Abstract
Reduced-intensity conditioning (RIC) allogeneic hematopoietic stem cell transplantation (RIC-alloHSCT) is associated with lower toxicity but higher rates of prolonged mixed chimerism than myeloablative conditioning. Decreased pretransplantation host T cell numbers are associated with less graft rejection and early full donor chimerism. To compensate for variability in pretransplantation host lymphocyte numbers and facilitate the achievement of rapid full donor chimerism, we tested a strategy of targeted lymphocyte depletion (TLD) using chemotherapy at conventional doses to provide cytoreduction and lymphocyte depletion before RIC-alloHSCT. In our study, 111 patients with advanced hematologic malignancies received 1 to 3 cycles of conventional-dose chemotherapy to reduce circulating lymphocytes to a predetermined level. Patients then underwent RIC-alloHSCT from HLA-matched siblings. Patients received a median of 2 cycles of TLD chemotherapy, resulting in a median 71% decline in CD4+ count. All patients engrafted; there were no late graft failures. By day +14, median CD3+ chimerism was 99% donor and was significantly associated with lower post-TLD CD4+ counts (P = .012). One- and 5-year treatment-related mortality were 15% and 21%, respectively. At 1-year follow-up, 66% of patients had achieved complete remission (CR) of which 92% were not in CR at the time of transplantation. Overall survival at 1 and 5 years post transplantation were 66% and 47%, respectively.
Keywords: Reduced-intensity, transplantation, Engraftment, Lymphocyte depletion
INTRODUCTION
Reduced-intensity conditioning (RIC) regimens are associated with decreased acute treatment-related toxicities, thereby broadening the general application of allogeneic hematopoietic stem cell transplantation (alloHSCT) [1–4]. Historically, RIC-alloHSCT has been associated with increased relapse rates compared with myeloablative conditioning regimens, particularly in patients with high-risk malignancies [5,6]. Both murine models and clinical trials have demonstrated that optimal graft-versus-tumor effects are achieved in states of full donor T-lymphoid chimerism [7–9]. Therefore, higher relapse rates may not be explained completely by the decreased antitumor effects of the conditioning regimen.
RIC often results in states of mixed donor and host chimerism in the early transplantation period [10,11]. Factors influencing engraftment include host immune status, histocompatibility, hematopoietic “space,” stem cell dose, and the population of T cells within the allograft [11–14]. Of these, host immune status is the least studied and clinically understood. Because of the significant differences in the type and amount of pretransplantation therapies of patients being considered for RIC-alloHSCT, host immune status immediately before transplantation is highly variable. To consistently achieve a uniform result of early full donor chimerism, patients most likely require different levels of conditioning intensity.
To address the issue of variability in host immune status, we developed a novel treatment approach, referred to as targeted lymphocyte depletion (TLD). The TLD approach is based on a preclinical murine engraftment model, in which the repetitive administration of moderate doses of disease-specific chemotherapy resulted in a significant depletion of circulating T cells with minimal myelosuppression [15]. This level of lymphocyte depletion, equivalent to that seen with lethal doses of myeloablative conditioning, was sufficient to permit the engraftment of fully MHC-mismatched allografts. Based on the these data, we hypothesized that: (1) circulating lymphocytes are a surrogate marker of host immune status; and (2) there exists a level of host T cells in humans that is sufficiently low enough to consistently permit the achievement of rapid and complete donor chimerism.
The TLD approach incorporates the standard clinical practice of administering “salvage” chemotherapy at conventional doses before transplantation to reduce tumor burden. However, TLD utilizes agents that are both disease-specific and lymphocyte depleting to reduce host T cells before RIC-alloHSCT. The number of chemotherapy cycles administered is based on reaching a target lymphocyte number; patients with higher starting lymphocyte counts receive more chemotherapy cycles. As such, TLD provides a personalized approach to adjust for highly variable pretransplantation patient lymphocyte numbers. We tested the TLD approach in patients with advanced hematologic malignancies undergoing RIC-alloHSCT to determine effects on engraftment and clinical outcomes.
METHODS
Patients
The TLD approach was evaluated in patients with advanced hematologic malignancies participating in 4 sequential National Cancer Institute (NCI) protocols NCT00019851, NCT00055744, NCT00077480, and NCT00074490 (http://clinicaltrials.gov/ct2/home). All 4 protocolsct were approved by the NCI Institutional Review Board, and informed written consent was obtained from each patient. Patients were eligible if they were between the ages of 18 and 75 years, had either primary treatment failure or relapse after salvage therapy for a hematologic malignancy, and had an HLA-matched sibling. Patients were not excluded based on disease chemotherapy-sensitivity, which was defined based on their response to their last pre-enrollment chemotherapy regimen (chemosensitive was defined as a complete response [CR] or partial response [PR]; chemoresistant was defined as stable disease [SD] or progressive disease [PD]). There were no competing protocols for this patient population during the time of enrollment at our institution. Engraftment was the primary objective of all the protocols that were included in the analysis.
TLD Chemotherapy Regimen
TLD chemotherapy was given to patients with CD4+ counts greater than 200 cells/µL before RIC and consisted of the lymphocyte-depleting agent fludarabine as either part of the dose adjusted (DA)-EPOCH-F regimen (etoposide, prednisone, vincristine, cyclophosphamide, and doxorubicin) [16,17] or as part of the FLAG regimen (fludarabine, cytarabine, G-CSF) [18], depending on whether the patient had a lymphoid or myeloid malignancy; the majority of the lymphoid malignancy patients had been included in our previously published DA-EPOCH-FR study, which focused on pretransplantation response to this novel chemotherapy regimen.
Chemotherapy cycles were repeated every 21 days with the goal of achieving a CD4+ T cell count less than 200 cells/µL. If CD4+ < 200/µL was achieved, no additional cycles were given (CD4+ count < 200 cells/µL was chosen based on the AIDS literature that demonstrated that individuals with CD4+ count < 200 cells/µL are at greatest risk of developing opportunistic infections because cell-mediated immunity is markedly diminished). Patients could receive a total of 3 cycles of TLD therapy, provided there was adequate hematological recovery and no progression of disease. If enrollment CD4+ count was < 200 cells/µL, no pretransplantation TLD chemotherapy was given.
Transplantation Regimen
All patients received an RIC regimen consisting of concurrent fludarabine (30 mg/m2/day) and cyclophosphamide (1200 or 400 mg/m2/day; protocol dependent) for 4 days (day −6 to day −3) followed by T cell–replete peripheral blood stem cell allografts from HLA-matched siblings. All patients received a cyclosporine-based GVHD prophylaxis regimen.
Lymphocyte Monitoring by Flow Cytometry
Peripheral blood was collected before treatment and after each cycle of TLD. Flow cytometry was performed using whole blood lysis in a Clinical Laboratory Improvement Amendments (CLIA)–certified laboratory (Science Applications International Corporation, Frederick, MD). The absolute number of CD3+, CD4+ and CD8+ T, B, and NK cells per liter of blood was calculated based upon the percentages of these cells in the lymphocyte gate, the percentage of lymphocytes (defined as CD45bright, CD14−, low side scatter) and the white blood cell counts (Beckman Coulter Inc, Fullerton, CA). CD4+ and CD8+ T cells were defined as CD3+CD4+ or CD3+CD8+, respectively. B cells were defined as CD3−CD19+; NK cells were defined as CD3−CD56+.
Serum Interluekin-7 and Interleukin-15
nterleukin (IL)-7 and IL-15 are Tcell homeostatic cytokines that have been demonstrated to correlate with the degree of lymphocyte depletion [19]. In a subset of patients, serum was obtained for IL-7 and IL-15 determination before treatment and after hematopoietic recovery from each cycle of TLD therapy, as well as after the RIC regimen. Serum samples were frozen at −80°C until analysis. Samples were thawed on ice and analyzed using a high-sensitivity colorimetric ELISA (R&D Systems, Minneapolis, MN) according to the manufacturer’s recommendations. A separate standard curve was prepared on each plate using serial dilutions. Samples exceeding the highest value on the standard curve were reanalyzed using serial dilutions. Plates were read with a microplate reader (Molecular Devices, Sunnyvale, CA) and analyzed using SOFTmax Pro 3.0 software. All samples were run in duplicate. Intra-assay and inter-assay variability was less than 15%.
Chimerism Evaluation
Hematopoietic chimerism was assessed using short-tandem-repeat amplification determined at baseline in the donor and patient. After transplantation, patient peripheral blood was collected for analysis of lymphoid, myeloid, and total chimerism on day +14 post transplantation and every 2 weeks thereafter until donor chimerism was greater than 95%.
Statistical Methods
A variety of exploratory statistical methods were used to identify potential associations between transplantation outcomes and demographic or clinical parameters. A comparison of continuous parameters between 2 groups was done using a Wilcoxon rank-sum test, whereas comparisons of continuous parameters between 3 or more unordered categories were done using a Kruskal-Wallis test. Comparisons of dichotomous parameters between 2 groups were done using a Fisher exact test, although comparisons of parameters with 2 or more categories and a second parameter with more than 2 categories were performed using Mehta’s modification to the Fisher exact test [20]. The significance of trends in ordered categorical parameters across a dichotomous parameter were determined using an exact Cochran-Armitage test [21].
The statistical significance of the trends in an ordered categorical or continuous parameter across an ordered categorical parameter were determined by a Jonckheere-Terpstra test for trend [22]. Correlations were determined by Spearman correlation analysis. Event-free survival (EFS) was determined from the on-study date until the date of progression or death, or until being censored at the date of last follow-up. Overall survival (OS) was determined from the on-study date until death, or last follow-up. The probability of OS or EFS as a function of time was determined by the Kaplan-Meier method, with a log-rank test used to determine the statistical significance of the difference of a pair of Kaplan-Meier curves [23]. A Cox proportional hazards model analysis was performed to determine the joint association of factors initially found to have potential association with outcome in the univariate analyses (those with unadjusted P < .10 from a log-rank test) [24]. Cumulative incidence curves of treatment-related mortality (TRM) and disease-related mortality were constructed using the method of Gooley et al. [25].
All P values are 2-tailed and presented without adjustment for multiple comparisons.
RESULTS
Patient Characteristics
One hundred and eleven patients were enrolled onto protocol between August 1999 and May 2006 (Table 1). Of these patients, 45% had chemotherapy-refractory disease; 61% had a high risk of relapse by Seattle criteria [26].
Table 1.
Patient Characteristics
| Characteristic | n |
|---|---|
| No. of patients | 111 |
| Age, median (range), yr. | 50 (19 to 71) |
| Female/male | 40/71 |
| Histological Findings | |
| Non-Hodgkins lymphoma | 70 |
| Indolent | 17 |
| Aggressive | 53 |
| Hodgkin lymphoma | 12 |
| Multiple myeloma | 16 |
| AML/MDS | 4 |
| CML | 3 |
| Other | 6 |
| No. of prior regimens, median (range) | 3 (0 to 7) |
| Disease status at enrollment | |
| Chemosensitive | 58 |
| Chemoresistant | 51 |
| CMV status of recipient | |
| Negative | 48 |
| Positive | 63 |
| Minor ABO mismatch | 76 |
| Major ABO mismatch | 22 |
| Risk of relapse | |
| Low | 23 |
| Medium | 18 |
| High | 66 |
AML indicates acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; CMV, cytomegalovirus. Data are presented as n unless otherwise indicated.
Lymphocyte Depletion and Chimerism
Ninety-four of 108 evaluable patients achieved CD4+ < 200 cells/µL with TLD chemotherapy. The other 14 patients either received the maximum 3 cycles without achieving CD4+ < 200 cells/µL or had progressive disease. The median CD3+, CD4+, and CD8+ counts at enrollment were 673 cells/µL (range, 5 to 3953), 286 cells/µL (range, 5 to 3888), and 277 cells/µL (range, 1 to 1763), respectively (Table 2). After a median number of 2 cycles of TLD chemotherapy, median CD3+, CD4+, and CD8+ counts were: 164 cells/µL (range, 1 to 1496), 82 cells/µL (range, 0 to 508), and 52 cells/µL (range, 1 to 1195), reflecting a median relative decrease of 76%, 71%, and 81%, respectively. Immediately before stem cell infusion (day 0) median host CD3+, CD4+, and CD8+ counts were 4 cells/µL (range, 0 to 69), 3 cells/µL (range, 0 to 65), and 1 cell/µL (range, 0 to 44), respectively.
Table 2.
Lymphocyte Depletion after TLD Chemotherapy and after Reduced-Intensity Conditioning
| Enrollment | Post TLD | Post FC (Day 0) | |
|---|---|---|---|
| CD3 + cells/µL Median (range) | 673 (5 to 3953) | 164 (1 to 1496) | 4 (0 to 69) |
| CD4 + cells/µL Median (range) | 286 (5 to 3888) | 82 (0 to 508) | 3 (0 to 65) |
| CD8 + cells/µL Median (range) | 277 (1 to 1763) | 52 (1 to 1195) | 1 (0 to 44) |
One hundred and nine patients were evaluable for engraftment, of which 100% engrafted; there were no late graft failures. At post-transplantation day +14, the median CD3+ chimerism was 98% (range, 60% to 100%), CD14+/15+ chimerism was 100% (range, 20% to 100%) and whole blood chimerism was 99% (range, 20% to 100%). Patients were able to maintain full donor chimerism (defined as ≥95% donor chimerism) as evidenced by median 99% chimerism in CD3+ and median 100% chimerism in CD14+/15+ and whole blood at day 28 and whole blood at day + 100 (Table 3).
Table 3.
Early Post-Transplantation Myeloid, Lymphoid, and Whole Blood Chimerism
| Day +14 | Day +28 | Day +100 | |
|---|---|---|---|
| CD14+/15+, median (range) | 100% (20% to 100%) | 100% (40% to 100%) | 100% (35% to 100%) |
| CD3+, median (range) | 98% (60% to 100%) | 99% (70% to 100%) | 100% (65% to 100%) |
| Whole blood, median (range) | 99% (20% to 100%) | 100% (10% to 100%) | 100% (0 to 100%) |
Full donor CD3+ chimerism at post-transplantation day +14 was associated with lower post-TLD CD4+ counts (median, 78 cells/µL versus 128 cells/µL in patients with versus without full donor chimerism; P = .012) and day 0 (before allograft infusion) CD3+, CD4+, and CD8+ counts (P < .0005). Full donor CD14+/15+ chimerism at day 14 and day 28 was associated with lower day 0 CD3+, CD4+, CD8+ counts (P < .05). Full donor whole blood chimerism at post-transplantation days +14, +28, and +100 were associated with lower day 0 CD3+, CD4+, and CD8+ counts (P < .05). The CD3+ and CD34+ dose contained in the allograft was associated with day +14 CD3+ chimerism (P = .04) and with day +28 CD14+/15+ chimerism (P = .03), respectively.
IL-7 and IL-15 Determination
A subset of 31 patients had IL-7 and IL-15 samples tested at baseline, after each sequential cycle of TLD chemotherapy and after completion of the conditioning chemotherapy before allograft infusion. The median IL-7 level at baseline from 27 observations was 12.9 pg/mL (range, 0.0 to 46.9). Median level of IL-7 after 1 to 3 cycles of TLD was 21.3 pg/mL (range, .8 to 63.5). Post conditioning, the median IL-7 level was 37.0 pg/mL (range, 13.3 to 79.2). The median IL-15 level of 26 evaluable patients at baseline was 1.9 pg/mL (range, 0.1 to 13.6). Median IL-15 level after 1 to 3 cycles of TLD was 7.7 pg/mL (range, .8 to 48.0). Post conditioning, the median IL-15 level was 61.6 pg/mL (range, 22.3 to 205.3). Increases in IL-7 levels were weakly associated with a decrease in CD3+ (r −.31; P = .13), CD4+ (r = .32; P = .12), and CD8+ (r = .18; P =.36) counts, respectively. Increases in IL-15 levels were weakly associated with decreases in CD3+ (r =.33; P = .11) and CD8+ (r = .25; P = .23) and moderately associated with a decrease in NK cell (r = .64; P =.0007) counts.
Acute and Chronic GVHD
The cumulative incidence of grades II to IV and grades III to IV acute GVHD (aGVHD) was 46% and 23%, respectively. The cumulative incidence of patients with chronic GVHD (cGVHD) was 59% (25% limited, 75% extensive). Patients with aGVHD II to IV had significantly higher CD4+ count after TLD (median, 110 cells/µL versus 77 cells/µL in patients with versus without aGVHD; P = .013). Patients with acute GVHD III to IV had significantly higher CD4+ and CD3+ counts at day 0 (P = .03 and P = .02, respectively).
Response, Mortality and Survival
By day +28 after transplantation, 38 patients (35%) achieved (n 27) or maintained a CR (n = 11) and 44 (40%) achieved (n = 21) or maintained (n = 23) a PR. Twelve additional CRs were observed at day + 100 without withdrawal of immunosuppression or donor lymphocyte infusion. Because many of the patients were enrolled on study before positron emission tomography (PET) scan was considered standard of care, the initial cohort of patients did not routinely get PET scans for their restaging. In the later cohorts, PET was used more frequently but not uniformly to assess response for lymphoproliferative diseases. EFS and OS at 1 year were 49% and 66% and were 31% and 47% at 5 years, respectively (Figure 1). One- and 5-year TRM were 15% and 21%, respectively. Seventy-three patients were alive at their 1-year follow-up, of which 48 patients (66%) were in CR. Ninety-two percent of these patients had not been in CR at the time of transplantation. By univariate analysis, younger age of recipient and lack of an ABO major mismatch were significantly associated (P < .05) with OS; number of prior therapies, disease status at the time of transplantation, and relapse risk by Seattle criteria were significantly associated (P < .05) with both EFS and OS. Positive cytomegalovirus serology was associated with both lymphoid (P = .022) and whole blood chimerism (P = .034) at day +14.
Figure 1.
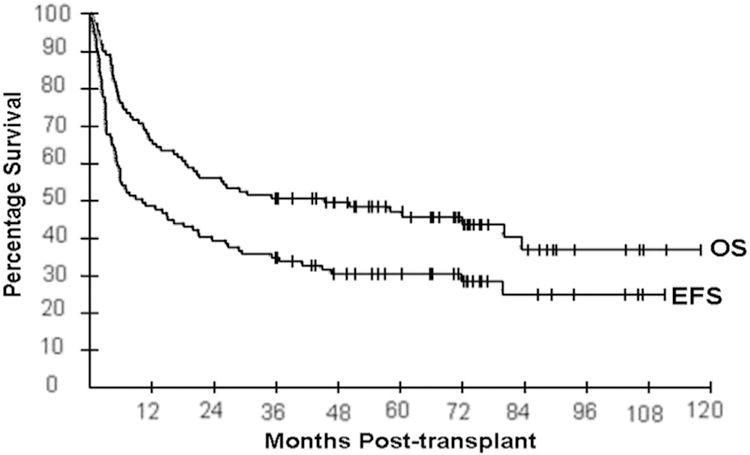
Event-free and overall survival for all patients enrolled in study.
DISCUSSION
Pretransplantation chemotherapy has been shown to facilitate engraftment by decreasing recipient immune competence and weakening the host-versus-graft reaction [27]. In a study by Childs et al., it was first established that control of malignant disease after nonmyeloablative conditioning was dependent on engraftment of donor T lymphocytes and occurrence of graft-versus-tumor effects [28]. This was corroborated by Keil et al., who demonstrated that patients with less than 90% donor T cell chimerism on day +28 had significantly higher risks of graft rejection and disease relapse than those with greater than 90% T cell donor chimerism [29].
The variability inherent in pretransplantation host immune status is evident by the large range of circulating T cell numbers in patients referred to our institution for transplantation. By using TLD, we were able to compensate for this inconsistency while achieving levels of host T cell depletion comparable to levels achieved with myeloablative conditioning. Although RIC regimens are associated with fewer toxicities and lower rates of early TRM, the risks of graft rejection are higher because residual recipient immunity is still present [30]. Whereas there is some evidence to suggest that prolonged antigen presentation by donor cells in mixed chimeras leads to enhanced graft-versus-tumor effect [31,32], several studies have clearly shown an increased risk of relapse in the setting of mixed chimerism [21,33]. Early CD3+ complete donor chimerism has been demonstrated to be more common in immunosuppressed hosts, and exposure to chemotherapy immediately before transplantation has been shown in several studies to correlate with better outcomes [27,34]. Using the strategy of TLD before RIC-alloHSCT, we confirmed our hypothesis that the degree of pretransplantation lymphocyte depletion is associated with the establishment of early post-transplantation full donor chimerism. In the current study, almost 100% of recipients had full donor T cell chimerism by post-transplantation day +14, which was sustained at days +28 and +100.
Under normal T cell homeostasis, fewer cytokines are available because of consumption by the endogenous lymphocyte population. After lymphocyte depletion, however, the consumptive cellular cytokine “sinks” are removed and adoptively transferred T cells have greater access to IL-7, IL-15, and IL-21 [35]. This is followed by preferential homeostatic expansion of adoptively transferred T cells. The TLD approach reduced circulating T cell numbers by 70% to 80%, which correlated with a significant increase in both serum IL-7 and IL-15. This is in contrast to recently published data by De Bock et al., who demonstrated that IL-7 and IL-15 levels did not increase after RIC-alloHSCT compared with the increase they demonstrated in myeloablative conditioining [36]. This corroborates the assertion that the reductions in circulating T cell numbers accomplished by TLD may enhance total body T cell depletion over and above what is achieved by RIC alone.
In patients with high risk of relapse by the Seattle criteria [26] and extensive amounts of prior chemotherapy, the long-term EFS and OS data reported in the current study are notable. It raises the possibility that by giving additional doses of disease-specific chemotherapy to achieve lymphocyte depletion, we are also eradicating minimal residual disease (MRD), which in multiple studies has been demonstrated to lead to a more favorable outcome [37–39]. In a prior paper that came out of our institution, the number of cycles of EPOCH-F/R was significantly associated with overall response rate: 1 cycle, 17%; 2 cycles, 33%; 3 cycles, 84%; P < .001 [16]. Therefore, it can be argued that patients should be treated until best response. This would be worth pursuing in future studies.
We conclude that TLD provides a personalized approach to pretransplantation host immune status resulting in absence of graft rejection and rapid and full donor chimerism after RIC-alloHSCT. This approach results in long-term survival in a high-risk population of patients with hematologic malignancies undergoing RIC-alloHSCT. Although the number of patients with myeloid malignancies was too small to draw any generalizable conclusions, pretransplantation MRD in this population is a subject of much current interest and the strategy of lymphocyte-depleting disease targeted chemotherapy before RIC-alloHSCT would be a strategy worth exploring. Limitations of this investigation include the variability inherent in the 4 protocols completed over a 10-year time span and the diversity of disease histologies included in the analysis. Future directions consist of a prospective assessment looking at a more uniform group of patients, such as those with lymphoproliferative disease, as well as expanding this paradigm to populations more likely to experience graft rejection, such as patients with unrelated donors and in cord blood transplantation.
Reduced-intensity transplantation is commonly used for patients with both myeloid and lymphoid malignancies who are older or have comorbidities. We have previously reported that pretransplantation disease-targeted chemotherapy before RIC-alloHSCT can be well tolerated by this patient population and results in the majority of patients entering transplantation with less disease. In this current report, we expand upon these results to demonstrate that pretransplantation disease-targeted chemotherapy also provides significant lymphocyte depletion, homeostatic cytokine expansion, and early full donor chimerism, which has not been previously reported with reduced-intensity conditioning alone. This approach avoids issues of mixed chimerism that may lead to early withdrawal of immune suppression or DLI that are necessitated by many RIC preparative regimens.
ACKNOWLEDGMENTS
Financial disclosure: All authors were employed by the National Institutes of Health at the time of the writing of this manuscript and have no financial disclosures to report.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
REFERENCES
- 1.Horwitz ME. Reduced intensity versus myeloablative allogeneic stem cell transplantation for the treatment of acute myeloid leukemia, myelodysplastic syndrome and acute lymphoid leukemia. Curr Opin Oncol. 2011;23:197–202. [DOI] [PubMed] [Google Scholar]
- 2.Khouri IF, Saliba RM, Giralt SA, et al. Nonablative allogeneic hematopoietic transplantation as adoptive immunotherapy for indolent lymphoma: low incidence of toxicity, acute graft-versus-host disease, and treatment-related mortality. Blood. 2001;98:3595–3599. [DOI] [PubMed] [Google Scholar]
- 3.Slavin S, Nagler A, Naparstek E, et al. Nonmyeloablative stem cell transplantation and cell therapy as an alternative to conventional bone marrow transplantation with lethal cytoreduction for the treatment of malignant and nonmalignant hematologic diseases. Blood. 1998;91: 756–763. [PubMed] [Google Scholar]
- 4.Khouri IF, Champlin RE. Nonmyeloablative allogeneic stem cell transplantation for non-hodgkin lymphoma. Cancer J. 2012;18:457–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringden O, Labopin M, Ehninger G, et al. Reduced intensity conditioning compared with myeloablative conditioning using unrelated donor transplants in patients with acute myeloid leukemia. J Clin Oncol. 2009;27:4570–4577. [DOI] [PubMed] [Google Scholar]
- 6.Hamadani M, Saber W, Ahn KW, et al. Impact of pretransplantation conditioning regimens on outcomes of allogeneic transplantation for chemotherapy-unresponsive diffuse large B cell lymphoma and grade III follicular lymphoma. Biol Blood Marrow Transplant. 2013;19: 746–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Truitt RL, Atasoylu AA. Impact of pretransplant conditioning and donor T cells on chimerism, graft-versus-host disease, graft-versus-leukemia reactivity, and tolerance after bone marrow transplantation. Blood. 1991;77:2515–2523. [PubMed] [Google Scholar]
- 8.Mackinnon S, Barnett L, Heller G, O’Reilly RJ. Minimal residual disease is more common in patients who have mixed T-cell chimerism after bone marrow transplantation for chronic myelogenous leukemia. Blood. 1994;83:3409–3416. [PubMed] [Google Scholar]
- 9.Orsini E, Alyea EP, Chillemi A, et al. Conversion to full donor chimerism following donor lymphocyte infusion is associated with disease response in patients with multiple myeloma. Biol Blood Marrow Transplant. 2000;6: 375–386. [DOI] [PubMed] [Google Scholar]
- 10.McSweeney PA, Niederwieser D, Shizuru JA, et al. Hematopoietic cell transplantation in older patients with hematologic malignancies: replacing high-dose cytotoxic therapy with graft-versus-tumor effects. Blood. 2001;97:3390–3400. [DOI] [PubMed] [Google Scholar]
- 11.Carvallo C, Geller N, Kurlander R, et al. Prior chemotherapy and allograft CD34+ dose impact donor engraftment following nonmyeloablative allogeneic stem cell transplantation in patients with solid tumors. Blood. 2004;103:1560–1563. [DOI] [PubMed] [Google Scholar]
- 12.Bishop MR, Hou JW, Wilson WH, et al. Establishment of early donor engraftment after reduced-intensity allogeneic hematopoietic stem cell transplantation to potentiate the graft-versus-lymphoma effect against refractory lymphomas. Biol Blood Marrow Transplant. 2003;9: 162–169. [DOI] [PubMed] [Google Scholar]
- 13.Bishop MR, Steinberg SM, Gress RE, et al. Targeted pretransplant host lymphocyte depletion prior to T-cell depleted reduced-intensity allogeneic stem cell transplantation. Br J Haematol. 2004;126:837–843. [DOI] [PubMed] [Google Scholar]
- 14.Craddock C, Bardy P, Kreiter S, et al. Short Report: Engraftment of T-cell-depleted allogeneic haematopoietic stem cells using a reduced intensity conditioning regimen. Br J Haematol. 2000;111:797–800. [PubMed] [Google Scholar]
- 15.Petrus MJ, Williams JF, Eckhaus MA, et al. An immunoablative regimen of fludarabine and cyclophosphamide prevents fully MHC-mismatched murine marrow graft rejection independent of GVHD. Biol Blood Marrow Transplant. 2000;6:182–189. [DOI] [PubMed] [Google Scholar]
- 16.Salit RB, Fowler DH, Wilson WH, et al. Dose-adjusted EPOCH-rituximab combined with fludarabine provides an effective bridge to reduced-intensity allogeneic hematopoietic stem-cell transplantation in patients with lymphoid malignancies. J Clin Oncol. 2012;30:830–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson WH, Grossbard ML, Pittaluga S, et al. Dose-adjusted EPOCH chemotherapy for untreated large B-cell lymphomas: a pharmacodynamic approach with high efficacy. Blood. 2002;99:2685–2693. [DOI] [PubMed] [Google Scholar]
- 18.Estey E, Thall P, Andreeff M, et al. Use of granulocyte colony-stimulating factor before, during, and after fludarabine plus cytarabine induction therapy of newly diagnosed acute myelogenous leukemia or myelodysplastic syndromes: comparison with fludarabine plus cytarabine without granulocyte colony-stimulating factor. J Clin Oncol. 1994; 12:671–678. [DOI] [PubMed] [Google Scholar]
- 19.Fry TJ, Mackall CL. Interleukin-7: from bench to clinic. Blood. 2002;99: 3892–3904. [DOI] [PubMed] [Google Scholar]
- 20.Mehta CR, Patel NR. A network algorithm for performing Fisher’s exact test in r x c contingency tables. J Am Stat Assoc. 1983;78:427–434. [Google Scholar]
- 21.Agresti A In: Categorical Data Analysis. New York: John Wiley and Sons, Inc.; 1990. p. 79–129. [Google Scholar]
- 22.Hollander M, Wolfe DA. Nonparametrical Statistical Methods Second ed. New York: John Wiley and Sons, Inc.; 1999. [Google Scholar]
- 23.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 24.Cox DR. Regression Models and Life-Tables. J Roy Stat Soc B. 1972;34: 187–220. [Google Scholar]
- 25.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat Med. 1999;18:695–706. [DOI] [PubMed] [Google Scholar]
- 26.Kahl C, Storer BE, Sandmaier BM, et al. Relapse risk in patients with malignant diseases given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2007;110:2744–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valcarcel D, Martino R, Caballero D, et al. Chimerism analysis following allogeneic peripheral blood stem cell transplantation with reduced-intensity conditioning. Bone Marrow Transplant. 2003;31:387–392. [DOI] [PubMed] [Google Scholar]
- 28.Childs R, Chernoff A, Contentin N, et al. Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N Engl J Med. 2000;343:750–758. [DOI] [PubMed] [Google Scholar]
- 29.Keil F, Kalhs P, Chen X, et al. Hematopoietic donor chimerism and graft-versus-myeloma effect in relapse of multiple myeloma after allogeneic bone marrow transplantation. Ann Hematol. 1999;78:376–379. [DOI] [PubMed] [Google Scholar]
- 30.Baron F, Baker JE, Storb R, et al. Kinetics of engraftment in patients with hematologic malignancies given allogeneic hematopoietic cell transplantation after nonmyeloablative conditioning. Blood. 2004;104: 2254–2262. [DOI] [PubMed] [Google Scholar]
- 31.Ting DT, Spitzer TR, Chaudhary A, et al. Clinical outcomes of late rather than early full-donor chimerism in patients with advanced lymphomas receiving nonmyeloablative allogeneic hematopoietic SCT. Bone Marrow Transplant. 2008;42:329–335. [DOI] [PubMed] [Google Scholar]
- 32.Mapara MY, Kim YM, Wang SP, et al. Donor lymphocyte infusions mediate superior graft-versus-leukemia effects in mixed compared to fully allogeneic chimeras: a critical role for host antigen-presenting cells. Blood. 2002;100:1903–1909. [DOI] [PubMed] [Google Scholar]
- 33.Childs R, Clave E, Contentin N, et al. Engraftment kinetics after nonmyeloablative allogeneic peripheral blood stem cell transplantation: full donor T-cell chimerism precedes alloimmune responses. Blood. 1999;94:3234–3241. [PubMed] [Google Scholar]
- 34.Saito B, Fukuda T, Yokoyama H, et al. Impact of T cell chimerism on clinical outcome in 117 patients who underwent allogeneic stem cell transplantation with a busulfan-containing reduced-intensity conditioning regimen. Biol Blood Marrow Transplant. 2008;14:1148–1155. [DOI] [PubMed] [Google Scholar]
- 35.Gattinoni L, Finkelstein SE, Klebanoff CA, et al. Removal of homeostatic cytokine sinks by lymphodepletion enhances the efficacy of adoptively transferred tumor-specific CD8+ T cells. J Exp Med. 2005; 202:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Bock M, Fillet M, Hannon M, et al. Kinetics of IL-7 and IL-15 levels after allogeneic peripheral blood stem cell transplantation following nonmyeloablative conditioning. PloS one. 2013;8:e55876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elorza I, Palacio C, Dapena JL, et al. Relationship between minimal residual disease measured by multiparametric flow cytometry prior to allogeneic hematopoietic stem cell transplantation and outcome in children with acute lymphoblastic leukemia. Haematologica. 2010;95: 936–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moreno C, Villamor N, Colomer D, et al. Clinical significance of minimal residual disease, as assessed by different techniques, after stem cell transplantation for chronic lymphocytic leukemia. Blood. 2006;107: 4563–4569. [DOI] [PubMed] [Google Scholar]
- 39.Bader P, Kreyenberg H, Henze GH, et al. Prognostic value of minimal residual disease quantification before allogeneic stem-cell transplantation in relapsed childhood acute lymphoblastic leukemia: the ALL-REZ BFM Study Group. J Clin Oncol. 2009;27:377–384. [DOI] [PubMed] [Google Scholar]


