Abstract
Stroke is a heterogeneous vascular disease. Carotid artery atherosclerosis is associated with almost one-quarter of ischemic strokes. Moreover, a large percentage of preventable strokes are currently attributed to carotid atherosclerosis. Over the past three decades, the management of carotid artery disease has evolved. The benefits of carotid revascularization alongside medical therapy have early been recognized. Nonetheless, the debate regarding the optimal strategy is still ongoing, particularly in patients with asymptomatic carotid artery disease. One of the challenges is the use of luminal stenosis to quantify the severity of the carotid artery disease and to guide decision-making regarding invasive revascularization. Characterizing carotid atherosclerotic plaque is a promising tool to identify vulnerable plaque. Certain features such as large lipid core have already been linked to acute vascular events, not only at the plaque level but also to predict systemic cardiovascular events. Recently, a quantitative T2 mapping magnetic resonance imaging technique was developed and validated against histology. The ability to accurately quantify plaque lipid content using this technique opens several new opportunities. In this review articles, we will discuss the current challenges in the management of carotid artery disease and the future roles of T2 mapping to aid therapeutic options. These roles may include how to determine the mode of invasive carotid revascularization in symptomatic patients. Moreover, there may be a rational to use T2 mapping as a risk stratification tool in asymptomatic patients with carotid artery stenosis. It may also provide an opportunity to stage atherosclerosis and identify patients with coronary atherosclerosis who may benefit maximally from intensive lipid interventions.
Keywords: Atherosclerosis, carotid, plaque characterisation
Introduction
Stroke is a prevalent vascular condition and is one of the leading causes of death, dementia, and chronic disability.[1] The prevalence of stroke is almost 3% of adult population, with six of seven strokes are of ischemic nature.[1,2] Over the past three decades, substantial advances have been made in managing stroke; nevertheless, there is remaining a significant proportion of strokes that are considered preventable.[3] A large percentage of these preventable strokes are attributed to carotid artery stenosis (CAS). In fact, almost one-quarter of ischemic strokes are caused by carotid artery atherosclerosis.[4] Moreover, CAS is associated with a higher risk of early recurrent ischemic events when compared with other stroke etiologies.[5]
The management of CAS has evolved over the past 30 years. Educating patients regarding stroke symptoms and the importance of timely seeking medical attention is the first step in modifying stroke risk.[6] Moreover, the current guidelines recommend regular exercise combined with healthy diet and weight control to mitigate stroke risk.[7] Better control of traditional risk factors such hypertension, diabetes, and smoking has been associated with a significant reduction in mortality associated with stroke.[1,8] Furthermore, the essential role of platelets in the pathogenesis of atherosclerosis was early recognized and was targeted using aspirin.[9] Nonetheless, the combination of dual antiplatelet did not manifest in incremental benefits but led to an increased in bleeding risk.[10] Finally, targeting lipoprotein-related risk, in particularly low-density lipoprotein cholesterol (LDL-c), using statin has been associated with carotid atherosclerosis regression.[11,12] This was translated into reduction of the incidence of strokes and cardiovascular events, with recent data suggesting better outcomes with more aggressive control of LDL-c (<70 mg/dl).[13,14] Moreover, nonstatin drugs, such as niacin, were shown to cause a significant reduction in carotid atherosclerosis over 1 year.[15]
The role of revascularization in the management of CAS was early established. Both the European Carotid Surgery Trial and the North American Symptomatic Carotid Endarterectomy Trial reported the efficacy of surgical revascularization in reducing stroke risk in patients with CAS.[16,17] Similarly, carotid artery stenting was shown to be a noninferior option to carotid endarterectomy (CEA) in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST).[18] Nonetheless, the precise management of CAS remains controversial, in particularly in patients with asymptomatic CAS.[19,20]
The recent development of novel imaging tools provided an opportunity to help in the management of CAS.[9,21,22] T2 mapping is a magnetic resonance imaging (MRI) technique which has the potential to guide clinicians to more rational treatments for patients with carotid atherosclerosis.[23] In this article, we will discuss the need for imaging tools to assist with the management of CAS and the promising role of T2 mapping in the spectrum of carotid disease.
Current Challenges in the Management of Carotid Disease
There is a wide consensus supporting the clinical benefits of carotid revascularization in symptomatic patients with CAS.[7,16,17,24] This was derived from three large randomized control trials that investigated the incremental benefits of CEA additional to medical therapy in the management of symptomatic CAS [Table 1].[16,17,24,25] Importantly, luminal stenosis, quantified using ultrasound duplex, was related to the magnitude of the exerted clinical benefits by CEA in these trials.[28] A substantial reduction in stroke risk was established in patients with CAS >70%, while surgical revascularization did not produce significant clinical benefits in individuals with CAS <50%.[7,16,17,24] Patients with “moderate” CAS (i.e., 50%–70%) had demonstrated clinical benefits which was marginally offset by the periprocedural stroke risk. Notably, the rate of periprocedural stroke and death has declined over time from 7% to 2% between 2000 and 2008.[29] Such reduction highlights the modifiable nature of periprocedural risk and the importance of factoring procedural risk and surgical experience in decision-making in this subgroup.[7,30]
Table 1.
Incidence of stroke across major studies of symptomatic and asymptomatic carotid artery stenosis comparing carotid revascularization and medical therapy
| Study name | Clinical presentation | Luminal stenosis (%) | Number of patients | Medical treatment (%) | Carotid revascularisation (%) | NNT |
|---|---|---|---|---|---|---|
| ACAS[26] | Asymptomatic | ≥60 | 1659 | 6.2* | 4* | 45 |
| ACST[27] | Asymptomatic | ≥60 | 3120 | 6.7 | 1.9 | 21 |
| <80 | 1284 | 7 | 1.4 | 18 | ||
| ≥80 | 1836 | 6.5 | 2.3 | 24 | ||
| ECST[16] | Symptomatic | 0-100 | 3018 | 17.8 | 17.4 | 250 |
| <50 | 1070 | 12 | 18.4 | - | ||
| 50-69 | 959 | 15.6 | 17.9 | 43 | ||
| ≥70 | 989 | 26.4 | 16 | 10 | ||
| NASCET[17] | Symptomatic | <70 | 2226 | 32.6 | 29.6 | 33 |
| <50 | 1368 | 26.2 | 25.7 | 200 | ||
| 50-69 | 858 | 32.3 | 23.9 | 12 | ||
| VA cooperative[25] | Symptomatic | 0-100 | 444 | 24.5 | 12.8 | 9 |
| 50-69¶ | 58 | 6.7 | 7.1* | - | ||
| ≥70¶ | 129 | 25.6 | 7.9* | 6 |
*Including death, ¶Early report of results stratified according to luminal stenosis. ACAS: Asymptomatic Carotid Artery Study, ACST: Asymptomatic Carotid Surgery Trial, ECST: European Carotid Surgery Trial, NASCET: North American Symptomatic Carotid Endarterectomy Trial, VA: Veterans affairs
The decision in the management of asymptomatic CAS is even more challenging.[19] The evidence of carotid revascularization in asymptomatic CAS was driven by the results from the Asymptomatic Carotid Artery Study and the Asymptomatic Carotid Surgery Trial (ACST) studies.[26,27] The absolute risk reduction with carotid revascularization in asymptomatic patients was modest when compared to symptomatic ones with comparable degree of luminal stenosis [Table 1]. This becomes more important given the diminishing risk of stroke with advancing medical therapy. The 10-year analysis from the ACST trial reported a significant reduction in stroke events in patients receiving statin and irrespective of their assigned therapy.[31] This poses challenges to the concept that “one size fits all” in the management of asymptomatic CAS and highlights the urgent need to identify a subgroup of patients who may benefit from revascularization without being offset by the procedure itself. Importantly, luminal stenosis did not serve as a reliable marker in predicting clinical benefits in the asymptomatic group.[19] Using ultrasound duplex to measure the severity of CAS does not directly reflect the burden of atherosclerotic disease embedded in the wall of the carotid artery. Luminal stenosis is considered as a surrogate of advanced atherosclerosis and a marker for plaque rupture. The variable rate for atherosclerotic plaque to impact on ultrasound signal was translated into a lack of correlation between luminal stenosis and plaque burden.[20]
Characterizing carotid atherosclerotic plaque is a promising tool to identify vulnerable plaque.[9,21,22] Certain features such as large lipid core has already been linked to acute vascular events, not only at the plaque level but also to predict systemic cardiovascular events.[32,33,34] Likewise, carotid intraplaque hemorrhage (IPH) was also demonstrated to be a significant predictor of stroke beyond clinical risk factors and may help tailoring treatments to patients with CAS.[35] Recent advances in technology have revolutionized in vivo imaging techniques, and the emergence of T2 mapping MRI technique would enable us, with a high degree of accuracy, to early characterize carotid atherosclerotic plaques.[21]
T2 Mapping Validation and Plaque Characterization
MRI was early recognized as a promising tool to characterize carotid atherosclerotic plaque.[36] Moreover, certain features, such as large lipid core, were identified as high-risk imaging markers of vascular events.[37] Such features were feasibly delineated using multi-contrast MRI whereby the combination of four in vivo sequences (T1 wieghted, T2 weighted, proton density, and time of flight) can characterize carotid plaque.[38] Nonetheless, the nonquantitative nature of estimating plaque components, alongside the insensitivities in detecting cholesterol deposits beyond discernible core, has restricted the widespread clinical applications of multi-contrast MRI.[15,39] In addition, the need for extensive postacquisition processing and interpretation was proved limiting.[15,39] T2 mapping is a novel and sensitive technique which was developed for vascular wall imaging. It quantifies lipid at a high resolution on a voxel by the voxel level based on the physical properties of the tissue in that voxel. It is generated from 14 images with incremental echo times between 9 and 127 ms, which are acquired using a DANTE-MESE sequence [Figure 1].[15,39,40] The slice thickness is 2 mm with a similar size slice gap, meaning two acquisitions are required to cover 2 cm of the carotid arteries with 10 interleaved slices.[15,39,40]
Figure 1.
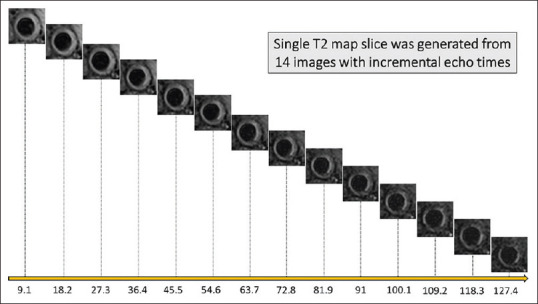
Acquisition of T2 mapping sequence using incremental echo times. The generation of an individual slice from 14 images with incremental echo times starting from 9 ms to 127 ms
This technique was validated against histology using 26 plaques with a total of 60 slice locations from patients scheduled for CEA.[23] A direct comparison was performed between lipid area derived from segmented T2 mapping and plaque lipid as identified by histology.[23] Lipid burden quantified using T2 mapping showed an excellent correlation with histology at both individual slice and plaque level. Moreover, the association between lipid area on T2 map and histology was identical irrespective of the symptomatic status of the plaque. The good reproducibility of this technique alongside its quantitative nature would overcome the limitations by current carotid wall imaging techniques. One of the challenges to T2 mapping MRI is its sensitivities to motion artifacts which initially resulted in relatively high rejection rate. Nonetheless, a significant improvement has been reported with more experience in using T2 mapping.[11,20,23] Moreover, ongoing work using motion correction strategies is promising which would allow this technique to fulfill its clinical potentials.[41]
The Clinical Utility of T2 Mapping in the Management of Carotid Disease
The ability to quantify plaque lipid accurately and reproducibly with high sensitivity opens several new opportunities [Figure 2]. Importantly, data from T2 mapping studies suggested that carotid plaques with recent symptoms contained higher plaque lipid content that asymptomatic ones.[20,23] Moreover, lipid distribution within the carotid wall also varied according to the symptomatic status of the plaque. The lipid aggregation index (LAI) was developed as a metric tool to quantify lipid distribution within the atherosclerotic plaque. High LAI reflected coalesced lipid and was associated with symptomatic patients. Notably, large carotid lipid volume, quantified using virtual histology intravascular ultrasound, was associated with a significant amount of debris during carotid stenting and was an important predictor of periprocedural stroke during carotid artery stenting.[42] Moreover, data from the CREST trial showed that symptomatic patients had better outcomes with CEA when compared to carotid stenting.[18] On the other hand, both the approaches had comparable outcomes in the asymptomatic group.[18] Such findings are plausibly related to carotid plaque composition, and therefore, T2 mapping may play a role in clinical decisions to determine the mode of invasive carotid revascularization strategy.
Figure 2.
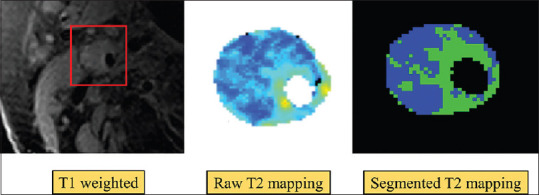
Lipid quantification using T2 mapping magnetic resonance imaging technique. Large plaque and lipid burden with matched raw T2 map (middle panel). The right panel was produced using a validated threshold to segment lipid with an estimated lipid content of 59% in this slice. Lipid is denoted in blue, while fibrous tissue is represented in green
The role of T2 mapping could also be extended beyond carotid revascularization. Its precision in quantifying carotid lipid content would allow identifying high-risk patients and may offer a platform to test whether these individuals would benefit from disease-modifying treatment.[21,22] In a substudy analysis from the AIM-HIGH, large carotid lipid content was associated with increased future risks; nonetheless, a significant proportion of patients had to be excluded due to the limitations of the used MRI technique.[33] T2 mapping, on the other hand, does not share these limitations, and therefore, there may be a rational based on previous observations to use T2 mapping as a risk stratification tool in asymptomatic patients with CAS. Patients with high-risk features may be candidates for intensive lipid interventions, including proprotein convertase subtilisin/kexin type 9 inhibitors to mitigate their future risk.
Finally, the ability of T2 mapping to quantify carotid lipid content without the need to prescreen patients for established carotid atherosclerosis unlocks the concept of staging atherosclerosis disease.[11,21,22] It would enable us to determine the extent and distribution of carotid plaque lipid content in patients presenting with coronary events. Thus, it would establish whether some patients have the propensity to develop lipid-rich plaques at multivascular sites. In fact, recent data showed that both evolocumab and alirocumab demonstrated larger clinical benefits in patients with polyvascualr atherosclerotic disease.[43,44] Moreover, T2 mapping would allow serial measurements with a high degree of accuracy, thus providing a useful tool to identify patients with a poor response to standard lipid therapy.[21,22] These patients may receive aggressive lipid-lowering treatment and rationalize the use of expensive and “biological” novel therapies.
The Role of Other High-Risk Atherosclerotic Plaque Features in Stroke Risk
Beside plaque lipid, the association of IPH with stroke was early recognized. IPH is suggested to destabilize atherosclerotic plaque by expanding necrotic core alongside triggering inflammatory response that contributes to plaque rupture.[45] Two opposing hypotheses were proposed to ascertain the source of IPH. The first one suggested that IPH occurred from the extravasation of blood from neovascularization process with advanced atherosclerotic plaques.[45,46] On the other hand, the second theory proposed that IPH is originated from the carotid lumen via intimal fissuring.[45,46] Recently, a novel theory suggested that IPH and angiogenesis are markers of inadequate plaque healing leading to a plaque rupture.[47] Nevertheless, IPH was a strong predictor of stroke in patients with CAS beyond any other clinical risk factors.[48] While IPH was present more frequently in patients with symptomatic CAS, the association with stroke was evident irrespective of the symptomatic status of the carotid plaque.[48]
Finally, an improvement in technology allowed the introduction of new imaging biomarker such as the perivascular adipose tissue (PVAT).[49] This marker has been strongly implicated in the initiation and progression of atherosclerosis.[50] It is proposed to reflect the inflammatory status of the atherosclerotic plaque and may serve as a predictor of plaque instability. The role of inflammation in the development of atherosclerosis is widely recognized. Certain blood biomarkers have been established to reflect the systemic inflammatory state of an individual.[51,52] PVAT, on the other hand, is associated with local inflammation and may help to tailor medical therapy and/or invasive revascularization in future.
Conclusion
The management of carotid artery disease remains challenging, particularly in patients with asymptomatic carotid plaque. The disconnect between plaque burden residing in the vascular wall and degree of stenosis poses difficulties in decision-making of treatment options. Plaque characterization using novel T2 mapping MRI technique may provide a new approach to risk stratify patients toward precision medicine.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. Heart disease and stroke statistics–2011 update: A report from the American Heart Association. Circulation. 2011;123:e18–209. doi: 10.1161/CIR.0b013e3182009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ovbiagele B, Nguyen-Huynh MN. Stroke epidemiology: Advancing our understanding of disease mechanism and therapy. Neurotherapeutics. 2011;8:319–29. doi: 10.1007/s13311-011-0053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mott M, Koroshetz W, Wright CB. CREST-2: Identifying the best method of stroke prevention for carotid artery stenosis: National Institute of Neurological Disorders and Stroke Organizational Update. Stroke. 2017;48:e130–1. doi: 10.1161/STROKEAHA.117.016051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nadareishvili ZG, Rothwell PM, Beletsky V, Pagniello A, Norris JW. Long-term risk of stroke and other vascular events in patients with asymptomatic carotid artery stenosis. Arch Neurol. 2002;59:1162–6. doi: 10.1001/archneur.59.7.1162. [DOI] [PubMed] [Google Scholar]
- 5.Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology. 2013;40:36–41. doi: 10.1159/000341410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Flynn D, Ford GA, Rodgers H, Price C, Steen N, Thomson RG. A time series evaluation of the FAST national stroke awareness campaign in England. PLoS One. 2014;9:e104289. doi: 10.1371/journal.pone.0104289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 8.Goldacre MJ, Duncan M, Griffith M, Rothwell PM. Mortality rates for stroke in England from 1979 to 2004: Trends, diagnostic precision, and artifacts. Stroke. 2008;39:2197–203. doi: 10.1161/STROKEAHA.107.509695. [DOI] [PubMed] [Google Scholar]
- 9.Alkhalil M. Mechanistic Insights to Target Atherosclerosis Residual Risk. Curr Probl Cardiol. 2019 Jun 24;:100432. doi: 10.1016/j.cpcardiol.2019.06.004. doi:10.1016/j.cpcardiol.2019.06.004. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 10.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–7. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 11.Alkhalil M, Biasiolli L, Akbar N, Galassi F, Chai JT, Robson MD, et al. T2 mapping MRI technique quantifies carotid plaque lipid, and its depletion after statin initiation, following acute myocardial infarction. Atherosclerosis. 2018;279:100–6. doi: 10.1016/j.atherosclerosis.2018.08.033. [DOI] [PubMed] [Google Scholar]
- 12.Lee JM, Wiesmann F, Shirodaria C, Leeson P, Petersen SE, Francis JM, et al. Early changes in arterial structure and function following statin initiation: Quantification by magnetic resonance imaging. Atherosclerosis. 2008;197:951–8. doi: 10.1016/j.atherosclerosis.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amarenco P, Bogousslavsky J, Callahan A, 3rd, Goldstein LB, Hennerici M, Rudolph AE, et al. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–59. doi: 10.1056/NEJMoa061894. [DOI] [PubMed] [Google Scholar]
- 14.Amarenco P, Kim JS, Labreuche J, Charles H, Abtan J, Bejot Y, et al. A Comparison of Two LDL Cholesterol Targets after Ischemic Stroke. N Engl J Med. 2019 doi: 10.1056/NEJMoa1910355. [DOI] [PubMed] [Google Scholar]
- 15.Lee JM, Robson MD, Yu LM, Shirodaria CC, Cunnington C, Kylintireas I, et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J Am Coll Cardiol. 2009;54:1787–94. doi: 10.1016/j.jacc.2009.06.036. [DOI] [PubMed] [Google Scholar]
- 16.Group ECSTC. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: Final results of the MRC European Carotid Surgery Trial (ECST) Lancet. 1998;351:1379–87. [PubMed] [Google Scholar]
- 17.Barnett HJ, Taylor DW, Eliasziw M, Fox AJ, Ferguson GG, Haynes RB, et al. Benefit of carotid endarterectomy in patients with symptomatic moderate or severe stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1998;339:1415–25. doi: 10.1056/NEJM199811123392002. [DOI] [PubMed] [Google Scholar]
- 18.Brott TG, Hobson RW, 2nd, Howard G, Roubin GS, Clark WM, Brooks W, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naylor AR. Time to rethink management strategies in asymptomatic carotid artery disease. Nat Rev Cardiol. 2011;9:116–24. doi: 10.1038/nrcardio.2011.151. [DOI] [PubMed] [Google Scholar]
- 20.Alkhalil M, Biasiolli L, Chai JT, Galassi F, Li L, Darby C, et al. Quantification of carotid plaque lipid content with magnetic resonance T2 mapping in patients undergoing carotid endarterectomy. PLoS One. 2017;12:e0181668. doi: 10.1371/journal.pone.0181668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alkhalil M, Chai JT, Choudhury RP. Plaque imaging to refine indications for emerging lipid-lowering drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3:58–67. doi: 10.1093/ehjcvp/pvw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alkhalil M. Proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, reality or dream in managing patients with cardiovascular disease. Curr Drug Metab. 2019;20:72–82. doi: 10.2174/1389200219666180816141827. [DOI] [PubMed] [Google Scholar]
- 23.Chai JT, Biasiolli L, Li L, Alkhalil M, Galassi F, Darby C, et al. Quantification of Lipid-rich core in carotid atherosclerosis using magnetic resonance T2 mapping: Relation to clinical presentation. JACC Cardiovasc Imaging. 2017;10:747–56. doi: 10.1016/j.jcmg.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mayberg MR, Wilson SE, Yatsu F, Weiss DG, Messina L, Hershey LA, et al. Carotid endarterectomy and prevention of cerebral ischemia in symptomatic carotid stenosis. Veterans Affairs Cooperative Studies Program 309 Trialist Group. JAMA. 1991;266:3289–94. [PubMed] [Google Scholar]
- 25.Hobson RW, 2nd, Weiss DG, Fields WS, Goldstone J, Moore WS, Towne JB, et al. Efficacy of carotid endarterectomy for asymptomatic carotid stenosis. The Veterans Affairs Cooperative Study Group. N Engl J Med. 1993;328:221–7. doi: 10.1056/NEJM199301283280401. [DOI] [PubMed] [Google Scholar]
- 26.Walker MD, Marler JR, Goldstein M, et al. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. [Last accessed on 2020 May 20];JAMA. 1995 273:1421–8. Available from: https://pubmed.ncbi.nlm.nih.gov/7723155/ [PubMed] [Google Scholar]
- 27.Halliday A, Mansfield A, Marro J, Peto C, Peto R, Potter J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363:1491–502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 28.Rothwell PM, Eliasziw M, Gutnikov SA, Warlow CP, Barnett HJ. Carotid Endarterectomy Trialists Collaboration. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet. 2004;363:915–24. doi: 10.1016/S0140-6736(04)15785-1. [DOI] [PubMed] [Google Scholar]
- 29.Müller MD, von Felten S, Algra A, Becquemin JP, Bulbulia R, Calvet D, et al. Secular trends in procedural stroke or death risks of stenting versus endarterectomy for symptomatic carotid stenosis. Circ Cardiovasc Interv. 2019;12:e007870. doi: 10.1161/CIRCINTERVENTIONS.119.007870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brott TG, Halperin JL, Abbara S, Bacharach JM, Barr JD, Bush RL, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: Executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124:489–532. doi: 10.1161/CIR.0b013e31820d8d78. [DOI] [PubMed] [Google Scholar]
- 31.Halliday A, Harrison M, Hayter E, Kong X, Mansfield A, Marro J, et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet. 2010;376:1074–84. doi: 10.1016/S0140-6736(10)61197-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zavodni AE, Wasserman BA, McClelland RL, Gomes AS, Folsom AR, Polak JF, et al. Carotid artery plaque morphology and composition in relation to incident cardiovascular events: The Multi-Ethnic Study of Atherosclerosis (MESA) Radiology. 2014;271:381–9. doi: 10.1148/radiol.14131020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun J, Zhao XQ, Balu N, Neradilek MB, Isquith DA, Yamada K, et al. Carotid plaque lipid content and fibrous cap status predict systemic CV outcomes: The MRI Substudy in AIM-HIGH. JACC Cardiovasc Imaging. 2017;10:241–9. doi: 10.1016/j.jcmg.2016.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Redgrave JN, Lovett JK, Gallagher PJ, Rothwell PM. Histological assessment of 526 symptomatic carotid plaques in relation to the nature and timing of ischemic symptoms: The Oxford plaque study. Circulation. 2006;113:2320–8. doi: 10.1161/CIRCULATIONAHA.105.589044. [DOI] [PubMed] [Google Scholar]
- 35.Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: Meta-analysis of individual patient data. JACC Cardiovasc Imaging. 2020;13:395–406. doi: 10.1016/j.jcmg.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 36.Zhao XQ, Yuan C, Hatsukami T, Frechette E, Kang X, Maravilla KR, et al. Effects of pronlonged intensive lipid-lowering therapy on the characteristics of carotid atherosclerotic plaques in vivo by MRI. Arterioscler Thromb Vasc Biol. 2001;21:1623–29. doi: 10.1161/hq1001.098463. [DOI] [PubMed] [Google Scholar]
- 37.Gupta A, Baradaran H, Schweitzer AD, Kamel H, Pandya A, Delgado D, et al. Carotid plaque MRI and stroke risk: A systematic review and meta-analysis. Stroke. 2013;44:3071–7. doi: 10.1161/STROKEAHA.113.002551. [DOI] [PubMed] [Google Scholar]
- 38.Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, et al. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051–6. doi: 10.1161/hc4201.097839. [DOI] [PubMed] [Google Scholar]
- 39.Biasiolli L, Lindsay AC, Chai JT, Choudhury RP, Robson MD. In vivo quantitative T2 mapping of carotid arteries in atherosclerotic patients: Segmentation and T2 measurement of plaque components. J Cardiovasc Magn Reson. 2013;15:69. doi: 10.1186/1532-429X-15-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Chai JT, Biasiolli L, Robson MD, Choudhury RP, Handa AI, et al. Black-blood multicontrast imaging of carotid arteries with DANTE-prepared 2D and 3D MR imaging. Radiology. 2014;273:560–9. doi: 10.1148/radiol.14131717. [DOI] [PubMed] [Google Scholar]
- 41.Frost R, Biasiolli L, Li L, Hurst K, Alkhalil M, Choudhury RP, et al. Navigator-based reacquisition and estimation of motion-corrupted data: Application to multi-echo spin echo for carotid wall MRI. Magn Reson Med. 2020;83:2026–41. doi: 10.1002/mrm.28063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Matsumoto S, Nakahara I, Higashi T, Iwamuro Y, Watanabe Y, Takezawa M, et al. Fibro-fatty volume of culprit lesions in Virtual Histology intravascular ultrasound is associated with the amount of debris during carotid artery stenting. Cerebrovasc Dis. 2010;29:468–75. doi: 10.1159/000297962. [DOI] [PubMed] [Google Scholar]
- 43.Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, et al. Low-density lipoprotein cholesterol lowering with evolocumab and outcomes in patients with peripheral artery disease: Insights from the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk) Circulation. 2018;137:338–50. doi: 10.1161/CIRCULATIONAHA.117.032235. [DOI] [PubMed] [Google Scholar]
- 44.Jukema JW, Szarek M, Zijlstra LE, de Silva HA, Bhatt DL, Bittner VA, et al. Alirocumab in patients with polyvascular disease and recent acute coronary syndrome: ODYSSEY OUTCOMES Trial. J Am Coll Cardiol. 2019;74:1167–76. doi: 10.1016/j.jacc.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Alkhalil M, Choudhury RP. Intraplaque hemorrhage as a marker of stroke risk. JACC Cardiovasc Imaging. 2020;13:407–9. doi: 10.1016/j.jcmg.2019.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Virmani R, Kolodgie FD, Burke AP, Finn AV, Gold HK, Tulenko TN, et al. Atherosclerotic plaque progression and vulnerability to rupture: Angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thromb Vasc Biol. 2005;25:2054–61. doi: 10.1161/01.ATV.0000178991.71605.18. [DOI] [PubMed] [Google Scholar]
- 47.Brezinski M, Willard F, Rupnick M. Inadequate intimal angiogenesis as a source of coronary plaque instability: Implications for healing. Circulation. 2019;140:1857–9. doi: 10.1161/CIRCULATIONAHA.119.042192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schindler A, Schinner R, Altaf N, Hosseini AA, Simpson RJ, Esposito-Bauer L, et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: Meta-analysis of individual patient data. JACC Cardiovasc Imaging. 2020;13:395–406. doi: 10.1016/j.jcmg.2019.03.028. [DOI] [PubMed] [Google Scholar]
- 49.Alkhalil M, Edmond E, Edgar L, Digby JE, Omar O, Robson MD, et al. The relationship of perivascular adipose tissue and atherosclerosis in the aorta and carotid arteries, determined by magnetic resonance imaging. Diab Vasc Dis Res. 2018;15:286–93. doi: 10.1177/1479164118757923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–39. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alkhalil M, Kearney A, Hegarty M, Stewart C, Devlin P, Owens CG, et al. Eosinopenia as an adverse marker of clinical outcomes in patients presenting with acute myocardial infarction. Am J Med. 2019;132:e827–34. doi: 10.1016/j.amjmed.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 52.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM., Jr Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of rosuvastatin: A prospective study of the JUPITER trial. Lancet. 2009;373:1175–82. doi: 10.1016/S0140-6736(09)60447-5. [DOI] [PubMed] [Google Scholar]


