Abstract
Autoinflammation is characterized by aberrant regulation of the innate immune system and often manifests as periodic fevers and systemic inflammation involving multiple organs, including the skin. Mutations leading to abnormal behavior or activity of the interleukin 1 beta (IL-1ß)-processing inflammasome complex have been found in several rare autoinflammatory syndromes, for which anticytokine therapy such as IL-1 or tumor necrosis factor-alfa inhibition may be effective. It is becoming clear that features of autoinflammation also affect common dermatoses, some of which were previously thought to be solely autoimmune in origin (eg, vitiligo, systemic lupus erythematosus). Recognizing the pathogenetic role of autoinflammation can open up new avenues for the targeted treatment of complex, inflammatory dermatoses.
Keywords: anakinra, autoinflammation, common dermatoses, inflammasomes, interleukin-1 beta, periodic fevers
The discovery of monogenic origins for seemingly unprovoked inflammatory episodes in patients with periodic fever syndromes has led to a new disease pathogenesis model known as autoinflammation. This concept is distinct from autoimmunity, in which lymphocyte-mediated immune responses are directed against specific self-antigens. Autoinflammation, by contrast, is characterized by aberrant regulation of the innate immune system. As a more complete understanding of autoinflammation emerges, it is also becoming clear that these pathways may play an important role in common dermatologic disease, leading to the possibility of new therapeutic approaches for these conditions.
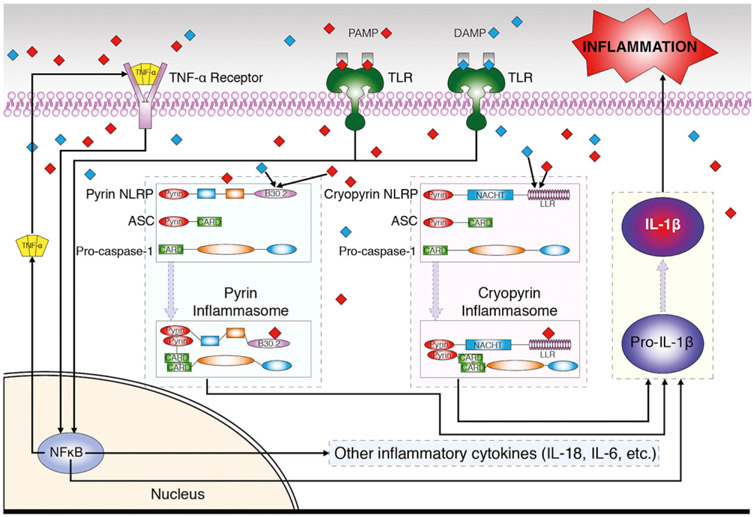
A family of genes known as the nucleotide-binding domain leucine-rich repeat-containing (NLR) genes are integral to autoinflammation.1 Thus far 22 human NLR genes have been identified.2 Most NLRs include a caspase-recruiting domain (CARD) or a pyrin domain at the N-terminal, a central nucleotide-binding domain (NACHT), and a C-terminal leucine-rich repeat domain (Fig 1). Each NLR encodes a NLR protein (NLRP), which interacts with the apoptosis-associated speck-like protein and the precursor form of caspase-1 to form a multiprotein structure known as an inflammasome. Upon formation of the inflammasome, caspase-1 becomes activated and hydrolyzes the interleukin (IL)-1 family precursors into their active cytokine counterparts.3 Caspase-1 can also mediate secretion of IL-1 alpha (IL-1α) and fibroblast growth factor 2.4
Fig 1.
Autoinflammatory syndromes. Illustration of commonly targeted pathways. ASC, Apoptosis-associated speck-like protein; DAMP, danger-associated molecular pattern; IL, interleukin; NFκB, nuclear factor kappa B; NLRP, nucleotide-binding domain leucine-rich repeat-containing protein; PAMP, pathogen-associated molecular pattern; TLR, Toll-like receptor, TNF-α, tumor necrosis factor alpha.
NLR mutations may lead to inappropriate activation of or failure to inhibit inflammasomes,5 resulting in abnormal secretion of inflammatory cytokines (primarily IL-1ß, IL-6, and IL-18). Although incompletely understood, active IL-1ß appears to prime the production of its precursor pro-IL-1ß, thereby perpetuating autoinflammatory responses that further damage affected tissues.6,7 Alternative pathways of autoinflammation have also been suggested, including inflammasome activation by mitochondria-derived reactive oxygen species in response to exogenous pathogens or endogenous danger signals.8
Both infectious and noninfectious stimuli are capable of triggering innate immune responses through membrane-bound pattern recognition receptors (PRRs) such as Toll-like receptors (TLRs) or cytosolic PRRs such as the aforementioned NLRPs.9 Binding of TLRs to pathogen- or danger-associated molecular patterns activates expression of inflammatory cytokines via nuclear gene transcription factors (Fig 1). Independent of the role of TLRs, NLRPs in the cytosol function as innate sensors of intracellular pathogen- and danger-associated molecular patterns. Their direct binding is responsible for the formation of inflammasomes, activation and secretion of inflammatory cytokines, and the subsequent cascade of extracellular downstream effects of inflammation (Fig 1). See Tables I and II for a summary of autoinflammatory syndromes and their therapies.
Table I.
Clinical features, molecular basis, histologic and laboratory findings of autoinflammatory syndromes
| Disease | Skin and nail findings | Systemic manifestations | Length of fevers |
Histology | Gene/protein | Laboratory findings |
|---|---|---|---|---|---|---|
| CAPS (AD) | Urticaria-like eruptions | Fevers, distal arthralgia, neurologic symptoms, eye disease, amyloidosis | Daily | Perivascular, interstitial, or perieccrine neutrophilic infiltrate |
NLRP3/CIAS1 Cryopyrin |
Leukocyte count (↑), CRP, ESR, and SAA (↑), creatinine (↑), IL-1ß (↑) |
| PAPA syndrome (AD) | Pyoderma gangrenosum, acne | Pyogenic arthritis | Variable | – |
PSTPIP1 PSTPIP1 |
CRP and ESR (↑), gammaglobulin (↓); IL-1ß and TNF-alfa (↑); joint culture (often –) |
| Blau syndrome (AD) | Densely populated, erythematous papular eruptions | Fevers, polyarthritis (±camptodactyly), eye disorders;granulomatous kidney, liver, lung, and CNS disease | Variable | Noncaseating granulomata | CARD15 CARD15 | ESR (↑), ACE (↑), IgA and IgG (↑); IL-1ß (↑) |
| TRAPS (AD) | Erysipelas-like macules and patches overlying myalgia | Fevers, focal myalgia, abdominal pain, conjunctivitis, periorbital edema, LAD | 7-21 d | Perivascular and interstitial lymphocytic infiltrate |
TNFRSF1A TNF receptor |
CRP and ESR (↑), haptoglobin, fibrinogen, and ferritin (↑) |
| HIDS (AR) | Intermittent erythematous macules or morbilliform papular eruptions | Fevers, arthralgia, severe abdominal pain, LAD, splenomegaly, amyloidosis | 1-2 d | Perivascular IgD and C3 complex deposits |
MVK Mevalonate kinase |
IgD and IgA (↑), IL-1ß and TNF-alfa (↑);urine mevalonic acid (↑) |
| FMF syndrome (AR) | Acral erysipelas-like erythema and purpuric lesions | Periodic fevers, synovitis, serositis, HSP, polyarteritis nodosa, protracted febrile myalgia, amyloidosis | 1-3 d | Perivascular lymphocytes, neutrophils, and histiocytes |
MEFV Pyrin |
CRP and ESR (↑), SAA (↑), creatinine (↑); IL-1ß and TNF-alfa (↑) |
| DIRA syndrome (likely AR) | Generalized pustulosis, nail changes (±) | Periostitis, osteomyelitis, hepatosplenomegaly, radiographic skeletal abnormalities | Variable | Neutrophilic infiltrate with hyperkeratosis, follicular pustules |
IL1RN IL-1 antagonist |
IL-1ß (↑);bone-tissue culture (often –) |
| CANDLE syndrome (likely AR) | Annular violaceous plaques | Fevers, edematous eyelids, progressive facial lipodystrophy, arthralgia, and delayed physical development | Daily | Perivascular and interstitial neutrophilic infiltrate |
PSMB8 PSMB8 |
ESR and hepatic transaminases (↑) |
| SAPHO syndrome | Palmoplantar pustulosis (±psoriasis), severe acne | Chronic synchondrosis inflammation, osteosclerosis, hypertrophic osteitis, and synovitis | Variable | – | – | CRP and ESR (↑); IL-1ß and TNF-alfa (↑); skin culture for Staphylococcus aureus and Propionibacterium acnes (often +) |
| Schnitzler syndrome | Nonpruritic urticarial plaques | Fevers, arthritis, hyperostosis, osteosclerosis, IgM gammopathy | – | Perivascular lymphocytes, histiocytes, and neutrophils | – | CRP and ESR (↑); IL-1ß, IL-6, and IL-18 (↑) |
| SOJIA | Morbilliform erythematous macules and papules | Spiking fevers, polyarticular arthritis | Daily | Perivascular and interstitial neutrophils and lymphocytes | – | IL-1ß, IL-6, and IL-18 (↑) |
ACE, Angiotensin-converting enzyme; AD, autosomal dominant; AR, autosomal recessive; CANDLE, chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperature; CAPS, cryopyrin-associated periodic syndrome; CARD, caspase-recruiting domain; CARD15, caspase-recruiting domain 15; CIAS1, cold-induced autoinflammatory syndrome 1; CNS, central nervous system; CRP, C-reactive protein; DIRA, deficiency of interleukin-1 receptor antagonist; ESR, erythrocyte sedimentation rate; FMF, familial Mediterranean fever; HIDS, hyper-IgD syndrome; HSP, Henoch-Schönlein purpura; IL, interleukin; IL1RN, interleukin 1 receptor antagonist; LAD, lymphadenopathy; MEFV, mediterrean fever; MVK, mevalonate kinase; NLRP, nucleotide-binding domain leucine-rich repeat-containing protein; NLRP3, nucleotide-binding domain leucine-rich repeat-containing protein 3; PAPA, pyogenic arthritis, pyoderma gangrenosum, and acne; PSMB8, proteasome subunit ß type 8; PSTPIP1, proline-serine-threonine phosphatase interacting protein 1; SAA, serum amyloid A; SAPHO, synovitis, acne, pustulosis, hyperostosis, and osteitis; SOJIA, systemic-onset juvenile idiopathic arthritis; TNF, tumor necrosis factor; TNFRSF1A, tumor necrosis factor receptor superfamily, member 1A; TRAPS, tumor necrosis factor receptor—associated periodic syndrome.
Table II.
Reported therapies for treatment of autoinflammatory disease
| Treatment (route) |
Anakinra, canakinumab, and rilonacept (all SC) |
Infliximab (IV), etanercept and adalimumab (SC) |
Prednisone (PO) | Colchicine (PO/IV) | Thalidomide (PO) | Simvastatin (PO) | Tocilizumab (IV) | Other |
|---|---|---|---|---|---|---|---|---|
| Mechanism | IL-1 inhibition | TNF inhibition | Decreases inflammation | Inhibits leukocyte migration | Down-regulates leukocyte migration | HMG-CoA reductase inhibitor | IL-6 receptor antagonist | |
| Adverse effects | Injection-site rxn, infections, URI, HA, nausea diarrhea, neutropenia | Injection-site rxn, infusion rxn (infliximab), infection, URI, abdominal pain, nausea, HA | Adrenal suppression, psychosis, insomnia, vertigo, acne, osteoporosis, myopathy | Diarrhea, nausea, vomiting | Rash, HA, polyneuropathy | (↑)CPK, (↑) transaminases, constipation, URI, flatulence | URI, HA, gastritis, HTN, (↑)ALT, (↑)lipids, (↓)neutrophils and platelets | |
| CAPS | -Anakinra 1-10 mg/kg/d up to 100 mg/d -Canakinumab 150 mg/8 wk -Rilonacept 300-320 mg loading, then 100-320 mg/wk |
|||||||
| PAPA syndrome | Anakinra 100 mg/d | -Infliximab 4 mg/kg ×4 doses -Etanercept 25 mg BIW |
2 mg/kg/d up to 60 mg/d | -IVIG 400 mg/kg -Isotretinoin PO 0.3-0.5 mg/kg/d |
||||
| Blau syndrome | -Infliximab 10 mg/kg/8 wk -Combined infliximab 5 mg/kg/6 wk, prednisolone 5 mg/d, and methotrexate 15.7 mg/wk |
-0.1 mg/kg up to 60 mg/d -0.5-2.5 mg/kg/2 d |
2 mg/kg/d up to 75 mg/d | -Azithromycin PO 10 mg/kg TIW -Eye surgery for advanced glaucoma |
||||
| TRAPS | Anakinra 1.5 mg/kg/d | Etanercept 0.4 mg/kg up to 25 mg BIW | 60 mg/d | 8 mg/kg/mo | ||||
| HIDS | -Anakinra 1-2 mg/kg/d -Canakinumab |
Etanercept 0.8 mg/kg/wk | 20-80 mg/d | |||||
| FMF syndrome | -Anakinra 100 mg/1-2 d -Canakinumab 2 mg/kg/8 wk |
Etanercept 0.8 mg/kg up to 25 mg BIW | -PO 1-20 mg/d -IV 1 mg/wk |
Sulfasalazine PO 50 mg/kg/d | ||||
| DIRA syndrome | Anakinra 1-3 mg/kg/d | |||||||
| CANDLE syndrome | Methotrexate IV 10 mg/m2/wk and PO 0.3 mg/kg/wk | |||||||
| SAPHO syndrome | Anakinra 100 mg/d | 5 mg/d | 1-1.5 mg/d | Etretinate PO 20-50 mg/d | ||||
| Schnitzler syndrome | -Anakinra 100 mg/d -Combined anakinra 100 mg/d and methotrexate 5 mg/wk |
Adalimumab 40 mg/2 wk | Combined prednisone 2 mg/d and anakinra 100 mg/d | 100 mg/d | 8 mg/kg/mo | -PUVA TIW -Rituximab 1 g/2 wk -Interferon alfa-2b 3 MIU TIW |
||
| SOJIA syndrome | -Anakinra 1-2 mg/kg/d up to 100 mg/d -Canakinumab 4 mg/kg/4 wk |
ALT, Alanine aminotransferase; BIW, biweekly; CANDLE, chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperature; CAPS, cryopyrin-associated periodic syndrome; CPK, creatine phosphokinase; DIRA, deficiency of interleukin-1 receptor antagonist; FMF, familial Mediterranean fever; HA, headache; HIDS, hyper-IgD syndrome; HMG-CoA, 3-hydroxy-3-methylglutaryl coenzyme A; HTN, hypertension; IL, interleukin; IV, intravenous; IVIG, intravenous immunoglobulin; MIU, million units; PAPA, pyogenic arthritis, pyoderma gangrenosum, and acne; PO, per os (by mouth); PUVA, psoralen plus ultraviolet A; rxn, reaction; SAPHO, synovitis, acne, pustulosis, hyperostosis, and osteitis; SC, subcutaneous; SOJIA, systemic-onset juvenile idiopathic arthritis; TIW, 3 times a week; TNF, tumor necrosis factor; TRAPS, tumor necrosis factor receptor—associated periodic syndrome; URI, upper respiratory tract infection.
MONOGENIC AUTOINFLAMMATORY SYNDROMES
Cryopyrin-associated periodic syndrome
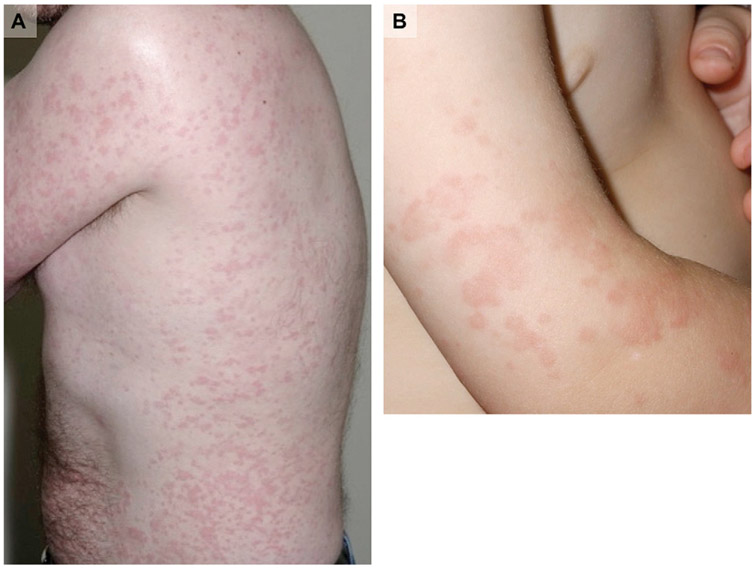
Cryopyrin-associated period syndrome (CAPS) is a rare childhood-onset disorder that presents with a wide spectrum of severity. In fact, CAPS encompasses 3 distinct phenotypes, listed in the order of increasing severity: familial cold autoinflammatory syndrome, Muckle-Wells syndrome, and neonatal-onset multisystem inflammatory disorder. As the name suggests, episodes of familial cold autoinflammatory syndrome may follow exposure to low ambient temperatures.10,11 The hallmarks of CAPS episodes are evanescent, nonpruritic, urticaria-like papules and confluent geographic plaques on the trunk and extremities, periodic fevers, and distal arthralgia (Fig 2).10,12,13 Skin histology reveals a sparse interstitial, perivascular, or perieccrine neutrophilic infiltrate.
Fig 2.
Cryopyrin-associated periodic syndrome. A, Familial cold autoinflammatory syndrome, urticaria-like eruption in adult. B, Muckle-Wells syndrome, urticaria-like dermatitis in child.
Less common features of CAPS are ocular involvement, including conjunctivitis, episcleritis, and uveitis, and neurologic manifestations, which encompass headaches, sensorineural hearing loss, and chronic meningitis.14,15 Secondary amyloid A amyloidosis most frequently affects the kidney and can lead to nephrotic syndrome. One case series reported 6 cases of reactive amyloidosis out of 22 patients.14 Leukocytosis and elevation of C-reactive protein (CRP) and serum protein amyloid A are almost always present, whereas the erythrocyte sedimentation rate (ESR) is variably elevated.16 IL-1ß expression is up-regulated in tissues of patients with CAPS.7,14,17 There are no known susceptibility markers in patients with CAPS for the development of amyloidosis.
Mutations in the NLRP3 gene [also referred to as the CIAS1 (cold-induced autoinflammatory syndrome 1) or NALP3 (nacht domain-, leucine-rich repeat-, and PYD-containing protein 3) gene], which codes for the cryopyrin NLRP, are dominantly inherited; however, de novo NLRP3 mutations have been reported.18-20 Targeted inhibition of IL-1ß has revolutionized the treatment of patients with CAPS. Treatment with anakinra, a recombinant-DNA analog of the human IL-1 receptor antagonist (RA), typically leads to rapid clearance of skin lesions and improvement of amyloidosis-induced nephrotic syndrome.21-25 Rilonacept, a “cytokine trap” antibody with high affinity for anti-IL-1, is also effective,26-28 and canakinumab, a fully human monoclonal antibody against IL-1ß, demonstrated a 97% complete response rate in a recent clinical trial.29
Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome
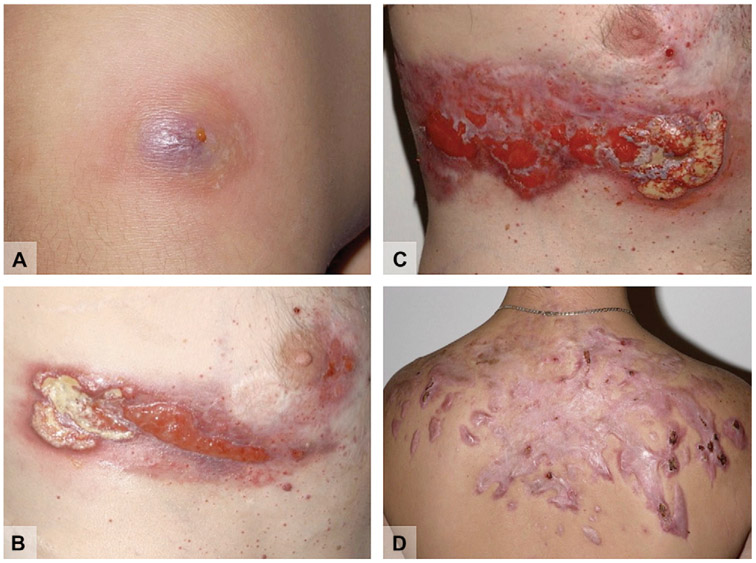
Pyogenic arthritis, pyoderma gangrenosum, and acne (PAPA) syndrome is a dominantly inherited disorder characterized by pyoderma gangrenosum (PG), acne vulgaris, and pyogenic arthritis primarily involving the appendicular skeleton.30 PG and arthritis typically present in early childhood, whereas acne often begins during puberty. PG lesions are characterized as single or multiple deep, “beefy red” ulcers with bluish, undermined borders (Fig 3). Common locations are the legs and face and occasionally the intertriginous regions. Skin ulcers in PAPA syndrome are indistinguishable from PG lesions secondary to other causes.
Fig 3.
Pyogenic arthritis, pyoderma gangrenosum (PG), and acne (PAPA) syndrome. A, PG in its early stage. B, Developing PG. C, Progression and scarring of the same PG. D, Extensive hypertrophic scarring at sites of severe acne involvement.
Mutations in PSTPIP1 (proline-serine-threonine phosphatase interacting protein 1), also known as CD2BP1, cause increased binding of the protein pyrin to the pyrin domain of NLRP, leading to inflammasome formation.31 Laboratory findings include elevated IL-1ß, tumor necrosis factor (TNF)-alfa, CRP, and ESR, as well as hypogammaglobulinemia.32-34 Acne and PG typically respond to infliximab and etanercept, respectively, whereas response to anakinra is variable.35-38 Control of inflammation can sometimes be achieved with prednisone (15-60 mg/d).39
Blau syndrome
Blau syndrome is an autosomal dominant disorder presenting in childhood with cutaneous granulomata, symmetric polyarthritis (with or without camptodactyly), and ocular manifestations, including uveitis, iritis, vitritis, and closed-angle glaucoma.40-43 Skin examination reveals nonpruritic, generalized, densely populated erythematous papules.44,45 Recalcitrant, tender leg ulcers with granulating bases and poorly demarcated flat borders have been described (in contrast to the well-defined undermined borders of PG ulcers).46 Histopathology reveals noncaseating granulomata.44,45,47 Granulomatous infiltration of the lungs, kidneys, liver, and of the arterial and nervous systems may also occur.48-52
Missense mutations in the CARD15 (caspase-recruiting domain 15) gene, also known as NOD2 (nucleotide-binding oligomerization domain) gene, are responsible for Blau syndrome.42,53 CARD15 serves as an activator of the nuclear factor kappa B pathway in monocytes, leading to expression of inflammatory cytokines that in turn contribute to the development of granulomata in affected tissues.54,55 Increased IgA, IgG, ESR, and angiotensin-converting enzyme levels have been documented.41 Response to targeted anti-IL-1 therapy is inconsistent, and serum IL-1ß levels do not necessarily correlate with disease severity.56,57 Infliximab and thalidomide have been used with moderate success,58,59 whereas treatment with prednisone (2 mg/kg/d) may be necessary to control ocular inflammation.40,43 Surgical intervention is an option for advanced glaucoma.43
TNF receptor—associated periodic syndrome
TNF receptor—associated periodic syndrome (TRAPS) is a dominantly inherited disorder that presents with prolonged periodic fevers (typically 7-21 days), erysipelas-like macules and patches overlying focal myalgia, abdominal pain, conjunctivitis, unilateral periorbital edema, and occasional lymphadenopathy.60,61 Most patients develop skin manifestations during early childhood: warm, blanchable, erythematous macules and patches with a tendency to migrate from the trunk to distal extremities. Other morphologies include widespread reticulate erythema or annular edematous plaques.61,62
Mutations in the TNFRSF1A (tumor necrosis factor receptor superfamily, member 1A) gene coding for a TNF receptor are associated with reduced concentrations of the cytosolic, soluble form of the receptor.63 This may be a result of “defective shedding” of the receptor from it position on the cell surface. However, some patients with TRAPS manifest normal levels of the membrane-bound TNF receptor.64 Plasma levels of ESR, CRP, haptoglobin, fibrinogen, and ferritin may be elevated during inflammatory attacks. Histology of skin specimens reveals perivascular and interstitial infiltrate of lymphocytes and monocytes—distinct from the neutrophilic infiltrate observed in CAPS.61
With regard to treatment, the respective use of anakinra and tocilizumab, a humanized monoclonal anti-IL-6 receptor antibody, has produced moderate success.65,66 Anecdotal reports of the efficacy of etanercept can be found, including improvement of amyloidosis-induced nephrotic syndrome.67,68 However, 2 studies involving 7 and 15 patients, respectively, did not show resolution of symptoms or normalization of laboratory parameters with etanercept.69,70 An in vitro study of cellular response to infliximab demonstrated no therapeutic benefits for patients with TRAPS. In this study, infliximab treatment paradoxically led to increased levels of IL-6, IL-8, and IL-12.71
Hyper-IgD syndrome
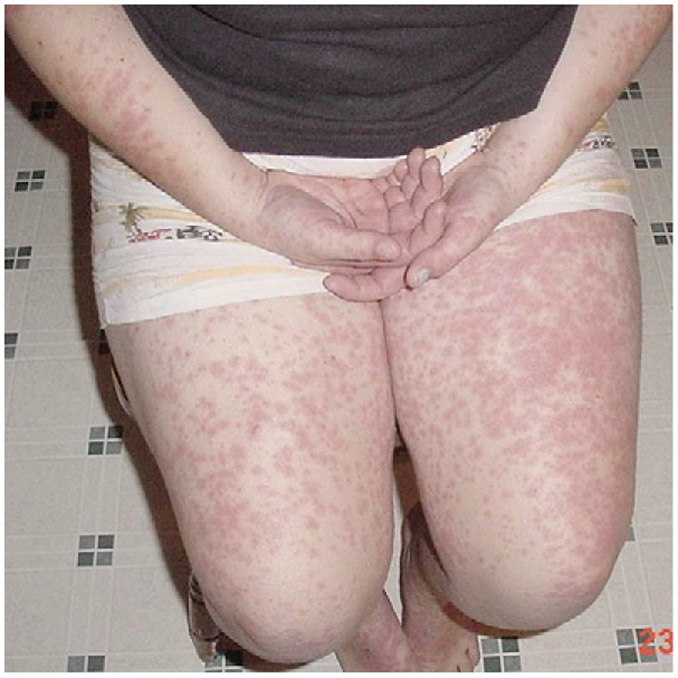
Hyper-IgD syndrome (HIDS), or mevalonate kinase deficiency, is an autosomal recessive disorder characterized by periodic fevers, arthralgia, gastrointestinal disturbances, lymphadenopathy, and splenomegaly.72-74 Skin findings range from intermittent, painful, ill-defined erythematous macules and papules to edematous, erythematous plaques with prominent borders and occasionally central clearing (Fig 4). Common areas of involvement are the trunk and extremities but can extend to the face, neck, and buttocks. Amyloidosis can be present in severe cases.73 Immunohistology of lesional skin reveals perivascular deposition of IgD and C3 complexes.75
Fig 4.
Hyper-IgD syndrome. Discrete, confluent pink papules and plaques. (Used with permission of Karyl S. Barron, MD, Deputy Director, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services.)
Mutations in the MVK (mevalonate kinase) gene, which codes for the enzyme mevalonate kinase, disrupt cholesterol synthesis, resulting in decreased serum cholesterol levels and an episodic increase in urinary mevalonic acid.76 Speculation about the pathogenetic role of inflammasomes has not been successful.77,78 The characteristic feature of HIDS is elevation of serum IgD,73,79 whereas IgA elevation is variable.80 Ex vivo expression of TNF-alfa and IL-1ß is up-regulated during primary attacks.81 Simvastatin dosed at 20 or 80 mg/d is efficacious for patients with HIDS, via inhibition of mevalonic acid production.82-86 HIDS might respond to anakinra (20-100 mg/d)86-89 or canakinumab.74 Therapeutic trials with etanercept and adalimumab have yielded mixed results, and colchicine is generally ineffective.82-85
Familial Mediterranean fever syndrome
Familial Mediterranean fever (FMF) syndrome is an early-onset, autosomal recessive disorder presenting with periodic fevers, an erysipelas-like rash, synovitis, and serositis in patients of Mediterranean descent. Other reported features include Henoch-Schönlein purpura, polyarteritis nodosa, and protracted febrile myalgia.90-100 The classic erysipeloid rash of FMF appears as tender, erythematous plaques with sharply demarcated, advancing borders localized to bilateral legs. Histopathology typically reveals dermal edema and a sparse perivascular infiltrate composed of lymphocytes, neutrophils, and histiocytes. Amyloidosis is a rare complication of FMF.90,101
FMF is caused by mutations in the MEFV (MEditerrean FeVer) gene, which encodes pyrin, a key protein involved in inflammasome activation.102-104 Patients with homozygous MEFV mutations from Armenia, Turkey, and Arabian countries are at high risk of developing amyloidosis and should be placed on long-term prophylactic colchicine.105 Intravenous or oral colchicine has been shown to reduce the severity of acute inflammatory episodes.106 IL-1 inhibition represents an alternative option for the treatment of FMF.107-111 Etanercept and sulfasalazine, respectively, may prove to be helpful, whereas thalidomide administration has yielded conflicting results.106,112-116
Deficiency of IL-1-RA syndrome
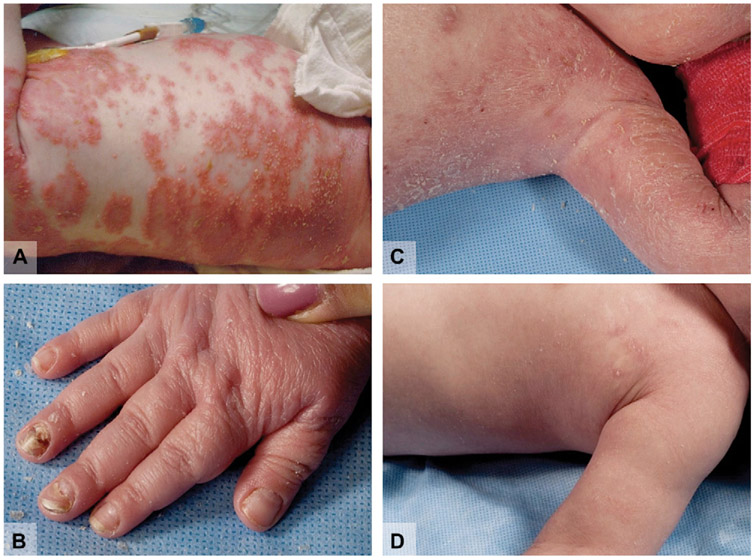
In 2009, Aksentijevich et al117 described a novel autoinflammatory syndrome characterized by neonatal-onset, generalized pustulosis, periostitis, and osteomyelitis with negative bone-tissue culture findings (Fig 5). Abnormal radiographic skeletal features were commonly observed, whereas nail changes and hepatosplenomegaly were intermittent findings. Therapy with disease-modifying antirheumatic drugs and prednisone at 2 mg/kg/d did not diminish symptoms or normalize levels of acute-phase reactants.117 Two of the 9 reported children died of multiorgan failure secondary to severe inflammation, and another died from complications of pulmonary hemosiderosis.
Fig 5.
Deficiency of interleukin (IL)-1 receptor antagonist (DIRA) syndrome. A, Generalized pustulosis. B, Nail dystrophy. C, Patient with DIRA before IL-1 blockade therapy. D, Same child after 5-day course of subcutaneous anakinra 100 mg/d.
The new entity was named “deficiency of the interleukin-1 receptor antagonist” (DIRA) based on the discovery of homozygous mutations in the IL1RN (interleukin 1 receptor antagonist) gene, which encodes a circulating antagonist to IL-1ß signaling.117 Monocytes of patients with DIRA secrete a truncated, nonfunctional version of the anti-IL-1ß antagonist, leading to hyperresponsiveness of inflammatory cells to IL-1ß stimulation. Not surprisingly, therapeutic response to anakinra was rapid.117
Chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperature syndrome
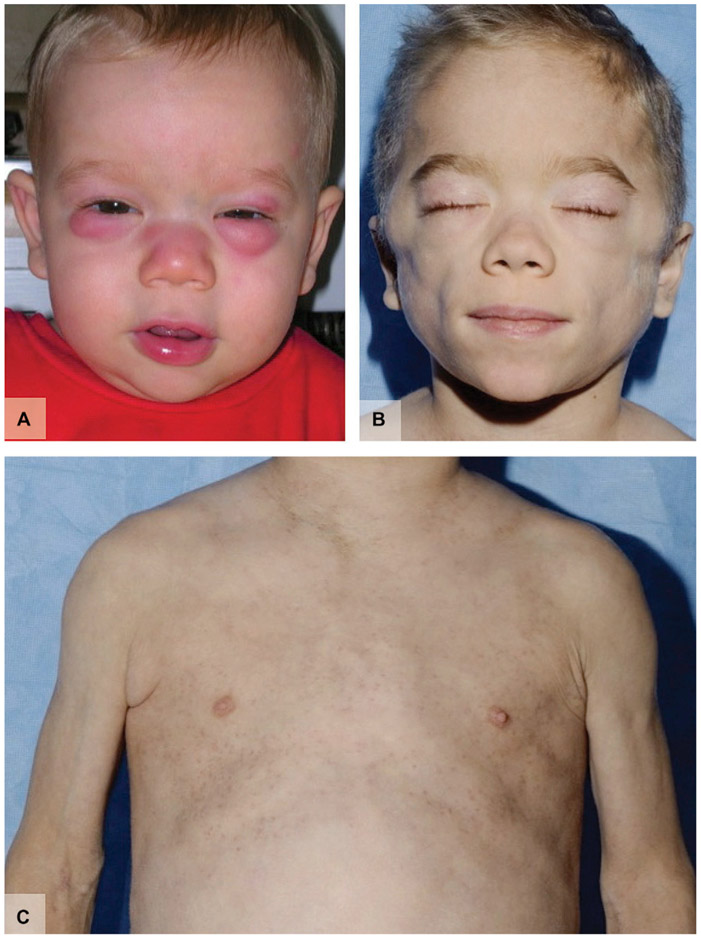
Chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperature (CANDLE) syndrome, first described by Torrelo et al118 in 2010, is characterized by generalized annular erythematous/violaceous plaques, edematous eyelids, progressive facial lipodystrophy, arthralgia, early-onset periodic fevers, and delayed physical development (Fig 6).119 Homozygous and heterozygous mutations in the PSMB8 (proteasome subunit ß type 8) gene have been identified.120 Impaired proteasome function means that damaged proteins serving as signals of cellular stress are not adequately degraded, leading to chronic inflammation.120 ESR and hepatic transaminase levels are consistently elevated in patients with CANDLE syndrome. Skin histology typically shows mature neutrophils and perivascular/interstitial infiltrates rich in myeloid cells.118 Lipodystrophy may be a result of chronic inflammation involving adipose tissue.121,122 Patients generally respond poorly to anakinra, intravenous immunoglobulin, infliximab, etanercept, cyclosporine, and prednisone.118,119 Partial response to methotrexate has been reported.118
Fig 6.
Chronic atypical neutrophilic dermatitis with lipodystrophy and elevated temperature (CANDLE) syndrome. A, Violaceous, edematous eyelids. B, Facial lipodystrophy of same patient several years later. C, Lipoatrophy of the torso.
OTHER AUTOINFLAMMATORY SYNDROMES
Synovitis, acne, pustulosis, hyperostosis, osteitis syndrome
Synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome is characterized by severe acne, palmoplantar pustulosis or palmoplantar pustular psoriasis, chronic inflammation of sternoclavicular and sternocostal synchondroses, osteosclerosis and hypertrophic osteitis of the vertebrae and femurs, as well as synovitis involving the elbows, knees, metacarpophalangeal, and proximal interphalangeal joints.123-128 Sterile pustules measuring 2 to 4 mm in diameter are localized to the palms and soles, sometimes studding scaly erythematous plaques. Inflamed comedones and pustules of acne vulgaris can be found on the face and upper aspect of the trunk. Acne conglobata, acne fulminans, and acne inversa appear as suppurating cysts, nodules, abscesses, and sinus tracts.123,129 Dermatologic and rheumatologic manifestations are not temporally related.130
Both Staphylococcus aureus and Propionibacterium acnes have been implicated in triggering the inflammatory attacks of SAPHO syndrome. P acnes has been cultured from bone specimens, a sternal osteosclerotic lesion, and intervertebral material from affected individuals.131 This has led to the hypothesis that the inflammation may be a result of multiple failed attempts to clear the bacterium.131 Serum levels of ESR and CRP are elevated. Successful treatment with anakinra has been reported.132,133 TNF-alfa blockade with infliximab, etanercept, or adalimumab can be helpful for some patients.134 Several case reports have documented response of skin manifestations to colchicine (1-1.5 mg/d), prednisone (5 mg/d), etretinate (20-50 mg/d), dapsone, and tonsillectomy.134-142
Schnitzler syndrome
Schnitzler syndrome is a rare disorder characterized by recurrent fevers, urticaria, arthritis, hyperostosis, osteosclerosis, and IgM gammopathy.143-145 Skin findings consist of asymptomatic erythematous, edematous plaques with prominent borders, primarily found on the trunk and lower extremities. Lymphadenopathy, hepatomegaly, polyclonal lymphoplasmacytic infiltration of the bone marrow, and rarely, severe anemia and life-threatening thrombophilia have been reported.146,147 Impaired renal function and Waldenstrom macroglobulinemia may occur as late sequelae.148-153
No genetic basis for Schnitzler syndrome has been found. Laboratory investigations reveal elevation of IL-1ß, IL-6, IL-18, ESR, and CRP levels.154-156 Histology of lesional skin demonstrates a perivascular infiltrate consisting of lymphocytes, histiocytes, and neutrophils.145 Immunofluorescence staining shows IgM deposits in the papillary dermis or at the basement membrane.145 Daily administration of anakinra has provided long-term control.157-160 Psoralen combined with ultraviolet A therapy and IL-6 blockade with tocilizumab may be effective, respectively.161,162 Other treatments that have been used with variable success include TNF-alfa inhibition, thalidomide, colchicine, systemic steroids, and interferon alfa-2b.163-171
Systemic-onset juvenile idiopathic arthritis
Systemic-onset juvenile idiopathic arthritis (SOJIA) is a childhood-onset, relapsing inflammatory disorder with spiking fevers, an evanescent morbilliform rash occurring daily, and polyarticular arthritis.172,173 Skin examination reveals diffuse erythematous macules and papules closely distributed on the trunk, the upper extremities, and less frequently, on the face.173-176 Histology of lesional skin shows a perivascular and interstitial infiltrate composed of (in order of decreasing frequency) neutrophils, monocytes, lymphocytes, and eosinophils.177 Neutrophils can also be visualized in perieccrine tissues and at the dermoepidermal junction.177
Elevated IL-6 levels, which have been found to parallel febrile episodes, suggest a possible role for IL-6 blockade therapy.172 No genetic mutations have been found in patients with SOJIA. Anakinra and canakinumab may reduce the severity of inflammatory attacks.178-180
AUTOINFLAMMATION IN COMMON SKIN DISEASES
Evidence of abnormal innate immunity can be found in common dermatoses, including atopic dermatitis, contact dermatitis, psoriasis, PG, neutrophilic dermatoses, acne, alopecia areata, vitiligo, and systemic lupus erythematosus (SLE). The pathogenesis of atopic dermatitis involves complex interactions among environmental triggers (eg, S. aureus), disruption of the epidermal barrier, IgE dysregulation, and genetic factors, including single nucleotide polymorphisms (SNPs) and de novo mutations in the NOD1, NLR, and CARD15 genes.181-183 Exactly which NLR polymorphisms predispose patients to atopic dermatitis is a topic deserving further investigation, as NLRP1 and NLRP3 SNPs have been not found to be associated with atopic dermatitis.184 Interestingly, the house dust mite allergen Dermatophagoides pteronyssinus has been shown to stimulate secretion of IL-1ß and IL-18 from human keratinocytes.185 Contact sensitizers can also activate the IL-1ß-processing inflammasomes in the hypersensitive reaction of contact dermatitis.186
Abnormal interactions between antigen-presenting cells and T-helper lymphocytes (helper T cells type 1 and type 17) in psoriasis lead to excessive keratinocyte proliferation and elevated serum levels of TNF-alfa, interferon-α, and IL-8.187 Recent discovery of other pathways and cytokines relevant to psoriatic inflammation has led to emerging targeted therapies (eg, IL-23, IL-17, JAK kinase signaling). A role of innate immunity in psoriasis has been suggested by increased expression of PRRs (eg, TLR-2, TLR-4, dectin-1) in patients with psoriasis compared with nonpsoriatic control subjects.188,189 For instance, expression of TLR-2 is positively correlated with levels of danger-associated molecular patterns and the aforementioned inflammatory cytokines, respectively.189
Decreased IL-1ß expression and increased IL-1-RA activity can be demonstrated in active psoriatic skin.190,191 The opposite situation has also been reported, where IL-1β expression is increased and IL-1-RA expression is decreased in lesional epidermis of patients with psoriasis.192,193 The use of anakinra (100 mg/d) in 9 patients with psoriatic arthritis led to improvement of psoriasis in 2 patients, new plaques in 1 patient, and worsening of plaques in 4 patients.194
Recently, a new autoinflammatory pathway has been described for patients with an early-onset heritable form of generalized pustular psoriasis. A whole-genome scan was conducted on 9 Tunisian families, revealing homozygous missense mutations in the IL36RN gene in affected patients.195 IL36RN encodes the IL-36-RA, which counters inflammation in an analogous manner to IL-1-RA in DIRA syndrome. Serum IL-1ß, IL-1α, IL-6, and IL-8 are elevated.195,196 Successful therapy with anakinra has been reported for pustular psoriasis and its variant, acrodermatitis continua of Hallopeau.196,197 These reports, along with the pustulosis characteristic of DIRA, suggest a role for innate immunity and autoinflammation in a subset of patients with pustular skin disease and a possible new avenue of treatment, particularly for patients with concurrent systemic inflammatory symptoms.
PG might in part be mediated by autoinflammation. Mutations in PSTPIP1 are characteristic of PAPA syndrome198,199 but have also been described in patients with PG who lack other features of PAPA syndrome.200 Both Crohn’s disease and Blau syndrome are associated with mutations that compromise function of the antibacterial factor CARD15.201,202 Interestingly, PG is a well-recognized manifestation of Crohn’s disease but to our knowledge has not been associated with Blau syndrome. One of the authors (K. S. L.) has observed 1 case of PG in a patient with CAPS. Reported response of PG to anakinra in 1 patient, who tested negative for PSTPIP1 mutations, warrants further investigation of the role of IL-1 inhibition.203
Although no genetic mutations have been directly linked to Sweet syndrome, it has been found in patients with CAPS and Crohn’s disease, and neutrophilic infiltrates are characteristic of many monogenic autoinflammatory diseases.14,204,205 Anakinra has been used anecdotally in patients with neutrophilic dermatoses, but its role for these conditions remains to be determined.206,207
The pathogenesis of acne involves microbial triggers, aberrant keratinocyte adhesion, hormonal imbalance, and genetic factors. Predisposition to severe acne vulgaris has been linked with TLR-2, TNF-2, and IL1RN polymorphisms.208,209 For both acne vulgaris and acne rosacea, expression of the PRR TLR-2 has been found to be up-regulated in response to microbial stimulation.210 PRRs are crucial gateways to innate immunity, and alteration of their activity is likely to have an impact on the normal inflammatory response in the epidermis.
Granulomatous lesions of acne rosacea have been documented in a patient with a known mutation predisposing to Crohn’s disease and Blau syndrome.211 The overlap of acne conglobata, hidradenitis suppurativa, and PG also suggests a common pathway involving innate immunity, which is further implicated by the favorable response of these disorders to IL-1 inhibition.203,212,213
Known MEFV and TNFRSF1A mutations responsible for FMF and TRAPS, respectively, have been found in patients with Behçet disease.214-217 Associations between patchy alopecia areata and IL1RNSNPs218,219 as well as between vitiligo and NALP1 SNPs220 have also been reported. One report documented improvement of vitiligo after administration of infliximab.221 However, an open-label, pilot study (N = 4) using etanercept at 50 mg weekly for 12 weeks and 25 mg weekly for an additional 4 weeks showed no repigmentation of vitiligo lesions.222
The past 2 decades of research have highlighted the important role of IL-1-RA polymorphisms in SLE.223-226 Affected individuals with high levels of IL-1-RA tend to be at a lower risk of developing lupus nephritis compared with those possessing normal IL-1-RA levels.227 In addition, certain NALP1 SNPs have been associated with susceptibility to the dermatitis, arthritis, and nephritis of SLE.228 The response of musculoskeletal and joint symptoms to anakinra, albeit transient, warrants further investigation into the potential of IL-1 blockade therapy.229
Our understanding of the innate immune system has expanded significantly in the last decade through the study of the rare monogenic autoinflammatory syndromes. It is also becoming clear that features of autoinflammation may affect several common dermatoses, including those previously thought to be solely autoimmune in origin (eg, SLE, vitiligo). It may be more helpful to view these syndromes and diseases as part of a spectrum of self-directed tissue injury mediated by adaptive and innate pathways. Recognition of aberrant activity of inflammasomes and other key mediators of the innate immune system opens up the possibility for new, targeted therapies for many complex and recalcitrant inflammatory dermatoses.
CAPSULE SUMMARY.
Autoinflammation is characterized by aberrant regulation of the innate immune system.
Pathways mediating innate immunity, many of which are related to the interleukin-1ß-processing inflammasome, are common targets in monogenic autoinflammatory syndromes.
Several common dermatoses have been found to be affected by features of autoinflammatory disease, leading to the possibility of new, targeted therapies.
Acknowledgments
The authors would like to acknowledge Dr Michael Rosenblum for his review and helpful comments, and Ms Kate Ganim for her help with composing Fig 1.
Funding sources: None.
Abbreviations used:
- CAPS
cryopyrin-associated periodic syndrome
- CARD
caspase-recruiting domain
- CRP
C-reactive protein
- DIRA
deficiency of the interleukin-1 receptor antagonist
- ESR
erythrocyte sedimentation rate
- FMF
familial Mediterranean fever
- HIDS
hyper-IgD syndrome
- IL
interleukin
- NLR
nucleotide-binding domain leucine-rich repeat-containing
- NLRP
nucleotide-binding domain leucine-rich repeat-containing protein
- PAPA
pyogenic arthritis, pyoderma gangrenosum, and acne
- PG
pyoderma gangrenosum
- PRR
pattern recognition receptor
- RA
receptor antagonist
- SLE
systemic lupus erythematosus
- SNPs
single nucleotide polymorphisms
- TLR
Toll-like receptor
- TNF
tumor necrosis factor
- TRAPS
tumor necrosis factor receptor—associated periodic syndrome
Footnotes
Conflicts of interest: None declared.
REFERENCES
- 1.Ye Z,Ting JP. NLR, the nucleotide-binding domain leucine-rich repeat containing gene family. Curr Opin Immunol 2008;20:3–9. [DOI] [PubMed] [Google Scholar]
- 2.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, et al. The NLR gene family: a standard nomenclature. Immunity 2008;28:285–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schroder K, Tschopp J. The inflammasomes. Cell 2010;140: 821–32. [DOI] [PubMed] [Google Scholar]
- 4.Keller M, Ruegg A, Werner S, Beer HD. Active caspase-1 is a regulator of unconventional protein secretion. Cell 2008;132: 818–31. [DOI] [PubMed] [Google Scholar]
- 5.Glaser RL, Goldbach-Mansky R. The spectrum of monogenic autoinflammatory syndromes: understanding disease mechanisms and use of targeted therapies. Curr Allergy Asthma Rep 2008;8:288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello CA, Ikejima T, Warner SJ, Orencole SF, Lonnemann G, Cannon JG, et al. Interleukin 1 induces interleukin 1, I: induction of circulating interleukin 1 in rabbits in vivo and in human mononuclear cells in vitro. J Immunol 1987;139:1902–10. [PubMed] [Google Scholar]
- 7.Lachmann HJ, Lowe P, Felix SD, Rordorf C, Leslie K, Madhoo S, et al. In vivo regulation of interleukin 1beta in patients with cryopyrin-associated periodic syndromes. J Exp Med 2009; 206:1029–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011;469:221–5. [DOI] [PubMed] [Google Scholar]
- 9.Mason DR, Beck PL, Muruve DA. Nucleotide-binding oligomerization domain-like receptors and inflammasomes in the pathogenesis of non-microbial inflammation and diseases. J Innate Immun 2012;4:16–30. [DOI] [PubMed] [Google Scholar]
- 10.Doeglas HM, Bleumink E. Familial cold urticaria: clinical findings. Arch Dermatol 1974;110:382–8. [PubMed] [Google Scholar]
- 11.Stych B, Dobrovolny D. Familial cold auto-inflammatory syndrome (FCAS): characterization of symptomatology and impact on patients’ lives. Curr Med Res Opin 2008;24: 1577–82. [DOI] [PubMed] [Google Scholar]
- 12.Yu JR, Leslie KS. Cryopyrin-associated periodic syndrome: an update on diagnosis and treatment response. Curr Allergy Asthma Rep 2011;11:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kubota T, Koike R. Cryopyrin-associated periodic syndromes: background and therapeutics. Mod Rheumatol 2010;20: 213–21. [DOI] [PubMed] [Google Scholar]
- 14.Leslie KS, Lachmann HJ, Bruning E, McGrath JA, Bybee A, Gallimore JR, et al. Phenotype, genotype, and sustained response to anakinra in 22 patients with autoinflammatory disease associated with CIAS-1/NALP3 mutations. Arch Dermatol 2006;142:1591–7. [DOI] [PubMed] [Google Scholar]
- 15.Montealegre Sanchez GA, Hashkes PJ. Neurological manifesttations of the Mendelian-inherited autoinflammatory syndromes. Dev Med Child Neurol 2009;51:420–8. [DOI] [PubMed] [Google Scholar]
- 16.Dowds TA, Masumoto J, Zhu L, Inohara N, Nunez G. Cryopyrin-induced interleukin 1beta secretion in monocytic cells: enhanced activity of disease-associated mutants and requirement for ASC. J Biol Chem 2004;279:21924–8. [DOI] [PubMed] [Google Scholar]
- 17.Aksentijevich I, Nowak M, Mallah M, Chae JJ, Watford WT, Hofmann SR, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis Rheum 2002;46:3340–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat Genet 2001;29:301–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldmann J, Prieur AM, Quartier P, Berquin P, Certain S, Cortis E, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. Am J Hum Genet 2002;71:198–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1beta-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 2004;20:319–25. [DOI] [PubMed] [Google Scholar]
- 21.Hawkins PN, Lachmann HJ, Aganna E, McDermott MF. Spectrum of clinical features in Muckle-Wells syndrome and response to anakinra. Arthritis Rheum 2004;50:607–12. [DOI] [PubMed] [Google Scholar]
- 22.Ramos E, Arostegui JI,Campuzano S, Rius J, Bousono C, Yague J. Positive clinical and biochemical responses to anakinra in a 3-yr-old patient with cryopyrin-associated periodic syndrome (CAPS). Rheumatology (Oxford) 2005;44:1072–3. [DOI] [PubMed] [Google Scholar]
- 23.Boschan C, Witt O, Lohse P, Foeldvari I, Zappel H, Schweigerer L. Neonatal-onset multisystem inflammatory disease (NOMID) due to a novel S331R mutation of the CIAS1 gene and response to interleukin-1 receptor antagonist treatment. Am J Med Genet A 2006;140:883–6. [DOI] [PubMed] [Google Scholar]
- 24.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med 2006;355:581–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neven B, Marvillet I, Terrada C, Ferster A, Boddaert N, Couloignier V, et al. Long-term efficacy of the interleukin-1 receptor antagonist anakinra in ten patients with neonatal-onset multisystem inflammatory disease/chronic infantile neurologic, cutaneous, articular syndrome. Arthritis Rheum 2010;62:258–67. [DOI] [PubMed] [Google Scholar]
- 26.Economides AN, Carpenter LR, Rudge JS, Wong V, Koehler-Stec EM, Hartnett C, et al. Cytokine traps: multi-component, high-affinity blockers of cytokine action. Nat Med 2003;9:47–52. [DOI] [PubMed] [Google Scholar]
- 27.Goldbach-Mansky R, Shroff SD, Wilson M, Snyder C, Plehn S, Barham B, et al. A pilot study to evaluate the safety and efficacy of the long-acting interleukin-1 inhibitor rilonacept (interleukin-1 Trap) in patients with familial cold autoinflammatory syndrome. Arthritis Rheum 2008;58:2432–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman HM, Throne ML, Amar NJ, Sebai M, Kivitz AJ, Kavanaugh A, et al. Efficacy and safety of rilonacept (interleukin-1 Trap) in patients with cryopyrin-associated periodic syndromes: results from two sequential placebo-controlled studies. Arthritis Rheum 2008;58:2443–52. [DOI] [PubMed] [Google Scholar]
- 29.Lachmann HJ, Kone-Paut I, Kuemmerle-Deschner JB, Leslie KS, Hachulla E, Quartier P, et al. Use of canakinumab in the cryopyrin-associated periodic syndrome. N Engl J Med 2009; 360:2416–25. [DOI] [PubMed] [Google Scholar]
- 30.Lindor NM, Arsenault TM, Solomon H, Seidman CE, McEvoy MT. A new autosomal dominant disorder of pyogenic sterile arthritis, pyoderma gangrenosum, and acne: PAPA syndrome. Mayo Clin Proc 1997;72:611–5. [DOI] [PubMed] [Google Scholar]
- 31.Shoham NG, Centola M, Mansfield E, Hull KM, Wood G, Wise CA, et al. Pyrin binds the PSTPIP1/CD2BP1 protein, defining familial Mediterranean fever and PAPA syndrome as disorders in the same pathway. Proc Natl Acad Sci U S A 2003;100:13501–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith EJ, Allantaz F, Bennett L, Zhang D, Gao X, Wood G, et al. Clinical, molecular, and genetic characteristics of PAPA syndrome: a review. Curr Genomics 2010;11:519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Edrees AF, Kaplan DL, Abdou NI. Pyogenic arthritis, pyoderma gangrenosum, and acne syndrome (PAPA syndrome) associated with hypogammaglobulinemia and elevated serum tumor necrosis factor-alpha levels. J Clin Rheumatol 2002;8:273–5. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs JC, Goetzl EJ. “Streaking leukocyte factor,” arthritis, and pyoderma gangrenosum. Pediatrics 1975;56:570–8. [PubMed] [Google Scholar]
- 35.Brenner M, Ruzicka T, Plewig G, Thomas P, Herzer P. Targeted treatment of pyoderma gangrenosum in PAPA (pyogenic arthritis, pyoderma gangrenosum and acne) syndrome with the recombinant human interleukin-1 receptor antagonist anakinra. Br J Dermatol 2009;161:1199–201. [DOI] [PubMed] [Google Scholar]
- 36.Stichweh DS, Punaro M, Pascual V. Dramatic improvement of pyoderma gangrenosum with infliximab in a patient with PAPA syndrome. Pediatr Dermatol 2005;22:262–5. [DOI] [PubMed] [Google Scholar]
- 37.Cortis E, De Benedetti F, Insalaco A, Cioschi S, Muratori F, D’Urbano LE, et al. Abnormal production of tumor necrosis factor (TNF)—alpha and clinical efficacy of the TNF inhibitor etanercept in a patient with PAPA syndrome [published correction appears in J Pediatr 2005;146:193]. J Pediatr 2004; 145:851–5. [DOI] [PubMed] [Google Scholar]
- 38.Tofteland ND, Shaver TS. Clinical efficacy of etanercept for treatment of PAPA syndrome. J Clin Rheumatol 2010;16: 244–5. [DOI] [PubMed] [Google Scholar]
- 39.Schellevis MA, Stoffels M, Hoppenreijs EP, Bodar E, Simon A, van der Meer JW. Variable expression and treatment of PAPA syndrome. Ann Rheum Dis 2011;70:1168–70. [DOI] [PubMed] [Google Scholar]
- 40.Pastores GM, Michels VV, Stickler GB, Su WP, Nelson AM, Bovenmyer DA. Autosomal dominant granulomatous arthritis, uveitis, skin rash, and synovial cysts. J Pediatr 1990;117:403–8. [DOI] [PubMed] [Google Scholar]
- 41.Raphael SA, Blau EB, Zhang WH, Hsu SH. Analysis of a large kindred with Blau syndrome for HLA, autoimmunity, and sarcoidosis. Am J Dis Child 1993;147:842–8. [DOI] [PubMed] [Google Scholar]
- 42.Snyers B, Dahan K. Blau syndrome associated with a CARD15/NOD2 mutation. Am J Ophthalmol 2006;142: 1089–92. [DOI] [PubMed] [Google Scholar]
- 43.Kurokawa T, Kikuchi T, Ohta K, Imai H, Yoshimura N. Ocular manifestations in Blau syndrome associated with a CARD15/Nod2 mutation. Ophthalmology 2003;110:2040–4. [DOI] [PubMed] [Google Scholar]
- 44.Alonso D, Elgart GW, Schachner LA. Blau syndrome: a new kindred. J Am Acad Dermatol 2003;49:299–302. [DOI] [PubMed] [Google Scholar]
- 45.Schaffer JV, Chandra P, Keegan BR, Heller P, Shin HT. Widespread granulomatous dermatitis of infancy: an early sign of Blau syndrome. Arch Dermatol 2007;143:386–91. [DOI] [PubMed] [Google Scholar]
- 46.Dhondt V, Hofman S, Dahan K, Beele H. Leg ulcers: a new symptom of Blau syndrome? Eur J Dermatol 2008;18:635–7. [DOI] [PubMed] [Google Scholar]
- 47.Stoevesandt J, Morbach H, Martin TM, Zierhut M, Girschick H, Hamm H. Sporadic Blau syndrome with onset of widespread granulomatous dermatitis in the newborn period. Pediatr Dermatol 2010;27:69–73. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Kuivaniemi H, Bonavita G, Mutkus L, Mau U, Blau E, et al. CARD15 mutations in familial granulomatosis syndromes: a study of the original Blau syndrome kindred and other families with large-vessel arteritis and cranial neuropathy. Arthritis Rheum 2002;46:3041–5. [DOI] [PubMed] [Google Scholar]
- 49.Ting SS, Ziegler J, Fischer E. Familial granulomatous arthritis (Blau syndrome) with granulomatous renal lesions. J Pediatr 1998;133:450–2. [DOI] [PubMed] [Google Scholar]
- 50.Saini SK, Rose CD. Liver involvement in familial granulomatous arthritis (Blau syndrome). J Rheumatol 1996;23:396–9. [PubMed] [Google Scholar]
- 51.Becker ML, Martin TM, Doyle TM, Rose CD. Interstitial pneumonitis in Blau syndrome with documented mutation in CARD15. Arthritis Rheum 2007;56:1292–4. [DOI] [PubMed] [Google Scholar]
- 52.Israel HL. Prognosis of sarcoidosis. Ann Intern Med 1970;73: 1038–9. [DOI] [PubMed] [Google Scholar]
- 53.Miceli-Richard C, Lesage S, Rybojad M, Prieur AM, Manouvrier-Hanu S, Hafner R, et al. CARD15 mutations in Blau syndrome. Nat Genet 2001;29:19–20. [DOI] [PubMed] [Google Scholar]
- 54.Hisamatsu T, Suzuki M, Reinecker HC, Nadeau WJ, McCormick BA, Podolsky DK. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology 2003;124:993–1000. [DOI] [PubMed] [Google Scholar]
- 55.Ogura Y, Inohara N, Benito A, Chen FF, Yamaoka S, Nunez G. Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-kappaB. J Biol Chem 2001;276: 4812–8. [DOI] [PubMed] [Google Scholar]
- 56.Martin TM, Zhang Z, Kurz P, Rose CD, Chen H, Lu H, et al. The NOD2 defect in Blau syndrome does not result in excess interleukin-1 activity. Arthritis Rheum 2009;60:611–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arostegui JI, Arnal C, Merino R, Modesto C, Antonia Carballo M, Moreno P, et al. NOD2 gene-associated pediatric granulomatous arthritis: clinical diversity, novel and recurrent mutations, and evidence of clinical improvement with interleukin-1 blockade in a Spanish cohort. Arthritis Rheum 2007;56:3805–13. [DOI] [PubMed] [Google Scholar]
- 58.Yasui K, Yashiro M, Tsuge M, Manki A, Takemoto K, Yamamoto M, et al. Thalidomide dramatically improves the symptoms of early-onset sarcoidosis/Blau syndrome: its possible action and mechanism. Arthritis Rheum 2010;62:250–7. [DOI] [PubMed] [Google Scholar]
- 59.Milman N, Andersen CB, Hansen A, van Overeem Hansen T, Nielsen FC, Fledelius H, et al. Favorable effect of TNF-alpha inhibitor (infliximab) on Blau syndrome in monozygotic twins with a de novo CARD15 mutation. APMIS 2006;114:912–9. [DOI] [PubMed] [Google Scholar]
- 60.Schmaltz R, Vogt T, Reichrath J. Skin manifestations in tumor necrosis factor receptor-associated periodic syndrome (TRAPS). Dermatoendocrinol 2010;2:26–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hull KM, Drewe E, Aksentijevich I, Singh HK, Wong K, McDermott EM, et al. The TNF receptor-associated periodic syndrome (TRAPS): emerging concepts of an autoinflammatory disorder. Medicine (Baltimore) 2002;81:349–68. [DOI] [PubMed] [Google Scholar]
- 62.Toro JR, Aksentijevich I, Hull K, Dean J, Kastner DL. Tumor necrosis factor receptor-associated periodic syndrome: a novel syndrome with cutaneous manifestations. Arch Dermatol 2000;136:1487–94. [DOI] [PubMed] [Google Scholar]
- 63.McDermott MF, Aksentijevich I, Galon J, McDermott EM, Ogunkolade BW, Centola M, et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 1999;97:133–44. [DOI] [PubMed] [Google Scholar]
- 64.Kimberley FC, Lobito AA, Siegel RM, Screaton GR. Falling into TRAPS—receptor misfolding in the TNF receptor 1-associated periodic fever syndrome. Arthritis Res Ther 2007;9:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gattorno M, Pelagatti MA, Meini A, Obici L, Barcellona R, Federici S, et al. Persistent efficacy of anakinra in patients with tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum 2008;58:1516–20. [DOI] [PubMed] [Google Scholar]
- 66.Vaitla PM, Radford PM, Tighe PJ, Powell RJ, McDermott EM, Todd I, et al. Role of interleukin-6 in a patient with tumor necrosis factor receptor-associated periodic syndrome: assessment of outcomes following treatment with the anti-interleukin-6 receptor monoclonal antibody tocilizumab. Arthritis Rheum 2011;63:1151–5. [DOI] [PubMed] [Google Scholar]
- 67.Jesus AA, Oliveira JB, Aksentijevich I, Fujihira E, Carneiro-Sampaio MM, Duarte AJ, et al. TNF receptor-associated periodic syndrome (TRAPS): description of a novel TNFRSF1A mutation and response to etanercept. Eur J Pediatr 2008;167:1421–5. [DOI] [PubMed] [Google Scholar]
- 68.Drewe E, McDermott EM, Powell RJ. Treatment of the nephrotic syndrome with etanercept in patients with the tumor necrosis factor receptor-associated periodic syndrome. N Engl J Med 2000;343:1044–5. [DOI] [PubMed] [Google Scholar]
- 69.Bulua AC, Mogul DB, Aksentijevich I, Singh H, He D, Muenz L, et al. Efficacy of etanercept in the tumor necrosis factor receptor-associated periodic syndrome (TRAPS). Arthritis Rheum 2012;64:908–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Drewe E, McDermott EM, Powell PT, Isaacs JD, Powell RJ. Prospective study of anti-tumor necrosis factor receptor superfamily 1B fusion protein, and case study of anti-tumor necrosis factor receptor superfamily 1A fusion protein, in tumor necrosis factor receptor associated periodic syndrome (TRAPS): clinical and laboratory findings in a series of seven patients. Rheumatology (Oxford) 2003;42:235–9. [DOI] [PubMed] [Google Scholar]
- 71.Nedjai B, Hitman GA, Quillinan N, Coughlan RJ, Church L, McDermott MF, et al. Proinflammatory action of the antiinflammatory drug infliximab in tumor necrosis factor receptor-associated periodic syndrome. Arthritis Rheum 2009;60:619–25. [DOI] [PubMed] [Google Scholar]
- 72.Drenth JP, Boom BW, Toonstra J, Van der Meer JW. Cutaneous manifestations and histologic findings in the hyperimmunoglobulinemia D syndrome: international hyper IgD study group. Arch Dermatol 1994;130:59–65. [PubMed] [Google Scholar]
- 73.van der Hilst JC, Bodar EJ, Barron KS,Frenkel J, Drenth JP,van der Meer JW, et al. Long-term follow-up, clinical features, and quality of life in a series of 103 patients with hyperimmunoglobulinemia D syndrome. Medicine (Baltimore) 2008;87:301–10. [DOI] [PubMed] [Google Scholar]
- 74.Bader-Meunier B, Florkin B, Sibilia J, Acquaviva C, Hachulla E, Grateau G, et al. Mevalonate kinase deficiency: a survey of 50 patients. Pediatrics 2011;128:e152–9. [DOI] [PubMed] [Google Scholar]
- 75.Boom BW, Daha MR, Vermeer BJ, van der Meer JW. IgD immune complex vasculitis in a patient with hyperimmunoglobulinemia D and periodic fever. Arch Dermatol 1990;126: 1621–4. [PubMed] [Google Scholar]
- 76.Milhavet F, Touitou I. Infevers: an online database for autoinflammatory mutations. Copyright 2001-2013. Available at http://fmf.igh.cnrs.fr/ISSAID/infevers/. Accessed April 5, 2012. [Google Scholar]
- 77.Normand S, Massonnet B, Delwail A, Favot L, Cuisset L, Grateau G, et al. Specific increase in caspase-1 activity and secretion of IL-1 family cytokines: a putative link between mevalonate kinase deficiency and inflammation. Eur Cytokine Netw 2009;20:101–7. [DOI] [PubMed] [Google Scholar]
- 78.Pontillo A, Paoluzzi E, Crovella S. The inhibition of mevalonate pathway induces up-regulation of NALP3 expression: new insight in the pathogenesis of mevalonate kinase deficiency. Eur J Hum Genet 2010;18:844–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, et al. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol 2009;10:889–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Drenth JP, van der Meer JW. Hereditary periodic fever. N Engl J Med 2001;345:1748–57. [DOI] [PubMed] [Google Scholar]
- 81.Drenth JP, van Deuren M, van der Ven-Jongekrijg J, Schalkwijk CG, van der Meer JW. Cytokine activation during attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Blood 1995;85:3586–93. [PubMed] [Google Scholar]
- 82.Attout H, Guez S, Ranaivo I, Jameerbaccus N, Series C. A patient with hyper-IgD syndrome responding to simvastatin treatment. Eur J Intern Med 2008;19:e82–3. [DOI] [PubMed] [Google Scholar]
- 83.Demirkaya E, Caglar MK, Waterham HR, Topaloglu R, Ozen S. A patient with hyper-IgD syndrome responding to anti-TNF treatment. Clin Rheumatol 2007;26:1757–9. [DOI] [PubMed] [Google Scholar]
- 84.Simon A, Drewe E, van der Meer JW, Powell RJ, Kelley RI, Stalenhoef AF, et al. Simvastatin treatment for inflammatory attacks of the hyperimmunoglobulinemia D and periodic fever syndrome. Clin Pharmacol Ther 2004;75: 476–83. [DOI] [PubMed] [Google Scholar]
- 85.Topaloglu R, Ayaz NA, Waterham HR, Yuce A, Gumruk F, Sanal O. Hyperimmunoglobulinemia D and periodic fever syndrome; treatment with etanercept and follow-up. Clin Rheumatol 2008;27:1317–20. [DOI] [PubMed] [Google Scholar]
- 86.Korppi M, Van Gijn ME, Antila K. Hyperimmunoglobulinemia D and periodic fever syndrome in children: review on therapy with biological drugs and case report. Acta Paediatr 2011; 100:21–5. [DOI] [PubMed] [Google Scholar]
- 87.Rigante D, Ansuini V, Bertoni B, Pugliese AL, Avallone L, Federico G, et al. Treatment with anakinra in the hyperimmunoglobulinemia D/periodic fever syndrome. Rheumatol Int 2006;27:97–100. [DOI] [PubMed] [Google Scholar]
- 88.Bodar EJ, Kuijk LM, Drenth JP, van der Meer JW, Simon A, Frenkel J. On-demand anakinra treatment is effective in mevalonate kinase deficiency. Ann Rheum Dis 2011;70: 2155–8. [DOI] [PubMed] [Google Scholar]
- 89.Bodar EJ, van der Hilst JC, Drenth JP, van der Meer JW, Simon A. Effect of etanercept and anakinra on inflammatory attacks in the hyper-IgD syndrome: introducing a vaccination provocation model. Neth J Med 2005;63:260–4. [PubMed] [Google Scholar]
- 90.Shohat M, Halpern GJ. Familial Mediterranean fever—a review. Genet Med 2011;13:487–98. [DOI] [PubMed] [Google Scholar]
- 91.Cabral M, Conde M, Brito MJ, Almeida H, Melo Gomes JA. Protracted febrile myalgia syndrome with Henoch-Schönlein purpura: an atypical presentation of familial Mediterranean fever [in Portuguese]. Acta Reumatol Port 2011;36:69–74. [PubMed] [Google Scholar]
- 92.Aydin F, Ozcelik C, Akpolat I, Turanli AY, Akpolat T. Erysipelas-like erythema with familial Mediterranean fever. J Dermatol 2011;38:513–5. [DOI] [PubMed] [Google Scholar]
- 93.Azizi E, Fisher BK. Cutaneous manifestations of familial Mediterranean fever. Arch Dermatol 1976;112:364–6. [PubMed] [Google Scholar]
- 94.Satta R, Obici L, Merlini G, Cottoni F. Late-onset familial Mediterranean fever: an atypical presentation of dermatologic interest. Arch Dermatol 2007;143:1080–1. [DOI] [PubMed] [Google Scholar]
- 95.Balbir-Gurman A, Nahir AM, Braun-Moscovici Y. Vasculitis in siblings with familial Mediterranean fever: a report of three cases and review of the literature. Clin Rheumatol 2007;26: 1183–5. [DOI] [PubMed] [Google Scholar]
- 96.Lange-Sperandio B, Mohring K, Gutzler F, Mehls O. Variable expression of vasculitis in siblings with familial Mediterranean fever. Pediatr Nephrol 2004;19:539–43. [DOI] [PubMed] [Google Scholar]
- 97.ten Oever J, de Munck DR. Recurrent pleurisy as sole manifestation of familial Mediterranean fever [in Dutch]. Ned Tijdschr Geneeskd 2008;152:887–90. [PubMed] [Google Scholar]
- 98.Lega JC, Khouatra C, Cottin V, Cordier JF. Isolated recurrent pleuritis revealing familial Mediterranean fever in adulthood. Respiration 2010;79:508–10. [DOI] [PubMed] [Google Scholar]
- 99.Okutur K, Seber S, Oztekin E, Bes C, Borlu F. Recurrent pericarditis as the initial manifestation of familial Mediterranean fever. Med Sci Monit 2008;14:CS139–41. [PubMed] [Google Scholar]
- 100.Senel K, Melikoglu MA, Baykal T, Melikoglu M, Erdal A, Ugur M. Protracted febrile myalgia syndrome in familial Mediterranean fever. Mod Rheumatol 2010;20:410–2. [DOI] [PubMed] [Google Scholar]
- 101.Shohat M, Magal N, Shohat T, Chen X, Dagan T, Mimouni A, et al. Phenotype-genotype correlation in familial Mediterranean fever: evidence for an association between Met694Val and amyloidosis. Eur J Hum Genet 1999;7: 287–92. [DOI] [PubMed] [Google Scholar]
- 102.Gershoni-Baruch R, Brik R, Shinawi M, Livneh A. The differential contribution of MEFV mutant alleles to the clinical profile of familial Mediterranean fever. Eur J Hum Genet 2002;10:145–9. [DOI] [PubMed] [Google Scholar]
- 103.Solak M, Yildiz H, Koken R, Erdogan M, Eser B, Sen T, et al. Analysis of familial Mediterranean fever gene mutations in 202 patients with familial Mediterranean fever. Genet Test 2008;12:341–4. [DOI] [PubMed] [Google Scholar]
- 104.Pras M Familial Mediterranean fever: from the clinical syndrome to the cloning of the pyrin gene. Scand J Rheumatol 1998;27:92–7. [DOI] [PubMed] [Google Scholar]
- 105.Touitou I, Sarkisian T, Medlej-Hashim M, Tunca M, Livneh A, Cattan D, et al. Country as the primary risk factor for renal amyloidosis in familial Mediterranean fever. Arthritis Rheum 2007;56:1706–12. [DOI] [PubMed] [Google Scholar]
- 106.Lidar M, Kedem R, Langevitz P, Pras M, Livneh A. Intravenous colchicine for treatment of patients with familial Mediterranean fever unresponsive to oral colchicine. J Rheumatol 2003;30:2620–3. [PubMed] [Google Scholar]
- 107.Gattringer R, Lagler H, Gattringer KB, Knapp S, Burgmann H, Winkler S, et al. Anakinra in two adolescent female patients suffering from colchicine-resistant familial Mediterranean fever: effective but risky. Eur J Clin Invest 2007;37:912–4. [DOI] [PubMed] [Google Scholar]
- 108.Belkhir R, Moulonguet-Doleris L, Hachulla E, Prinseau J, Baglin A, Hanslik T. Treatment of familial Mediterranean fever with anakinra. Ann Intern Med 2007;146:825–6. [DOI] [PubMed] [Google Scholar]
- 109.Kuijk LM, Govers AM, Frenkel J, Hofhuis WJ. Effective treatment of a colchicine-resistant familial Mediterranean fever patient with anakinra. Ann Rheum Dis 2007;66:1545–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Meinzer U, Quartier P, Alexandra JF, Hentgen V, Retornaz F, Kone-Paut I. Interleukin-1 targeting drugs in familial Mediterranean fever: a case series and a review of the literature. Semin Arthritis Rheum 2011;41:265–71. [DOI] [PubMed] [Google Scholar]
- 111.Moser C, Pohl G, Haslinger I, Knapp S, Rowczenio D, Russel T, et al. Successful treatment of familial Mediterranean fever with anakinra and outcome after renal transplantation. Nephrol Dial Transplant 2009;24:676–8. [DOI] [PubMed] [Google Scholar]
- 112.Bakkaloglu SA, Aksu T, Goker B, Unlusoy A, Peru H, Fidan K, et al. Sulfasalazine treatment in protracted familial Mediterranean fever arthritis. Eur J Pediatr 2009;168:1017–9. [DOI] [PubMed] [Google Scholar]
- 113.Seyahi E, Ozdogan H, Masatlioglu S, Yazici H. Successful treatment of familial Mediterranean fever attacks with thalidomide in a colchicine resistant patient. Clin Exp Rheumatol 2002;20:S43–4. [PubMed] [Google Scholar]
- 114.Seyahi E, Ozdogan H, Celik S, Ugurlu S, Yazici H. Treatment options in colchicine resistant familial Mediterranean fever patients: thalidomide and etanercept as adjunctive agents. Clin Exp Rheumatol 2006;24:S99–103. [PubMed] [Google Scholar]
- 115.Sakallioglu O, Duzova A, Ozen S. Etanercept in the treatment of arthritis in a patient with familial Mediterranean fever. Clin Exp Rheumatol 2006;24:435–7. [PubMed] [Google Scholar]
- 116.Mor A, Pillinger MH, Kishimoto M, Abeles AM, Livneh A. Familial Mediterranean fever successfully treated with etanercept. J Clin Rheumatol 2007;13:38–40. [DOI] [PubMed] [Google Scholar]
- 117.Aksentijevich I, Masters SL, Ferguson PJ, Dancey P, Frenkel J, van Royen-Kerkhoff A, et al. An autoinflammatory disease with deficiency of the interleukin-1-receptor antagonist. N Engl J Med 2009;360:2426–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Torrelo A, Patel S, Colmenero I, Gurbindo D, Lendinez F, Hernandez A, et al. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature (CANDLE) syndrome. J Am Acad Dermatol 2010;62:489–95. [DOI] [PubMed] [Google Scholar]
- 119.Ramot Y, Czarnowicki T, Maly A, Navon-Elkan P, Zlotogorski A. Chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature syndrome: a case report. Pediatr Dermatol 2011;28:538–41. [DOI] [PubMed] [Google Scholar]
- 120.Liu Y, Ramot Y, Torrelo A, Paller AS, Si N, Babay S, et al. Mutations in proteasome subunit beta type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum 2012;64:895–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Brown TT. Approach to the human immunodeficiency virus-infected patient with lipodystrophy. J Clin Endocrinol Metab 2008;93:2937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Mallewa JE, Wilkins E, Vilar J, Mallewa M, Doran D, Back D, et al. HIV-associated lipodystrophy: a review of underlying mechanisms and therapeutic options. J Antimicrob Chemother 2008;62:648–60. [DOI] [PubMed] [Google Scholar]
- 123.Kahn MF, Khan MA. The SAPHO syndrome. Baillieres Clin Rheumatol 1994;8:333–62. [DOI] [PubMed] [Google Scholar]
- 124.Matzaroglou C, Velissaris D, Karageorgos A, Marangos M, Panagiotopoulos E, Karanikolas M. SAPHO syndrome diagnosis and treatment: report of five cases and review of the literature. Open Orthop J 2009;3:100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jurik AG, Graudal H. Monarthritis of the manubriosternal joint: a follow-up study. Rheumatol Int 1987;7:235–41. [DOI] [PubMed] [Google Scholar]
- 126.Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO): a new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 1988;6:109–12. [PubMed] [Google Scholar]
- 127.Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Acne-pustulosis-hyperostosis-osteitis syndrome: results of a national survey, 85 cases [in French]. Rev Rhum Mal Osteoartic 1987;54:187–96. [PubMed] [Google Scholar]
- 128.Sonozaki H, Mitsui H, Miyanaga Y, Okitsu K, Igarashi M, Hayashi Y, et al. Clinical features of 53 cases with pustulotic arthro-osteitis. Ann Rheum Dis 1981;40:547–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ehrenfeld M, Samra Y, Kaplinsky N. Acne conglobata and arthritis: report of a case and review of the literature. Clin Rheumatol 1986;5:407–9. [DOI] [PubMed] [Google Scholar]
- 130.Kahn MF, Bouvier M, Palazzo E, Tebib JG, Colson F. Sternoclavicular pustulotic osteitis (SAPHO): 20-year interval between skin and bone lesions. J Rheumatol 1991;18:1104–8. [PubMed] [Google Scholar]
- 131.Govoni M, Colina M, Massara A, Trotta F. SAPHO syndrome and infections. Autoimmun Rev 2009;8:256–9. [DOI] [PubMed] [Google Scholar]
- 132.Colina M, Pizzirani C, Khodeir M, Falzoni S, Bruschi M, Trotta F, et al. Dysregulation of P2X7 receptor-inflammasome axis in SAPHO syndrome: successful treatment with anakinra. Rheumatology (Oxford) 2010;49:1416–8. [DOI] [PubMed] [Google Scholar]
- 133.Wendling D, Prati C, Aubin F. Anakinra treatment of SAPHO syndrome: short-term results of an open study. Ann Rheum Dis 2012;71:1098–100. [DOI] [PubMed] [Google Scholar]
- 134.Ben Abdelghani K, Dran DG, Gottenberg JE, Morel J, Sibilia J, Combe B. Tumor necrosis factor-alpha blockers in SAPHO syndrome. J Rheumatol 2010;37:1699–704. [DOI] [PubMed] [Google Scholar]
- 135.Bjorksten B, Gustavson KH, Eriksson B, Lindholm A, Nordstrom S. Chronic recurrent multifocal osteomyelitis and pustulosis palmoplantaris. J Pediatr 1978;93:227–31. [DOI] [PubMed] [Google Scholar]
- 136.Patterson AC, Bentley-Corbett K. Pustulotic arthroosteitis. J Rheumatol 1985;12:611–4. [PubMed] [Google Scholar]
- 137.Berbis P, Deharo C, Privat Y. Osteite rhumatismale aseptique associee la pustulose palmoplantaire. Interet de la colchicine. Rev Rhum Mal Osteoartic 1988;17:1410–1. [PubMed] [Google Scholar]
- 138.Edlund E, Johnsson U, Lidgren L, Pettersson H, Sturfelt G, Svensson B, et al. Palmoplantar pustulosis and sternocostoclavicular arthro-osteitis. Ann Rheum Dis 1988;47:809–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Siegel D, Strosberg JM, Wiese F, Chen J. Acne fulminans with a lytic bone lesion responsive to dapsone. J Rheumatol 1982; 9:344–6. [PubMed] [Google Scholar]
- 140.Andersson R Effective treatment with interferon-alpha in chronic recurrent multifocal osteomyelitis. J Interferon Cytokine Res 1995;15:837–8. [DOI] [PubMed] [Google Scholar]
- 141.Tsubota H, Kataura A, Kukuminato Y, Hamamoto M, Ohguro S, Shido F, et al. Efficacy of tonsillectomy for improving skin lesions of pustulosis palmaris et plantaris—evaluation of 289 cases at the Department of Otolaryngology of Sapporo Medical University [in Japanese]. Nihon Jibiinkoka Gakkai Kaiho 1994;97:1621–30. [DOI] [PubMed] [Google Scholar]
- 142.Nakamura T, Oishi M, Johno M, Ono T, Honda M. Serum levels of interleukin 6 in patients with pustulosis palmaris et plantaris. J Dermatol 1993;20:763–6. [DOI] [PubMed] [Google Scholar]
- 143.Tinazzi E, Puccetti A, Patuzzo G, Sorleto M, Barbieri A, Lunardi C. Schnitzler syndrome, an autoimmune-autoinflammatory syndrome: report of two new cases and review of the literature. Autoimmun Rev 2011;10:404–9. [DOI] [PubMed] [Google Scholar]
- 144.de Koning HD, Bodar EJ, van der Meer JW, Simon A. Schnitzler syndrome: beyond the case reports; review and follow-up of 94 patients with an emphasis on prognosis and treatment. Semin Arthritis Rheum 2007; 37:137–48. [DOI] [PubMed] [Google Scholar]
- 145.Janier M, Bonvalet D, Blanc MF, Lemarchand F, Cavelier B, Ribrioux A, et al. Chronic urticaria and macroglobulinemia (Schnitzler’s syndrome): report of two cases. J Am Acad Dermatol 1989;20:206–11. [DOI] [PubMed] [Google Scholar]
- 146.Berdy SS, Bloch KJ. Schnitzler’s syndrome: a broader clinical spectrum. J Allergy Clin Immunol 1991;87:849–54. [DOI] [PubMed] [Google Scholar]
- 147.Famularo G, Barracchini A, Minisola G. Severe thrombophilia with antiphospholipid syndrome and hyperhomocysteinemia in a patient with Schnitzler’s syndrome. Clin Exp Rheumatol 2003;21:366–8. [PubMed] [Google Scholar]
- 148.Westhoff TH, Zidek W, Uharek L, Steinhoff-Georgieva J, van der Giet M. Impairment of renal function in Schnitzler’s syndrome. J Nephrol 2006;19:660–3. [PubMed] [Google Scholar]
- 149.Lim W, Shumak KH, Reis M, Perez-Ordonez B, Sauder D, Fam A, et al. Malignant evolution of Schnitzler’s syndrome—chronic urticaria and IgM monoclonal gammopathy: report of a new case and review of the literature. Leuk Lymphoma 2002;43:181–6. [DOI] [PubMed] [Google Scholar]
- 150.Cream JJ, Porter D. Urticaria in Waldenstrom’s macroglobulinemia. J R Soc Med 1979;72:858–9. [PMC free article] [PubMed] [Google Scholar]
- 151.Pujol RM, Barnadas MA, Brunet S, de Moragas JM. Urticarial dermatosis associated with Waldenstrom’s macroglobulinemia. J Am Acad Dermatol 1989;20:855–7. [DOI] [PubMed] [Google Scholar]
- 152.Machet L, Vaillant L, Machet MC, Reisenleiter M, Goupille P, Lorette G. Schnitzler’s syndrome (urticaria and macroglobulinemia): evolution to Waldenstrom’s disease is not uncommon. Acta Derm Venereol 1996;76:413. [DOI] [PubMed] [Google Scholar]
- 153.Welsh B, Tate B. Schnitzler’s syndrome: report of a case with progression to Waldenstrom’s macroglobulinemia. Australas J Dermatol 1999;40:201–3. [DOI] [PubMed] [Google Scholar]
- 154.Migliorini P, Del Corso I, Tommasi C, Boraschi D. Free circulating interleukin-18 is increased in Schnitzler syndrome: a new autoinflammatory disease? Eur Cytokine Netw 2009; 20:108–11. [DOI] [PubMed] [Google Scholar]
- 155.Asahina A, Sakurai N, Suzuki Y, Narushima K. Schnitzler’s syndrome with prominent neutrophil infiltration misdiagnosed as Sweet’s syndrome: a typical example of urticarial neutrophilic dermatosis. Clin Exp Dermatol 2010;35: e123–6. [DOI] [PubMed] [Google Scholar]
- 156.Pizzirani C, Falzoni S, Govoni M, La Corte R, Donadei S, Di Virgilio F, et al. Dysfunctional inflammasome in Schnitzler’s syndrome. Rheumatology (Oxford) 2009;48:1304–8. [DOI] [PubMed] [Google Scholar]
- 157.Cascavilla N, Bisceglia M, D’Arena G. Successful treatment of Schnitzler’s syndrome with anakinra after failure of rituximab trial. Int J Immunopathol Pharmacol 2010;23:633–6. [DOI] [PubMed] [Google Scholar]
- 158.Besada E, Nossent H. Dramatic response to IL1-RA treatment in longstanding multidrug resistant Schnitzler’s syndrome: a case report and literature review. Clin Rheumatol 2010;29: 567–71. [DOI] [PubMed] [Google Scholar]
- 159.Kluger N, Riviere S, Guillot B, Bessis D. Efficacy of interleukin 1 receptor antagonist (anakinra) on a refracttory case of Schnitzler’s syndrome. Acta Derm Venereol 2008;88:287–8. [DOI] [PubMed] [Google Scholar]
- 160.Klemmer N, Lenain P, Balguerie X, Le Loet X. Effectiveness of anti-IL1 in Schnitzler’s syndrome. Joint Bone Spine 2007;74: 509–10. [DOI] [PubMed] [Google Scholar]
- 161.Cianchini G, Colonna L, Bergamo F, Angelo C, Puddu P. Efficacy of psoralen-UV-A therapy in 3 cases of Schnitzler syndrome. Arch Dermatol 2001;137:1536–7. [PubMed] [Google Scholar]
- 162.Krause K, Feist E, Fiene M, Kallinich T, Maurer M. Complete remission in 3 of 3 anti-IL-6-treated patients with Schnitzler syndrome. J Allergy Clin Immunol 2012;129:848–50. [DOI] [PubMed] [Google Scholar]
- 163.Martinez-Taboada VM, Fontalba A, Blanco R, Fernandez-Luna JL. Successful treatment of refractory Schnitzler syndrome with anakinra: comment on the article by Hawkins et al. Arthritis Rheum 2005;52:2226–7. [DOI] [PubMed] [Google Scholar]
- 164.Aikawa NE, Silva CA, Bonfa E, Carvalho JF. Schnitzler’s syndrome improvement after anti-TNF-alpha therapy. Joint Bone Spine 2010;77:491. [DOI] [PubMed] [Google Scholar]
- 165.Lipsker D, Veran Y, Grunenberger F, Cribier B, Heid E, Grosshans E. The Schnitzler syndrome: four new cases and review of the literature. Medicine (Baltimore) 2001;80:37–44. [DOI] [PubMed] [Google Scholar]
- 166.Germain P, Fach J, Bui N, Traissac T, Delbrel X, Le Bras M, et al. Schnitzler syndrome: a rare cause of systemic urticaria [in French]. Rev Med Interne 2000;21:285–9. [DOI] [PubMed] [Google Scholar]
- 167.Pascual-Lopez M, Hernandez-Nunez A, Sanchez-Perez J, Fernandez-Herrera J, Garcia-Diez A. Schnitzler’s syndrome with monoclonal IgG kappa gammopathy: good response to cyclosporin. J Eur Acad Dermatol Venereol 2002;16:267–70. [DOI] [PubMed] [Google Scholar]
- 168.Worm M, Kolde G. Schnitzler’s syndrome: successful treatment of two patients using thalidomide. Br J Dermatol 2003; 148:601–2. [DOI] [PubMed] [Google Scholar]
- 169.de Koning HD, Bodar EJ, Simon A, van der Hilst JC, Netea MG, van der Meer JW. Beneficial response to anakinra and thalidomide in Schnitzler’s syndrome. Ann Rheum Dis 2006; 65:542–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Schartz NE, Buder S, Sperl H, Audring H, Paus R, Tebbe B, et al. Report of a case of Schnitzler’s syndrome treated successfully with interferon alpha 2b. Dermatology 2002;205: 54–6. [DOI] [PubMed] [Google Scholar]
- 171.Kuenzli S, Buchet S, Saurat JH. Successful treatment of Schnitzler’s syndrome with interferon alfa-2b. Dermatology 2002;205:74. [DOI] [PubMed] [Google Scholar]
- 172.Yokota S, Miyamae T, Imagawa T, Iwata N, Katakura S, Mori M. Inflammatory cytokines and systemic-onset juvenile idiopathic arthritis. Mod Rheumatol 2004;14:12–7. [DOI] [PubMed] [Google Scholar]
- 173.Frosch M, Roth J. New insights in systemic juvenile idiopathic arthritis—from pathophysiology to treatment. Rheumatology (Oxford) 2008;47:121–5. [DOI] [PubMed] [Google Scholar]
- 174.Pay S, Turkcapar N, Kalyoncu M, Simsek I, Beyan E, Ertenli I, et al. A multicenter study of patients with adult-onset Still’s disease compared with systemic juvenile idiopathic arthritis. Clin Rheumatol 2006;25:639–44. [DOI] [PubMed] [Google Scholar]
- 175.Ghosh JB, Gupta D, Chattopadhyay N. Systemic onset juvenile idiopathic arthritis—its unusual presentation. Indian J Pediatr 2008;75:400–2. [DOI] [PubMed] [Google Scholar]
- 176.Muskardin TW, Binstadt BA. Malar rash in systemic juvenile idiopathic arthritis. J Rheumatol 2010;37:2187. [DOI] [PubMed] [Google Scholar]
- 177.Frosch M, Metze D, Foell D, Vogl T, Sorg C, Sunderkotter C, et al. Early activation of cutaneous vessels and epithelial cells is characteristic of acute systemic onset juvenile idiopathic arthritis. Exp Dermatol 2005;14:259–65. [DOI] [PubMed] [Google Scholar]
- 178.Lequerre T, Quartier P, Rosellini D, Alaoui F, De Bandt M, Mejjad O, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis 2008;67:302–8. [DOI] [PubMed] [Google Scholar]
- 179.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med 2005;201:1479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Quartier P, Allantaz F, Cimaz R, Pillet P, Messiaen C, Bardin C, et al. A multicenter, randomized, double-blind, placebo-controlled trial with the interleukin-1 receptor antagonist anakinra in patients with systemic-onset juvenile idiopathic arthritis (ANAJIS trial). Ann Rheum Dis 2011;70:747–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Weidinger S, Klopp N, Rummler L, Wagenpfeil S, Novak N, Baurecht HJ, et al. Association of NOD1 polymorphisms with atopic eczema and related phenotypes. J Allergy Clin Immunol 2005;116:177–84. [DOI] [PubMed] [Google Scholar]
- 182.Kabesch M, Peters W, Carr D, Leupold W, Weiland SK, von Mutius E. Association between polymorphisms in caspase recruitment domain containing protein 15 and allergy in two German populations. J Allergy Clin Immunol 2003;111: 813–7. [DOI] [PubMed] [Google Scholar]
- 183.Macaluso F, Nothnagel M, Parwez Q, Petrasch-Parwez E, Bechara FG, Epplen JT, et al. Polymorphisms in NACHT-LRR (NLR) genes in atopic dermatitis. Exp Dermatol 2007;16: 692–8. [DOI] [PubMed] [Google Scholar]
- 184.Pontillo A, Brandao L, Guimaraes R, Segat L, Araujo J, Crovella S. Two SNPs in NLRP3 gene are involved in the predisposition to type-1 diabetes and celiac disease in a pediatric population from northeast Brazil. Autoimmunity 2010;43:583–9. [DOI] [PubMed] [Google Scholar]
- 185.Dai X, Sayama K, Tohyama M, Shirakata Y, Hanakawa Y, Tokumaru S, et al. Mite allergen is a danger signal for the skin via activation of inflammasome in keratinocytes. J Allergy Clin Immunol 2011;127:806–14.e1-4. [DOI] [PubMed] [Google Scholar]
- 186.Watanabe H, Gaide O, Petrilli V, Martinon F, Contassot E, Roques S, et al. Activation of the IL-1beta-processing inflammasome is involved in contact hypersensitivity. J Invest Dermatol 2007;127:1956–63. [DOI] [PubMed] [Google Scholar]
- 187.Barker CL, McHale MT, Gillies AK, Waller J, Pearce DM, Osborne J, et al. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol 2004;123: 892–901. [DOI] [PubMed] [Google Scholar]
- 188.de Koning HD, Rodijk-Olthuis D, van Vlijmen-Willems IM, Joosten LA, Netea MG, Schalkwijk J, et al. A comprehensive analysis of pattern recognition receptors in normal and inflamed human epidermis: up-regulation of dectin-1 in psoriasis. J Invest Dermatol 2010;130:2611–20. [DOI] [PubMed] [Google Scholar]
- 189.Garcia-Rodriguez S, Arias-Santiago S, Perandres-Lopez R, Castellote L, Zumaquero E, Navarro P, et al. Increased gene expression of Toll-like receptor 4 on peripheral blood mononuclear cells in patients with psoriasis. J Eur Acad Dermatol Venereol doi: 10.1111/j.1468-3083.2011.04372.x. Published online December 6, 2011. [DOI] [PubMed] [Google Scholar]
- 190.Hammerberg C, Bata-Csorgo Z, Voorhees JJ, Cooper KD. IL-1 and IL-1 receptor antagonist regulation during keratinocyte cell cycle and differentiation in normal and psoriatic epidermis. Arch Dermatol Res 1998;290:367–74. [DOI] [PubMed] [Google Scholar]
- 191.Anderson KS, Petersson S, Wong J, Shubbar E, Lokko NN, Carlstrom M, et al. Elevation of serum epidermal growth factor and interleukin 1 receptor antagonist in active psoriasis vulgaris. Br J Dermatol 2010;163:1085–9. [DOI] [PubMed] [Google Scholar]
- 192.Debets R, Hegmans JP, Croughs P, Troost RJ, Prins JB, Benner R, et al. The IL-1 system in psoriatic skin: IL-1 antagonist sphere of influence in lesional psoriatic epidermis. J Immunol 1997;158:2955–63. [PubMed] [Google Scholar]
- 193.Kristensen M, Deleuran B, Eedy DJ, Feldmann M, Breathnach SM, Brennan FM. Distribution of interleukin 1 receptor antagonist protein (IRAP), interleukin 1 receptor, and interleukin 1 alpha in normal and psoriatic skin: decreased expression of IRAP in psoriatic lesional epidermis. Br J Dermatol 1992;127:305–11. [DOI] [PubMed] [Google Scholar]
- 194.Jung N, Hellmann M, Hoheisel R, Lehmann C, Haase I, Perniok A, et al. An open-label pilot study of the efficacy and safety of anakinra in patients with psoriatic arthritis refractory to or intolerant of methotrexate (MTX). Clin Rheumatol 2010;29: 1169–73. [DOI] [PubMed] [Google Scholar]
- 195.Marrakchi S, Guigue P, Renshaw BR, Puel A, Pei XY, Fraitag S, et al. Interleukin-36-receptor antagonist deficiency and generalized pustular psoriasis. N Engl J Med 2011;365:620–8. [DOI] [PubMed] [Google Scholar]
- 196.Viguier M, Guigue P, Pages C, Smahi A, Bachelez H. Successful treatment of generalized pustular psoriasis with the interleukin-1-receptor antagonist anakinra: lack of correlation with IL1RN mutations. Ann Intern Med 2010;153:66–7. [DOI] [PubMed] [Google Scholar]
- 197.Lutz V, Lipsker D. Acitretin- and tumor necrosis factor inhibitor-resistant acrodermatitis continua of Hallopeau responsive to the interleukin 1 receptor antagonist anakinra. Arch Dermatol 2012;148:297–9. [DOI] [PubMed] [Google Scholar]
- 198.Wise CA, Gillum JD, Seidman CE, Lindor NM, Veile R, Bashiardes S, et al. Mutations in CD2BP1 disrupt binding to PTP PEST and are responsible for PAPA syndrome, an autoinflammatory disorder. Hum Mol Genet 2002;11:961–9. [DOI] [PubMed] [Google Scholar]
- 199.Yu JW, Fernandes-Alnemri T, Datta P, Wu J, Juliana C, Solorzano L, et al. Pyrin activates the ASC pyroptosome in response to engagement by autoinflammatory PSTPIP1 mutants. Mol Cell 2007;28:214–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Nesterovitch AB, Hoffman MD, Simon M, Petukhov PA, Tharp MD, Glant TT. Mutations in the PSTPIP1 gene and aberrant splicing variants in patients with pyoderma gangrenosum. Clin Exp Dermatol 2011;36:889–95. [DOI] [PubMed] [Google Scholar]
- 201.Ogura Y, Bonen DK, Inohara N, Nicolae DL, Chen FF, Ramos R, et al. A frameshift mutation in NOD2 associated with susceptibility to Crohn’s disease. Nature 2001;411:603–6. [DOI] [PubMed] [Google Scholar]
- 202.Girardin SE, Boneca IG, Viala J, Chamaillard M, Labigne A, Thomas G, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem 2003;278:8869–72. [DOI] [PubMed] [Google Scholar]
- 203.Hsiao JL, Antaya RJ, Berger T, Maurer T, Shinkai K, Leslie KS. Hidradenitis suppurativa and concomitant pyoderma gangrenosum: a case series and literature review. Arch Dermatol 2010;146:1265–70. [DOI] [PubMed] [Google Scholar]
- 204.Mustafa NM, Lavizzo M. Sweet’s syndrome in a patient with Crohn’s disease: a case report. J Med Case Rep 2008; 2:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kemmett D, Gawkrodger DJ, Wilson G, Hunter JA. Sweet’s syndrome in Crohn’s disease. BMJ 1988;297:1513–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Delluc A, Limal N, Puechal X, Frances C, Piette JC, Cacoub P. Efficacy of anakinra, an IL1 receptor antagonist, in refractory Sweet syndrome. Ann Rheum Dis 2008;67:278–9. [DOI] [PubMed] [Google Scholar]
- 207.Kluger N, Gil-Bistes D, Guillot B, Bessis D. Efficacy of anti-interleukin-1 receptor antagonist anakinra (Kineret®) in a case of refractory Sweet’s syndrome. Dermatology 2011; 222:123–7. [DOI] [PubMed] [Google Scholar]
- 208.Tian LM, Xie HF, Yang T, Hu YH, Li J, Wang WZ. Association study of tumor necrosis factor receptor type 2 M196R and toll-like receptor 2 Arg753Gln polymorphisms with acne vulgaris in a Chinese Han ethnic group. Dermatology 2010; 221:276–84. [DOI] [PubMed] [Google Scholar]
- 209.Szabo K, Tax G, Kis K, Szegedi K, Teodorescu-Brinzeu DG, Dioszegi C, et al. Interleukin-1A +4845(G> T) polymorphism is a factor predisposing to acne vulgaris. Tissue Antigens 2010;76:411–5. [DOI] [PubMed] [Google Scholar]
- 210.Shibata M, Katsuyama M, Onodera T, Ehama R, Hosoi J, Tagami H. Glucocorticoids enhance Toll-like receptor 2 expression in human keratinocytes stimulated with Propionibacterium acnes or proinflammatory cytokines. J Invest Dermatol 2009;129:375–82. [DOI] [PubMed] [Google Scholar]
- 211.van Steensel MA, Badeloe S, Winnepenninckx V, Vreeburg M, Steijlen PM, van Geel M. Granulomatous rosacea and Crohn’s disease in a patient homozygous for the Crohn-associated NOD2/CARD15 polymorphism R702W. Exp Dermatol 2008; 17:1057–8. [DOI] [PubMed] [Google Scholar]
- 212.Moschella SL. Is there a role for infliximab in the current therapy of hidradenitis suppurativa? A report of three treated cases. Int J Dermatol 2007;46:1287–91. [DOI] [PubMed] [Google Scholar]
- 213.Braun-Falco M, Kovnerystyy O, Lohse P, Ruzicka T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)—a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol 2012;66: 409–15. [DOI] [PubMed] [Google Scholar]
- 214.Touitou I, Magne X, Molinari N, Navarro A, Quellec AL, Picco P, et al. MEFV mutations in Behçet’s disease. Hum Mutat 2000;16:271–2. [DOI] [PubMed] [Google Scholar]
- 215.Livneh A, Aksentijevich I, Langevitz P, Torosyan Y, G-Shoham N, Shinar Y, et al. A single mutated MEFV allele in Israeli patients suffering from familial Mediterranean fever and Behçet’s disease (FMF-BD). Eur J Hum Genet 2001;9:191–6. [DOI] [PubMed] [Google Scholar]
- 216.Amoura Z, Dode C, Hue S, Caillat-Zucman S, Bahram S, Delpech M, et al. Association of the R92Q TNFRSF1A mutation and extracranial deep vein thrombosis in patients with Behçet’s disease. Arthritis Rheum 2005;52:608–11. [DOI] [PubMed] [Google Scholar]
- 217.Kone-Paut I, Sanchez E, Le Quellec A, Manna R, Touitou I. Autoinflammatory gene mutations in Behçet’s disease. Ann Rheum Dis 2007;66:832–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Barahmani N, de Andrade M, Slusser J, Zhang Q, Duvic M. Interleukin-1 receptor antagonist allele 2 and familial alopecia areata. J Invest Dermatol 2002;118:335–7. [DOI] [PubMed] [Google Scholar]
- 219.Tazi-Ahnini R, Cox A, McDonagh AJ, Nicklin MJ, di Giovine FS, Timms JM, et al. Genetic analysis of the interleukin-1 receptor antagonist and its homologue IL-1L1 in alopecia areata: strong severity association and possible gene interaction. Eur J Immunogenet 2002;29:25–30. [DOI] [PubMed] [Google Scholar]
- 220.Jin Y, Mailloux CM, Gowan K, Riccardi SL, LaBerge G, Bennett DC, et al. NALP1 in vitiligo-associated multiple autoimmune disease. N Engl J Med 2007;356:1216–25. [DOI] [PubMed] [Google Scholar]
- 221.Simon JA, Burgos-Vargas R. Vitiligo improvement in a patient with ankylosing spondylitis treated with infliximab. Dermatology 2008;216:234–5. [DOI] [PubMed] [Google Scholar]
- 222.Rigopoulos D, Gregoriou S, Larios G, Moustou E, Belayeva-Karatza E, Kalogeromitros D. Etanercept in the treatment of vitiligo. Dermatology 2007;215:84–5. [DOI] [PubMed] [Google Scholar]
- 223.Gabay C, Cakir N, Moral F, Roux-Lombard P, Meyer O, Dayer JM, et al. Circulating levels of tumor necrosis factor soluble receptors in systemic lupus erythematosus are significantly higher than in other rheumatic diseases and correlate with disease activity. J Rheumatol 1997;24:303–8. [PubMed] [Google Scholar]
- 224.Tjernstrom F, Hellmer G, Nived O, Truedsson L, Sturfelt G. Synergetic effect between interleukin-1 receptor antagonist allele (IL1RN*2) and MHC class II (DR17, DQ2) in determining susceptibility to systemic lupus erythematosus. Lupus 1999; 8:103–8. [DOI] [PubMed] [Google Scholar]
- 225.Parks CG, Cooper GS, Dooley MA, Treadwell EL, St Clair EW, Gilkeson GS, et al. Systemic lupus erythematosus and genetic variation in the interleukin 1 gene cluster: a population-based study in the southeastern United States. Ann Rheum Dis 2004;63:91–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tsai LJ, Lan JL, Lin CY, Hsiao SH, Tsai LM, Tsai JJ. The different expression patterns of interleukin-1 receptor antagonist in systemic lupus erythematosus. Tissue Antigens 2006;68: 493–501. [DOI] [PubMed] [Google Scholar]
- 227.Brugos B, Kiss E, Dul C, Gubisch W, Szegedi G, Sipka S, et al. Measurement of interleukin-1 receptor antagonist in patients with systemic lupus erythematosus could predict renal manifestation of the disease. Hum Immunol 2010;71:874–7. [DOI] [PubMed] [Google Scholar]
- 228.Pontillo A, Girardelli M, Kamada AJ, Pancotto JA, Donadi EA, Crovella S, et al. Polymorphisms in inflammasome genes are involved in the predisposition to systemic lupus erythematosus. Autoimmunity 2012;45:271–8. [DOI] [PubMed] [Google Scholar]
- 229.Moosig F, Zeuner R, Renk C, Schroder JO. IL-1RA in refractory systemic lupus erythematosus. Lupus 2004;13:605–6. [DOI] [PubMed] [Google Scholar]