Abstract
Purpose
While the significance of circulating tumor cells in clinically localized cancer remains controversial, it has been reported that surgical tumor manipulation can increase circulating tumor cells, including during open prostatectomy. To our knowledge it is unknown whether this cell shedding also occurs during minimally invasive prostatectomy, which minimizes tumor palpation and uses earlier vascular control. We tested the impact of robotic assisted laparoscopic radical prostatectomy on intraoperative circulating tumor cell levels.
Materials and Methods
Circulating tumor cell counts were compared in peripheral blood specimens from 25 patients treated with robotic assisted laparoscopic radical prostatectomy preoperatively vs intraoperatively after prostate excision, in addition to 11 healthy blood donors. Circulating tumor cell detection was performed using EpCAM immunomagnetic enrichment and multiparametric flow cytometry quantification of viable EpCAM positive/prostate specific membrane antigen positive/CD45 negative cells. Intraoperative cell counts and increases were tested in univariable analyses for associations with perioperative variables, histopathology and postoperative progression.
Results
Circulating tumor cells were detected in 0% of healthy controls compared to 48% and 52% of prostatectomy cases preoperatively and intraoperatively, respectively (range 1 to 8 cells). There was no difference in the incidence or mean number of circulating tumor cells preoperatively vs intraoperatively. Of the patients 60% had no intraoperative change from preoperative levels. Intraoperative cell increases vs decreases were equally infrequent (each 20%) with no intraoperative increase greater than 1 circulating tumor cell. Intraoperative circulating tumor cell detection was not significantly associated with prostatectomy operative characteristics, histopathology or early postoperative progression at a median 21-month followup.
Conclusions
Robotic assisted laparoscopic radical prostatectomy does not cause significant intraoperative increases in circulating tumor cells in contrast to historical reports of open prostatectomy. These findings may aid urologists in counseling candidates for robotic assisted laparoscopic radical prostatectomy regarding the possibility of intraoperative tumor cell shedding.
Keywords: prostatic neoplasms, neoplastic cells, circulating, prostatectomy, robotics, laparoscopy
For a tumor cell to generate a metastasis it must complete a series of well-defined physiological steps known as the metastatic cascade. The steps include intravasation into the circulation, transit in the circulation, extravasation into the distant organ parenchyma and proliferation at the distant site. While the metastatic cascade is highly inefficient as a whole,1 the ability of tumor cells to reach the circulation is an early and common clinical event, although alone it is inadequate for metastasis.2 Contemporary strategies to detect these CTCs lack standardization but typically exploit 1 or more cancer specific nucleic acid or protein marker using PCR or antibody based methods, respectively, with the latter necessary for CTC enumeration. Technical challenges to CTC detection include the miniscule circulating concentrations of CTCs and their overlapping morphology with hematopoietic cells.3
CTC detection has known clinical usefulness in patients with metastatic prostate, breast and colon cancers. For these cancers CTC counts with the antibody based detection platform CellSearch® outperform histopathology, radiology and tumor markers, including PSA, to predict patient survival.3 However, in patients with nonmetastatic cancer CellSearch may lack adequate sensitivity and when using other platforms, detection rates vary widely.3 Thus, the clinical significance of perioperative CTCs in nonmetastatic cancer cases remains controversial, although an increasing body of literature supports a greater risk of progression, including after radical prostatectomy.4–6
Given the potentially adverse impact of CTCs in localized disease, the question has been raised of whether surgical manipulation can provoke hematogenous tumor shedding and trigger systemic involvement. Numerous studies of various cancer types agree as to the common occurrence of intraoperative CTC shedding with tumor manipulation. For example, in patients with early stage breast cancer CTC detection rates increase from 5% to 13% preoperatively to 33% to 44% intraoperatively by PCR based or antibody based detection.7–10 Similar findings were reported in patients with lung, colorectal, pancreatic and hepatocellular cancers.11–18
Although the clinical significance of intraoperative CTC shedding is unclear, an association with worse survival outcomes has been suggested and, therefore, methods to limit this event are of interest.12,16 Operative technical modifications may be useful, including a no-touch approach, which decreases tumor manipulation prior to vascular control or throughout resection.11,14,19–22 The ability of such modifications to limit intraoperative hematogenous tumor cell dissemination has been confirmed in animal models and randomized clinical patient trials.14,22,23 Use of minimally invasive surgery as another strategy to reduce intraoperative tumor manipulation and tumor cell shedding has been proposed but not yet thoroughly investigated for this purpose.24
Few reports have addressed hematogenous tumor dissemination during radical prostatectomy. These studies generally support the common occurrence of intraoperative CTC shedding but they are limited primarily to open prostatectomy series and PCR based detection without CTC enumeration.4,25–28 No prior study to our knowledge has investigated intraoperative CTC levels in a purely robotic or laparoscopic prostatectomy cohort. Given the early arterial control and avoidance of digital tumor palpation with minimally invasive radical prostatectomy, it is feasible that a robotic or laparoscopic approach might effectively limit prostatic CTC shedding.
We investigated the impact of RALRP on CTC levels in patients with clinically localized prostate cancer. For CTC detection we used an antibody based approach combining immunomagnetic enrichment for the EpCAM epithelial specific cell surface protein with flow cytometric enumeration of viable cells expressing EpCAM and prostate specific membrane antigen but lacking the CD45 pan-leukocyte cell surface protein. Our findings confirmed the common presence of CTCs in nonmetastatic prostate cancer cases at radical prostatectomy but challenge the occurrence of significant intraoperative hematogenous tumor cell dissemination using a robotic approach.
METHODS
Patients and Blood Specimens
Institutional review board approval was obtained for patient participation in this study. Peripheral blood was procured from 25 consenting patients with clinically localized prostate cancer who underwent RALRP between August 2011 and March 2013 at the NCI (National Cancer Institute), NIH (National Institutes of Health), Bethesda, Maryland. For each patient an 8 ml peripheral blood specimen was procured in a BD Vacutainer® K2 EDTA Blood Collection Tube immediately before skin incision and again after prostate removal prior to skin closure. Fresh peripheral blood (8 ml) from 11 healthy donors was obtained from the NIH Clinical Center Department of Transfusion Medicine.
All RALRP operations included prostatic arterial pedicle ligation before prostate mobilization and any nerve sparing dissection. Bilateral pelvic lymphadenectomy was performed in all cases. Tumors were staged according to AJCC (American Joint Committee on Cancer) and graded using Gleason score according to ISUP (International Society of Urological Pathology) guidelines. Initial postoperative PSA was measured at 6 to 8 weeks with detectable PSA defined as 0.03 ng/dl or greater. Postoperative progression was defined as biochemical failure according to National Comprehensive Cancer Network® criteria or as a secondary prostate cancer treatment, including pelvic radiation or androgen deprivation therapy.
CTC Isolation and Enumeration
Blood specimens were processed at 4C and analyzed on the day of procurement. Osmotic lysis of erythrocytes was performed with ACK lysis buffer (Gibco™). Subsequent CTC detection was performed using EpCAM based immunomagnetic enrichment with human anti-CD326 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) followed by flow cytometric detection using the MACSQuant® Analyzer as described previously.29 For prostatic CTC detection cells enriched for EpCAM expression were analyzed by multiparametric flow cytometry with APC (allophycocyanin), FITC (fluorescein isothiocyanate) and PE-Cy7 fluorophore conjugated antibodies to EpCAM (clone HEA-125, Miltenyi Biotec), PSMA (clone FOLH1, BioLegend®) and CD45 (clone HI30, BioLegend), respectively, and with Hoechst 33342 (Invitrogen™), a fluorescent dye marker of nucleated cells. Prostate CTCs were quantified by 6-parameter flow cytometry using the MACSQuant Analyzer based on an EpCAM positive/PSMA positive/CD45 negative/H33342 positive staining signature after forward (size) and side (granularity) scatter gating to exclude nonviable cells and debris.
Statistical Analysis
As the primary outcome measures of this study CTC detection rates and mean CTC counts were compared between preoperative and intraoperative blood specimens using the Fisher exact test and the Student t-test, respectively. Assuming 25 paired observations, a SD of 1.5 CTCs and 2-sided significance of 5% the study was powered at 80% to detect a mean difference of 0.88 CTC or of 1.17 CTCs if assuming a SD of 2.0 CTCs. Assuming a preoperative detection rate of approximately 40% the study was powered at 80% to detect a 40% increase in the detection rate intraoperatively. As secondary outcome measures of this study CTC detection rates were tested for associations with clinical and perioperative variables, including patient age, obesity, prostate size, operative blood loss and time, extent of nerve dissection, tumor histopathology (stage, grade, percent gland involvement and perineural invasion) and detectable postoperative PSA using the Fisher exact test. Mean CTC counts were tested for associations with these clinicopathological variables using the Student t-test. Statistical significance was considered at p <0.05.
RESULTS
Patient Characteristics
Table 1 lists the characteristics of patients treated with RALRP. Median PSA was 7.3 ng/dl and 76% of patients underwent a bilateral nerve sparing approach. Of the patients 56% had high risk surgical pathology based on Gleason score 8 or greater, or tumor stage pT3 or greater. Three patients (12%) had pathologically confirmed pelvic lymph node involvement with prostate cancer. PSA remained detectable postoperatively in 6 patients (24%) at a range of 0.04 to 0.82 ng/dl. At a median postoperative followup of 21 months 5 patients (20%) had disease progression.
Table 1.
Patient characteristics
| No. pts (%) | 25 (100) |
| Mean/median age | 62/64 |
| No. ethnicity (%): | |
| White | 19 (76) |
| Black | 5 (20) |
| Other | 1 (4) |
| Mean/median body mass index (kg/m2) | 29.1/28.4 |
| Mean/median PSA (ng/dl) | 12.2/7.3 |
| Mean/median operative time (mins) | 355/344 |
| Mean/median estimated blood loss (ml) | 428/300 |
| No. nerve sparing extent (%): | |
| Bilat | 19 (76) |
| Unilat | 5 (20) |
| None | 1 (4) |
| No. pT stage (%): | |
| pT2 | 16 (64) |
| pT3/T4 | 9 (36) |
| No. Gleason score (%): | |
| 6 | 1 (4) |
| 7 | 13 (52) |
| 8–10 | 11 (44) |
| Mean/median Ca vol (% of gland) | 28/23 |
| No. perineural invasion (%) | 16 (64) |
| No. pos surgical margin (%) | 4 (16) |
| No. pos lymph node (%) | 3 (12) |
CTC Detection
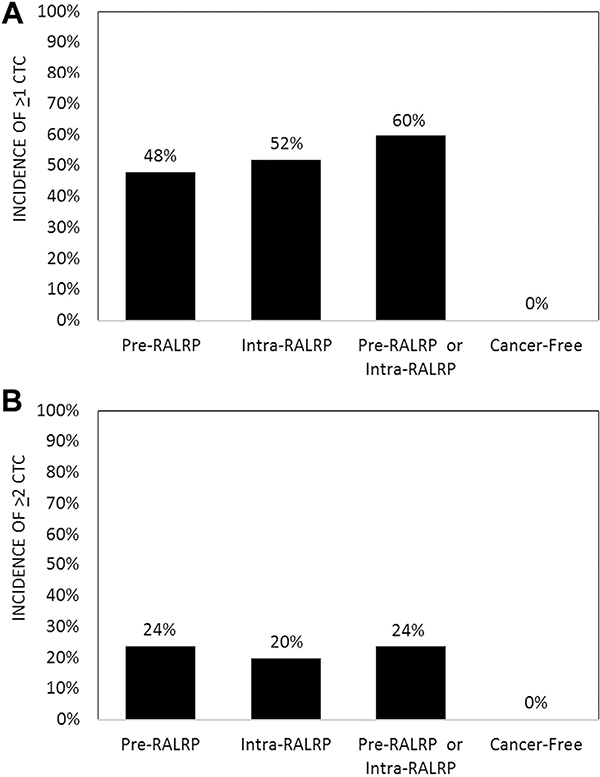
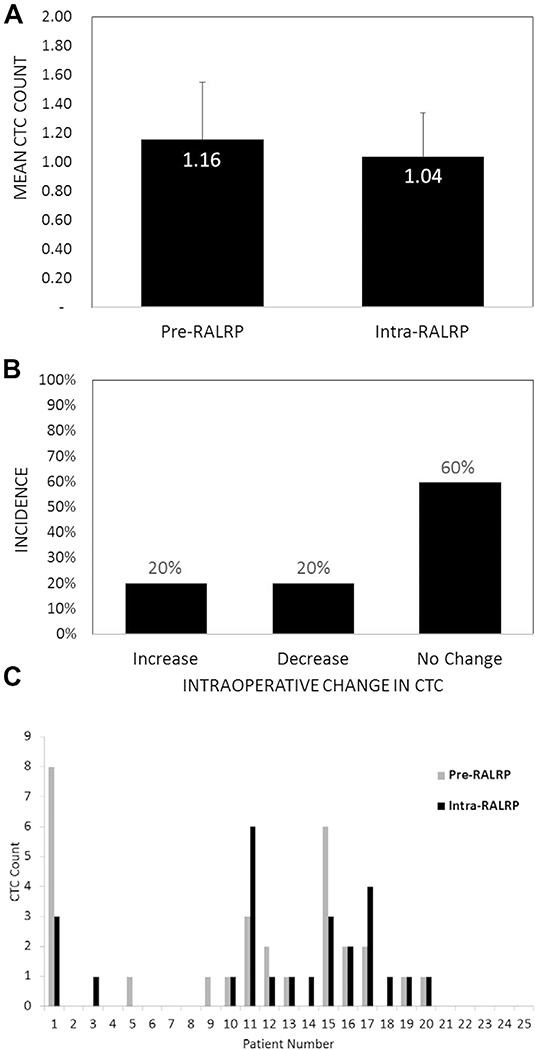
Prostate CTCs were identified in the peripheral blood of 15 of 25 patients (60%) treated with RALRP overall, including 12 preoperatively (48%) and 13 intraoperatively (52%) who showed a range of 0 to 8 and 0 to 6 cells, respectively (fig. 1, A). In contrast CTCs were detectable in 0 of 11 cancer-free blood donors (0%) (vs preoperative and postoperative detection rates p <0.01), yielding 100% specificity for cancer cell detection. When using a minimal criterion of 2 CTCs or greater, detection rates were 0% (0 of 11 cases), 24% (6 of 25) and 20% (5 of 25) for cancer-free, preoperative and intraoperative blood specimens, respectively (fig. 1, B). There was no significant difference between the preoperative and intraoperative incidence of CTC detection using a CTC criterion of 1 or greater, or 2 or greater (fig. 1). There was also no significant difference in the mean CTC count preoperatively vs intraoperatively (fig. 2, A). An intraoperative increase in CTCs occurred in the same number of patients (5 of 25 or 20%) as did an intraoperative decrease while 60% of patients showed no change relative to preoperative baseline counts (fig. 2, B). No patient had an intraoperative increase of greater than 1 CTC relative to the preoperative CTC level (fig. 2, C).
Figure 1.
Preoperative and intraoperative CTC detection incidence in patients treated with RALRP and incidence in cancer-free controls. A, rates using criterion greater than 1 CTC (preRALRP or intraRALRP vs cancer free p <0.01 and preRALRP vs intraRALRP p = 1.0). B, rates using criterion greater than 2 CTCs (preRALRP or intraRALRP vs cancer free p <0.01 and preRALRP vs intraRALRP p = 1.0).
Figure 2.
Intraoperative vs preoperative CTC counts in patients treated with RALRP. A, mean counts preoperatively vs intraoperatively (p = 0.81). Error bar represents SEM. B, incidence of increase, decrease or no change in intraoperative vs preoperative count in same patient (increase or decrease vs no change p <0.01 and increase vs decrease p = 1.0). C, preoperative and intraoperative counts in each of 25 patients treated with RALRP.
CTC Association with Perioperative Outcomes
There was no association between the intraoperative CTC count or increase and any clinical or operative variable, including blood loss or extent of nerve dissection (table 2). Intraoperative CTC detection was also not associated with tumor histopathology or early oncologic outcomes after RALRP (table 2). Preoperative CTC detection similarly was not associated with any patient variable in table 2 (data not shown).
Table 2.
Relationship of intraoperative CTC levels to patient characteristics
| Intraop CTC |
||||
|---|---|---|---|---|
| % Increase | p Value | Mean Count | p Value | |
| Age (greater than 60 vs 60 yrs or less) | 31 vs 0 | 0.12 | 1.4 vs 0.3 | 0.08 |
| Ethnicity (white vs nonwhite) | 21 vs 17 | 0.64 | 1.1 vs 1.0 | 0.82 |
| Body mass index (greater than 30 vs 30 kg/m2 or less) | 25 vs 15 | 1.00 | 1.3 vs 0.8 | 0.36 |
| Prostate size (greater than 50 vs 50 cc or less) | 17 vs 23 | 1.00 | 1.2 vs 1.0 | 0.77 |
| Estimated blood loss (500 or greater vs less than 500 ml) | 13 vs 24 | 1.00 | 0.8 vs 1.2 | 0.52 |
| Operative time (greater than 5 vs 5 hrs or less) | 12 vs 38 | 0.28 | 0.6 vs 1.9 | 0.06 |
| Nerve sparing extent (bilat vs unilat or nonnerve sparing) | 21 vs 17 | 1.00 | 1.0 vs 1.2 | 0.82 |
| pT stage (pT3 or greater vs pT2) | 11 vs 25 | 0.62 | 0.8 vs 1.2 | 0.52 |
| Gleason score (greater than 7 vs 7 or less) | 18 vs 21 | 1.00 | 0.8 vs 1.2 | 0.53 |
| Prostate Ca vol (25% or greater vs less than 25% of gland) | 17 vs 23 | 1.00 | 0.8 vs 1.3 | 0.37 |
| Perineural invasion (yes vs no) | 25 vs 11 | 0.62 | 1.1 vs 0.9 | 0.72 |
| Detectable postop PSA (yes vs no) | 29 vs 17 | 0.60 | 1.0 vs 1.1 | 0.94 |
| Clinical stage (cT1c vs cT2) | 25 vs 0 | 0.54 | 1.2 vs 0.6 | 0.48 |
| Initial PSA detectable (yes vs no) | 17 vs 21 | 1.00 | 1.0 vs 1.0 | 0.94 |
| Postop progression (yes vs no) | 0 vs 25 | 0.54 | 1.2 vs 1.0 | 0.80 |
DISCUSSION
It is not uncommon for surgeons to face patient inquiry regarding intraoperative tumor cell shedding. It is important that urologists are aware of current understanding on this topic to appropriately counsel operative candidates. While there is general agreement in the scientific community regarding the common occurrence of intraoperative CTC shedding with tumor manipulation,7–18 there is also accumulating evidence that early vascular control and limitation of tumor manipulation can prevent this event.11,14,19–23 However, few groups have investigated intraoperative shedding in patients with prostate cancer. Studies are limited primarily to open prostatectomy series using nonenumerating CTC detection methodology.4,25–28 While most prostatectomy studies support common intraoperative CTC shedding, it is conceivable that dissemination may be provoked by techniques of the open approach that are avoided in the robotic operation, such as digital tumor palpation or prostatic mobilization and nerve dissection prior to arterial vascular control.
To our knowledge the current study is the first to investigate intraoperative hematogenous tumor cell dissemination during RALRP. Using a multimarker antibody based approach to CTC enumeration we detected CTCs in 60% of patients treated with RALRP compared to 0% of cancer-free controls, indicating 100% specificity for detection. In contrast to prior open prostatectomy series we observed no significant increases in CTC counts or detection rates intraoperatively vs preoperatively despite adequate statistical power. Individual cases of increased intraoperative CTCs were infrequent and no more common than intraoperative decreases. No patient had an intraoperative increase of greater than 1 CTC relative to the preoperative CTC count. Thus, these findings confirm the frequent presence of CTCs in patients with clinically localized prostate cancer but challenge the common occurrence of CTC increases during RALRP.
Many studies of various cancer types have shown higher CTC detection rates intraoperatively compared to preoperatively.7–11,14–18 In some cases intraoperative CTC shedding has been dramatic. For example, Yamashita16 and Hayashi11 et al identified CTCs in almost all patients with localized cancer intraoperatively despite a 0% preoperative detection rate. Wong et al noted that their patients with hepatocellular carcinoma had 10 to 1,000,000 times higher levels of a tumor specific mRNA transcript in the blood intraoperatively compared to preoperatively.18 Intraoperative increases in CTC positivity are also well documented during metastatectomy.12,13 In most of these reports intraoperative CTC shedding was demonstrated by intraoperative increases in CTC detection rates but without CTC enumeration. In a rare case of CTC enumeration Papavasiliou et al found a mean of 28 CTCs during resection of colon cancer liver metastases compared to only 4 CTCs preoperatively.13
Currently the clinical significance of perioperative CTCs in clinically localized cancers is controversial. Many reports support an increased risk of postoperative progression with perioperative CTC detection.4–6 Thus, there is theoretical potential that iatrogenic CTC shedding with tumor manipulation might convert localized disease to systemic disease. On the other hand several studies suggest that increases in intraoperative CTCs normalize postoperatively within days to weeks.9,10,12,13,18 While postoperative CTC clearance is reassuring, it does not ensure that viable CTCs have not already lodged and initiated micrometastatic colonization of distant sites. A link between CTC increases during lobectomy and overall survival in patients with lung cancer has been reported16 but research addressing survival in association with intraoperative CTC levels is otherwise scarce.
In the current study we found no relation between intraoperative CTCs and postoperative disease progression at short-term followup. However, our study was not designed to detect associations with this and other secondary outcome measures and it was likely underpowered to do so. Additional study is needed to more definitively address the prognostic significance of intraoperative CTC levels.
Few groups have investigated intraoperative CTC shedding specifically during prostatectomy and they generally support the common occurrence of intraoperative prostate CTC shedding. In studies with cohorts of similar size as that in the current study Oefelein25 and Ogawa27 et al detected circulating PSA mRNA in open prostatectomy cases almost twice as often intraoperatively as preoperatively. Similarly using PCR detection of PSMA and PSA mRNA Eschwege et al identified CTCs in 66% of patients intraoperatively compared to 37% preoperatively.4 In the only prior report to enumerate intraoperative prostate CTCs Planz et al reported an increase in mean CTC levels from 2 preoperatively to 19 intraoperatively in patients who underwent open prostatectomy using cytokeratin immunostaining.26 In contrast Schmidt et al noted no difference in preoperative and intraoperative CTC detection rates (each 32%) in 77 open prostatectomy cases using quantitative PCR for PSMA mRNA.28
Reasons for the discrepancy between our findings and those of most prior prostatectomy series may relate to differences in patient selection, operative approach and/or CTC detection methodology. A relatively high proportion of patients in our study had adverse surgical pathology. However, there is no validated association between CTCs and localized prostate tumor stage or grade and no intuitive reason why a higher risk cohort would be less likely to shed CTCs intraoperatively. Regarding our detection strategy antibody based platforms, including CellSearch, have been challenged due to low detection rates in patients with nonmetastatic cancer.3 However, our baseline (ie preoperative) detection rate of 48% is as high as that in any prior prostatectomy series using PCR based detection (range 27% to 48%)4,25,27,28 and similar to the 53% baseline detection rate in the open prostatectomy study by Planz et al using antibody based detection.26 Newer antibody based platforms with 100% reported sensitivity in nonmetastatic prostate cancer cases, including the CTC-chip, require external validation and are yet to be investigated intraoperatively during prostatectomy.3
Intraoperative manual (digital) palpation of the prostate is generally considered useful during open prostatectomy, aiding in the delineation of tumor margins. The necessary omission of this step during minimally invasive prostatectomy has been challenged as a disadvantage relative to the open approach. Given the relationship between tumor manipulation and intraoperative CTC shedding, the lack of tumor palpation during RALRP may limit intraoperative CTC shedding and account for the discrepancy between our findings and those of open prostatectomy series. Timing of arterial control may also be a factor since pedicle ligation is typically performed before prostate mobilization and nerve sparing during RALRP but it is done after these steps using the open approach.
By limiting tumor manipulation and using early vascular control minimally invasive prostatectomy may be analogous to the no-touch approaches confirmed in prior randomized clinical trials to prevent intraoperative hematogenous dissemination of other cancer types.11,14,19–23 A no-touch approach to colectomy, which avoids tumor manipulation before lymphovascular ligation, was found by Turnbull et al to be associated with a reduction in colon CTC detection rates as well as with improved survival outcomes but conclusions were limited by the retrospective study design.19 Similar findings in an independent followup study of colectomy cases patients were also limited by the retrospective design.11 More recently Gall et al performed a prospective, randomized comparison of the conventional and no-touch approaches to distal pancreatectomy using the CellSearch platform and concluded that there was a significant reduction in intraoperative pancreatic CTCs as a result of the no-touch approach.22 In another randomized study Ge et al found that intraoperative CTC levels in patients with nonsmall cell lung cancer decreased as a result of the timing of vascular control.14 The group advocated turning over and squeezing the mass as rarely as possible to limit hematogenous dissemination.
To our knowledge the oncologic outcome of intraoperative CTC shedding is unknown. In a rabbit model of squamous cell carcinoma a decrease in intraoperative CTC levels during laparotomy using a no-touch approach was associated with longer animal survival.23 However, clinical studies that address survival are few and limited by small patient cohorts.20,22 Wiggers et al compared the outcomes of 236 colon cases randomly assigned to no-touch vs conventional colectomy operations and discovered a lower median time to metastasis (12.6 vs 22.4 months) in the no-touch cohort but without statistical significance.20 The power of this study has been questioned and a larger randomized trial with target accrual of greater than 800 patients with colon cancer is currently under way.30
Limitations of the current study include a relatively small patient cohort size, although the study was adequately powered to detect meaningful intraoperative changes in CTC levels. Lack of direct comparison with an open approach prevents one from drawing definitive conclusions regarding discrepant findings between our study and prior prostatectomy studies in which an open approach and different CTC detection strategies were used. CTC detection rates can vary based on detection methodology and our approach was not directly compared with other detection strategies.
CONCLUSIONS
Urologists should be aware of current understanding on intraoperative CTC shedding to appropriately counsel patients considering radical prostatectomy. Current literature supports the common occurrence of intraoperative CTC shedding but also indicates that this event can be prevented by operative technical modifications. As what is to our knowledge the first study to investigate the impact of RALRP on intraoperative CTC shedding the current findings confirm that CTCs are frequently present in patients with clinically localized prostate cancer. However, in contrast to historical open prostatectomy reports our study does not support significant intraoperative CTC increases during RALRP. Reasons for the discrepancy may include differences in CTC detection strategies and/or technical differences between the open and robotic approaches, such as the degree of tumor palpation and timing of vascular control. While the prognostic significance of intraoperative shedding in clinically localized disease remains under active investigation, it is reassuring that RALRP does not appear to significantly increase CTCs, which may or may not have the potential to trigger systemic disease.
Abbreviations and Acronyms
- CTC
circulating tumor cell
- PCR
polymerase chain reaction
- PSA
prostate specific antigen
- RALRP
robotic assisted laparoscopic radical prostatectomy
Footnotes
No direct or indirect commercial incentive associated with publishing this article.
The corresponding author certifies that, when applicable, a statement(s) has been included in the manuscript documenting institutional review board, ethics committee or ethical review board study approval; principles of Helsinki Declaration were followed in lieu of formal ethics committee approval; institutional animal care and use committee approval; all human subjects provided written informed consent with guarantees of confidentiality; IRB approved protocol number; animal approved project number.
Contributor Information
Eric C. Kauffman, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland; Departments of Urology and Cancer Genetics, Roswell Park Cancer Institute, Buffalo.
Min-Jung Lee, Developmental Therapeutics Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Sylvia V. Alarcon, Developmental Therapeutics Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Sunmin Lee, Developmental Therapeutics Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Anthony N. Hoang, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Annerleim Walton Diaz, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Raju Chelluri, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland.
Srinivas Vourganti, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland; Department of Urology, State University of New York, Upstate Medical University, Syracuse, New York.
Jane B. Trepel, Developmental Therapeutics Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
Peter A. Pinto, Urologic Oncology Branch, National Cancer Institute, National Institutes of Health, Bethesda, Maryland
REFERENCES
- 1.Kauffman EC, Robinson VL, Stadler WM et al. : Metástasis suppression: the evolving role of metastasis suppressor genes for regulating cancer cell growth at the secondary site. J Urol 2003; 169: 1122. [DOI] [PubMed] [Google Scholar]
- 2.Weinberg RA: Leaving home early: reexamination of the canonical models of tumor progression. Cancer Cell 2008; 14: 283. [DOI] [PubMed] [Google Scholar]
- 3.Small AC, Gong Y, Oh WK et al. : The emerging role of circulating tumor cell detection in genitourinary cancer. J Urol 2012; 188: 21. [DOI] [PubMed] [Google Scholar]
- 4.Eschwege P, Moutereau S, Droupy S et al. : Prognostic value of prostate circulating cells detection in prostate cancer patients: a prospective study. Br J Cancer 2009; 100: 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rack B, Schindlbeck C, Jückstock J et al. : Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014; 106: dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch M, Kienle P, Kastrati D et al. : Prognostic impact of hematogenous tumor cell dissemination in patients with stage II colorectal cancer. Int J Cancer 2006; 118: 3072. [DOI] [PubMed] [Google Scholar]
- 7.Brown DC, Purushotham AD, Birnie GD et al. : Detection of intraoperative tumor cell dissemination in patients with breast cancer by use of reverse transcription and polymerase chain reaction. Surgery 1995; 117: 95. [DOI] [PubMed] [Google Scholar]
- 8.Mori M, Mimori K, Ueo H et al. : Molecular detection of circulating solid carcinoma cells in the peripheral blood: the concept of early systemic disease. Int J Cancer 1996; 68: 739. [DOI] [PubMed] [Google Scholar]
- 9.McCulloch P, Choy A and Martin L: Association between tumour angiogenesis and tumour cell shedding into effluent venous blood during breast cancer surgery. Lancet 1995; 346: 1334. [DOI] [PubMed] [Google Scholar]
- 10.Choy A and McCulloch P: Induction of tumour cell shedding into effluent venous blood breast cancer surgery. Br J Cancer 1996; 73: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi N, Egami H, Kai M et al. : No-touch isolation technique reduces intraoperative shedding of tumor cells into the portal vein during resection of colorectal cancer. Surgery 1999; 125: 369. [PubMed] [Google Scholar]
- 12.Koch M, Kienle P Hinz U et al. : Detection of hematogenous tumor cell dissemination predicts tumor relapse in patients undergoing surgical resection of colorectal liver metastases. Ann Surg 2005; 241: 199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Papavasiliou P, Fisher T, Kuhn J et al. : Circulating tumor cells in patients undergoing surgery for hepatic metastases from colorectal cancer. Proc (Bayl Univ Med Cent) 2010; 23: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ge MJ, Shi D, Wu QC et al. : Observation of circulating tumour cells in patients with nonsmall cell lung cancer by real-time fluorescent quantitative reverse transcriptase-polymerase chain reaction in peroperative period. J Cancer Res Clin Oncol 2006; 132: 248. [DOI] [PubMed] [Google Scholar]
- 15.Hashimoto M, Tanaka F, Yoneda K et al. : Significant increase in circulating tumour cells in pulmonary venous blood during surgical manipulation in patients with primary lung cancer. Interact Cardiovasc Thorac Surg 2014; 18: 775. [DOI] [PubMed] [Google Scholar]
- 16.Yamashita JI, Kurusu Y, Fujino N et al. : Detection of circulating tumor cells in patients with nonsmall cell lung cancer undergoing lobectomy by video-assisted thoracic surgery: a potential hazard for intraoperative hematogenous tumor cell dissemination. J Thorac Cardiovasc Surg 2000; 119: 899. [DOI] [PubMed] [Google Scholar]
- 17.Miyazono F, Takao S, Natsugoe S et al. : Molecular detection of circulating cancer cells during surgery in patients with biliary-pancreatic cancer. Am J Surg 1999; 177: 475. [DOI] [PubMed] [Google Scholar]
- 18.Wong IH, Lau WY, Leung T et al. : Hematogenous dissemination of hepatocytes and tumor cells after surgical resection of hepatocellular carcinoma: a quantitative analysis. Clin Cancer Res 1999; 5: 4021. [PubMed] [Google Scholar]
- 19.Turnbull RB Jr, Kyle K, Watson FR et al. : Cancer of the colon: the influence of the no-touch isolation technic on survival rates. Ann Surg 1967; 166: 420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiggers T, Jeekel J, Arends JW et al. : No-touch isolation technique in colon cancer: a controlled prospective trial. Br J Surg 1988; 75: 409. [DOI] [PubMed] [Google Scholar]
- 21.Liu CL, Fan ST, Lo CM et al. : Anterior approach for major right hepatic resection for large hepatocellular carcinoma. Ann Surg 2000; 232: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gall TM, Jacob J, Frampton AE et al. : Reduced dissemination of circulating tumor cells with notouch isolation surgical technique in patients with pancreatic cancer. JAMA Surg 2014; 149: 482. [DOI] [PubMed] [Google Scholar]
- 23.Nishizaki T, Matsumata T, Kanematsu T et al. : Surgical manipulation of VX2 carcinoma in the rabbit liver evokes enhancement of metastasis. J Surg Res 1990; 49: 92. [DOI] [PubMed] [Google Scholar]
- 24.Li WW, Klomp HM, van Boven WJ et al. : eComment. Circulating tumour cells caused by surgical manipulation in patients with lung cancer. Is minimally invasive “no-touch” surgery the solution? Interact Cardiovasc Thorac Surg 2014; 18: 783. [DOI] [PubMed] [Google Scholar]
- 25.Oefelein MG, Kaul K, Ignatoff JM et al. : Haematogenous dissemination of prostate epithelial cells during surgery. Lancet 1996; 347: 324. [DOI] [PubMed] [Google Scholar]
- 26.Planz B, Szyska P, Valdor M et al. : Detection of circulating prostatic cells during radical prostatectomy. Urol Res 1997; 25: 385. [DOI] [PubMed] [Google Scholar]
- 27.Ogawa O, Iinuma M, Sato K et al. : Circulating prostate-specific antigen mRNA during radical prostatectomy in patients with localized prostate cancer: with special reference to neoadjuvant hormonal therapy. Urol Res 1999; 27: 291. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt B, Anastasiadis AG, Seifert HH et al. : Detection of circulating prostate cells during radical prostatectomy by standardized PSMA RT-PCR: association with positive lymph nodes and high malignant grade. Anticancer Res 2003; 23: 3991. [PubMed] [Google Scholar]
- 29.Thomas A, Rajan A, Berman A et al. : Sunitinib in patients with chemotherapy-refractory thymoma and thymic carcinoma: an open-label phase 2 trial. Lancet Oncol 2015; 16: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takii Y, Shimada Y, Moriya Y et al. : A randomized controlled trial of the conventional technique versus the no-touch isolation technique for primary tumor resection in patients with colorectal cancer: Japan Clinical Oncology Group Study JCOG1006. Jpn J Clin Oncol 2014; 44: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]