18 authors. From these, in 14 individuals, the recovery time was <1 week, and in 2, it was <1 month (recovery range: 1 day–6 months). All the individuals where the follow-up was reported had a full recovery.
The management was the PGB withdrawal in 80.85% (38/47). The other options were PGB dose increase, decrease, or maintenance. Three studies did not report or were not detailed in their management. In some of the reports where individuals had MCL, after PGB was withdrawn, benzodiazepines were started in an attempt to accelerate the recovery process.
Discussion
General
The general data extracted from the 305 cases encountered were divided into ''with'' and ''without tremor and ataxia'' due to the different backgrounds of the study populations involved in these two categories. For example, individuals with ataxia and tremor were all participants of clinical trials. Usually, the goal of this type of study is to report adverse effects in a general manner; in case reports, generally, more attention is given to clinical details and side effects.[61]
The cases about tremor or ataxia did not clearly report the clinical characteristics of the patients such as sex, age, and management. Only the PGB dose when the individual developed the tremor or ataxia side effect was provided. Pulman et al. did a systematic review with meta-analysis of ataxia after PGB, and they encountered a relative risk of 3.90 (99% confidence interval: 2.05–7.42) for this side effect.[62] In this way, the main data of the present literature review are from sixty individuals with a MD that was not only tremor or ataxia.
Based on the results, we could illustrate the average person who developed a MD secondary to PGB as an old adult male, with North American origin, who used a PGB dose from 150 to 300 mg a day that was prescribed for the treatment of neuropathic pain. This individual presented with multifocal large-amplitude myoclonic jerks, mainly in the bilateral upper limb, with onset after < 1 month of the PGB start. The management was the withdrawal of the medication. One day after, he had excellent improvement, and within a week, he had a full recovery of the motor symptoms.
Herein, we would like to discuss some of the MDs in subtopics.
Myoclonus
The MCL was the first reported MD secondary to PGB and the most commonly reported in the literature. In this context, we believe that it was more published than the other abnormal movements due to its severity and remarkable presentation characteristics.[63] Besides, MCL significantly affects activities of daily living and causes immeasurable social disabilities. It was specified as multifocal in 48.71% (19/39) of the patients with bilateral upper limb involvement. The individuals found can probably be classified as MCL with a subcortical origin, but a more detailed analysis could not be done because the majority of the reports did not describe electrodiagnostic studies.[64] With this background, three facts can support the subcortical as the presumed source of MCL generation. First, in some reports who did describe electrodiagnostic studies and MCL occurring in many muscle groups, the electroencephalogram had normal results, which is the clinical definition for subcortical MCL.[65] Second, in the cases where benzodiazepines were used, the recovery time was much shorter; in this context, it is known that the subcortical MCL has the best response to benzodiazepines among the MCL types.[64] The last fact is that asterixis, which was clearly described in at least five patients, is known as a subcortical source of MCL.[66] It is crucial to note that there was a small number of asterixis, probably because this abnormal finding needs careful decomposition of the movement during the examination. Moreover, most of the patients have not been examined by a MD specialist which could have improved the description of the cases with minutiae.[67]
An interesting finding was that in some studies where the PGB dose was reduced, the frequency of the MCL decreased. However, in other patients, the dose was increased without affecting the frequency of the MCL.[36] Hence, we can hypothesize that the PGB-induced MCL is more likely to be a threshold effect rather than a linear dose-dependent adverse effect, in which, when the critical level is achieved, there will be an all-or-none process.
Desai et al. did a case report and literature review of patients who developed MCL after the use of gabapentin and PGB. They concluded that drug-resistant epilepsy, higher PGB doses, and renal impairment were risk factors for the development of MCL.[24] We believe that none of these risk factors are highly associated with MCL secondary to PGB, but they could be related to the gabapentin use. Based on our records, the percentage of patients with drug-resistant epilepsy who developed MCL after the use of PGB was only 20% (8/39). These studies occurred previous to the approval of PGB for the treatment of epilepsy by the FDA, and in the majority of the cases, the PGB doses were low to moderate. Moreover, the renal function was normal, and the comorbidities, when present, were controlled in more than 80% of the individuals. Therefore, we believe in the existence of a probable genetic predisposition for the occurrence of MCL with PGB, but no other risk factors can be assumed with the existing reports.
The management of MCL was the PGB discontinuation as the first option in about 90% of the cases. Other reported option was the decrease of PGB dose, but no study showed complete recovery of MCL when this choice was tried. If the patient is on dialysis program, more sessions could be done to decrease the recovery time and improve the symptoms of the MD. Furthermore, the addition of benzodiazepines could reduce MCL based on some reports where the prescription of these drugs led to a fast recovery.[68]
Several neurotransmitter pathways seem to be involved in drug-induced MCL, including glutamate, glycine, GABA, dopamine, and serotonin.[69] In animal models, the use of PGB prolonged application led to an increased density of GABA transporter protein and functional rate in cultured neurons.[70] In this way, we hypothesized that in predisposed individuals, the use of PGB may lead to an increased concentration of GABA in specific areas of the central nervous system. Furthermore, the cortical areas are protected due to the transmission block of seizure activity through the subcortical networks. However, to support this increase, the subcortical area loses GABA becoming unprotected and susceptible to insults that could lead to seizures.[71]
Parkinsonism
Parkinsonism was the second most commonly encountered MD. Perez Lloret et al. reported the first case in an adult female who presented with bradykinesia, bilateral symmetric tremor, and rigidity 3 months after PGB start. The medication was withdrawn with the gradual improvement of the symptoms.[52] An interesting fact was that the described cases in Table 1 had a long time from drug onset to the symptom's development and the time from drug withdrawal until recovery. It is worthy of mentioning that the mean age in this group of individuals was of about 63 years, which is related to an increased percentage of Parkinson's disease.[72] We believe that an important feature that needs to be described in upcoming reports is a longer follow-up observation to assess a correlation with Parkinson's disease development throughout the time.
One pathophysiological mechanism assumed is the same as with the other medications that have an affinity for L-type voltage-dependent calcium channels such as cinnarizine.[73] However, another possible hypothesis proposed by Perez Lloret et al. is based on substance P.[52] The substance P is found in the basal ganglia and is being studied in Parkinson's disease pathogenesis.[74] The neurokinin 1 has a high affinity for the substance P, and when they interact, the substance P leads to an increase of inhibitory neurotransmission to the globus pallidus, which decreases the thalamic inhibition facilitating the movement.[75] In this context, when PGB is added, a reduction of these mammalian tachykinins is observed, and this can lead to a decreased inhibition of the internal globus pallidus, which will increase the thalamic inhibition difficulting the movement.[76]
The most common management was the withdrawal of PGB. In one of the patients reported by Matsuki et al., a benzodiazepine was started, but the recovery time was not reported, so we were not able to analyze if this option could change the disease course.[44]
Dystonia, restless legs syndrome, dyskinesia, and akathisia
There is only one case study of oromandibular dystonia reported in the literature occurring after PGB use. The case was poorly described, and the clinical history and management are missing important facts.[40] Giray et al. reported another possible case of dystonia that they diagnosed as being a stutter, but the neurological examination about the muscles of the face and even the tongue was not described.[26] It has been suggested that the pathophysiology of dystonia may be opposite to that of stuttering [Figure 3].[77,78] One of the mechanisms to explain dystonia is an impaired inhibition of the surrounding muscles that can be assessed by somatosensory cortex potential alterations. In this context, Vreeswijk et al. studied the somatosensory evoked potential in stutter individuals, and the achieved results were normal without the description of abnormalities in these individuals. These findings in the stuttering group showed that the inhibition was normal, so what probably happens is the compensatory facilitation of surrounding muscles.[79] Therefore, PGB may be related to both mechanisms, but we believe that it is more likely that some piece of the puzzle is missing. One hypothesis that explains stutter and dystonia may be the GABA density involved in MCL; in susceptible individuals, there is an assumed increase of GABAergic transmission in a specific area.[80]
Figure 3.
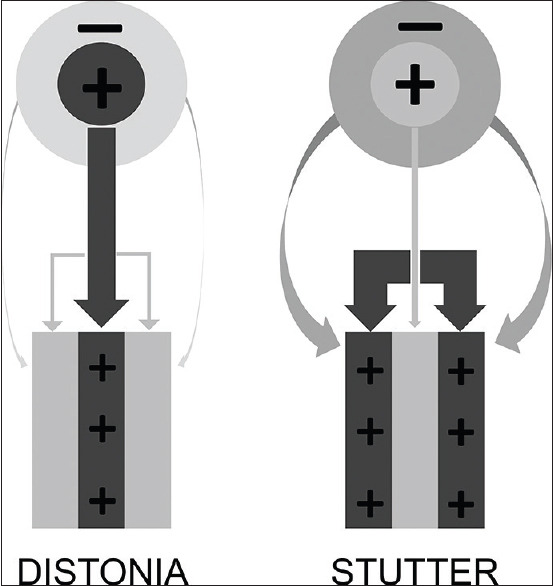
Schematic diagram about the proposed mechanisms underlying dystonia and stutter. In dystonia, the inhibition of the surrounding muscles is impaired. In the stutter, the inhibition is normal, so what probably happens is the compensatory facilitation of surrounding muscles by a center not adequately facilitated. +: Excitation/active, -: Inhibition/inactive
Park et al. reported a patient using mirtazapine to which treatment regimen PGB was added, and she developed restless legs syndrome symptoms. The management was the replacement of mirtazapine by bupropion with symptomatic recovery.[51] We believe that the presence of PGB probably did not influence in any way the development of the patient's symptoms, but we cannot exclude an interaction between these drugs. It is worthy of mentioning that PGB is one of the medications most commonly prescribed off-label for the management of restless legs syndrome.[81]
A case of dyskinesia was reported by Aksoy et al., in which the patient had abnormal movements with pramipexole, PGB, and gabapentin on different occasions. Only when clonazepam was added in the regimen, his symptoms completely improved.[17] We believe that pramipexole led to an abnormal adaptation of the striatal neuronal organization by oxidative stress leading to an overactivation of the direct pathway.[82] The inflammatory hypothesis could be supported by the recovery of symptoms after discontinuation of medication and recurrence at the beginning of a new drug. Furthermore, the reported time was probably sufficient for the development of these processes.
Dag et al. reported a case of akathisia after a single dose of PGB. The medication was replaced by gabapentin with a full recovery.[23] Another case by Hamlyn et al. described a male taking PGB that stopped the medication abruptly without medical advice, and after 1 week, the patient started with akathisia; when the drug was reintroduced, the patient symptoms improved.[28] In this way, both cases are contradictory: in one, the akathisia was caused by an increase of PGB and, in the other, by the decrease of PGB. We can assume, in the same way as with MCL, that the changes of PGB concentration in predisposed individuals may lead to increased or decreased inhibition in the internal and external globus pallidus.[80]
Conclusion
In sum, PGB-associated MD could be divided into “with” and “without tremor and ataxia” due to the different backgrounds of the study populations involved in these two categories. The abnormal movements encountered were ataxia, tremor, MCL, parkinsonism, restless legs syndrome, dystonia, dyskinesia, stuttering, and akathisia. Further studies are warranted to a more detailed report of the clinical data such as timeline events, neurological examination details, and especially electrodiagnostic studies. Furthermore, future reports need to fully investigate the influence of these adverse events during the entire patients' life as well as a long-term follow-up for the development of other MDs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Taylor C. Profile of the anticonvulsant activity of CI-1008 (pregabalin) in animal models. Epilepsia. 1997;38:8. [Google Scholar]
- 2.Asconape J. Pregabalin-associated myoclonus. Epilepsia. 1999;40:143. [Google Scholar]
- 3.French JA, Kugler AR, Robbins JL, Knapp LE, Garofalo EA. Dose-response trial of pregabalin adjunctive therapy in patients with partial seizures. Neurology. 2003;60:1631–7. doi: 10.1212/01.wnl.0000068024.20285.65. [DOI] [PubMed] [Google Scholar]
- 4.Dworkin RH, Kirkpatrick P. Pregabalin. Nat Rev Drug Discov. 2005;4:455–6. doi: 10.1038/nrd1756. doi:10.1038/nrd1756. [DOI] [PubMed] [Google Scholar]
- 5.ClinCalc. The Top 300 of 2020. [Last accessed on 2020 May 23]. Available from: https://clincalccom/DrugStats/Top300Drugsaspx .
- 6.Cross AL, Viswanath O, Sherman Al. StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. Jan- [Last updated 2020 Mar 26]. Pregabalin. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470341/ [Google Scholar]
- 7.Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A. The diverse therapeutic actions of pregabalin: Is a single mechanism responsible for several pharmacological activities? Trends Pharmacol Sci. 2013;34:332–9. doi: 10.1016/j.tips.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Dolphin AC. Calcium channel auxiliary α 2 δ and β subunits: Trafficking and one step beyond. Nat Rev Neurosci. 2012;13:542. doi: 10.1038/nrn3311. [DOI] [PubMed] [Google Scholar]
- 9.Moore RA, Straube S, Wiffen PJ, Derry S, McQuay HJ. Pregabalin for acute and chronic pain in adults. Cochrane Database Syst Rev. 2009;3:CD007076. doi: 10.1002/14651858.CD007076.pub2. Published 2009 Jul 8. doi:10.1002/14651858.CD007076.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA, et al. Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology. 2005;64:475–80. doi: 10.1212/01.WNL.0000150932.48688.BE. [DOI] [PubMed] [Google Scholar]
- 11.Rissardo JP, Caprara AL. Buspirone-associated movement disorder: A literature review. Prague Med Rep. 2020;121:5–24. doi: 10.14712/23362936.2020.1. [DOI] [PubMed] [Google Scholar]
- 12.Rissardo JP, Caprara AL. Carbamazepine-, oxcarbazepine-, eslicarbazepine-associated movement disorder: A literature review. Clin Neuropharmacol. 2020;43:66–80. doi: 10.1097/WNF.0000000000000387. [DOI] [PubMed] [Google Scholar]
- 13.Rissardo JP, Caprara AL. The link between amitriptyline and movement disorders: Clinical profile and outcome. Ann Acad Med Singapore. 2020;49:236–51. [PubMed] [Google Scholar]
- 14.Jankovic J, Tolosa E. Parkinson's Disease and Movement Disorders. Philadelphia: Lippincott Williams & Wilkins; 2007. [Google Scholar]
- 15.Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. doi: 10.1038/clpt.1981.154. [DOI] [PubMed] [Google Scholar]
- 16.De Vries E, Schoonvelde M, Schumacher G. No longer lost in translation: Evidence that Google translate works for comparative bag-of-words text applications. Polit Anal. 2018;26:417–30. [Google Scholar]
- 17.Aksoy D, Çevik B, Solmaz V, Kurt SG, Sümbül O. A dyskinesia case induced by pramipexole, pregabalin and gabapentin after cardiopulmonary resuscitation. Turk J Neurol. 2013;19:148–50. [Google Scholar]
- 18.Arroyo S, Anhut H, Kugler AR, Lee CM, Knapp LE, Garofalo EA, et al. Pregabalin add-on treatment: A randomized, double-blind, placebo-controlled, dose-response study in adults with partial seizures. Epilepsia. 2004;45:20–7. doi: 10.1111/j.0013-9580.2004.31203.x. [DOI] [PubMed] [Google Scholar]
- 19.Baulac M, Leon T, O'Brien TJ, Whalen E, Barrett J. A comparison of pregabalin, lamotrigine, and placebo as adjunctive therapy in patients with refractory partial-onset seizures. Epilepsy Res. 2010;91:10–9. doi: 10.1016/j.eplepsyres.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Baysal Kirac L, Aydogdu I, Acarer A, Alpaydin S, Bayam FE, Onbasi H, et al. Myoclonic status epilepticus in six patients without epilepsy. Epilepsy Behav Case Rep. 2013;1:10–3. doi: 10.1016/j.ebcr.2012.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ari BC, Domac FM, Kenangil GO. A Case of Pregabalin induced Parkinsonism [abstract] [Last accessed 2020 Jun 11];Mov Disord. 2019 34(Suppl 2) doi: 10.4103/0028-3886.304071. Available from: https://www.mdsabstracts.org/abstract/a-case-of-pregabalin-induced-parkinsonism/ [DOI] [PubMed] [Google Scholar]
- 22.Courtois F, Borrey D, Haufroid V, Hantson P. Pregabalin-associated myoclonic encephalopathy without evidence of drug accumulation in a patient with acute renal failure. Indian J Nephrol. 2014;24:48–50. doi: 10.4103/0971-4065.125102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dag E, Gokce B, Buturak SV, Tiryaki D, Erdemoglu AK. Pregabalin-induced akathisia. Ann Pharmacother. 2013;47:592–3. doi: 10.1345/aph.1R699. [DOI] [PubMed] [Google Scholar]
- 24.Desai A, Kherallah Y, Szabo C, Marawar R. Gabapentin or pregabalin induced myoclonus: A case series and literature review. J Clin Neurosci. 2019;61:225–34. doi: 10.1016/j.jocn.2018.09.019. [DOI] [PubMed] [Google Scholar]
- 25.Elger CE, Brodie MJ, Anhut H, Lee CM, Barrett JA. Pregabalin add-on treatment in patients with partial seizures: A novel evaluation of flexible-dose and fixed-dose treatment in a double-blind, placebo-controlled study. Epilepsia. 2005;46:1926–36. doi: 10.1111/j.1528-1167.2005.00341.x. [DOI] [PubMed] [Google Scholar]
- 26.Giray E, Toprak CS, Saçaklidir R, Gündüz OH. Pregabalin-associated stuttering in a patient with complex regional pain syndrome: A case report. J Clin Psychopharmacol. 2016;36:740–2. doi: 10.1097/JCP.0000000000000609. [DOI] [PubMed] [Google Scholar]
- 27.Gosavi T. Myoclonus in an elderly patient: Case report. Reactions. 2011;1365:20. [Google Scholar]
- 28.Hamlyn A, Foo K, Bhatia A, Bobrin B. Manifestations of pregabalin withdrawal. J Psychiatry. 2017;20:418. [doi:10.4172/2378-5756.1000418] [Google Scholar]
- 29.Shneker BF, McAuley JW. Pregabalin: A new neuromodulator with broad therapeutic indications. Ann Pharmacother. 2005;39:2029–37. doi: 10.1345/aph.1G078. [DOI] [PubMed] [Google Scholar]
- 30.Han S. Asterixis in elderly patients: 2 case reports. Reactions. 2008;1218:6. [Google Scholar]
- 31.Healy DG, Ingle GT, Brown P. Pregabalin- and gabapentin-associated myoclonus in a patient with chronic renal failure. Mov Disord. 2009;24:2028–9. doi: 10.1002/mds.22286. [DOI] [PubMed] [Google Scholar]
- 32.Heckmann JG, Ulrich K, Dütsch M, Neundörfer B. Pregabalin associated asterixis. Am J Phys Med Rehabil. 2005;84:724. doi: 10.1097/01.phm.0000176355.97155.f5. [DOI] [PubMed] [Google Scholar]
- 33.Hellwig S, Amtage F. Pregabalin-induced cortical negative myoclonus in a patient with neuropathic pain. Epilepsy Behav. 2008;13:418–20. doi: 10.1016/j.yebeh.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 34.Hernández SC, Grullón MC, Carbonell FR, Soto MB, Panicot JE, Villa MJ. Abdominal pain challenge to manage in an elderly patient. Dolor. 2019;34:63–8. [Google Scholar]
- 35.Huber B, Bocchicchio M, Feuerbaum E, May T, Meinert T, Robertson E, et al. Efficacy and tolerability of pregabalin in patients with difficult-to-treat epilepsy and intellectual disability. Epilepsy Behav. 2008;13:397–401. doi: 10.1016/j.yebeh.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 36.Huppertz HJ, Feuerstein TJ, Schulze-Bonhage A. Myoclonus in epilepsy patients with anticonvulsive add-on therapy with pregabalin. Epilepsia. 2001;42:790–2. doi: 10.1046/j.1528-1157.2001.44000.x. [DOI] [PubMed] [Google Scholar]
- 37.Kim JB, Jung JM, Park MH, Lee EJ, Kwon DY. Negative myoclonus induced by gabapentin and pregabalin: A case series and systematic literature review. J Neurol Sci. 2017;382:36–9. doi: 10.1016/j.jns.2017.09.019. [DOI] [PubMed] [Google Scholar]
- 38.Knake S, Klein KM, Hattemer K, Wellek A, Oertel WH, Hamer HM, et al. Pregabalin-induced generalized myoclonic status epilepticus in patients with chronic pain. Epilepsy Behav. 2007;11:471–3. doi: 10.1016/j.yebeh.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 39.Kwon D, Park M. Regardless of impaired renal function, negative myoclonus can be induced by gabapetin and pregabalin. Mov Disord. 2019;34:1461. [Google Scholar]
- 40.Kwong YL, Leung AY, Cheung RT. Pregabalin-induced trismus in a leukemia patient. J Hosp Med. 2011;6:103. doi: 10.1002/jhm.701. [DOI] [PubMed] [Google Scholar]
- 41.Lee BI, Yi S, Hong SB, Kim MK, Lee SA, Lee SK, et al. Pregabalin add-on therapy using a flexible, optimized dose schedule in refractory partial epilepsies: A double-blind, randomized, placebo-controlled, multicenter trial. Epilepsia. 2009;50:464–74. doi: 10.1111/j.1528-1167.2008.01954.x. [DOI] [PubMed] [Google Scholar]
- 42.Lee DW, Lee HJ, Kim HJ, Chang SH, Park DJ. Two cases of pregabalin neurotoxicity in chronic kidney disease patients. NDT Plus. 2011;4:138. doi: 10.1093/ndtplus/sfq219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masmoudi I, Gras-Champel V, Barbieux-Vaquez D, Masmoudi K. Pregabalin-induced Parkinsonism: A case report. Thérapie. 2017;72:395–6. doi: 10.1016/j.therap.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 44.Matsuki Y, Tabata M, Nobukawam Y, Sakai M, Yasuca Y, Mizogam M, et al. Muscle rigidity associated with pregabalin. Pain Physician. 2012;15:E349–351. [PubMed] [Google Scholar]
- 45.Modur PN, Milteer WE. Adjunctive pregabalin therapy in mentally retarded, developmentally delayed patients with epilepsy. Epilepsy Behav. 2008;13:554–6. doi: 10.1016/j.yebeh.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Murphy N, Mosher L, editors. Clinical Toxicology. New York, NY 10017 USA: Informa Healthcare 52 Vanderbilt Ave; 2008. Severe myoclonus from pregabalin (lyrica) due to chronic renal insufficiency. [Google Scholar]
- 47.Olszewska DA, Chalissery AJ, Williams J, Lynch T, Smyth S. Speech myoclonus due to probable pregabalin adverse drug-reaction. Parkinsonism Relat Disord. 2015;21:823–4. doi: 10.1016/j.parkreldis.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 48.Ozturk HM, Morkavuk G. Nasal pregabalin overdose and myclonus: A new way of misuse. Psychiatry Clin Psychopharmacol. 2019;29:216–9. [Google Scholar]
- 49.Pacheco-Paez T, Montastruc F, Rousseau V, Chebane L, Lapeyre-Mestre M, Renoux C, et al. Parkinsonism associated with gabapentinoid drugs: A pharmacoepidemiologic study. Mov Disord. 2020;35:176–80. doi: 10.1002/mds.27876. [DOI] [PubMed] [Google Scholar]
- 50.Park KD, Kim MK, Lee SJ. Negative myoclonus associated with pregabalin. Yeungnam Univ J Med. 2018;35:240–3. doi: 10.12701/yujm.2018.35.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Park YM, Lee HJ, Kang SG, Cho JH, Kim L. Resolution of pregabalin and mirtazapine associated restless legs syndrome by bupropion in a patient with major depressive disorder. Psychiatry Investig. 2009;6:313–5. doi: 10.4306/pi.2009.6.4.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perez Lloret S, Amaya M, Merello M. Pregabalin-induced Parkinsonism: A case report. Clin Neuropharmacol. 2009;32:353–4. doi: 10.1097/WNF.0b013e3181a9eb1b. [DOI] [PubMed] [Google Scholar]
- 53.Prado-Mel E, Gil-López M, Navarro-Corrales C. Pregabalin-induced Parkinsonism 72 hours after iodinated contrast administration. J Pharm Pract Res. 2018;48:368–71. [Google Scholar]
- 54.Reyad RM, Omran AF, Abbas DN, Kamel MA, Shaker EH, Tharwat J, et al. The possible preventive role of pregabalin in postmastectomy pain syndrome: A double-blinded randomized controlled trial. J Pain Symptom Manage. 2019;57:1–9. doi: 10.1016/j.jpainsymman.2018.10.496. [DOI] [PubMed] [Google Scholar]
- 55.Saif MW, Syrigos K, Kaley K, Isufi I. Role of pregabalin in treatment of oxaliplatin-induced sensory neuropathy. Anticancer Res. 2010;30:2927–33. [PubMed] [Google Scholar]
- 56.Shimizu T, Yoshida T, Kitamura K, Hamada O. Disturbance of consciousness and involuntary movements caused by pregabalin. BMJ Case Rep. 2012;2012:bcr2012007559. doi: 10.1136/bcr-2012-007559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slocum GW, Schult RF, Gorodetsky RM, Wiegand TJ, Kamali M, Acquisto NM. Pregabalin and paradoxical reaction of seizures in a large overdose. Toxicol Commun. 2018;2:19–20. [Google Scholar]
- 58.Sluyts I. Myoclonus in an elderly patient: Case report. Reactions. 2007;1158:30. [Google Scholar]
- 59.Yoo L, Matalon D, Hoffman RS, Goldfarb DS. Treatment of pregabalin toxicity by hemodialysis in a patient with kidney failure. Am J Kidney Dis. 2009;54:1127–30. doi: 10.1053/j.ajkd.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 60.Zaccara G, Gangemi P, Perucca P, Specchio L. The adverse event profile of pregabalin: A systematic review and meta-analysis of randomized controlled trials. Epilepsia. 2011;52:826–36. doi: 10.1111/j.1528-1167.2010.02966.x. [DOI] [PubMed] [Google Scholar]
- 61.Friedman LM, Furberg C, DeMets DL, Reboussin DM, Granger CB. Fundamentals of Clinical Trials. 5th ed. New York: Springer; 2010. [Google Scholar]
- 62.Pulman J, Hemming K, Marson AG. Pregabalin add-on for drug-resistant partial epilepsy. Cochrane Database Syst Rev. 2014;3:CD005612. doi: 10.1002/14651858.CD005612.pub3. Published 2014 Mar 12. doi:10.1002/14651858.CD005612.pub3. [DOI] [PubMed] [Google Scholar]
- 63.Shibasaki H, Yamashita Y, Kuroiwa Y. Electroencephalographic studies myoclonus. Brain. 1978;101:447–60. doi: 10.1093/brain/101.3.447. [DOI] [PubMed] [Google Scholar]
- 64.Caviness JN, Brown P. Myoclonus: Current concepts and recent advances. Lancet Neurol. 2004;3:598–607. doi: 10.1016/S1474-4422(04)00880-4. [DOI] [PubMed] [Google Scholar]
- 65.Levy A, Chen R. Myoclonus: Pathophysiology and treatment options. Curr Treat Options Neurol. 2016;18:21. doi: 10.1007/s11940-016-0404-7. [DOI] [PubMed] [Google Scholar]
- 66.Young RR, Shahani BT. Asterixis: One type of negative myoclonus. Adv Neurol. 1986;43:137–56. [PubMed] [Google Scholar]
- 67.Hughes AJ, Daniel SE, Ben-Shlomo Y, Lees AJ. The accuracy of diagnosis of parkinsonian syndromes in a specialist movement disorder service. Brain. 2002;125:861–70. doi: 10.1093/brain/awf080. [DOI] [PubMed] [Google Scholar]
- 68.Stahl CM, Frucht SJ. An update on myoclonus management. Expert Rev Neurother. 2019;19:325–31. doi: 10.1080/14737175.2019.1592676. [DOI] [PubMed] [Google Scholar]
- 69.Eberhardt O, Topka H. Myoclonic disorders. Brain Sci. 2017;7:103. doi: 10.3390/brainsci7080103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Marks DM, Patkar AA, Masand PS, Pae CU. Does pregabalin have neuropsychotropic effects? A short perspective. Psychiatry Investig. 2009;6:55–8. doi: 10.4306/pi.2009.6.2.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zareba G. New treatment options in the management of fibromyalgia: Role of pregabalin. Neuropsychiatr Dis Treat. 2008;4:1193–201. doi: 10.2147/ndt.s3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ben-Shlomo Y, Sieradzan K. Idiopathic Parkinson's disease: Epidemiology, diagnosis and management. Br J Gen Pract. 1995;45:261–8. [PMC free article] [PubMed] [Google Scholar]
- 73.Martí-Massó JF, Poza JJ. Cinnarizine-induced Parkinsonism: Ten years later. Mov Disord. 1998;13:453–6. doi: 10.1002/mds.870130313. [DOI] [PubMed] [Google Scholar]
- 74.Cui QL, Yung WH, Chen L. Effects of substance Pon neuronal firing of pallidal neurons in parkinsonian rats. Neurosci Res. 2008;60:162–9. doi: 10.1016/j.neures.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Cui QL, Yung WH, Xue Y, Chen L. SubstancePexcites globus pallidus neurons in vivo. Eur J Neurosci. 2007;26:1853–61. doi: 10.1111/j.1460-9568.2007.05803.x. [DOI] [PubMed] [Google Scholar]
- 76.Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB. Loss of brainstem serotonin- and substance P-containing neurons in Parkinson's disease. Brain Res. 1990;510:104–7. doi: 10.1016/0006-8993(90)90733-r. [DOI] [PubMed] [Google Scholar]
- 77.Vreeswijk SM, Hoang TL, Neef NE, Gudenberg A, Paulus W, Sommer M. T47.Is stuttering a focal dystonia? Clin Neurophysiol. 2018;129:e19. [Google Scholar]
- 78.Mink JW. The basal ganglia: Focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- 79.Vreeswijk SM, Hoang TN, Korzeczek A, Neef NE, Wolff von Gudenberg A, Paulus W, et al. No evidence for dystonia-like sensory overflow of tongue representations in adults who stutter. Front Hum Neurosci. 2019;13:336. doi: 10.3389/fnhum.2019.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: Not just for reuptake anymore. J Neurophysiol. 2003;90:1363–74. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- 81.Sommer M, Bachmann CG, Liebetanz KM, Schindehütte J, Tings T, Paulus W. Pregabalin in restless legs syndrome with and without neuropathic pain. Acta Neurol Scand. 2007;115:347–50. doi: 10.1111/j.1600-0404.2007.00796.x. [DOI] [PubMed] [Google Scholar]
- 82.Lepping P, Delieu J, Mellor R, Williams JH, Hudson PR, Hunter-Lavin C. Antipsychotic medication and oxidative cell stress: A systematic review. J Clin Psychiatry. 2011;72:273–85. doi: 10.4088/JCP.09r05268yel. [DOI] [PubMed] [Google Scholar]


