Abstract
Background
Senescence refers to spontaneous and progressive irreversible degenerative changes in which both the physical and psychological power diminish significantly. Hypertension is the most common cardiovascular disease in the elderly. Several studies have been conducted regarding the effect of exercise on reducing the blood pressure of the elderly, which have found contradictory results. One of the uses of meta-analysis study is responding to these assumptions and resolving the discrepancies. Accordingly, the aim of the present study is to determine the impact of exercise on the blood pressure of older adults.
Method
In this research, in order to find electronic published papers from 1992 to 2019, the papers published in both domestic and foreign databases including SID, MagIran, IranMedex, IranDox, Gogole Scholar, Cohrane, Embase, Science Direct, Scopus, PubMed, and Web of Science (ISI) were used. Heterogeneity index between the studies was determined based on Cochran test Q(c) and I2. Considering existence of heterogeneity, random effects model was employed to estimate the standardized subtraction of the mean exercise test score for reduction of blood pressure in the older adults across the intervention group before and after the test.
Results
In this meta-analysis and systematic review, eventually 69 papers met the inclusion criteria. The total number of participants was 2272 in the pre- and postintervention groups when examining the systolic changes and 2252 subjects in the pre- and postintervention groups when inspecting the diastolic changes. The standardized mean difference in examining the systolic changes before the intervention was 137.1 ± 8.09 and 132.98 ± 0.96 after the intervention; when exploring the diastolic changes, the pre- and postintervention values were 80.3 ± 0.85 and 76.0 ± 6.56, respectively, where these differences were statistically significant (P < 0.01).
Conclusion
The results of this study indicated that exercise leads to significant reduction in both systolic and diastolic blood pressure. Accordingly, regular exercise can be part of the treatment plan for hypertensive elderly.
1. Background
Senescence is a natural course of development in which special physical, psychological, and social changes occur [1]. In other words, senescence refers to spontaneous and irreversible progressive degenerative changes in which both psychological and physical power significantly decline [2]. In the elderly, all organs of the body undergo some degree of degeneration in all of their tasks; for this reason, various chronic diseases occur in the older adults including cardiovascular disease such as hypertension, coronary artery disease, and skeletal diseases such as arthritis, osteoporosis, and cancer [3].
Hypertension is the most common cardiovascular disease in the older adults [4], claiming high healthcare costs [4]. Since pharmacotherapy among the older adults necessitates adhering to various issues, today researchers tend to recommend nonpharmacological methods instead of pharmacotherapy considering the pathological mechanism of hypertension. Nonpharmacological methods include modifying the lifestyle through low sodium diet, low fat diet, increasing potassium as well as calcium intake, weight reduction in obese individuals, daily exercise, and reducing anxiety and fear [5]. Regular exercise at a moderate level for three days per week 30 min/day results in increased longevity, reduced mortality, and reduced development of cardiovascular disease, heart attack, hypertension, arthritis, osteoporosis, depression, and different types of cancer [6]. Regular aerobic exercise leads to reduction of both systolic and diastolic blood pressure by 11 and 8 mmHg. A regular physical activity program should start gradually and sustain for 30–45 min in most days of the week. This level of activity can control hypertension without pharmacotherapy [7].
The impact of aerobic exercises on hypertension has mostly been tested in long-term exercise programs (at least three months) with high intensity and high number of sessions per week (5 days/week). Increase in the number of exercise sessions per week and high intensity of exercise in individuals who are not able to do high intensity activities may be an obstacle to participating in such exercise programs [8]. There are different and sometimes contradictory responses to the numerous questions about the effect of different exercises and their varying intensities on the elderly's blood pressure. Various research studies have reported different results about the impact of exercise on blood pressure considering the type of exercise, its conditions, duration, and frequency within a specific period, and its relationship with blood pressure reduction [9].
In the research by Moraes et al., after three days of aerobic exercise per week for three months in the intervention group, the mean systolic and diastolic blood pressure diminished by 3.2 and 1.2 mmHg, respectively, but no significant change was observed in the mean blood pressure of the control group [10]. In the research by Ferrier et al., the arterial compliance showed resistance against a short aerobic exercise program, and no reduction was found in the blood pressure of patients [11]. The study by Tabara et al. with the aim of comparing aerobic short-term and long-term exercise programs with mild and moderate intensities on cardiovascular indicators of the older adults indicated that the short-term program had no impact on reducing systolic blood pressure, but it decreased the diastolic blood pressure. Long-term program resulted in diminished mean systolic and diastolic blood pressure from 136 to 129 and from 87 to 83, respectively. Also, both the mild and moderate intensity programs were influential for blood pressure reduction [12]. In the research by Westhoff et al., the impact of moderate intensity long-term exercise program was tested on patients with hypertension. The results showed blood pressure decline in the samples, though it was not statistically significant [13].
With regard to the impact of exercise on the blood pressure of the older adults with hypertension, some preliminary studies have been conducted across Asia, Europe, and America, which have found contradictory results. One of the uses of meta-analysis study is to address these assumptions and resolve the contradictions. Although Herrod et al. [14] conducted a meta-analysis study investigating the impact of exercise and other nonpharmacological measures on the blood pressure of the elderly, and this study has not tested the influence of exercise on the blood pressure of the older adults across different continents. Thus, the aim of this study is to determine the impact of exercise on the blood pressure of the older adults with hypertension across meta-analysis.
2. Methods
2.1. The Methods for Searching Papers
In this investigation, the search was performed on Persian databases including SID, MagIran, IranMedex, and IranDoc along with the international databases of Google Scholar, Cochrane, Embase, Science Direct, Scopus, PubMed, and Web of Science (ISI) with the aim of finding relevant papers without any time constraint (from 1992 to 2019). The list of the references utilized in all papers and the relevant reports found in the previously mentioned electronic search was assessed manually so that other possible references could also be found. The keywords used for searching the references were chosen from The Medical Subject Headings (MeSH) thesaurus. The keywords searched were exercise, resistance training, circuit-based exercise, plyometric exercise, exercise therapy, exercise training, physical activity, and hypertension (both in English and Persian).
((((((((((((Exercise[Title/Abstract]) OR Physical Activity[Title/Abstract]) OR Exercise Training[Title/Abstract]) AND Resistance Training[Title/Abstract]) OR Strength Training[Title/Abstract]) OR Weight-Bearing Exercise Program[Title/Abstract]) AND Circuit-Based Exercise[Title/Abstract]) OR Circuit Training[Title/Abstract]) AND Plyometric Exercise[Title/Abstract]) OR Plyometric Drill[Title/Abstract]) OR Plyometric Training[Title/Abstract]) OR Stretch-Shortening Cycle Exercise [Title/Abstract])))))))))))
2.2. The Criteria of Selection of Papers
The papers with the following characteristics were chosen for the meta-analysis: (1) original research papers, (2) clinical trials studies, and (3) availability of full text of papers. For the objectives of this investigation, physical exercise is any bodily activity that enhances or maintains physical fitness and overall health and wellness. It is performed for various reasons including strengthening muscles and the cardiovascular system, honing athletic skills, and weight loss or maintenance, as well as for the purpose of enjoyment [15]. The older adults were defined as individuals above 60 years of age, while hypertensive patients was defined as the patients with a medical diagnosis of hypertension for more than six months (it includes patients with a definite diagnosis of hypertension and does not include patients with prehypertension).
2.3. Exclusion Criteria
The selected studies were investigated more accurately. Those conducted as review or those whose sample had not been chosen from the older adults with hypertension or the studies repeated with previous data were removed from the meta-analysis. Eventually, 76 studies entered the third stage, qualitative assessment. Each article was separately reviewed by two reviewers. If the article was rejected by them, they expressed the reason, and if there was any controversy between the reviewers, the article was reviewed by a third referee whose opinion was considered as the final decision. Duplicate publication and multiple publications from the same population were removed using citation management software EndNote (version X7, for Windows, Thomson Reuters).
2.4. Qualitative Assessment of the Studies
The quality of the papers was evaluated based on the selected and relevant items of CONSORT checklist, which could be assessed in this study and already mentioned in previous studies (design of study, background and review of literature, place and time of the study, consequence, inclusion criteria, sample size, and statistical analysis). The papers mentioning six to seven criteria were considered of high quality, while those citing two or less of the seven mentioned items were considered as moderate and low quality papers in terms of their methodology [16]. In the present study, 69 papers were included in this systematic review and meta-analysis as being of high and moderate quality, while seven papers which were of low quality were excluded.
2.5. Data Extraction
All of the final papers introduced into the meta-analysis process were prepared for extraction by a premade checklist. The checklist included title of paper, name of the first author, year of publication, place of study, sample size of the intervention group, mean systolic and diastolic blood pressure before the intervention, mean systolic and diastolic blood pressure after the intervention, and standard deviation of systolic and diastolic blood pressure both before and after the intervention.
2.6. Statistical Analysis
Since the studied index was the impact of exercise on the blood pressure of the elderly, in order to combine the results of different studies, frequency and percentage were used along with standardized mean difference index in every study. In order to investigate homogeneity across studies, I2 index was used; considering the heterogeneity in the studies, random effects model was used to combine the studies and conduct the meta-analysis. Note that I2 < 25%, 25–75%, and greater than 75% represent low, medium [16], and high heterogeneity, respectively. P < 0.05 was considered as statistically significant. Also, to investigate publication bias, funnel plot and Egger test were used.
3. Results
In this study, all studies conducted over the impact of exercise on the blood pressure of older adults were examined systematically without any time constraint and based on the PRISMA instructions. In the preliminary search, 1386 papers were identified; eventually, 69 studies published from 1992 to March 2019 were included in the final analysis (Figure 1).
Figure 1.
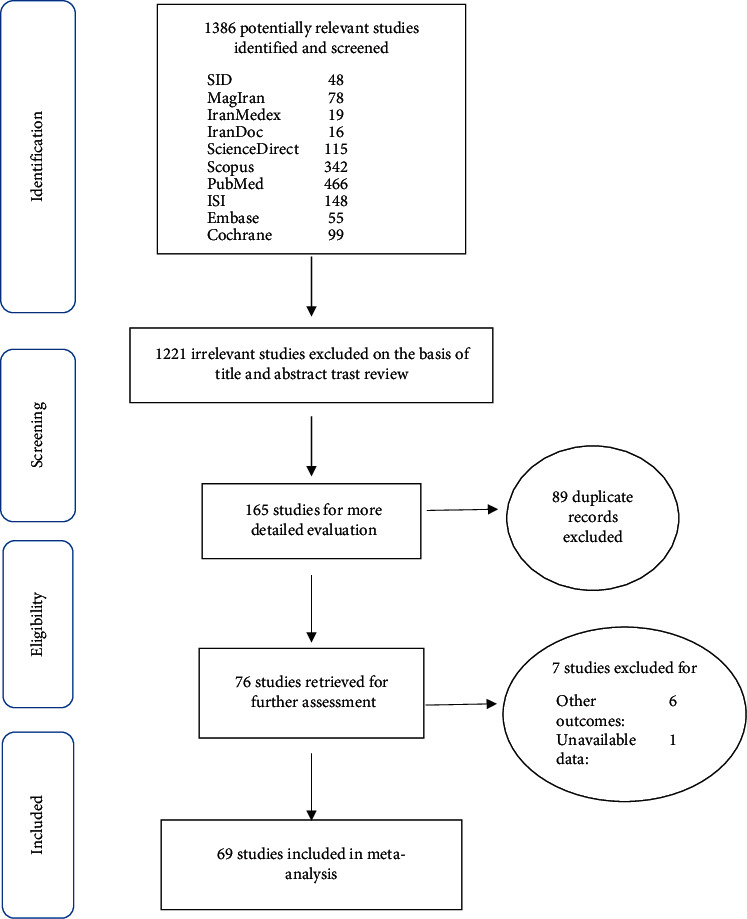
Flow diagram of study selection.
The total number of participants was 2272 in the pre- and postintervention groups for investigating systolic changes, and 2252, for investigating diastolic changes. The characteristics of the studies included in this systematic review are shown in Table 1.
Table 1.
Specifications of studies entered into the meta-analysis.
| Author, year, and reference | Place of study | Sample size | Mean ± SD of before SBP | Mean ± SD of after SBP | Mean ± SD of before DBP | Mean ± SD of after DBP | Quality |
|---|---|---|---|---|---|---|---|
| Haidari, 2014, [17] | Iran | 46 | 149.8 ± 4.63 | 144.9 ± 5.21 | 94.03 ± 3.66 | 85.9 ± 6.38 | High |
| Amooali, 2015, [18] | Iran | 20 | 132.8 ± 13.66 | 121.9 ± 9.17 | 77.6 ± 7.89 | 74.9 ± 3.66 | High |
| Hosseiny, 2007, [8] | Iran | 36 | 150.04 ± 12 | 149.5 ± 11 | 88.6 ± 6 | 84.6 ± 5 | High |
| Tabara, 2007, [12] | Japan | 40 | 136 ± 19 | 129 ± 17 | 75 ± 11 | 70 ± 10 | High |
| Noroalahi, 2019, [19] | Iran | 18 | 140.33 ± 18.1 | 125.33 ± 15.1 | 83.94 ± 13.26 | 75.94 ± 11.33 | High |
| Faramarzi, 2012, [20] | Iran | 20 | 131.13 ± 18.5 | 123.63 ± 11.1 | 86.48 ± 3.5 | 81.61 ± 1.8 | High |
| Behjati Ardakani, 2018, [21] | Iran | 24 | 131.1 ± 18 | 123.5 ± 11.1 | 86.4 ± 3.45 | 81.6 ± 1.8 | High |
| Ghasemian, 2013, [22] | Iran | 20 | 131.3 ± 10.5 | 121.63 ± 11 | 86.88 ± 3.5 | 80.1 ± 0.8 | High |
| Kawasaki, 2011, [23] | Japan | 35 | 136.6 ± 3.2 | 127 ± 2.7 | 81 ± 1.6 | 77.5 ± 1.3 | Medium |
| Yin, 1998, [24] | Japan | 25 | 137.0 ± 10 | 135 ± 14 | 80 ± 7 | 78 ± 8 | Medium |
| Wong, 2019, [25] | Korea | 52 | 146 ± 8.1 | 135 ± 1 | 88 ± 1 | 79 ± 1 | High |
| Ruangthai, 2019, [26] | Thailand | 13 | 141 ± 15.9 | 128.3 ± 15.4 | 84.1 ± 10 | 76.6 ± 7.5 | High |
| Hamdorf, 1999, [27] | Australia | — | 144.6 ± 4.9 | 139.8 ± 4.2 | 72.6 ± 2.2 | 74 ± 1.8 | Medium |
| Lee, 2007, [28] | Taiwan | 102 | 152 ± 10.5 | 136.2 ± 16.7 | 83.5 ± 11.2 | 76.7 ± 12.3 | High |
| Lim, 2015, [29] | Korea | 10 | 129.4 ± 12.7 | 124.5 ± 9.2 | 80.8 ± 6.7 | 78.1 ± 6.7 | High |
| Miura, 2015, [30] | Japan | 45 | 150 ± 9.1 | 145 ± 8.9 | 83.5 ± 5.9 | 80.2 ± 6.2 | High |
| Ohkubo, 2001, [31] | Japan | 121 | 134.2 ± 2.4 | 127.6 ± 2.3 | 79.1 ± 1.3 | 76.9 ± 1.3 | High |
| Okumiya, 1996, [32] | Japan | 21 | 136.4 ± 22.6 | 140.9 ± 22.8 | 78.1 ± 11.8 | 73.6 ± 11.2 | High |
| Patil, 2015, [33] | India | 30 | 146.87 ± 5.72 | 154.83 ± 6.33 | 74.2 ± 4.6 | 75.57 ± 5.68 | High |
| Sunami, 1999, [34] | Japan | 20 | 142 ± 22 | 140 ± 19 | 83 ± 11 | 81 ± 11 | Medium |
| Thomas, 2005, [35] | Hong Kong | 64 | 142 ± 17 | 142 ± 23 | 72 ± 13 | 72 ± 14 | Medium |
| Pitsavos, 2011, [36] | Greece | 52 | 131.5 ± 13.48 | 119.45 ± 6.87 | 83 ± 4.97 | 76.55 ± 4.88 | High |
| Deiseroth, 2019, [37] | Switzerland | 25 | 132 ± 15 | 128 ± 16 | 88 ± 10 | 78 ± 8 | High |
| del Pozo-Cruz, 2012, [38] | España | 21 | 140.55 ± 12.9 | 133.3 ± 20.26 | 86.1 ± 8.16 | 77.65 ± 6.64 | High |
| Di Mauro, 1998, [39] | Italy | 35 | 156.1 ± 15.3 | 154.4 ± 13.6 | 97 ± 7.7 | 94.7 ± 6.5 | Medium |
| Leibovitz, 2005, [40] | Israel | 25 | 131 ± 3 | 130 ± 2 | 73 ± 1 | 74 ± 1 | High |
| Broman, 2006, [41] | Sweden | 15 | 138 ± 9 | 138 ± 14 | 79 ± 8 | 75 ± 7 | High |
| Chomiuk, 2013, [42] | Poland | 44 | 134.61 ± 13.1 | 129.67 ± 11.7 | 75.67 ± 8.46 | 73.22 ± 8.04 | High |
| Dimeo, 2012, [43] | Germany | 24 | 138.4 ± 14.1 | 132.5 ± 10.8 | 78.3 ± 10.2 | 75 ± 9.8 | High |
| Faulkner, 2013, [44] | New Zealand | 36 | 141 ± 16 | 136 ± 16 | 82 ± 9 | 80 ± 9 | Medium |
| Finucane, 2010, [45] | UK | 50 | 138.8 ± 15 | 135.8 ± 12.5 | 76.8 ± 8.6 | 75.2 ± 9.1 | High |
| Kallinen, 2002, [46] | Finland | 12 | 174 ± 15 | 164 ± 16 | 83 ± 3 | 80 ± 2 | Medium |
| Niederseer, 2011, [47] | Austria | 22 | 123.1 ± 11.9 | 122.6 ± 13.4 | 82.4 ± 11.2 | 78.1 ± 9.5 | High |
| Puggaard, 2000, [48] | Denmark | 22 | 153 ± 37 | 161 ± 31 | 77 ± 16 | 84 ± 22 | High |
| Sousa, 2013, [49] | Portugal | 16 | 149.4 ± 25.1 | 148.5 ± 15.1 | 80.4 ± 7.6 | 82.8 ± 9.6 | High |
| Westhoff, 2008, [13] | Germany | 12 | 134 ± 20 | 127 ± 16.4 | 73 ± 21.6 | 67.1 ± 8.2 | High |
| Peters, 2006, [50] | USA | 10 | 146 ± 11 | 133 ± 14 | 90 ± 7 | 88 ± 11 | High |
| Ditor, 2005, [51] | Canada | 8 | 117 ± 20.3 | 114.8 ± 15 | 73.3 ± 10.6 | 71.8 ± 9.4 | High |
| Cunha, 2011, [52] | Brazil | 30 | 134.41 ± 17.5 | 126.81 ± 16.1 | 81.65 ± 10.57 | 76.35 ± 10.28 | High |
| Cunha, 2012, [53] | Brazil | 16 | 135.46 ± 7.42 | 126.93 ± 11.5 | 76.09 ± 6.49 | 72.84 ± 5.08 | High |
| de Freitas Brito, 2014, [54] | Brazil | 10 | 147 ± 4 | 146 ± 3 | 93 ± 4 | 91 ± 3 | Medium |
| Júnior, 2019, [55] | Brazil | 10 | 162.5 ± 11.2 | 160 ± 9.1 | 90 ± 5.9 | 80 ± 7.6 | High |
| Lowenthal, 2004, [56] | USA | 25 | 128 ± 3 | 126 ± 4 | 86 ± 4 | 78 ± 3 | High |
| Moreira, 2016, [57] | Brazil | 23 | 125.2 ± 9.3 | 114.7 ± 5.3 | 72 ± 6.8 | 71 ± 5.7 | High |
| Mota, 2013, [58] | Brazil | 32 | 134.5 ± 14.6 | 122.4 ± 13.8 | 80.9 ± 11.1 | 73.2 ± 9.5 | High |
| Santana, 2011, [59] | Brazil | 10 | 129 ± 17 | 125 ± 14 | 77.0 ± 8 | 77 ± 7 | High |
| Scher, 2010, [60] | Brazil | 16 | 130 ± 15 | 128 ± 10 | 80 ± 8 | 76 ± 8 | High |
| Toth, 2006, [5] | USA | 50 | 148.3 ± 22.8 | 140.5 ± 27.2 | — | — | Medium |
| Chan, 2018, [61] | USA | 82 | 142.39 ± 17.3 | 138.15 ± 17.3 | 81.83 ± 11.65 | 79.74 ± 10.51 | High |
| Costa, 2019, [62] | Brazil | 23 | 121.5 ± 11.2 | 119.2 ± 6.9 | 71.1 ± 8.4 | 70 ± 7.8 | High |
| Nascimento, 2019, [63] | Brazil | 27 | 126.04 ± 11.04 | 118.08 ± 9.09 | 72.95 ± 6.47 | 69.02 ± 6.54 | High |
| Kling, 2019, [64] | USA | 10 | 137.1 ± 22.3 | 124.7 ± 19.4 | 76.3 ± 12.2 | 76 ± 9.5 | High |
| Leandro, 2019, [65] | Brazil | 24 | 142.96 ± 20.91 | 124.12 ± 9.95 | 79.48 ± 19.48 | 61.68 ± 11.07 | High |
| Braith, 1994, [66] | USA | 19 | 120 ± 9 | 112 ± 11 | 75 ± 7 | 68 ± 6 | Medium |
| Applegate, 1992, [67] | USA | 56 | 145 ± 10 | 143 ± 12 | 88 ± 4 | 87 ± 2 | Medium |
| Barone, 2009, [68] | USA | 51 | 140 ± 8 | 134.7 ± 10.1 | 77 ± 7 | 73.4 ± 4.8 | High |
| Bouchonville, 2014, [69] | USA | 26 | 131.2 ± 11.7 | 130 ± 15.5 | 70.9 ± 8.3 | 68.8 ± 6.1 | High |
| Dusek, 2008, [70] | USA | 61 | 146.3 ± 5.5 | 145.3 ± 4.7 | 77.3 ± 7.1 | 77.6 ± 7.8 | High |
| Gerage, 2013, [71] | Brazil | 15 | 125 ± 8 | 120 ± 7 | 81 ± 6 | 80 ± 6 | Medium |
| Goncalves, 2014, [72] | Brazil | 17 | 126 ± 5.2 | 122.9 ± 4.4 | 80.9 ± 3.3 | 81.9 ± 4.4 | High |
| Jessup, 1998, [73] | USA | 11 | 134.7 ± 11.9 | 129.4 ± 11.5 | 76.9 ± 5.1 | 72.1 ± 7.4 | High |
| Li, 2005, [74] | USA | 54 | 135.02 ± 13.51 | 125.98 ± 13.1 | 78.59 ± 11.06 | 72.83 ± 10.63 | Medium |
| Madden, 2010, [75] | Canada | 20 | 143 ± 3 | 139 ± 4 | 85 ± 2 | 86 ± 2 | High |
| Millar, 2008, [76] | Canada | 25 | 122 ± 2.8 | 117 ± 2.8 | 70 ± 1.3 | 68 ± 1.6 | High |
| Simons, 2006, [77] | USA | 64 | 133 ± 12 | 128 ± 9 | 68 ± 6 | 70 ± 4 | Medium |
| Wang, 2011, [78] | USA | 180 | 131.9 ± 17.6 | 128.2 ± 17.8 | 68.7 ± 10.7 | 67.5 ± 9.4 | High |
| Wood, 2001, [79] | USA | 11 | 153.3 ± 10.8 | 146.6 ± 15.6 | 81.3 ± 7.2 | 70.9 ± 8.2 | High |
| Yassine, 2009, [80] | USA | 24 | 135.6 ± 11.2 | 121.1 ± 11.2 | 81.6 ± 11.2 | 71.6 ± 9.6 | High |
| Mortimer, 2011, [81] | South Africa | 15 | 130.6 ± 6.4 | 123 ± 9.1 | 83.88 ± 4.1 | 78 ± 5.1 | High |
Based on the available data, for final estimation of the effects of studies, standardized mean difference indices were used in the papers. In the studies that had reported standard deviation ± mean, standardized mean difference index was used in the meta-analysis. The results obtained from meta-analysis showed that across the studies, heterogeneity in investigating systolic changes pre- and postintervention was obtained as I2 = 98.8 and 98.6, while it was 99.2 and 98.6, respectively, for diastolic changes. Thus, for combination of studies and the final results, random method was used.
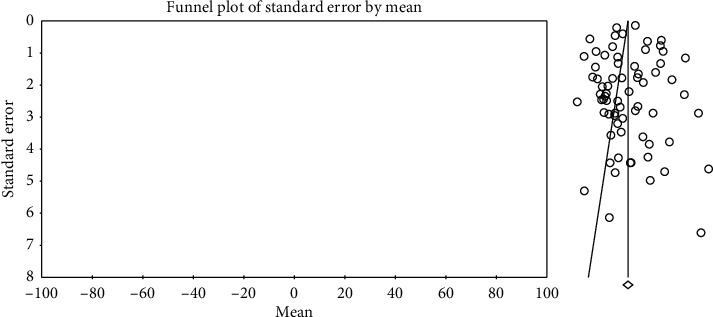
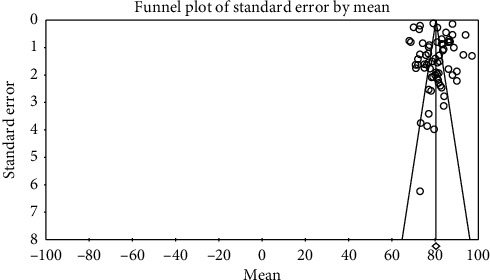
In order to investigate publication bias in the studies, Egger test was used. According to the results of this test, publication bias did not exist in investigating systolic changes pre- (P=0.057) and postintervention (P=0.713) and investigation of diastolic changes before (P=0.943) and after the intervention (P=0.522) (Figures 2–5).
Figure 2.
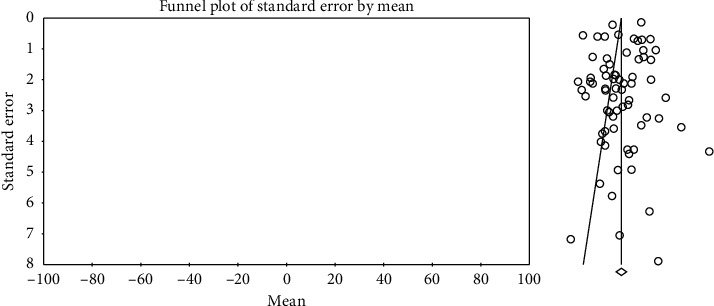
Funnel plot obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for systolic changes preintervention.
Figure 3.
Funnel plot obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for systolic changes postintervention.
Figure 4.
Funnel plot obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for diastolic changes preintervention.
Figure 5.
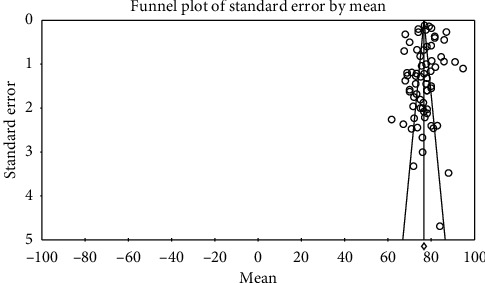
Funnel plot obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for diastolic changes postintervention.
Based on the results obtained from the meta-analysis, the standardized mean difference in examining the systolic changes pre- and postintervention was obtained as 137.8 ± 1.09 and 132.08 ± 0.96, while, for diastolic changes, they were 80.3 ± 0.85 and 76.6 ± 0.56, respectively. All of these suggest that exercise leads to diminished hypertension during advanced ages. In the cumulative figures, the standardized mean difference index, confidence interval of 95% in each study, and the final result of the index obtained from combining the studies have been shown. In this diagram, the weight of each study has been shown in the final combined value, where the size of each square is in proportion with the weight that the study has had in the meta-analysis. The horizontal line of each square represents the confidence interval of 95% (Figures 6–9).
Figure 6.
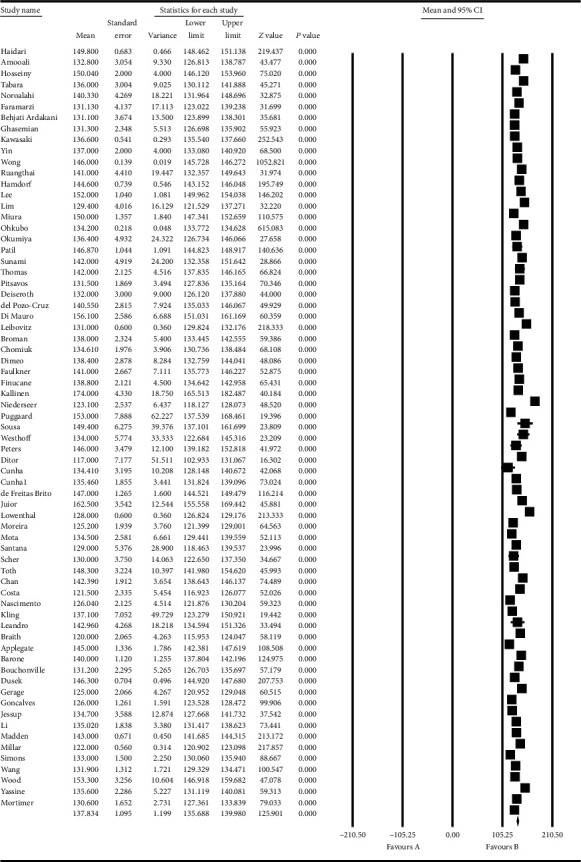
Cumulative diagram obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for systolic changes preintervention.
Figure 7.
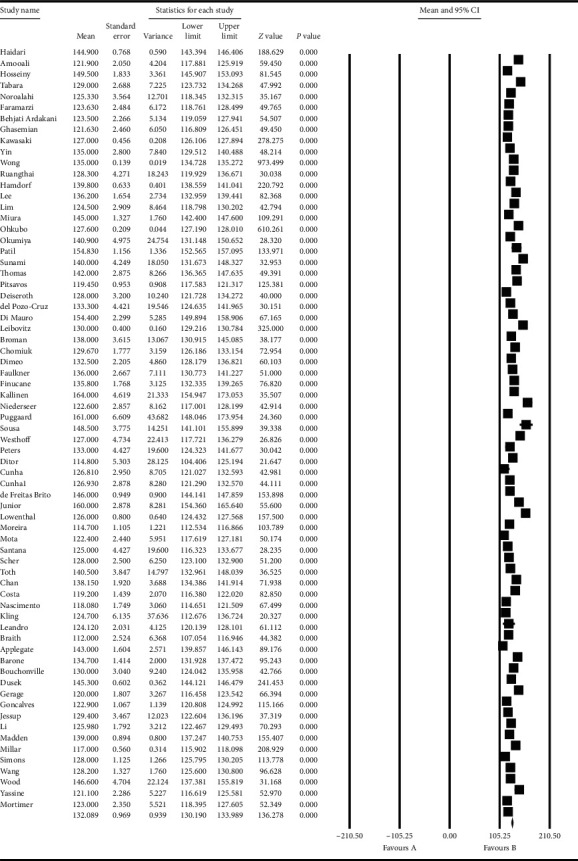
Cumulative diagram obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for systolic changes postintervention.
Figure 8.
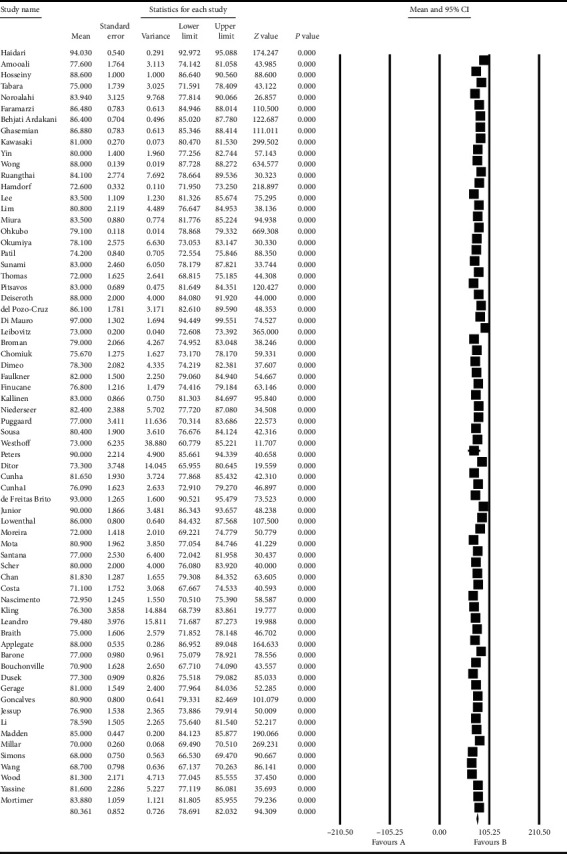
Cumulative diagram obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for diastolic changes preintervention.
Figure 9.
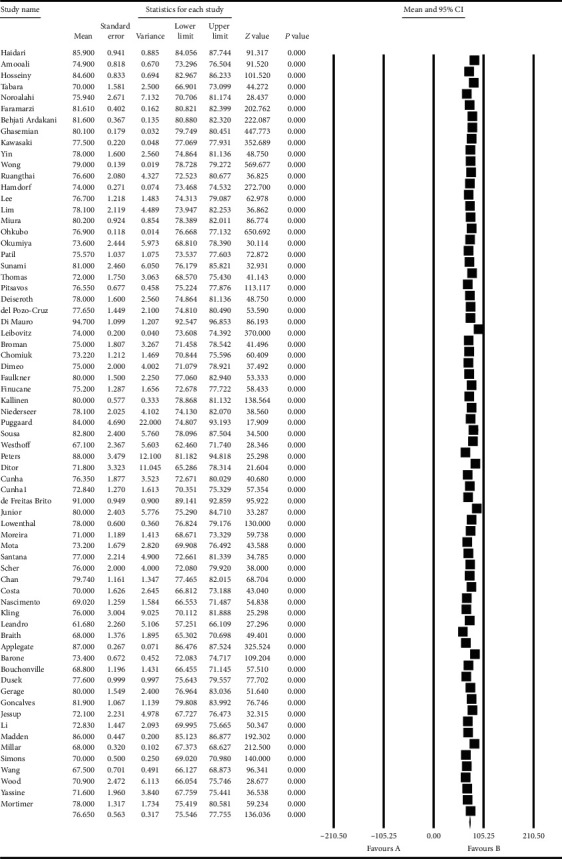
Cumulative diagram obtained from the studies introduced in the meta-analysis based on the standardized mean difference index for diastolic changes postintervention.
According to Table 2 reporting the mean and standard deviation of pre-/postintervention in systolic and diastolic blood pressure changes in terms of different continents (Asia, Europe, Africa, and America), 21, 15, 1, and 32 papers were analyzed in the meta-analysis from Asia, Europe, Africa, and America, respectively. In all of the investigations in terms of the studies carried out in the mentioned continents, exercise led to reduced age-induced hypertension (Figures 10 and 11).
Table 2.
The mean and standard deviation of pre-/postintervention in systolic and diastolic blood pressure changes across different continents.
| Blood pressure changes | Continents | Number of articles | I 2 | Sample size | Egger test | Mean ± SD |
|---|---|---|---|---|---|---|
| Systole (before intervention) | Asia | 21 | 99.2 | 806 | 0.706 | 140.4 ± 1.7 |
| Europe | 15 | 94.1 | 411 | 0.051 | 140.2 ± 2.4 | |
| America | 32 | 98 | 1040 | 0.503 | 135.3 ± 1.8 | |
| Africa | 1 | 0 | 15 | — | 130.6 ± 1.6 | |
|
| ||||||
| Systole (after intervention) | Asia | 21 | 99 | 806 | 0.627 | 134.2 ± 1.8 |
| Europe | 15 | 96.1 | 411 | 0.119 | 136.5 ± 2.4 | |
| America | 32 | 98.6 | 1040 | 0.793 | 129.1 ± 2.1 | |
| Africa | 1 | 0 | 15 | — | 123 ± 2.3 | |
|
| ||||||
| Diastole (before intervention) | Asia | 21 | 99.5 | 806 | 0.990 | 81.8 ± 1.3 |
| Europe | 15 | 98 | 411 | 0.051 | 81.2 ± 2.01 | |
| America | 31 | 98.6 | 990 | 0.138 | 78.7 ± 1.5 | |
| Africa | 1 | 0 | 15 | — | 83.8 ± 1.05 | |
|
| ||||||
| Diastole (after intervention) | Asia | 21 | 97.7 | 806 | 0.797 | 78.1 ± 0.56 |
| Europe | 15 | 97 | 411 | 0.111 | 77.9 ± 1.4 | |
| America | 31 | 99.1 | 990 | 0.207 | 75.06 ± 1.6 | |
| Africa | 1 | 0 | 15 | — | 78 ± 1.3 | |
Figure 10.
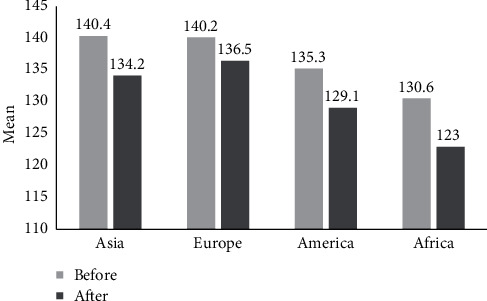
The results of mean systolic blood pressure across different continents for pre-/postintervention.
Figure 11.
The results of mean diastolic blood pressure across different continents for pre-/postintervention.
3.1. Standard Difference in Mean
In the study of the mean difference between systolic and diastolic blood pressure changes pre- and postintervention, it was reported that the difference between the systolic blood pressure changes pre- and postintervention was 0.65 ± 0.09, which showed a decrease in the systolic blood pressure after exercise (Figure 12), and the difference between the diastolic blood pressure changes pre- and postintervention was 0.64 ± 0.3609, which showed a decrease in the diastolic blood pressure after exercise (Figure 13).
Figure 12.
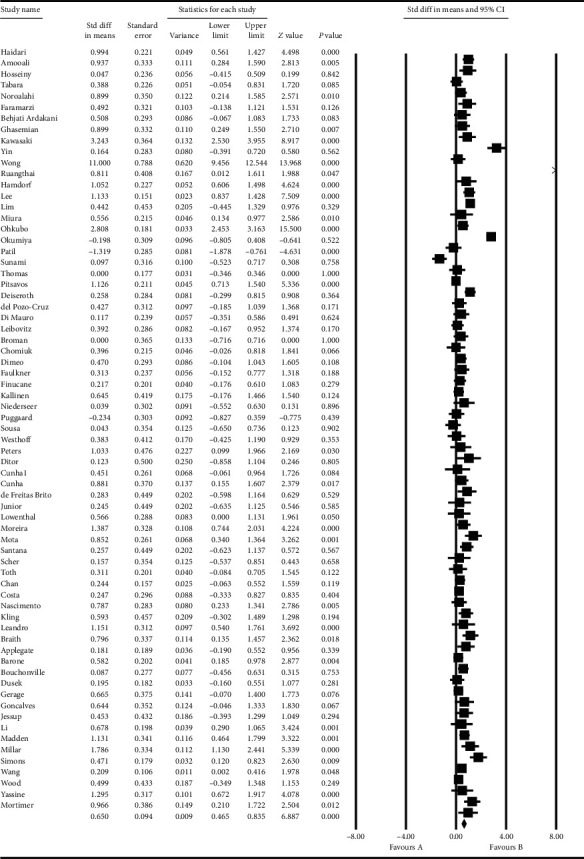
Standard difference in mean between systolic blood pressure changes pre- and postintervention.
Figure 13.
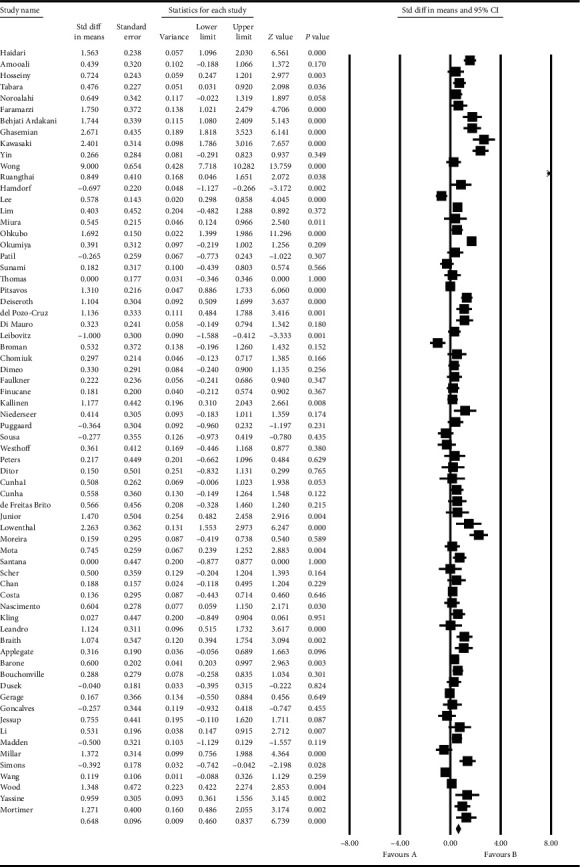
Standard difference in mean between diastolic blood pressure changes pre- and postintervention.
3.2. Subgroup Analysis Based on the Type of Exercise
Subgroup analysis based on the standard difference in mean before and after the intervention according to the type of exercise shows that resistance exercises reduces systolic (0.69 ± 0.1) and diastolic blood pressure (0.73 ± 0.16) more than aerobic exercise (Table 3).
Table 3.
Subgroup analysis based on type of exercise.
| Type of exercise | Number of articles | Sample size | I 2 | Egger test | Std. difference in mean (before and after intervention) | |
|---|---|---|---|---|---|---|
| Aerobic exercise | Systolic blood pressure | 48 | 1734 | 94.4 | 0.715 | 0.69 ± 0.16 |
| Diastolic blood pressure | 89.5 | 0.178 | 0.64 ± 0.11 | |||
|
| ||||||
| Resistance exercises | Systolic blood pressure | 20 | 494 | 54.6 | 0.051 | 0.69 ± 0.1 |
| Diastolic blood pressure | 82.8 | 0.053 | 0.73 ± 0.16 | |||
4. Discussion
Hypertension is one of the most common diseases in industrial countries [82] and one of the important causes of atherosclerosis, which can cause different problems. In case the treatment is not received, 50% of patients with hypertension die because of coronary artery diseases and congestive heart failure, 33%, because of stroke, and 10–15%, due to renal complications. Further, other organs including the eyes and larger vessels can also be affected [83]. Thus, the aim of the present study is to determine the impact of exercise on the blood pressure of the older adults with hypertension across Asia, Europe, Africa, and America through meta-analysis.
Based on the results obtained from the meta-analysis here, the standardized mean difference in investigating the systolic changes before and after the intervention was 137.8 and 132.08, respectively, and, for diastolic changes, 80.3 and 76.6, respectively. All these suggest that exercise causes a significant decline in age-induced hypertension.
Chronic hypertension adversely affects the myocardial structure and function, inducing a concentric hypertrophy [84]. It seems that the hypertrophic cardiac response to the overpressure is an attempt for normalizing the ventricular walls, thus helping preserve the heart function when undergoing an increased hemodynamic load. This process of hypertrophy is called compensatory hypertrophy [85]. Physical exercise leads to proper adaptation in the cardiovascular system, thereby reducing heart rate, resting heart rate, and increased left ventricle filling, venous return, and stroke volume [86]. In a research by Hinderliter et al., they concluded that after a six-month aerobic exercise program, the left ventricle hypertrophy of patients with hypertension diminished significantly. This reduction of hypertrophy was associated with reduced blood pressure and weight loss of patients. These researchers also found that weight loss is an important factor in mitigating the left ventricle hypertrophy [87]. In addition, Kokkinos et al. observed that aerobic exercise can lead to diminished hypertrophy in hypertensive individuals [88]. Notwithstanding, following aerobic exercise, eccentric contractions occur, whereby the ventricle volume grows; hypertrophy in the ventricle is also possible to occur though to a little extent (in healthy subjects). However, when the subjects are hypertensive, since they have pathologic hypertrophy in the ventricle wall, the mechanism is different. This means that, upon physiological increase in the ventricle dimensions (resulting from aerobic activity) and according to Frank-Starling law, the stroke volume increases and thus pathologic hypertrophy of the ventricular wall diminishes [89].
The present study indicates the results of mean and standard deviation before and after the intervention in changes of systolic and diastolic blood pressure for different continents. These changes have been reported for Asia, Europe, Africa, and America. In all of the investigations conducted across different continents, it was reported that exercise leads to significant reduction of age-induced hypertension. The real mechanism of postactivity hypotension is unknown, and most probably the mechanism is multifactorial. Studies suggest that acute hypotension is mostly associated with diminished peripheral resistance of vessels rather than cardiac output [90]. According to animal and human studies, diminished sympathetic activity occurs after physical exercise [91–93]. Changes in reactivity of vessels are associated with reduced sympathetic conduction for vessel resistance and release of local vasodilator substances (e.g., nitric oxide) in response to muscle contraction and increased blood flow to the muscles. After a heavy physical exercise, reactivity of vessels to alpha-adrenergic stimulation diminishes [94]. The local release of nitric oxide, prostaglandins, and adenosine increases during physical activity, thus facilitating peripheral postactivity vasodilation [95].
Postexercise hypertension is a result of physical activity, and daily changes of blood pressure do not affect its reduction. The density and volume of physical exercise play an important role in hemodynamic and thermal regulation as well as regulation of neurological reactions of the body during activity [96]. Also, Syme et al. reported that the intensity of physical activity influences the duration of hypotension and has a direct relationship with it [97]. Studies show that possibly factors such as diminished plasma volume, increased vasodilation substances, changes in the hormones affecting blood pressure including vasopressin, angiotensin 2 and renin, and peripheral vasodilation resulting from elevated central temperature are effective in inducing hypotension [98].
Use of physical exercise in the long run functions as a no pharmacological method for blood pressure reduction at rest or during daily physical activities [99]. In the elderly, exercise may be a more suitable method for controlling blood pressure because of low cost and not interfering with other treatments. Through exercise and physical activity, the adverse physiological effects that occur with the aging can be mitigated and the quality of life can be improved [100]. One session of mild or moderate intensity exercise can lead to blood pressure fall after exercise in hypertensive individuals, which is called postexercise hypotension [101].
In the human, postexercise hypotension is observed in response to several types of exercise in which large muscles are active including jogging, cycling, running, and swimming [102].
Through exercise, the oxidation capacity of muscles increases, whereby the aerobic biochemical system is stimulated to create adaptation. All these result in enhanced oxygen uptake in the body. Some diseases cause inhibition of oxygen in any of the above stages and reduce the functional capacity. However, aerobic exercises are able to create physiological adaptation in the efficiency of the aerobic energy system. They also enhance the functional ability of the person and improve the functional capacity even under progression conditions of the disease. Other advantages of regular exercise in this group of patients include increased power, improved body posture, diminished fatigue, improved mood, increased self-confidence, and sense of well-being. Doing physical exercise increases the persons' independence thereby leading to improved quality of life [103, 104].
Since these methods are easy to learn and possible to perform for almost all patients and there is no need to special equipment or cost, and even the patient can perform them in a lying position, patients can learn these methods easily and do them at home. By benefiting from the impacts of exercise methods such as greater blood perfusion to the muscles and reduction of stress and anxiety, their hypertension would diminish and their performance would be boosted. Also, through training patients on these methods and supervision on the way they should be practiced, the disturbing symptoms of this disease can be mitigated. Thus, by educating this method to both the healthcare team and patients, effective steps can be taken to alleviate this disorder.
It is recommended that relevant specialists benefit from regular aerobic exercise as a complementary treatment alongside pharmacotherapy to help patients with hypertension.
One of the limitations of the present research was completing the sheet of doing the exercise at home by the patients in the papers introduced to this meta-analysis, which may have been affected by the psychological status or inadequate care of the samples. Nevertheless, in some papers, checking whether exercise was done was followed up through a weekly in-person meeting with the patients.
5. Conclusion
The results of this study indicated that exercise significantly reduces blood pressure in the older adults across different continents. Accordingly, regular physical exercise can be part of the healthcare program of older adults with hypertension.
Acknowledgments
This work was funded by the Student Research Committee of Kermanshah University of Medical Sciences Deputy for Research and Technology, Kermanshah University of Medical Sciences (IR) (3009270).
Abbreviations
- SID:
Scientific information database
- CONSORT:
Consolidated standards of reporting trials
- PRISMA:
Preferred Reporting Items for Systematic Reviews and Meta-analysis.
Data Availability
Datasets are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
MK and NR contributed to the design, and MM and RJ were responsible of statistical analysis and participated in most of the study steps. AVR and AD prepared the manuscript. NS and MM assisted in designing the study and helped in the interpretation of the study. All authors have read and approved the content of the manuscript.
References
- 1.Allender J., Rector C., Rector C., Warner K. Community & Public Health Nursing: Promoting the Public’s Health. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. [Google Scholar]
- 2.Fiori K. L., Smith J., Antonucci T. C. Social network types among older adults: a multidimensional approach. The Journals of Gerontology Series B: Psychological Sciences and Social Sciences. 2007;62(6):P322–P330. doi: 10.1093/geronb/62.6.p322. [DOI] [PubMed] [Google Scholar]
- 3.Hamidizadeh S., Ahmadi F., Fallahi M. The effect of progressive muscle relaxation on blood pressure in older adultspatients with primary hypertension in older adultspeople living in the center of the Kahrizak in 2004. Journal of Rehabilitation Medicine. 2004;5(4):48–52. in Persian. [Google Scholar]
- 4.Schneider R., Alexander C., Staggers F., et al. A randomized controlled trial of stress reduction in African Americans treated for hypertension for over one year. American Journal of Hypertension. 2005;18(1):88–98. doi: 10.1016/j.amjhyper.2004.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Toth P. P. Short-term aerobic exercise in the older adults promotes blood pressure reduction. Journal of Applied Research In Clinical And Experimental Therapeutics. 2006;6(3):p. 186. [Google Scholar]
- 6.Craven R. F., Hirnle C. J., Jensen S. Fundamentals of Nursing: Human Health and Function. 7th. Philadelphia, PA, USA: Wolters Kluwer Health/Lippincott Williams & Wilkins; 2012. p. p. 653. [Google Scholar]
- 7.Cooper A., Moore L., McKenna J., Riddoch C. What is the magnitude of blood pressure response to a programme of moderate intensity exercise? Randomised controlled trial among sedentary adults with unmedicated hypertension. British Journal of General Practice. 2000;50(461):958–962. [PMC free article] [PubMed] [Google Scholar]
- 8.Hosseiny M., Farahani Z., Shiri H., AbedSaeidi J., AlaviMajd H., Hamidizadeh S. The effects of low intensity aerobic exercise on blood pressure. Journal of Shahrekord University of Medical Sciences. 2007;9(2):14–19. in Persian. [Google Scholar]
- 9.Owen A., Wiles J., Swaine I. Effect of isometric exercise on resting blood pressure: a meta analysis. Journal of Human Hypertension. 2010;24(12):p. 796. doi: 10.1038/jhh.2010.13. [DOI] [PubMed] [Google Scholar]
- 10.Moraes W. M. D., Souza P. R. M., Pinheiro M. H. N. P., Irigoyen M. C., Medeiros A., Koike M. K. Exercise training program based on minimum weekly frequencies: effects on blood pressure and physical fitness in elderly hypertensive patients. Brazilian Journal of Physical Therapy. 2012;16(2):114–121. doi: 10.1590/s1413-35552012005000013. [DOI] [PubMed] [Google Scholar]
- 11.Ferrier K. E., Waddell T. K., Gatzka C. D., Cameron J. D., Dart A. M., Kingwell B. A. Aerobic exercise training does not modify large-artery compliance in isolated systolic hypertension. Hypertension. 2001;38(2):222–226. doi: 10.1161/01.hyp.38.2.222. [DOI] [PubMed] [Google Scholar]
- 12.Tabara Y., Yuasa T., Oshiumi A., et al. Effect of acute and long-term aerobic exercise on arterial stiffness in the elderly. Hypertension Research. 2007;30(10):p. 895. doi: 10.1291/hypres.30.895. [DOI] [PubMed] [Google Scholar]
- 13.Westhoff T. H., Schmidt S., Gross V., et al. The cardiovascular effects of upper-limb aerobic exercise in hypertensive patients. Journal of Hypertension. 2008;26(7):1336–1342. doi: 10.1097/hjh.0b013e3282ffac13. [DOI] [PubMed] [Google Scholar]
- 14.Herrod P. J. J., Doleman B., Blackwell J. E. M., et al. Exercise and other nonpharmacological strategies to reduce blood pressure in older adults: a systematic review and meta-analysis. Journal of the American Society of Hypertension. 2018;12(4):248–267. doi: 10.1016/j.jash.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Kirk-Sanchez N. J., McGough E. L. Physical exercise and cognitive performance in the elderly: current perspectives. Clinical Interventions in Aging. 2014;9:51–62. doi: 10.2147/cia.s39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaisi-Raygani A., Mohammadi M., Jalali R., Ghobadi A., Salari N. The prevalence of obesity in older adults in Iran: a systematic review and meta-analysis. BMC Geriatrics. 2019;19(1):p. 371. doi: 10.1186/s12877-019-1396-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haidari H., Bolurchifard F., Yaghmaei F., Naseri N., Hamadzadeh S. The effect of short- terobic exercise on the blood pressure in older adultsclients urith hypertension. Medical- Surgical Nursing Journal. 2014;3(1):45–51. in Persian. [Google Scholar]
- 18.Amooali N., Daryanoosh F., Babaee Baigi M. A., Mohammadi M. The Impact of 12 Weeks of Aerobic Exercise on Serum Levels of Cardiotrophin-1, Blood Pressure and Left Ventricular Hypertrophy in Hypertensive Older adultsWomen. Zanjan, Iran: Zanjan University of Medical Sciences & Health Services; 2015. in Persian. [Google Scholar]
- 19.Noroalahi Z., Valipour V., Eslami R. The effect of eight weeks of exercise training on body weight, blood pressure, and cholesterol levels and liver enzymes in older adultswomen with metabolic syndrome. Journal of Obstetrics and Gynecology. 2019;22(2):63–72. in Persian. [Google Scholar]
- 20.Faramarzi M., Azamian A., Ghasemian A. The effect of a resistance training course on endothelin-1 concentration and systolic and diastolic blood pressure in older adults women. Applied Research in Management and Life Sciences in Sport. 2012;2:95–104. [Google Scholar]
- 21.Behjati Ardakani A., Qassemian A., Koushki M., Shakour E., Mehrez A. The effect of a resistance training course on blood pressure and nitric oxide levels in older adults women. Salmand: Iranian Journal of Ageing. 2018;13(1):16–27. [Google Scholar]
- 22.Ghasemian A., Salesi M. The effect of a combination of resistance and aerobic exercise training on endothelin-1 concentration and blood pressure in older adults women. Journal of Kerman University of Medical Sciences. 2013;21(1):11–24. [Google Scholar]
- 23.Kawasaki T., Sullivan C. V., Ozoe N., Higaki H., Kawasaki J. A long-term, comprehensive exercise program that incorporates a variety of physical activities improved the blood pressure, lipid and glucose metabolism, arterial stiffness, and balance of middle-aged and elderly Japanese. Hypertension Research. 2011;34(9):1059–1066. doi: 10.1038/hr.2011.81. [DOI] [PubMed] [Google Scholar]
- 24.Yin D., Ijiri H., Kohno I., et al. Differences in exersice blood pressure response between dipper and non-dipper elderly patients with essential hypertension. Japanese Journal of Geriatrics. 1998;35(11):845–850. doi: 10.3143/geriatrics.35.845. [DOI] [PubMed] [Google Scholar]
- 25.Wong A., Kwak Y.-S., Scott S. D., et al. The effects of swimming training on arterial function, muscular strength, and cardiorespiratory capacity in postmenopausal women with stage 2 hypertension. Menopause. 2019;26(6):653–658. doi: 10.1097/gme.0000000000001288. [DOI] [PubMed] [Google Scholar]
- 26.Ruangthai R., Phoemsapthawee J. Combined exercise training improves blood pressure and antioxidant capacity in elderly individuals with hypertension. Journal of Exercise Science & Fitness. 2019;17(2):67–76. doi: 10.1016/j.jesf.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamdorf P. A., Penhall R. K. Walking with its training effects on the fitness and activity patterns of 79–91 year old females. Australian and New Zealand Journal of Medicine. 1999;29(1):22–28. doi: 10.1111/j.1445-5994.1999.tb01584.x. [DOI] [PubMed] [Google Scholar]
- 28.Lee L.-L., Arthur A., Avis M. Evaluating a community-based walking intervention for hypertensive older people in Taiwan: a randomized controlled trial. Preventive Medicine. 2007;44(2):160–166. doi: 10.1016/j.ypmed.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Lim S.-T., Min S.-K., Park H., Park J.-H., Park J.-K. Effects of a healthy life exercise program on arteriosclerosis adhesion molecules in elderly obese women. Journal of Physical Therapy Science. 2015;27(5):1529–1532. doi: 10.1589/jpts.27.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura H., Takahashi Y., Maki Y., Sugino M. Effects of exercise training on arterial stiffness in older hypertensive females. European Journal of Applied Physiology. 2015;115(9):1847–1854. doi: 10.1007/s00421-015-3168-y. [DOI] [PubMed] [Google Scholar]
- 31.Ohkubo T., Hozawa A., Nagatomi R., et al. Effects of exercise training on home blood pressure values in older adults: a randomized controlled trial. Journal of Hypertension. 2001;19(6):1045–1052. doi: 10.1097/00004872-200106000-00008. [DOI] [PubMed] [Google Scholar]
- 32.Okumiya K., Matsubayashi K., Wada T., Kimura S., Doi Y., Ozawa T. Effects of exercise on neurobehavioral function in community-dwelling older people more than 75 Years of age. Journal of the American Geriatrics Society. 1996;44(5):569–572. doi: 10.1111/j.1532-5415.1996.tb01444.x. [DOI] [PubMed] [Google Scholar]
- 33.Patil S. G., Aithala M. R., Das K. K. Effect of yoga on arterial stiffness in elderly subjects with increased pulse pressure: a randomized controlled study. Complementary Therapies in Medicine. 2015;23(4):562–569. doi: 10.1016/j.ctim.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 34.Sunami Y., Motoyama M., Kinoshita F., et al. Effects of low-intensity aerobic training on the high-density lipoprotein cholesterol concentration in healthy elderly subjects. Metabolism. 1999;48(8):984–988. doi: 10.1016/s0026-0495(99)90194-4. [DOI] [PubMed] [Google Scholar]
- 35.Thomas G. N., Hong A. W. L., Tomlinson B., et al. Effects of Tai Chi and resistance training on cardiovascular risk factors in elderly Chinese subjects: a 12-month longitudinal, randomized, controlled intervention study. Clinical Endocrinology. 2005;63(6):663–669. doi: 10.1111/j.1365-2265.2005.02398.x. [DOI] [PubMed] [Google Scholar]
- 36.Pitsavos C., Chrysohoou C., Koutroumbi M., et al. The impact of moderate aerobic physical training on left ventricular mass, exercise capacity and blood pressure response during treadmill testing in borderline and mildly hypertensive males. Hellenic Journal of Cardiology. 2011;52(1):6–14. [PubMed] [Google Scholar]
- 37.Deiseroth A., Streese L., Köchli S., et al. Exercise and arterial stiffness in the elderly: a combined cross-sectional and randomized controlled trial (EXAMIN AGE) Frontiers in Physiology. 2019;10 doi: 10.3389/fphys.2019.01119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.del Pozo-Cruz J., del Pozo-Cruz B., Rodríguez Bies E. C., Alfonso-Rosa R. M., Navas P., López-Lluch G. Hypotensive acute effect of a combined resistance and walk-based exercise among over 65-year old community-dwelling women. Revista Andaluza de Medicina del Deporte. 2012;5(2):41–47. doi: 10.1016/s1888-7546(12)70007-2. [DOI] [Google Scholar]
- 39.Di Mauro S., Spallina G., Leotta C., et al. The effects of caloric restriction and controlled physical exercise on hypertension in the elderly. Archives of Gerontology and Geriatrics. 1998;27(1):1–8. doi: 10.1016/s0167-4943(98)00009-0. [DOI] [PubMed] [Google Scholar]
- 40.Leibovitz E., Manevich D., Viskoper R., Gavish D. Physical exercise does not help reduce blood pressure in very elderly populations. American Journal of Hypertension. 2005;18(5):p. A110. doi: 10.1016/j.amjhyper.2005.03.307. [DOI] [Google Scholar]
- 41.Broman G., Quintana M., Lindberg T., Jansson E., Kaijser L. High intensity deep water training can improve aerobic power in elderly women. European Journal of Applied Physiology. 2006;98(2):117–123. doi: 10.1007/s00421-006-0237-2. [DOI] [PubMed] [Google Scholar]
- 42.Chomiuk T., Folga A., Mamcarz A. The influence of systematic pulse-limited physical exercise on the parameters of the cardiovascular system in patients over 65 years of age. Archives of Medical Science. 2013;2(2):p. 201. doi: 10.5114/aoms.2013.34559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimeo F., Pagonas N., Seibert F., Arndt R., Zidek W., Westhoff T. H. Aerobic exercise reduces blood pressure in resistant hypertension. Hypertension. 2012;60(3):653–658. doi: 10.1161/hypertensionaha.112.197780. [DOI] [PubMed] [Google Scholar]
- 44.Faulkner J., McGonigal G., Woolley B., Stoner L., Wong L., Lambrick D. The effect of a short-term exercise programme on haemodynamic adaptability; a randomised controlled trial with newly diagnosed transient ischaemic attack patients. Journal of Human Hypertension. 2013;27(12):p. 736. doi: 10.1038/jhh.2013.43. [DOI] [PubMed] [Google Scholar]
- 45.Finucane F. M., Sharp S. J., Purslow L. R., et al. The effects of aerobic exercise on metabolic risk, insulin sensitivity and intrahepatic lipid in healthy older people from the Hertfordshire Cohort Study: a randomised controlled trial. Diabetologia. 2010;53(4):624–631. doi: 10.1007/s00125-009-1641-z. [DOI] [PubMed] [Google Scholar]
- 46.Kallinen M., Sipilä S., Alen M., Suominen H. Improving cardiovascular fitness by strength or endurance training in women aged 76–78 years. A population-based, randomized controlled trial. Age and Ageing. 2002;31(4):247–254. doi: 10.1093/ageing/31.4.247. [DOI] [PubMed] [Google Scholar]
- 47.Niederseer D., Ledl-Kurkowski E., Kvita K., et al. Salzburg skiing for the elderly study: changes in cardiovascular risk factors through skiing in the elderly. Scandinavian Journal of Medicine & Science in Sports. 2011;21:47–55. doi: 10.1111/j.1600-0838.2011.01341.x. [DOI] [PubMed] [Google Scholar]
- 48.Puggaard L., Larsen J. B., Støvring H., Jeune B. Maximal oxygen uptake, muscle strength and walking speed in 85-year-old women: effects of increased physical activity. Aging Clinical and Experimental Research. 2000;12(3):180–189. doi: 10.1007/bf03339835. [DOI] [PubMed] [Google Scholar]
- 49.Sousa N., Mendes R., Abrantes C., Sampaio J., Oliveira J. Long-term effects of aerobic training versus combined aerobic and resistance training in modifying cardiovascular disease risk factors in healthy elderly men. Geriatrics & Gerontology International. 2013;13(4):928–935. doi: 10.1111/ggi.12033. [DOI] [PubMed] [Google Scholar]
- 50.Peters P. G., Alessio H. M., Hagerman A. E., Ashton T., Nagy S., Wiley R. L. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: possible role of reactive oxygen species. International Journal of Cardiology. 2006;110(2):199–205. doi: 10.1016/j.ijcard.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 51.Ditor D. S., Kamath M. V., MacDonald M. J., Bugaresti J., McCartney N., Hicks A. L. Effects of body weight-supported treadmill training on heart rate variability and blood pressure variability in individuals with spinal cord injury. Journal of Applied Physiology. 2005;98(4):1519–1525. doi: 10.1152/japplphysiol.01004.2004. [DOI] [PubMed] [Google Scholar]
- 52.Cunha R. M., MacEdo C. B., Araujo S. F., Borges V. M., Soares A. A. Subacute blood pressure behavior in older adults hypertensive women after aquatic exercise session. Hypertension. 2011;58(5):e147–e148. [Google Scholar]
- 53.Cunha R. M., Macedo C. B., Araújo S. F. M., et al. Subacute blood pressure response in elderly hypertensive women after a water exercise session. High Blood Pressure & Cardiovascular Prevention. 2012;19(4):223–227. doi: 10.1007/bf03297634. [DOI] [PubMed] [Google Scholar]
- 54.Brito A. F., de Oliveira C. V., Brasileiro-Santos M. S., Santos A. C. Resistance exercise with different volumes: blood pressure response and forearm blood flow in the hypertensive elderly. Clinical Interventions in Aging. 2014;9:2151–2158. doi: 10.2147/cia.s53441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Júnior F. A., Gomes S. G., da Silva F. F., et al. The effects of aquatic and land exercise on resting blood pressure and post-exercise hypotension response in older adults hypertensives. Cardiovascular Journal of Africa. 2019;30:1–7. doi: 10.5830/CVJA-2019-051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lowenthal D. T., Vincent K. R. Clinical physiology and pharmacology conference: a nonpharmacological, evidence based medical approach using exercise method to lower and maintain blood pressure control in the elderly. International Urology and Nephrology. 2004;36(3):473–476. doi: 10.1007/s11255-004-1241-2. [DOI] [PubMed] [Google Scholar]
- 57.Moreira S. R., Cucato G. G., Terra D. F., Ritti-Dias R. M. Acute blood pressure changes are related to chronic effects of resistance exercise in medicated hypertensives elderly women. Clinical Physiology and Functional Imaging. 2016;36(3):242–248. doi: 10.1111/cpf.12221. [DOI] [PubMed] [Google Scholar]
- 58.Mota M. R., De Oliveira R. J., Dutra M. T., et al. Acute and chronic effects of resistive exercise on blood pressure in hypertensive elderly women. Journal of Strength and Conditioning Research. 2013;27(12):3475–3480. doi: 10.1519/jsc.0b013e31828f2766. [DOI] [PubMed] [Google Scholar]
- 59.Santana H. A. P., Moreira S. R., Neto W. B., et al. The higher exercise intensity and the presence of allele I of ACE gene elicit a higher post-exercise blood pressure reduction and nitric oxide release in older adults women: an experimental study. BMC Cardiovascular Disorders. 2011;11(1) doi: 10.1186/1471-2261-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Scher L. M. D. L., Ferriolli E., Moriguti J. C., Lima N. K. C. Pressão arterial obtida pelos métodos oscilométrico e auscultatório antes e após exercício em idosos. Arquivos Brasileiros de Cardiologia. 2010;94(5):656–662. doi: 10.1590/s0066-782x2010005000031. [DOI] [PubMed] [Google Scholar]
- 61.Chan A. W. K., Chair S. Y., Lee D. T. F., et al. Tai Chi exercise is more effective than brisk walking in reducing cardiovascular disease risk factors among adults with hypertension: a randomised controlled trial. International Journal of Nursing Studies. 2018;88:44–52. doi: 10.1016/j.ijnurstu.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 62.Costa I. B. B., Schwade D., Macêdo G. A. D., et al. Acute antihypertensive effect of self-selected exercise intensity in older women with hypertension: a crossover trial. Clinical Interventions in Aging. 2019;14:1407–1418. doi: 10.2147/cia.s207254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Da Cunha Nascimento D., Silva C. R., Leite Vieira D. C., Schoenfeld B. J., Prestes J. New insights for statistical analysis of blood pressure response to resistance training in older adultshypertensive women. Journal of Physical Education (Maringa) 2019;30(1) doi: 10.4025/jphyseduc.v30i13025. [DOI] [Google Scholar]
- 64.Kling H. E., D’Agostino E. M., Booth J. m. V., et al. The effect of a park-based physical activity program on cardiovascular, strength, and mobility outcomes among a sample of racially/ethnically diverse adults aged 55 or older. Preventing Chronic Disease. 2018;15:p. E166. doi: 10.5888/pcd15.180326. [DOI] [PubMed] [Google Scholar]
- 65.Leandro M. P. G., Moura J. L. S. d., Barros G. W. P., Silva Filho A. P. d., Farias A. C. d. O., Carvalho P. R. C. Effect of the aerobic component of combined training on the blood pressure of hypertensive elderly women. Revista Brasileira de Medicina do Esporte. 2019;25(6):469–473. doi: 10.1590/1517-869220192506214228. [DOI] [Google Scholar]
- 66.Braith R. W., Pollock M. L., Lowenthal D. T., Graves J. E., Limacher M. C. Moderate- and high-intensity exercise lowers blood pressure in normotensive subjects 60 to 79 years of age. The American Journal of Cardiology. 1994;73(15):1124–1128. doi: 10.1016/0002-9149(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 67.Applegate W. B., Miller S. T., Elam J. T., et al. Nonpharmacologic intervention to reduce blood pressure in older patients with mild hypertension. Archives of Internal Medicine. 1992;152(6):1162–1166. doi: 10.1001/archinte.152.6.1162. [DOI] [PubMed] [Google Scholar]
- 68.Barone B. B., Wang N. Y., Bacher A. C., Stewart K. J. Decreased exercise blood pressure in older adults after exercise training: contributions of increased fitness and decreased fatness. British Journal of Sports Medicine. 2009;43(1):52–56. doi: 10.1136/bjsm.2008.050906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bouchonville M., Armamento-Villareal R., Shah K., et al. Weight loss, exercise or both and cardiometabolic risk factors in obese older adults: results of a randomized controlled trial. International Journal of Obesity. 2014;38(3):p. 423. doi: 10.1038/ijo.2013.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dusek J. A., Hibberd P. L., Buczynski B., et al. Stress management versus lifestyle modification on systolic hypertension and medication elimination: a randomized trial. The Journal of Alternative and Complementary Medicine. 2008;14(2):129–138. doi: 10.1089/acm.2007.0623. [DOI] [PubMed] [Google Scholar]
- 71.Gerage A., Forjaz C. L., Nascimento M., Januário R. S., Polito M., Cyrino E. Cardiovascular adaptations to resistance training in elderly postmenopausal women. International Journal of Sports Medicine. 2013;34(9):806–813. doi: 10.1055/s-0032-1331185. [DOI] [PubMed] [Google Scholar]
- 72.Goncalves C. G., Nakamura F. Y., Polito M. D., et al. Functional and physiological effects of a 12-week programme of resistance training in older adults hypertensive women. International SportMed Journal. 2014;15(1):50–61. [Google Scholar]
- 73.Jessup J. V., Lowenthal D. T., Pollock M. L., Turner T. The effects of endurance exercise training on ambulatory blood pressure in normotensive older adults. Geriatric Nephrology and Urology. 1998;8(2):103–109. doi: 10.1023/a:1008287320868. [DOI] [PubMed] [Google Scholar]
- 74.Li F., Fisher K. J., Harmer P. Improving physical function and blood pressure in older adults through cobblestone mat walking: a randomized trial. Journal of the American Geriatrics Society. 2005;53(8):1305–1312. doi: 10.1111/j.1532-5415.2005.53407.x. [DOI] [PubMed] [Google Scholar]
- 75.Madden K. M., Lockhart C., Potter T. F., Cuff D. Aerobic training restores arterial baroreflex sensitivity in older adults with type 2 diabetes, hypertension, and hypercholesterolemia. Clinical Journal of Sport Medicine. 2010;20(4):p. 312. doi: 10.1097/jsm.0b013e3181ea8454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Millar P. J., Bray S. R., MacDonald M. J., McCartney N. The hypotensive effects of isometric handgrip training using an inexpensive spring handgrip training device. Journal of Cardiopulmonary Rehabilitation and Prevention. 2008;28(3):203–207. doi: 10.1097/01.hcr.0000320073.66223.a7. [DOI] [PubMed] [Google Scholar]
- 77.Simons R., Andel R. The effects of resistance training and walking on functional fitness in advanced old age. Journal of Aging and Health. 2006;18(1):91–105. doi: 10.1177/0898264305281102. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Hsu F.-C., Isom S., et al. Effects of a 12-month physical activity intervention on prevalence of metabolic syndrome in elderly men and women. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2011;67A(4):417–424. doi: 10.1093/gerona/glr187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wood R. H., Reyes R., Welsch M. A., et al. Concurrent cardiovascular and resistance training in healthy older adults. Medicine & Science in Sports & Exercise. 2001;33(10):1751–1758. doi: 10.1097/00005768-200110000-00021. [DOI] [PubMed] [Google Scholar]
- 80.Yassine H. N., Marchetti C. M., Krishnan R. K., Vrobel T. R., Gonzalez F., Kirwan J. P. Effects of exercise and caloric restriction on insulin resistance and cardiometabolic risk factors in older obese adults--A randomized clinical trial. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2009;64A(1):90–95. doi: 10.1093/gerona/gln032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mortimer J., Mckune A. J. Effect of short-term isometric handgrip training on blood pressure in middle-aged females. Cardiovascular Journal of Africa. 2011;22(5):p. 257. doi: 10.5830/cvja-2010-090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al Sowielem L., El Zubier A. Compliance and knowledge of hypertensive patients attending PHC centres in Al-Khobar, Saudi Arabia. 1998.
- 83.Braunwald E. Heart Disease, a Textbook of Cardiovascular Medicine. Philadelphia, PA, USA: WB Saunders Company; 2001. [Google Scholar]
- 84.Buss S. J., Riffel J. H., Malekar P., et al. Chronic Akt blockade aggravates pathological hypertrophy and inhibits physiological hypertrophy. American Journal of Physiology-Heart and Circulatory Physiology. 2011;302(2):H420–H430. doi: 10.1152/ajpheart.00211.2011. [DOI] [PubMed] [Google Scholar]
- 85.Finsen A. V., Lunde I. G., Sjaastad I., et al. Syndecan-4 is essential for development of concentric myocardial hypertrophy via stretch-induced activation of the calcineurin-NFAT pathway. PLoS One. 2011;6(12) doi: 10.1371/journal.pone.0028302.e28302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dawson E., George K., Shave R., Whyte G., Ball D. Does the human heart fatigue subsequent to prolonged exercise? Sports Medicine. 2003;33(5):365–380. doi: 10.2165/00007256-200333050-00003. [DOI] [PubMed] [Google Scholar]
- 87.Hinderliter A., Sherwood A., Gullette E. C. D., et al. Reduction of left ventricular hypertrophy after exercise and weight loss in overweight patients with mild hypertension. Archives of Internal Medicine. 2002;162(12):1333–1339. doi: 10.1001/archinte.162.12.1333. [DOI] [PubMed] [Google Scholar]
- 88.Kokkinos P. F., Narayan P., Colleran J. A., et al. Effects of regular exercise on blood pressure and left ventricular hypertrophy in African-American men with severe hypertension. New England Journal of Medicine. 1995;333(22):1462–1467. doi: 10.1056/nejm199511303332204. [DOI] [PubMed] [Google Scholar]
- 89.Cornelis J., Beckers P., Taeymans J., Vrints C. Comparing exercise training modalities in heart failure: a systematic review and meta-analysis. International Journal of Cardiology. 2016;221:867–876. doi: 10.1016/j.ijcard.2016.07.105. [DOI] [PubMed] [Google Scholar]
- 90.Ciolac E. G., Guimarães G. V., Dàvila V. M., Doria E. L., Bocchi E. A. Acute effects of continuous and interval aerobic exercise on 24-h ambulatory blood pressure in long-term treated hypertensive patients. International Journal of Cardiology. 2009;133(3):381–387. doi: 10.1016/j.ijcard.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 91.Floras J. S., Sinkey C. A., Aylward P. E., Seals D. R., Thoren P. N., Mark A. L. Postexercise hypotension and sympathoinhibition in borderline hypertensive men. Hypertension. 1989;14(1):28–35. doi: 10.1161/01.hyp.14.1.28. [DOI] [PubMed] [Google Scholar]
- 92.Halliwill J. R., Taylor J. A., Eckberg D. L. Impaired sympathetic vascular regulation in humans after acute dynamic exercise. The Journal of Physiology. 1996;495(1):279–288. doi: 10.1113/jphysiol.1996.sp021592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kulics J. M., Collins H. L., DiCarlo S. E. Postexercise hypotension is mediated by reductions in sympathetic nerve activity. American Journal of Physiology-Heart and Circulatory Physiology. 1999;276(1):H27–H32. doi: 10.1152/ajpheart.1999.276.1.h27. [DOI] [PubMed] [Google Scholar]
- 94.Halliwill J. R. Mechanisms and clinical implications of post-exercise hypotension in humans. Exercise and Sport Sciences Reviews. 2001;29(2):65–70. doi: 10.1249/00003677-200104000-00005. [DOI] [PubMed] [Google Scholar]
- 95.Franklin P. J., Green D. J., Cable N. T. The influence of thermoregulatory mechanisms on post-exercise hypotension in humans. The Journal of Physiology. 1993;470(1):231–241. doi: 10.1113/jphysiol.1993.sp019856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Melo C. n. M., Alencar Filho A. t. C., Tinucci T., Mion D. c., Jr., Forjaz C. u. L. M. Postexercise hypotension induced by low-intensity resistance exercise in hypertensive women receiving captopril. Blood Pressure Monitoring. 2006;11(4):183–189. doi: 10.1097/01.mbp.0000218000.42710.91. [DOI] [PubMed] [Google Scholar]
- 97.Syme A. N., Blanchard B. E., Guidry M. A., et al. Peak systolic blood pressure on a graded maximal exercise test and the blood pressure response to an acute bout of submaximal exercise. The American Journal of Cardiology. 2006;98(7):938–943. doi: 10.1016/j.amjcard.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 98.MacDonald J. R., Rosenfeld J. M., Tarnopolsky M. A., Hogben C. D., Ballantyne C. S., MacDougall J. D. Post exercise hypotension is not mediated by the serotonergic system in borderline hypertensive individuals. Journal of Human Hypertension. 2002;16(1):p. 33. doi: 10.1038/sj.jhh.1001290. [DOI] [PubMed] [Google Scholar]
- 99.Arazi H., Rohani H. Blood pressure responses after resistance training methods, and double extra times. Journal of Sport Sciences, University of Guilan. 2009;27:123–136. in Persian. [Google Scholar]
- 100.Canuto P. M. d. B. C., Nogueira I. D. B., Cunha E. S. d., et al. Influência do treinamento resistido realizado em intensidades diferentes e mesmo volume de trabalho sobre a pressão arterial de idosas hipertensas. Revista Brasileira de Medicina do Esporte. 2011;17(4):246–249. doi: 10.1590/s1517-86922011000400006. [DOI] [Google Scholar]
- 101.Kenney M. J., Seals D. R. Postexercise hypotension. key features, mechanisms, and clinical significance. Hypertension. 1993;22(5):653–664. doi: 10.1161/01.hyp.22.5.653. [DOI] [PubMed] [Google Scholar]
- 102.Chen C. Y., Bonham A. C. Postexercise hypotension. Exercise and Sport Sciences Reviews. 2010;38(3):122–127. doi: 10.1097/jes.0b013e3181e372b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Randall T., Schapiro M. Managing symptoms of multiple sclerosis. Neurologic Clinics. 2007;23:177–187. doi: 10.1016/j.ncl.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 104.Schulz K. F., Altman D. G., Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Medicine. 2010;8(1):p. 18. doi: 10.1186/1741-7015-8-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets are available from the corresponding author upon reasonable request.


