Abstract
Introduction.
Membrane transporters are integral components to the maintenance of cellular integrity of all tissue and cell types. While transporters play an established role in the systemic pharmacokinetics of therapeutic drugs, tissue specific expression of uptake transporters can serve as an initiating mechanism that governs the accumulation and impact of cytotoxic drugs.
Areas covered.
This review provides an overview of organic cation transporters as determinants to chemotherapy-induced toxicities. We also provide insights into the recently updated FDA guidelines for in vitro drug interaction studies, with a particular focus on the class of tyrosine kinase inhibitors as perpetrators of transporter-mediated drug interactions.
Expert opinion.
Studies performed over the last few decades have highlighted the important role of basolateral uptake and apical efflux transporters in the pathophysiology of drug-induced organ damage. Increased understanding of the mechanisms that govern the accumulation of cytotoxic drugs has provided insights into the development of novel strategies to prevent debilitating toxicities. Furthermore, we argue that current regulatory guidelines provide inadequate recommendations for in vitro studies to identify substrates or inhibitors of drug transporters. Therefore, the translational and predictive power of FDA-approved drugs as modulators of transport function remains ambiguous and warrants further revision of the current guidelines.
Keywords: organic cation transporters, solute carriers, pharmacokinetics, toxicity, tyrosine kinase inhibitors, FDA, transporter-drug interactions
1. Introduction
It has become evident that the expression and localization of drug transporters contribute to a dynamic interplay between the maintenance of cellular integrity via the removal of toxic metabolites or entry of essential nutrients [1]. Moreover, members of the ATP-binding cassette (ABC) and solute carrier (SLC) superfamilies are recognized as important determinants governing the therapeutic response of many drugs [1–3]. Disruption of these sensitive transport systems through changes in gene expression, genetic variations or drug-drug interaction, has the potential to contribute to chemoresistance, endogenous toxin-mediated diseases, diminished therapeutic efficacy, and increased sensitivity to drug-induced organ damage [4–7]. The following review focuses on specific examples within the SLC subfamily of organic cation transporters (OCTs) and multidrug and toxin extrusion proteins (MATEs) as critical determinants of the dose-limiting toxicity profiles associated with chemotherapeutic agents.
2. Organic cation-type transporters
ABC and SLC family members comprise of over 400 genes encoded in the human genome [7,8]. While the function of these gene families is to regulate the entry of essential nutrients and the removal of endogenous toxins, the SLC family also contributes to the cellular and systemic distribution profile of many therapeutic drugs [3,9,10]. Specifically, OCT1 [SLC22A1], OCT2 [SLC22A2], OCT3 [SLC22A3], OCTN1 [SLC22A4], and OCTN2 [SLC22A5] are the major organic cation-type transporters encoded by the SLC22A subfamily that facilitate drug accumulation. Due to their expression on the basolateral membranes of enterocytes, hepatocytes, proximal tubules and other endothelial peripheral tissues, OCTs play a critical role in the vectorial transport of organic cations [3,11–13]. Independent of Na+ and H+, OCTs translocate substrates across a biological membrane driven by membrane potential and the electrochemical gradient of the protonated molecule [3]. Additionally, the subfamily of MATEs, including MATE1 [SLC47A1] and MATE2-K [SLC47A2], mediate the efflux of diverse substrates consisting primarily of organic cations, but also certain anions and zwitterions, from the liver and kidney [14,15]. MATEs have emerged as clinically relevant efflux transporters that work in concert with OCTs to mediate apical efflux into the bile and urine. Unlike OCTs, the bi-directionality of MATE transport is dependent on the proton concentration gradient, and driven by exchange of protons in the bile or urine [16]. It is important to note that other members within this family, including OCTL2 [SLC22A14], OCT6 [SLC22A16], OCTN3 [SLC22A21], and UST6 [SLC22A25] [17,18] as well as members of the ABC family such as MDR1 [ABCB1], MRP1 [ABCC1], MRP2 [ABCC2], MRP4 [ABCC4], and BCRP [ABCG2] [19,20], are also capable of governing intracellular levels of organic cations. Since 40% of FDA-approved therapeutic drugs exist as organic cations under physiological conditions [2], OCTs and MATEs are recommended for evaluation during drug development by the Food and Drug Administration (FDA) [21].
2.1. Organic cation transporter 1 (OCT1).
OCT1 is the most abundant cationic uptake transporter expressed in the liver in humans [13]. The localization of OCT1 is predominantly expressed on the sinusoidal membrane (blood facing) of hepatocytes, and it serves as the rate-determining step in the translocation of endogenous [22,23] and xenobiotic cationic compounds from the systemic circulation into the liver [24], and thus contributes as an initial step in hepatic metabolism and excretion. In addition to OCT1, efflux transporters of ABC superfamily of transporters such as MDR1 [19], and MATE1 [16] work in a concerted manner to assist in the biliary excretion and detoxification of endogenous cationic toxins. OCT1 has been implicated in drug-drug interactions, and contributes to pharmacokinetic and pharmacodynamic variability of substrates in humans [25,26]. OCT1 is also expressed on the bronchial epithelial cells [27], endothelial cells of the heart and brain, and enterocytes [3,28]. In contrast to the dominant OCT1 expression in human livers, rodents also express Oct1 on enterocytes [29] and epithelial cells found in the S1 and S2 segments of proximal tubular cells. In the rodent kidney, Oct1 is redundant with Oct2, and these proteins jointly fulfill a function that is equivalent to that of OCT2 in humans. Targeted disruption of Oct1 in rodents reduced liver accumulation of prototypical transport substrates such as tetraethylammonium (TEA) and the neurotoxin 1-methyl-4-phenylpyridinium (MPP) [30,31].
2.2. Organic cation transporter 2 (OCT2)
Unlike OCT1, OCT2 presents a more restricted expression pattern. OCT2 is highest on the basolateral membrane (blood facing) along S2 and S3 segments of proximal tubule cells [12,30,31]. OCT2 plays a key role in facilitating the translocation of cationic compounds from systemic circulation into renal proximal tubule cells and working in concert with efflux transporters (e.g., primarily MATE1 and MATE2-K) to mediate excretion of substrates into the urine. Although OCTs share many overlapping substrates, OCT2 also efficiently transports monoamine neurotransmitters, as evidenced by moderate expression across different types of neuronal cells [3,28]. Due to its high expression in proximal tubule cells, OCT2 has been implicated in mediating drug-drug interactions and it therefore recommended for evaluation during development by the FDA [21].
2.3. Organic cation transporter 3 (OCT3)
In contrast to the wealth of knowledge accumulated in recent years on OCT1 and OCT2, relatively little is known about the third member of the SLC22A family, OCT3. OCT3 exhibits a promiscuous expression pattern in most major organs and peripheral tissues, with highest expression in the heart, placenta and skeletal muscles [12,32,33]. Although OCT3 is generally not considered a significant contributor to drug-drug interactions, recent studies have highlighted OCT3 as an important transporter in the heart and cardiovascular disease [34], and carriers of polymorphic variants in OCT3 (rs2048327, rs1810126, rs1810126 and rs3088442) are at reduced risk of coronary artery disease development [35–37]. While OCT3 is present in most tissues, the genetic deletion of Oct3 in mice does not affect viability and fertility, and does not lead to any overt and/or abnormal pathology [38]. Oct3 deficiency in mice is associated with diminished accumulation of prototypical substrates such as metformin and MPP in homogenized hearts [34,38].
2.4. Organic cation/carnitine transporter 1 and 2 (OCTN1 and OCTN2)
In comparison with OCT1–3, OCTN1 and OCTN2 can transport substrates either dependently or independently of Na+. Driven by a proton gradient, OCTNs can recognize zwitterions as well as organic cations and even certain anions, and share a high degree of membrane topology [39,40]. OCTN1 is the only known transporter of ergothioneine, whereas OCTN2 is believed to be an essential carnitine transporter. Both OCTN1 and OCTN2 are widely expressed in human tissues. Localization of OCTNs in proximal tubular cells is restricted to the apical membrane (urine side) [3,12], where it may function in the reabsorption of essential cations from the urine. OCTNs are also expressed on the apical membrane of enterocytes, and OCTN2 is expressed in skeletal muscles, heart, lung and eye, where it may be involved in regulating L-carnitine uptake and tissue homeostasis [41–43]. Genetic deletion of Octn1 provides viable offspring [44], whereas Octn2 deletion displays embryonic lethality without L-carnitine supplementation [45]. The clinical relevance of OCTN1- and OCTN2-mediated drug-drug interactions remains largely unstudied but is likely limited due to the narrow substrate specificity of these transporters.
2.5. Multidrug and extrusion protein 1 and 2-K (MATE1 and MATE2-K)
MATE1 and MATE2-K are poly-specific efflux transporters that translocate organic cations, and certain anions, in a Na+ independent and pH-dependent manner [3,10]. MATE1 is apically expressed on the cannicular and brush-border membrane of hepatocytes as well as on proximal tubular cells, and mediates the efflux of organic cations into the bile and urine, respectively [46]. MATE2-K, a splice variant, is restricted to kidneys in humans and the redundant expression of this additional antiporter on the luminal membrane of proximal tubules in humans suggests that MATEs evolutionary adapted to mediate the clearance of endogenous toxins or xenobiotics [16]. The tissue distribution of Mate1 in rodents is generally consistent with that observed for MATE1 in humans, with the exception of MATE2-K being absent in the kidney of mice and rats, while Mate1 is additionally absent in the liver of rats [15,47].
3. Dose-limiting toxicities associated with chemotherapeutic agents
3.1. Nephrotoxicity
Cisplatin is a standard of care drug for the treatment of various malignant solid tumors in both pediatric and adult populations [48–51]. The clinical utility of cisplatin is limited by the onset of debilitating toxicities, including nephrotoxicity [52–54], which can cause severe renal impairment. Approximately one-third of patients receiving cisplatin develop acute kidney injury (AKI) during the course of treatment, and in severe cases, patients display a permanent incomplete recovery of renal function despite discontinuation of treatment. More than 50 percent of the drug is excreted into the urine within the first 24 hours [55,56], and renal cortical concentrations of platinum are orders of magnitude greater than that observed in the systemic circulation and compared to other organs [57], suggesting a key renal-mediated mechanism of accumulation. The pathophysiology of cisplatin-induced nephrotoxicity involves renal tubular apoptosis and necrosis, particularly in the S3 segment of proximal tubule cells and of the distal nephron, manifesting clinically as imbalances in salt and essential nutrients, tubular acidosis and a reduced glomerular filtration rate (GFR) [58–60]. Although proper hydration of patients and prophylactic treatment with diuretics can manage the symptoms of nephrotoxicity, the prevalence of this side effect remains high and greatly diminishes quality of life [59,61].
Renal concentrations of platinum are highest in the S2 and S3 segment of proximal tubular cells [52,62], highlighting a key cell-specific and transporter-mediated process. In rodents, the deficiency of both Oct1 and Oct2 conferred protection against cisplatin-induced nephrotoxicity [63] while the OCT2 808G>T (rs316019) genetic variant in humans was associated with reduced changes in serum creatinine, a hallmark of acute kidney injury, after one cycle of cisplatin treatment as compared to baseline and to patients lacking this variant [64–66]. Furthermore, sexually dimorphic expression of Oct2 in proximal tubular cells of male rodents correlated with a greater propensity and susceptibility to cisplatin-mediated tubular injury compared to female rodents [12,67]. Cisplatin is also a transported substrate of MATE1, and rodent deficiency for Mate1 was associated with diminished urinary excretion of total platinum, potentiating nephrotoxicity [68]. The pharmacologic targeting of these transport mechanisms using OCT2 and MATE1 inhibitors protected or exacerbated nephrotoxicity, respectively [69–71]. The translational significance of OCT2-dependent intervention strategies, in which cisplatin is administered after an OCT2 inhibitor to restrict access of the chemotherapy to tubular cells, remains somewhat disappointing. This may be explained by the partial reduction in biomarkers of cisplatin-induced nephrotoxicity (e.g., serum creatinine and blood urea nitrogen), the use of non-specific OCT2 inhibitors lacking potency at clinically-achievable concentrations, the incomplete recovery of pathological damage in the kidneys that occurs despite the OCT2 inhibition [63], and/or the failure of the intervention strategy to modulate uptake mechanisms that mediate cisplatin-induced AKI in an OCT2-independent manner. Regarding the latter, it should be pointed out that several reports have suggested the existence of a negatively charged N-acetylcysteine S-conjugate (NAC-1) derived from cisplatin that can act as an intermediate, highly reactive nephrotoxic thiol. NAC-1 is generated by cisplatin conjugation with glutathione and cysteine before accumulation in proximal tubular cells [72]. NAC-1 was recently found to be a transported substrate of the organic anion transporters OAT1 [SLC22A6] and OAT3 [SLC22A8], which are also expressed on the basolateral membrane of proximal tubules, and can contribute to cisplatin nephrotoxicity independently of Oct2 in a signaling pathway that is upstream of p53 [73]. However, similar to Oct2-deficient rodents, genetic deficiency of Oat1 and Oat3 or pharmacological inhibition with probenecid only offers partial reduction in serum markers and pathological damage [74]. This study further demonstrated that concomitant administration of the Bcr-Abl tyrosine kinase inhibitor nilotinib, which can simultaneously inhibit both Oct2-mediated uptake of cisplatin [75] and Oat1/Oat3-mediated uptake of NAC-1 through non-competitive mechanisms, afforded complete protection against cisplatin-induced proximal tubular injury in vivo [74].
Compared to cisplatin, the related drug oxaliplatin lacks significant nephrotoxic activity, despite being a transported substrate of the rodent and human OCT2, MATE1 and MATE2-K [76]. While Oct2 transports both platinum agents with similar kinetics, the extent of total platinum accumulation in the kidney was far greater in cisplatin-treated animals compared to oxaliplatin [77]. The differences observed in the accumulation and elimination of these platinum agents is potentially related to differences in transport kinetics for Mate1 in mice, and MATE1 and MATE2-K in humans [77], suggesting higher luminal efflux of oxaliplatin into the urine compared to cisplatin. However, this explanation of differential tubular secretion rates for cisplatin and oxaliplatin as a driving discriminatory mechanism for their different nephrotoxic properties is not completely satisfactory, and is inconsistent with comparative in vivo studies in mice indicating that the renal excretion of both agents is strongly dependent on Mate1. It also largely ignores intrinsic differences in renal metabolic activation pathways that results in the formation of potent nephrotoxins in the case of cisplatin but not oxaliplatin, and fails to recognize the differing contribution of other potentially critical renal ABC efflux transporters in the process, such as MRP2 and MRP4.
Similar to oxaliplatin, the novel platinum derivative, phenanthriplatin, is a high affinity substrate for both OCT2 and MATE1/2-K, and it has been argued that these are kinetically favorable attributes that would be efficacious in the treatment of OCT-expressing tumors, while efficient MATE-mediated efflux may decrease the renal tubular residence time and thereby prevent the debilitating toxicity profiles observed in the kidneys following cisplatin treatment [78]. Similar observations have been documented with the alkylating agent, ifosfamide, which is associated uniquely with nephrotoxic activity and the development of renal Fanconi syndrome [79]. Bioactivation of ifosfamide mediated by OCT2 transport into proximal tubular cells has been proposed as a differentiating mechanism between ifosfamide on the one hand and cyclophosphamide’s lack of nephrotoxic activity on the other [80]. Although the nephrotoxic properties of these chemotherapeutic agents is predominantly attributed to originate with basolateral OCT2-mediated uptake and subsequent apical MATE1- and MATE2-K-mediated efflux, it should be pointed out that significant differences in transport kinetics and transporter-mediated interactions involving MATEs could potentially contribute to lower or higher incidences of nephrotoxicity in patients, depending on relative interindividual differences in functional expression of these proteins. While interindividual differences in expression may predict susceptibility to drug-induced organ damage in tissues with a clear functional role of uptake transporters, it is important to note that these expression differences also contribute as mechanisms of chemoresistance in tumor cells. Consistent with this notion, several studies have demonstrated that the down-regulation of OCT6 in lung and colon cancer tissues is associated with cellular resistance to cisplatin and oxaliplatin [81,82]. Thus, intervention strategies aimed at targeting uptake transporters to ameliorate drug-induced toxicity must be evaluated for antagonized therapeutic efficacy due to unintended inhibition of transport mechanisms regulating entry into tumor cells.
3.2. Neurotoxicity
In addition to their varying degrees to which cisplatin and oxaliplatin induce AKI, platinum agents can also cause severe and debilitating, dose-limiting peripheral neurotoxicity, and this has resulted in extensive preventative efforts as evidenced by the increasing emergence of large numbers of neuroprotective clinical trials [83]. Clinical manifestation of neurotoxicity occurs immediately after infusion and is characterized by paresthesia, ataxia and dysesthesia in the extremities of the body [84,85]. Within the nervous system, platinum agents are unable to penetrate the blood-brain barrier, but rather preferentially accumulate in peripheral sensory neurons present along dorsal root ganglia (DRG) of the spinal cord [86]. In peripheral sensory neurons, induction of nuclear and mitochondrial DNA damage, disruption of ion channels while glial activation of astrocytes and microglial cells are mechanisms of pharmacological interest in preventing mechanical and thermal neuropathic pain [87]. At higher cumulative doses, incompletely recovery and permanent dysfunction of sensory neurons represents a major clinical problem [86].
Somewhat unexpectedly, several studies have demonstrated that OCT2 transcripts are detectable in rodent and human DRG samples, suggesting a carrier-mediated mechanism of accumulation into these structures [88]. Consistently, the genetic deletion of Oct2 in mice prevented the onset of acute oxaliplatin-induced thermal and mechanical allodynia, while this phenotypic preservation occurred in the absence of detectable changes across structurally similar and putative SLC or ABC transporters of relevance to oxaliplatin [88]. This protection can also be recapitulated in wild-type animals using pretreatment with dasatinib, a pharmacological SRC-family kinase inhibitor, which inhibits both mouse and human OCT2 through a noncompetitive mechanism that regulates the post-translational modification of OCT2 through tyrosine phosphorylation by the kinase YES1 [89]. Interestingly, other reports have suggested oxaliplatin is also a transported substrate of rat Octn1 and the use of genetic targeting constructs or pharmacologic inhibition with ergothioneine, an Octn1-specific substrate, protected rats from chronic forms of oxaliplatin neurotoxicity [90–92]. However, due to the reported expression of Octn1 on the mitochondrial membrane and intrinsic antioxidant activity of ergothioneine, the observed neuroprotection may be independent of a direct contribution to the transmembrane transport of oxaliplatin into target cells. This hypothesis would be consistent with data suggesting that ergothioneine can reduce cellular concentrations of oxaliplatin in Octn1-deficient DRG neurons of mice [44]. Furthermore, it is unknown whether ergothioneine competes or exhibit inhibitory activity against putative transporters of relevance to oxaliplatin in neurons. The discrepant findings reported for the involvement of transporters involved in oxaliplatin-induced peripheral neurotoxicity in rodents may also be attributed to differences in transport kinetics or expression between the two species, and further studies are required to delineate the translational contribution of human OCT2, OCTN1, and other SLC family members to this side effect.
3.3. Ototoxicity
Cisplatin is unique among platinum chemotherapeutics in its ability to frequently induce severe hearing-loss (ototoxicity), where the incidence ranges from 20 to 80% in pediatric patients, and this side effect may permanently affect early speech and ultimately hamper social development [93,94]. Excessive generation of reactive oxygen species (ROS), increases in cochlear inflammatory responses and induction of apoptosis mediated by platinum-DNA adduct formation have been proposed as mechanisms of cisplatin ototoxicity [95,96]. However, antioxidant or anti-inflammatory drugs used prophylactically do not prevent permanent damage, and only offer moderate protection in the management of cisplatin-induced cochlear injury [95,96]. It is possible that the initial accumulation of cisplatin into the cochlea cells represents a key trigger for the following pathophysiological changes in cisplatin-induced hearing loss. Indeed, both mouse Oct2 and human OCT2 are expressed in the organ of Corti and stria vascularis of the cochlea, and genetic deficiency or pharmacological targeting of Oct2 with cimetidine protected rodents against toxicity [71]. A retrospective and exploratory analysis of 11 SNPs in the human SLC22A2 gene identified a genetic variant, c.808G>T; Ser270Ala (rs316019), that conferred significant protection against cisplatin-induced ototoxicity in a pediatric cohort [97]. In vitro insertion of the 808G>T allele into OCT2, located within the transmembrane domain [98], reduced transport kinetics (e.g., Vm and Km) of OCT2 substrates (e.g., MPP and dopamine) [98–100] and a moderate increase in serum creatinine [101], supporting the notion that the observed human variant functionally reduces transport affinity of cisplatin and cellular injury. Of note, pantoprazole, an inhibitor of OCT2 [102], did not ameliorate ototoxicity or nephrotoxicity in a pediatric cohort of osteosarcoma patients treated with cisplatin [103]. While prediction of the applied dosing schedules for pantoprazole-mediated OCT2 inhibition was performed using pharmacokinetic modeling and simulations, these strategies were not adequately validated and ultimately lack rationally designed preclinical and clinical studies to document the local (cochlea or kidney) and systemic (blood) changes in endogenous substrates of OCT2 as a pharmacodynamic biomarker of OCT2 function. While the reported intervention study with pantoprazole was negative, caution is warranted against the conclusion that the proposed OCT2 inhibition concept is intrinsically flawed, and different results could have been obtained with a more potent inhibitor given at a dose and schedule known to affect cochlear biomarkers of OCT2 function.
In connection with MATE1 function, it is worth pointing out that, in an adult cohort of patients with head and neck squamous cell carcinoma (HNSCC) receiving cisplatin-containing therapy, the presence of one or two copies of the SLC47A1 variant (rs2289669) significantly predisposed to cisplatin-induced ototoxicity [104]. However, it is uncertain whether this variant produces a gain or loss of function phenotype. Pharmacogenomic analysis of this same variant in patients with type 2 diabetes receiving metformin indicated a reduced function phenotype, as demonstrated by an increase in glucose lowering, a marker of pharmacodynamic response to metformin [105]. On the other hand, the protective effects observed among HNSCC patients suggested a gain of function since MATE1-mediated cisplatin efflux and treatment response was unaffected in the HNSCC cohort [104]. The unique bi-directional antiporter characteristics of MATE1, compared to other SLCs, suggest that the function of this variant may be dependent on tissue localization and cellular polarity of MATE1-expressing cells.
3.4. Cardiotoxicity
Anthracycline agents (e.g., doxorubicin, daunorubicin, epirubicin and idarubicin) are among standard of care chemotherapeutic agents in the treatment of various solid tumors and hematological malignancies in children and adults. Severe and debilitating cardiotoxicity associated with anthracyclines is of significant clinical concern due to the risk of detrimental and irreversible cardiac damage that is accompanied with a high incidence of cardiovascular mortality, especially in young adults and adolescent survivors [106,107]. Initial studies evaluating mechanisms of transmembrane transport of doxorubicin concluded that passive diffusion of the unionized drug is a dominant pathway of uptake [108]. However, the existence of saturation kinetics in vitro and the apparent cationic nature of the drug at physiological pH are supportive of the involvement of a carrier-mediated mechanism [109,110]. Extensive pharmacogenomics studies have identified the class of ABC transporters as important determinants of doxorubicin-induced cardiac injury. Polymophoric variants in MDR1, MRP2, and BCRP have been linked with altered risks of cardiotoxicity, presumably due to reduced function and increased intracellular levels of doxorubicin and its metabolite doxorubicinol in cardiomyocytes [111]. Furthermore, OCT6 polymorphisms have been associated in preliminary studies with pharmacokinetic variability of doxorubicin in humans [112,113]. Despite these observations, direct evidence supporting a role of OCT6 and other OCTs in anthracycline disposition, particularly in the myocardium, remains lacking.
Among the class of OCTs, mRNA transcript and immuno-reactivity is present in the vasculature, endothelial cells, and cardiomyocytes [34,42]. OCTN2 is the highest OCT expressed in the myocardium, and this is not surprising given the cardio-protective role of L-carnitine against oxidative stress and inflammation, and in regulating cardiac energy metabolism [114]. Following OCTN2, expression levels were in decreasing order observed for OCT3 > OCTN1 > OCT1, although their precise localization and expression pattern in these cell types remain controversial [42]. In vitro overexpression of OCTN1, OCT1, and OCT6 indicate a role of cationic-type transporters in facilitating doxorubicin and daunorubicin accumulation in leukemic and ovarian cancer cells [17,115]. The contribution of these transporters to doxorubicin-induced cardiotoxicity remains to be elucidated, though preliminary studies have indicated an important independent contribution of Oct3 in mice [116]. Since OCT3 is known to transport important cardiac signaling hormones (e.g., catecholamines) and given that genetic variants in the SLC22A3 gene locus are associated with the development of coronary artery disease [35], further studies are required to substantiate these findings.
4. Tyrosine kinase inhibitors and OCT/MATEs: Implications for drug interaction studies
In the past decade, the development and approval of small molecule targeted therapies, especially tyrosine kinase inhibitors (TKIs) for the treatment of various solid tumors and hematological malignancies has revolutionized the conventional landscape of cancer treatment [117]. TKIs largely act by competing with adenosine 5’- triphosphate (ATP) on binding sites of tyrosine kinases, such that the displacement of ATP results in the diminished activity of mutated or overexpressed protein kinases [117]. Drug-drug interactions and the high degree of interindividual pharmacokinetic variability observed with TKIs present a great risk for the development of toxicity or suboptimal therapeutic efficacy, especially for drugs with narrow therapeutic windows [118,119].
Mechanistic insights into the contribution of OCTs to the systemic pharmacokinetic profile of TKIs remain largely unstudied. Recently, several reports proposed that tumoral expression of OCT1 mRNA may be a prognostic biomarker for sorafenib treatment and response in patients with hepatocellular carcinoma (HCC) or cholangiocarcinoma [120]. Consistent with this observation, inherited polymorphic variants in OCT1 or pharmacological targeting by quinidine, an OCT1 inhibitor, diminished sorafenib transport activity in tumor cells and overexpressed model systems [120–123]. This notion is further supported by evidence demonstrating that localization of OCT1 protein at the plasma membrane, rather than transcript levels, correlated with improved outcomes in HCC patients receiving sorafenib [122]. Although these reports conclude that OCT1 expression may influence sorafenib response, others studies have suggested that sorafenib transport may be facilitated through a transporter-independent mechanism or mediated by transporters other than OCT1 [124–126]. For example, a recent study demonstrated that genetic deletion of Oct1 in mice did neither diminish liver accumulation of sorafenib nor influence sorafenib plasma pharmacokinetics, and was not associated with compensatory gene expression changes [124]. The possibility that OCT1 may not play an important role in the uptake of sorafenib in cancer cells is further supported by the findings that sorafenib can extensively accumulate in liver cancer cell lines with low levels of OCT1 transcripts or in cells with impaired OCT1 functional activity [125,126]. It is conceivable that clinical associations of OCT1 transcript and protein levels only represent a prognostic tool rather than a predictive biomarker for efficacy in patients receiving sorafenib treatment. In addition, genetic variants in OCT1 could be a mere surrogate biomarker of poor response due to linkage disequilibrium with variants in genes that are causally connected with the pharmacologic action of sorafenib. It is also possible that differences in OCT1 expression observed in HCC patient populations and sorafenib response is an indirect result of sorafenib-mediated OCT1 inhibition, resulting in reduced accumulation of endogenous OCT1 substrates or metabolites that may be essential for liver function (e.g., thiamine) [22]. Similarly, functional activity of OCT1 has been suggested as a prognostic marker for long-term resistance in patients with chronic myeloid leukemia receiving imatinib [127]. However, follow-up preclinical studies have demonstrated an OCT1-independent mechanism of imatinib accumulation in hepatocytes and CD34+ mononuclear cells [128–130]. Similar observations have been made for other putative OCT1-TKI interactions, including those with erlotinib, gefitinib, and pazopanib [131,132]. The lack of consistent preclinical and clinical findings on the role of OCT1-mediated liver clearance of TKIs warrant further investigation using properly validated in vivo models.
The recently updated FDA guidance for industry in January 2020 recommends the evaluation of renal OAT1, OAT3, OCT2, MATE1 and MATE2-K, and hepatic OATP1B1, OATP1B3, MDR1, and BCRP for transporter-mediated drug interactions. If preclinical ADME data indicate active renal secretion (CLr – [funbound, plasma * GFR]) is responsible for ≥25% of systemic clearance, in vitro transporter-mediated drug interaction studies are required to be performed for the renal transporters OAT1, OAT3, OCT2, MATE1 and MATE2-K. In this context, a drug is considered a substrate if (1) accumulation in a cell-based overexpression system is ≥2-fold compared to accumulation observed in cells transfected with an empty vector; and (2) a known inhibitor decreases uptake by ≥50% at a concentration that is 10-times the predicted inhibition constant (Ki) value. Similarly, a drug is considered an inhibitor if the ratio of IC50 or Ki is 10-fold greater than the average maximum unbound inhibitor concentration (Imax,u) observed in the systemic circulation in patients receiving approved doses [21]. Additionally, the need for in vivo clinical drug interaction as a victim or perpetrator is determined based on the drug’s putative site of action, concomitant use within the patient population, or is dependent on a drug’s narrow therapeutic index [133]. Furthermore, supportive evidence from a pharmacodynamic marker of OAT2, OCT2 and MATE function (e.g., serum creatinine) is required in mechanistic studies [134–136]. However, in the regulatory guidance documents, OCT1 or MATE1-mediated hepatic interactions are not required for evaluation.
Structural and physiochemical analysis of TKIs suggest that many of these agents are cationic and highly lipophilic in nature, and are thus themselves potential transported substrates or inhibitors for OCTs and MATEs. Comprehensive interrogation of the available prescribing information and package inserts showed that 7 of the 48 FDA-approved TKIs are claimed to be inhibitors of OCT2 or MATE1 transport function, respectively [137]. Of note, the presence or absence of an OCT2- or MATE1-mediated drug-drug interaction is not mentioned in the prescribing information for a majority of FDA-approved TKIs. An additional search of relevant literature evidence on TKIs identified 15/48 as confirmed inhibitors of OCT2 (Ki range: 0.02 – 10 μM) and 17/48 as inhibitors of MATE1 (Ki range: 0.09 – 10 μM) (Table 1), while a subset of TKIs also potently inhibit OAT1 and OAT3 function [74,89,137,138]. According to the prescribing information, clinical elevations of increases in serum creatinine (all grades) are observed with the majority of TKIs; however, whether this observation is a consequence of the TKI’s direct influence on cationic-type transport or related to intrinsic nephrotoxic activity associated with TKIs, as documented for example with vemurafenib, remains to be elucidated. With the emerging and ever increasing number of TKIs approved, it is plausible that a large subset of TKIs in Table 1 are actually undocumented substrates or inhibitors of OCT2 and MATE1, and remain to be verified as such.
Table 1. FDA-approved tyrosine kinase inhibitors.
List of FDA-approved tyrosine kinase inhibitors and their indications. ✓ represents TKI as either an OCT2 or MATE1 inhibitor described in the prescribing information. X represents TKI is not an inhibitor of OCT2 or MATE1 at clinically-relevant concentrations, as provided by the prescribing information. Fecal/urine recovery and incidence of creatinine elevations are also provided by the prescribing information. IC50 or Ki values are either exact values or estimates after an exhaustive literature search. The list also contains the reference number and citations that provided all table data or values.
| Indication | OCT2 | IC50 | MATE1 | IC50 | Fecal/Urine (% recovered) |
Creatinine (% incidence) |
Reference/ Citation |
|
|---|---|---|---|---|---|---|---|---|
| Acalabrutinib | BTK | X | X | 84/12 | ↑ SCr; 5% | 4523127 | ||
| Afatinib | EGFR HER2/4 |
< 10 μM | 85/4 | ↑ SCr; 49% | 4207081, [89] | |||
| Alectinib | ALK | X | 98/0.5 | ↑ SCr; 28 – 38% | 4273596 | |||
| Avapratinib | PDGFRa KIT |
✓ | 70/18 | ↑ SCr; 29% | 4544122 | |||
| Axitinib | C-KIT PDGFR |
< 10 μM | < 10 μM | 41/23 | ↑ SCr; 55% | 3078397, [89] | ||
| Baricitinib | JAK | ✓ | 20/75 | ↑ SCr | 4271150 | |||
| Binimetinib | MEK1 BRAF |
62/31 | ↑ SCr; 93% | 4283608 | ||||
| Bosutinib | BCR-ABL SRC |
≤ 50 nM | < 10 μM | 91/3 | ↑Renal Impairment; 13% |
4083804, [89] | ||
| Brigatinib | ALK | ✓ | 65/25 | 4090797 | ||||
| Cabozantinib | C-MET | < 10 μM | < 10 μM | 54/21 | ↑ SCr; 58% | 4375294, [89] | ||
| Ceritinib | ALK | 92/1 | ↑ SCr; 58% | 4104052 | ||||
| Cobimetinib | MEK1/2 BRAF |
76/18 | ↑ SCr; 99% | 3845167 | ||||
| Crizotinib | ALK ROS1 MET |
✓ | 0.34 μM | 0.34 μM | 63/22 | ↑ SCr; 98% | 4127887, [89,137] | |
| Dabrafenib | BRAF | ✓ | 71/23 | ↑ SCr; 21% | 4255750 | |||
| Dacomitinib | EGFR HER2/4 |
79/3 | ↑ SCr; 24% | 4327054 | ||||
| Dasatinib | BCR-ABL SRC |
0.02 μM | 0.84 μM | 85/4 | ↑ SCr observed | 4179887, [89,138] | ||
| Encorafenib | BRAF | ✓ | 47/47 | ↑ SCr; 93% | 4283598 | |||
| Entrectinib | TRKs ROS1 ALK |
83/3 | ↑ SCr; 73% | 4477931 | ||||
| Erdafitinib | FGFRs | ✓ | X | 69/19 | ↑ SCr; 52% | 4418725 | ||
| Erlotinib | EGFR | 4.21 μM | 7.93 μM | 83/8 | 3308430, [89,138] | |||
| Fedratinib | JAK2 | ✓ | ✓ | 77/5 | ↑ SCr; 10% | 4478261 | ||
| Fostamatinib | SYK | 80/20 | 4249943 | |||||
| Gefitinib | EGFR | 24.4 μM | 1.92 μM | 86/4 | ↑ SCr; 3% | 4310729, [89,137,138] | ||
| Gilteritinib | FLT3 | ✓ | 64/16 | ↑ SCr; 3% | 4440577 | |||
| Ibrutinib | BTK | 80/10 | ↑ SCr; 9% | 4222705 | ||||
| Imatinib | BCR-ABL | 2.37 μM | 0.05 μM | 68/13 | ↑ SCr | 3993828, [89,137,138] | ||
| Lapatinib | EGFR HER2 |
27/2 | 4359049 | |||||
| Larotrectinib | TRKs | X | X | 58/39 | 4354331 | |||
| Lenvatinib | VEGFRs | 64/25 | ↑Renal impairment; 18% | 4274001 | ||||
| Lorlatinib | ALK ROS1 |
X | ✓ | 41/48 | 4344940 | |||
| Midostaurin | FLT3 | 95/5 | ↑ SCr; 25% | 4090671 | ||||
| Neratinib | EGFR HER2/4 |
97/1 | ↑Renal impairment; 0.4% | 4566456 | ||||
| Nilotinib | BCR-ABL | 0.1 μM | 3.38 μM | 93/ | ↑ SCr; <1% | 4200046, [89,138] | ||
| Nintedanib | PDGFRs FGFRs VEGFRs SRC |
93/1 | 4487848 | |||||
| Osimertinib | EGFR | X | X | 68/14 | 4174859 | |||
| Pazopanib | VEGFRs PDGFRs KIT |
≤ 1 μM | < 0.47 μM | 90/4 | 3823458, [89,137,152] | |||
| Pexidartinib | CSFR1 KIT FLT3 |
X | ✓ | 65/27 | 4471832 | |||
| Ponatinib | ABL | X | < 10 μM | < 10 μM | 85/5 | ↑ SCr; 21% | 4019587, [89] | |
| Regorafenib | VEGFR | < 10 μM | 71/19 | 4090114, [89] | ||||
| Ruxolitinib | JAK1/2 | X | < 10 μM | < 10 μM | 22/74 | 4191667, [89] | ||
| Sorafenib | BRAF FLT3 |
40.8 μM | 1.43 μM | 77/19 | 4357655, [137,138] | |||
| Sunitinib | PDFGRs VEGFRs FLT3 |
1.73 μM | 0.28 μM | 61/16 | ↑ SCr; 27% | 4182165, [89,137,138] | ||
| Tofacitinib | JAK1/3 | 70/30 | ↑ SCr observed | 4269956 | ||||
| Trametinib | MEK1/2 BRAF |
X | X | 80/20 | ↑ SCr; 21% | 4255758 | ||
| Upadacitinib | JAKs | X | X | 38/24 | 4478363 | |||
| Vandetinib | EGFR VEGFR |
✓ | ≤ 1 μM | < 10 μM | 44/25 | ↑ SCr; 16% | 3480537, [89] | |
| Vemurafenib | BRAF | 94/1 | ↑ SCr; 27% | 4084937 | ||||
| Zanubrutinib | BTK | X | 87/8 | 4520008 |
Diminished efficacy and increase risk of toxicity are major potential consequences of drug-drug interactions, especially for agents that have a narrow therapeutic window. One example highlighting this risk is the commonly prescribed anti-arrhythmic drug, dofetilide, where a mere 1-ng/mL increase in plasma concentration is associated with an average increase in the QTc prolongation of 15 ms. Previous work demonstrated that concurrent administration of dofetilide with the OCT2 and MATE1 inhibitor, cimetidine, dose-dependently increased the area under the curve of dofetilide due to impaired renal tubular secretion [139], increased dofetilide-induced QTc prolongation, and increased the risk for Torsades de Pointes. The concurrent use of certain FDA-approved TKIs, such as vandetanib and vemurafenib, is contraindicated with dofetilide, presumably due to an unacceptable risk associated with anticipated increases in systemic exposure or local inhibition of efflux from cardiomyocytes [140]. Although the mechanistic underpinning of these potentially life-threatening, albeit hypothetical scenarios remains further study, it is likely that some off-target effects of TKIs, including inhibition of clinically-important transporters creates a liability by possibly potentiating drug-induced toxicities. In addition to the fact that OCT-inhibitory TKIs can influence the function of uptake transporters, several studies have also demonstrated that many TKIs also inhibit ABC transporters that are relevant to drug efflux in tumor cells and as well as excretory pathways of xenobiotics in the liver and kidney [19,20,141,142]. Although the strategic use of TKIs as modulators of transport function may reduce tissue injury, this intervention approach will only be feasible if there are no detrimental effects on the pharmacokinetics and no negative impact on the antitumor efficacy due to concurrent inhibition of relevant ABC transporters. Detailed preclinical studies evaluating the systemic and distribution changes to on- and off-target (tumor vs. healthy cells) must thus be conducted to ensure drug safety.
A particularly poorly studied area of potentially fruitful research is related to the extent to which the inhibitory potential of TKIs against drug transporters and the predictive ability of in vitro test systems is dependent on specific experimental conditions of the employed models. This not only refers to the type of prototypical transport substrate (e.g., TEA, MPP or metformin) and concentration range of the substrate(s) used, but also to culture media and incubation conditions. These details are not provided in the regulatory guidelines, but rather left at the discretion of investigators. TKI-transport inhibition studies have often remained unpublished and unverified, and this is an unsustainable situation in need of an appropriate and cost-effective solution. This is because implementation of dependable and reproducible approaches to unequivocally establish TKI-transporter interactions -or the lack thereof- in a statistically sound and unbiased manner is required to avoid the generation of conflicting results and should ultimately be utilized to inform the design of subsequent in vivo validation studies.
An additional concern pertains to a general lack of information in the prescribing information of TKIs on the mechanism by which these agents can inhibit transport function (non-competitive vs. competitive; reversible vs. irreversible). The presence or absence of either pre- and co-incubation of TKIs with probe substrates for the transporter(s) of interest could further contribute to their lack of reported inhibitory potential of a drug, and result in false negative observations. For example, dasatinib potently inhibits OCT2 function (IC50 = 15.9 nM) through a non-competitive mechanism that is dependent on the phosphorylating kinase YES1 (Kd = 0.3 nM). However, co-incubation, based on a presumed competitive mechanism of inhibition, with the prototypical transport substrate TEA identified dasatinib as only a weak inhibitor of OCT2. In contrast, pre-incubation was sufficient to significantly influence OCT2 function [89]. Of the 48 TKIs investigated (Table 1), only for crizotinib and vandetanib is there strict concordance between the prescribing information and published literature. This is not surprising given the differential capacity for TKIs to inhibit OCT/MATE function and the discrepancy in published reports, prescribing information, as well as interindividual variations among different laboratory settings. Recently, it was suggested that previously reported conflicting findings are due, in part, to the fact that the potency of transport inhibitors can be highly dependent on the substrate used in the overexpressed model systems [143]. More specifically, this study demonstrated that the prototypical OCT/MATE substrate MPP is the least sensitive to inhibition compared to other probe substrates: metformin, TEA, NBD-MTMA and ASP, with an average of 6-fold higher IC50 values, while metformin produced the lowest variability compared to non-MPP substrates [144–146]. Given that OCTs and MATEs share a high degree of substrate and inhibitor specificity, the choice of model substrates used in in vitro cationic-type transport assays should become an integral component of a rationally-designed strategy to make informative, translationally-relevant predictions.
The absence of regulatory guidelines regarding the design and interpretation of in vitro studies aimed at demonstrating an inhibitory potential of drugs with transporters has likely contributed to this dismal state of affairs. Since TKIs are most commonly prescribed on a chronic basis (e.g., once or twice daily) and given together with multiple other medications, it is hoped that investigators will be encouraged to re-evaluate the potential liability of TKIs as perpetrators of transporter-mediated drug-drug interactions to gain new mechanistic insights and to improve the safety of currently used polypharmacy regimens.
5. Expert opinion
Over the past few decades, the contribution of transporters to the accumulation and efflux of therapeutic drugs has been a focus for many academic institutions and pharmaceutical industries [1]. The preclinical utility of heterologous overexpression systems and genetically engineered model systems has substantially contributed to the in vitro and in vivo understanding of the initial pathophysiology of drug-induced organ damage [18]. Many conventional chemotherapeutic agents are now known to hitchhike on the tissue-specific drug transporters and this process can contribute to the incidence and severity of debilitating cellular injuries that could result in partial or complete tissue dysfunction. Many currently ongoing efforts in the field are focused on the treatment and management of symptoms by targeting an intracellular signaling cascade that influences this cellular dysfunction and injury [147], and the targeting of these intracellular mechanisms may antagonize antitumor properties due to overlapping signaling pathways between normal and cancer cells. In contrast, the targeting of transmembrane transport mechanisms unique to healthy tissues may offer preventative approaches that can be exploited strategically (Figure 1), without negatively influencing antitumor properties and disease management [148]. The class of TKIs is of particular interest in this context due to their inhibitory potency towards members of the SLC and ABC family and because of their already approved use in the treatment of a wide array of solid tumors and hematological malignancies [117]. The success of these approaches is also dependent on the lack of unintended inhibition in off-target and healthy cells. The strategy of integrating transport inhibitors to prevent drug-induced toxicity can be optimized to inhibit local exposure without altering the systemic pharmacokinetics of the chemotherapeutic agent. Rationally-designed preclinical and clinical studies that document the local and systemic changes in endogenous substrates of OCT/MATE should also be integrated to serve as a pharmacodynamic biomarker of transport function to achieve an optimal degree of temporary inhibition.
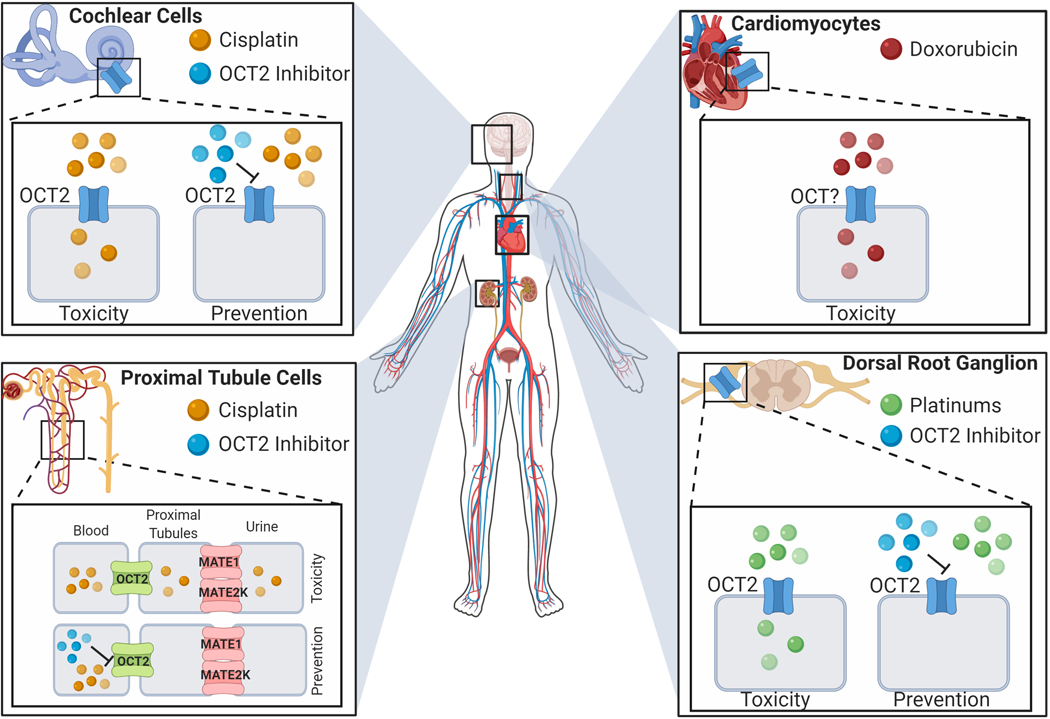
Figure 1.
Proposed mechanism of chemotherapy-induced cellular injury and clinical intervention. Expression and localization of cationic transporters in cochlear and proximal tubular cells, and dorsal root ganglion as mediators of established toxicity profiles associated with chemotherapeutic agents. Putative transport of doxorubicin in cardiomyocytes.
The recently updated FDA guidelines for in vitro drug interaction studies do not necessarily predict the probability of transporter-mediated drug interactions, and further revision of these guidelines is warranted. The absence of detailed guidance on experimental methodology and study design, which are now at the discretion of investigators, can contribute to the generation of false negative data, as evidenced by the noted discrepancies between data contained in package inserts and those available in the published literature (Table 1). It is our contention that the number of currently FDA-approved TKIs that is able to substantially affect the function of OCTs and/or MATEs is underestimated [116,140], and in that context the following recommendations are offered:
1). Background Uptake.
The identification of transporters involved in TKI uptake with the use of currently available in vitro model systems is difficult due to high background accumulation in most mammalian cells, such as HEK293, HeLa and MDCK-II cells [124]. Oocytes from Xenopus laevis are a potentially useful alternative overexpression system for such studies due to their ability to translate foreign genetic material (including post-translational modifications), correct localization to subcellular compartments, and have a low number of endogenous transport systems expressed on their plasma membranes that may differentiate between active uptake and signal noise [149]. Many TKIs also preferentially accumulate at high concentrations within lysosomes and/or bind extracellularly, and these properties may further artificially increase the background reading and be mistaken for mediated uptake. Manipulation of proton concentrations in incubation buffers and known inhibitors that are absent of lysosomal properties can potentially delineate between lysosomal trapping and transporter-mediated uptake [150,151].
2). Identification of inhibitors.
Transport of prototypical substrates (e.g., TEA, MPP and metformin) is dependent on their kinetic affinity for OCTs and MATEs. Although the guidelines outline Imax, unbound /IC50 or Ki ≥ 0.1 as the criterion for consideration, a drug’s putative inhibition potential could also be dependent on the duration of uptake studies, and this is rarely considered in the experimental design. A requirement should be outlined regarding different inhibitor incubation methods, as transport function might be sensitive to the presence or absence of pre-incubation conditions. This also helps delineate between non-competitive and competitive mechanisms of inhibition. If a drug is identified as an inhibitor of a transporter, reversibility of the property should also be verified in order to allow translationally-relevant predictions to be made, depending on the chosen dose and frequency of administration of the agent to humans. Indeed, irreversible inhibition or prolonged times required for recovery of transport function are important considerations in order to arrive at meaningful predictions regarding the long-term functional consequences of TKI-mediated transport inhibition and to properly predict interactions with concomitant medications.
More comprehensive evaluation of drug-transporter interactions should become an integrated approach to human studies aimed at understanding basic pharmacokinetic properties of approved and investigational oncology drugs, and it is anticipated that such studies will continue to make significant contributions to the design of clinical studies aimed at improving the safety of patients afflicted with cancer.
Article highlights.
Organic cation-type transporters are integral membrane proteins that facilitate the translocation of endogenous substrates and xenobiotics across a biological membrane.
Debilitating toxicities associated with several chemotherapeutic agents are dependent on the tissue-specific expression of organic cation transporters that governs their cellular accumulation.
Modulating transport function to prevent toxicity without antagonizing therapeutic benefits of anticancer agents represents a novel intervention strategy that can be translationally exploited.
Tyrosine kinase inhibitors are an emerging class of anticancer therapeutics that may influence transport function to a greater extent than held presently, and these agents are thus liable to perpetrate drug-drug interactions and affect drug-induced toxicity.
Recently updated regulatory guidelines on the conduct of in vitro drug interaction studies remain ambiguous and warrant further revision.
Funding
This review is supported in part by the NIH grants R01CA215802 (AS), R01CA187176 (AS) and R01CA238946 (MBL and SH), by the OSU Comprehensive Cancer Center using Pelotonia funds (KMH), and by the American Heart Association (MEU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Articles of special interest have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- 1.Cesar-Razquin A, Snijder B, Frappier-Brinton T, et al. A Call for Systematic Research on Solute Carriers. Cell. 2015. July 30;162(3):478–87. [DOI] [PubMed] [Google Scholar]
- 2.International Transporter C, Giacomini KM, Huang SM, et al. Membrane transporters in drug development. Nat Rev Drug Discov. 2010. March;9(3):215–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koepsell H, Lips K, Volk C. Polyspecific organic cation transporters: structure, function, physiological roles, and biopharmaceutical implications. Pharm Res. 2007. July;24(7):1227–51. [DOI] [PubMed] [Google Scholar]
- 4.Schaller L, Lauschke VM. The genetic landscape of the human solute carrier (SLC) transporter superfamily. Hum Genet. 2019. December;138(11–12):1359–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller F, Fromm MF. Transporter-mediated drug-drug interactions. Pharmacogenomics. 2011. July;12(7):1017–37. [DOI] [PubMed] [Google Scholar]
- 6.Giacomini KM, Balimane PV, Cho SK, et al. International Transporter Consortium commentary on clinically important transporter polymorphisms. Clin Pharmacol Ther. 2013. July;94(1):23–6. [DOI] [PubMed] [Google Scholar]
- 7.Hediger MA, Clemencon B, Burrier RE, et al. The ABCs of membrane transporters in health and disease (SLC series): introduction. Mol Aspects Med. 2013. April-Jun;34(2–3):95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hediger MA, Romero MF, Peng JB, et al. The ABCs of solute carriers: physiological, pathological and therapeutic implications of human membrane transport proteinsIntroduction. Pflugers Arch. 2004. February;447(5):465–8. [DOI] [PubMed] [Google Scholar]
- 9.Zhang L, Brett CM, Giacomini KM. Role of organic cation transporters in drug absorption and elimination. Annu Rev Pharmacol Toxicol. 1998;38:431–60. [DOI] [PubMed] [Google Scholar]
- 10.Grundemann D, Gorboulev V, Gambaryan S, et al. Drug excretion mediated by a new prototype of polyspecific transporter. Nature. 1994. December 8;372(6506):549–52. [DOI] [PubMed] [Google Scholar]
- 11.Zhang S, Lovejoy KS, Shima JE, et al. Organic cation transporters are determinants of oxaliplatin cytotoxicity. Cancer Res. 2006. September 1;66(17):8847–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alnouti Y, Petrick JS, Klaassen CD. Tissue distribution and ontogeny of organic cation transporters in mice. Drug Metab Dispos. 2006. March;34(3):477–82. [DOI] [PubMed] [Google Scholar]
- 13.Gorboulev V, Ulzheimer JC, Akhoundova A, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997. July;16(7):871–81. [DOI] [PubMed] [Google Scholar]
- 14.Hiasa M, Matsumoto T, Komatsu T, et al. Wide variety of locations for rodent MATE1, a transporter protein that mediates the final excretion step for toxic organic cations. Am J Physiol Cell Physiol. 2006. October;291(4):C678–86. [DOI] [PubMed] [Google Scholar]
- 15.Ohta KY, Inoue K, Hayashi Y, et al. Molecular identification and functional characterization of rat multidrug and toxin extrusion type transporter 1 as an organic cation/H+ antiporter in the kidney. Drug Metab Dispos. 2006. November;34(11):1868–74. [DOI] [PubMed] [Google Scholar]
- 16.Yonezawa A, Inui K. Importance of the multidrug and toxin extrusion MATE/SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and pharmacogenomics. Br J Pharmacol. 2011. December;164(7):1817–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okabe M, Unno M, Harigae H, et al. Characterization of the organic cation transporter SLC22A16: a doxorubicin importer. Biochem Biophys Res Commun. 2005. August 5;333(3):754–62. [DOI] [PubMed] [Google Scholar]
- 18.Koepsell H. Organic Cation Transporters in Health and Disease. Pharmacol Rev. 2020. January;72(1):253–319.** Koepsell summarizes an updated overarching review of human organic cation transporters (OCT1–3, OCTN1/2, MATE1 and MATE2-K) as critical determinants of energy metabolism, pharmacokinetics, drug-drug interactions, drug response, and toxicity of drugs. Furthermore, this review also focuses on the molecular mechanisms of substrate specificity recognize by cationic-type transporters.
- 19.Nicolaou M, Andress EJ, Zolnerciks JK, et al. Canalicular ABC transporters and liver disease. J Pathol. 2012. January;226(2):300–15. [DOI] [PubMed] [Google Scholar]
- 20.Robey RW, Pluchino KM, Hall MD, et al. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018. July;18(7):452–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Research FaDACfDEa. In Vitro Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry. FDA Maryland; 2020. [Google Scholar]
- 22.Chen L, Shu Y, Liang X, et al. OCT1 is a high-capacity thiamine transporter that regulates hepatic steatosis and is a target of metformin. Proc Natl Acad Sci U S A. 2014. July 8;111(27):9983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denk GU, Soroka CJ, Mennone A, et al. Down-regulation of the organic cation transporter 1 of rat liver in obstructive cholestasis. Hepatology. 2004. May;39(5):1382–9. [DOI] [PubMed] [Google Scholar]
- 24.Nies AT, Koepsell H, Winter S, et al. Expression of organic cation transporters OCT1 (SLC22A1) and OCT3 (SLC22A3) is affected by genetic factors and cholestasis in human liver. Hepatology. 2009. October;50(4):1227–40. [DOI] [PubMed] [Google Scholar]
- 25.Zamek-Gliszczynski MJ, Giacomini KM, Zhang L. Emerging Clinical Importance of Hepatic Organic Cation Transporter 1 (OCT1) in Drug Pharmacokinetics, Dynamics, Pharmacogenetic Variability, and Drug Interactions. Clin Pharmacol Ther. 2018. May;103(5):758–760. [DOI] [PubMed] [Google Scholar]
- 26.Shu Y, Sheardown SA, Brown C, et al. Effect of genetic variation in the organic cation transporter 1 (OCT1) on metformin action. J Clin Invest. 2007. May;117(5):1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lips KS, Volk C, Schmitt BM, et al. Polyspecific cation transporters mediate luminal release of acetylcholine from bronchial epithelium. Am J Respir Cell Mol Biol. 2005. July;33(1):79–88. [DOI] [PubMed] [Google Scholar]
- 28.Lin CJ, Tai Y, Huang MT, et al. Cellular localization of the organic cation transporters, OCT1 and OCT2, in brain microvessel endothelial cells and its implication for MPTP transport across the blood-brain barrier and MPTP-induced dopaminergic toxicity in rodents. J Neurochem. 2010. August;114(3):717–27. [DOI] [PubMed] [Google Scholar]
- 29.Chen JJ, Li Z, Pan H, et al. Maintenance of serotonin in the intestinal mucosa and ganglia of mice that lack the high-affinity serotonin transporter: Abnormal intestinal motility and the expression of cation transporters. J Neurosci. 2001. August 15;21(16):6348–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karbach U, Kricke J, Meyer-Wentrup F, et al. Localization of organic cation transporters OCT1 and OCT2 in rat kidney. Am J Physiol Renal Physiol. 2000. October;279(4):F679–87. [DOI] [PubMed] [Google Scholar]
- 31.Sugawara-Yokoo M, Urakami Y, Koyama H, et al. Differential localization of organic cation transporters rOCT1 and rOCT2 in the basolateral membrane of rat kidney proximal tubules. Histochem Cell Biol. 2000. September;114(3):175–80. [DOI] [PubMed] [Google Scholar]
- 32.Wu X, Kekuda R, Huang W, et al. Identity of the organic cation transporter OCT3 as the extraneuronal monoamine transporter (uptake2) and evidence for the expression of the transporter in the brain. J Biol Chem. 1998. December 4;273(49):32776–86. [DOI] [PubMed] [Google Scholar]
- 33.Hayer-Zillgen M, Bruss M, Bonisch H. Expression and pharmacological profile of the human organic cation transporters hOCT1, hOCT2 and hOCT3. Br J Pharmacol. 2002. July;136(6):829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Solbach TF, Grube M, Fromm MF, et al. Organic cation transporter 3: expression in failing and nonfailing human heart and functional characterization. J Cardiovasc Pharmacol. 2011. October;58(4):409–17. [DOI] [PubMed] [Google Scholar]
- 35.Tregouet DA, Konig IR, Erdmann J, et al. Genome-wide haplotype association study identifies the SLC22A3-LPAL2-LPA gene cluster as a risk locus for coronary artery disease. Nat Genet. 2009. March;41(3):283–5. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Hong C, Chen EC, et al. Genetic and epigenetic regulation of the organic cation transporter 3, SLC22A3. Pharmacogenomics J. 2013. April;13(2):110–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao Q, Wei H, Liu D, et al. PHACTR1 and SLC22A3 gene polymorphisms are associated with reduced coronary artery disease risk in the male Chinese Han population. Oncotarget. 2017. January 3;8(1):658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zwart R, Verhaagh S, Buitelaar M, et al. Impaired activity of the extraneuronal monoamine transporter system known as uptake-2 in Orct3/Slc22a3-deficient mice. Mol Cell Biol. 2001. July;21(13):4188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu X, Prasad PD, Leibach FH, et al. cDNA sequence, transport function, and genomic organization of human OCTN2, a new member of the organic cation transporter family. Biochem Biophys Res Commun. 1998. May 29;246(3):589–95. [DOI] [PubMed] [Google Scholar]
- 40.Grundemann D, Harlfinger S, Golz S, et al. Discovery of the ergothioneine transporter. Proc Natl Acad Sci U S A. 2005. April 5;102(14):5256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T, Nakanishi T, Haruta T, et al. Transport of ipratropium, an anti-chronic obstructive pulmonary disease drug, is mediated by organic cation/carnitine transporters in human bronchial epithelial cells: implications for carrier-mediated pulmonary absorption. Mol Pharm. 2010. February 1;7(1):187–95. [DOI] [PubMed] [Google Scholar]
- 42.Grube M, Ameling S, Noutsias M, et al. Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am J Pathol. 2011. June;178(6):2547–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tachikawa M, Takeda Y, Tomi M, et al. Involvement of OCTN2 in the transport of acetyl-L-carnitine across the inner blood-retinal barrier. Invest Ophthalmol Vis Sci. 2010. January;51(1):430–6. [DOI] [PubMed] [Google Scholar]
- 44.Jong NN-J. The role of the organic cation/carnitine transporters in the uptake and neurotoxicity of oxaliplatin: The University of Auckland; 2012. [Google Scholar]
- 45.Shekhawat PS, Sonne S, Matern D, et al. Embryonic lethality in mice due to carnitine transporter OCTN2 defect and placental carnitine deficiency. Placenta. 2018. September;69:71–73. [DOI] [PubMed] [Google Scholar]
- 46.Otsuka M, Matsumoto T, Morimoto R, et al. A human transporter protein that mediates the final excretion step for toxic organic cations. Proc Natl Acad Sci U S A. 2005. December 13;102(50):17923–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsuda M, Terada T, Mizuno T, et al. Targeted disruption of the multidrug and toxin extrusion 1 (mate1) gene in mice reduces renal secretion of metformin. Mol Pharmacol. 2009. June;75(6):1280–6. [DOI] [PubMed] [Google Scholar]
- 48.Go RS, Adjei AA. Review of the comparative pharmacology and clinical activity of cisplatin and carboplatin. J Clin Oncol. 1999. January;17(1):409–22. [DOI] [PubMed] [Google Scholar]
- 49.Planting AS, van der Burg ME, de Boer-Dennert M, et al. Phase I/II study of a short course of weekly cisplatin in patients with advanced solid tumours. Br J Cancer. 1993. October;68(4):789–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jassem J, Gyergyay F, Kerpel-Fronius S, et al. Combination of daily 4-h infusion of 5-fluorouracil and cisplatin in the treatment of advanced head and neck squamous-cell carcinoma: a South-East European Oncology Group study. Cancer Chemother Pharmacol. 1993;31(6):489–94. [DOI] [PubMed] [Google Scholar]
- 51.Meijer S, Mulder NH, Sleijfer DT, et al. Nephrotoxicity of cis-diamminedichloride platinum (CDDP) during remission-induction and maintenance chemotherapy of testicular carcinoma. Cancer Chemother Pharmacol. 1982;8(1):27–30. [DOI] [PubMed] [Google Scholar]
- 52.Safirstein R, Winston J, Goldstein M, et al. Cisplatin nephrotoxicity. Am J Kidney Dis. 1986. November;8(5):356–67. [DOI] [PubMed] [Google Scholar]
- 53.Weiner MW, Jacobs C. Mechanism of cisplatin nephrotoxicity. Fed Proc. 1983. October;42(13):2974–8. [PubMed] [Google Scholar]
- 54.Meijer S, Sleijfer DT, Mulder NH, et al. Cisplatin-induced nephrotoxicity. Neth J Med. 1982;25(8):262–9. [PubMed] [Google Scholar]
- 55.de Jongh FE, Gallo JM, Shen M, et al. Population pharmacokinetics of cisplatin in adult cancer patients. Cancer Chemother Pharmacol. 2004. August;54(2):105–12. [DOI] [PubMed] [Google Scholar]
- 56.Johnsson A, Hoglund P, Grubb A, et al. Cisplatin pharmacokinetics and pharmacodynamics in patients with squamous-cell carcinoma of the head/neck or esophagus. Cancer Chemother Pharmacol. 1996;39(1–2):25–33. [DOI] [PubMed] [Google Scholar]
- 57.Stewart DJ, Mikhael NZ, Nanji AA, et al. Renal and hepatic concentrations of platinum: relationship to cisplatin time, dose, and nephrotoxicity. J Clin Oncol. 1985. September;3(9):1251–6. [DOI] [PubMed] [Google Scholar]
- 58.Zhang P, Chen JQ, Huang WQ, et al. Renal Medulla is More Sensitive to Cisplatin than Cortex Revealed by Untargeted Mass Spectrometry-Based Metabolomics in Rats. Sci Rep. 2017. March 16;7:44804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Volarevic V, Djokovic B, Jankovic MG, et al. Molecular mechanisms of cisplatin-induced nephrotoxicity: a balance on the knife edge between renoprotection and tumor toxicity. J Biomed Sci. 2019. March 13;26(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Price PM, Safirstein RL, Megyesi J. Protection of renal cells from cisplatin toxicity by cell cycle inhibitors. Am J Physiol Renal Physiol. 2004. February;286(2):F378–84. [DOI] [PubMed] [Google Scholar]
- 61.Hayati F, Hossainzadeh M, Shayanpour S, et al. Prevention of cisplatin nephrotoxicity. J Nephropharmacol. 2016;5(1):57–60. [PMC free article] [PubMed] [Google Scholar]
- 62.Pabla N, Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008. May;73(9):994–1007. [DOI] [PubMed] [Google Scholar]
- 63.Filipski KK, Mathijssen RH, Mikkelsen TS, et al. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009. October;86(4):396–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Filipski KK, Loos WJ, Verweij J, et al. Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res. 2008. June 15;14(12):3875–80. [DOI] [PubMed] [Google Scholar]
- 65.Song IS, Shin HJ, Shim EJ, et al. Genetic variants of the organic cation transporter 2 influence the disposition of metformin. Clin Pharmacol Ther. 2008. November;84(5):559–62. [DOI] [PubMed] [Google Scholar]
- 66.Song IS, Shin HJ, Shin JG. Genetic variants of organic cation transporter 2 (OCT2) significantly reduce metformin uptake in oocytes. Xenobiotica. 2008. September;38(9):1252–62. [DOI] [PubMed] [Google Scholar]
- 67.Nematbakhsh M, Ebrahimian S, Tooyserkani M, et al. Gender difference in Cisplatin-induced nephrotoxicity in a rat model: greater intensity of damage in male than female. Nephrourol Mon. 2013. July 1;5(3):818–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakamura T, Yonezawa A, Hashimoto S, et al. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol. 2010. December 1;80(11):1762–7. [DOI] [PubMed] [Google Scholar]
- 69.Katsuda H, Yamashita M, Katsura H, et al. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol Pharm Bull. 2010;33(11):1867–71. [DOI] [PubMed] [Google Scholar]
- 70.Tanihara Y, Masuda S, Katsura T, et al. Protective effect of concomitant administration of imatinib on cisplatin-induced nephrotoxicity focusing on renal organic cation transporter OCT2. Biochem Pharmacol. 2009. November 1;78(9):1263–71. [DOI] [PubMed] [Google Scholar]
- 71.Ciarimboli G, Deuster D, Knief A, et al. Organic cation transporter 2 mediates cisplatin-induced oto- and nephrotoxicity and is a target for protective interventions. Am J Pathol. 2010. March;176(3):1169–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Townsend DM, Marto JA, Deng M, et al. High pressure liquid chromatography and mass spectrometry characterization of the nephrotoxic biotransformation products of Cisplatin. Drug Metab Dispos. 2003. June;31(6):705–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sprowl JA, Lancaster CS, Pabla N, et al. Cisplatin-induced renal injury is independently mediated by OCT2 and p53. Clin Cancer Res. 2014. August 1;20(15):4026–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hu S, Leblanc AF, Gibson AA, et al. Identification of OAT1/OAT3 as Contributors to Cisplatin Toxicity. Clin Transl Sci. 2017. September;10(5):412–420.* This particular study by Hu et al., demonstrated the importance of dual cationic and anionic transport mechanisms that both dependently and independently contribute cisplatin-induced acute kidney injury. The study also identified tyrosine kinase inhibitors as modulators of both organic cation- and anion-type transport function.
- 75.Sprowl JA, Mathijssen RH, Sparreboom A. Can erlotinib ameliorate cisplatin-induced toxicities? J Clin Oncol. 2013. September 20;31(27):3442–3. [DOI] [PubMed] [Google Scholar]
- 76.Yonezawa A, Masuda S, Yokoo S, et al. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1–3 and multidrug and toxin extrusion family). J Pharmacol Exp Ther. 2006. November;319(2):879–86. [DOI] [PubMed] [Google Scholar]
- 77.Yokoo S, Yonezawa A, Masuda S, et al. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007. August 1;74(3):477–87. [DOI] [PubMed] [Google Scholar]
- 78.Hucke A, Park GY, Bauer OB, et al. Interaction of the New Monofunctional Anticancer Agent Phenanthriplatin With Transporters for Organic Cations. Front Chem. 2018;6:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aleksa K, Halachmi N, Ito S, et al. Renal ontogeny of ifosfamide nephrotoxicity. J Lab Clin Med. 2004. December;144(6):285–93. [DOI] [PubMed] [Google Scholar]
- 80.Ciarimboli G, Holle SK, Vollenbrocker B, et al. New clues for nephrotoxicity induced by ifosfamide: preferential renal uptake via the human organic cation transporter 2. Mol Pharm. 2011. February 7;8(1):270–9. [DOI] [PubMed] [Google Scholar]
- 81.Kunii E, Oguri T, Kasai D, et al. Organic cation transporter OCT6 mediates cisplatin uptake and resistance to cisplatin in lung cancer. Cancer Chemother Pharmacol. 2015. May;75(5):985–91. [DOI] [PubMed] [Google Scholar]
- 82.Oguri T, Kunii E, Fukuda S, et al. Organic cation transporter 6 directly confers resistance to anticancer platinum drugs. Biomed Rep. 2016. November;5(5):639–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cavaletti G. Peripheral neurotoxicity of platinum-based chemotherapy. Nat Rev Cancer. 2008. January;8(1):1p following 71; author reply 1p following 71. [DOI] [PubMed] [Google Scholar]
- 84.Argyriou AA, Polychronopoulos P, Iconomou G, et al. A review on oxaliplatin-induced peripheral nerve damage. Cancer Treat Rev. 2008. June;34(4):368–77. [DOI] [PubMed] [Google Scholar]
- 85.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009. January;8(1):10–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pachman DR, Qin R, Seisler DK, et al. Clinical course of oxaliplatin-induced neuropathy: results from the randomized phase III trial N08CB (Alliance). J Clin Oncol. 2015. October 20;33(30):3416–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Di Cesare Mannelli L, Pacini A, Micheli L, et al. Glial role in oxaliplatin-induced neuropathic pain. Exp Neurol. 2014. November;261:22–33. [DOI] [PubMed] [Google Scholar]
- 88.Sprowl JA, Ciarimboli G, Lancaster CS, et al. Oxaliplatin-induced neurotoxicity is dependent on the organic cation transporter OCT2. Proc Natl Acad Sci U S A. 2013. July 2;110(27):11199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sprowl JA, Ong SS, Gibson AA, et al. A phosphotyrosine switch regulates organic cation transporters. Nat Commun. 2016. March 16;7:10880.** Studies by Sprowl et al., have demonstrated the importance of organic cation transport systems to the toxicity profile of platinum agents (cisplatin and oxaliplatin) using validated in vitro and in vivo model systems. This current highlighted review sheds light onto the etiology of tyrosine kinase inhibitor-mediated inhibition of cationic-type transport function through post-translational modification of OCT2 by the kinase YES1. This particular study identifies potential off-target effects through unintended inhibition of transporter function.
- 90.Jong NN, Nakanishi T, Liu JJ, et al. Oxaliplatin transport mediated by organic cation/carnitine transporters OCTN1 and OCTN2 in overexpressing human embryonic kidney 293 cells and rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 2011. August;338(2):537–47. [DOI] [PubMed] [Google Scholar]
- 91.Nishida K, Takeuchi K, Hosoda A, et al. Ergothioneine ameliorates oxaliplatin-induced peripheral neuropathy in rats. Life Sci. 2018. August 15;207:516–524. [DOI] [PubMed] [Google Scholar]
- 92.Fujita S, Hirota T, Sakiyama R, et al. Identification of drug transporters contributing to oxaliplatin-induced peripheral neuropathy. J Neurochem. 2019. February;148(3):373–385. [DOI] [PubMed] [Google Scholar]
- 93.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005. December 1;23(34):8588–96. [DOI] [PubMed] [Google Scholar]
- 94.Coradini PP, Cigana L, Selistre SG, et al. Ototoxicity from cisplatin therapy in childhood cancer. J Pediatr Hematol Oncol. 2007. June;29(6):355–60. [DOI] [PubMed] [Google Scholar]
- 95.Rybak LP, Mukherjea D, Jajoo S, et al. Cisplatin ototoxicity and protection: clinical and experimental studies. Tohoku J Exp Med. 2009. November;219(3):177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sheth S, Mukherjea D, Rybak LP, et al. Mechanisms of Cisplatin-Induced Ototoxicity and Otoprotection. Front Cell Neurosci. 2017;11:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lanvers-Kaminsky C, Sprowl JA, Malath I, et al. Human OCT2 variant c.808G>T confers protection effect against cisplatin-induced ototoxicity. Pharmacogenomics. 2015;16(4):323–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Leabman MK, Huang CC, Kawamoto M, et al. Polymorphisms in a human kidney xenobiotic transporter, OCT2, exhibit altered function. Pharmacogenetics. 2002. July;12(5):395–405. [DOI] [PubMed] [Google Scholar]
- 99.Zolk O, Solbach TF, Konig J, et al. Functional characterization of the human organic cation transporter 2 variant p.270Ala>Ser. Drug Metab Dispos. 2009. June;37(6):1312–8. [DOI] [PubMed] [Google Scholar]
- 100.Zhang J, Zhou W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem Toxicol. 2012. July;50(7):2289–93. [DOI] [PubMed] [Google Scholar]
- 101.Iwata K, Aizawa K, Kamitsu S, et al. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin Exp Nephrol. 2012. December;16(6):843–51. [DOI] [PubMed] [Google Scholar]
- 102.Hacker K, Maas R, Kornhuber J, et al. Substrate-Dependent Inhibition of the Human Organic Cation Transporter OCT2: A Comparison of Metformin with Experimental Substrates. PLoS One. 2015;10(9):e0136451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fox E, Levin K, Zhu Y, et al. Pantoprazole, an Inhibitor of the Organic Cation Transporter 2, Does Not Ameliorate Cisplatin-Related Ototoxicity or Nephrotoxicity in Children and Adolescents with Newly Diagnosed Osteosarcoma Treated with Methotrexate, Doxorubicin, and Cisplatin. Oncologist. 2018. July;23(7):762–e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Teft WA, Winquist E, Nichols AC, et al. Predictors of cisplatin-induced ototoxicity and survival in chemoradiation treated head and neck cancer patients. Oral Oncol. 2019. February;89:72–78. [DOI] [PubMed] [Google Scholar]
- 105.Tkac I, Klimcakova L, Javorsky M, et al. Pharmacogenomic association between a variant in SLC47A1 gene and therapeutic response to metformin in type 2 diabetes. Diabetes Obes Metab. 2013. February;15(2):189–91. [DOI] [PubMed] [Google Scholar]
- 106.Synold TW, Doroshow JH. Anthracycline dose intensity: clinical pharmacology and pharmacokinetics of high-dose doxorubicin administered as a 96-hour continuous intravenous infusion. J Infus Chemother. 1996. Spring;6(2):69–73. [PubMed] [Google Scholar]
- 107.Kremer LC, van Dalen EC, Offringa M, et al. Anthracycline-induced clinical heart failure in a cohort of 607 children: long-term follow-up study. J Clin Oncol. 2001. January 1;19(1):191–6. [DOI] [PubMed] [Google Scholar]
- 108.Peterson C, Trouet A. Transport and storage of daunorubicin and doxorubicin in cultured fibroblasts. Cancer Res. 1978. December;38(12):4645–9. [PubMed] [Google Scholar]
- 109.Dalmark M, Storm HH. A Fickian diffusion transport process with features of transport catalysis. Doxorubicin transport in human red blood cells. J Gen Physiol. 1981. October;78(4):349–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Raghunand N, He X, van Sluis R, et al. Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer. 1999. June;80(7):1005–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang KM, Hu S, Sparreboom A. Drug transporters and anthracycline-induced cardiotoxicity. Pharmacogenomics. 2018. July 1;19(11):883–888. [DOI] [PubMed] [Google Scholar]
- 112.Lal S, Wong ZW, Jada SR, et al. Novel SLC22A16 polymorphisms and influence on doxorubicin pharmacokinetics in Asian breast cancer patients. Pharmacogenomics. 2007. June;8(6):567–75. [DOI] [PubMed] [Google Scholar]
- 113.Faraji A, Dehghan Manshadi HR, Mobaraki M, et al. Association of ABCB1 and SLC22A16 Gene Polymorphisms with Incidence of Doxorubicin-Induced Febrile Neutropenia: A Survey of Iranian Breast Cancer Patients. PLoS One. 2016;11(12):e0168519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang ZY, Liu YY, Liu GH, et al. l-Carnitine and heart disease. Life Sci. 2018. February 1;194:88–97. [DOI] [PubMed] [Google Scholar]
- 115.Okabe M, Szakacs G, Reimers MA, et al. Profiling SLCO and SLC22 genes in the NCI-60 cancer cell lines to identify drug uptake transporters. Mol Cancer Ther. 2008. September;7(9):3081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang K, Magdy T, Hu S, et al. , editors. Carrier-mediated transport of doxorubicin in cardiomyocytes Clinical Pharmacology & Therapeutics 2018: WILEY 111 RIVER ST, HOBOKEN 07030–5774, NJ USA. [Google Scholar]
- 117.Wu P, Nielsen TE, Clausen MH. FDA-approved small-molecule kinase inhibitors. Trends Pharmacol Sci. 2015. July;36(7):422–39. [DOI] [PubMed] [Google Scholar]
- 118.Baker SD, Hu S. Pharmacokinetic considerations for new targeted therapies. Clin Pharmacol Ther. 2009. February;85(2):208–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jeon JY, Sparreboom A, Baker SD. Kinase Inhibitors: The Reality Behind the Success. Clin Pharmacol Ther. 2017. November;102(5):726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Grimm D, Lieb J, Weyer V, et al. Organic Cation Transporter 1 (OCT1) mRNA expression in hepatocellular carcinoma as a biomarker for sorafenib treatment. BMC Cancer. 2016. February 12;16:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Herraez E, Lozano E, Macias RI, et al. Expression of SLC22A1 variants may affect the response of hepatocellular carcinoma and cholangiocarcinoma to sorafenib. Hepatology. 2013. September;58(3):1065–73. [DOI] [PubMed] [Google Scholar]
- 122.Geier A, Macias RI, Bettinger D, et al. The lack of the organic cation transporter OCT1 at the plasma membrane of tumor cells precludes a positive response to sorafenib in patients with hepatocellular carcinoma. Oncotarget. 2017. February 28;8(9):15846–15857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Al-Abdulla R, Lozano E, Macias RIR, et al. Epigenetic events involved in organic cation transporter 1-dependent impaired response of hepatocellular carcinoma to sorafenib. Br J Pharmacol. 2019. March;176(6):787–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen M, Neul C, Schaeffeler E, et al. Sorafenib Activity and Disposition in Liver Cancer Does Not Depend on Organic Cation Transporter 1. Clin Pharmacol Ther. 2020. January;107(1):227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swift B, Nebot N, Lee JK, et al. Sorafenib hepatobiliary disposition: mechanisms of hepatic uptake and disposition of generated metabolites. Drug Metab Dispos. 2013. June;41(6):1179–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zimmerman EI, Hu S, Roberts JL, et al. Contribution of OATP1B1 and OATP1B3 to the disposition of sorafenib and sorafenib-glucuronide. Clin Cancer Res. 2013. March 15;19(6):1458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.White DL, Dang P, Engler J, et al. Functional activity of the OCT-1 protein is predictive of long-term outcome in patients with chronic-phase chronic myeloid leukemia treated with imatinib. J Clin Oncol. 2010. June 1;28(16):2761–7. [DOI] [PubMed] [Google Scholar]
- 128.Engler JR, Frede A, Saunders V, et al. The poor response to imatinib observed in CML patients with low OCT-1 activity is not attributable to lower uptake of imatinib into their CD34+ cells. Blood. 2010. October 14;116(15):2776–8. [DOI] [PubMed] [Google Scholar]
- 129.Nies AT, Schaeffeler E, van der Kuip H, et al. Cellular uptake of imatinib into leukemic cells is independent of human organic cation transporter 1 (OCT1). Clin Cancer Res. 2014. February 15;20(4):985–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blanc Mettral J, Faller N, Cruchon S, et al. Imatinib Uptake into Cells is Not Mediated by Organic Cation Transporters OCT1, OCT2, or OCT3, But is Influenced by Extracellular pH. Drug Metab Lett. 2019;13(2):102–110. [DOI] [PubMed] [Google Scholar]
- 131.Arbitrio M, Di Martino MT, Barbieri V, et al. Identification of polymorphic variants associated with erlotinib-related skin toxicity in advanced non-small cell lung cancer patients by DMET microarray analysis. Cancer Chemother Pharmacol. 2016. January;77(1):205–9. [DOI] [PubMed] [Google Scholar]
- 132.Ellawatty WEA, Masuo Y, Fujita KI, et al. Organic Cation Transporter 1 Is Responsible for Hepatocellular Uptake of the Tyrosine Kinase Inhibitor Pazopanib. Drug Metab Dispos. 2018. January;46(1):33–40. [DOI] [PubMed] [Google Scholar]
- 133.Research FaDACfDEa. Clinical Drug Interaction Studies — Cytochrome P450 Enzyme- and Transporter-Mediated Drug Interactions Guidance for Industry FDA Maryland; 2020. [Google Scholar]
- 134.Chu X, Bleasby K, Chan GH, et al. Transporters affecting biochemical test results: Creatinine-drug interactions. Clin Pharmacol Ther. 2016. November;100(5):437–440. [DOI] [PubMed] [Google Scholar]
- 135.Chu X, Bleasby K, Chan GH, et al. The Complexities of Interpreting Reversible Elevated Serum Creatinine Levels in Drug Development: Does a Correlation with Inhibition of Renal Transporters Exist? Drug Metab Dispos. 2016. September;44(9):1498–509. [DOI] [PubMed] [Google Scholar]
- 136.Mathialagan S, Rodrigues AD, Feng B. Evaluation of Renal Transporter Inhibition Using Creatinine as a Substrate In Vitro to Assess the Clinical Risk of Elevated Serum Creatinine. J Pharm Sci. 2017. September;106(9):2535–2541. [DOI] [PubMed] [Google Scholar]
- 137.Omote S, Matsuoka N, Arakawa H, et al. Effect of tyrosine kinase inhibitors on renal handling of creatinine by MATE1. Sci Rep. 2018. June 18;8(1):9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Minematsu T, Giacomini KM. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther. 2011. March;10(3):531–9.* This initial study by Giacomini et al., is the first to demonstrate the inhibition potential of tyrosine kinase inhibitors on organic cation transport function. As TKIs are an emerging class of cancer therapeutics and given the ever increasing number of FDA-approvals, the absence of regulatory guidelines on the precise in vitro methodology to assess transport function warrants additional studies like these to ensure drug safety.
- 139.Abel S, Nichols DJ, Brearley CJ, et al. Effect of cimetidine and ranitidine on pharmacokinetics and pharmacodynamics of a single dose of dofetilide. Br J Clin Pharmacol. 2000. January;49(1):64–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Uddin M, Gibson A, Chen M, et al. , editors. Identification of MATE1 as a high-affinity carrier of dofetlide Clinical Pharmacology & Therapeutics 2018: WILEY 111 RIVER ST, HOBOKEN 07030–5774, NJ USA. [Google Scholar]
- 141.Shukla S, Chen ZS, Ambudkar SV. Tyrosine kinase inhibitors as modulators of ABC transporter-mediated drug resistance. Drug Resist Updat. 2012. February-Apr;15(1–2):70–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Beretta GL, Cassinelli G, Pennati M, et al. Overcoming ABC transporter-mediated multidrug resistance: The dual role of tyrosine kinase inhibitors as multitargeting agents. Eur J Med Chem. 2017. December 15;142:271–289. [DOI] [PubMed] [Google Scholar]
- 143.Sandoval PJ, Zorn KM, Clark AM, et al. Assessment of Substrate-Dependent Ligand Interactions at the Organic Cation Transporter OCT2 Using Six Model Substrates. Mol Pharmacol. 2018. September;94(3):1057–1068.** Cumulative studies by Wright et al., have illustrated the importance of substrate-dependent interactions with OCT2 and MATEs. This particular study uses six different probe substrates to categorize a library of inhibitors whose inhibitory profile is dependent on substrate identity. This body of work describes a reason why the choice of probe substrate is important in order to draw informative and translationally-relevant predictions.
- 144.Belzer M, Morales M, Jagadish B, et al. Substrate-dependent ligand inhibition of the human organic cation transporter OCT2. J Pharmacol Exp Ther. 2013. August;346(2):300–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Martinez-Guerrero LJ, Morales M, Ekins S, et al. Lack of Influence of Substrate on Ligand Interaction with the Human Multidrug and Toxin Extruder, MATE1. Mol Pharmacol. 2016. September;90(3):254–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Severance AC, Sandoval PJ, Wright SH. Correlation between Apparent Substrate Affinity and OCT2 Transport Turnover. J Pharmacol Exp Ther. 2017. September;362(3):405–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hu S, Huang KM, Adams EJ, et al. Recent Developments of Novel Pharmacologic Therapeutics for Prevention of Chemotherapy-Induced Peripheral Neuropathy. Clin Cancer Res. 2019. November 1;25(21):6295–6301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Leblanc AF, Sprowl JA, Alberti P, et al. OATP1B2 deficiency protects against paclitaxel-induced neurotoxicity. J Clin Invest. 2018. February 1;128(2):816–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sobczak K, Bangel-Ruland N, Leier G, et al. Endogenous transport systems in the Xenopus laevis oocyte plasma membrane. Methods. 2010. May;51(1):183–9. [DOI] [PubMed] [Google Scholar]
- 150.de Klerk DJ, Honeywell RJ, Jansen G, et al. Transporter and Lysosomal Mediated (Multi)drug Resistance to Tyrosine Kinase Inhibitors and Potential Strategies to Overcome Resistance. Cancers (Basel). 2018. December 10;10(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Morse BL, Kolur A, Hudson LR, et al. Pharmacokinetics of Organic Cation Transporter 1 (OCT1) Substrates in Oct1/2 Knockout Mice and Species Difference in Hepatic OCT1-Mediated Uptake. Drug Metab Dispos. 2020. February;48(2):93–105. [DOI] [PubMed] [Google Scholar]
- 152.Sauzay C, White-Koning M, Hennebelle I, et al. Inhibition of OCT2, MATE1 and MATE2-K as a possible mechanism of drug interaction between pazopanib and cisplatin. Pharmacol Res. 2016. August;110:89–95. [DOI] [PubMed] [Google Scholar]